Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes
Abstract
1. Introduction
2. Materials and Methods
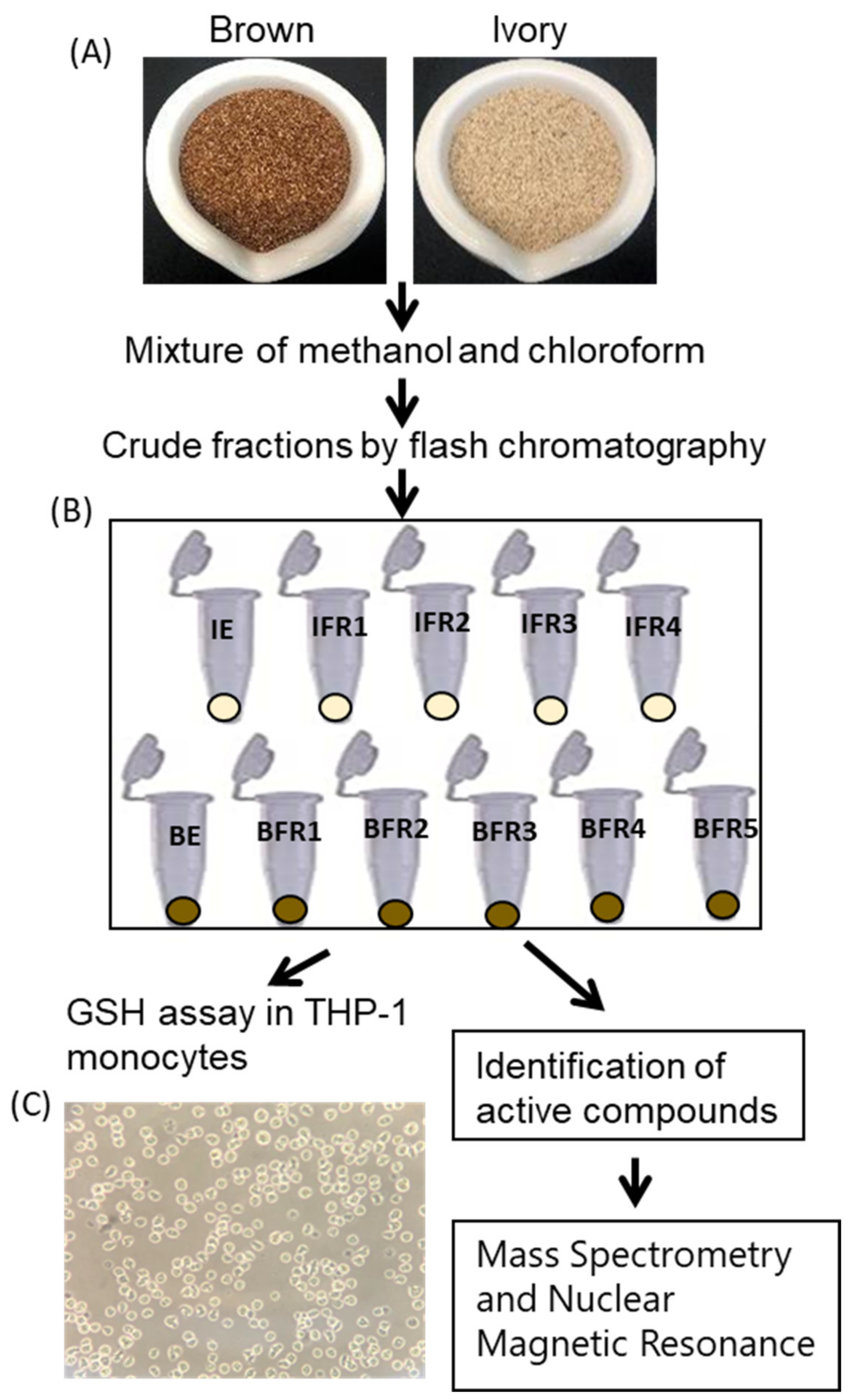
2.1. Preparation of Tef Extracts
2.2. Mammalian Cell Culture
2.3. Cell Viability Assay
2.4. Glutathione (GSH) Assay
2.5. qPCR Analysis of GSH-Pathway Genes
2.6. Identification of Active Compounds from Tef Extracts
2.7. Bioassay Data Analysis
3. Results
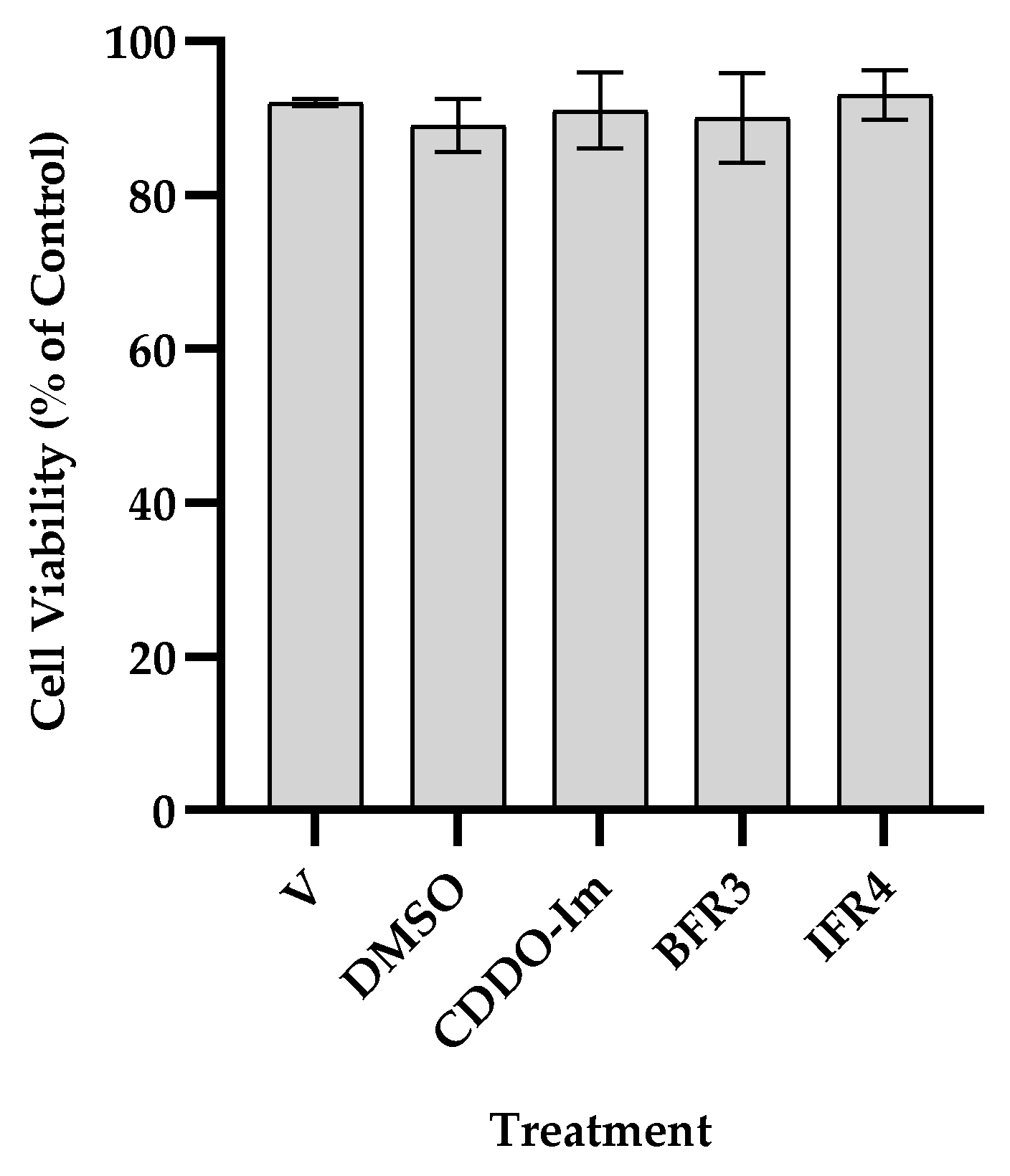
3.1. Effect of Tef Extracts on Cell Viability
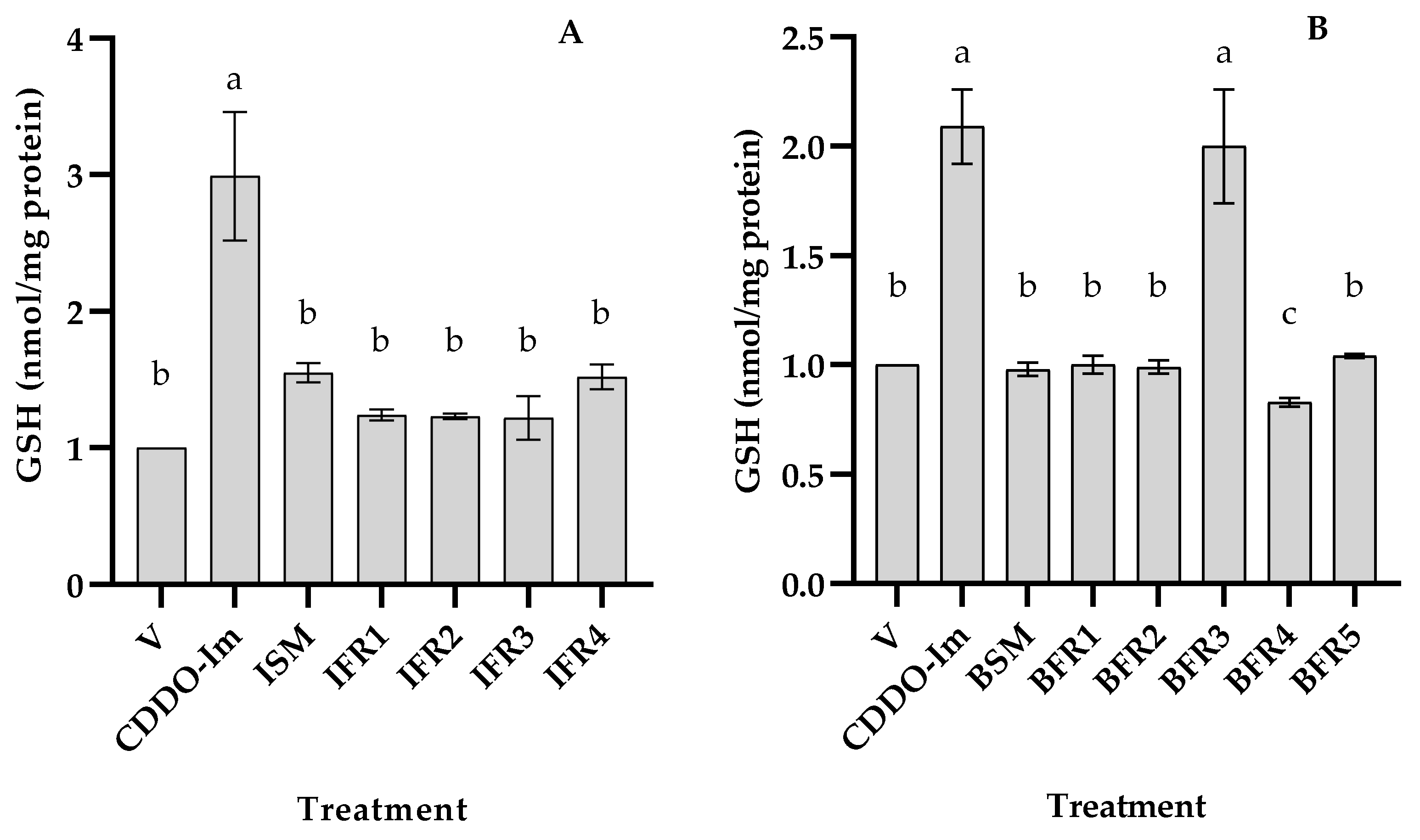
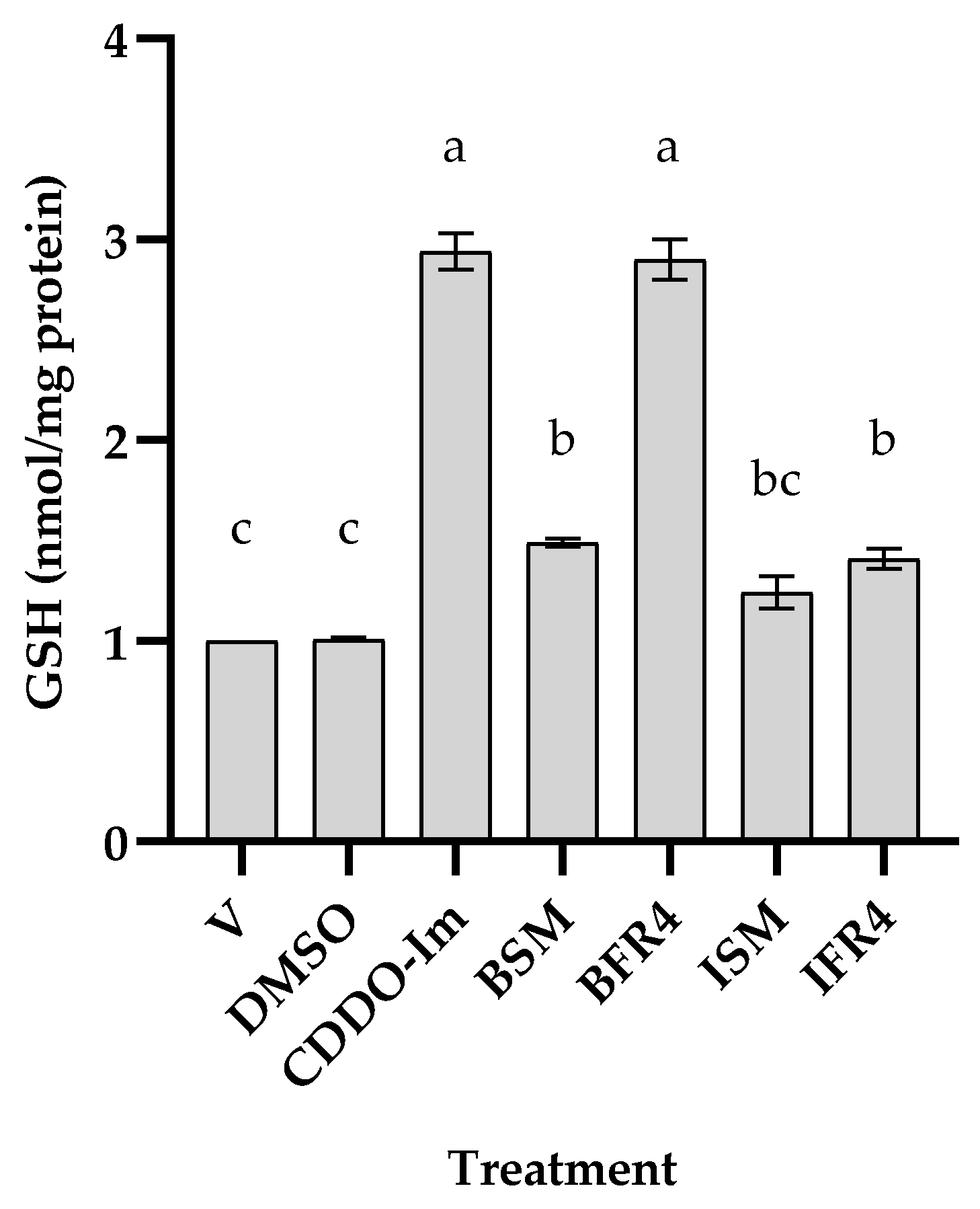
3.2. Antioxidant Activity of Tef Seed Extract
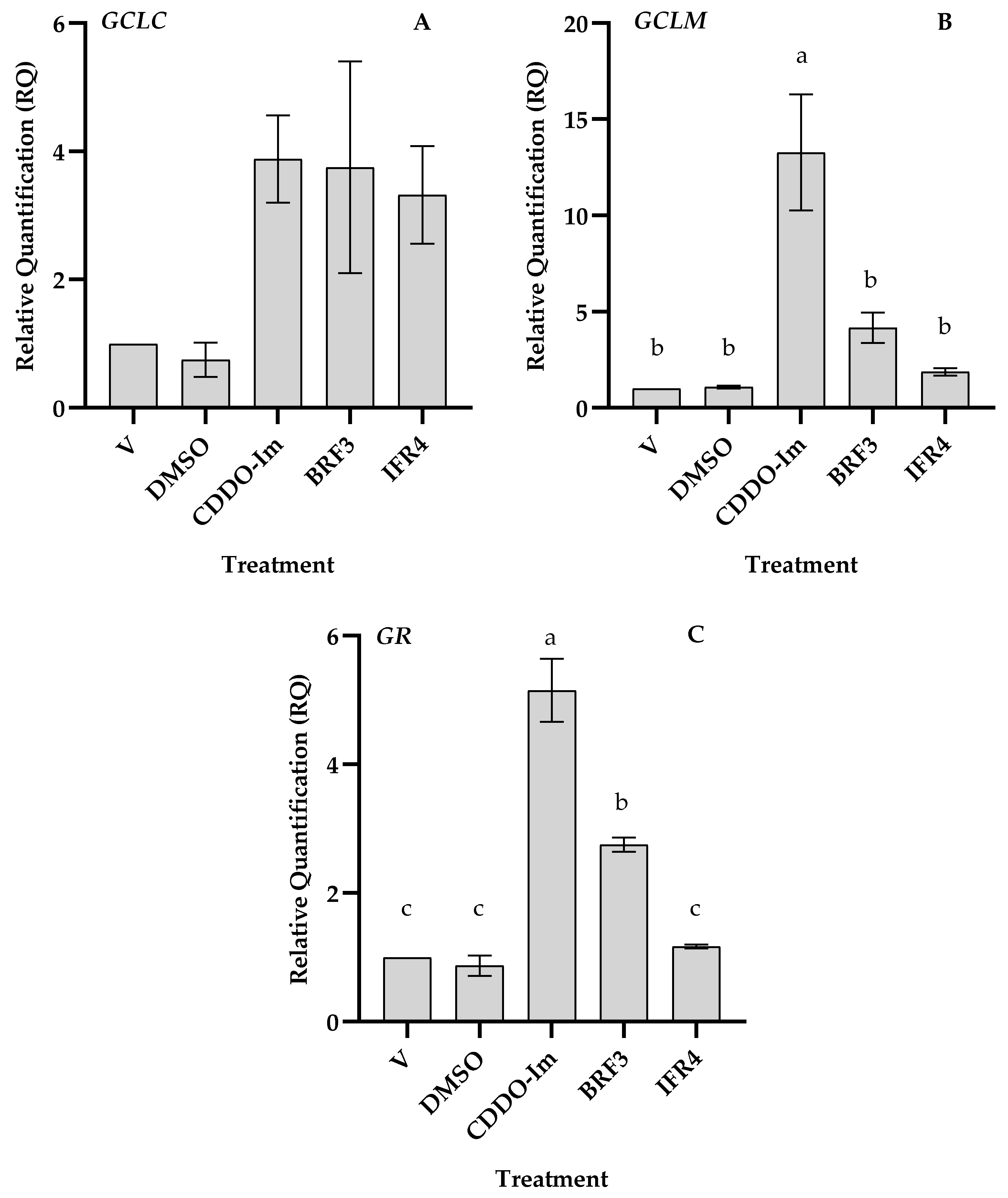
3.3. Effects of Tef Active Fractions on GSH-Pathway Genes
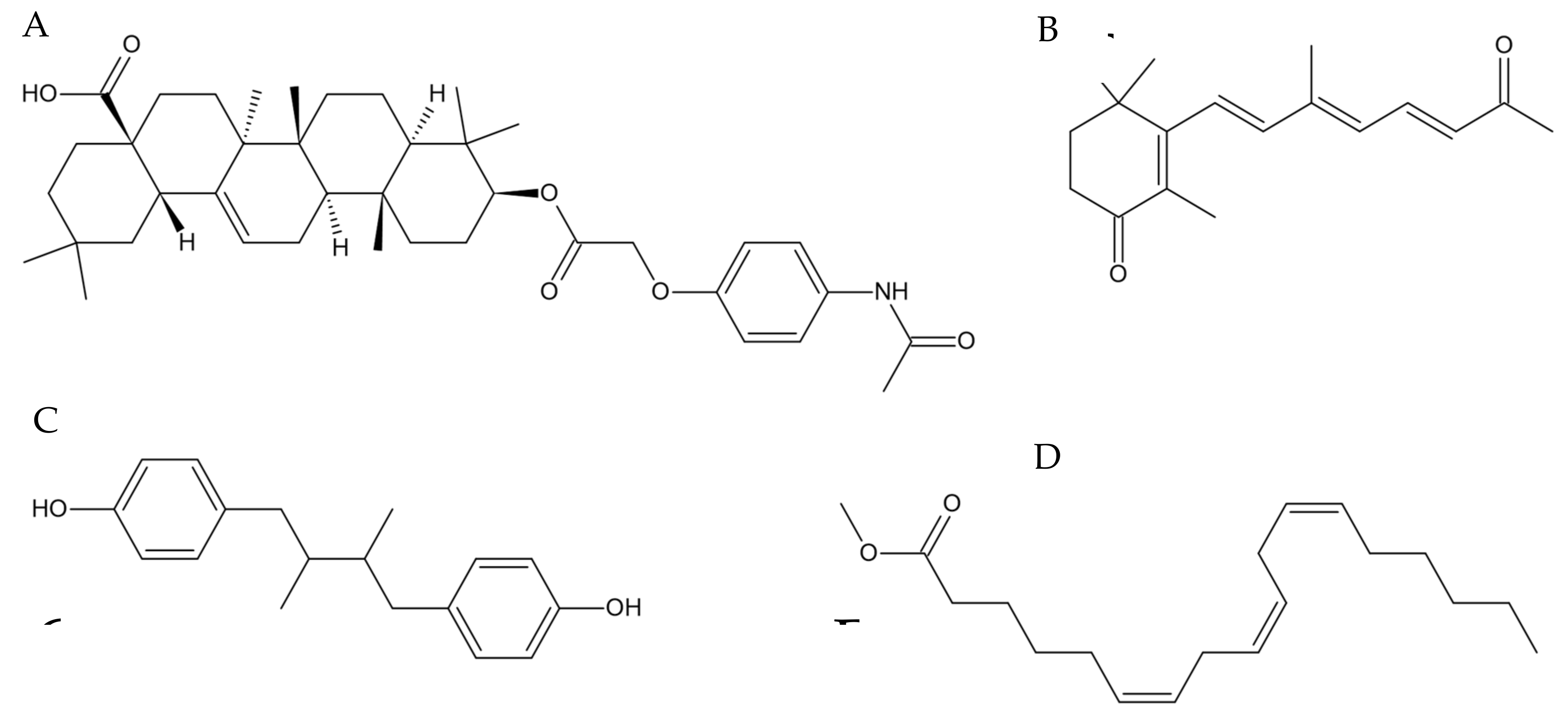
3.4. Identification of Active Compounds in Tef Extracts
4. Discussion
4.1. Observed Cell Activity with Respect to the Mass-Spectrometry Analysis
4.2. Features Identified from the Mass-Spectrometry Analysis
4.3. Expressions of GSH Biosynthetic Genes and Cellular GSH
4.4. Observed Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in Health and Disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of Oxidative Stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.-P.-P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.J.; Pappa, A.; Panayiotidis, M.I. The Central Role of Glutathione in the Pathophysiology of Human Diseases. Arch. Physiol. Biochem. 2007, 113, 234–258. [Google Scholar] [CrossRef]
- Pizzorno, J. Glutathione! Integr. Med. 2014, 13, 8–12. [Google Scholar]
- Lu, S.C. Glutathione Synthesis. Biochim. Biophys. Acta 2013, 1830, 3143–3153. [Google Scholar] [CrossRef]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione Dysregulation and the Etiology and Progression of Human Diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Wild, A.C.; Gipp, J.J.; Mulcahy, T. Overlapping Antioxidant Response Element and PMA Response Element Sequences Mediate Basal and Beta-Naphthoflavone-Induced Expression of the Human Gamma-Glutamylcysteine Synthetase Catalytic Subunit Gene. Biochem. J. 1998, 332, 373–381. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Smale, S.T. Hierarchies of NF-ΚB Target Gene Regulation. Nat. Immunol. 2011, 12, 689–694. [Google Scholar] [CrossRef]
