Myeloperoxidase Alters Lung Cancer Cell Function to Benefit Their Survival
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell line and Cell Culture
2.2. Cell Treatment
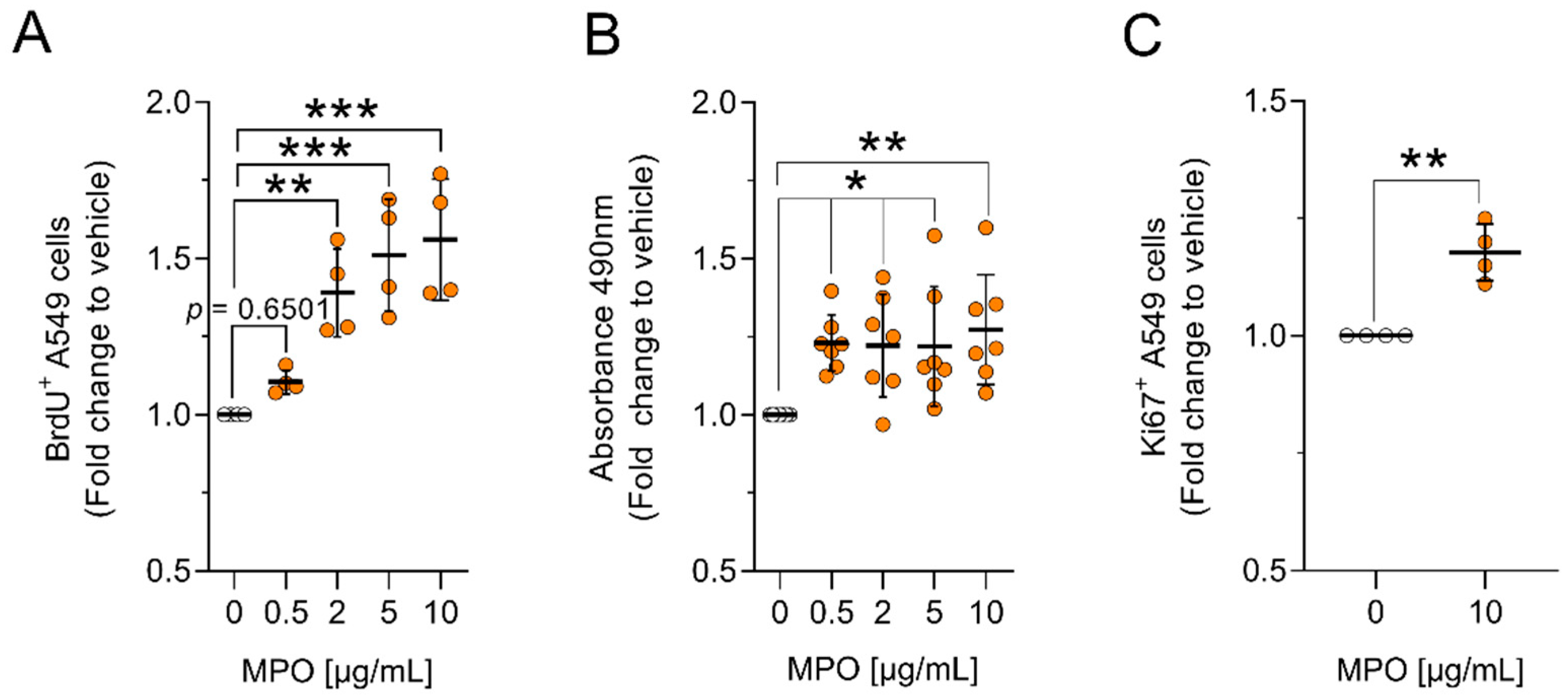
2.3. Bromodeoxyuridine (BrdU) Cell Proliferation Assay
2.4. Ki67 Cell Proliferation Assay
2.5. MTS Cell Proliferation Assay
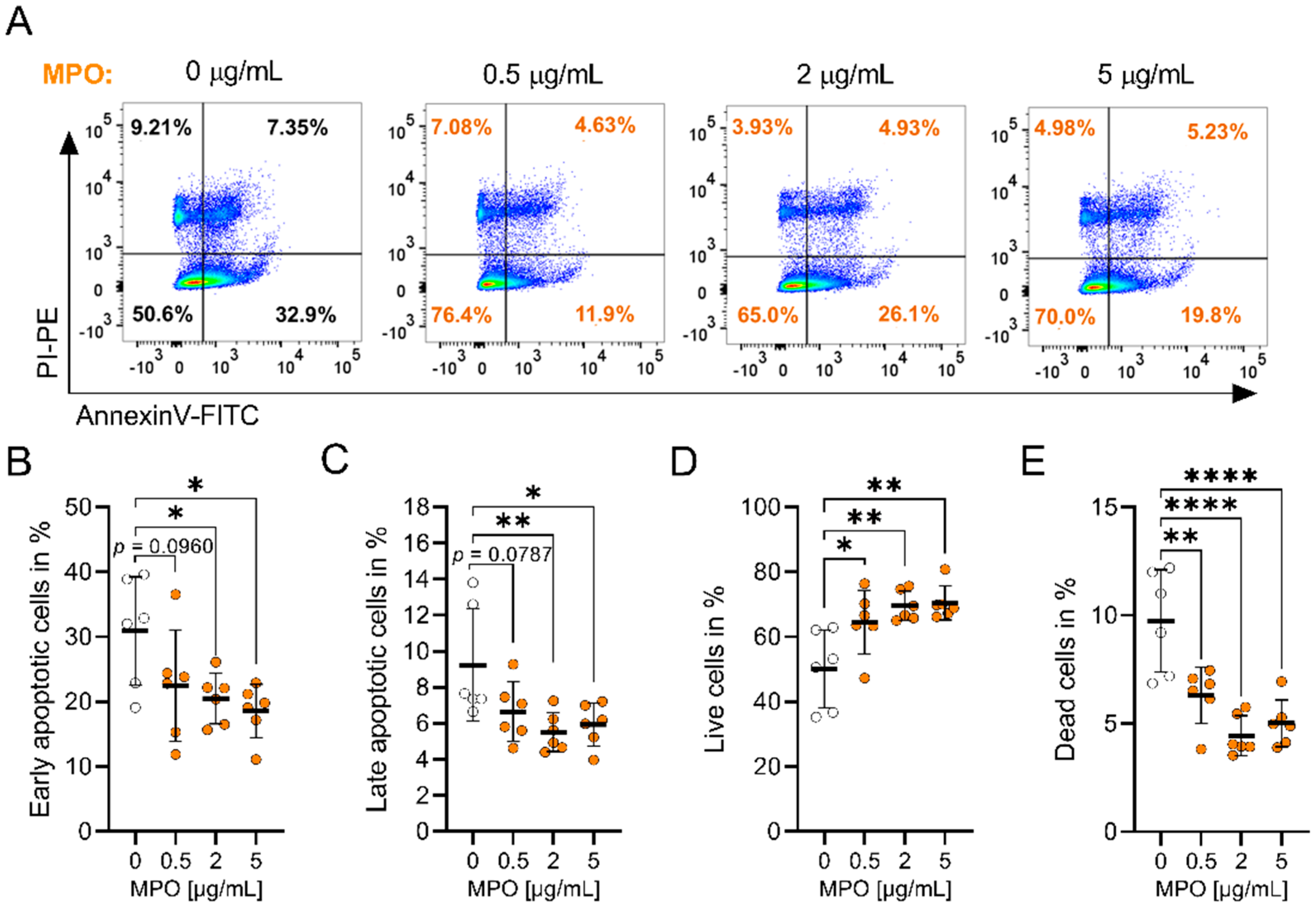
2.6. Annexin V and Propidium Iodide (PI) Apoptosis Assay
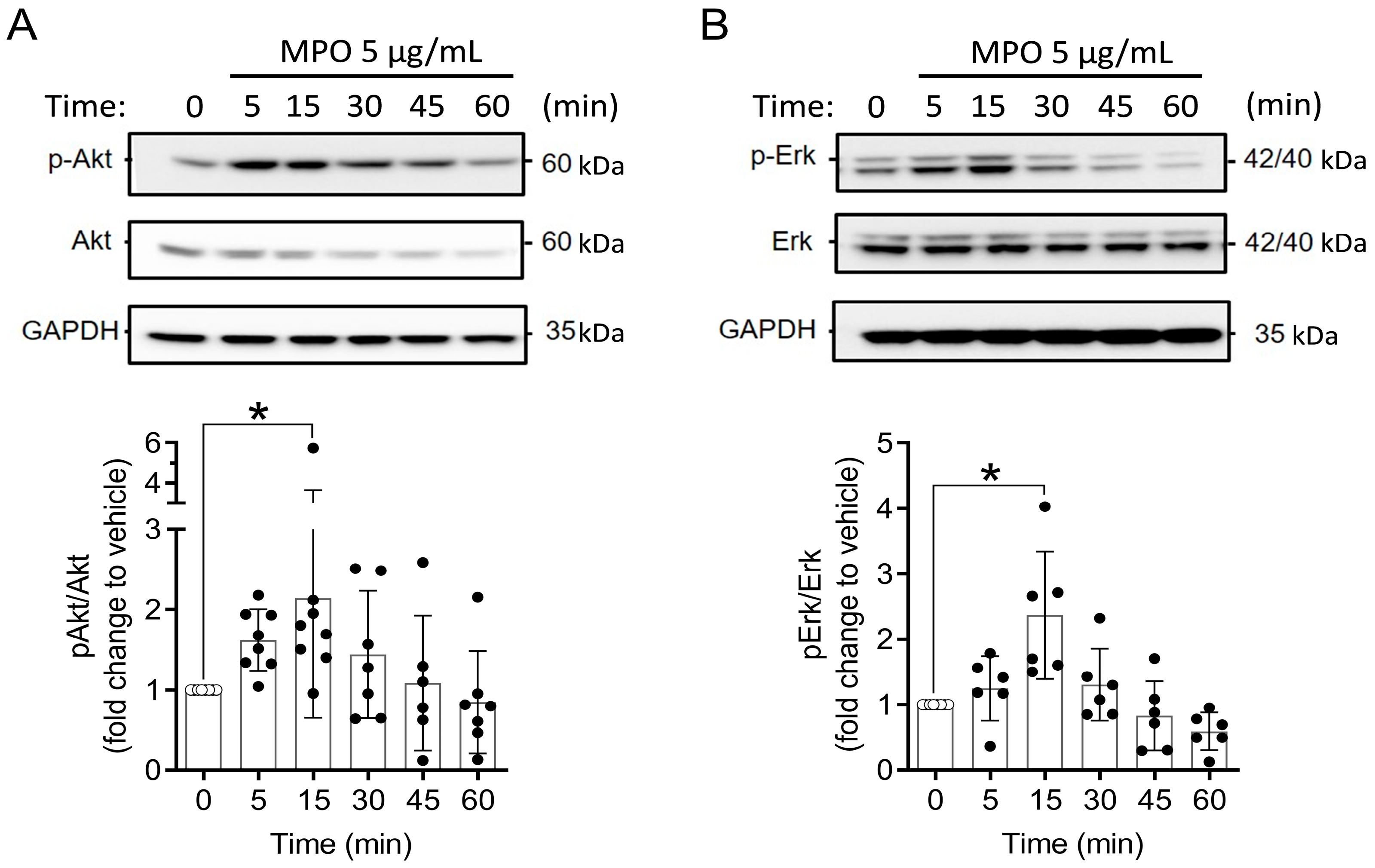
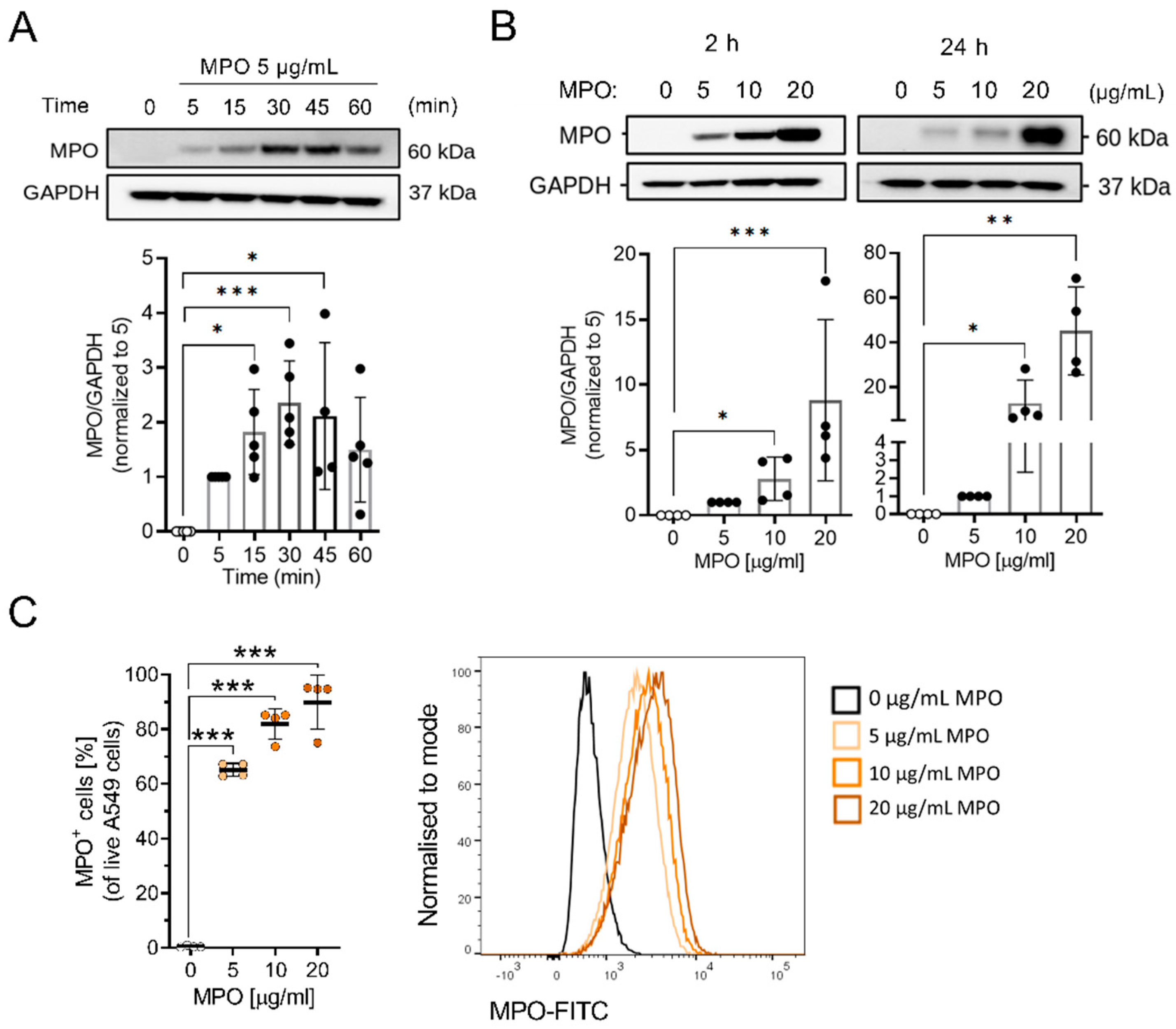
2.7. Western Blot Analysis
2.8. Immunofluorescence Staining of MPO
2.9. Intracellular MPO Flow Cytometry Staining
2.10. Basal Production of Hydrogen Peroxide (H2O2) by A549 Cells
2.11. Measurement of HOCl Production Using the “R19-S” Sensor
2.12. Murine Tumour Model and ABAH Application
2.13. Statistical Analyses
3. Results
3.1. MPO Alters A549 Proliferation and Apoptosis In Vitro
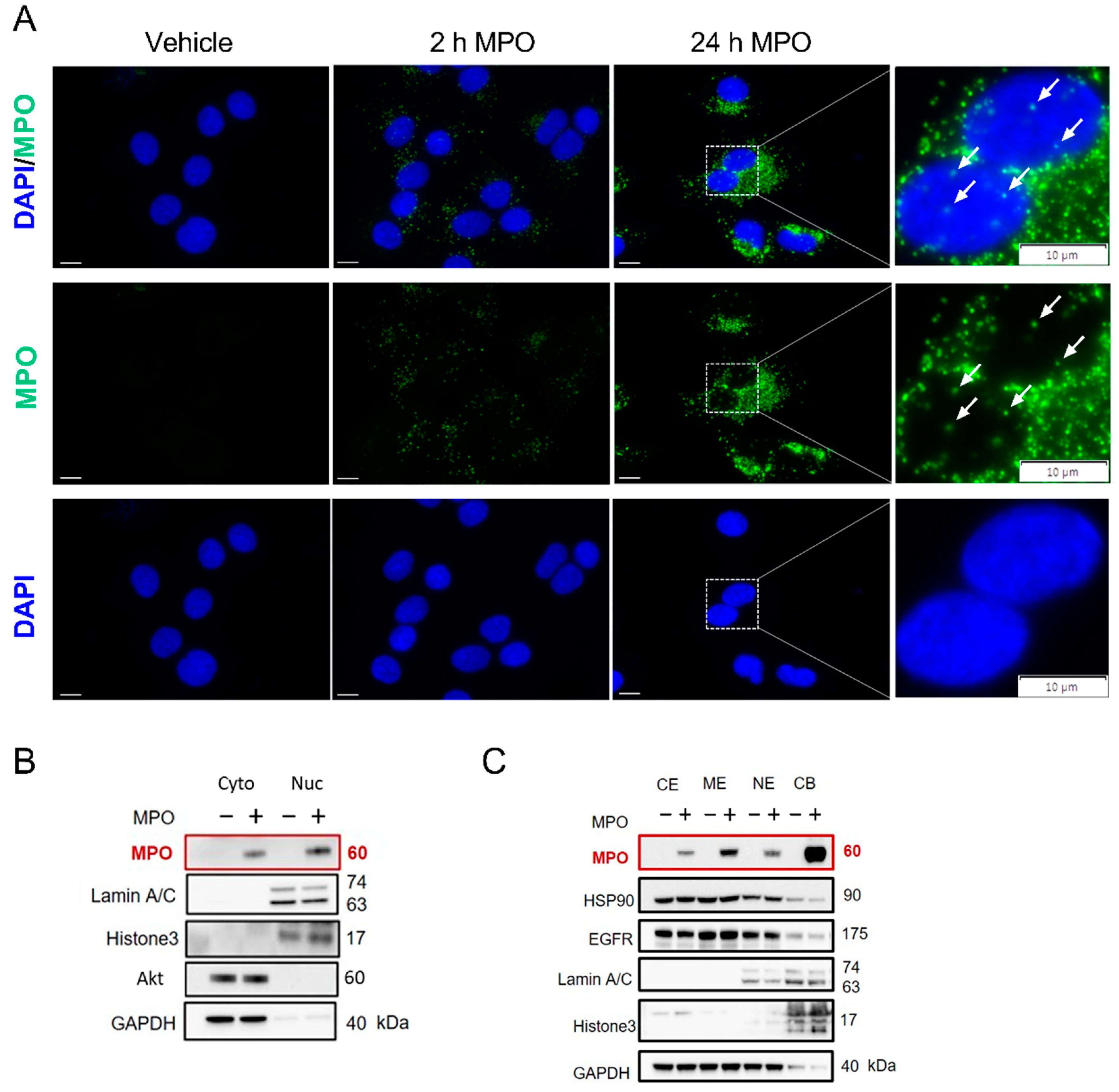
3.2. MPO Binds to and Is Taken Up by A549 Cells
3.3. Blocking MPO Internalisation with Heparin Abolishes MPO Effects on Apoptosis in A549 Cells
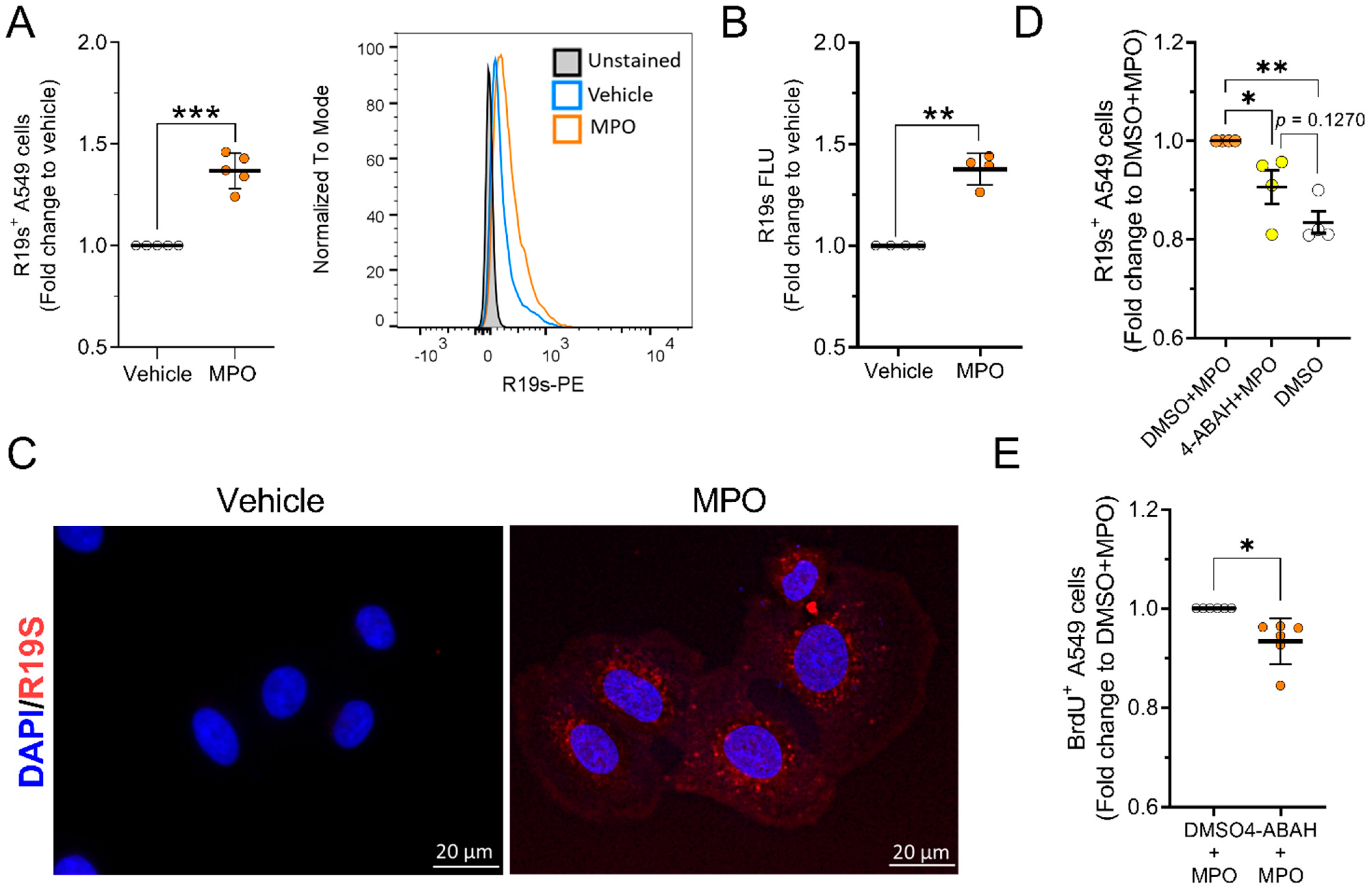
3.4. MPO Is Active after Internalising A549 Cells
3.5. 4-ABAH Reduces Tumour Growth In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Callaghan, D.S.; O’Donnell, D.; O’Connell, F.; O’Byrne, K.J. The Role of Inflammation in the Pathogenesis of Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2010, 5, 2024–2036. [Google Scholar] [CrossRef]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-Small Cell Lung Cancer: Epidemiology, Risk Factors, Treatment, and Survivorship. In Mayo Clinic Proceedings; Elsevier Ltd.: Amsterdam, The Netherlands, 2008; Volume 83, pp. 584–594. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Bade, B.C.; Dela Cruz, C.S. Lung Cancer 2020: Epidemiology, Etiology, and Prevention. Clin. Chest. Med. 2020, 41, 1–24. [Google Scholar] [CrossRef]
- Hedrick, C.C.; Malanchi, I. Neutrophils in Cancer: Heterogeneous and Multifaceted. Nat. Rev. Immunol. 2022, 22, 173–187. [Google Scholar] [CrossRef]
- Kargl, J.; Busch, S.E.; Yang, G.H.Y.; Kim, K.; Hanke, M.L.; Metz, H.E.; Hubbard, J.J.; Lee, S.M.; Madtes, D.K.; Mcintosh, M.W.; et al. Neutrophils Dominate the Immune Cell Composition in Non-Small Cell Lung Cancer. Nat. Commun. 2017, 8, 14381. [Google Scholar] [CrossRef]
- Cools-Lartigue, J.; Spicer, J.; McDonald, B.; Gowing, S.; Chow, S.; Giannias, B.; Bourdeau, F.; Kubes, P.; Ferri, L. Neutrophil Extracellular Traps Sequester Circulating Tumor Cells and Promote Metastasis. J. Clin. Investig. 2013, 123, 3446–3458. [Google Scholar] [CrossRef]
- Szczerba, B.M.; Castro-Giner, F.; Vetter, M.; Krol, I.; Gkountela, S.; Landin, J.; Scheidmann, M.C.; Donato, C.; Scherrer, R.; Singer, J.; et al. Neutrophils Escort Circulating Tumour Cells to Enable Cell Cycle Progression. Nature 2019, 566, 553–557. [Google Scholar] [CrossRef]
- Eruslanov, E.B.; Bhojnagarwala, P.S.; Quatromoni, J.G.; Stephen, T.L.; Ranganathan, A.; Deshpande, C.; Akimova, T.; Vachani, A.; Litzky, L.; Hancock, W.W.; et al. Tumor-Associated Neutrophils Stimulate T Cell Responses in Early-Stage Human Lung Cancer. J. Clin. Investig. 2014, 124, 5466–5480. [Google Scholar] [CrossRef] [PubMed]
