Increased Blood Concentrations of Malondialdehyde in Plasmodium Infection: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Data Syntheses
3. Results
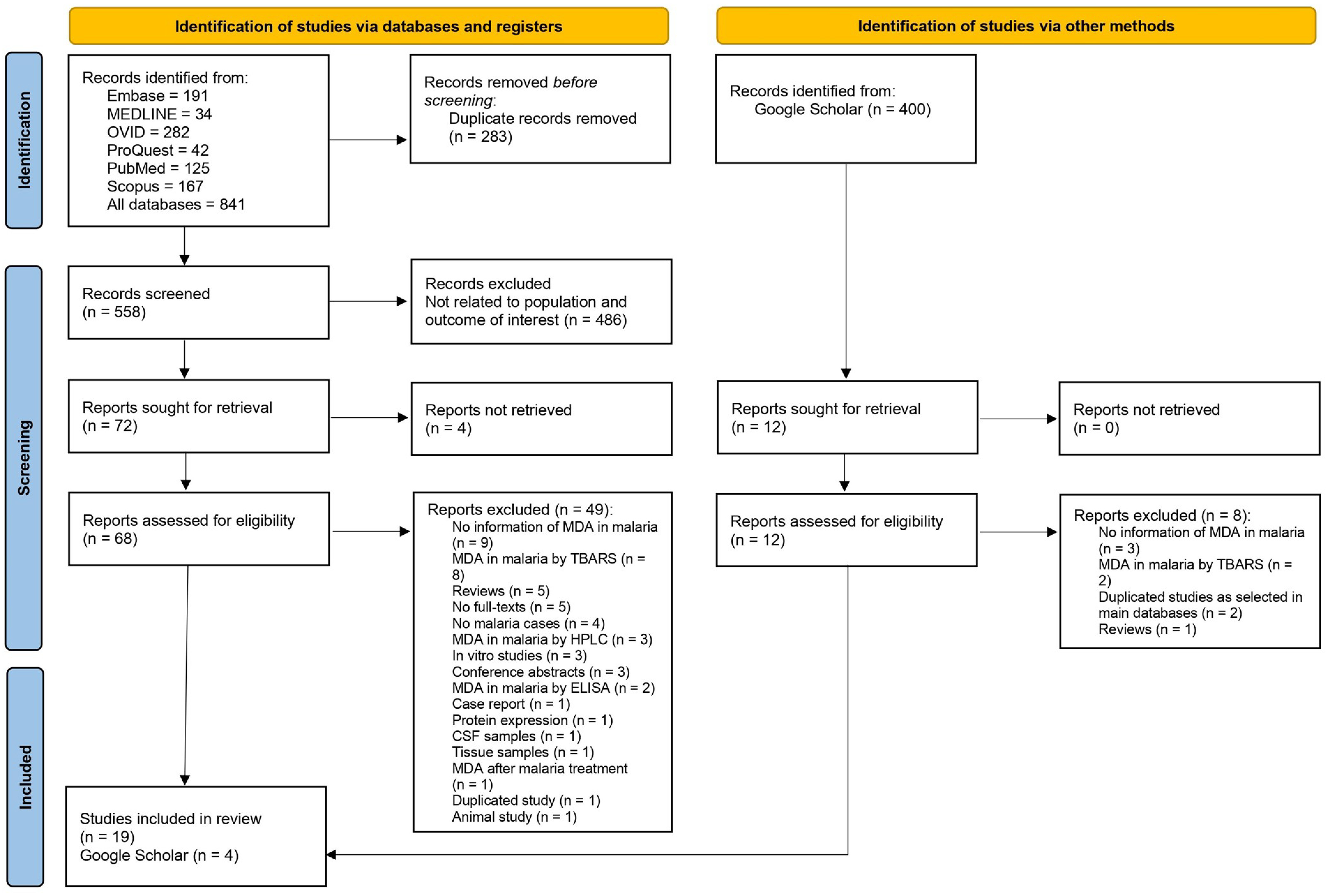
3.1. Search Results
3.2. Summary Characteristics of the Included Studies
3.3. Quality of the Included Studies
3.4. MDA Concentrations in Malaria and Uninfected Controls
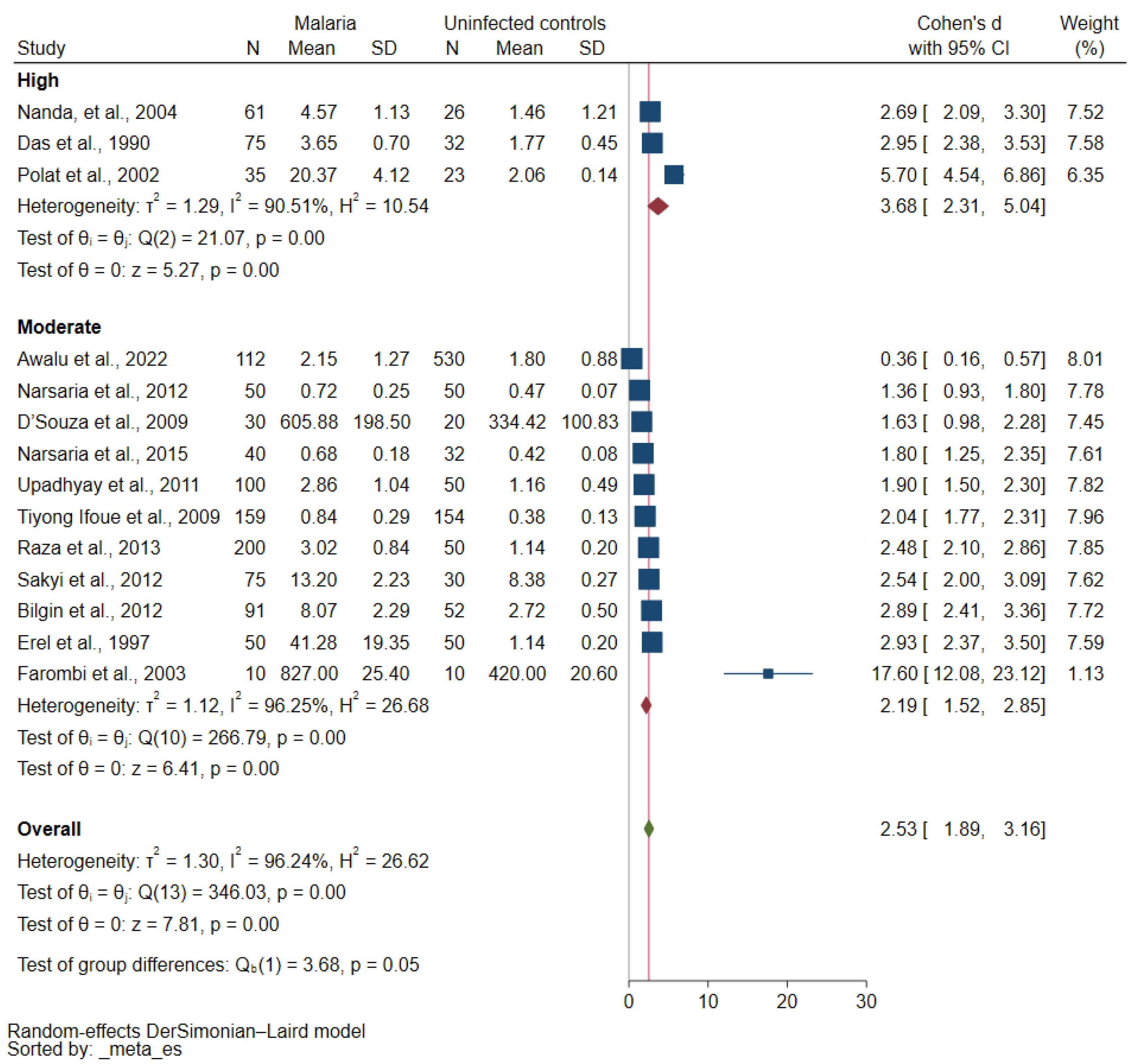
3.5. MDA Concentrations in Severe and Nonsevere Malaria
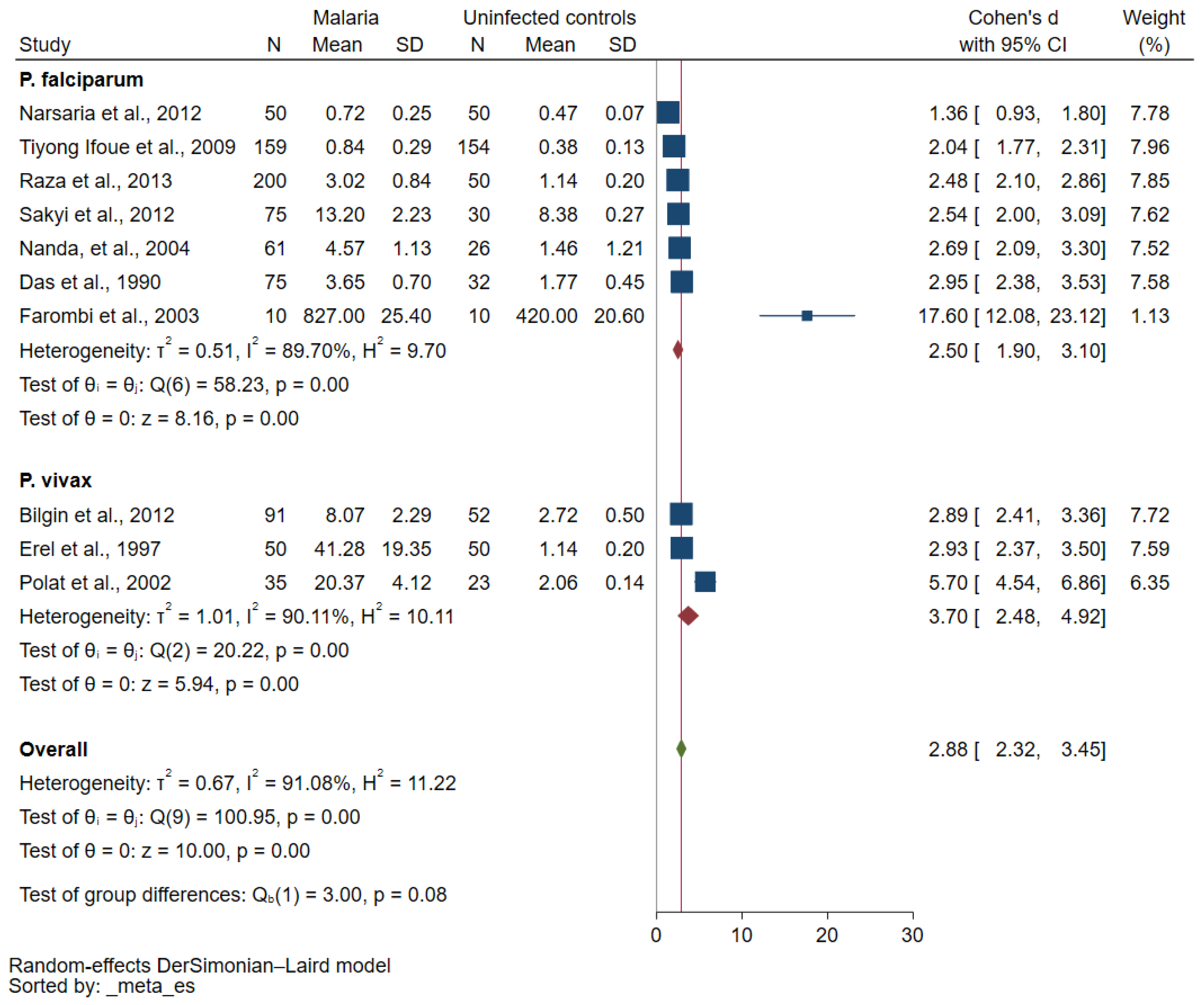
3.6. MDA in P. falciparum and P. vivax Malaria
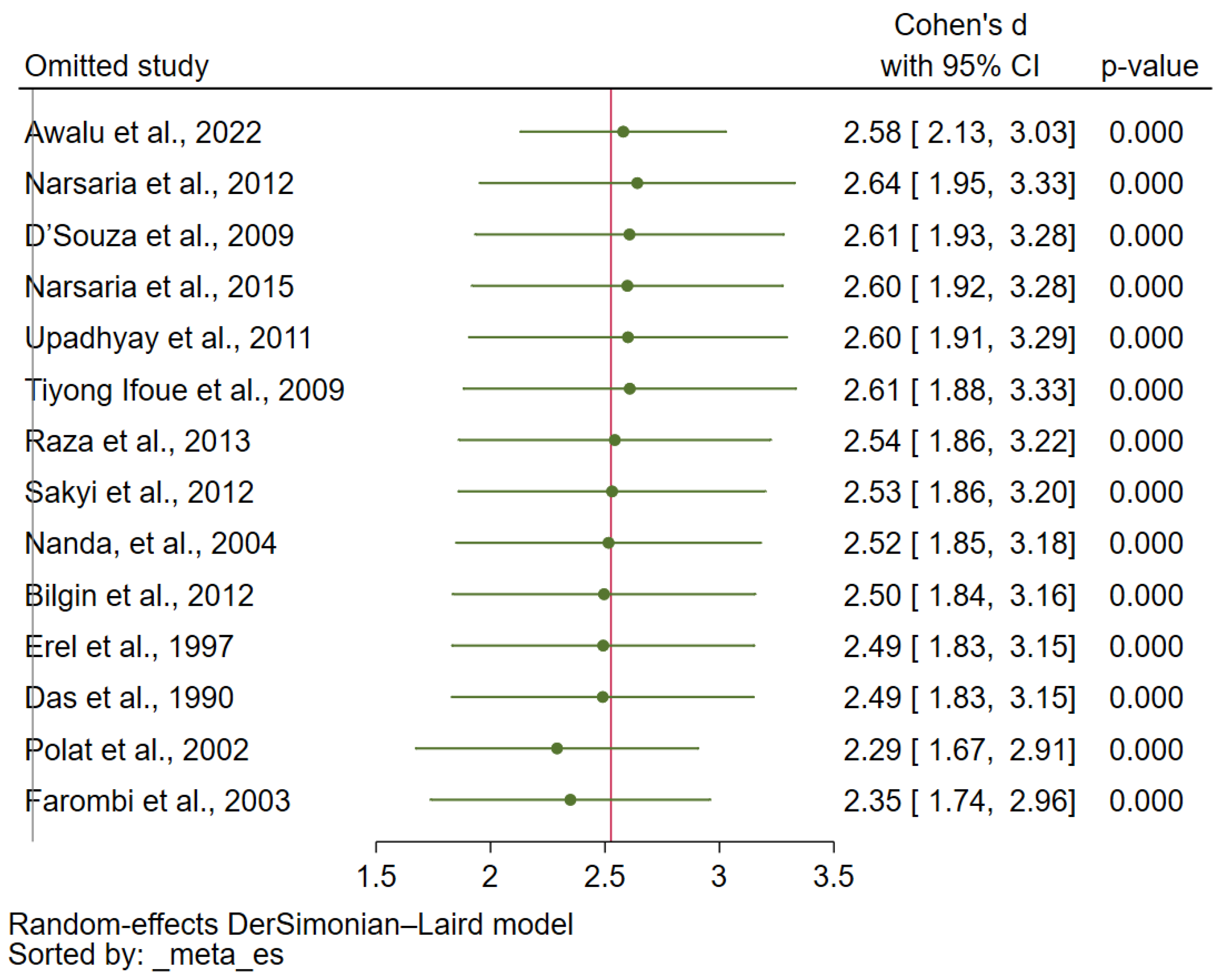
