Effects of Supplementation with the Standardized Extract of Saffron (affron®) on the Kynurenine Pathway and Melatonin Synthesis in Rats
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. In Vivo Study
2.2.1. Animals
2.2.2. Treatment
2.3. Measurement of Subsantances in the Serum
2.4. RNA Extraction and Quantification
2.5. Quantitative Real-Time PCR
2.6. Statistical Analysis
3. Results
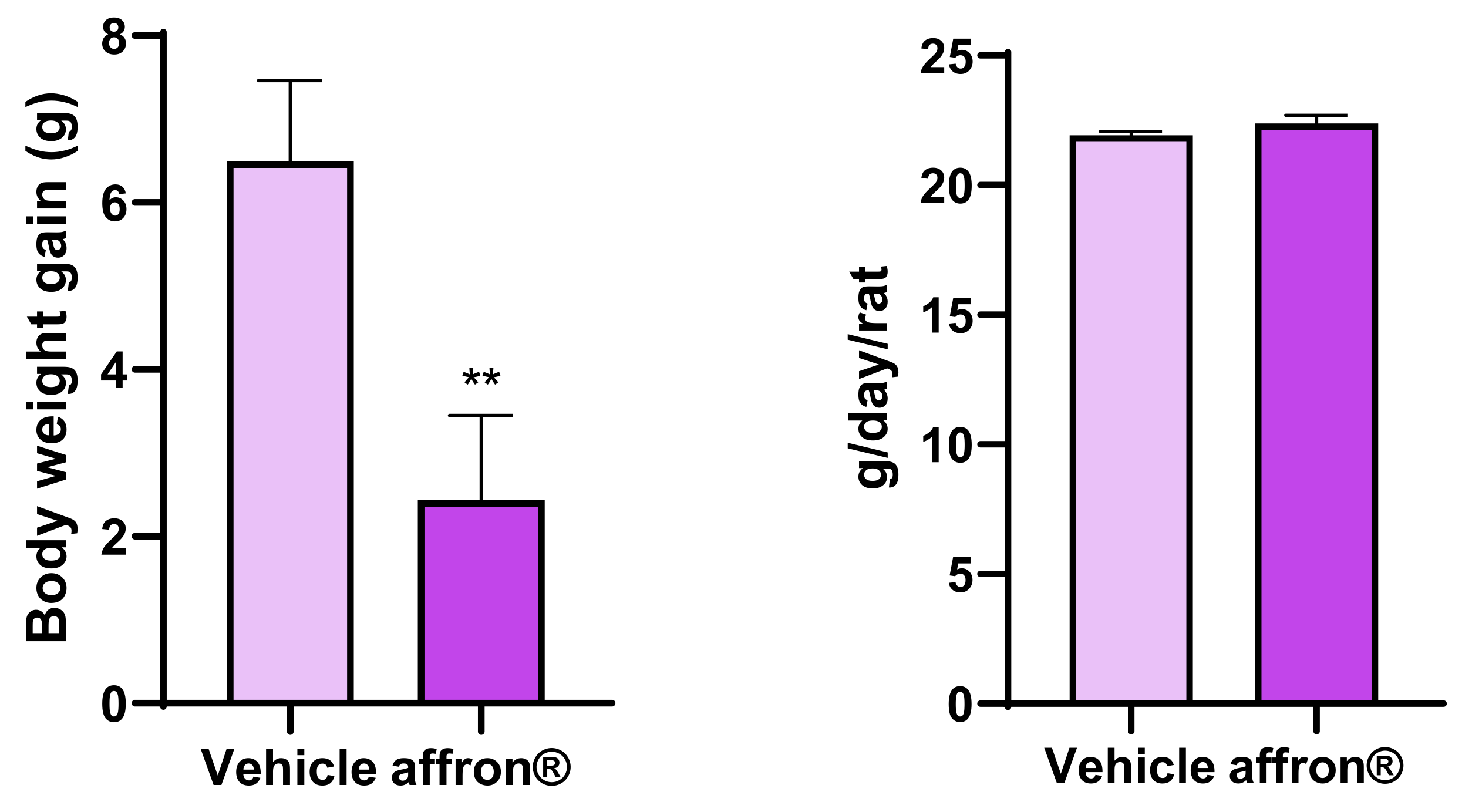
3.1. Body Weight Gain and Food Intake
3.2. Serum Measurements
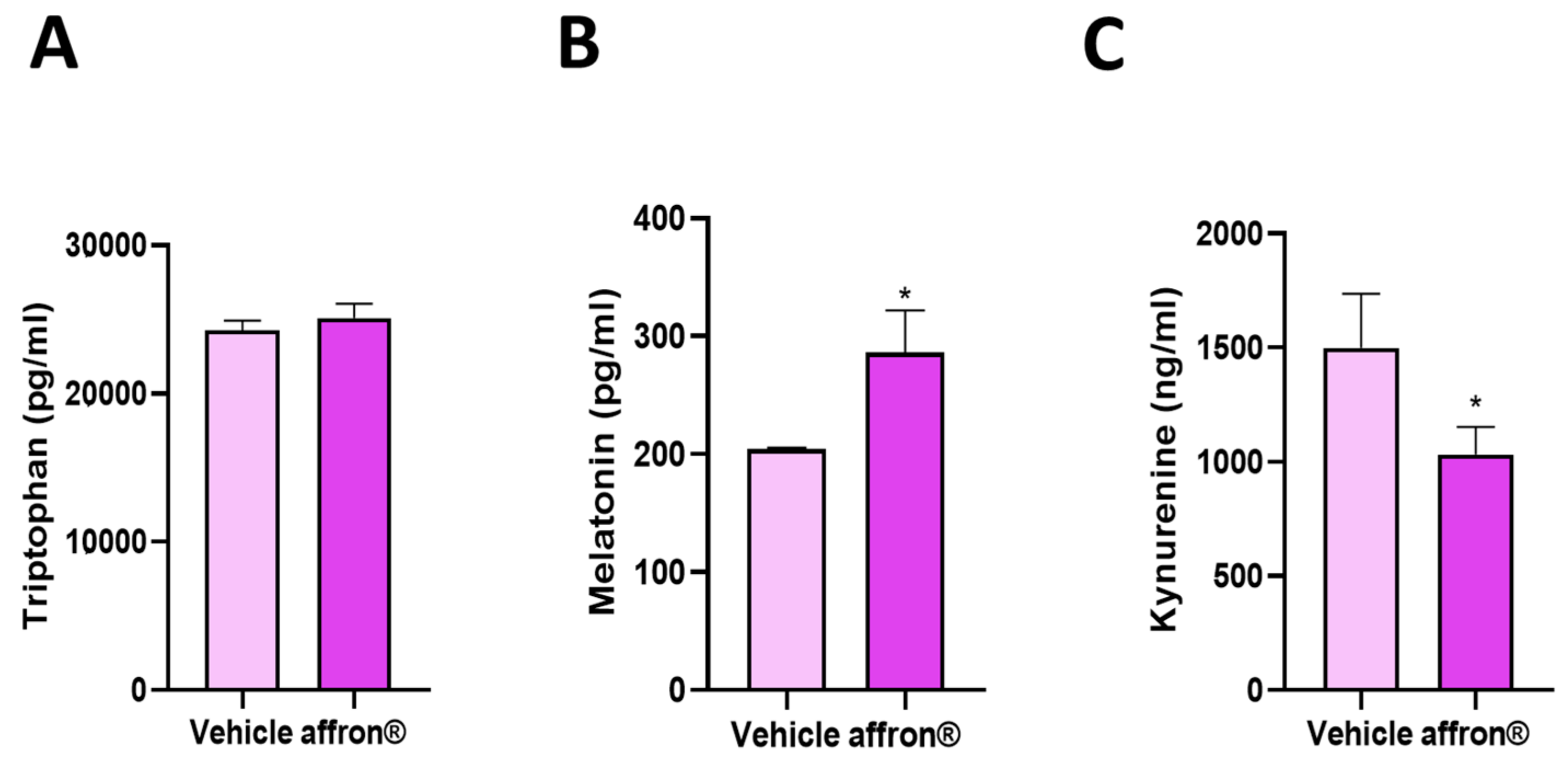
3.3. Effects of affron® Supplementation on the Circulating Levels of Tryptophan, Melatonin, and Kynurenine
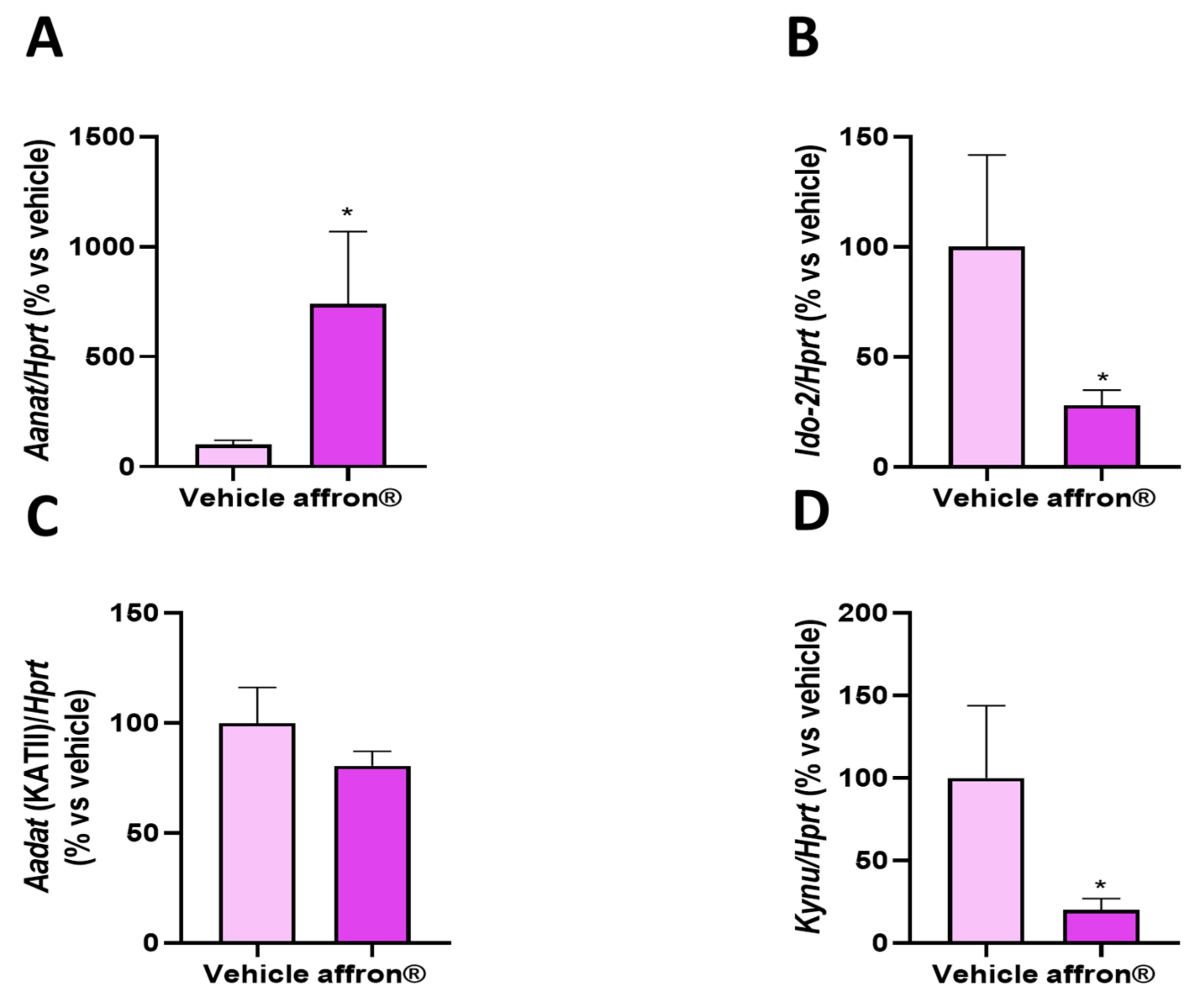
3.4. Gene Expression of Enzymes of the Kynurenine Pathway in the Pineal Gland
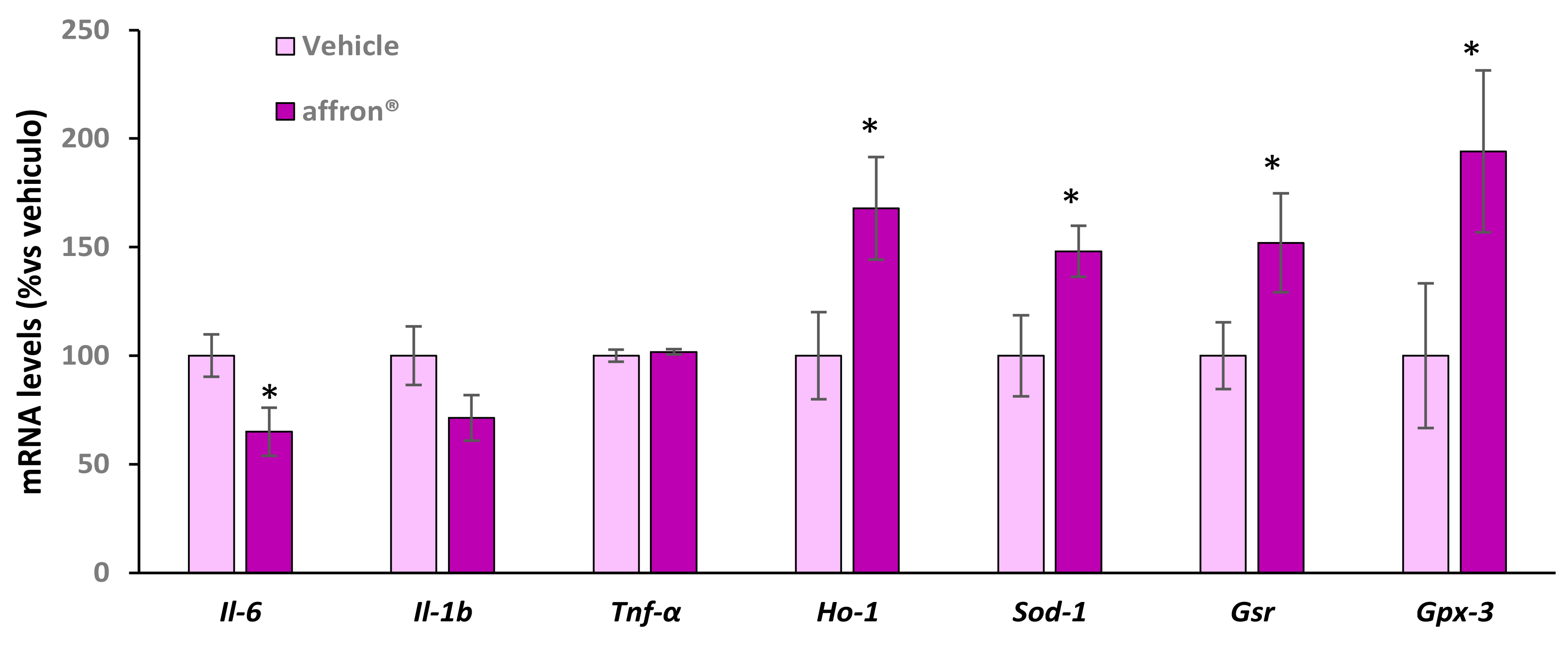
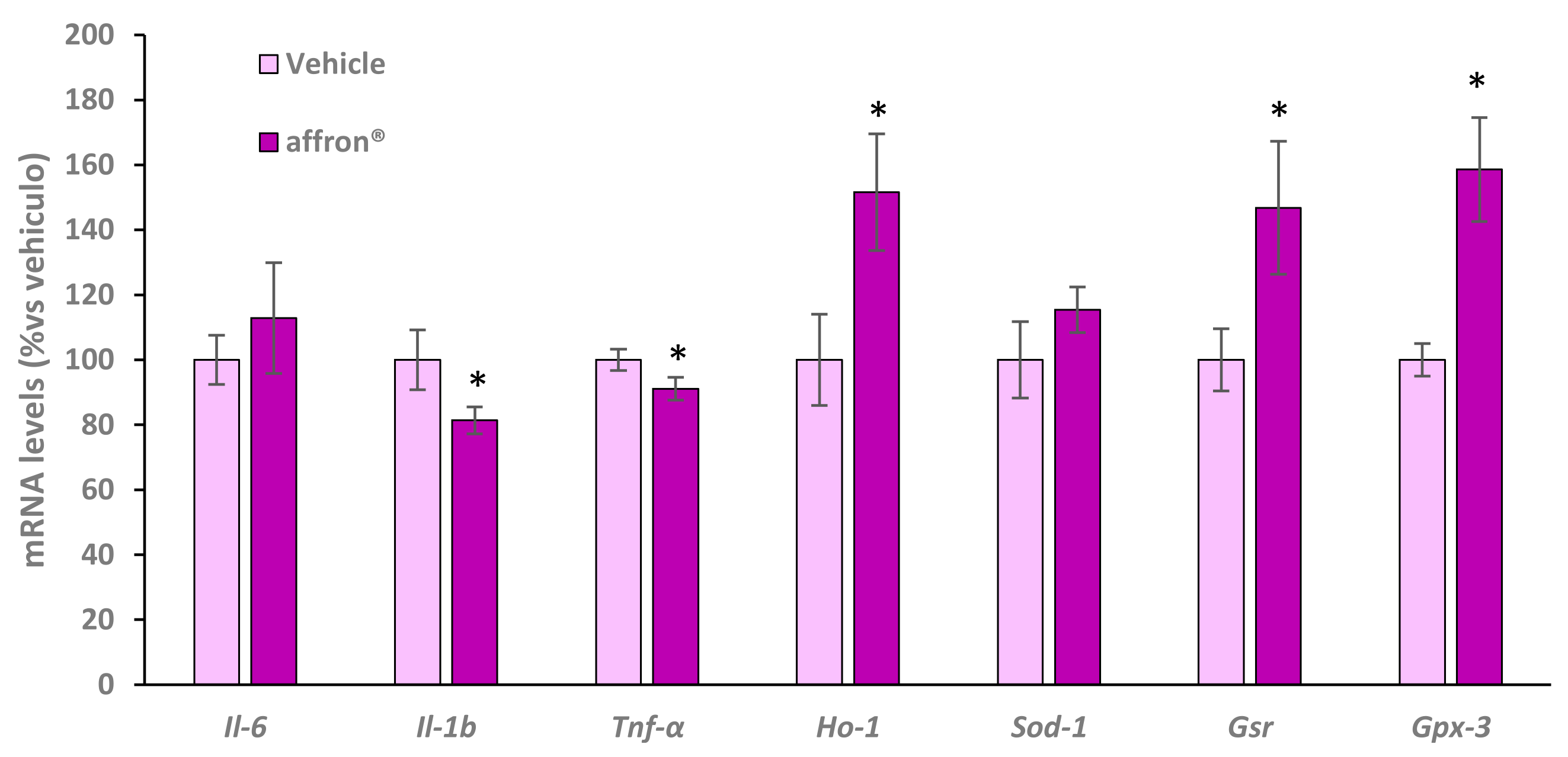
3.5. Gene Expression of Proinflammatory Cytokines and Antioxidant Enzymes in the Pineal Gland
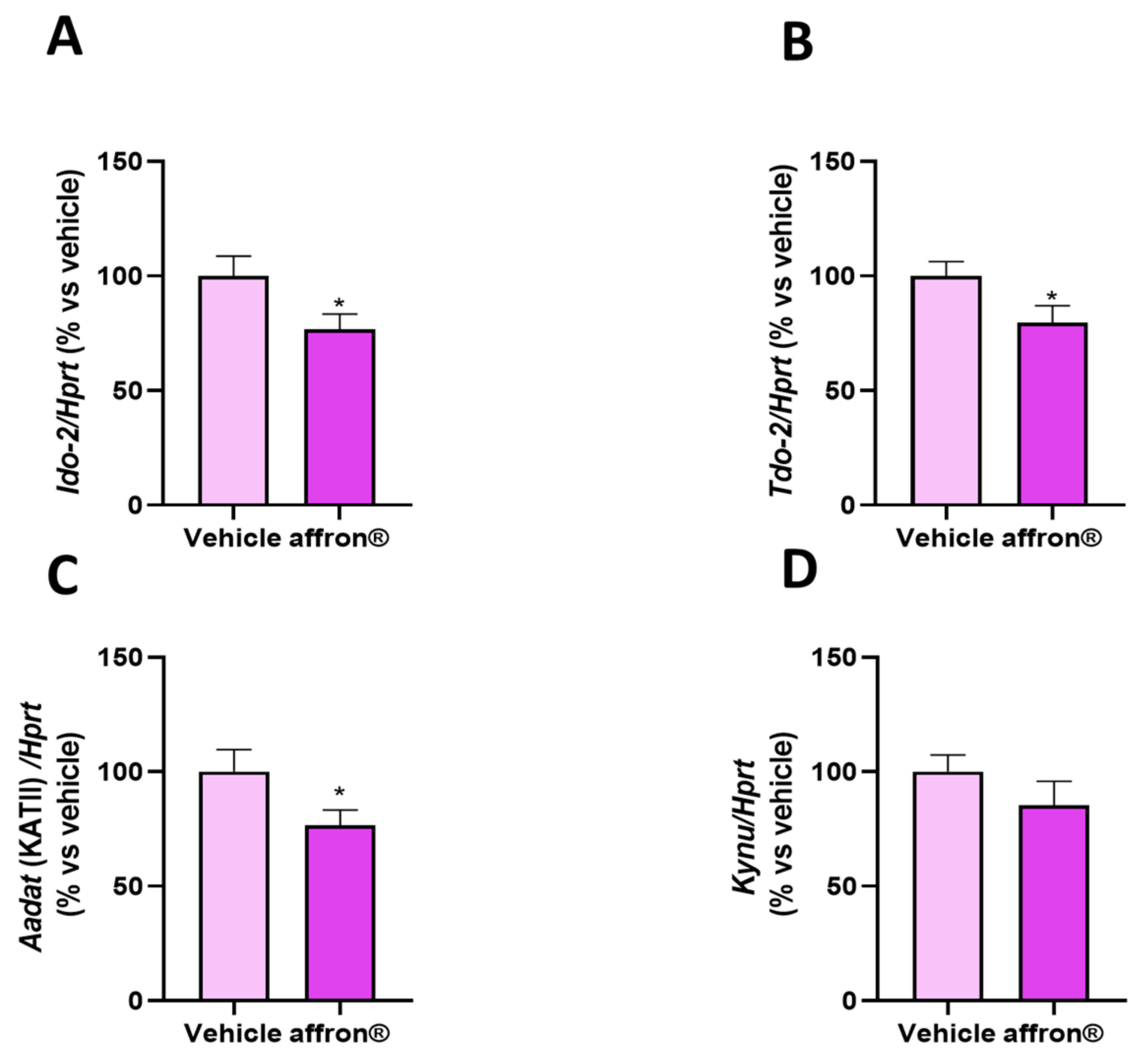
3.6. Gene Expression of Enzymes of the Kynurenine Pathway in the Liver
3.7. Gene Expression of Proinflammatory Cytokines and Antioxidant Enzymes in the Liver
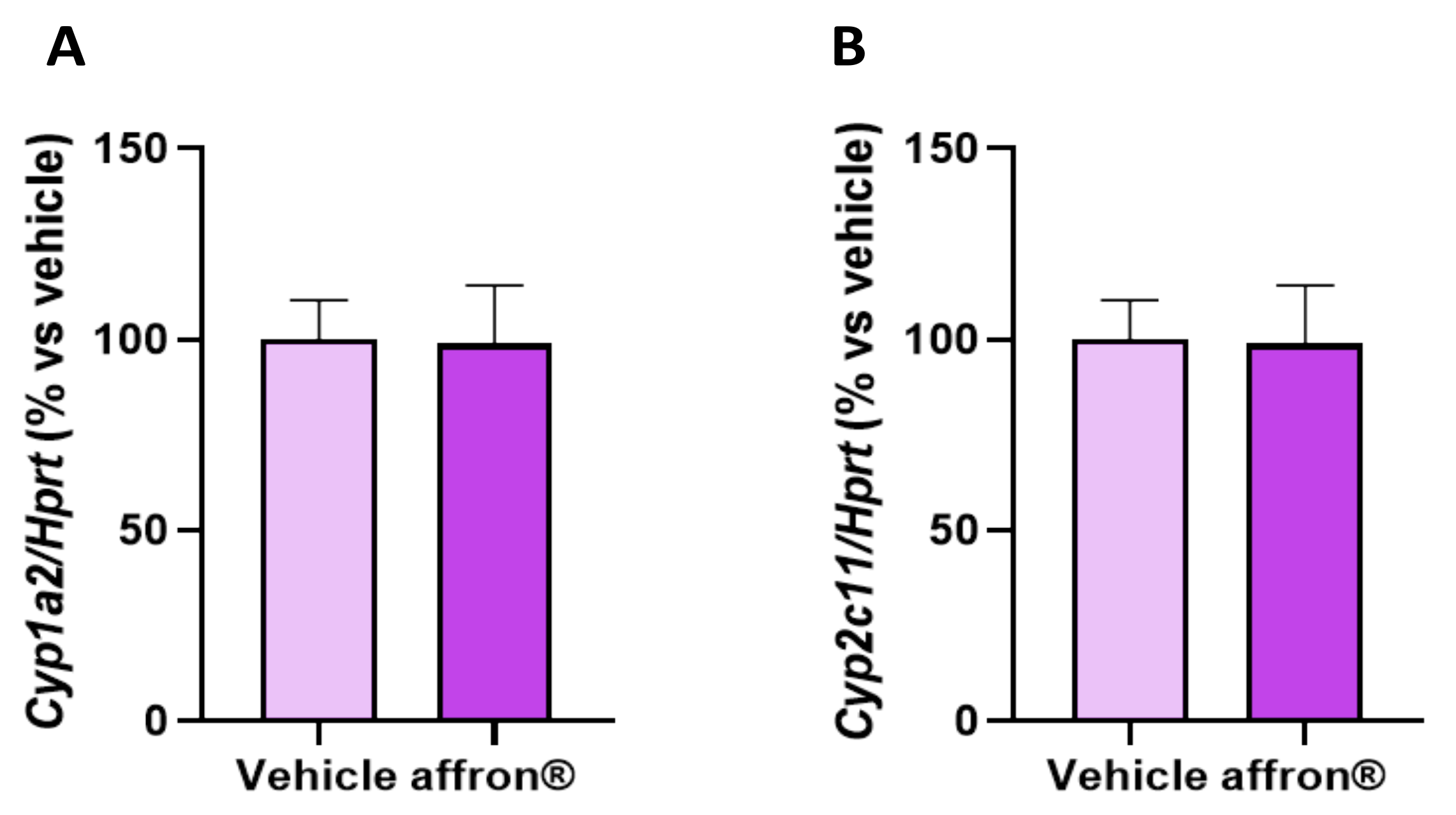
3.8. Gene Expression of Enzymes of the Cytochrome P450 Superfamily in the Liver
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- El Midaoui, A.; Ghzaiel, I.; Vervandier-Fasseur, D.; Ksila, M.; Zarrouk, A.; Nury, T.; Khallouki, F.; El Hessni, A.; Ibrahimi, S.O.; Latruffe, N.; et al. Saffron (Crocus sativus L.): A Source of Nutrients for Health and for the Treatment of Neuropsychiatric and Age-Related Diseases. Nutrients 2022, 14, 597. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.E.; Stasinopoulou, M.; Konstandi, O.A.; Kenoutis, C.; Kakazanis, Z.I.; Rizakou, A.; Kostomitsopoulos, N.; Valsami, G. Crocus sativus L. aqueous extract reduces atherogenesis, increases atherosclerotic plaque stability and improves glucose control in diabetic atherosclerotic animals. Atherosclerosis 2018, 268, 207–214. [Google Scholar] [CrossRef]
- Chichiricco, G.; Ferrante, C.; Menghini, L.; Recinella, L.; Leone, S.; Chiavaroli, A.; Brunetti, L.; Di Simone, S.; Ronci, M.; Piccone, P.; et al. Crocus sativus by-products as sources of bioactive extracts: Pharmacological and toxicological focus on anthers. Food Chem. Toxicol. 2019, 126, 7–14. [Google Scholar] [CrossRef]
- Menghini, L.; Leporini, L.; Vecchiotti, G.; Locatelli, M.; Carradori, S.; Ferrante, C.; Zengin, G.; Recinella, L.; Chiavaroli, A.; Leone, S.; et al. Crocus sativus L. stigmas and byproducts: Qualitative fingerprint, antioxidant potentials and enzyme inhibitory activities. Food Res. Int. 2018, 109, 91–98. [Google Scholar] [CrossRef]
- Almodovar, P.; Briskey, D.; Rao, A.; Prodanov, M.; Inarejos-Garcia, A.M. Bioaccessibility and Pharmacokinetics of a Commercial Saffron (Crocus sativus L.) Extract. Evid. Based Complement. Alternat. Med. 2020, 2020, 1575730. [Google Scholar] [CrossRef]
- Moratalla-Lopez, N.; Bagur, M.J.; Lorenzo, C.; Salinas, M.; Alonso, G.L. Bioactivity and Bioavailability of the Major Metabolites of Crocus sativus L. Flower. Molecules 2019, 24, 2827. [Google Scholar] [CrossRef]
