Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde
Abstract
1. Introduction
2. Material and Methods
2.1. Materials
2.2. RAW 264.7 Cell Culture and Lipid Treatments
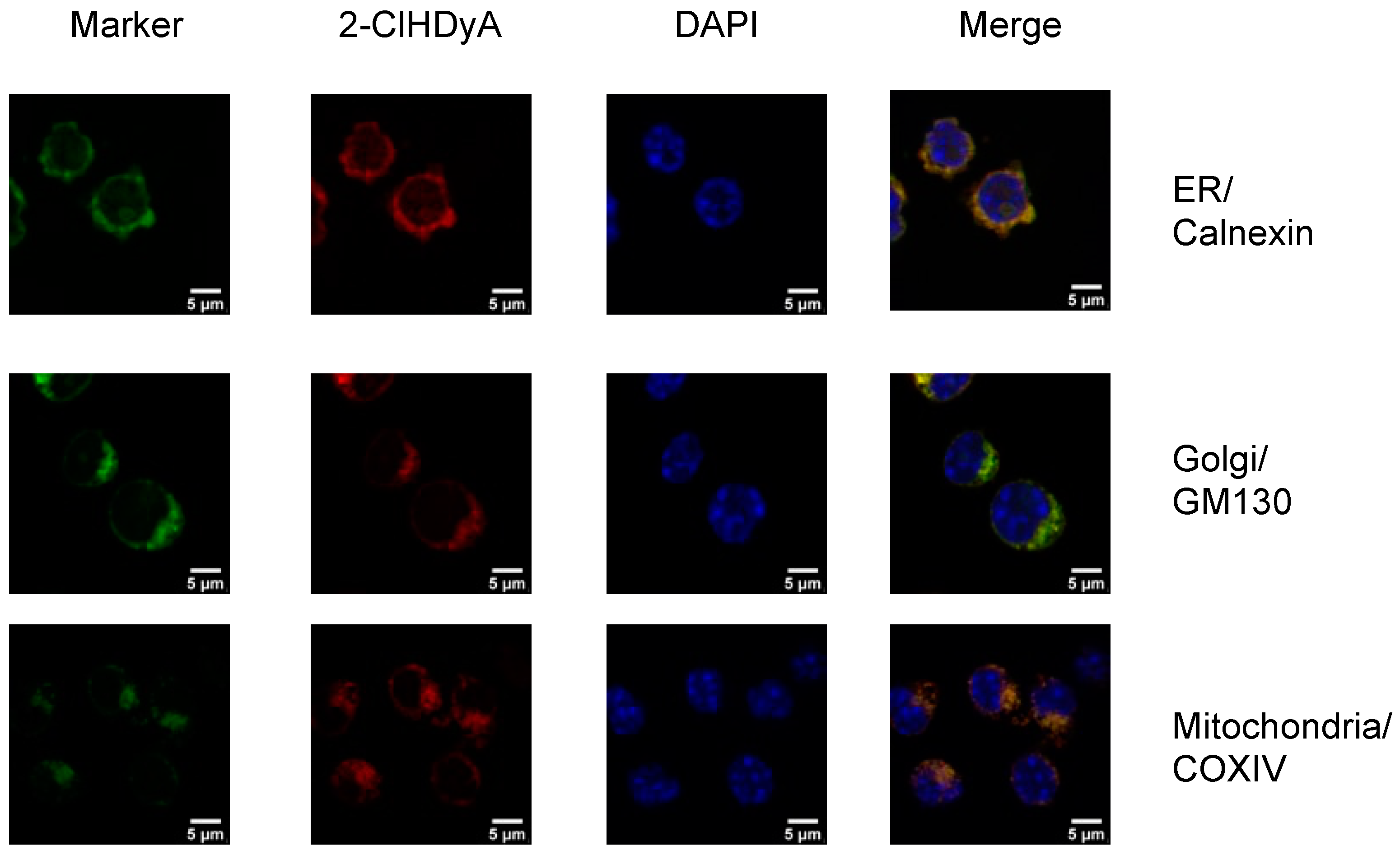
2.3. Detection of 2-ClHDyA by Immunofluorescence
2.4. Confocal Microscopy
2.5. Cell Metabolic Activity Assessed by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium Bromide Assay
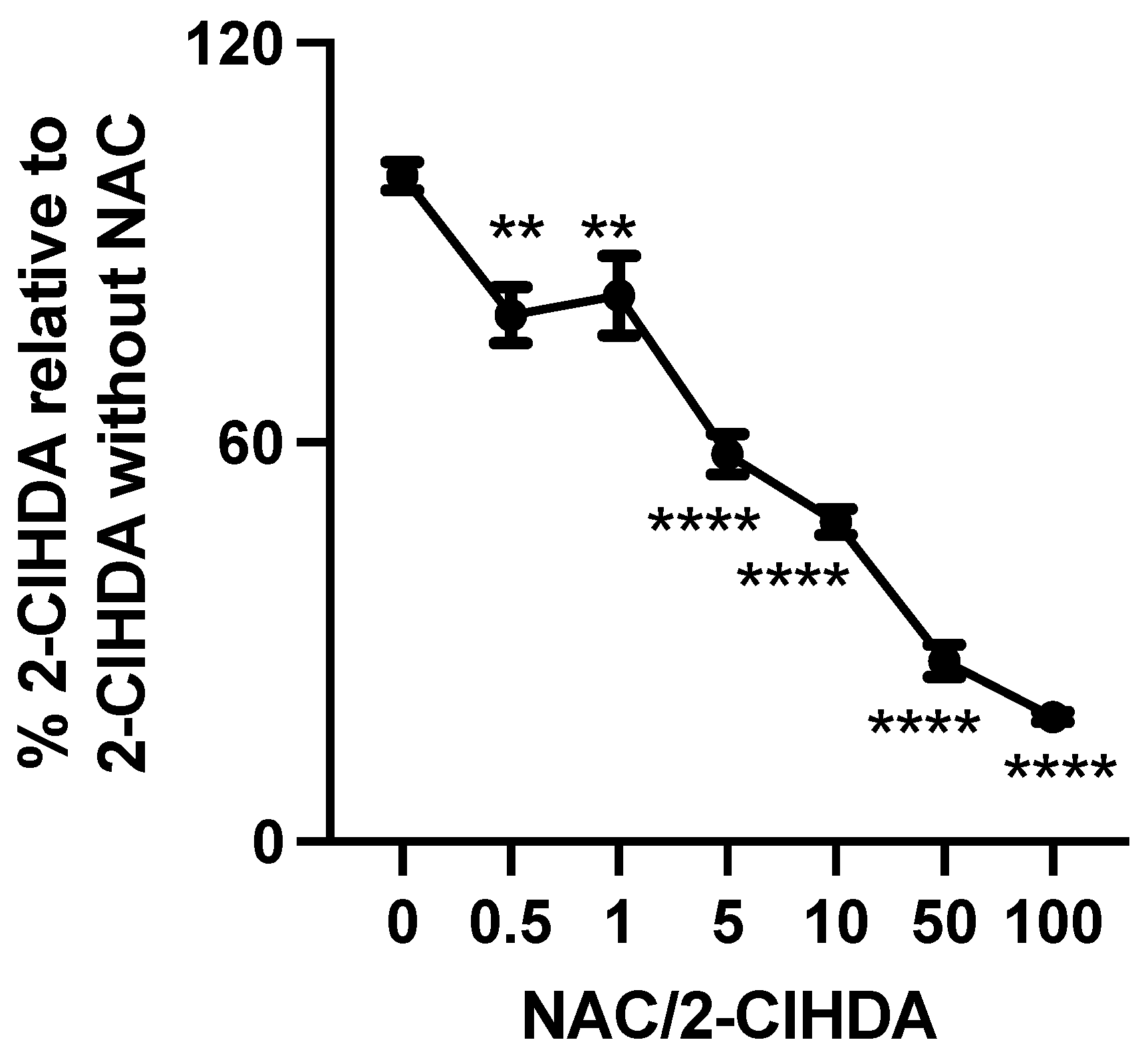
2.6. Sequestering 2-ClHDA with NAC
2.7. Human Neutrophil Studies
2.8. Detection of Protein Modification by Lipid
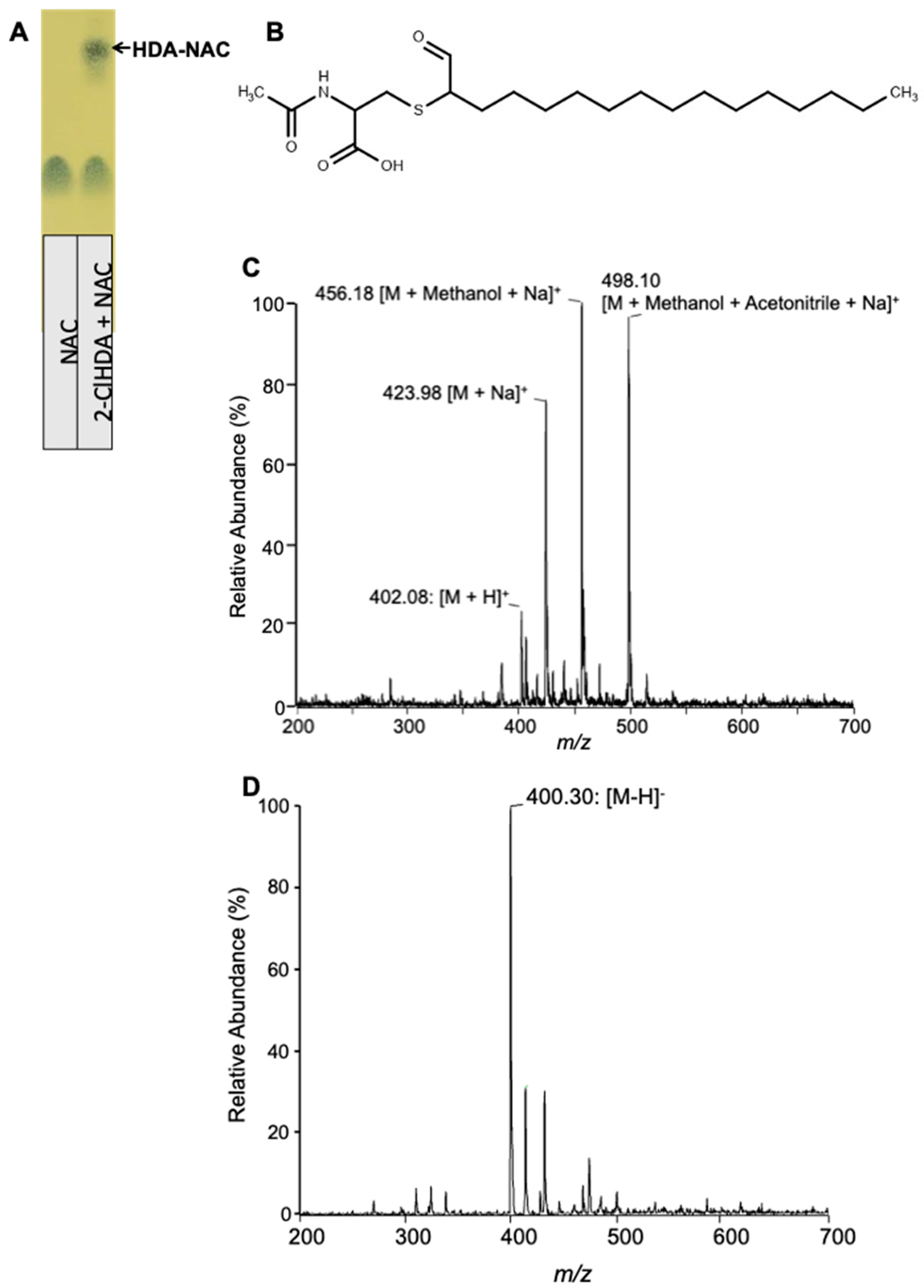
2.9. 2-ClHDA and NAC In Vitro Reaction Products and Purification
2.10. ESI/MS/MS Characterization of 2-ClHDA and NAC In Vitro Reaction Products
2.11. Extraction and Quantification of HDA–GSH and HDA–NAC
2.12. Statistical Analyses
3. Results
3.1. Effect of 2-ClHDA on Cell Metabolic Activity
3.2. Subcellular Localization of 2-ClHDyA
3.3. N-Acetyl Cysteine Quenches 2-ClFALD
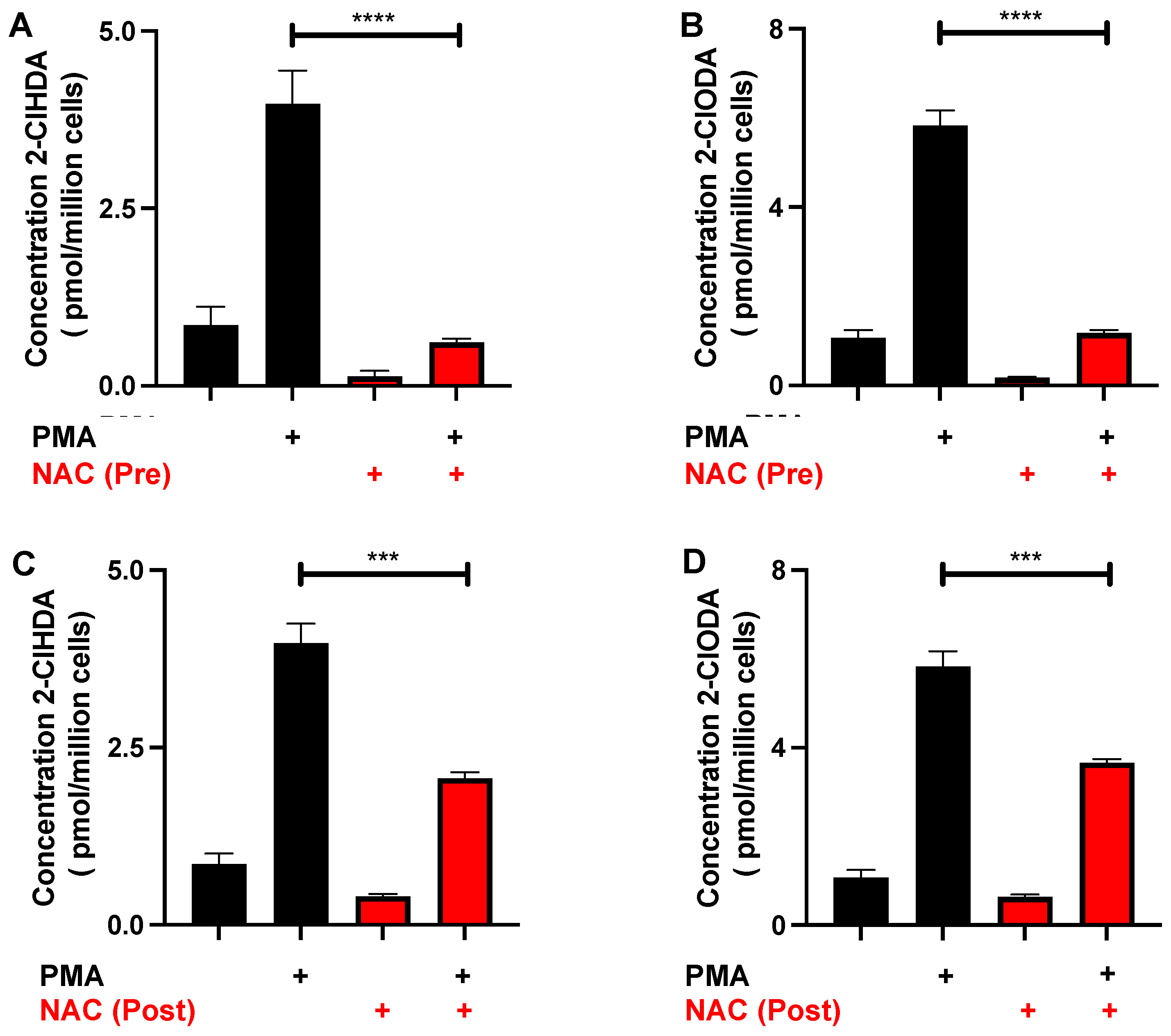
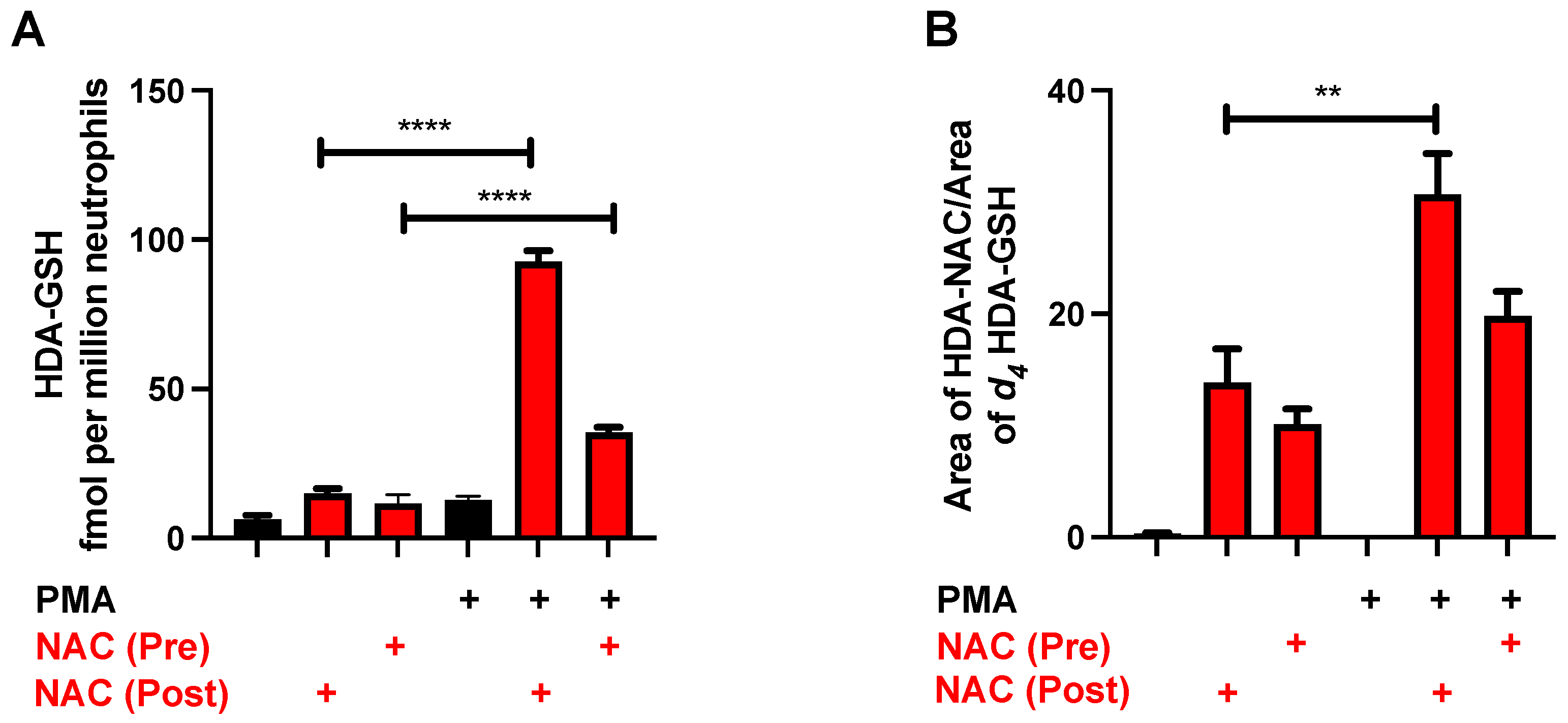
3.4. NAC Reduction of 2-ClFALD in Activated Neutrophils
3.5. Protective Effects of NAC
3.6. Characterization of 2-ClHDA Adduct with NAC
3.7. Formation of HDA-GSH and HDA–NAC in Neutrophils
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albert, C.J.; Crowley, J.R.; Hsu, F.F.; Thukkani, A.K.; Ford, D.A. Reactive chlorinating species produced by myeloperoxidase target the vinyl ether bond of plasmalogens: Identification of 2-chlorohexadecanal. J. Biol. Chem. 2001, 276, 23733–23741. [Google Scholar] [CrossRef] [PubMed]
