Abstract
Due to the limitations in the use of antibiotic agents, researchers are constantly seeking natural bioactive compounds that could benefit udder health status but also milk quality characteristics in dairy animals. The aim of the current study was therefore to examine the effects of a standardized mixture of plant bioactive components (MPBC) originated from thyme, anise and olive on milk yield, composition, oxidative stability and somatic cell count in dairy ewes. Thirty-six ewes approximately 75 days after parturition were randomly allocated into three experimental treatments, which were provided with three diets: control (C); without the addition of the mixture, B1; supplemented with MPBC at 0.05% and B2; supplemented with rumen protected MPBC at 0.025%. The duration of the experiment was 11 weeks, and milk production was weekly recorded, while individual milk samples for the determination of composition, oxidative stability, somatic cell count (SCC), pH and electric conductivity were collected. Every two weeks, macrophage, lymphocyte, and polymorphonuclear leukocyte counts were also determined in individual milk samples. It was observed that milk yield was the greatest in the B2 group, with significant differences within the seventh and ninth week (p < 0.05), whereas no significant differences were found for milk composition, with the exception of the seventh week, when protein, lactose and non-fat solid levels were lower in MPBC groups (p < 0.05). Oxidative stability was improved in the groups that received the MPBC, with significant differences at the third, seventh, tenth and eleventh week (p < 0.05). SCC was also significantly lower at the second, eighth and ninth week in B2 compared to the other groups (p < 0.05), while no significant effects on the macrophage, lymphocyte, and polymorphonuclear leukocyte counts were observed. In conclusion, the MPBC addition had a positive effect on sheep milk yield, oxidative stability and somatic cell count, without any negative effect on its composition.
1. Introduction
There is an increased public awareness of potential health hazards induced by the excessive use of in-feed antibiotics that is reflected in the legislation of many countries, such as the EU [1] and the efforts of animal scientists to find alternative safe natural feed additives [2,3]. Consumer concerns refer to toxicity, residues and metabolites in milk that can induce bacterial resistance in human infections. In general, farmers and industry comply with the legislation and several food safety controls are routinely carried out; however, these concerns remain possibly as a result of incorrect information [4,5]. Currently, research efforts are focused on the development of functional dairy products that fortify human health and are in harmony with the concept of sustainable production, green economy, environmental protection, and proper health and welfare status of dairy animals [2]. Bioactive compounds of plants, well known as phytobiotics, possess strong antioxidant and anti-inflammatory properties that depend upon their type and amount and are generally cheaper and safer compared to synthetic antibiotic agents [6]. Their dietary supplementation generally improves performance and ameliorates the health status of ruminants due to their multifaceted properties [6]. Milk Somatic Cell Count (SCC) is often used as an indirect index of mammary health, since high values are strongly related with changes in milk quality, poor udder health and inflammatory damage of mammary tissue leading to significant economic losses in modern dairy farms [7].
At the same time, the dietary inclusion of plants’ bioactive compounds improves milk oxidative stability, resulting in dairy products of high-quality and safety [8,9]. An antioxidant is defined as any substance that when present in low concentrations compared to the oxidizable substrate (i.e., proteins, lipids, carbohydrates and DNA) significantly delays or prevents oxidation of this substrate through its radical scavenging, metal ion chelation, and singlet oxygen quenching properties [10]. A compound exerts its antioxidant activities by inhibiting the creation of reactive oxygen species (ROS), or directly cleaning free radicals [10]. ROS are small molecules that contain active oxygen and are produced as by-products in sub-cellular organelles such as mitochondria. A high concentration of ROS in any normal cell can turn it into a malignant cell [10]. Phytobiotic supplementation may be more efficient in animals that are under physiologic stress, such as the peak of milk production in dairy animals [11]. In addition, the combination of phytobiotics from different plants may exert an increased antioxidant activity due to their synergism [12]. However, several parameters, namely area of origin, period of harvesting within the year, used part of the plant (leaf, bark, seeds or root) and method of isolation (steam distillation, extraction with non-aqueous solvents, cold expression, etc.) modify the antioxidant capacity and the efficacy of each phytogenic compound. Discrepancies are also observed due to the type of the phytobiotic, its level of dietary supplementation, the composition and the digestibility of the basal diet, the level of feed intake and hygiene and environmental conditions [2].
The objective of the present study was to determine if the supplementation with a mixture of plant bioactive components (MPBC) to high-producing dairy ewes that are prone to subclinical udder health disorders may alleviate this stressful condition and, consequently, improve their lactation performance and health status.
2. Materials and Methods
2.1. Animals
Thirty-six 2-year-old Chios ewes with similar body condition scores (2.5–3.0) and a mean weight of 52.3 ± 1.9 kg that were at their second parity and thirty days after lamb weaning (75 ± 5 days after lamb birth) were randomly selected from the sheep herd of the experimental farm of the Agricultural University of Athens and allocated into three experimental groups of twelve ewes each based on their milk yield and body weight. The ewes of the flock were mated following estrus synchronization with Ovigest intravaginal progestogen sponges ( Hipra S.A., Girona, Spain).
All animals initially consumed alfalfa hay and the same concentrate basal diet (Table 1) without the addition of MPBC for one week in order to become acclimatized to the experimental conditions. After this adaptive pre-experimental period, they received the three experimental diets for 11 weeks. One of the groups served as a control (C) and was fed with the previous concentrate diet, whereas the other two groups were offered the same concentrated diet further supplemented with MPBC (B1) at the level of 0.05% or with rumen protected MPBC at the level of 0.025% (B2). These levels were selected based on our preliminary studies. Furthermore, we attempted to evaluate whether a lower level of supplementation in a rumen protected form would return comparable results with the 0.05% level in the framework of precision livestock feeding. The MBPC used in this trial (NuPhoria, Nuevo S.A., Schimatari, Viotia, Greece) was a proprietary mixture of phytogenic substances originating from thyme (Thymus vulgaris), anise (Pimpinella anisum) and olive (Olea europea) at an approximate ratio of 20, 35 and 45% with a standardized active ingredient concentration of 100 g/kg. In detail, the levels of thymol, anethole and hydroxytyrosol in thyme, anise and olive were 24, 210 and 50 g/kg, respectively. Rumen-protected MPBC was obtained by freeze drying with maltodextrin at a rate of 50:50. Maltodextrins of different dextrose equivalents are commonly used as encapsulating agents due to their high-water solubility, low viscosity and colorless solutions [13,14]. Ewes in the present study consumed on average 2.0 kg of feed (concentrate and forage at a mean ratio of 50:50). Quantities of concentrates were constantly adjusted to the milk yield of each ewe and the additional demanded dry matter for high yielding animals was individually provided.

Table 1.
Composition and analysis of dairy ewes’ diet.
Ewes were housed in 3 different pens (one pen per treatment) at the premises of the Agricultural University of Athens. Each pen consisted of an indoor and outdoor area and had the same direction and orientation, the same covered area (3 m2/ewe) and was equipped with similar troughs for feeding (12 individual feeders indoors for concentrate and 1 feeder outdoors for alfalfa hay per pen). Water was available ad libitum and the diet that was formulated according to ewes’ individual requirements, based on their body weight and milk yield, was provided twice daily at 8 a.m. and 15 p.m. Forage was offered to the animals after assuring that the concentrate was completely consumed. No refusals of forage and/or concentrates were observed.