- Mykhailenko, O.; Kovalyov, V.; Goryacha, O.; Ivanauskas, L.; Georgiyants, V. Biologically active compounds and pharmacological activities of species of the genus Crocus: A review. Phytochemistry 2019, 162, 56–89. [Google Scholar] [CrossRef]
- Christodoulou, E.; Kadoglou, N.P.; Kostomitsopoulos, N.; Valsami, G. Saffron: A natural product with potential pharmaceutical applications. J. Pharm. Pharmacol. 2015, 67, 1634–1649. [Google Scholar] [CrossRef]
- Poma, A.; Fontecchio, G.; Carlucci, G.; Chichiricco, G. Anti-inflammatory properties of drugs from saffron crocus. Antiinflamm Antiallergy Agents Med. Chem. 2012, 11, 37–51. [Google Scholar] [CrossRef]
- Mzabri, I.; Addi, M.; Berrichi, A. Traditional and Modern Uses of Saffron (Crocus sativus). Cosmetics 2019, 6, 63. [Google Scholar] [CrossRef]
- Jose Bagur, M.; Alonso Salinas, G.L.; Jimenez-Monreal, A.M.; Chaouqi, S.; Llorens, S.; Martinez-Tome, M.; Alonso, G.L. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2017, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Ali Redha, A.; Snoeck, E.R.; Singh, S.; Simal-Gandara, J.; Ibrahim, S.A.; Jafari, S.M. Anti-Depressant Properties of Crocin Molecules in Saffron. Molecules 2022, 27, 2076. [Google Scholar] [CrossRef] [PubMed]
- Munirah, M.P.; Norhayati, M.N.; Noraini, M. Crocus sativus for Insomnia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public. Health 2022, 19, 11658. [Google Scholar] [CrossRef]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep. 2017, 9, 151–161. [Google Scholar] [CrossRef]
- Buysse, D.J. Insomnia. J. Am. Med. Assoc. 2013, 309, 706–716. [Google Scholar] [CrossRef] [PubMed]
- Meerlo, P.; Sgoifo, A.; Suchecki, D. Restricted and disrupted sleep: Effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep. Med. Rev. 2008, 12, 197–210. [Google Scholar] [CrossRef]
- McCoy, J.G.; Strecker, R.E. The cognitive cost of sleep lost. Neurobiol. Learn. Mem. 2011, 96, 564–582. [Google Scholar] [CrossRef]
- Phillips, B.; Mannino, D.M. Do insomnia complaints cause hypertension or cardiovascular disease? J. Clin. Sleep. Med. 2007, 3, 489–494. [Google Scholar] [CrossRef]
- Cedernaes, J.; Schioth, H.B.; Benedict, C. Determinants of shortened, disrupted, and mistimed sleep and associated metabolic health consequences in healthy humans. Diabetes 2015, 64, 1073–1080. [Google Scholar] [CrossRef]
- Ali, T.; Choe, J.; Awab, A.; Wagener, T.L.; Orr, W.C. Sleep, immunity and inflammation in gastrointestinal disorders. World J. Gastroenterol. 2013, 19, 9231–9239. [Google Scholar] [CrossRef]
- Sigurdardottir, L.G.; Valdimarsdottir, U.A.; Mucci, L.A.; Fall, K.; Rider, J.R.; Schernhammer, E.; Czeisler, C.A.; Launer, L.; Harris, T.; Stampfer, M.J.; et al. Sleep disruption among older men and risk of prostate cancer. Cancer Epidemiol. Biomarkers Prev. 2013, 22, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Klein, T.; Rodenbeck, A.; Feige, B.; Horny, A.; Hummel, R.; Weske, G.; Al-Shajlawi, A.; Voderholzer, U. Nocturnal cortisol and melatonin secretion in primary insomnia. Psychiatry Res. 2002, 113, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Meerlo, P.; Havekes, R.; Steiger, A. Chronically restricted or disrupted sleep as a causal factor in the development of depression. Curr. Top. Behav. Neurosci. 2015, 25, 459–481. [Google Scholar] [CrossRef]