- Anbukumar, D.S.; Shornick, L.P.; Albert, C.J.; Steward, M.M.; Zoeller, R.A.; Neumann, W.L.; Ford, D.A. Chlorinated lipid species in activated human neutrophils: Lipid metabolites of 2-chlorohexadecanal. J. Lipid Res. 2010, 51, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; Hsu, F.F.; Crowley, J.R.; Wysolmerski, R.B.; Albert, C.J.; Ford, D.A. Reactive chlorinating species produced during neutrophil activation target tissue plasmalogens: Production of the chemoattractant, 2-chlorohexadecanal. J. Biol. Chem. 2002, 277, 3842–3849. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; Martinson, B.D.; Albert, C.J.; Vogler, G.A.; Ford, D.A. Neutrophil-mediated accumulation of 2-ClHDA during myocardial infarction: 2-ClHDA-mediated myocardial injury. Am. J. Physiol.-Heart Circ. Physiol. 2005, 288, H2955–H2964. [Google Scholar] [CrossRef] [PubMed]
- Thukkani, A.K.; McHowat, J.; Hsu, F.F.; Brennan, M.L.; Hazen, S.L.; Ford, D.A. Identification of alpha-chloro fatty aldehydes and unsaturated lysophosphatidylcholine molecular species in human atherosclerotic lesions. Circulation 2003, 108, 3128–3133. [Google Scholar] [CrossRef]