- Templeton, A.J.; McNamara, M.G.; Šeruga, B.; Vera-Badillo, F.E.; Aneja, P.; Ocaña, A.; Leibowitz-Amit, R.; Sonpavde, G.; Knox, J.J.; Tran, B.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2014, 106, dju124. [Google Scholar] [CrossRef]
- Aloe, C.; Wang, H.; Vlahos, R.; Irving, L.; Steinfort, D.; Bozinovski, S. Emerging and Multifaceted Role of Neutrophils in Lung Cancer. Transl. Lung Cancer Res. 2021, 10, 2806–2818. [Google Scholar] [CrossRef]
- Houghton, A.M.G.; Rzymkiewicz, D.M.; Ji, H.; Gregory, A.D.; Egea, E.E.; Metz, H.E.; Stolz, D.B.; Land, S.R.; Marconcini, L.A.; Kliment, C.R.; et al. Neutrophil Elastase–Mediated Degradation of IRS-1 Accelerates Lung Tumor Growth. Nat. Med. 2010, 16, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Coussens, L.M.; Tinkle, C.L.; Hanahan, D.; Werb, Z. MMP-9 Supplied by Bone Marrow-Derived Cells Contributes to Skin Carcinogenesis. Cell 2000, 103, 481–490. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Raftopoulou, S.; Mihalic, Z.N.; Marsche, G.; Kargl, J. Myeloperoxidase: Growing Importance in Cancer Pathogenesis and Potential Drug Target. Pharmacol. Ther. 2022, 236, 108052. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J.; Hawkins, C.L. The Role of Myeloperoxidase in Biomolecule Modification, Chronic Inflammation, and Disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [PubMed]
- Van Der Veen, B.S.; De Winther, M.P.J.; Heeringa, P. Myeloperoxidase: Molecular Mechanisms of Action and Their Relevance to Human Health and Disease. Antioxid. Redox Signal. 2009, 11, 2899–2937. [Google Scholar] [CrossRef]
- Vanhamme, L.; Zouaoui Boudjeltia, K.; Van Antwerpen, P.; Delporte, C. The Other Myeloperoxidase: Emerging Functions. Arch. Biochem. Biophys. 2018, 649, 1–14. [Google Scholar] [CrossRef]
- Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Neutrophil-Mediated Regulation of Innate and Adaptive Immunity: The Role of Myeloperoxidase Immunity: The Role of MPO. J. Immunol. Res. 2016, 2016, 2349817. [Google Scholar] [CrossRef]
- Davies, M.J. Myeloperoxidase: Mechanisms, Reactions and Inhibition as a Therapeutic Strategy in Inflammatory Diseases. Pharmacol. Ther. 2021, 218, 107685. [Google Scholar] [CrossRef]
- Cai, H.; Chuang, C.Y.; Hawkins, C.L.; Davies, M.J. Binding of Myeloperoxidase to the Extracellular Matrix of Smooth Muscle Cells and Subsequent Matrix Modification. Sci. Rep. 2020, 10, 666. [Google Scholar] [CrossRef]
- Lockhart, J.S.; Sumagin, R. Non-Canonical Functions of Myeloperoxidase in Immune Regulation, Tissue Inflammation and Cancer. Int. J. Mol. Sci. 2022, 23, 12250. [Google Scholar] [CrossRef]
- Kolarova, H.; Klinke, A.; Kremserova, S.; Adam, M.; Pekarova, M.; Baldus, S.; Eiserich, J.P.; Kubala, L. Myeloperoxidase Induces the Priming of Platelets. Free Radic. Biol. Med. 2013, 61, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Medfai, H.; Poelvoorde, P.; Kazan, M.F.; Delporte, C.; Van Antwerpen, P.; EL-Makhour, Y.; Biston, P.; Delrée, P.; Badran, B.; et al. Myeloperoxidase Promotes Tube Formation, Triggers ERK1/2 and Akt Pathways and Is Expressed Endogenously in Endothelial Cells. Arch. Biochem. Biophys. 2018, 654, 55–69. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Eiserich, J.P.; Mani, A.; Castro, L.; Figueroa, M.; Chumley, P.; Ma, W.; Tousson, A.; White, C.R.; Bullard, D.C.; et al. Endothelial Transcytosis of Myeloperoxidase Confers Specificity to Vascular ECM Proteins as Targets of Tyrosine Nitration. J. Clin. Investig. 