Anti-Inflammatory and Anti-Oxidative Effects of Polysaccharides Extracted from Unripe Carica papaya L. Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Extraction of Polysaccharides
2.3. Isolation and Purification of PPs
2.4. Chemical Analysis
2.5. Determination of Monosaccharide Components
2.6. Determination of the Molecular Weight of Polysaccharide Fractions
2.7. Fourier Transform Infrared (FTIR) Spectroscopic Analysis
2.8. Determination of Antioxidant Capacities
2.8.1. Hydroxyl Radical Scavenging Activity
2.8.2. Chelating Capacity of Ferrous Ions
2.8.3. Reducing Power Assay
2.9. Cell Viability Assay
2.10. Intracellular ROS Production Assay
2.11. Western Blot Analysis
2.12. Evaluation of IL-6 and TNF-α Levels in HaCaTs
2.13. Statistical Analysis
3. Results
3.1. Purification of Crude PP-URS
3.2. Composition and Molecular Weights of Polysaccharide Fractions
3.3. Determination of the Functional Groups of PPs
3.4. Analysis of the Antioxidant Activity
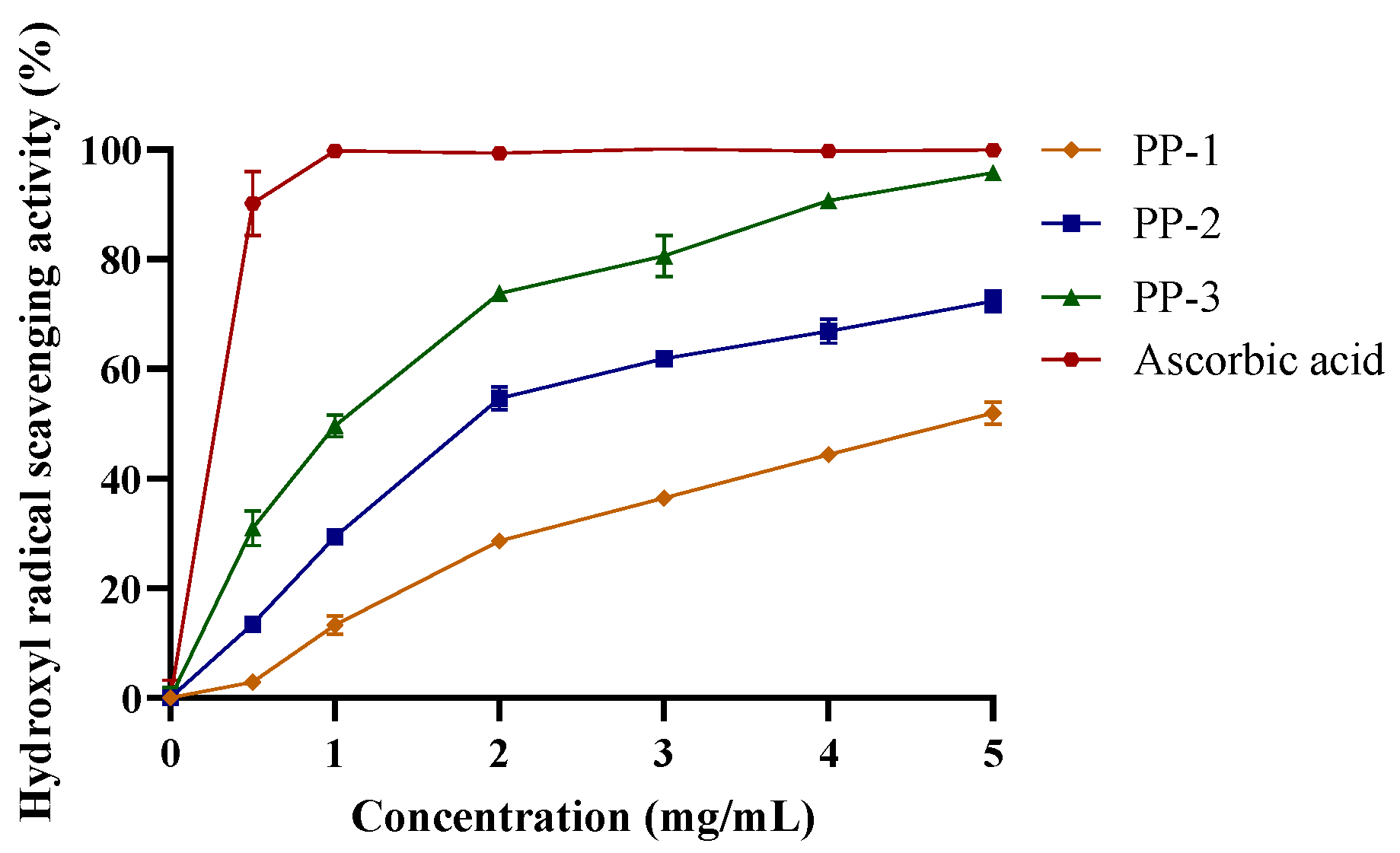
3.4.1. Hydroxyl Radical Scavenging Activity of PPs
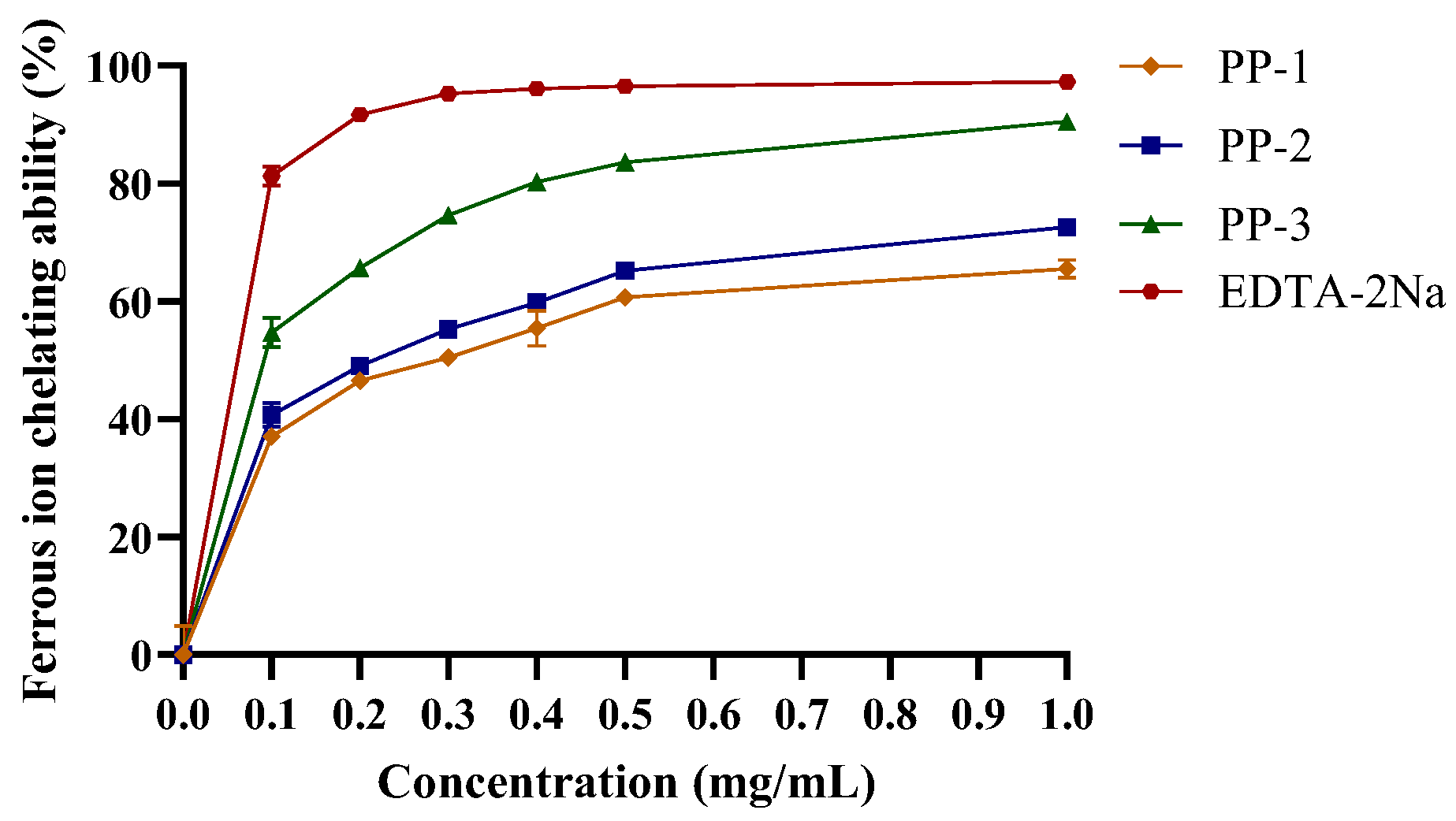
3.4.2. Ferrous Ion-Chelating Capacity of PPs
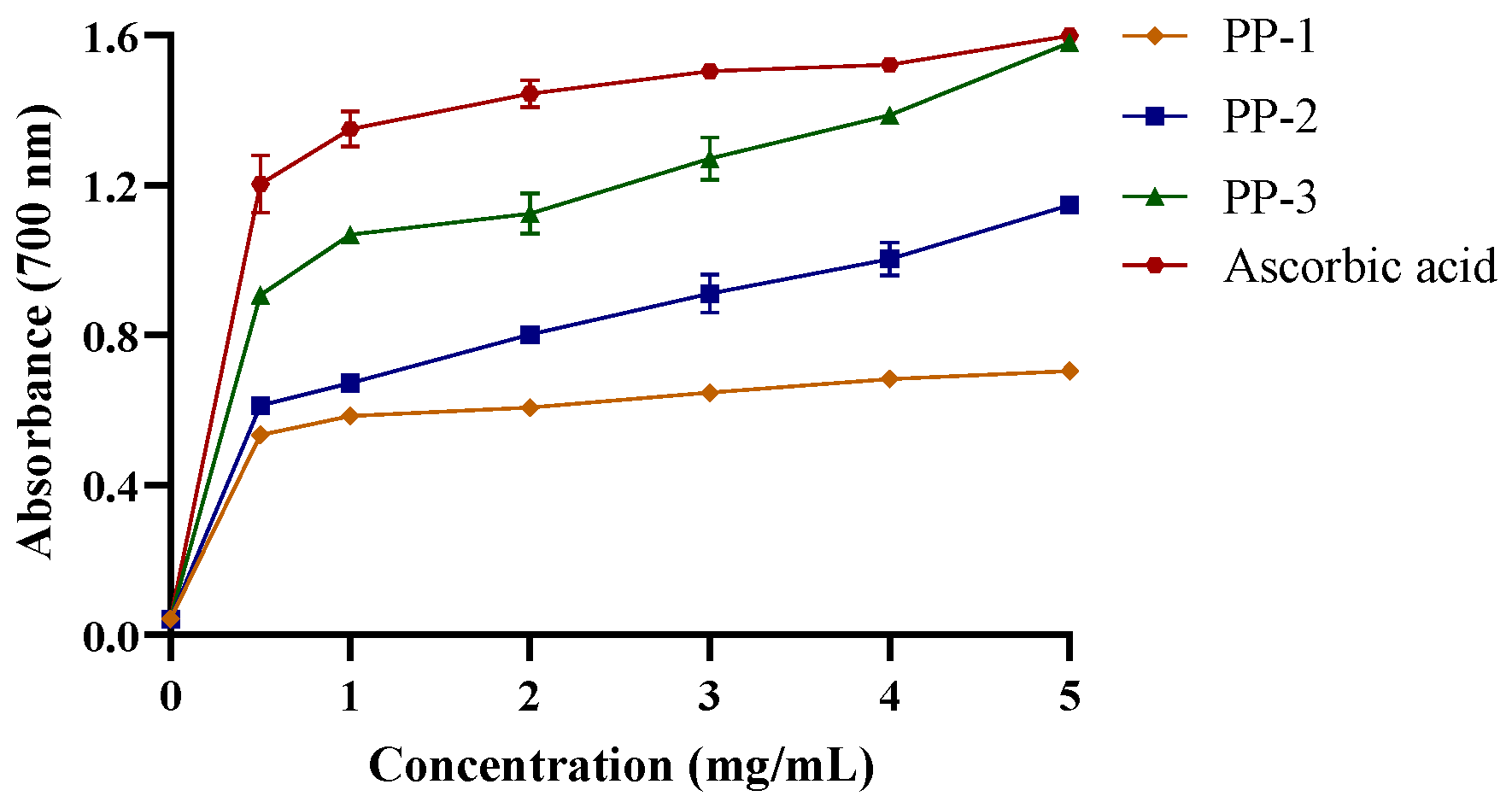
3.4.3. PPs Exhibit Reducing Power in a Concentration-Dependent Manner
3.5. PP-3 Improves Cell Viability by Reversing the Antiproliferative Effect of ROS
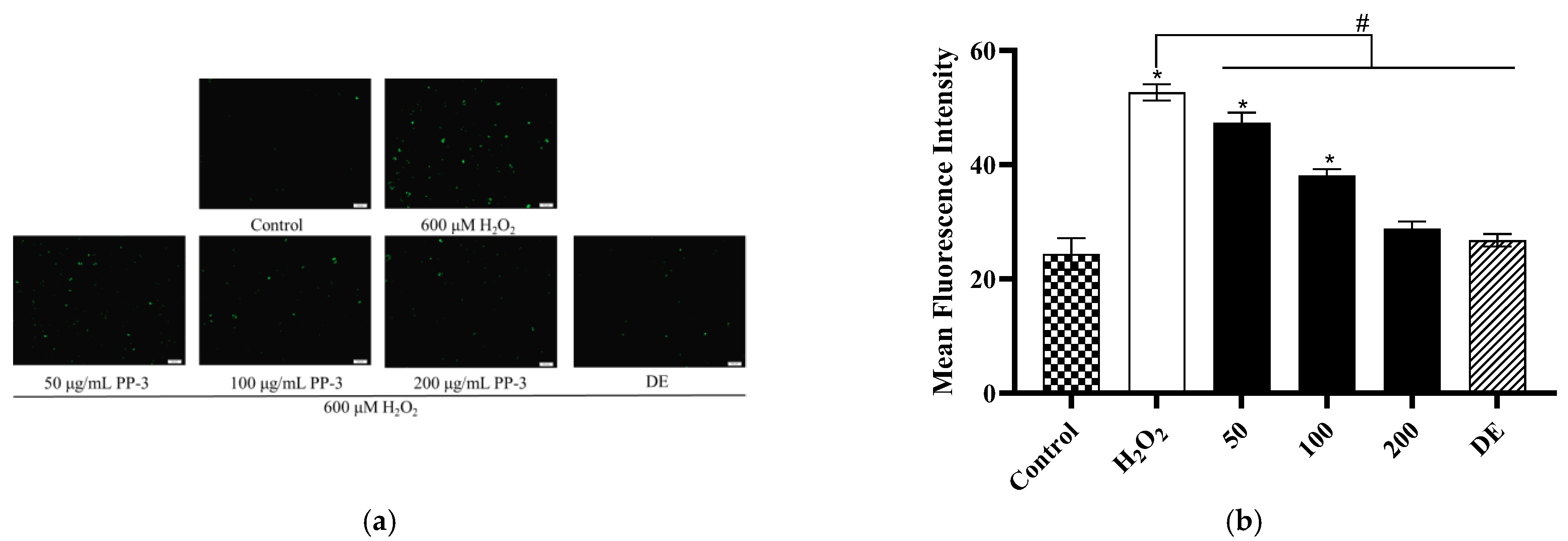
3.6. PP-3 Mitigates Intracellular ROS Production
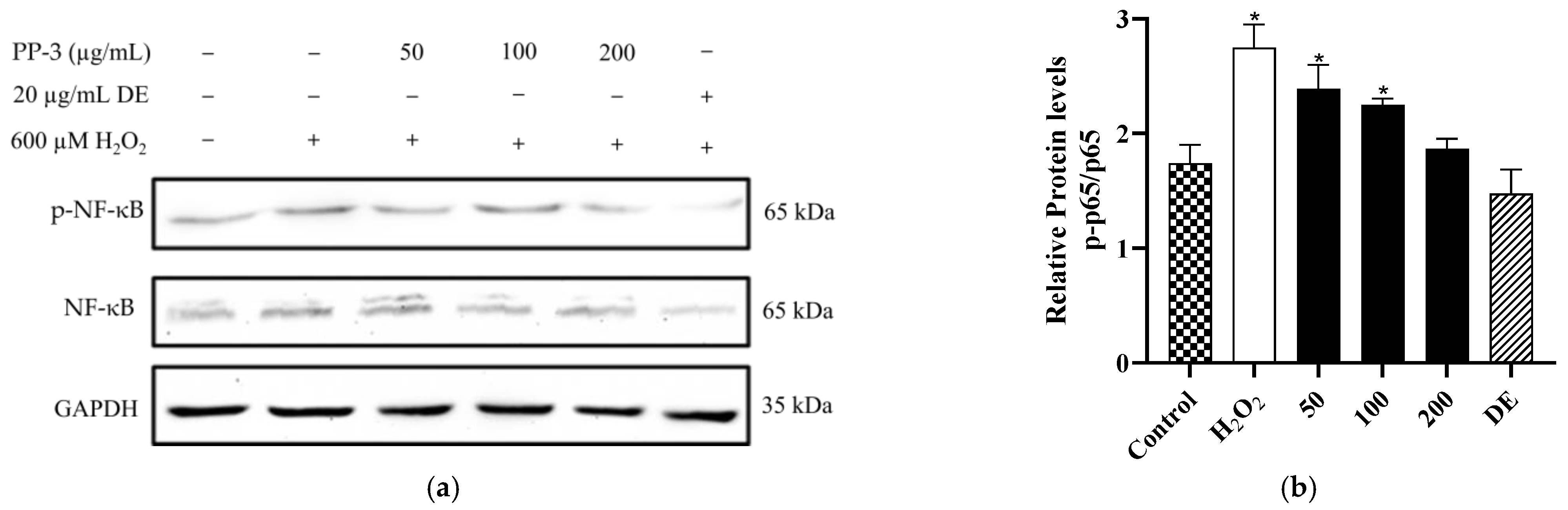
3.7. PP-3 Mediates the Reduction of NF-κB Phosphorylation
3.8. PP-3 Regulates the Level of Inflammatory Cytokines
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Zhang, X.; Wang, S.; Guo, Q.; Li, Z.; Liu, H.; Wang, C. Structural characterisation and immunomodulatory activity of polysaccharides from white asparagus skin. Carbohydr. Polym. 2019, 227, 115314. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Xiong, Y.; Fan, J.; Zheng, Z.; Li, Y.; Dong, L.; Zhao, Z. Extraction optimization, separation and antioxidant activity of Luffa cylindrica polysaccharides. Food Bioprod. Process. 2019, 116, 98–104. [Google Scholar] [CrossRef]
- Hwang, K.C.; Shin, H.Y.; Kim, W.J.; Seo, M.S.; Kim, H. Effects of a High-Molecular-Weight Polysaccharides Isolated from Korean Persimmon on the Antioxidant, Anti-Inflammatory, and Antiwrinkle Activity. Molecules 2021, 26, 1600. [Google Scholar] [CrossRef]