- Meyer, N.J.; Reilly, J.P.; Feng, R.; Christie, J.D.; Hazen, S.L.; Albert, C.J.; Franke, J.D.; Hartman, C.L.; McHowat, J.; Ford, D.A. Myeloperoxidase-derived 2-chlorofatty acids contribute to human sepsis mortality via acute respiratory distress syndrome. JCI Insight 2017, 2, pii:96432. [Google Scholar] [CrossRef]
- Palladino, E.N.D.; Hartman, C.L.; Albert, C.J.; Ford, D.A. The chlorinated lipidome originating from myeloperoxidase-derived HOCl targeting plasmalogens: Metabolism, clearance, and biological properties. Arch. Biochem. Biophys. 2018, 641, 31–38. [Google Scholar] [CrossRef]
- Palladino, E.N.D.; Katunga, L.A.; Kolar, G.R.; Ford, D.A. 2-Chlorofatty acids: Lipid mediators of neutrophil extracellular trap formation. J. Lipid Res. 2018, 59, 1424–1432. [Google Scholar] [CrossRef]
- Ford, D.A.; Honavar, J.; Albert, C.J.; Duerr, M.A.; Oh, J.Y.; Doran, S.; Matalon, S.; Patel, R.P. Formation of chlorinated lipids post-chlorine gas exposure. J. Lipid Res. 2016, 57, 1529–1540. [Google Scholar] [CrossRef]
- Nusshold, C.; Ullen, A.; Kogelnik, N.; Bernhart, E.; Reicher, H.; Plastira, I.; Glasnov, T.; Zangger, K.; Rechberger, G.; Kollroser, M.; et al. Assessment of electrophile damage in a human brain endothelial cell line utilizing a clickable alkyne analog of 2-chlorohexadecanal. Free Radic. Biol. Med. 2016, 90, 59–74. [Google Scholar] [CrossRef]