- Yue, J.L.; Chang, X.W.; Zheng, J.W.; Shi, L.; Xiang, Y.J.; Que, J.Y.; Yuan, K.; Deng, J.H.; Teng, T.; Li, Y.Y.; et al. Efficacy and tolerability of pharmacological treatments for insomnia in adults: A systematic review and network meta-analysis. Sleep. Med. Rev. 2023, 68, 101746. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, F.; Li, W.A.; Geng, X.; Li, C.; Meng, X.; Feng, Y.; Liu, W.; Yu, F. A review of sleep disorders and melatonin. Neurol. Res. 2017, 39, 559–565. [Google Scholar] [CrossRef]
- Andersen, L.P.; Gogenur, I.; Rosenberg, J.; Reiter, R.J. The Safety of Melatonin in Humans. Clin. Drug Investig. 2016, 36, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Neurobiology, pathophysiology, and treatment of melatonin deficiency and dysfunction. Sci. World J. 2012, 2012, 640389. [Google Scholar] [CrossRef]
- Cohen, P.A.; Avula, B.; Wang, Y.H.; Katragunta, K.; Khan, I. Quantity of Melatonin and CBD in Melatonin Gummies Sold in the US. J. Am. Med. Assoc. 2023, 329, 1401–1402. [Google Scholar] [CrossRef]
- Lelak, K.; Vohra, V.; Neuman, M.I.; Toce, M.S.; Sethuraman, U. Pediatric Melatonin Ingestions—United States, 2012–2021. MMWR Morb. Mortal. Wkly. Rep. 2022, 71, 725–729. [Google Scholar] [CrossRef]
- Scholtens, R.M.; van Munster, B.C.; van Kempen, M.F.; de Rooij, S.E. Physiological melatonin levels in healthy older people: A systematic review. J. Psychosom. Res. 2016, 86, 20–27. [Google Scholar] [CrossRef]
- Amaral, F.G.D.; Cipolla-Neto, J. A brief review about melatonin, a pineal hormone. Arch. Endocrinol. Metab. 2018, 62, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, S.C.; Clarkson, A.N.; Cakmak, Y.O. Neuromodulation of the Pineal Gland via Electrical Stimulation of Its Sympathetic Innervation Pathway. Front. Neurosci. 2020, 14, 264. [Google Scholar] [CrossRef] [PubMed]
- Muneer, A. Kynurenine Pathway of Tryptophan Metabolism in Neuropsychiatric Disorders: Pathophysiologic and Therapeutic Considerations. Clin. Psychopharmacol. Neurosci. 2020, 18, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10, 1603. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.; Liu, A. What is the tryptophan kynurenine pathway and why is it important to neurotherapeutics? Expert. Rev. Neurother. 2015, 15, 719–721. [Google Scholar] [CrossRef]
- Toth, F.; Cseh, E.K.; Vecsei, L. Natural Molecules and Neuroprotection: Kynurenic Acid, Pantethine and alpha-Lipoic Acid. Int. J. Mol. Sci. 2021, 22, 403. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Pires, A.S.; Tan, V.; Babu Chidambaram, S.; Guillemin, G.J. Effects of Sleep Deprivation on the Tryptophan Metabolism. Int. J. Tryptophan Res. 2020, 13, 1178646920970902. [Google Scholar] [CrossRef]
- Monchaux De Oliveira, C.; De Smedt-Peyrusse, V.; Morael, J.; Vancassel, S.; Capuron, L.; Gaudout, D.; Pourtau, L.; Castanon, N. Prevention of Stress-Induced Depressive-like Behavior by Saffron Extract Is Associated with Modulation of Kynurenine Pathway and Monoamine Neurotransmission. Pharmaceutics 2021, 13, 2155. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. An investigation into an evening intake of a saffron extract (affron(R)) on sleep quality, cortisol, and melatonin concentrations in adults with poor sleep: A randomised, double-blind, placebo-controlled, multi-dose study. Sleep. Med. 2021, 86, 7–18. [Google Scholar] [CrossRef]
