Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals
Abstract
1. Introduction
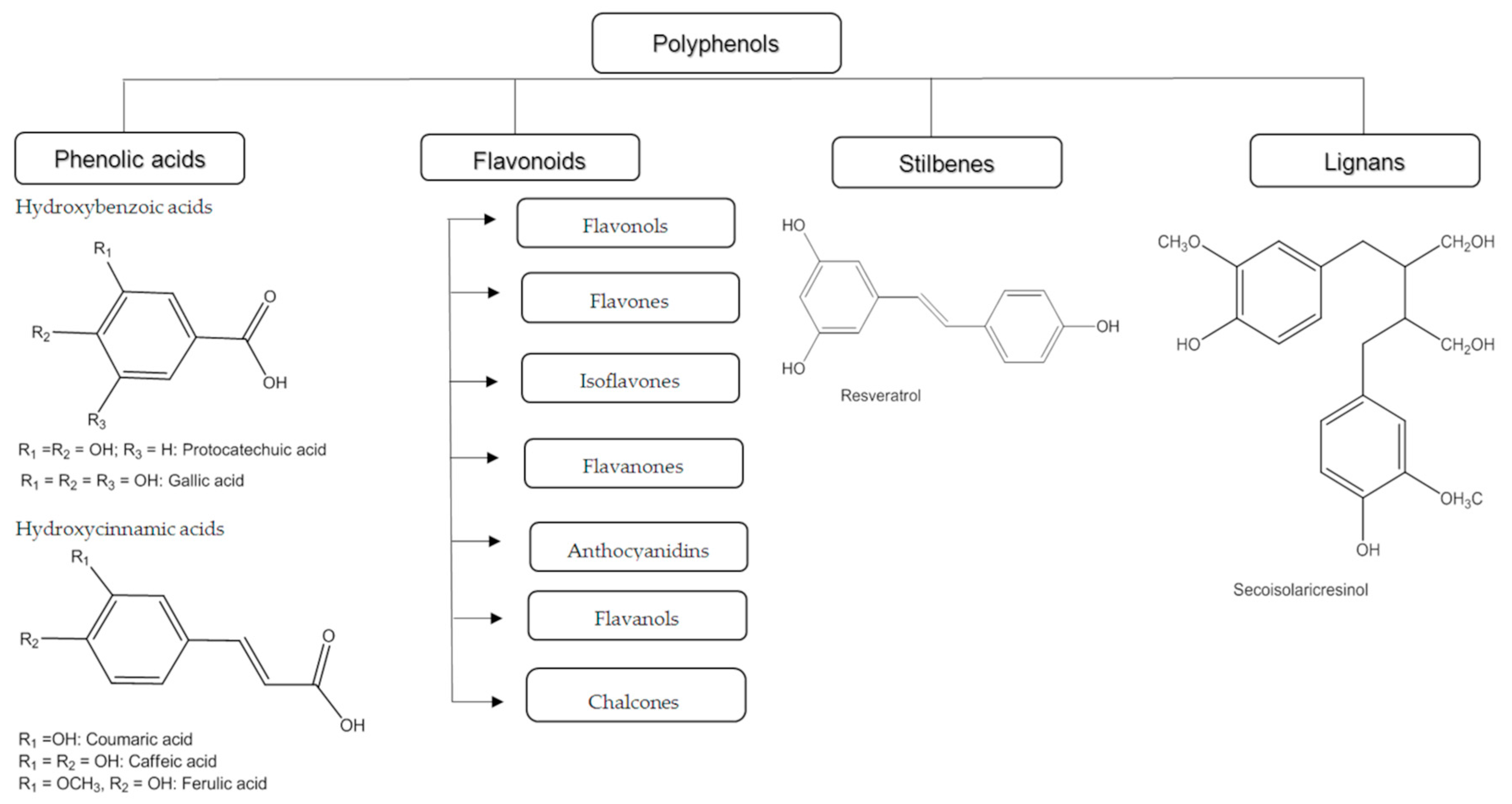
2. Classification of Polyphenols
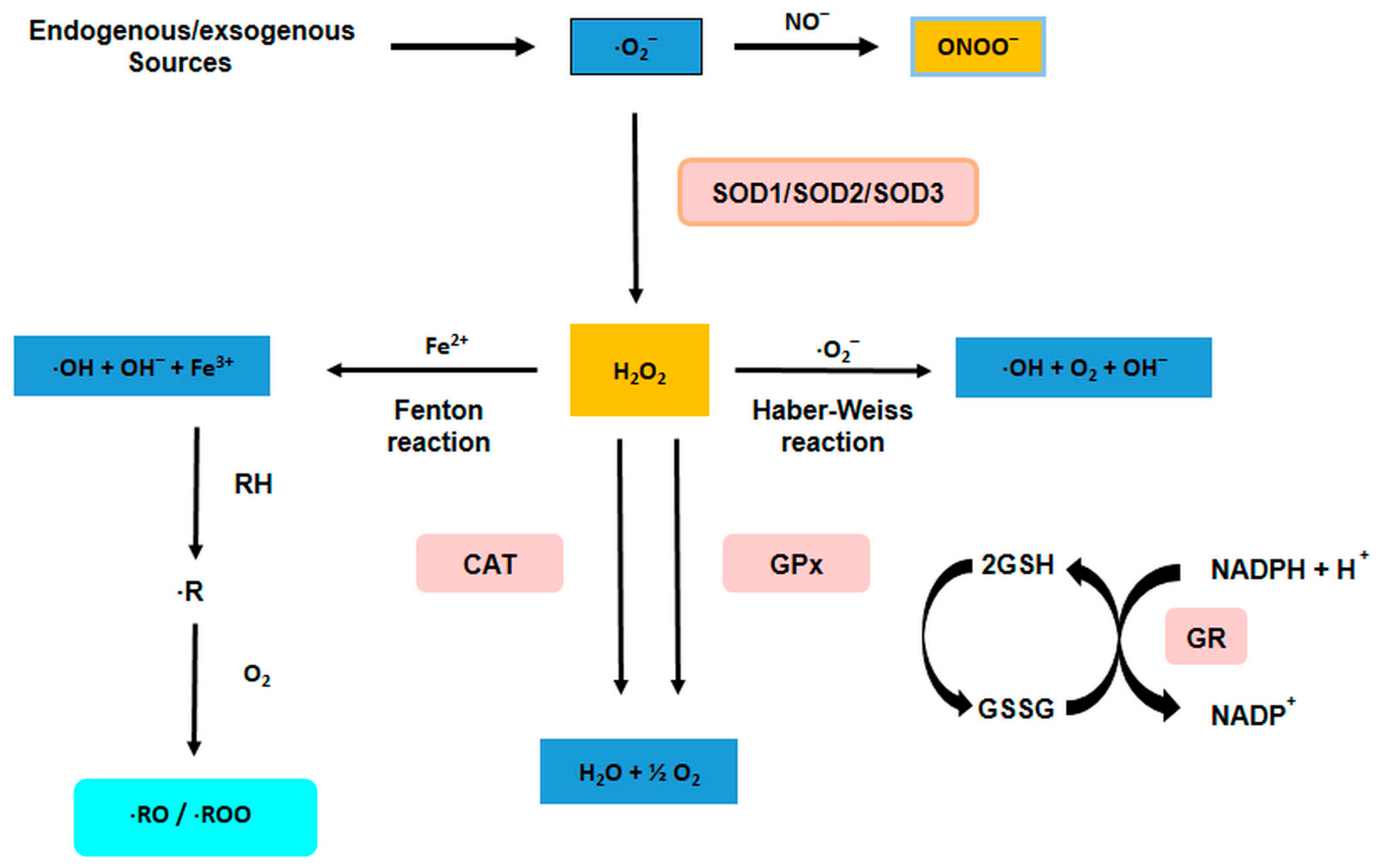
3. Reactive Oxygen Species and Reactive Nitrogen Species
3.1. Sources of ROS
3.2. Nitrosative Stress
3.3. Free Radicals and Internal Defense
3.4. Exogenous Molecules in Defense against Oxidative Stress
4. Distribution of Polyphenols in Nature
5. Digestion, Absorption, and Metabolism of Polyphenols
5.1. Digestion/Metabolism/Biotransformation and Bioavailability of Polyphenols in Animals
5.2. Biotransformation of Polyphenols by the Gut/Colon Microbiota
5.2.1. Fate of Polyphenols in the Stomach and Small Intestine
5.2.2. Polyphenols and the Gut Microbiome
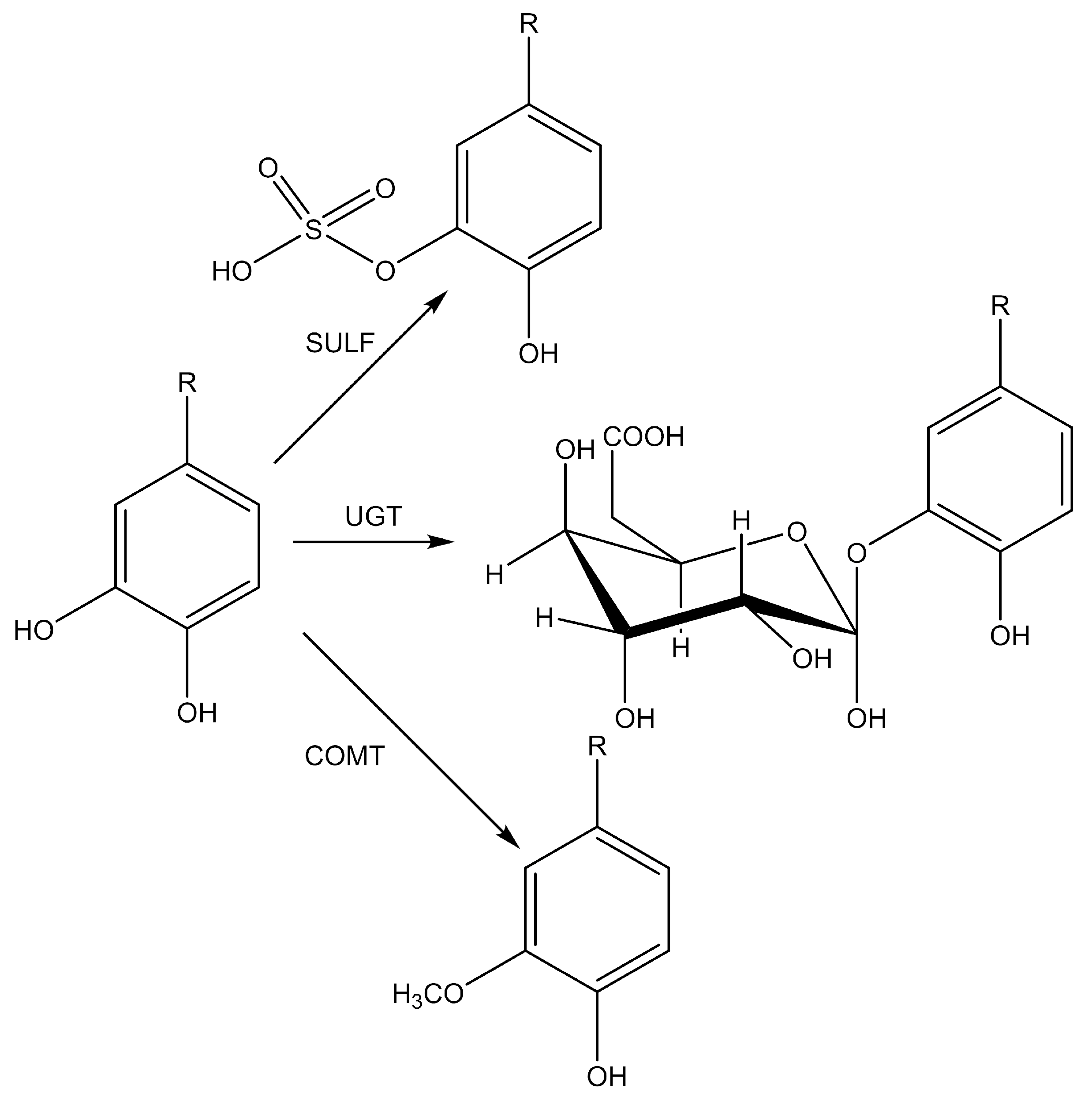
5.2.3. Reaction Phases of the Biotransformation of Polyphenols
5.2.4. The Most Important Metabolic Reactions in the Biotransformation of Polyphenols
5.2.5. Uptake of Polyphenol Metabolites into the Body
5.3. The Relationship between the Intake of Food Rich in Polyphenols and the Change in Intestinal Microbiota
6. Antioxidant and Pro-Oxidant Activity of Polyphenols
6.1. Antioxidant Activity of Polyphenols
6.2. Pro-oxidative Activity of Polyphenols
7. The Influence of Polyphenols on Animals
7.1. Effect of the Addition of Polyphenols on Animals
7.2. Immunomodulatory Effect of Polyphenols on Intestinal Health of Animals
7.3. The Effectiveness of Polyphenols on the Quality of Products of Animal Origin
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PUFA | polyunsaturated fatty acid |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| SOD | superoxide dismutase |
| CAT | catalase |
| GTx | glutathion peroxidase |
| SFA | saturation fatty acid |
| CTC | glucocorticoide |
| GP | grape pomace |
| ETC | electron transport chain |
| CYP | cytochrome P450 |
| NOX | NADPH oxidase |
| NF-kB | nuclear factor kappa B |
| UGT | uridine-5-diphosphate glucuronosyltranspherase |
| SULT | sulphotransferase |
| COMT | catechol-O-methyltransferase |
| OMTs | O-methyltransferases |
| HAT | hydrogen atom transfer |
| PCET | one-electron transfer-proton transfer |
| SPLET | sequential proton loss electron transfer |
References
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Lipiński, K.; Mazur, M.; Antoszkiewicz, Z.; Purwin, C. Polyphenols in monogastric nutrition. Ann. Anim. Sci. 2017, 17, 41–58. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.M.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; Abd El-Hack, M. The role of polyphenols in poultry nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Serra, V.; Salvatori, G.; Pastorelli, G. Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals 2021, 11, 401. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, J.D. Flavonoid antioxidants: Chemistry, metabolism and structure-activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sies, H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. Engl. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Lauridsen, C.; Hojsgaard, S.; Sørensen, M.T. Influence of dietary rapeseed oil, vitamin E and copper on the performance and the antioxidative and oxidative status in pigs. J. Anim. Sci. 1999, 77, 906–916. [Google Scholar] [CrossRef]
- Basu, S.; Eriksson, M. Oxidative injury and survival during endotoxemia. FEBS Lett. 1998, 438, 159–160. [Google Scholar] [CrossRef]
- Campbell, J.M.; Crenshaw, J.D.; Polo, J. The biological stress of early weaned piglets. J. Anim. Sci. Biotechn. 2013, 4, 19. [Google Scholar] [CrossRef]
- Wijtten, P.J.; van der Meulen, J.; Verstegen, M.W. Intestinal barrier function and absorption in pigs after weaning: A review. Brit. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef]
- Ohtsuka, A.; Ohtani, T.; Horiguchi, H.; Kojima, H.; Hayashi, K. Vitamin E reduces glucocorticoidinduced growth inhibition and lipid peroxidation inrats. J. Nutr. Sci. Vitaminol. 1998, 44, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Tanigucha, N.; Ohtsuka, A.; Hayashi, K. Effect of dietary corticosterone and vitamin E on growth and oxidative stress in broiler chickens. Anim. Sci. J. 1999, 70, 195–200. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 26. [Google Scholar] [CrossRef]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Cadet, J.; Davies, K.J.A.; Medeiros, M.H.G.; Di Mascio, P.; Wagner, J.R. Formation and repair of oxidatively generated damage in cellular DNA. Free Radic. Biol. Med. 2017, 107, 13–34. [Google Scholar] [CrossRef]
- Chamorro, S.A.; Viveros, A.A.; Rebolé, A.; Rica, B.D.I.; Arija, I.; Brenes, A. Influence of dietary enzyme addition on polyphenol utilization andmeat lipid oxidation of chicks fed grape pomace. Food Res. Int. 2015, 73, 197–203. [Google Scholar] [CrossRef]
- Rymer, C.; Given, D.I. Effects of vitamin E and fish oil inclusion in broiler diets on meat fatty acid composition and on the flavour of a composite sample of breast meat. J. Sci. Food Agric. 2010, 90, 1628–1633. [Google Scholar] [CrossRef]