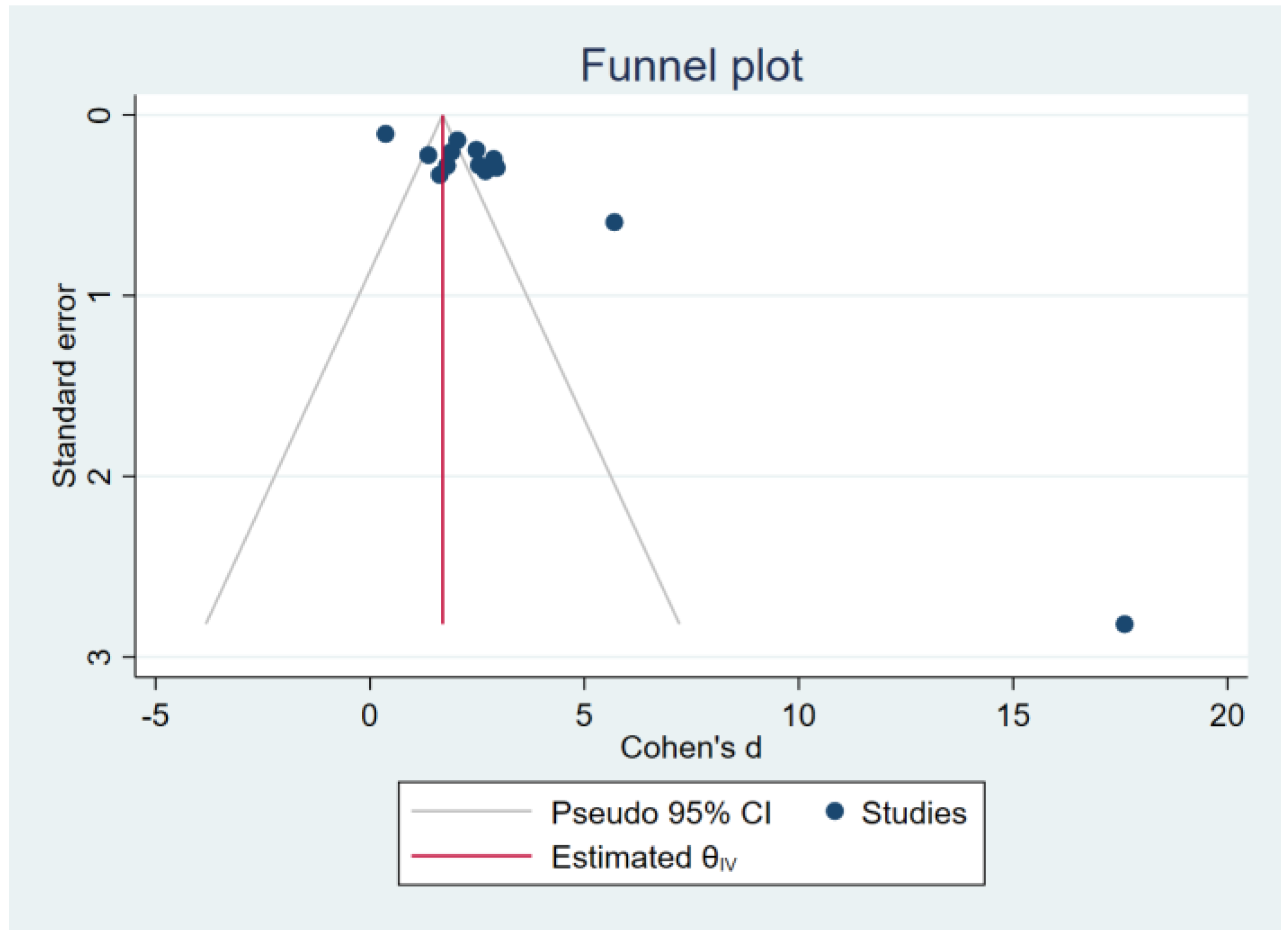
3.7. Sensitivity Analysis and Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Poli, G.; Albano, E.; Dianzani, M.U. The role of lipid peroxidation in liver damage. Chem. Phys. Lipids 1987, 45, 117–142. [Google Scholar] [CrossRef]
- Njie-Mbye, Y.F.; Kulkarni-Chitnis, M.; Opere, C.A.; Barrett, A.; Ohia, S.E. Lipid peroxidation: Pathophysiological and pharmacological implications in the eye. Front. Physiol. 2013, 4, 366. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, T.; Li, J.; Xia, M.; Li, Y.