- Wei, H.; Shi, Y.; Yuan, Z.; Huang, Z.; Cai, F.; Zhu, J.; Zhang, W.; Li, J.; Xiong, Q.; Wang, Y.; et al. Isolation, Identification, and Anti-Inflammatory Activity of Polysaccharides of Typha angustifolia. Biomacromolecules 2021, 22, 2451–2459. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Huang, H. Preparation, analysis, antioxidant activities in vivo of phosphorylated polysaccharide from Momordica charantia. Carbohydr. Polym. 2021, 252, 117179. [Google Scholar] [CrossRef]
- Yang, Y.; Qiu, Z.; Li, L.; Vidyarthi, S.K.; Zheng, Z.; Zhang, R. Structural characterization and antioxidant activities of one neutral polysaccharide and three acid polysaccharides from Ziziphus jujuba cv. Hamidazao: A comparison. Carbohydr. Polym. 2021, 261, 117879. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, G.; Wang, M.; Lv, Z.; Du, P. A selenium polysaccharide from Platycodon grandiflorum rescues PC12 cell death caused by H2O2 via inhibiting oxidative stress. Int. J. Biol. Macromol. 2017, 104, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Gong, P.; Cui, D.; Guo, Y.; Wang, M.; Wang, Z.; Huang, Z.; Yang, W.; Chen, F.; Chen, X. A novel polysaccharide obtained from Siraitia grosvenorii alleviates inflammatory responses in a diabetic nephropathy mouse model via the TLR4-NF-κB pathway. Food Funct. 2021, 12, 9054–9065. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Zhang, X.; Meng, M.; Han, L.; Li, C.; Hou, L.; Qi, W.; Wang, C. Inhibitory effect on HT-29 colon cancer cells of a water-soluble polysaccharide obtained from highland barley. Int. J. Biol. Macromol. 2016, 92, 88–95. [Google Scholar] [CrossRef]
- Laurora, A.; Bingham, J.-P.; Poojary, M.M.; Wall, M.M.; Ho, K.K. Carotenoid composition and bioaccessibility of papaya cultivars from Hawaii. J. Food Compos. Anal. 2021, 101, 103984. [Google Scholar] [CrossRef]
- Ali, A.; Devarajan, S.; Waly, M.; Essa, M.M.; Ragman, M.S. Nutritional and medicinal value of papaya (Carica papaya L.). In Natural Products and Bioactive Compounds in Disease Prevention; Nova Science Publisher: New York, NY, USA, 2011; pp. 307–324. [Google Scholar]
- Singh, S.P.; Mathan, S.V.; Dheeraj, A.; Tailor, D.; Singh, R.P.; Acharya, A. Abstract 3004: Anticancer effects and associated molecular changes of Carica papaya against prostate cancer. Cancer Res. 2019, 79, 3004. [Google Scholar] [CrossRef]