- Aziza, A.E.; Quezada, N.; Cherian, G. Antioxidative effect of dietary camelina meal in fresh, stored, or cooked broiler chicken meat. Poult. Sci. 2010, 89, 2711–2718. [Google Scholar] [CrossRef]
- Brenes, A.A.; Viveros, I.; Goñi, C.; Centeno, S.G.; Sáyago-Ayerdi, I.; Saura-Calixto, A.F. Effect of grape pomace concentrate and vitamin E on digestibility of polyphenols and antioxidant activity in chickens. Poult. Sci. 2008, 87, 307–316. [Google Scholar] [CrossRef]
- Pascual, J.V.M.; Rafecas, M.A.; Canela, J.; Boatella, R.; Bou, A.C.; Barroeta, C.; Codony, R. Effect of increasing amounts of a linoleic-rich dietary fat on the fat composition of four pig breeds. Part II: Fatty acid composition in muscle and fat tissues. Food Chem. 2007, 100, 1639–1648. [Google Scholar] [CrossRef]
- Bešlo, D.; Došlić, G.; Agić, D.; Rastija, V.; Šperanda, M.; Gantner, V.; Lučić, B. Polyphenols in Ruminant Nutrition and Their Effects on Reproduction. Antioxidants 2022, 11, 970. [Google Scholar] [CrossRef] [PubMed]
- Dretcanu, G.; Stirbu, I.; Leoplold, N.; Cruceriu, D.; Danciu, C.; Stănilă, A.; Fărcas, A.; Monica Borda, I.; Iuhas, C.; Diaconeasa, Z. Chemical Structure, Sources and Role of Bioactive Flavonoids in Cancer Prevention: A Review. Plants 2022, 11, 1117. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizv, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Gomes, M.N.; Muratov, E.N.; Pereira, M.; Peixoto, J.C.; Rosseto, L.P.; Cravo, P.V.L.; Andrade, C.; Neves, B.J. Chalcone Derivatives: Promising Starting Points for Drug Design. Molecules 2017, 22, 1210. [Google Scholar] [CrossRef]
- Halliev, B. Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant. Physiol. 2006, 141, 312–322. [Google Scholar] [CrossRef]
- Aruoma, O.I. Nutrition and health aspects of free radicals and antioxidants. Food Chem. Toxicol. 1994, 32, 671–683. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.-Y.; Wang, N.; Zhang, Z.-J.; Lao, L.; Wong, C.-W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Free radicals, antioxidants, nuclear factor-E2-related factor-2 and liver damage. J. Appl. Toxicol. 2020, 40, 151–168. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Mark, T.D.; Cronin, M.T.D. Free radical and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell. Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Lee, J.; Giordano, S.; Zhang, J. Autophagy, mitochondria and oxidative stress: Cross-talk and redox signalling. Biochem. J. 2012, 441, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Tovar, E.; Muriel, P. Molecular Mechanisms That Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants 2020, 9, 1279; [Google Scholar] [CrossRef]
- Cichoz-Lach, H. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free Radicals, Antioxidants in Disease and Health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 3rd ed.; Oxford Science Publications: Oxford, UK, 1999. [Google Scholar]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Liang, X.; Wang, S.; Wang, L.; Ceylan, A.F.; Ren, J.; Zhang, Y. Mitophagy inhibitor liensinine suppresses doxorubicin-induced cardiotoxicity through inhibition of drp1-mediated maladaptive mitochondrial fission. Pharmacol. Res. 2020, 157, 104846. [Google Scholar] [CrossRef]
- Edeas, M.; Attaf, D.; Mailfert, A.S.; Nasu, M.; Joubet, R. Maillard reaction, mitochondria and oxidative stress: Potential role of antioxidants. Pathol. Biol. 2010, 58, 220–225. [Google Scholar] [CrossRef]
- Majima, H.J.; Indo, H.P.; Suenaga, S.; Matsui, H.; Yen, H.C.; Ozawa, T. Mitochondria as possible pharmaceutical targets for the effects of vitamin E and its homologues in oxidative stressrelated diseases. Curr. Pharm. Des. 2011, 17, 2190–2195. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Kraus, G.A.; Kim, I.; Spurlock, M.E.; Bailey, T.B.; Beitz, D.C. Effect of a mitochondria-targeted vitamin E derivative on mitochondrial alteration and systemic oxidative stress in mice. Br. J. Nutr. 2011, 106, 87–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Snezhkina, A.V.; Kurdymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxid. Med. Cell Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, B.D.; Seth, V.; Ahmed, R.S. Pesticide-Induced Oxidative Stress: Perspectives and Trends. Rev. Environ. Health 2001, 16, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Pantano, C.; Reynaert, N.L.; van der Vliet, A.; Janssen-Heininger, Y.M. Redox-sensitive kinases of the nuclear factor-kappaB signaling pathway. Antioxid. Redox Signal. 2006, 8, 1791–1806. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of Plant Polyphenols to Combat Oxidative Stress and Inflammatory Processes in Farm Animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S.; Saxena, U. Role of oxidative stress and inflammation in the origin of Type 2 diabetes—A paradigm shift. Expert. Opin. Ther. Targets. 2004, 8, 401–408. [Google Scholar] [CrossRef]