- Pachikian, B.D.; Copine, S.; Suchareau, M.; Deldicque, L. Effects of Saffron Extract on Sleep Quality: A Randomized Double-Blind Controlled Clinical Trial. Nutrients 2021, 13, 1473. [Google Scholar] [CrossRef]
- Taherzadeh, Z.; Khaluyan, H.; Iranshahy, M.; Rezaeitalab, F.; Eshaghi Ghalibaf, M.H.; Javadi, B. Evaluation of sedative effects of an intranasal dosage form containing saffron, lettuce seeds and sweet violet in primary chronic insomnia: A randomized, double-dummy, double-blind placebo controlled clinical trial. J. Ethnopharmacol. 2020, 262, 113116. [Google Scholar] [CrossRef] [PubMed]
- Tajaddini, A.; Roshanravan, N.; Mobasseri, M.; Aeinehchi, A.; Sefid-Mooye Azar, P.; Hadi, A.; Ostadrahimi, A. Saffron improves life and sleep quality, glycaemic status, lipid profile and liver function in diabetic patients: A double-blind, placebo-controlled, randomised clinical trial. Int. J. Clin. Pract. 2021, 75, e14334. [Google Scholar] [CrossRef] [PubMed]
- Nishide, A.; Fujita, T.; Nagaregawa, Y.; Shoyama, Y.; Ohnuki, K.; Shimizu, K.; Yamamoto, T.; Watanabe, T.; Ohnuki, K. Sleep enhancement by saffron extract in randomized control trial. Jpn. Pharmacol. Ther. 2018, 46, 1407–1415. [Google Scholar]
- Lopresti, A.L.; Smith, S.J.; Metse, A.P.; Drummond, P.D. Effects of saffron on sleep quality in healthy adults with self-reported poor sleep: A randomized, double-blind, placebo-controlled trial. J. Clin. Sleep. Med. 2020, 16, 937–947. [Google Scholar] [CrossRef]
- Orio, L.; Alen, F.; Ballesta, A.; Martin, R.; Gomez de Heras, R. Antianhedonic and Antidepressant Effects of affron®, a Standardized Saffron (Crocus sativus L.) Extract. Molecules 2020, 25, 3207. [Google Scholar] [CrossRef]
- Jang, H.S.; Jung, J.Y.; Jang, I.S.; Jang, K.H.; Kim, S.H.; Ha, J.H.; Suk, K.; Lee, M.G. L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharmacol. Biochem. Behav. 2012, 101, 217–221. [Google Scholar] [CrossRef]
- Kim, S.; Hong, K.B.; Jo, K.; Suh, H.J. Quercetin-3-O-glucuronide in the Ethanol Extract of Lotus Leaf (Nelumbo nucifera) Enhances Sleep Quantity and Quality in a Rodent Model via a GABAergic Mechanism. Molecules 2021, 26, 3023. [Google Scholar] [CrossRef]
- Gonzalez-Hedstrom, D.; Garcia-Villalon, A.L.; Amor, S.; de la Fuente-Fernandez, M.; Almodovar, P.; Prodanov, M.; Priego, T.; Martin, A.I.; Inarejos-Garcia, A.M.; Granado, M. Olive leaf extract supplementation improves the vascular and metabolic alterations associated with aging in Wistar rats. Sci. Rep. 2021, 11, 8188. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Sánchez, S.; Sánchez, C.L.; Paredes, S.D.; Rodriguez, A.B.; Barriga, C. The effect of tryptophan administration on the circadian rhythms of melatonin in plasma and the pineal gland of rats. J. Appl. Biomed. 2008, 6, 177–186. [Google Scholar] [CrossRef]
- Cipolla-Neto, J.; Amaral, F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018, 39, 990–1028. [Google Scholar] [CrossRef] [PubMed]