2.2. Determination of Milk Yield Composition and Oxidative Stability
Ewes were milked twice per day (6:00 a.m. and 18:00 p.m.) in a 12 stall milking parlor (GEA Westfalia, Düsseldorf, Germany). A pulsation ratio of 50:50 was applied; pulsation rate was 150 cycles min−1 with 37.5 kPa vacuum level. Milk yield, determined as the sum of the morning and afternoon milking, was recorded on day 1 prior to and on week 1–11 after MPBC dietary supplementation. Fat corrected (FCM6%) milk yield was also calculated using the following formula:
Fat corrected milk (FCM) in 6% (FCM6%) = (0.28 + 0.12 × milk fat concentration (%)) × milk yield (kg/d).
Individual milk samples were also collected on acclimation week and on week 1–11 after MPBC dietary supplementation and analyzed for fat, protein, lactose, total solids-not-fat, pH, electric conductivity and somatic cell count by using the Lactoscan COMBO Milk Cell Analyser (Lactoscan, Nova Zagora, Bulgaria) in accordance with international standard protocol guidelines. Milk oxidative stability was evaluated by measuring the levels of malondialdehyde (MDA), a secondary lipid oxidation product formed by hydrolysis of lipid hydroperoxides. MDA concentration (ng/mL) was determined by applying a selective third-order derivative spectrophotometric method, previously developed by Botsoglou et al. [15].
2.3. Isolation of Milk Somatic Cells and Milk Somatic Cell Immunophenotyping
Every two weeks, 15 mL individual milk samples were also collected and kept on ice for the determination of macrophage, lymphocyte, and polymorphonuclear leukocytes count. Milk somatic cells (MSC) were isolated following a modified protocol of Koess and Hamann [16] optimized for sheep milk. Briefly, milk samples were centrifuged at 400× g for 15 min at 4 °C. Pellets were resuspended in 15 mL of dilution buffer, i.e., phosphate-buffered saline (PBS; pH 7.4) containing 0.01% sodium azide (NaN3) and 0.2% bovine serum albumin (BSA). Samples were centrifuged at 400× g for 10 min at 4 °C. Pellets were resuspended in 4 mL of dilution buffer and centrifuged at 400× g for 10 min at 4 °C. Cell pellets were resuspended in 1 mL of dilution buffer and filtered through 40 μm cell strainers.
Cell surface labelling was performed with anti-CD11b, anti-CD8 and anti-Cytokeratins 4 + 5 + 6 + 8 + 10 + 13 + 18 (anti-Pan Cytokeratins) for the identification of granulocytes and macrophages, T-cytotoxic and epithelial cells, respectively. In addition, propidium iodide (PI) staining was used to differentiate live from dead cells. Aliquots of MSC prepared as described above containing approximately 2 × 105 cells were centrifuged at 400× g for 5 min at 4 °C and cell pellets were resuspended in 50 μL ice cold antibody solutions containing combinations of 0.002 mg/mL anti-CD11b conjugated to Fluorescein isothiocyanate (FITC), 0.005 mg/mL anti-pan Cytokeratins conjugated to Allophycocyanin (APC) and 0.002 mg/mL CD8 R-PE antibodies. Cells in the staining solutions were incubated on ice and in the dark for 30 min, then a 2 mL dilution buffer was added, samples were centrifuged at 400× g for 5 min and cell pellets were resuspended in 100 μL dilution buffer. DNA staining was performed by addition of PI at a final concentration of 5 ng/μL. Following 10 min incubation at room temperature in the dark, 100 μL of PBS were added and samples were analyzed by flow cytometry (Cytomics FC 500, California, Beckman Coulter Inc., Fullerton, CA, USA).
Instrument voltage/gain for detectors FS, SS, FL1, FL2, FL3, FL4 and FL5 were set at 700/2.0, 680/20.0, 550/1.0, 650/1.0, 650/1.0, 614/1.0, and 601/1.0, respectively. The samples were run at medium speed and approximately 65,000 events were collected per sample. Data were stored as list mode files. Events that were identified as being of appropriate size and granularity based on their position on an FS/SS dotplot and were negative for PI were considered as live cells. PI stains nucleic acids only in cells with disrupted cell membranes, i.e., necrotic cells and thus cells that were negative for PI staining were considered as