- Kong, Y.R.; Jong, Y.X.; Balakrishnan, M.; Bok, Z.K.; Weng, J.K.K.; Tay, K.C.; Goh, B.H.; Ong, Y.S.; Chan, K.G.; Lee, L.H.; et al. Beneficial Role of Carica papaya Extracts and Phytochemicals on Oxidative Stress and Related Diseases: A Mini Review. Biology 2021, 10, 287. [Google Scholar] [CrossRef]
- Nafiu, A.B.; Rahman, M.T. Anti-inflammatory and antioxidant properties of unripe papaya extract in an excision wound model. Pharm. Biol. 2015, 53, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Jarisarapurin, W.; Sanrattana, W.; Chularojmontri, L.; Kunchana, K.; Wattanapitayakul, S.K. Antioxidant Properties of Unripe Carica papaya Fruit Extract and Its Protective Effects against Endothelial Oxidative Stress. Evid.-Based Complement. Altern. Med. 2019, 2019, 4912631. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-S.; Wang, X.-M.; Han, Z.-P.; Zhao, M.-X.; Yin, L. Purification, antioxidant and moisture-preserving activities of polysaccharides from papaya. Carbohydr. Polym. 2012, 87, 2332–2337. [Google Scholar] [CrossRef]
- Shi, L. Bioactivities, isolation and purification methods of polysaccharides from natural products: A review. Int. J. Biol. Macromol. 2016, 92, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pasparakis, M.; Haase, I.; Nestle, F.O. Mechanisms regulating skin immunity and inflammation. Nat. Rev. Immunol. 2014, 14, 289–301. [Google Scholar] [CrossRef]
- Sollberger, G.; Strittmatter, G.E.; Grossi, S.; Garstkiewicz, M.; Keller, U.A.D.; French, L.E.; Beer, H.-D. Caspase-1 Activity Is Required for UVB-Induced Apoptosis of Human Keratinocytes. J. Investig. Dermatol. 2015, 135, 1395–1404. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2020, 47, 170–180. [Google Scholar] [CrossRef]
- da Silva, J.M.; Conegundes, J.L.M.; Pinto, N.C.C.; Mendes, R.F.; Castañon, M.C.M.N.; Scio, E. Comparative analysis of Lacistema pubescens and dexamethasone on topical treatment of skin inflammation in a chronic disease model and side effects. J. Pharm. Pharmacol. 2018, 70, 576–582. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, F.; Li, R.; Wu, Y.; Liu, S.; Liang, Q. Purification, characterization, antioxidant and moisture-preserving activities of polysaccharides from Rosa rugosa petals. Int. J. Biol. Macromol. 2019, 124, 938–945. [Google Scholar] [CrossRef]