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Kan, D.; et al. Oxidative stress and 4-hydroxy-2-nonenal (4-HNE): Implications in the pathogenesis and treatment of aging-related diseases. J. Immunol. Res. 2022, 2022, 2233906. [Google Scholar] [CrossRef]
- Jove, M.; Mota-Martorell, N.; Pradas, I.; Martin-Gari, M.; Ayala, V.; Pamplona, R. The advanced lipoxidation end-product malondialdehyde-lysine in aging and longevity. Antioxidants 2020, 9, 1132. [Google Scholar] [CrossRef]
- Nair, V.; O’Neil, C.; Wang, P. Malondialdehyde. Encycl. Reag. Org. Synth. 2008, 542, 78–79. [Google Scholar]
- Davey, M.; Stals, E.; Panis, B.; Keulemans, J. High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem. 2005, 347, 201–207. [Google Scholar]
- Megnekou, R.; Djontu, J.C.; Bigoga, J.D.; Medou, F.M.; Tenou, S.; Lissom, A. Impact of placental Plasmodium falciparum malaria on the profile of some oxidative stress biomarkers in women living in Yaoundé, Cameroon. PLoS ONE 2015, 10, e0134633. [Google Scholar] [CrossRef]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical relevance of biomarkers of oxidative stress. Antioxid. Redox Signal 2015, 23, 144–170. [Google Scholar] [CrossRef] [PubMed]
- Mehri, F.; Rahbar, A.H.; Ghane, E.T.; Souri, B.; Esfahani, M. Changes in oxidative markers in COVID-19 patients. Arch. Med. Res. 2021, 52, 843–849. [Google Scholar] [CrossRef]
- Phillips, M.A.; Burrows, J.N.; Manyando, C.; van Huijsduijnen, R.H.; Van Voorhis, W.C.; Wells, T.N.C. Malaria. Nat. Rev. Dis. Primers 2017, 3, 17050. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2022; World Health Organization: Gneva, Switzerland, 2022. Available online: https://www.who.int/publications/i/item/9789240064898 (accessed on 23 January 2023).