- Lykkesfeldt, J.; Svendsen, O. Oxidants and antioxidants in disease: Oxidative stress in farm animals. Vet. J. 2007, 173, 502–511. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS- generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Oxygen metabolism and the toxic properties of phagocytes. Ann. Intern. Med. 1980, 93, 480–489. [Google Scholar] [CrossRef]
- Nauser, T.; Koppenol, W.H. The rate constant of the reaction of superoxide with nitrogen monoxide: Approaching the diffusion limit. J. Phys. Chem. A 2002, 106, 4084. [Google Scholar] [CrossRef]
- Francis, S.H.; Busch, J.L.; Corbin, J.D.; Sibley, D. cGMP-dependent protein kinases and cGMP phosphodiesterases in nitric oxide and cGMP action. Pharmacol. Rev. 2010, 62, 525–563. [Google Scholar] [CrossRef]
- McDonald, L.J.; Murad, F. Nitric oxide and cGMP signaling. Adv. Pharmacol. 1995, 34, 263–275. [Google Scholar] [PubMed]
- Gilbert, R.S.; Herschman, H.R. Transforming growth factor beta differentially modulates the inducible nitric oxide synthase gene in distinct cell types. Biochem. Biophys. Res. Commun. 1993, 195, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Carreras, M.C.; Pargament, G.A.; Catz, S.D.; Poderoso, J.J.; Boveris, A. Kinetics of nitric oxide and hydrogen peroxide production and formation of peroxynitrite during the respiratory burst of human neutrophils. FEBS Lett. 1994, 341, 65–68. [Google Scholar] [CrossRef]
- Allen, R.G.; Lafuse, W.P.; Powell, N.D.; Webster Marketon, J.I.; StinerJones, L.M.; Sheridan, J.F.; Bailey, M.T. Stressor-induced increase in microbicidal activity of splenic macrophages is dependent upon peroxynitrite production. Infect. Immun. 2012, 80, 3429–3437. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods 1999, 232, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.; Franco, M.C.; Estevez, A.G. Reactive nitrogen species in cellular signaling. Exp. Biol. Med. 2015, 240, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharm. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef]
- Beeridge, M.V.; Tan, A.S.; McCoy, K.D.; Wang, R. The biochemical and cellular basis of cell proliferation assay that use tetrazolium salts. Biochemica 1996, 4, 15–20. [Google Scholar]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncola, J.; Izakovic, M.; Mazura, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Lučić, B.; Amić, D.; Trinajstić, N. Antioxidant QSAR Modeling as Exemplified on Polyphenols. In Advanced Protocols in Oxidative Stress I; Armstrong, D., Ed.; Humana Press: Totowa, NJ, USA, 2008; Volume 477, pp. 207–218. ISBN 978-1-60327-218-6. [Google Scholar]
- Filipović, M.; Marković, Z.; Đorović, J.; Marković, J.D.; Lučić, B.; Amić, D. QSAR of the Free Radical Scavenging Potency of Selected Hydroxybenzoic Acids and Simple Phenolics. Comptes Rendus Chim. 2015, 18, 492–498. [Google Scholar] [CrossRef]
- Amić, D.; Davidović-Amić, D.; Bešlo, D.; Rastija, V.; Lučić, B.; Trinajstić, N. SAR and QSAR of the antioxidant activity of flavonoids. Curr. Med. Chem. 2007, 14, 827–845. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Signal. 2007 19, 1807–1819. [CrossRef]
- Muriel, P. Role of free radicals in liver diseases. Hepatol. Int. 2009, 3, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Casas-Grajales, S.; Muriel, P. The Liver, Oxidative Stress, and Antioxidants. In Liver Pathophysiology; Elsevier BV: Berlin, Germany, 2017; pp. 583–604. [Google Scholar]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef]
- Abrams, G.A.; Trauner, M.; Nathanson, M.H. Nitric oxide and liver disease. Gastroenterologist 1995, 3, 220–233. [Google Scholar]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free Radicals: Properties, Sources, Targets, and Their Implication in Various Diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Venza, I.; Venza, M.; Visalli, M.; Lentini, G.; Teti, D.; d’Alcontres, F.S. ROS as Regulators of Cellular Processes in Melanoma. Oxid. Med. Cell. Longev. 2021, 2021, 1–19. [Google Scholar] [CrossRef]
- Di Ferdinandoa, M.; Brunetti, C.; Agati, G.; Tattini, M. Multiple functions of polyphenols in plants inhabiting unfavorable Mediterranean areas. Environ. Exp. Bot. 2014, 103, 107–116. [Google Scholar] [CrossRef]
- Amrit, B.K.; Ponnampalam, E.N.; Macwan, S.; Wu, H.; Aziz, A.; Muir, S.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive screening and characterization of polyphenol compounds from pasture grasses used for livestock production under temperate region. Anim. Feed. Sci. Technol. 2023, 300, 115657. [Google Scholar] [CrossRef]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tar, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Wink, M. Compartmentation of secondary metabolites and xenobiotics in plant vacuoles. Adv. Bot. Res. 1997, 25, 141–169. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Asfaw, D.E. Antioxidant Properties of Phenolic Compounds to Manage Oxidative Stress—A Review. J. Adv. Agron. Crop. Sci. 2022, 1, 1–16. [Google Scholar]
- Rice-Evans, C. Flavonoid antioxidants. Curr. Med. Chem. 2001, 8, 797–807. [Google Scholar] [CrossRef]