- Bellezza, I.; Mierla, A.L.; Minelli, A. Nrf2 and NF-ΚB and Their Concerted Modulation in Cancer Pathogenesis and Progression. Cancers 2010, 2, 483–497. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.H.; Sethi, G.; Bishayee, A. Oleanolic Acid and Its Synthetic Derivatives for the Prevention and Therapy of Cancer: Preclinical and Clinical Evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef]
- Jia, Z.; Hallur, S.; Zhu, H.; Li, Y.; Misra, H.P. Potent Upregulation of Glutathione and NAD(P)H: Quinone Oxidoreductase 1 by Alpha-Lipoic Acid in Human Neuroblastoma SH-SY5Y Cells: Protection against Neurotoxicant-Elicited Cytotoxicity. NeuroChem. Res. 2008, 33, 790–800. [Google Scholar] [CrossRef]
- Sussan, T.E.; Rangasamy, T.; Blake, D.J.; Malhotra, D.; El-Haddad, H.; Bedja, D.; Yates, M.S.; Kombairaju, P.; Yamamoto, M.; Liby, K.T.; et al. Targeting Nrf2 with the Triterpenoid CDDO-Imidazolide Attenuates Cigarette Smoke-Induced Emphysema and Cardiac Dysfunction in Mice. Proc. Natl. Acad. Sci. USA 2009, 106, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuźniak, V.; Baer-Dubowska, W. Modulation of Nrf2 and NF-ΚB Signaling Pathways by Naturally Occurring Compounds in Relation to Cancer Prevention and Therapy. Are Combinations Better Than Single Compounds? Int. J. Mol. Sci. 2021, 22, 8223. [Google Scholar] [CrossRef] [PubMed]
- Forsido, S.F.; Rupasinghe, H.P.V.; Astatkie, T. Antioxidant Capacity, Total Phenolics and Nutritional Content in Selected Ethiopian Staple Food Ingredients. Int. J. Food Sci. Nutr. 2013, 64, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Salawu, S.O.; Salimon, Y.A. Evaluation of the Effect of Sorghum Bicolor Aqueous Extract on the Haematological, Renal and Hepatic Parameters in Rats Fed with Low and High Iron Diet. Eur. J. Med. Plants 2014, 4, 783–793. [Google Scholar] [CrossRef]
- Kotásková, E.; Sumczynski, D.; Mlček, J.; Valášek, P. Determination of Free and Bound Phenolics Using HPLC-DAD, Antioxidant Activity and In Vitro Digestibility of Eragrostis tef. J. Food Compos. Anal. 2015, 46, 15–21. [Google Scholar] [CrossRef]