- Yan, J.-K.; Wu, L.-X.; Qiao, Z.-R.; Cai, W.-D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-H.; Lai, M.-N.; Ng, L.-T. Effects of different extraction temperatures on the physicochemical properties of bioactive polysaccharides from Grifola frondosa. Food Chem. 2017, 220, 400–405. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhang, H.; Guo, B.; Chen, W.; Wu, Z.; Wu, Y. Preparation, partial characterization and bioactivity of exopolysaccharides from Lactobacillus casei LC2W. Carbohydr. Polym. 2008, 74, 353–357. [Google Scholar] [CrossRef]
- Wang, W.; Li, X.; Bao, X.; Gao, L.; Tao, Y. Extraction of polysaccharides from black mulberry fruit and their effect on enhancing antioxidant activity. Int. J. Biol. Macromol. 2018, 120, 1420–1429. [Google Scholar] [CrossRef]
- Chou, C.-H.; Sung, T.-J.; Hu, Y.-N.; Lu, H.-Y.; Yang, L.-C.; Cheng, K.-C.; Lai, P.-S.; Hsieh, C.-W. Chemical analysis, moisture-preserving, and antioxidant activities of polysaccharides from Pholiota nameko by fractional precipitation. Int. J. Biol. Macromol. 2019, 131, 1021–1031. [Google Scholar] [CrossRef]
- Han, Q.-H.; Liu, W.; Li, H.-Y.; He, J.-L.; Guo, H.; Lin, S.; Zhao, L.; Chen, H.; Liu, Y.-W.; Wu, D.-T.; et al. Extraction Optimization, Physicochemical Characteristics, and Antioxidant Activities of Polysaccharides from Kiwifruit (Actinidia chinensis Planch.). Molecules 2019, 24, 461. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, H.; Zhou, P.; Liu, L.; Yu, J.; Dai, P.; Qu, M. RETRACTED: Angelica polysaccharide alleviates oxidative response damage in HaCaT cells through up-regulation of miR-126. Exp. Mol. Pathol. 2019, 110, 104281. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Mao, X. Chitopentaose protects HaCaT cells against H2O2-induced oxidative damage through modulating MAPKs and Nrf2/ARE signaling pathways. J. Funct. Foods 2020, 72, 104086. [Google Scholar] [CrossRef]
- Jia, X.; Zhang, C.; Qiu, J.; Wang, L.; Bao, J.; Wang, K.; Zhang, Y.; Chen, M.; Wan, J.; Su, H.; et al. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohydr. Polym. 2015, 132, 67–71. [Google Scholar] [CrossRef]