- Kell, G.; Rao, A.; Beccaria, G.; Clayton, P.; Inarejos-Garcia, A.M.; Prodanov, M. affron® a novel saffron extract (Crocus sativus L.) improves mood in healthy adults over 4 weeks in a double-blind, parallel, randomized, placebo-controlled clinical trial. Complement. Ther. Med. 2017, 33, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Masaki, M.; Aritake, K.; Tanaka, H.; Shoyama, Y.; Huang, Z.L.; Urade, Y. Crocin promotes non-rapid eye movement sleep in mice. Mol. Nutr. Food Res. 2012, 56, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Umigai, N.; Takeda, R.; Mori, A. Effect of crocetin on quality of sleep: A randomized, double-blind, placebo-controlled, crossover study. Complement. Ther. Med. 2018, 41, 47–51. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Noraei, N.B. Anxiolytic and hypnotic effect of Crocus sativus aqueous extract and its constituents, crocin and safranal, in mice. Phytother. Res. 2009, 23, 768–774. [Google Scholar] [CrossRef]
- Halataei, B.A.; Khosravi, M.; Arbabian, S.; Sahraei, H.; Golmanesh, L.; Zardooz, H.; Jalili, C.; Ghoshooni, H. Saffron (Crocus sativus) aqueous extract and its constituent crocin reduces stress-induced anorexia in mice. Phytother. Res. 2011, 25, 1833–1838. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Sadeghi, O.; Rigi, S.; Tan, S.C.; Shokri, A.; Mousavi, S.M. Effects of saffron (Crocus sativus L.) supplementation on inflammatory biomarkers: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 20–32. [Google Scholar] [CrossRef]
- Moallem, S.A.; Hariri, A.T.; Mahmoudi, M.; Hosseinzadeh, H. Effect of aqueous extract of Crocus sativus L. (saffron) stigma against subacute effect of diazinon on specific biomarkers in rats. Toxicol. Ind. Health 2014, 30, 141–146. [Google Scholar] [CrossRef]
- Li, L.; Zhang, H.; Jin, S.; Liu, C. Effects of crocin on inflammatory activities in human fibroblast-like synoviocytes and collagen-induced arthritis in mice. Immunol. Res. 2018, 66, 406–413. [Google Scholar] [CrossRef]
- Hazman, O.; Ovali, S. Investigation of the anti-inflammatory effects of safranal on high-fat diet and multiple low-dose streptozotocin induced type 2 diabetes rat model. Inflammation 2015, 38, 1012–1019. [Google Scholar] [CrossRef]
- Shaheen, M.J.; Bekdash, A.M.; Itani, H.A.; Borjac, J.M. Saffron extract attenuates neuroinflammation in rmTBI mouse model by suppressing NLRP3 inflammasome activation via SIRT1. PLoS ONE 2021, 16, e0257211. [Google Scholar] [CrossRef]
- Hamdi, E.; Muniz-Gonzalez, A.B.; Hidouri, S.; Bermejo, A.M.; Sakly, M.; Venero, C.; Amara, S. Prevention of neurotoxicity and cognitive impairment induced by zinc nanoparticles by oral administration of saffron extract. J. Anim. Physiol. Anim. Nutr. 2023; ahead of print. [Google Scholar] [CrossRef]
- Samarghandian, S.; Azimi-Nezhad, M.; Farkhondeh, T. Immunomodulatory and antioxidant effects of saffron aqueous extract (Crocus sativus L.) on streptozotocin-induced diabetes in rats. Indian. Heart J. 2017, 69, 151–159. [Google Scholar] [CrossRef]
- Elseady, W.S.; Keshk, W.A.; Negm, W.A.; Elkhalawany, W.; Elhanafy, H.; Ibrahim, M.A.A.; Radwan, D.A. Saffron extract attenuates Sofosbuvir-induced retinal neurodegeneration in albino rat. Anat. Rec. 2023, 306, 422–436. [Google Scholar] [CrossRef]