live cells. Dead cells were not included in further analysis as they often exhibit non-specific staining with cell surface antibodies and may be misclassified. Live cells that stained positive for CD11b (FL1) with higher SS values were classified as polymorphonuclear granulocytes (PMN) and live cells that stained positive for CD11b with lower SS values were classified as monocytes/macrophages (MPh). Epithelial cells were identified from the CD11b−/CD8− live cells that stained positive for pan-cytokeratins. Lymphocytes were identified as the live cells that were positioned in the FS/SS dotplot in the area identified by CD8+ cells. Proportions of each cell subset in the MSC were estimated as a percentage of the live cells.
2.4. Statistical Analysis
The experimental unit was the animal since it was the smallest unit upon which either the treatment was applied or the measurements were made. Data were subjected to repeated measures analysis of variance using the MIXED procedure of SAS software, with dietary treatment as the fixed factor and sampling week as the repeated factor. SCC was log transformed prior to statistical analysis in order to achieve normal distribution. Significant differences were tested with Bonferroni adjustment at 0.05 significance level and the results are presented as least square means ± S.E.M.
3. Results
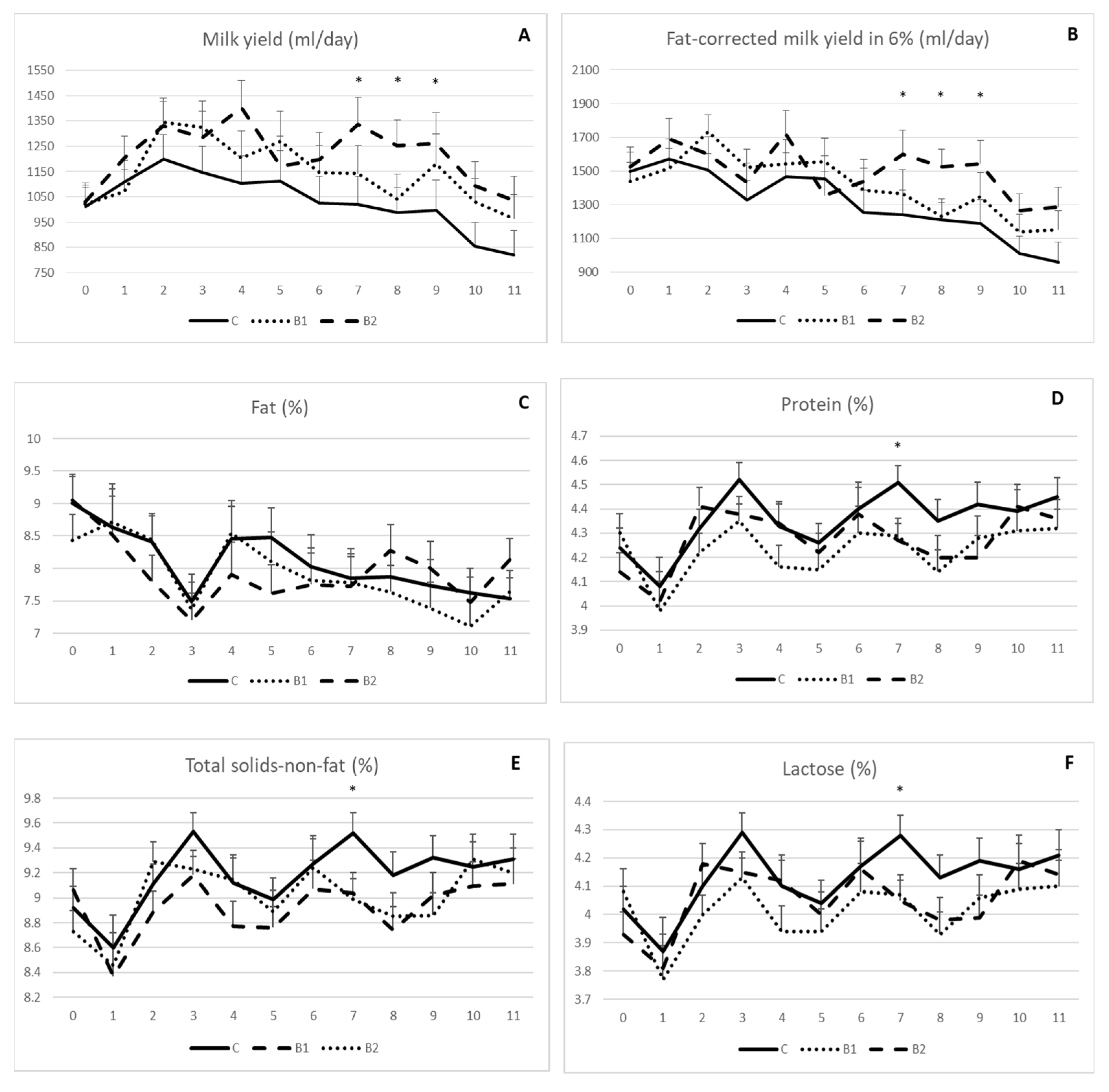
As shown, the milk yield (Figure 1A) and fat-corrected milk yield in 6% (Figure 1B) were generally higher in the MPBC supplemented groups than the controls with significant differences between the 7th and 9th week of the experiment for the B2 compared to the other groups (p < 0.05). In detail, the milk yield was 1335, 1021 and 1142 (±109) mL/day on week 7, 1253, 988 and 1042 (±99) mL/day on week 8 and 1262, 996 and 1179 (±121) mL/day on week 9 for the B2, C and B1 groups, respectively (p < 0.05; Figure 1A). The respective values for the fat-corrected milk yield were 1599, 1243 and 1367 (±142) mL/day on week 7, 1525, 1209 and 1230 (±104) mL/day on week 8 and 1543, 1187 and 1348 (±14,121) mL/day on week 9 for the B2, C and B1 groups, respectively (p < 0.05; Figure 1B).
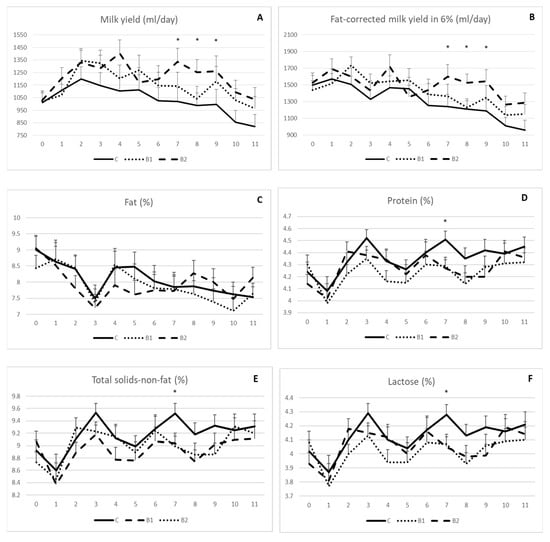
Figure 1.
Effect of MPBC dietary supplementation on milk yield (A), fat-corrected milk yield in 6% (B), fat (C), protein (D), total solids-not-fat (E) and lactose (F). Control group was fed with the basal concentrate diet (C), whereas the other two groups were offered the same concentrated diet further with MPBC (B1) at the levels of 0.05% or with rumen protected MPBC (B2) at the levels of 0.025%. The use of (*) indicates significant difference at p < 0.05.
Milk composition was generally not affected by MPBC dietary supplementation. No significant differences were shown for milk fat throughout the experiment (Figure 1C). Similar findings were observed for milk protein (Figure 1D), total solids-non-fat (Figure 1E) and lactose (Figure 1F) with the only exception at the 7th week, when the values for these parameters were higher in the control compared with the MPBC supplemented groups (p < 0.05). In detail, milk protein (%) was 4.51, 4.29 and 4.27 (±0.07), milk total solids-non-fat (%) were 9.52, 9.04 and 8.99 (±0.16) and milk lactose was 4.28, 4.07 and 4.05 (±0.07) for the C, B1 and B2 groups, respectively.
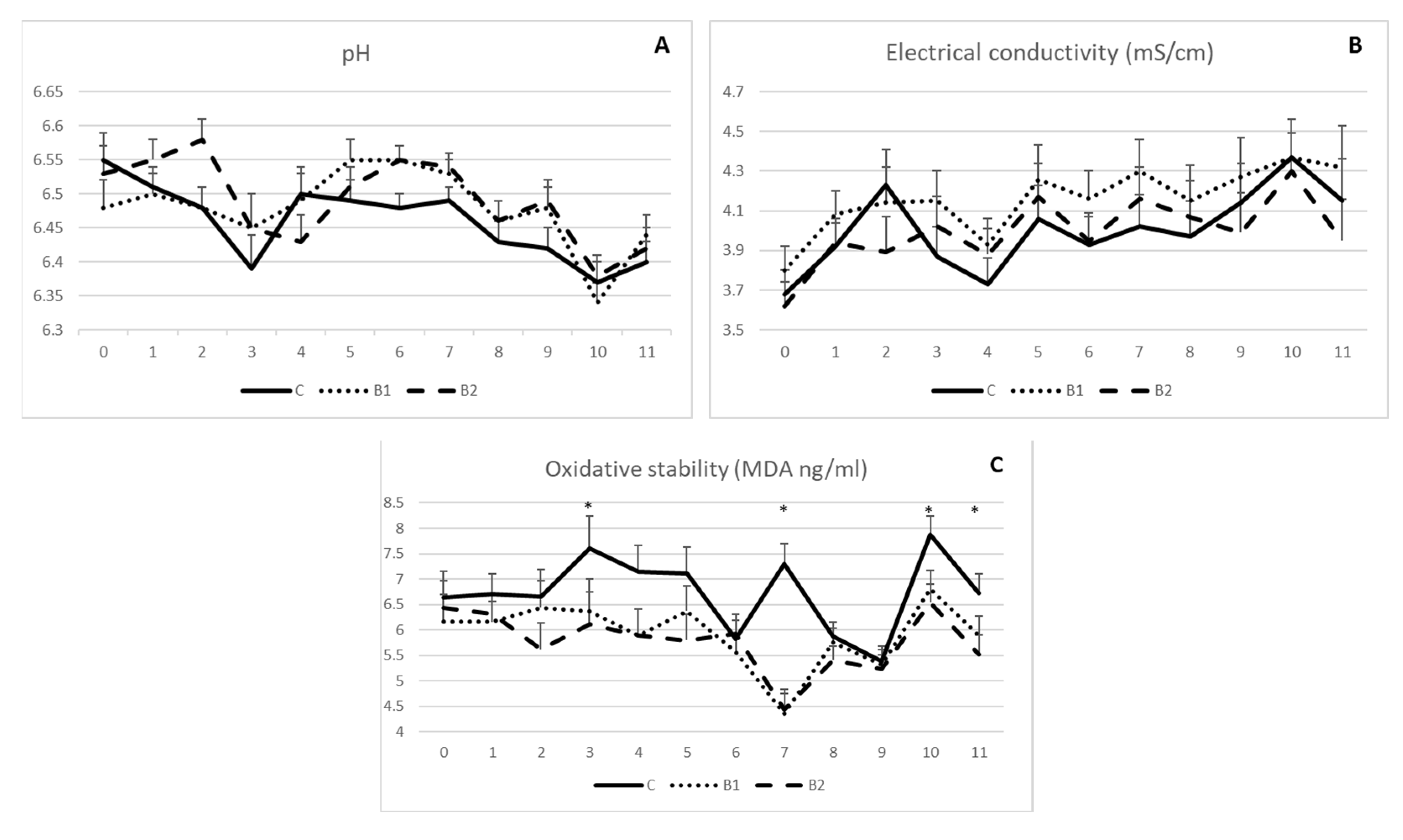
As indicated in Figure 2A,B, milk pH and electrical conductivity were not influenced by MPBC dietary supplementation, since no significant differences were observed among the experimental groups during the 11-week experimental period. On the other hand, milk oxidative stability was in general improved in MPBC supplemented groups as shown by the reduced MDA values. Significant differences were shown on week 3, 7, 10 and 11 (p < 0.05; Figure 2C). The respective MDA values (ng/g) were 6.36 and 6.11 vs. 7.60 (±0.64) on week 3, 4.35 and 4.44 vs. 7.29 (±0.40) on week 7, 6.81 and 6.54 vs. 7.87 (±0.36) on week 10 and 5.89 and 5.52 vs. 6.72 (±0.29) on week 11 for the B1, B2 and C groups, respectively (p < 0.05; Figure 2C).