- Ullen, A.; Fauler, G.; Bernhart, E.; Nusshold, C.; Reicher, H.; Leis, H.J.; Malle, E.; Sattler, W. Phloretin ameliorates 2-chlorohexadecanal-mediated brain microvascular endothelial cell dysfunction in vitro. Free Radic. Biol. Med. 2012, 53, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Üllen, A.; Nusshold, C.; Glasnov, T.; Saf, R.; Cantillo, D.; Eibinger, G.; Reicher, H.; Fauler, G.; Bernhart, E.; Hallstrom, S.; et al. Covalent adduct formation between the plasmalogen-derived modification product 2-chlorohexadecanal and phloretin. Biochem. Pharmacol. 2015, 93, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Albert, C.J.; Ford, D.A. Alpha-chlorofatty acid accumulates in activated monocytes and causes apoptosis through reactive oxygen species production and endoplasmic reticulum stress. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Duerr, M.A.; Aurora, R.; Ford, D.A. Identification of glutathione adducts of alpha-chlorofatty aldehydes produced in activated neutrophils. J. Lipid Res. 2015, 56, 1014–1024. [Google Scholar] [CrossRef] [PubMed]
- Duerr, M.A.; Palladino, E.N.D.; Hartman, C.L.; Lambert, J.A.; Franke, J.D.; Albert, C.J.; Matalon, S.; Patel, R.P.; Slungaard, A.; Ford, D.A. Bromofatty aldehyde derived from bromine exposure and myeloperoxidase and eosinophil peroxidase modify GSH and protein. J. Lipid Res. 2018, 59, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Wildsmith, K.R.; Albert, C.J.; Hsu, F.F.; Kao, J.L.-F.; Ford, D.A. Myeloperoxidase-derived 2-chlorohexadecanal forms Schiff bases with primary amines of ethanolamine glycerophospholipids and lysine. Chem. Phys. Lipids 2006, 139, 157–170. [Google Scholar] [CrossRef]