- Mahmoudzadeh, L.; Najafi, H.; Ashtiyani, S.C.; Yarijani, Z.M. Anti-inflammatory and protective effects of saffron extract in ischaemia/reperfusion-induced acute kidney injury. Nephrology 2017, 22, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Faridi, S.; Delirezh, N.; Abtahi Froushani, S.M. Beneficial Effects of Hydroalcoholic Extract of Saffron in Alleviating Experimental Autoimmune Diabetes in C57bl/6 Mice. Iran. J. Allergy Asthma Immunol. 2019, 18, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Omidi, A.; Riahinia, N.; Montazer Torbati, M.B.; Behdani, M.A. Hepatoprotective effect of Crocus sativus (saffron) petals extract against acetaminophen toxicity in male Wistar rats. Avicenna J. Phytomed 2014, 4, 330–336. [Google Scholar] [PubMed]
- Rezaee-Khorasany, A.; Razavi, B.M.; Taghiabadi, E.; Tabatabaei Yazdi, A.; Hosseinzadeh, H. Effect of saffron (stigma of Crocus sativus L.) aqueous extract on ethanol toxicity in rats: A biochemical, histopathological and molecular study. J. Ethnopharmacol. 2019, 237, 286–299. [Google Scholar] [CrossRef]
- Boozari, M.; Hosseinzadeh, H. Crocin molecular signaling pathways at a glance: A comprehensive review. Phytother. Res. 2022, 36, 3859–3884. [Google Scholar] [CrossRef]
- Troubat, R.; Barone, P.; Leman, S.; Desmidt, T.; Cressant, A.; Atanasova, B.; Brizard, B.; El Hage, W.; Surget, A.; Belzung, C.; et al. Neuroinflammation and depression: A review. Eur. J. Neurosci. 2021, 53, 151–171. [Google Scholar] [CrossRef]
- Garbarino, S.; Lanteri, P.; Bragazzi, N.L.; Magnavita, N.; Scoditti, E. Role of sleep deprivation in immune-related disease risk and outcomes. Commun. Biol. 2021, 4, 1304. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, Q.; Wang, H.; Xiu, W.; Xu, P.; Deng, Y.; Huang, W.; Wang, D.O. Crocetin antagonizes parthanatos in ischemic stroke via inhibiting NOX2 and preserving mitochondrial hexokinase-I. Cell Death Dis. 2023, 14, 50. [Google Scholar] [CrossRef]
- Khoshandam, A.; Razavi, B.M.; Hosseinzadeh, H. Interaction of saffron and its constituents with Nrf2 signaling pathway: A review. Iran. J. Basic. Med. Sci. 2022, 25, 789–798. [Google Scholar]
- Akbari, G.; Ali Mard, S.; Veisi, A. A comprehensive review on regulatory effects of crocin on ischemia/reperfusion injury in multiple organs. Biomed. Pharmacother. 2018, 99, 664–670. [Google Scholar] [CrossRef]
- Asdaq, S.M.; Inamdar, M.N. Potential of Crocus sativus (saffron) and its constituent, crocin, as hypolipidemic and antioxidant in rats. Appl. Biochem. Biotechnol. 2010, 162, 358–372. [Google Scholar] [CrossRef]
- Tawfik, S.S.; Elkady, A.A.; El Khouly, W.A. Crocin mitigates gamma-rays-induced hepatic toxicity in rats. Environ. Sci. Pollut. Res. Int. 2019, 26, 15414–15419. [Google Scholar] [CrossRef]
- Alayunt, O.N.; Aksoy, L.; Karafakioglu, Y.S.; Sevimli, S. Assessment of Anti-inflammatory and Antioxidant Properties of Safranal on CCI4-Induced Oxidative Stress and Inflammation in Rats. An. Acad. Bras. Cienc. 2019, 91, e20181235. [Google Scholar] [CrossRef]