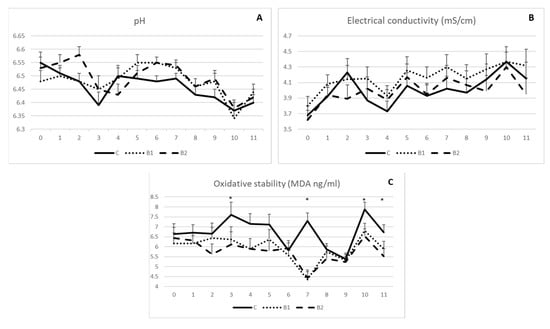
Figure 2.
Effect of MPBC dietary supplementation on milk pH (A), electrical conductivity (B) and oxidative stability (MDA levels) (C). Control group was fed with the basal concentrate diet (C), whereas the other two groups were offered the same concentrated diet further supplemented with MPBC (B1) at the levels of 0.05% or with rumen protected MPBC (B2) at the levels of 0.025%. The use of (*) indicates significant difference at p < 0.05.
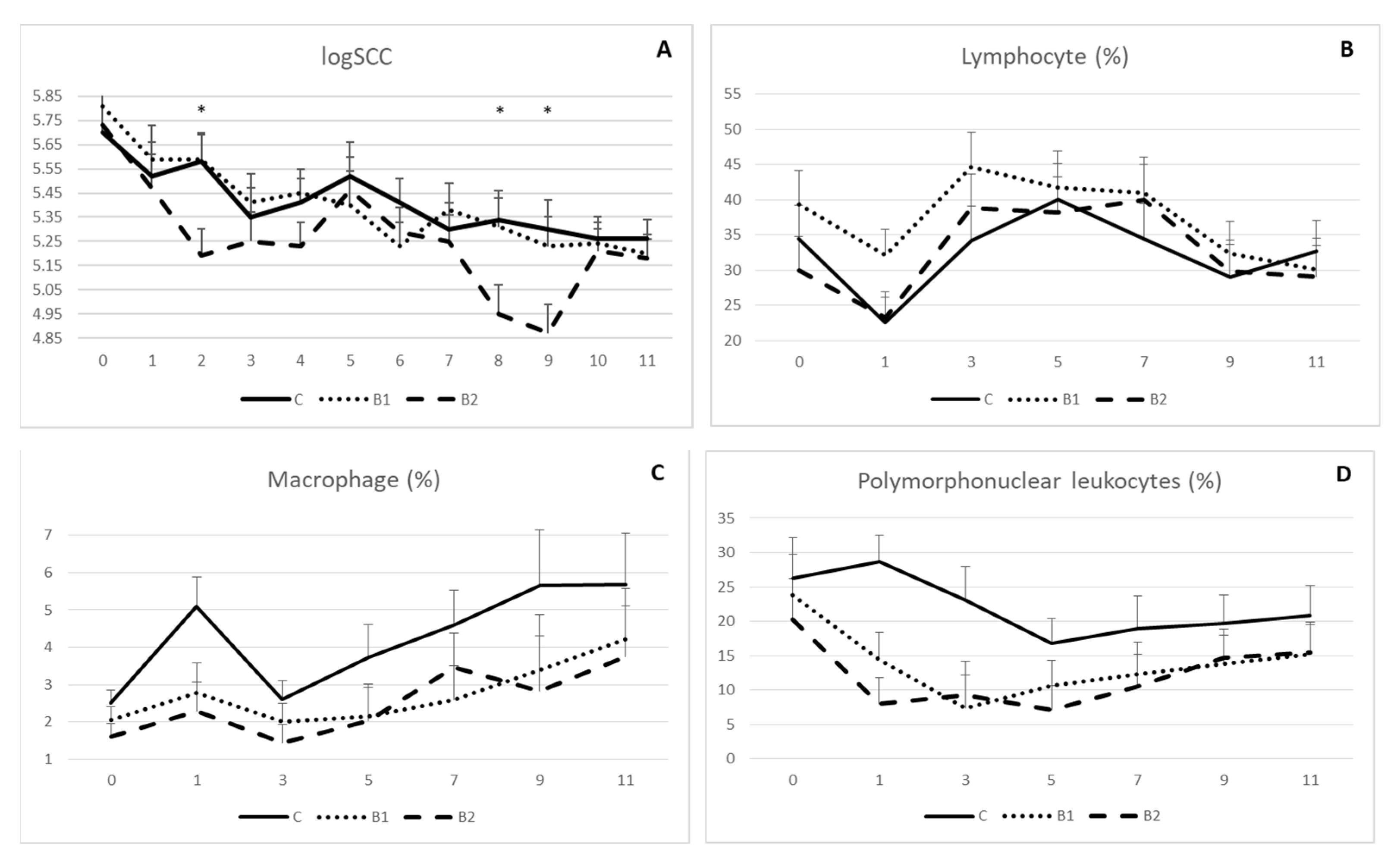
Rumen protected MPBC dietary supplementation decreased milk somatic cell count, as indicated in Figure 3A. However, significant differences were shown on week 2, 8 and 9. In detail, logSCC was 5.19 vs. 5.58 and 5.59 (±0.11) on week 2, 4.95 vs. 5.34 and 5.31 (±0.12) on week 8 and 4.87 vs. 5.30 and 5.23 (±0.12) on week 9 for B2, C and B1 group, respectively (p < 0.05; Figure 3A). On the other hand, proportions for lymphocyte (Figure 3B), macrophage (Figure 3C) and polymorphonuclear leucocytes (Figure 3D) were not significantly different among the experimental groups, although values for macrophage and polymorphonuclear leucocytes were numerically higher in controls compared to the MPBC dietary supplemented groups throughout the experiment.
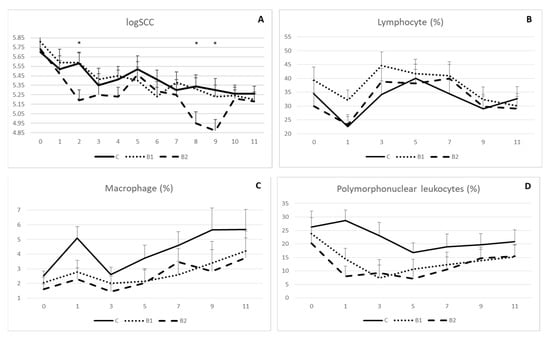
Figure 3.