- Chen, D.; Mubeen, B.; Hasnain, A.; Rizwan, M.; Adrees, M.; Naqvi, S.A.H.; Iqbal, S.; Kamran, M.; El-Sabrout, A.M.; Elansary, H.O.; et al. Role of Promising Secondary Metabolites to Confer Resistance Against Environmental Stresses in Crop Plants: Current Scenario and Future Perspectives. Front. Plant. Sci. 2022, 13, 881032. [Google Scholar] [CrossRef]
- Rein, M.J.; Renouf, M.; Cruz-Hernandez, C.; Actis-Goretta, L.; Thakkar, S.K.; da Silva Pinto, M. Bioavailability of bioactive food compounds: A challenging journey to bioefficacy. Br. J. Clin. Pharmacol. 2013, 75, 588–602. [Google Scholar] [CrossRef]
- Eseberri, I.; Trepiana, J.; Léniz, A.; Gómez-García, I.; Carr-Ugarte, H.; González, M.; María, P.; Portillo, M.P. Variability in the Beneficial Effects of Phenolic Compounds: A Review. Nutrients. 2022, 14, 1925. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activitiesof Flavonoids: An Overview. Sci. World J. 2013, 16, 162750. [Google Scholar] [CrossRef]
- Rice-Evans, C.; Spencer, J.P.E.; Schroeter, H.; Rechner, A.R. Bioavailability of flavonoids and potential bioactive forms in vivo. Drug. Metab. Drug. Interact. 2000, 17, 291–310. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols 1. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.P.; Kong, X.Y.; Kong, A.Y.; Han, M. Pharmacokinetics and biotransformation of tea polyphenols. Curr. Drug Metab. 2014, 15, 30–36. [Google Scholar] [CrossRef]
- Gonzales, G.B.M.; Smagghe, G.; Grootaert, C.; Zotti, M.; Raes, K.; Van Camp, J. Flavonoid interactions during digestion, absorption, distribution and metabolism: A sequential structure -activity/property relationship-based approach in the study of bioavailability and bioactivity. Drug Metab. Rev. 2015, 47, 175–190. [Google Scholar] [CrossRef]
- Monagas, M.; Urpi-Sarda, M.; Sánchez-Patán, F.; Lorach, R.; Garrido, I.; Gómez-Cordovés, C.; Andrés-Lacueva, C.; Bartolomé, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef]
- Kay, C.D.; Pereira-Caro, G.; Ludwig, I.A.; Clifford, M.N.; Crozier, A. Anthocyanins and Flavanones Are More Bioavailable than Previously Perceived: A Review of Recent Evidence. Annu. Rev. Food Sci. Technol. 2017, 8, 155–180. [Google Scholar] [CrossRef]
- Day, A.J.; Cañada, F.J.; Díaz, J.C.; Kroon, P.A.; McLauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 468, 166–170. [Google Scholar] [CrossRef]
- Costa, M.; Sezgin-Bayindir, Z.; Losada-Barreiro, S.; Paiva-Martins, F.; Saso, L.; Bravo-Díaz, C. Polyphenols as Antioxidants for Extending Food Shelf-Life and in the Prevention of Health Diseases: Encapsulation and Interfacial Phenomena. Biomedicines 2021, 9, 1909. [Google Scholar] [CrossRef]
- Jiamboonsri, P.; Pithayanukul, P.; Bavovada, R.; Leanpolchareanchai, J.; Gao, S.; Hu, M. In Vitro Glucuronidation of Methyl Gallate and Pentagalloyl Glucopyranose by Liver Microsomes. Drug Metab. Pharmacokinet. 2016, 31, 292–303. [Google Scholar] [CrossRef]
- Zong, L.; Inoue, M.; Nose, M.; Kojima, K.; Sakaguchi, N.; Isuzugawa, K.; Takeda, T.; Ogihara, Y. Metabolic fate of gallic acid orally administered to rats. Biol. Pharm. Bull. 1999, 22, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Serreli, G.; Deiana, M. Biological Relevance of Extra Virgin Olive Oil Polyphenols Metabolites. Antioxidants. 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed]
- Selma, M.V.; Espin, J.C.; Tomás-Barberán, F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef] [PubMed]
- Brenes, A.; Viveros, A.; Chamorro, S.; Arija, I. Use of Polyphenol-Rich Grape by-Products in Monogastric Nutrition: A Review. Anim. Feed. Sci. Technol. 2016, 211, 1–17. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targetted analysis of precursor compounds, intermediate metabolites and end-products. Food Chem. 2012, 131, 337–347. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Hossain, M.E.; Kim, G.M.; Hwang, J.A.; Ji, H.; Yang, C.J. Effects of resveratrol and essential oils on growth performance, immunity and microbial shedding in challenged piglets. Asian-Aust. J. Anim. Sci. 2013, 26, 683–690. [Google Scholar] [CrossRef]
- Fiesel, A.; Gessner, D.K.; Erika Most, E.; Eder, K. Effects of dietary polyphenol-rich plant products from grape or hop on pro-inflammatory gene expression in the intestine, nutrient digestibility and faecal microbiota of weaned pigs. BMC Vet. Res. 2014, 10, 196–206. [Google Scholar] [CrossRef]
- Qui, N.H.; Thu, N.T.A. Recent Advances of Using Polyphenols to Extenuate Oxidative Stress in Animal Production: Evidence from Poultry. Kafkas Univ. Vet. Fak. Derg. 2022, 28, 535–541. [Google Scholar] [CrossRef]
- Parkinson, A.; Ogilvie, B.W. Biotransformation of xenobiotics. In Casarett & Doull’s Essentials of Toxicology; Klaassen, C.D., Watkins, J.B., Eds.; McGraw-Hill Medical: Chicago, IL, USA, 2010. [Google Scholar]
- Kroon, P.A.; Clifford, M.N.; Crozier, A.; Day, A.J.; Donovan, J.L.; Manach, C.; Williamson, G. How should we assess the effects of exposure to dietary polyphenols in vitro? Am. J. Clin. Nut. 2004, 80, 15–21. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, S.; Chen, S.; Zeng, S. Glucuronidation of flavonoids by recombinant UGT1A3 and UGT1A9. Biochem. Pharmacol. 2008, 76, 416–425. [Google Scholar] [CrossRef]