- Ravisankar, S.; Abegaz, K.; Awika, J.M. Structural Profile of Soluble and Bound Phenolic Compounds in Teff (Eragrostis tef) Reveals Abundance of Distinctly Different Flavones in White and Brown Varieties. Food Chem. 2018, 263, 265–274. [Google Scholar] [CrossRef]
- Ghose, S.; Varshney, S.; Chakraborty, R.; Sengupta, S. Dietary Antioxidants in Mitigating Oxidative Stress in Cardiovascular Diseases. In Oxidative Stress in Heart Diseases; Springer: Singapore, 2019; pp. 83–139. ISBN 978-981-13-8272-7. [Google Scholar]
- Bultosa, G. Physicochemical Characteristics of Grain and Flour in 13 Tef [Eragrostis tef (Zucc.) Trotter] Grain Varieties. J. Appl. Sci. Res. 2007, 3, 2042–2051. [Google Scholar]
- Assefa, K.; Yu, J.-K.; Zeid, M.; Belay, G.; Tefera, H.; Sorrells, M.E. Breeding Tef [Eragrostis tef (Zucc.) Trotter]: Conventional and Molecular Approaches. Plant Breed. 2011, 130, 1–9. [Google Scholar] [CrossRef]
- Belay, G.; Tefera, H.; Tadesse, B.; Metaferia, G.; Jarra, D.; Tadesse, T. Participatory Variety Selection in the Ethiopian Cereal tef (Eragrostis tef). Exp. Agric. 2006, 42, 91–101. [Google Scholar] [CrossRef]
- Gebremariam, M.M.; Zarnkow, M.; Becker, T. Teff (Eragrostis tef) as a Raw Material for Malting, Brewing and Manufacturing of Gluten-Free Foods and Beverages: A Review. J. Food Sci. Technol. 2014, 51, 2881–2895. [Google Scholar] [CrossRef]
- Crymes, A. The International Footprint of Teff: Resurgence of an Ancient Ethiopian Grain. Master’s Dissertations, University College, Washington University in St. Louis, St. Louis, MO, USA, 2015. [Google Scholar]
- Teff: Nutrient Composition and Health Benefits|IFPRI: International Food Policy Research Institute. Available online: https://www.ifpri.org/publication/teff-nutrient-composition-and-health-benefits (accessed on 25 May 2023).
- Fantaye, D.; Mekonnen, S.; Kore, T.; Gebre, B.; Asamenew, G.; Shiferaw, L. Nutrition of Tef (Eragrostis tef) Recipes. Food Sci. Qual. Manag. 2015, 45, 18–22. [Google Scholar]