Effect of MPBC dietary supplementation on logSCC (A), lymphocyte (B), macrophage (C) and polymorphonuclear leukocytes (D) count of sheep milk. Control group was fed with the basal concentrate diet (C), whereas the other two groups were offered the same concentrated diet further with MPBC (B1) at the levels of 0.05% or with rumen protected MPBC (B2) at the levels of 0.025%. The use of (*) indicates significant difference at p < 0.05.
4. Discussion
As indicated by the findings of the present study, the milk yield and fat-corrected milk yield in 6% were generally higher in the MPBC supplemented groups than the controls with significant differences between the 7th and 9th week of the experiment for the B2 compared to the other groups. However, milk composition was not significantly affected by MPBC dietary supplementation, while oxidative stability was improved in B1 and B2 groups. According to the existing literature, thyme and/or celery seed mixture [17], thyme or celery essential oil [18], cornus extract enriched with EOs of oregano and thyme [19], orange peel essential oil (EO) [20] and EO components mixture (thymol, eugenol, vanillin, guaiacol and limonene) [21] induced an increase in the milk yield in dairy ewes. Moreover, Kholif et al. [22] reported that capsicum/thymus essential oils blend at 2 mL and/or enzymes cocktail at 4 g per day enhanced milk yield and milk fat levels in dairy ewes. On the other hand, no effect of citral oil [23] or anise, clove, and thyme EO [24] on milk yield was observed, while an increase in milk yield, protein and fat levels was observed in dairy goats as a result of Boswellia sacra resin [25] and rosemary or lemon grass [26] dietary supplementation. Feed efficiency, milk yield and levels of protein, fat, total solids were increased as a result of Lippia alba hay inclusion in the diet of dairy goats [27]. Choubey et al. [28] observed that the dietary supplementation with flowers, shoots and leaves of Woodfordia fruticosa, the whole plant of Solanum nigrum and the seeds of Trigonella foenum-graecum improves antioxidant status in adult goats. In contrast, Leparmarai et al. [29] showed that grape seed dietary supplementation did not influence milk yield, milk composition and blood antioxidant status in dairy sheep and goats.
Shabtay et al. [30] reported that the addition of pomegranate extract to dairy cow diets resulted in higher milk production. Enhanced daily outputs of milk, energy corrected milk and fat were also observed after coriander oil dietary supplementation without any negative effect on cow health [31]. Greater milk yield, total solids, protein, lactose and fat and decreased malondialdehyde values were observed in dairy cows supplemented with a phytogenic feed additives mixture that contained menthol, anethole and other terpinenes [32]. Moreover, according to a meta-analysis by Belanche et al. [33], long-term exposure to a commercial blend of EOs (Agolin) resulted in a slight increase in milk yield at the level of 4%, while no effects on feed intake and milk composition were evident. Braun et al. [34] suggested that the aforementioned effects on milk production in dairy cattle could be attributed to the improved rumen fermentation, feed efficiency, nutrients’ absorption and utilization, and increased uptake of cations like calcium and ammonium as a result of phytobiotics’ dietary supplementation. As indicated, herbs and their extracts can accelerate digestion by reducing residence time in the digestive tract [35], while the observation of similar values in feed intake among treatments may indicate that although the MPBC dietary supplementation improved nutrient digestibility, did not negatively affect feed palatability and acceptance. However, no effects of thyme oil and thymol [36], eucalyptus, thyme and anise oil [37], eugenol [38], cinnamaldehyde and eugenol [39], cinnamaldehyde and garlic oil [40], garlic or juniper berry EO [41], oregano leaves [42,43], EOs components mixture (thymol, eugenol, vanillin, guaiacol and limonene) [44,45,46,47], blend of oregano, cinnamon, thyme and orange peel EOs [48] and mixture of eugenol, geranyl acetate and coriander oil [49] on milk yield and composition of dairy cattle are observed. In a study carried out with a mixture of plant bioactive components, a decrease in milk fat content was observed, while the other milk components were not affected in dairy cows [50]. In water buffaloes, dietary supplementation with a phytogenic mix containing seeds of fennel, ajwain and fenugreek, tubers of ginger, leaves of Swertia chirata, roots of licorice, fruits of Citrullus colocynthis, Terminalia chebula and turmeric did not affect milk yield and rumen fermentation parameters, apart from pH [51].
Moreover, the improved milk oxidative stability as suggested by the reduced MDA levels indicating that the provision of polyphenols with potent antioxidant or co-antioxidant activity might be a beneficial strategy to protect mammary cells against the adverse effects of free radicals. Part of the molecular multifunctionality of the natural bioactive compounds is their antioxidant capacity, which improves the immune status and reduces the oxidative stress of animals [52,53,54]. Phenolic compounds have been found to in vitro modify immune status via the downregulation of the inflammatory response, since they reduce the production of cytokines and reactive oxygen species, as well as the functionality of cytotoxic T-lymphocytes and natural killer cells [55]. The anti-inflammatory and antioxidant activity of polyphenols has been confirmed by in vivo studies in dairy cows, goats and ewes [52,56,57]. The aforementioned properties are attributed to their ability to chelate with free radicals, inhibit the enzymes actions associated with the mechanisms of oxidative stress, reinforce the functionality of antioxidant mechanisms, and prevent the lipid oxidation [53,58,59,60].
As indicated, SCC was reduced in dairy ewes that were dietary supplemented with the rumen protected MPBC with significant differences on week 2, 8 and 9. The decreased levels of SCC in milk from ewes dietary supplemented with MPBC is associated with an ameliorated udder health status, since milk SCC is considered as an index of mammary health. This finding could be attributed to the provision of several hydrophylic and lipophylic phenols that are included in the MBPC and through their antioxidant properties fortify mammary cells against the adverse effects of free radicals produced as a result of oxidative stress. Similar findings were reported by Hashemzadeh-Cigari et al. [50] in dairy cows after their supplementation with a phytobiotics-rich herbal mixture (185 g/cow) that contained cinnamon bark, turmeric roots, rosemary leaves and clove buds. Moreover, supplementation of rosemary extract [59] or a mixture of essential oils [21] to lactating ewes and concentrated pomegranate extract to dairy cows [30] resulted in reduced milk SCC. Jaquezeski et al. [60] also found that curcumin dietary supplementation improved milk yield and antioxidant capacity, while a reduction in somatic cell count and protein oxidation was reported in dairy sheep. In dairy cows, thyme essential oil supplementation via esophageal tube decreased the standard plate count, while no differences were observed in the raw milk composition [61]. Moreover, although Rodrigues et al. [62] observed an increase in milk yield after dietary supplementation with a phytogenic mix, no effect on milk composition and incidence of clinical mastitis was observed in dairy cows.
Although SCC was decreased as an effect of rumen-protected MPBC dietary supplementation, no significant differences were observed among the treatments concerning the macrophage, lymphocyte, and polymorphonuclear leukocyte (PMN) counts. However, although there was not a clear tendency for the lymphocyte count, macrophages were increased and polymorphonuclear leukocytes were decreased from the beginning till the end of the experimental period. Although only numerical, MPBC supplemented groups had a lower PMN count compared to the controls, indicating a tendency for a healthier mammary gland, since PMN being the principal leucocytes that are increased during pathogen invasion, are closely correlated with high SCC [63] and oxidation stress in mammary gland [64]. On the other hand, macrophages represents 5–7% of leucocytes in ewe milk and are found to minimally contribute to the proteolytic activity in ewe milk [65]. Finally, lymphocytes represents approximately 40% of leucocyte population [66] and an absence of differences in the lymphocyte count as an effect of SCC suggests that in ewe milk this population is quite stable [63].