- Wu, B.; Kulkarni, K.; Basu, S.; Zhang, S.; Hu, M. First-pass metabolism via UDP glucuronosyltransferase: A barrier to oral bioavailability of phenolics. J. Pharm. Sci. 2011, 100, 3655–3681. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Morrow, J.K.; Singh, R.; Zhang, S.; Hu, M. Three-dimensional quantitative structure-activity relationship studies on UGT1A9- mediated 3-O-glucuronidation of natural flavonols using a pharmacophore- based Comparative Molecular Field Analysis model. J. Pharm. Exp. Ther. 2011, 336, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Basu, S.; Meng, S.; Wang, X.; Hu, M. Regioselective sulfation and glucuronidation of phenolics: Insights into the structural basis. Curr. Drug Metab. 2011, 12, 900–916. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Wu, B.; Singh, R.; Yin, T.; Morrow, J.K.; Zhang, S.; Hu, M. SULT1A3-mediated regiospecific 7-O-sulfation of flavonoids in Caco-2 cells can be explained by the relevant molecular docking studies. Mol. Pharm. 2012, 9, 862–873. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Chen, S.C.; Senthil Kumar, K.J.; Yu, K.N.; Lee Chao, P.D.; Tsai, S.Y.; Hou, Y.C.; Hseu, Y.C. Antioxidant and anti-inflammatory potential of hesperetin metabolites obtained from hesperetin-administered rat serum: An ex vivo approach. J. Agric. Food Chem. 2012, 60, 522–532. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, G.; Zuo, Z. Position preference on glucuronidation of mono-hydroxylflavones in human intestine. Life Sci. 2006, 78, 2772–2780. [Google Scholar] [CrossRef]
- Papuc, C.; Gheorghe, V.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1267. [Google Scholar] [CrossRef]
- Hosny, M. Microbial transformation of 2,5 dihydroxycinnamic acid by Aspergillus niger and Rhizopus oryzae. Az. J. Pharm. Sci. 2014, 49, 104–116. [Google Scholar] [CrossRef][Green Version]
- Spencer, J.P. Metabolism of tea flavonoids in the gastrointestinal tract. J. Nutr. 2003, 133, 3255–3261. [Google Scholar] [CrossRef]
- Walle, T. Methylation of Dietary Flavones Increases Their Metabolic Stability and Chemopreventive Effects. Int. J. Mol. Sci. 2009, 10, 5002–5019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qi, S.; Xue, X.; Al Naggar, Y.; Wu, L.; Wang, K. Understanding the Gastrointestinal Protective Effects of Polyphenols Using Foodomics-Based Approaches. Front. Immunol. 2021, 12, 671150. [Google Scholar] [CrossRef] [PubMed]
- Aura, A.M. Microbial metabolism of dietary phenolic compounds in the colon. Phytochem. Rev. 2008, 7, 407–429. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef]
- Belkaid, Y.; Hand, W.T. Role of the Microbiota in Immunity and Inflammation. Cell 2014, 157, 121–141. [Google Scholar] [CrossRef]
- Kim, H.B.; Isaacson, R.E. The pig gut microbial diversity: Understanding the pig gut microbial ecology through the next generation high throughput sequencing. Vet. Microbiol. 2015, 177, 242–251. [Google Scholar] [CrossRef]
- Makarewicz, M.; Drożdż, I.; Tarko, T.; Duda-Chodak, A. The Interactions between Polyphenols and Microorganisms, Especially Gut Microbiota. Antioxidants 2021, 10, 188. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of dietary compounds, especially polyphenols, with the intestinal microbiota: A review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef]
- Kaulmann, A.; Bohn, T. Bioactivity of Polyphenols: Preventive and Adjuvant Strategies toward Reducing Inflammatory Bowel Diseases—Promises, Perspectives, and Pitfalls. Oxid. Med. Cell. Longev. 2016, 2016, 9346470. [Google Scholar] [CrossRef]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Bommannan Santhakumar, A. Bioaccessibility and Bioactivity of Cereal Poly-phenols: A Review. Foods 2021, 10, 1595. [Google Scholar] [CrossRef] [PubMed]
- Axling, U.; Olsson, C.; Xu, J.; Fernandez, C.; Larsson, S.; Ström, K.; Ahrné, S.; Holm, C.; Molin, G.; Berger, K. Green Tea Powder and Lactobacillus Plantarum Affect Gut Microbiota, Lipid Metabolism and Inflammation in High-Fat Fed C57BL/6J Mice. Nutr. Metab. 2012, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef]
- Rummun, N.; Rondeau, P.; Bourdon, E.; Pires, E.; McCullagh, J.; Claridge, T.D.W.; Bahorun, T.; Li, W.-W.; Neergheen, V.S. Terminalia Bentzoë, a Mascarene Endemic Plant, Inhibits Human Hepatocellular Carcinoma Cells Growth In Vitro via G0/G1 Phase Cell Cycle Arrest. Pharmaceuticals 2020, 13, 303. [Google Scholar] [CrossRef] [PubMed]
- Rummun, N.; Pires, E.; McCullagh, J.; Claridge, T.W.D.; Bahorun, T.; Li, W.-W.; Neergheen, V.S. Methyl Gallate—Rich Fraction of Syzygium Coriaceum Leaf Extract Induced Cancer Cell Cytotoxicity via Oxidative Stress. S. Afr. J. Bot. 2021, 137, 149–158. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef]
- D’Archivio, M.; Filesi, C.; Varì, R.; Scazzocchio, B.; Masella, R. Bioavailability of the Polyphenols: Status and Controversies. Int. J. Mol. Sci. 2010, 11, 1321–1342. [Google Scholar] [CrossRef]