- Taylor, J. Chapter 4—Millets: Their Unique Nutritional and Health-Promoting Attributes. In Gluten-Free Ancient Grains: Cereals, Pseudocereals, and Legumes: Sustainable, Nutritious, and Health-Promoting Foods for the 21st Century; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2017; pp. 55–103. ISBN 978-0-08-100866-9. [Google Scholar]
- Shumoy, H.; Raes, K. Tef: The Rising Ancient Cereal: What Do We Know about Its Nutritional and Health Benefits? Plant Foods Hum. Nutr. 2017, 72, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Ligaba, O.A.; Mengistu, M.; Beyene, G.; Cushman, J.; Glahn, R.; Pineros, M. Grain Mineral Nutrient Profiling and Iron Bioavailability of an Ancient Crop Tef (‘Eragrostis tef’). Aust. J. Crop. Sci. 2021, 15, 1314–1324. [Google Scholar] [CrossRef]
- Michalska, M.; Gluba, A.; Mikhailidis, D.P.; Nowak, P.; Bielecka-Dabrowa, A.; Rysz, J.; Banach, M. The Role of Polyphenols in Cardiovascular Disease. Med. Sci. Monit. 2010, 16, RA110–RA119. [Google Scholar]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef]
- Shumoy, H.; Pattyn, S.; Raes, K. Tef Protein: Solubility Characterization, in-Vitro Digestibility and Its Suitability as a Gluten Free Ingredient. LWT-Food Sci. Technol. 2017, 89, 697–703. [Google Scholar] [CrossRef]
- Spaenij-Dekking, L.; Kooy-Winkelaar, Y.; Koning, F. The Ethiopian Cereal Tef in Celiac Disease. N. Engl. J. Med. 2005, 353, 1748–1749. [Google Scholar] [CrossRef]
- Bergamo, P.; Maurano, F.; Mazzarella, G.; Iaquinto, G.; Vocca, I.; Rivelli, A.R.; De Falco, E.; Gianfrani, C.; Rossi, M. Immunological Evaluation of the Alcohol-Soluble Protein Fraction from Gluten-Free Grains in Relation to Celiac Disease. Mol. Nutr. Food Res. 2011, 55, 1266–1270. [Google Scholar] [CrossRef]
- Shumoy, H.; Raes, K. Antioxidant Potentials and Phenolic Composition of Tef Varieties: An Indigenous Ethiopian Cereal. Cereal Chem. 2016, 93, 465–470. [Google Scholar] [CrossRef]