2001, 108, 1759–1770. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, D.L.; Gelderman, M.P.; Fuhrmann, S.R.; Graham, S.; Starnes, J.D.; Lefkowitz, S.S.; Bollen, A.; Moguilevsky, N. Neutrophilic Myeloperoxidase–Macrophage Interactions Perpetuate Chronic Inflammation Associated with Experimental Arthritis. Clin. Immunol. 1999, 91, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mejiba, S.E.; Zhai, Z.; Gimenez, M.S.; Ashby, M.T.; Chilakapati, J.; Kitchin, K.; Mason, R.P.; Ramirez, D.C. Myeloperoxidase-Induced Genomic DNA-Centered Radicals. J. Biol. Chem. 2010, 285, 20062–20071. [Google Scholar] [CrossRef] [PubMed]
- Güngör, N.; Knaapen, A.M.; Munnia, A.; Peluso, M.; Haenen, G.R.; Chiu, R.K.; Godschalk, R.W.L.; Van Schooten, F.J. Genotoxic Effects of Neutrophils and Hypochlorous Acid. Mutagenesis 2010, 25, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Van Schooten, F.J.; Boots, A.W.; Knaapen, A.M.; Godschalk, R.W.L.; Maas, L.M.; Borm, P.J.A.; Drent, M.; Jacobs, J.A. Myeloperoxidase (MPO)−463G→A Reduces MPO Activity and DNA Adduct Levels in Bronchoalveolar Lavages of Smokers. Cancer Epidemiol. Biomark. Prev. 2004, 13, 828–833. [Google Scholar] [CrossRef]
- Valadez-Cosmes, P.; Maitz, K.; Kindler, O.; Mujkanovic, N.C.; Lueger, A.; Raftopoulou, S.; Kienzl, M.; Mihalic, Z.N.; Santiso, A.; Sarsembayeva, A.; et al. Myeloperoxidase Promotes a Tumorigenic Microenvironment in Non-Small Cell Lung Cancer. bioRxiv 2023. [Google Scholar] [CrossRef]