- Farahmand, S.K.; Samini, F.; Samini, M.; Samarghandian, S. Safranal ameliorates antioxidant enzymes and suppresses lipid peroxidation and nitric oxide formation in aged male rat liver. Biogerontology 2013, 14, 63–71. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, Y.; Zheng, B.; Chu, L.; Ma, D.; Wang, H.; Chu, X.; Zhang, J. Protective Effects of Crocetin on Arsenic Trioxide-Induced Hepatic Injury: Involvement of Suppression in Oxidative Stress and Inflammation Through Activation of Nrf2 Signaling Pathway in Rats. Drug Des. Devel. Ther. 2020, 14, 1921–1931. [Google Scholar] [CrossRef]
- Sreekanth, G.P.; Chuncharunee, A.; Yenchitsomanus, P.T.; Limjindaporn, T. Crocetin Improves Dengue Virus-Induced Liver Injury. Viruses 2020, 12, 825. [Google Scholar] [CrossRef]
- Claria, J.; Moreau, R.; Fenaille, F.; Amoros, A.; Junot, C.; Gronbaek, H.; Coenraad, M.J.; Pruvost, A.; Ghettas, A.; Chu-Van, E.; et al. Orchestration of Tryptophan-Kynurenine Pathway, Acute Decompensation, and Acute-on-Chronic Liver Failure in Cirrhosis. Hepatology 2019, 69, 1686–1701. [Google Scholar] [CrossRef]
- Atrooz, F.; Salim, S. Sleep deprivation, oxidative stress and inflammation. Adv. Protein Chem. Struct. Biol. 2020, 119, 309–336. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Andolina, G.; Cannata, A.; Costanzo, M.G.; Rizzo, V.; Curro, M.; Ientile, R.; Caccamo, D. Is Melatonin the Cornucopia of the 21st Century? Antioxidants 2020, 9, 1088. [Google Scholar] [CrossRef]
- Rahmani, J.; Manzari, N.; Thompson, J.; Clark, C.C.T.; Villanueva, G.; Varkaneh, H.K.; Mirmiran, P. The effect of saffron on weight and lipid profile: A systematic review, meta-analysis, and dose-response of randomized clinical trials. Phytother. Res. 2019, 33, 2244–2255. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Koushyar, H.; Byrami, G.; Boskabady, M.H. The Extract of Crocus sativus and Its Constituent Safranal, Affect Serum Levels of Endothelin and Total Protein in Sensitized Guinea Pigs. Iran. J. Basic Med. Sci. 2013, 16, 1022–1026. [Google Scholar]
- Hooshmand-Moghadam, B.; Eskandari, M.; Shabkhiz, F.; Mojtahedi, S.; Mahmoudi, N. Saffron (Crocus sativus L.) in combination with resistance training reduced blood pressure in the elderly hypertensive men: A randomized controlled trial. Br. J. Clin. Pharmacol. 2021, 87, 3255–3267. [Google Scholar] [CrossRef]
- Zamani, M.; Zarei, M.; Nikbaf-Shandiz, M.; Gholami, F.; Hosseini, A.M.; Nadery, M.; Shiraseb, F.; Asbaghi, O. The effects of saffron supplementation on cardiovascular risk factors in adults: A systematic review and dose-response meta-analysis. Front. Nutr. 2022, 9, 1055517. [Google Scholar] [CrossRef]
- Moshfegh, F.; Balanejad, S.Z.; Shahrokhabady, K.; Attaranzadeh, A. Crocus sativus (saffron) petals extract and its active ingredient, anthocyanin improves ovarian dysfunction, regulation of inflammatory genes and antioxidant factors in testosterone-induced PCOS mice. J. Ethnopharmacol. 2022, 282, 114594. [Google Scholar] [CrossRef]
- Hu, Q.; Jin, J.; Zhou, H.; Yu, D.; Qian, W.; Zhong, Y.; Zhang, J.; Tang, C.; Liu, P.; Zhou, Y.; et al. Crocetin attenuates DHT-induced polycystic ovary syndrome in mice via revising kisspeptin neurons. Biomed. Pharmacother. 2018, 107, 1363–1369. [Google Scholar] [CrossRef]