- Simons, A.L.; Renouf, M.; Hendrich, S.; Murphy, P.A. Human Gut Microbial Degradation of Flavonoids: Structure-function Relationships. J. Agric. Food Chem. 2005, 53, 4258–4263. [Google Scholar] [CrossRef]
- Rastmanesh, R. High Polyphenol, Low Probiotic Diet forWeight Loss Because of Intestinal Microbiota Interaction. Chem. Biol. Interact. 2011, 189, 1–8. [Google Scholar] [CrossRef]
- Di Meo, F.; Anouar, E.; Podloucka, P.; Fabre, G.; Trouillas, P. Understanding antioxidant properties of natural compounds at the atomic scale. J. Serb. Soc. Comput. 2013, 7, 58–70. [Google Scholar]
- Majewska, M.; Czeczot, H. Flavonoids in prevention and therapy of diseases. Pol. Merkur. Lekarski. 2012, 32, 50–54. (In Polish) [Google Scholar]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, E.G.; Gibson, M.R.G.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.P.; Martín-Álvarez, P.; Moreno-Arribas, J.M.V.; Bartolomé, B. In Vitro Fermentation of a Red Wine Extract by Human Gut Microbiota: Changes in Microbial Groups and Formation of Phenolic Metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef]
- Kafantaris, I.; Kotsampasi, B.; Christodoulou, V.; Kokka, E.; Kouka, P.; Terzopoulou, Z.; Gerasopoulos1, K.; Stagos, D.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves antioxidant capacity and faecal microflora of lambs. J. Anim. Physiol. Anim. Nutr. 2016, 101, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L.; Lila, M.A.J.; Mawson, J.; De, S. In vitro and in vivo evaluation of the prebiotic activity of watersoluble blueberry extracts. World J. Microbiol. Biotechnol. 2009, 25, 1243–1249. [Google Scholar] [CrossRef]
- Cueva, C.; Sánchez-Patán, F.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. In vitro fermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol. Ecol. 2013, 83, 792–805. [Google Scholar] [CrossRef]
- Xu, Q.; Cheng, M.; Jiang, R.; Zhao, X.; Zhu, J.; Liu, M.; Chao, X.; Zhang, C.; Zhou, B. Effects of dietary supplement with a Chinese herbal mixture on growth performance, antioxidant capacity, and gut microbiota in weaned pigs. Front. Vet. Sci. 2022, 9, 971647. [Google Scholar] [CrossRef]
- Dolara, P.; Luceri, C.; De Filippo, C.; Femia, A.P.; Giovannelli, L.; Caderni, G.; Cecchini, C.; Silvi, S.; Orpianesi, C.; Cresci, A. Red wine polyphenols influence carcinogenesis, intestinal microflora, oxidative damage and gene expression profiles of colonicmucosa in F344 rats. Mutat. Res. 2005, 591, 237–246. [Google Scholar] [CrossRef]
- Viveros, A.; Chamorro, S.; Pizarro, M.; Arija, I.; Centeno, C.; Brenes, A. Effects of dietary polyphenol-rich grape products on intestinal microflora and gut morphology in broiler chicks. Poult. Sci. 2011, 90, 566–578. [Google Scholar] [CrossRef]
- Lacombe, A.; Li, R.W.; Klimis-Zacas, D.; Kristo, A.S.; Shravani Tadepalli, S.; Krauss, E.; Young, R.; Vivian, C.; Wu, H. Lowbush wild blueberries have the potential to modify gut microbiota and xenobiotic metabolism in the rat colon. PLoS ONE 2013, 8, e67497. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.U.; Tuohy, K.M. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an In Vitro Colonic Model. Nutrients 2017, 9, 533; [Google Scholar] [CrossRef]
- Gasaly, N.; Gottelan, M. Interference of dietary polyphenols with potentially toxic amino acid metabolites derived from the colonic microbiota. Amino Acids 2022, 54, 311–324. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Hu, L.; Huang, T.; Liu, X.J.; Cai, Y.D. Predicting protein phenotypes based on protein-protein interaction network. PLoS ONE 2011, 6, e17668. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Are polyphenols antioxidants or prooxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Halliwell, B. Dietary polyphenols: Good, bad, or indifferent for your health? Cardiovasc. Res. 2007, 73, 341–347. [Google Scholar] [CrossRef]
- Irina, I.; Mohamed, G. Biological Activities and Effects of Food Processing on Flavonoids as Phenolic Antioxidants. In Advances in Applied Biotechnology; Petre, M., Ed.; University of Pitesti: Pitesti, Romania, 2012; ISBN 978-953-307-820-5. [Google Scholar]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Rohn, S.; Petzke, K.J.; Rawel, H.M.; Kroll, J. Reactions of chlorogenic acid and quercetin with a soy protein isolate–Influence on the in vivo food protein quality in rats. Mol. Nutr. Food. Res. 2006, 50, 696–704. [Google Scholar] [CrossRef]
- Brenes, A.; Viveros, A.; Goñi, I.; Centeno, C.; Saura-Calixto, F.; Arija, I. Effect of grape seed extract on growth performance, protein and polyphenol digestibilities, and antioxidant activity in chickens. Span. J. Agric. Res. 2010, 8, 326–333. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Nkukwana, T.T.; Muchenje, V.; Pieterse, E.; Masika, P.J.; Mabusela, T.P.; Hoffman, L.C.; Dzama, K. Effect of Moringa oleifera leaf meal on growth performance, apparent digestibility, digestive organ size and carcass yield in broiler chickens. Livest. Sci. 2014, 161, 139–146. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.N.; Chu, G.M.; Jin, S.K. Growth performance, blood cell profiles, and meat quality properties of broilers fed with Saposhnikovia divaricata, Lonicera japonica, and Cheli-donium majus extracts. Livest. Sci. 2014, 165, 87–94. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the chicken/animal diet: From the pastto the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- El–Iraqi, K.G.; Abdelgawad, E.M.; Ibrahim, H.M.; El Sawe, A.E. Effect of Ginkgo biloba, dry peppermint and vitamin C as anti-stress on broiler welfare during summer heat stress. Glob. Vet. 2013, 10, 770–778. [Google Scholar]