- Kienzl, M.; Hasenoehrl, C.; Maitz, K.; Sarsembayeva, A.; Taschler, U.; Valadez-Cosmes, P.; Kindler, O.; Ristic, D.; Raftopoulou, S.; Santiso, A.; et al. Monoacylglycerol Lipase Deficiency in the Tumor Microenvironment Slows Tumor Growth in Non-Small Cell Lung Cancer. Oncoimmunology 2021, 10, 1965319. [Google Scholar] [CrossRef] [PubMed]
- Endl, E.; Hollmann, C.; Gerdes, J. Antibodies against the Ki-67 Protein: Assessment of the Growth Fraction and Tools for Cell Cycle Analysis. Methods Cell Biol. 2001, 63, 399–418. [Google Scholar] [CrossRef] [PubMed]
- Saed, G.M.; Ali-Fehmi, R.; Jiang, Z.L.; Fletcher, N.M.; Diamond, M.P.; Abu-Soud, H.M.; Munkarah, A.R. Myeloperoxidase Serves as a Redox Switch That Regulates Apoptosis in Epithelial Ovarian Cancer. Gynecol. Oncol. 2010, 116, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grǎdinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt Pathway in Oncology Therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, H.; Gagnon, J.; Therrien, M. ERK Signalling: A Master Regulator of Cell Behaviour, Life and Fate. Nat. Rev. Mol. Cell Biol. 2020, 21, 607–632. [Google Scholar] [CrossRef] [PubMed]
- Haegens, A.; Vernooy, J.H.J.; Heeringa, P.; Mossman, B.T.; Wouters, E.F.M. Myeloperoxidase Modulates Lung Epithelial Responses to Pro-Inflammatory Agents. Eur. Respir. J. 2008, 31, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, K.; Kolarova, H.; Kerkenpaß, C.; Mollenhauer, M.; Vitecek, J.; Rudolph, V.; Kubala, L.; Baldus, S.; Adam, M.; Klinke, A. MPO (Myeloperoxidase) Reduces Endothelial Glycocalyx Thickness Dependent on Its Cationic Charge. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1859–1867. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, C.L.; Davies, M.J. Role of Myeloperoxidase and Oxidant Formation in the Extracellular Environment in Inflammation-Induced Tissue Damage. Free Radic. Biol. Med. 2021, 172, 633–651. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lee, K.A.; Ren, X.; Ryu, J.C.; Kim, G.; Ryu, J.H.; Lee, W.J.; Yoon, J. Synthesis of a Highly HOCl-Selective Fluorescent Probe and Its Use for Imaging HOCl in Cells and Organisms. Nat. Protoc. 2016, 11, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Kettle, A.J.; Gedye, C.A.; Winterbourn, C.C. Mechanism of Inactivation of Myeloperoxidase by 4-Aminobenzoic Acid Hydrazide. Biochem. J. 1997, 321, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kolářová, H.; Víteček, J.; Černá, A.; Černík, M.; Přibyl, J.; Skládal, P.; Potěšil, D.; Ihnatová, I.; Zdráhal, Z.; Hampl, A.; et al. Myeloperoxidase Mediated Alteration of Endothelial Function Is Dependent on Its Cationic Charge. Free Radic. Biol. Med. 2021, 162, 14–26. [Google Scholar] [CrossRef] [PubMed]
- El Kebir, D.; József, L.; Pan, W.; Filep, J.G. Myeloperoxidase Delays Neutrophil Apoptosis Through CD11b/CD18 Integrins and Prolongs Inflammation. Circ. Res. 2008, 103, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Slater, T.W.; Finkielsztein, A.; Mascarenhas, L.A.; Mehl, L.C.; Butin-Israeli, V.; Sumagin, R. Neutrophil Microparticles Deliver Active Myeloperoxidase to Injured Mucosa To Inhibit Epithelial Wound Healing. J. Immunol. 2017, 198, 2886–2897. [Google Scholar] [CrossRef] [PubMed]
- Papayannopoulos, V.; Metzler, K.D.; Hakkim, A.; Zychlinsky, A. Neutrophil Elastase and Myeloperoxidase Regulate the Formation of Neutrophil Extracellular Traps. J. Cell Biol. 2010, 191, 677–691. [Google Scholar] [CrossRef] [PubMed]