5. Conclusions
Plant extracts have been widely recognized as potential functional alternatives to antibiotics due to their green, safe, and efficient properties. Dietary supplementation with a mixture of phytogenic substances originated from thyme, anise and olive was effective in improving milk oxidative stability (third, seventh, tenth and eleventh week), enhancing performance (seventh–ninth week) and lowering SCC (second, eighth and ninth week) when provided in its rumen protected form in mid-lactation high-producing dairy ewes. Considering the positive outcome on milk yield, oxidative stability and SCC and the lack of any side effects on the other milk properties, the MPBC used in the present study and especially in its rumen protected form appeared as a promising candidate for a feed additive for dairy ewes. The use of the protected form of MPBC may be successfully adapted in a production system that incorporates precision livestock feeding. However, a further in-depth analysis is necessary regarding its production cost before establishing its regular use.
Author Contributions
Conceptualization, P.S. and M.G.; methodology, P.S. and A.H.-T.; validation, P.S., M.G. and A.H.-T.; formal analysis, M.G.; investigation, A.K., M.T., K.P. and P.S.; resources, P.S.; writing—original draft preparation, A.K. and P.S.; writing—review and editing, M.G., M.T., K.P. and A.H.-T.; visualization, P.S.; supervision, P.S.; project administration, P.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Nuevo SA, AUA grant no: 35.0054.
Institutional Review Board Statement
The animal study protocol was approved by Research Ethics Committee of the Agricultural University of Athens (protocol code 46/09.05.2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used to support the findings of this study are included within the article.
Acknowledgments
The authors would like to thank Athanasios Gelasakis from the Laboratory of Anatomy and Physiology of Farm Animals, Department of Animal Science, School of Animal Biosciences, Agricultural University of Athens for his technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript and in the decision to publish the results.
References
- EU Regulation 1831/2003/EC of 22 September 2003 on Additives for Use in Animal Nutrition, Replacing Directive 70/524/EEC on Additives in Feeding-Stuffs. Available online: https://eur-lex.europa.eu/legal-content/en/ALL/?uri=CELEX%3A32003R1831 (accessed on 15 June 2023).
- Simitzis, P.E. Enrichment of animal diets with essential oils—A great perspective on improving animal performance and quality characteristics of the derived products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals 2021, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of rumen fermentation and methane production with plant secondary metabolites. Anim. Feed Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Elghalid, O.A.; Kholif, A.E.; El-Ashry, G.M.; Matloup, O.H.; Olafadehan, O.A.; El-Raffa, A.M.; Abd El-Hady, A.M. Oral supplementation of the diet of growing rabbits with a newly developed mixture of herbal plants and spices enriched with special extracts and essential oils affects their productive performance and immune status. Livest. Sci. 2020, 238, 104082. [Google Scholar] [CrossRef]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant bioactives for ruminant health and productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef]
- Schukken, Y.; Wilson, D.; Welcome, F.; Garrison-Tikofsky, L.; Gonzalez, R. Monitoring udder health and milk quality using somatic cell counts. Vet. Res. 2003, 34, 579–596. [Google Scholar] [CrossRef]
- Salami, S.; Guinguina, A.; Agboola, J.; Omede, A.; Agbonlahor, E.; Tayyab, U. In vivo and postmortem effects of feed antioxidants in livestock: A review of the implications on authorization of antioxidant feed additives. Animal 2016, 10, 1375–1390. [Google Scholar] [CrossRef]
- Nudda, A.; Cannas, A.; Correddu, F.; Atzori, A.S.; Lunesu, M.F.; Battacone, G.; Pulina, G. Sheep and goats respond differently to feeding strategies directed to improve the fatty acid profile of milk fat. Animals 2020, 10, 1290. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K. Reactive oxygen species (ROS) and cancer: Role of antioxidative nutraceuticals. Cancer Lett. 2017, 387, 95–105. [Google Scholar] [CrossRef]
- Gobert, M.; Martin, B.; Ferlay, A.; Chilliard, Y.; Graulet, B.; Pradel, P.; Bauchart, D.; Durand, D. Plant polyphenols associated with vitamin E can reduce plasma lipoperoxidation in dairy cows given n-3 polyunsaturated fatty acids. J. Dairy Sci. 2009, 92, 6095–6104. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Parada, J.; Martinez-Ferez, A.; Segura-Carretero, A.; Robert, P. Role of maltodextrin and inulin as encapsulating agents on the protection of oleuropein during in vitro gastrointestinal digestion. Food Chem. 2020, 310, 125976. [Google Scholar] [CrossRef] [PubMed]
- Todorović, A.; Šturm, L.; Salević-Jelić, A.; Lević, S.; Osojnik Črnivec, I.G.; Prislan, I.; Skrt, M.; Bjekovic, A.; Poklar Ulrih, N.; Nedović, V. Encapsulation of bilberry extract with maltodextrin and gum arabic by freeze-drying: Formulation, characterisation, and storage stability. Processes 2022, 10, 1991. [Google Scholar] [CrossRef]
- Botsoglou, N.A.; Fletouris, D.J.; Papageorgiou, G.E.; Vassilopoulos, V.N.; Mantis, A.J.; Trakatellis, A.G. A rapid, sensitive andspecific thiobarbituric acid method for measuring lipid peroxidation in animal tissues, food and feedstuff samples. J. Agric. Food Chem. 1994, 42, 1931–1937. [Google Scholar] [CrossRef]
- Koess, C.; Hamann, J. Detection of mastitis in the bovine mammary gland by flow cytometry at early stages. J. Dairy Res. 2008, 75, 225–232. [Google Scholar] [CrossRef]
- Khattab, M.S.A.; Kholif, A.E.; Abd El Tawab, A.M.; Shaaban, M.M.; Hadhoud, F.I.; El-Fouly, H.A.; Olafadehan, O.A. Effect of replacement of antibiotics with thyme and celery seed mixture on the feed intake and digestion, ruminal fermentation, blood chemistry, and milk lactation of lactating Barki ewes. Food Funct. 2020, 11, 6889–6898. [Google Scholar] [CrossRef]
- Abd El Tawab, A.M.; Kholif, A.E.; Khattab, M.S.A.; Shaaban, M.M.; Hadhoud, F.I.; Mostafa, M.M.M.; Olafadehan, O.A. Feed utilization and lactational performance of Barki sheep fed diets containing thyme or celery. Small Rumin. Res. 2020, 192, 106249. [Google Scholar] [CrossRef]
- Kalaitsidis, K.; Sidiropoulou, E.; Tsiftsoglou, O.; Mourtzinos, I.; Moschakis, T.; Basdagianni, Z.; Vasilopoulos, S.; Chatzigavriel, S.; Lazari, D.; Giannenas, I. Effects of cornus and its mixture with oregano and thyme essential oils on dairy sheep performance and milk, yoghurt and cheese quality under heat stress. Animals 2021, 11, 1063. [Google Scholar] [CrossRef]