- Zhou, L.-L.; Lin, Z.-X.; Fung, K.-P.; Cheng, C.H.K.; Che, C.-T.; Zhao, M.; Wu, S.-H.; Zuo, Z. Celastrol-induced apoptosis in human HaCaT keratinocytes involves the inhibition of NF-κB activity. Eur. J. Pharmacol. 2011, 670, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.K.; Tanna, B.; Gupta, H.; Mishra, A.; Jha, B. Physicochemical, scavenging and anti-proliferative analyses of polysaccharides extracted from psyllium (Plantago ovata Forssk) husk and seeds. Int. J. Biol. Macromol. 2019, 133, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.-H.; Xie, M.-Y.; Nie, S.-P.; Shen, M.-Y.; Wang, Y.-X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Li, J.; Fan, Y.; Huang, G.; Huang, H. Extraction, structural characteristics and activities of Zizylphus vulgaris polysaccharides. Ind. Crop. Prod. 2022, 178, 114675. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Qu, Z.; Xie, B. Antioxidant activities of different fractions of polysaccharide conjugates from green tea (Camellia sinensis). Food Chem. 2008, 106, 559–563. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, P.; Zhao, H.; Qiu, J.; Mac Regenstein, J.; Yang, X. Isolation, purification, structure and antioxidant activity of polysaccharide from pinecones of Pinus koraiensis. Carbohydr. Polym. 2021, 251, 117078. [Google Scholar] [CrossRef]
- Meng, L.; Sun, S.; Li, R.; Shen, Z.; Wang, P.; Jiang, X. Antioxidant activity of polysaccharides produced by Hirsutella sp. and relation with their chemical characteristics. Carbohydr. Polym. 2015, 117, 452–457. [Google Scholar] [CrossRef]
- Taoerdahong, H.; Zhou, K.; Yang, F.; Dong, C.-X. Structure, immunostimulatory activity, and the effect of ameliorating airway inflammation of polysaccharides from Pyrus sinkiangensis Yu. Int. J. Biol. Macromol. 2022, 195, 246–254. [Google Scholar] [CrossRef]
- Yan, X.; Ye, R.; Chen, Y. Blasting extrusion processing: The increase of soluble dietary fiber content and extraction of soluble-fiber polysaccharides from wheat bran. Food Chem. 2015, 180, 106–115. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Q.; Pang, Z.; Xie, J.; Lin, L.; Yao, Q. Optimization extraction, characterization and anticancer activities of polysaccharides from mango pomace. Int. J. Biol. Macromol. 2018, 117, 1314–1325. [Google Scholar] [CrossRef]
- Zhang, W.; Zeng, G.; Pan, Y.; Chen, W.; Huang, W.; Chen, H.; Li, Y. Properties of soluble dietary fiber-polysaccharide from papaya peel obtained through alkaline or ultrasound-assisted alkaline extraction. Carbohydr. Polym. 2017, 172, 102–112. [Google Scholar] [CrossRef]