- Baldus, S.; Rudolph, V.; Roiss, M.; Ito, W.D.; Rudolph, T.K.; Eiserich, J.P.; Sydow, K.; Lau, D.; Szöcs, K.; Klinke, A.; et al. Heparins Increase Endothelial Nitric Oxide Bioavailability by Liberating Vessel-Immobilized Myeloperoxidase. Circulation 2006, 113, 1871–1878. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosic-Mujkanovic, N.; Valadez-Cosmes, P.; Maitz, K.; Lueger, A.; Mihalic, Z.N.; Runtsch, M.C.; Kienzl, M.; Davies, M.J.; Chuang, C.Y.; Heinemann, A.; et al. Myeloperoxidase Alters Lung Cancer Cell Function to Benefit Their Survival. Antioxidants 2023, 12, 1587. https://doi.org/10.3390/antiox12081587
Cosic-Mujkanovic N, Valadez-Cosmes P, Maitz K, Lueger A, Mihalic ZN, Runtsch MC, Kienzl M, Davies MJ, Chuang CY, Heinemann A, et al. Myeloperoxidase Alters Lung Cancer Cell Function to Benefit Their Survival. Antioxidants. 2023; 12(8):1587. https://doi.org/10.3390/antiox12081587
Chicago/Turabian StyleCosic-Mujkanovic, Nejra, Paulina Valadez-Cosmes, Kathrin Maitz, Anna Lueger, Zala N. Mihalic, Marah C. Runtsch, Melanie Kienzl, Michael J. Davies, Christine Y. Chuang, Akos Heinemann, and et al. 2023. "Myeloperoxidase Alters Lung Cancer Cell Function to Benefit Their Survival" Antioxidants 12, no. 8: 1587. https://doi.org/10.3390/antiox12081587
APA StyleCosic-Mujkanovic, N., Valadez-Cosmes, P., Maitz, K., Lueger, A., Mihalic, Z. N., Runtsch, M. C., Kienzl, M., Davies, M. J., Chuang, C. Y., Heinemann, A., Schicho, R., Marsche, G., & Kargl, J. (2023). Myeloperoxidase Alters Lung Cancer Cell Function to Benefit Their Survival. Antioxidants, 12(8), 1587. https://doi.org/10.3390/antiox12081587