- Kotsampasi, B.; Tsiplakou, E.; Christodoulou, C.; Mavrommatis, A.; Mitsiopoulou, C.; Karaiskou, C.; Sossidou, E.; Fragioudakis, N.; Kapsomenos, I.; Bampidis, V.A.; et al. Effects of dietary orange peel essential oil supplementation on milk yield and composition, and blood and milk antioxidant status of dairy ewes. Anim. Feed Sci. Technol. 2018, 245, 20–31. [Google Scholar] [CrossRef]
- Giannenas, I.; Skoufos, J.; Giannakopoulos, C.; Wiemann, M.; Gortzi, O.; Lalas, S.; Kyriazakis, I. Effects of essential oils on milk production, milk composition, and rumen microbiota in Chios dairy ewes. J. Dairy Sci. 2011, 94, 5569–5577. [Google Scholar] [CrossRef]
- Kholif, A.E.; Kassab, A.Y.; Azzaz, H.H.; Matloup, O.H.; Hamdon, H.A.; Olafadehan, O.A.; Morsy, T.A. Essential oils blend with a newly developed enzyme cocktail works synergistically to enhance feed utilization and milk production of Farafra ewes in the subtropics. Small Rumin. Res. 2018, 161, 43–50. [Google Scholar] [CrossRef]
- Canaes, T.S.; Zanferari, F.; Maganhe, B.L.; Takiya, C.S.; Silva, T.H.; Del Valle, T.A.; Rennó, F.P. Increasing dietary levels of citral oil on nutrient total tract digestibility, ruminal fermentation, and milk composition in Saanen goats. Anim. Feed Sci. Technol. 2017, 229, 47–56. [Google Scholar] [CrossRef]
- El-Essawy, A.M.; Anele, U.Y.; Abdel-Wahed, A.M.; Abdou, A.R.; Khattab, I.M. Effects of anise, clove and thyme essential oils supplementation on rumen fermentation, blood metabolites, milk yield and milk composition in lactating goats. Anim. Feed Sci. Technol. 2021, 271, 114760. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Morsy, A.S.; Hashem, N.M.; Sallam, S.M. Boswellia sacra resin as a phytogenic feed supplement to enhance ruminal fermentation, milk yield, and metabolic energy status of early lactating goats. Anim. Feed Sci. Technol. 2021, 277, 114963. [Google Scholar] [CrossRef]
- Kholif, A.E.; Matloup, O.H.; Morsy, T.A.; Abdo, M.M.; Abu Elella, A.A.; Anele, U.Y.; Swanson, K.C. Rosemary and lemongrass herbs as phytogenic feed additives to improve efficient feed utilization, manipulate rumen fermentation and elevate milk production of Damascus goats. Livest. Sci. 2017, 204, 39–46. [Google Scholar] [CrossRef]
- da Silva, N.I.S.; de Moura, J.F.P.; de Lucena Nascimento, M.E.; Machado, F.C.G.; Costa, T.G.P.; de Araújo Filho, J.M.; Cordão, M.A.; Edvan, R.L.; Oliveira, R.L.; Bezerra, L.R. Effect of Lippia alba hay as phytogenic feed additive on the lactation performance, milk composition, and rumen and blood parameters of Alpine goats. Small Rumin. Res. 2022, 215, 106767. [Google Scholar] [CrossRef]
- Choubey, M.; Pattanaik, A.K.; Baliyan, S.; Dutta, N.; Jadhav, S.E.; Sharma, K. Dietary supplementation of a novel phytogenic feed additive: Effects on nutrient metabolism, antioxidant status and immune response of goats. Anim. Prod. Sci. 2015, 56, 1612–1621. [Google Scholar] [CrossRef]
- Leparmarai, P.T.; Sinz, S.; Kunz, C.; Liesegang, A.; Ortmann, S.; Kreuzer, M.; Marquardt, S. Transfer of total phenols from a grapeseed-supplemented diet to dairy sheep and goat milk, and effects on performance and milk quality. J. Anim. Sci. 2019, 97, 1840–1851. [Google Scholar] [CrossRef]
- Shabtay, A.; Nikbachat, M.; Zenou, A.; Yosef, E.; Arkin, O.; Sneer, O.; Shwimmer, A.; Yaari, A.; Budman, E.; Agmon, G.; et al. Effects of adding a concentrated pomegranate extract to the ration of lactating cows on performance and udder health parameters. Anim. Feed Sci. Technol. 2012, 175, 24–32. [Google Scholar] [CrossRef]
- Matloup, O.H.; Abd El Tawab, A.M.; Hassan, A.A.; Hadhoud, F.I.; Khattab, M.S.A.; Khalel, M.S.; Sallam, S.M.A.; Kholif, A.E. Performance of lactating Friesian cows fed a diet supplemented with coriander oil: Feed intake, nutrient digestibility, ruminal fermentation, blood chemistry, and milk production. Anim. Feed Sci. Technol. 2017, 226, 88–97. [Google Scholar] [CrossRef]
- Kholif, A.E.; Hassan, A.A.; El Ashry, G.M.; Bakr, M.H.; El-Zaiat, H.M.; Olafadehan, O.A.; Matloup, O.H.; Sallam, S.M.A. Phytogenic feed additives mixture enhances the lactational performance, feed utilization and ruminal fermentation of Friesian cows. Anim. Biotechnol. 2021, 32, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A meta-analysis describing the effects of the essential oils blend agolin ruminant on performance, rumen fermentation and methane emissions in dairy cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.S.; Schrapers, K.T.; Mahlkow-Nerge, K.; Stumpff, F.; Rosendahl, J. Dietary supplementation of essential oils in dairy cows: Evidence for stimulatory effects on nutrient absorption. Animal 2019, 13, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivasan, K. Studies on the in vitro absorption of spice principles—Curcumin, capsaicin and piperine in rat intestines. Food Chem. Toxicol. 2007, 45, 1437–1442. [Google Scholar] [CrossRef]
- Benchaar, C. Diet supplementation with thyme oil and its main component thymol failed to favorably alter rumen fermentation, improve nutrient utilization, or enhance milk production in dairy cows. J. Dairy Sci. 2021, 104, 324–336. [Google Scholar] [CrossRef]
- Giller, K.; Rilko, T.; Manzocchi, E.; Hug, S.; Bolt, R.; Kreuzer, M. Effects of mixed essential oils from eucalyptus, thyme and anise on composition, coagulation properties and antioxidant capacity of the milk of dairy cows. J. Anim. Feed Sci. 2020, 29, 3–10. [Google Scholar] [CrossRef]
- Benchaar, C.; Lettat, A.; Hassanat, F.; Yang, W.Z.; Forster, R.J.; Petit, H.V.; Chouinard, P.Y. Eugenol for dairy cows fed low or high concentrate diets: Effects on digestion, ruminal fermentation characteristics, rumen microbial populations and milk fatty acid profile. Anim. Feed Sci. Technol. 2012, 178, 139–150. [Google Scholar] [CrossRef]
- Tager, L.R.; Krause, K.M. Effects of essential oils on rumen fermentation, milk production, and feeding behavior in lactating dairy cows. J. Dairy Sci. 2011, 94, 2455–2464. [Google Scholar] [CrossRef]
- Blanch, M.; Carro, M.D.; Ranilla, M.J.; Viso, A.; Vazquez-Anon, M.; Bach, A. 2016. Influence of a mixture of cinnamaldehyde and garlic oil on rumen fermentation, feeding behaviour and performance of lactating dairy cows. Anim. Feed Sci. Technol. 2016, 219, 313–323. [Google Scholar] [CrossRef]
- Yang, W.Z.; Benchaar, C.; Ametaj, B.N.; Chaves, A.V.; He, M.L.; McAllister, T.A. Effects of garlic and juniper berry essential oils on ruminal fermentation and on the site and extent of digestion in lactating cows. J. Dairy Sci. 2007, 90, 5671–5681. [Google Scholar] [CrossRef]