- Nie, X.-R.; Li, H.-Y.; Du, G.; Lin, S.; Hu, R.; Li, H.-Y.; Zhao, L.; Zhang, Q.; Chen, H.; Wu, D.-T.; et al. Structural characteristics, rheological properties, and biological activities of polysaccharides from different cultivars of okra (Abelmoschus esculentus) collected in China. Int. J. Biol. Macromol. 2019, 139, 459–467. [Google Scholar] [CrossRef]
- Cui, S.W.; Phillips, G.O.; Blackwell, B.; Nikiforuk, J. Characterisation and properties of Acacia senegal (L.) Willd. var. senegal with enhanced properties (Acacia (sen) SUPERGUM™): Part 4. Spectroscopic characterisation of Acacia senegal var. senegal and Acacia (sen) SUPERGUM™ arabic. Food Hydrocoll. 2007, 21, 347–352. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, Y.; Ma, X.; Liu, X.; Yang, M.; Fan, W.; Ren, H.; Efehi, N.; Wang, X.; Zhu, X. Extraction, purification, characterization and antioxidant activities of polysaccharides from Zizyphus jujuba cv. Linzexiaozao. Int. J. Biol. Macromol. 2018, 118, 2138–2148. [Google Scholar] [CrossRef]
- Yuan, J.-F.; Zhang, Z.-Q.; Fan, Z.-C.; Yang, J.-X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr. Polym. 2008, 74, 822–827. [Google Scholar] [CrossRef]
- Cheng, H.; Feng, S.; Shen, S.; Zhang, L.; Yang, R.; Zhou, Y.; Ding, C. Extraction, antioxidant and antimicrobial activities of Epimedium acuminatum Franch. polysaccharide. Carbohydr. Polym. 2013, 96, 101–108. [Google Scholar] [CrossRef]
- Salvail, H.; Massé, E. Regulating iron storage and metabolism with RNA: An overview of posttranscriptional controls of intracellular iron homeostasis. Wiley Interdiscip. Rev. RNA 2012, 3, 26–36. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Yu, Y.; Liang, Y.-Z.; Chen, X.-Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef]
- Chen, R.; Liu, Z.; Zhao, J.; Chen, R.; Meng, F.; Zhang, M.; Ge, W. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011, 127, 434–440. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, R.; Hu, L.; Maffucci, K.G.; Ren, X.; Qu, Y. In vivo wound healing and in vitro antioxidant activities of Bletilla striata phenolic extracts. Biomed. Pharmacother. 2017, 93, 451–461. [Google Scholar] [CrossRef]
- Dou, J.; Meng, Y.; Liu, L.; Li, J.; Ren, D.; Guo, Y. Purification, characterization and antioxidant activities of polysaccharides from thinned-young apple. Int. J. Biol. Macromol. 2015, 72, 31–40. [Google Scholar] [CrossRef]
- You, Q.; Yin, X.; Zhang, S.; Jiang, Z. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr. Polym. 2014, 99, 1–10. [Google Scholar] [CrossRef]