- Tietel, Z.; Simhon, E.; Gashu, K.; Ananth, D.; Schwartz, B.; Saranga, Y.; Yermiyahu, U. Nitrogen Availability and Genotype Affect Major Nutritional Quality Parameters of Tef Grain Grown under Irrigation. Sci. Rep. 2020, 10, 14339. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, X.; Wang, S.; Zhang, Z.; Li, F.; Zhao, A.Z. Therapeutic Potential of ω-3 Polyunsaturated Fatty Acids in Human Autoimmune Diseases. Front. Immunol. 2019, 10, 2241. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.L.; Leonard, A.E.; Mukerji, P. Recent Advances in the Study of Fatty Acid Desaturases from Animals and Lower Eukaryotes. Prostaglandins Leukot. Essent. Fat. Acids 2003, 68, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Froyen, E.; Burns-Whitmore, B. The Effects of Linoleic Acid Consumption on Lipid Risk Markers for Cardiovascular Disease in Healthy Individuals: A Review of Human Intervention Trials. Nutrients 2020, 12, 2329. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, Y.; Wu, M.; Li, X.; Mai, K.; Ai, Q. ω-6 Polyunsaturated Fatty Acids (Linoleic Acid) Activate Both Autophagy and Antioxidation in a Synergistic Feedback Loop via TOR-Dependent and TOR-Independent Signaling Pathways. Cell. Death Dis. 2020, 11, 607. [Google Scholar] [CrossRef]
- Assefa, K.; Cannarozzi, G.; Girma, D.; Kamies, R.; Chanyalew, S.; Plaza-Wüthrich, S.; Blösch, R.; Rindisbacher, A.; Rafudeen, S.; Tadele, Z. Genetic Diversity in Tef [Eragrostis tef (Zucc.) Trotter]. Front. Plant. Sci. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-Free Flours from Cereals, Pseudocereals and Legumes: Phenolic Fingerprints and in Vitro Antioxidant Properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef]
- Shukla, H.; Lee, H.Y.; Koucheki, A.; Bibi, H.A.; Gaje, G.; Sun, X.; Zhu, H.; Li, Y.R.; Jia, Z. Targeting Glutathione with the Triterpenoid CDDO-Im Protects against Benzo-a-Pyrene-1,6-Quinone-Induced Cytotoxicity in Endothelial Cells. Mol. Cell. Biochem. 2020, 474, 27–39. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Deponte, M. Glutathione Catalysis and the Reaction Mechanisms of Glutathione-Dependent Enzymes. Biochim. Biophys. Acta 2013, 1830, 3217–3266. [Google Scholar] [CrossRef]