- Hristov, A.N.; Lee, C.; Cassidy, T.; Heyler, K.; Tekippe, J.A.; Varga, G.A.; Corl, B.; Brandt, R.C. Effect of Origanum vulgare L. leaves on rumen fermentation, production, and milk fatty acid composition in lactating dairy cows. J. Dairy Sci. 2013, 96, 1189–1202. [Google Scholar] [CrossRef]
- Tekippe, J.A.; Hristov, A.N.; Heyler, K.S.; Cassidy, T.W.; Zheljazkov, V.D.; Ferreira, J.F.S.; Karnati, S.K.; Varga, G.A. Rumen fermentation and production effects of Origanum vulgare L. leaves in lactating dairy cows. J. Dairy Sci. 2011, 94, 5065–5079. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Ouellet, D.R.; Chiquette, J.; Chouinard, P.Y. Effects of essential oils on digestion, ruminal fermentation, rumen microbial populations, milk production, and milk composition in dairy cows fed alfalfa silage or corn silage. J. Dairy Sci. 2007, 90, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; Petit, H.V.; Berthiaume, R.; Whyte, T.D.; Chouinard, P.Y. Effects of addition of essential oils and monensin premix on digestion, ruminal fermentation, milk production, and milk composition in dairy cows. J. Dairy Sci. 2006, 89, 4352–4364. [Google Scholar] [CrossRef] [PubMed]
- Kung, L.; Williams, P.; Schmidt, R.J.; Hu, W. A blend of essential plant oils used as an additive to alter silage fermentation or used as a feed additive for lactating dairy cows. J. Dairy Sci. 2008, 91, 4793–4800. [Google Scholar] [CrossRef] [PubMed]
- Joch, M.; Kudrna, V.; Hakl, J.; Božik, M.; Homolka, P.; Illek, J.; Tyrolova, Y.; Výborná, A. In vitro and in vivo potential of a blend of essential oil compounds to improve rumen fermentation and performance of dairy cows. Anim. Feed Sci. Technol. 2019, 251, 176–186. [Google Scholar] [CrossRef]
- Spanghero, M.; Robinson, P.H.; Zanfi, C.; Fabbro, E. Effect of increasing doses of a microencapsulated blend of essential oils on performance of lactating primiparous dairy cows. Anim. Feed Sci. Technol. 2009, 153, 153–157. [Google Scholar] [CrossRef]
- Santos, M.B.; Robinson, P.H.; Williams, P.; Losa, R. Effects of addition of an essential oil complex to the diet of lactating dairy cows on whole tract digestion of nutrients and productive performance. Anim. Feed Sci. Technol. 2010, 157, 64–71. [Google Scholar] [CrossRef]
- Hashemzadeh-Cigari, F.; Khorvash, M.; Ghorbani, G.R.; Kadivar, M.; Riasi, A.; Zebeli, Q. Effects of supplementation with a phytobiotics-rich herbal mixture on performance, udder health, and metabolic status of Holstein cows with various levels of milk somatic cell counts. J. Dairy Sci. 2014, 97, 7487–7497. [Google Scholar] [CrossRef]
- Hassan, F.U.; Ebeid, H.M.; Tang, Z.; Li, M.; Peng, L.; Peng, K.; Liang, X.; Yang, C. A Mixed Phytogenic Modulates the Rumen Bacteria Composition and Milk Fatty Acid Profile of Water Buffaloes. Front. Vet. Sci. 2020, 7, 569. [Google Scholar] [CrossRef]
- Salem, A.Z.M.; López, S.; Robinson, P.H. Plant bioactive compounds in ruminant agriculture—Impacts and opportunities. Anim. Feed Sci. Technol. 2012, 176, 1–4. [Google Scholar] [CrossRef]
- Tayengwa, T.; Mapiye, C. Citrus and winery wastes: Promising dietary supplements for sustainable ruminant animal nutrition, health, production, and meat quality. Sustainability 2018, 10, 3718. [Google Scholar] [CrossRef]
- Piao, M.; Tu, Y.; Zhang, N.; Diao, Q.; Bi, Y. Advances in the Application of Phytogenic Extracts as Antioxidants and Their Potential Mechanisms in Ruminants. Antioxidants 2023, 12, 879. [Google Scholar] [CrossRef] [PubMed]
- Provenza, F.D.; Villalba, J.J. The role of natural plant products in modulating the immune system: An adaptable approach for combating disease in grazing animals. Small Rumin. Res. 2010, 89, 131–139. [Google Scholar] [CrossRef]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can agro-industrial by-products rich in polyphenols be advantageously used in the feeding and nutrition of dairy small ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Niderkorn, V.; Jayanegara, A. Opportunities offered by plant bioactive compounds to improve silage quality, animal health and product quality for sustainable ruminant production: A review. Agronomy 2021, 11, 86. [Google Scholar] [CrossRef]
- Soldado, D.; Bessa, R.J.B.; Jerónimo, E. Condensed tannins as antioxidants in ruminants—Effectiveness and action mechanisms to improve animal antioxidant status and oxidative stability of products. Animals 2021, 11, 3243. [Google Scholar] [CrossRef]
- Chiofalo, V.; Liotta, L.; Fiumanò, R.; Riolo, E.B.; Chiofalo, B. Influence of dietary supplementation of Rosmarinus officinalis L. on performances of dairy ewes organically managed. Small Rumin. Res. 2012, 104, 122–128. [Google Scholar] [CrossRef]
- Jaguezeski, A.M.; Perin, G.; Bottari, N.B.; Wagner, R.; Fagundes, M.B.; Schetinger, M.R.C.; Morsch, V.M.; Stein, C.S.; Moresco, R.N.; Barreta, D.A.; et al. Addition of curcumin to the diet of dairy sheep improves health, performance and milk quality. Anim. Feed Sci. Technol. 2018, 246, 144–157. [Google Scholar] [CrossRef]
- Castelani, L.; Pfrimer, K.; Giglioti, R.; van Cleef, E.H.C.B.; Salles, M.S.V.; Júnior, L.C.R. Effects of thyme (Thymus vulgaris L.) essential oil supplementation on the microbiological quality of raw milk of lactating dairy cows. Res. Vet. Sci. 2023, 161, 118–121. [Google Scholar] [CrossRef]
- Rodrigues, R.O.; Cooke, R.F.; Firmino, F.C.; Moura, M.K.; Angeli, B.F.; Ferreira, H.A.; Brandao, A.P.; Gex-Farby, M.; Ostrensky, A.; Vasconcelos, J.L. Productive and physiological responses of lactating dairy cows supplemented with phytogenic feed ingredients. Transl. Anim. Sci. 2019, 3, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Albenzio, M.; Caroprese, M. Differential leucocyte count for ewe milk with low and high somatic cell count. J. Dairy Res. 2011, 78, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Hamed, H.; El Feki, A.; Gargouri, A. Total and differential bulk cow milk somatic cell counts and their relation with antioxidant factors. Comptes Rendus Biol. 2008, 331, 144–151. [Google Scholar] [CrossRef]
- Caroprese, M.; Marzano, A.; Schena, L.; Marino, R.; Santillo, A.; Albenzio, M. Contribution of macrophages to proteolysis and plasmin activity in ewe bulk milk. J. Dairy Sci. 2007, 90, 2767–2772. [Google Scholar] [CrossRef] [PubMed]
- Albenzio, M.; Santillo, A.; Caroprese, M.; d’Angelo, F.; Marino, R.; Sevi, A. Role of endogenous enzymes in proteolysis of sheep milk. J. Dairy Sci. 2009, 92, 79–86. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).