- Gessner, D.K.; Fiesel, A.; Most, E.; Dinges, J.; Wen, G.; Ringseis, R.; Eder, K. Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress-responsive transcription factors NF-κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet. Scand. 2013, 55, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Chedea, V.S.; Palade, L.M.; Pelmus, R.S.; Dragomir, C.; Taranu, I. Red Grape Pomace Rich in Polyphenols Diet Increases the Antioxidant Status in Key Organs—Kidneys, Liver, and Spleen of Piglets. Animals 2019, 9, 149. [Google Scholar] [CrossRef]
- Lipiński, K.; Korniewicz, D.; Antoszkiewicz, Z.; Mazur, M. Effect of onion and grape seed extracts on performance and the vitamin E and antioxidant status in sows. In Proceedings of the XLIV Scientific Session: Nutrition of Livestock, Companion and Wild Animals, Warsaw, Poland, 16–17 June 2015; pp. 16–17. [Google Scholar]
- Flis, M.; Sobotka, W.; Antoszkiewicz, Z.; Lipiński, K.; Zduńczyk, Z. Effect of husked and naked oat used in the diets supplemented with linseed oil on the growth performance of pigs, carcass and meat quality. Arch. Spec. Issue. 2007, 50, 161–171. [Google Scholar] [CrossRef]
- Leusink, G.; Rempel, H.; Skura, B.; Berkyto, M.; White, W.; Yang, Y.; Fitzpatrick, S. Growth performance, meat quality, and gut microflora of broiler chickens fed with cranberry extract. Poult. Sci. 2010, 89, 1514–1523. [Google Scholar] [CrossRef]
- Simitzis, P.E.; Symeon, G.K.; Charismiadou, M.A.; Ayoutanti, A.G.; Deligeorgis, S.G. The effects of dietary hesperidin supplementation on broiler performance and chicken meat characteristics. Can. J. Anim. Sci. 2011, 91, 275–282. [Google Scholar] [CrossRef]
- Clark, R.; Kupper, T. Old meets new: The interaction between innate and adaptive immunity. J. Invest. Dermatol. 2005, 125, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Hachimura, S.; Totsuka, M.; Hosono, A. Immunomodulation by food: Impact on gut immunity and immune cell function. Biosci. Biotechnol. Biochem. 2018, 82, 584–599. [Google Scholar] [CrossRef] [PubMed]
- Serra, D.; Almeida, L.M.; Dinis, T.C. Dietary polyphenols: A novel strategy to modulate microbiota-gut-brain axis. Trends Food Sci. Technol. 2018, 78, 224–233. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Qiao, Y.; Sun, J.; Xia, S.; Tang, X.; Shi, Y.; Le, G. Effects of Resveratrol on Gut Microbiota and Fat Storage in a Mouse Model with High-Fat-Induced Obesity. Food Funct. 2014, 5, 1241–1249. [Google Scholar] [CrossRef]
- Rodríguez, M.; Rebollar, P.G.; Mattioli, S.; Castellini, C. N-3 PUFA Sources (Precursor/Products): A Review of Current Knowledge on Rabbit. Animals 2019, 9, 806. [Google Scholar] [CrossRef]
- Shimizu, M. Multifunctions of dietary polyphenols in the regulation of intestinal inflammation. J. Food Drug Anal. 2017, 25, 93–99. [Google Scholar] [CrossRef]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]
- Scislowski, V.; Bauchart, D.; Gruffat, D.; Laplaud, P.M.; Durand, D. Effects of dietary n−6 or n−3 polyunsaturated fatty acids protected or not against ruminal hydrogenation on plasma lipids and their susceptibility to peroxidation in fattening steers. J. Anim. Sci. 2005, 83, 2162–2174. [Google Scholar] [CrossRef]
- Wood, J.D.; Enser, M. Factors influencing fatty acids in meat and the role of antioxidants in improving meat quality. Br. J. Nutr. 1997, 78, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Gladine, C.; Morand, C.; Rock, E.; Bauchart, D.; Durand, D. Plant extracts rich in polyphenols (PERP) are efficient antioxidants to prevent lipoperoxidation in plasma lipids from animals fed n-3 PUFA supplemented diets. Anim. Feed Sci. Technol. 2007, 136, 281–296. [Google Scholar] [CrossRef]
- Branciari, R.; Ranucci, D.; Trabalza-Marinucci, M.; Codini, M.; Orru, M.; Ortenzi, R.; Forte, C.; Ceccarini, M.R.; Valiani, A. Evaluation of the antioxidant properties and oxidative stability of Pecorino cheese made from the raw milk of ewes fed Rosmarinus officinalis L. leaves. Int. J. Food Sci. Technol. 2015, 50, 558–565. [Google Scholar] [CrossRef]
- Todaro, M.; Alabiso, M.; Scatassa, M.L.; Di Grigoli, A.; Mazza, F.; Maniaci, G.; Bonanno, A. Effect of the inclusion of fresh lemon pulp in the diet of lactating ewes on the properties of milk and cheese. Anim. Feed Sci. Tech. 2017, 225, 213–223. [Google Scholar] [CrossRef]