- Chang, C.-S.; Sun, H.-L.; Lii, C.-K.; Chen, H.-W.; Chen, P.-Y.; Liu, K.-L. Gamma-Linolenic Acid Inhibits Inflammatory Responses by Regulating NF-ΚB and AP-1 Activation in Lipopolysaccharide-Induced RAW 264.7 Macrophages. Inflammation 2010, 33, 46–57. [Google Scholar] [CrossRef]
- El-Alfy, T.S.; Ezzat, S.M.; Sleem, A.A. Chemical and Biological Study of the Seeds of Eragrostis tef (Zucc.) Trotter. Nat. Prod. Res. 2012, 26, 619–629. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Yang, Y.; Wang, Y.; Xia, S.; Gong, H.; Zhang, B.-K.; Yan, M. Dissecting the Crosstalk Between Nrf2 and NF-ΚB Response Pathways in Drug-Induced Toxicity. Front. Cell. Dev. Biol. 2022, 9, 809952. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.; Liu, R.; Chen, H.-L.; Bai, H.; Liang, X.; Zhang, X.-D.; Wang, Z.; Li, W.; Hai, C.-X. Antioxidant Activities of Oleanolic Acid in Vitro: Possible Role of Nrf2 and MAP Kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.P.; Reis, M.A.; Leão, P.N. 4-Oxo-β-Apo-13-Carotenone from the Cyanobacterium Anabaena Cylindrica PCC 7122. Chem. Biodivers. 2018, 15, e1800076. [Google Scholar] [CrossRef] [PubMed]
- Harrison, E.H. Carotenoids, β-Apocarotenoids, and Retinoids: The Long and the Short of It. Nutrients 2022, 14, 1411. [Google Scholar] [CrossRef]
- Ben-Dor, A.; Steiner, M.; Gheber, L.; Danilenko, M.; Dubi, N.; Linnewiel, K.; Zick, A.; Sharoni, Y.; Levy, J. Carotenoids Activate the Antioxidant Response Element Transcription System. Mol. Cancer Ther. 2005, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Park, S.L.; de la Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Systemic Administration of the Apocarotenoid Bixin Protects Skin against Solar UV-Induced Damage through Activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef]
- Lee, W.S.; Baek, Y.-I.; Kim, J.-R.; Cho, K.-H.; Sok, D.-E.; Jeong, T.-S. Antioxidant Activities of a New Lignan and a Neolignan from Saururus Chinensis. Bioorg. Med. Chem. Lett. 2004, 14, 5623–5628. [Google Scholar] [CrossRef]
- Fauré, M.; Lissi, E.; Torres, R.; Videla, L.A. Antioxidant Activities of Lignans and Flavonoids. Phytochemistry 1990, 29, 3773–3775. [Google Scholar] [CrossRef]