- Shakya, S.; Pyles, K.D.; Albert, C.J.; Patel, R.P.; McCommis, K.S.; Ford, D.A. Myeloperoxidase-derived hypochlorous acid targets human airway epithelial plasmalogens liberating protein modifying electrophilic 2-chlorofatty aldehydes. Redox Biol. 2022, 59, 102557. [Google Scholar] [CrossRef]
- Shakya, S.; Herr, R.A.; Carlson, H.L.; Zoeller, R.A.; Albert, C.J.; Ford, D.A. Endothelial cell protein targeting by myeloperoxidase-derived 2-chlorofatty aldehyde. Antioxidants 2022, 11, 940. [Google Scholar] [CrossRef]
- Albert, C.J.; Anbukumar, D.S.; Messner, M.C.; Ford, D.A. Chromatographic methods for the analyses of 2-halofatty aldehydes and chlorohydrin molecular species of lysophosphatidylcholine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2009, 877, 2768–2777. [Google Scholar] [CrossRef][Green Version]
- Hartman, C.L.; Duerr, M.A.; Albert, C.J.; Neumann, W.L.; McHowat, J.; Ford, D.A. 2-Chlorofatty acids induce Weibel-Palade body mobilization. J. Lipid Res. 2018, 59, 113–122. [Google Scholar] [CrossRef]
- Brahmbhatt, V.V.; Nold, C.; Albert, C.J.; Ford, D.A. Quantification of pentafluorobenzyl oxime derivatives of long chain aldehydes by GC-MS analysis. Lipids 2008, 43, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Wacker, B.K.; Albert, C.J.; Ford, B.A.; Ford, D.A. Strategies for the analysis of chlorinated lipids in biological systems. Free Radic. Biol. Med. 2013, 59, 92–99. [Google Scholar] [CrossRef][Green Version]