- Wu, D.-T.; Liu, W.; Han, Q.-H.; Du, G.; Li, H.-Y.; Yuan, Q.; Fu, Y.; Zhao, L.; Zhang, Q.; Li, S.-Q.; et al. Physicochemical characteristics and antioxidant activities of non-starch polysaccharides from different kiwifruits. Int. J. Biol. Macromol. 2019, 136, 891–900. [Google Scholar] [CrossRef]
- Gerlier, D.; Thomasset, N. Use of MTT colorimetric assay to measure cell activation. J. Immunol. Methods 1986, 94, 57–63. [Google Scholar] [CrossRef]
- Oliveira-Marques, V.; Marinho, H.S.; Cyrne, L.; Antunes, F. Role of hydrogen peroxide in NF-κB activation: From inducer to modulator. Antioxid. Redox Signal. 2009, 11, 2223–2243. [Google Scholar] [CrossRef]
- Eom, S.H.; Lee, E.H.; Park, K.; Kwon, J.-Y.; Kim, P.H.; Jung, W.-K.; Kim, Y.M. Eckol from Eisenia bicyclis inhibits inflammation through the Akt/NF-κB signaling in Propionibacterium acnes-induced human keratinocyte Hacat cells. J. Food Biochem. 2017, 41, e12312. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wen, X.; Hao, D.; Zhang, N.; He, G.; Jiang, X. NF-κB signaling in skin aging. Mech. Ageing Dev. 2019, 184, 111160. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Dias, M.K.H.M.; Madusanka, D.M.D.; Han, E.J.; Kim, M.J.; Heo, S.-J.; Lee, K.; Cheong, S.H.; Ahn, G. Low molecular weight fucoidan fraction ameliorates inflammation and deterioration of skin barrier in fine-dust stimulated keratinocytes. Int. J. Biol. Macromol. 2021, 168, 620–630. [Google Scholar] [CrossRef]
- Han, E.-J.; Fernando, I.P.S.; Kim, H.-S.; Lee, D.-S.; Kim, A.; Je, J.-G.; Seo, M.-J.; Jee, Y.H.; Jeon, Y.-J.; Kim, S.-Y.; et al. (–)-Loliolide Isolated from Sargassum horneri Suppressed Oxidative Stress and Inflammation by Activating Nrf2/HO-1 Signaling in IFN-γ/TNF-α-Stimulated HaCaT Keratinocytes. Antioxidants 2021, 10, 856. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, K.-C.; Lin, J.-A.; Hsieh, L.-P.; Chou, C.-H.; Wang, Y.-Y.; Lai, P.-S.; Chu, P.-C.; Hsieh, C.-W. Pholiota nameko Polysaccharides Protect against Ultraviolet A-Induced Photoaging by Regulating Matrix Metalloproteinases in Human Dermal Fibroblasts. Antioxidants 2022, 11, 739. [Google Scholar] [CrossRef]
- Liao, N.; Chen, S.; Ye, X.; Zhong, J.; Ye, X.; Yin, X.; Tian, J.; Liu, D. Structural Characterization of a Novel Glucan from Achatina fulica and Its Antioxidant Activity. J. Agric. Food Chem. 2014, 62, 2344–2352. [Google Scholar] [CrossRef]