- Kay, H.Y.; Kim, Y.W.; Ryu, D.H.; Sung, S.H.; Hwang, S.J.; Kim, S.G. Nrf2-Mediated Liver Protection by Sauchinone, an Antioxidant Lignan, from Acetaminophen Toxicity through the PKCδ-GSK3β Pathway. Br. J. Pharmacol. 2011, 163, 1653–1665. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Espada, S.; Orozco-Ibarra, M.; Pedraza-Chaverri, J.; Cuadrado, A. Nordihydroguaiaretic Acid Activates the Antioxidant Pathway Nrf2/HO-1 and Protects Cerebellar Granule Neurons against Oxidative Stress. Neurosci. Lett. 2008, 447, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.H.; Cho, S.S.; Kim, J.H.; Xu, J.; Seo, K.; Ki, S.H. 5-Caffeoylquinic Acid Ameliorates Oxidative Stress-Mediated Cell Death via Nrf2 Activation in Hepatocytes. Pharm. Biol. 2020, 58, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-Regulated Glutathione Recycling Independent of Biosynthesis Is Critical for Cell Survival during Oxidative Stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- McMahon, M.; Thomas, N.; Itoh, K.; Yamamoto, M.; Hayes, J.D. Dimerization of Substrate Adaptors Can Facilitate Cullin-Mediated Ubiquitylation of Proteins by a “Tethering” Mechanism: A Two-Site Interaction Model for the Nrf2-Keap1 Complex. J. Biol. Chem. 2006, 281, 24756–24768. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Llères, D.; Swift, S.; Dinkova-Kostova, A.T. Regulatory Flexibility in the Nrf2-Mediated Stress Response Is Conferred by Conformational Cycling of the Keap1-Nrf2 Protein Complex. Proc. Natl. Acad. Sci. USA 2013, 110, 15259–15264. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamamoto, M. Molecular Basis of the Keap1-Nrf2 System. Free Radic. Biol. Med. 2015, 88, 93–100. [Google Scholar] [CrossRef]
- Baird, L.; Yamamoto, M. The Molecular Mechanisms Regulating the KEAP1-NRF2 Pathway. Mol. Cell. Biol. 2020, 40, e00099-20. [Google Scholar] [CrossRef]
- Zhang, D.D. Mechanistic Studies of the Nrf2-Keap1 Signaling Pathway. Drug Metab. Rev. 2006, 38, 769–789. [Google Scholar] [CrossRef]
- Kensler, T.W.; Wakabayashi, N.; Biswal, S. Cell Survival Responses to Environmental Stresses via the Keap1-Nrf2-ARE Pathway. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 89–116. [Google Scholar] [CrossRef]