- Wang, W.Y.; Albert, C.J.; Ford, D.A. Approaches for the analysis of chlorinated lipids. Anal. Biochem. 2013, 443, 148–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prasch, J.; Bernhart, E.; Reicher, H.; Kollroser, M.; Rechberger, G.N.; Koyani, C.N.; Trummer, C.; Rech, L.; Rainer, P.P.; Hammer, A.; et al. Myeloperoxidase-Derived 2-Chlorohexadecanal Is Generated in Mouse Heart during Endotoxemia and Induces Modification of Distinct Cardiomyocyte Protein Subsets In Vitro. Int. J. Mol. Sci. 2020, 21, 9235. [Google Scholar] [CrossRef] [PubMed]
- McHowat, J.; Shakya, S.; Ford, D.A. 2-Chlorofatty aldehyde elicits endothelial cell activation. Front. Physiol. 2020, 11, 460. [Google Scholar] [CrossRef]
- Aldini, G.; Carini, M.; Yeum, K.J.; Vistoli, G. Novel molecular approaches for improving enzymatic and nonenzymatic detoxification of 4-hydroxynonenal: Toward the discovery of a novel class of bioactive compounds. Free Radic. Biol. Med. 2014, 69, 145–156. [Google Scholar] [CrossRef]
- Schreck, R.; Rieber, P.; Baeuerle, P.A. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991, 10, 2247–2258. [Google Scholar] [CrossRef]
- Verhasselt, V.; Vanden Berghe, W.; Vanderheyde, N.; Willems, F.; Haegeman, G.; Goldman, M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: Association with NF-kappaB inhibition. J. Immunol. 1999, 162, 2569–2574. [Google Scholar] [CrossRef]