- Quarto, G.; Cola, A.; Perdona, S. Efficacy of a formulation containing Serenoa repens, Crocus sativus and Pinus massoniana extracts in men with concomitant LUTS and erectile dysfunction. Minerva Urol. Nefrol. 2017, 69, 300–306. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Carradori, S.; Macchione, N.; Zengin, G.; Leporini, L.; Leone, S.; Martinotti, S.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Biol. Regul. Homeost. Agents 2017, 31, 531–541. [Google Scholar]
- Dovrtelova, G.; Noskova, K.; Jurica, J.; Turjap, M.; Zendulka, O. Can bioactive compounds of Crocus sativus L. influence the metabolic activity of selected CYP enzymes in the rat? Physiol. Res. 2015, 64, S453–S458. [Google Scholar] [CrossRef]
- Begas, E.; Bounitsi, M.; Kilindris, T.; Kouvaras, E.; Makaritsis, K.; Kouretas, D.; Asprodini, E.K. Effects of short-term saffron (Crocus sativus L.) intake on the in vivo activities of xenobiotic metabolizing enzymes in healthy volunteers. Food Chem. Toxicol. 2019, 130, 32–43. [Google Scholar] [CrossRef]
| Genes | Reference |
|---|---|
| Aadat | Rn00567882_m1 |
| Aanat | Rn00664873_g1 |
| Cyp1a2 | Rn00561082_m1 |
| Cyp2c11 | Rn01502203_m1 |
| Gpx-3 | Rn00574703_m1 |
| Gsr | Rn01482159_m1 |
| Ho-1 | Rn00561387_m1 |
| Hprt-1 | Rn01527840_m1 |
| Il-1β | Rn00580432_m1 |
| Il-6 | Rn01489669_m1 |
| Tnf-α | Rn01525859_g1 |
| Kynu | Rn01449532_m1 |
| Ido-2 | Rn01482543_m1 |
| Sod-1 | Rn00566938_m1 |
| Tod-2 | Rn00574499_m1 |
| Vehicle | affron® | |
|---|---|---|
| Triglycerides (mg/dL) | 100 ± 4.3 | 121 ± 19.3 |
| Total cholesterol (mg/dL) | 87.7 ± 4.6 | 89.6± 5.8 |
| LDL-c (mg/dL) | 23.2 ± 1.8 | 19.2 ± 1.1 * |
| HDL-c (mg/dL) | 25.3 ± 6.3 | 38.5 ± 3.3 * |
| Insulin (ng/mL) | 2.7 ± 0.5 | 2.6 ± 0.5 |
| ET-1 (pg/mL) | 1.5 ± 0.1 | 1.3 ± 0.07 * |
| NA (ng/mL) | 1.06 ± 0.1 | 0.92 ± 0.1 |
| IL-6 (ng/mL) | 3.2 ± 0.4 | 3.3 ± 0.2 |
| Corticosterone (pg/mL) | 258 ± 120 | 300 ± 102 |
| Testosterone (ng/mL) | 4.5 ± 2.1 | 15.2 ± 4.8 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Fuente Muñoz, M.; Román-Carmena, M.; Amor, S.; García-Villalón, Á.L.; Espinel, A.E.; González-Hedström, D.; Granado García, M. Effects of Supplementation with the Standardized Extract of Saffron (affron®) on the Kynurenine Pathway and Melatonin Synthesis in Rats. Antioxidants 2023, 12, 1619. https://doi.org/10.3390/antiox12081619
De la Fuente Muñoz M, Román-Carmena M, Amor S, García-Villalón ÁL, Espinel AE, González-Hedström D, Granado García M. Effects of Supplementation with the Standardized Extract of Saffron (affron®) on the Kynurenine Pathway and Melatonin Synthesis in Rats. Antioxidants. 2023; 12(8):1619. https://doi.org/10.3390/antiox12081619
Chicago/Turabian StyleDe la Fuente Muñoz, Mario, Marta Román-Carmena, Sara Amor, Ángel Luís García-Villalón, Alberto E. Espinel, Daniel González-Hedström, and Miriam Granado García. 2023. "Effects of Supplementation with the Standardized Extract of Saffron (affron®) on the Kynurenine Pathway and Melatonin Synthesis in Rats" Antioxidants 12, no. 8: 1619. https://doi.org/10.3390/antiox12081619
APA StyleDe la Fuente Muñoz, M., Román-Carmena, M., Amor, S., García-Villalón, Á. L., Espinel, A. E., González-Hedström, D., & Granado García, M. (2023). Effects of Supplementation with the Standardized Extract of Saffron (affron®) on the Kynurenine Pathway and Melatonin Synthesis in Rats. Antioxidants, 12(8), 1619. https://doi.org/10.3390/antiox12081619