| Gene Target | Gene ID | Amplicon Size | Forward Primer | Reverse Primer |
|---|---|---|---|---|
| GCLM | NM_002061 V4 | 246 bp | 5’-CTCCCTCTCGGGTCTCTCTC-3’ | 5’-ATCATGAAGCTCCTCGCTGT-3’ |
| GCLC | NM_001197115 | 182 bp | 5’-ACCATCATCAATGGGAAGGA-3’ | 5’-GCGATAAACTCCCTCATCCA-3’ |
| GR | NM_000637 V5 | 205 bp | 5’-CAGTGGGACTCACGGAAGAT-3’ | 5’-AAACCCTGCAGCATTTCATC-3’ |
| Feature * | [M+H]+ m/z | Calculated m/z | Molecular Formula | Potential Compounds |
|---|---|---|---|---|
| 2 | 273.1848 | 272.1769 | C18H24O2 | 4-Oxo-β-apo-13-carotenone |
| 18 | 271.1692 | 270.1612 | C18H22O2 | 4,4′-(2,3-dimethyl-1,4-butanediyl)bis-phenol |
| 31 | 648.4251 | 647.4172 | C40H57NO6 | (3β)-3-[[2-[4-(Acetylamino)phenoxy]acetyl]oxy]olean-12-en-28-oic acid |
| 36 | 293.2478 | 292.2398 | C19H32O2 | γ-Linolenic acid, methyl ester |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotter, C.J.; Wright, A.J.; Romanov, A.V.; Graf, T.N.; Whisnant, E.D.; Flores-Bocanegra, L.; Doldron, M.S.; Oberlies, N.H.; Jia, Z.; Ligaba-Osena, A. Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes. Antioxidants 2023, 12, 1561. https://doi.org/10.3390/antiox12081561
Cotter CJ, Wright AJ, Romanov AV, Graf TN, Whisnant ED, Flores-Bocanegra L, Doldron MS, Oberlies NH, Jia Z, Ligaba-Osena A. Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes. Antioxidants. 2023; 12(8):1561. https://doi.org/10.3390/antiox12081561
Chicago/Turabian StyleCotter, Christopher J., Allison J. Wright, Anastasia V. Romanov, Tyler N. Graf, Eric D. Whisnant, Laura Flores-Bocanegra, Megan S. Doldron, Nicholas H. Oberlies, Zhenquan Jia, and Ayalew Ligaba-Osena. 2023. "Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes" Antioxidants 12, no. 8: 1561. https://doi.org/10.3390/antiox12081561
APA StyleCotter, C. J., Wright, A. J., Romanov, A. V., Graf, T. N., Whisnant, E. D., Flores-Bocanegra, L., Doldron, M. S., Oberlies, N. H., Jia, Z., & Ligaba-Osena, A. (2023). Evaluating the Antioxidant Properties of the Ancient-Crop Tef (Eragrostis tef) Grain Extracts in THP-1 Monocytes. Antioxidants, 12(8), 1561. https://doi.org/10.3390/antiox12081561