- Zhitkovich, A. N-Acetylcysteine: Antioxidant, Aldehyde Scavenger, and More. Chem. Res. Toxicol. 2019, 32, 1318–1319. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinyagam, R.; Rajamanickam, V.; Sankaran, V.; Venkatesan, S.; David, E. Pharmacological Aspects and Potential Use of Phloretin: A Systemic Review. Mini Rev. Med. Chem. 2019, 19, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Hanna, P.E.; Anders, M.W. The mercapturic acid pathway. Crit. Rev. Toxicol. 2019, 49, 819–929. [Google Scholar] [CrossRef] [PubMed]
- Atkuri, K.R.; Mantovani, J.J.; Herzenberg, L.A.; Herzenberg, L.A. N-Acetylcysteine--a safe antidote for cysteine/glutathione deficiency. Curr. Opin. Pharmacol. 2007, 7, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Agren, L.; Elfsmark, L.; Akfur, C.; Hagglund, L.; Ekstrand-Hammarstrom, B.; Jonasson, S. N-acetyl cysteine protects against chlorine-induced tissue damage in an ex vivo model. Toxicol. Lett. 2020, 322, 58–65. [Google Scholar] [CrossRef]
- Wigenstam, E.; Koch, B.; Bucht, A.; Jonasson, S. N-acetyl cysteine improves the effects of corticosteroids in a mouse model of chlorine-induced acute lung injury. Toxicology 2015, 328, 40–47. [Google Scholar] [CrossRef]
- Pike, D.P.; Vogel, M.J.; McHowat, J.; Mikuzis, P.A.; Schulte, K.A.; Ford, D.A. 2-Chlorofatty acids are biomarkers of sepsis mortality and mediators of barrier dysfunction in rats. J. Lipid Res. 2020, 61, 1115–1127. [Google Scholar] [CrossRef]