- Habeanu, M.; Lefter, N.A.; Ropota, M.; Chedea, V.S.; Gheorghe, A.; Toma, S.M.; Ciurescu, G.; Dragomir, C. Dried grape pomace influenced fatty acids composition of Longissimus dorsi muscle and plasma polyphenols spectrum in finishing pigs. Indian J. Anim. Sci. 2015, 85, 786–789. [Google Scholar]
- Muñoz-González, I.; Chamorro, S.; Pérez-Jiménez, J.; López-Andrés, P.; Álvarez-Acero, I.; Herrero, A.M.; Nardoia, M.; Brenes, A.; Viveros, A.; Arija, I.; et al. Phenolic metabolites in plasma and thigh meat of chickens supplemented with grape byproducts. J. Agric. Food Chem. 2019, 67, 4463–4471. [Google Scholar] [CrossRef]
- Atwood, S.B.; Provenza, F.D.; Wiedmeier, R.D.; Banner, R.E. Influence of Free-Choice vs Mixed-Ration Diets on Food Intake and Performance of Fattening Calves. J. Anim. Sci. 2001, 79, 3034. [Google Scholar] [CrossRef]
- Catanese, F.; Obelar, M.; Villalba, J.J.; Distel, R.A. The Importance of Diet Choice on Stress-Related Responses by Lambs. Appl. Anim. Behav. Sci. 2013, 148, 37–45. [Google Scholar] [CrossRef]
- Pichler, J.; Schwarz, C.; Gierus, M.; Schedle, K. Choice Feeding in Fattening Pigs: Effect of Diets Differing in Nutrient Density on Feeding Behaviour and Fattening Performance. Czech J. Anim. Sci. 2020, 65, 247–257. [Google Scholar] [CrossRef]
- Meier, J.S.; Kreuzer, M.; Marquardt, S. Design and Methodology of Choice Feeding Experiments with Ruminant Livestock. Appl. Anim. Behav. Sci. 2012, 140, 105–120. [Google Scholar] [CrossRef]


| Name of the Substance | Symbol | Half-Life b | Characteristic | |
|---|---|---|---|---|
| Radicals | Superoxide | O2· | 10−6 s | unstable molecule, signaling molecule |
| Hydroxyl | OH· | 10−10 s | very reactive and unstable species, it is created in the Fenton and Haber–Weiss reaction with an iron catalyst | |
| Alkoxyl radical | RO· | 10−6 s | organic (lipid) radical | |
| Peroxyl Radical | ROO· | 17 s | it is formed from organic hydroperoxide (ROOH), by removing hydrogen | |
| Nitric oxide | NO· | s | environmental toxin, endogenous signaling molecule | |
| Nitrogen dioxide | NO2· | s | highly reactive species, environmental toxin | |
| Non radical | Hydrogen peroxide | H2O2 | Stable | a cellular toxin, signaling role, generation of other free radicals |
| Singlet oxygen | 1O2 | 10−6 s | the first excited form of oxygen | |
| Ozone | O3 | s | environmental toxin | |
| Organic peroxide | ROOH | Stabile | it easily decomposes into radicals, so it serves as a catalyst for radical reactions | |
| Peroxynitrous | ONOO− | Stabile | highly reactive species, environmental toxin | |
| nitrogen oxides | NOx | s | environmental toxin, including NO and NO2 derivatives formed in the combustion process | |
| Polyphenols and Source | Sample | Impact on Microbiome | References |
|---|---|---|---|
| Red wine extract | Fecal (human) in vitro | Increase: Bifidobacterium spp. | [138] |
| Lactobacillus/Enterococcus spp. | |||
| Bacteroides spp. | |||
| Inhibit: Clostridin hystolyticum | |||
| Grape pomace | Fecal (human) in vitro | Increase: Bifidobacterium spp. | [139] |
| Clostrida | |||
| Campylobacter | |||
| Inhibit: Escherichia coli | |||
| Salmonella | |||
| Blueberry extract | Fecal (human) in vitro | Increase: Lactobacillus | [140] |
| Bifidobacterium spp. | |||
| Grape seed extract fraction | Fecal (human) in vitro | Increase: Lactobacillus/Enterococcus spp. | [141] |
| Inhibit: Clostridin hystolyticum | |||
| Tea polyphenols | Fecal (pigs) in vitro | Increase: Lactobacillus | [142] |
| Inhibit: Bacteroidaceae | |||
| Clostridium perfringens | |||
| Red wine polyphenols powder | Fecal (rats) in vitro | Increase: Lactobacilli | [143] |
| Bifidobacteria | |||
| Inhibit: Propionibacteria | |||
| Bacteriodes, Clostridia | |||
| Grape pomace concentrate, Grape seed extract | Fecal (broiler chicks) in vitro | Increase: E. Coli, Enterococcus spp., Lactobacillus spp. | [144] |
| Lowbush wild blueberries | Fecal (rats) in vitro | Incerase: Thermonospora spp., Corynebacteria spp. | [145] |
| Slackia spp. | |||
| Inhibit: Lactobacillus spp. Enterococcus spp. | |||
| Apple pomace | In vivo (rats) | Increase: Lactobacillus | [146] |
| Bifidobacterium | |||
| Bacteriodoceae | |||
| Eubacterium | |||
| Inhibit: | |||
| Bacteroides spp. | |||
| Tannin supplementation | In vivo (mouse) | Increase: Bacteroides, Lactobacillus | [147] |
| Inhibit: Clostridium |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. https://doi.org/10.3390/antiox12061141
Bešlo D, Golubić N, Rastija V, Agić D, Karnaš M, Šubarić D, Lučić B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants. 2023; 12(6):1141. https://doi.org/10.3390/antiox12061141
Chicago/Turabian StyleBešlo, Drago, Nataša Golubić, Vesna Rastija, Dejan Agić, Maja Karnaš, Domagoj Šubarić, and Bono Lučić. 2023. "Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals" Antioxidants 12, no. 6: 1141. https://doi.org/10.3390/antiox12061141
APA StyleBešlo, D., Golubić, N., Rastija, V., Agić, D., Karnaš, M., Šubarić, D., & Lučić, B. (2023). Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants, 12(6), 1141. https://doi.org/10.3390/antiox12061141