- Wullaert, A.; Bonnet, M.C.; Pasparakis, M. NF-κB in the regulation of epithelial homeostasis and inflammation. Cell Res. 2011, 21, 146–158. [Google Scholar] [CrossRef] [PubMed]
- Gudjonsson, J.; Johnston, A.; Sigmundsdottir, H.; Vladimarsson, H. Immunopathogenic mechanisms in psoriasis. Clin. Exp. Immunol. 2004, 135, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]




| Chemical Composition (%) | |||||
|---|---|---|---|---|---|
| Yield | Polysaccharide | Protein | Uronic Acid | Polyphenol | |
| PP-1 | 2.76% | 84.90 ± 1.70 a | N.D. | 43.49 ± 0.06 b | 0.16 ± 0.01 b |
| PP-2 | 12.47% | 81.60 ± 0.73 b | N.D. | 76.52 ± 1.39 a | 0.35 ± 0.01 a |
| PP-3 | 31.21% | 83.51 ± 0.64 ab | N.D. | 76.69 ± 0.57 a | 0.06 ± 0.01 c |
| Constituent Monosaccharides (Molar Ratios) | Molecular Weight (kDa) | |||||||
|---|---|---|---|---|---|---|---|---|
| Mannose | Rhamnose | Galacturonic Acid | Glucose | Galactose | Xylose | Arabinose | ||
| PP-1 | 1.58 | 1.00 | 2.62 | 1.12 | 1.81 | 2.79 | 0.99 | 2252 |
| PP-2 | N.D. | 1.00 | 3.09 | 2.03 | 2.22 | 2.21 | 1.05 | 2448 |
| PP-3 | 4.47 | 1.00 | 2.99 | 2.06 | 2.47 | N.D. | 2.05 | 3741 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-Y.; Wu, Y.-T.; Chang, H.-J.; Huang, C.-C.; Cheng, K.-C.; Hsu, H.-Y.; Hsieh, C.-W. Anti-Inflammatory and Anti-Oxidative Effects of Polysaccharides Extracted from Unripe Carica papaya L. Fruit. Antioxidants 2023, 12, 1506. https://doi.org/10.3390/antiox12081506
Lin T-Y, Wu Y-T, Chang H-J, Huang C-C, Cheng K-C, Hsu H-Y, Hsieh C-W. Anti-Inflammatory and Anti-Oxidative Effects of Polysaccharides Extracted from Unripe Carica papaya L. Fruit. Antioxidants. 2023; 12(8):1506. https://doi.org/10.3390/antiox12081506
Chicago/Turabian StyleLin, Ting-Yun, Yun-Ting Wu, Hui-Ju Chang, Chun-Chen Huang, Kuan-Chen Cheng, Hsien-Yi Hsu, and Chang-Wei Hsieh. 2023. "Anti-Inflammatory and Anti-Oxidative Effects of Polysaccharides Extracted from Unripe Carica papaya L. Fruit" Antioxidants 12, no. 8: 1506. https://doi.org/10.3390/antiox12081506
APA StyleLin, T.-Y., Wu, Y.-T., Chang, H.-J., Huang, C.-C., Cheng, K.-C., Hsu, H.-Y., & Hsieh, C.-W. (2023). Anti-Inflammatory and Anti-Oxidative Effects of Polysaccharides Extracted from Unripe Carica papaya L. Fruit. Antioxidants, 12(8), 1506. https://doi.org/10.3390/antiox12081506