- Villa, P.; Ghezzi, P. Effect of N-acetyl-L-cysteine on sepsis in mice. Eur. J. Pharmacol. 1995, 292, 341–344. [Google Scholar] [CrossRef]
- Zhang, H.; Spapen, H.; Nguyen, D.N.; Benlabed, M.; Buurman, W.A.; Vincent, J.L. Protective effects of N-acetyl-L-cysteine in endotoxemia. Am. J. Physiol. 1994, 266, H1746–H1754. [Google Scholar] [CrossRef]
- Kamoshida, G.; Kikuchi-Ueda, T.; Nishida, S.; Tansho-Nagakawa, S.; Kikuchi, H.; Ubagai, T.; Ono, Y. Spontaneous formation of neutrophil extracellular traps in serum-free culture conditions. FEBS Open Bio 2017, 7, 877–886. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakya, S.; McGuffee, R.M.; Ford, D.A. Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde. Antioxidants 2023, 12, 504. https://doi.org/10.3390/antiox12020504
Shakya S, McGuffee RM, Ford DA. Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde. Antioxidants. 2023; 12(2):504. https://doi.org/10.3390/antiox12020504
Chicago/Turabian StyleShakya, Shubha, Reagan M. McGuffee, and David A. Ford. 2023. "Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde" Antioxidants 12, no. 2: 504. https://doi.org/10.3390/antiox12020504
APA StyleShakya, S., McGuffee, R. M., & Ford, D. A. (2023). Characterization of N-Acetyl Cysteine Adducts with Exogenous and Neutrophil-Derived 2-Chlorofatty Aldehyde. Antioxidants, 12(2), 504. https://doi.org/10.3390/antiox12020504