- Vasquez, M.; Zuniga, M.; Rodriguez, A. oxidative stress and pathogenesis in malaria. Front. Cell Infect. Microbiol. 2021, 11, 768182. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.N.; Musa, S.; Emeribe, A.U.; Muhammed, M.; Mustapha, J.O.; Shuwa, H.A.; Haruna, S.; Abubakar, S.D.; Billyrose, O.M.A.; Bakare, M. Immunological and anti-oxidant profiles of malarial children in Abuja, Nigeria. Biomedicine 2021, 11, 41–50. [Google Scholar] [CrossRef]
- Abubakar, M.; Usman, S.; Dandare, S. Oxidant status of children infected with Plasmodium falciparum malaria in Katsina Metropolis, Northwestern Nigeria. Afr. J. Infect. Dis. 2016, 10, 17–20. [Google Scholar]
- Akanbi, O.; Odaibo, A.; Olatoregun, R.; Ademowo, A. Role of malaria induced oxidative stress on anaemia in pregnancy. Asian Pac. J. Trop. Med. 2010, 3, 211–214. [Google Scholar]
- Araujo, C.; Lacerda, M.; Abdalla, D.; Lima, E. The role of platelet and plasma markers of antioxidant status and oxidative stress in thrombocytopenia among patients with vivax malaria. Mem. Inst. Oswaldo Cruz. 2008, 103, 517–521. [Google Scholar] [PubMed]
- Atiku, S.; Louise, N.; Kasozi, D. Severe oxidative stress in sickle cell disease patients with uncomplicated Plasmodium falciparum malaria in Kampala, Uganda. BMC Infect. Dis. 2019, 19, 600. [Google Scholar]
- Moxon, C.A.; Grau, G.E.; Craig, A.G. Malaria: Modification of the red blood cell and consequences in the human host. Br. J. Haematol. 2011, 154, 670–679. [Google Scholar] [CrossRef]
- Sisquella, X.; Nebl, T.; Thompson, J.K.; Whitehead, L.; Malpede, B.M.; Salinas, N.D.; Rogers, K.; Tolia, N.H.; Fleig, A.; O’Neill, J.; et al. Plasmodium falciparum ligand binding to erythrocytes induce alterations in deformability essential for invasion. Elife 2017, 6, e21083. [Google Scholar] [CrossRef]
- Kapoor, G.; Banyal, H.S. Glutathione reductase and thioredoxin reductase: Novel antioxidant enzymes from Plasmodium berghei. Korean J. Parasitol. 2009, 47, 421–424. [Google Scholar]
- Kawazu, S.; Yasuda-Komaki, K.; Oku, H.; Kano, S. Peroxiredoxins in malaria parasites: Parasitologic aspects. Parasitol. Int. 2008, 57, 1–7. [Google Scholar]
- Dey, S.; Guha, M.; Alam, A.; Goyal, M.; Bindu, S.; Pal, C.; Maity, P.; Mitra, K.; Bandyopadhyay, U. Malarial infection develops mitochondrial pathology and mitochondrial oxidative stress to promote hepatocyte apoptosis. Free Radic. Biol. Med. 2009, 46, 271–281. [Google Scholar] [PubMed]
- Guha, M.; Kumar, S.; Choubey, V.; Maity, P.; Bandyopadhyay, U. Apoptosis in liver during malaria: Role of oxidative stress and implication of mitochondrial pathway. FASEB J. 2006, 20, 339–449. [Google Scholar]
- Easton, J.; Eckman, J.; Berger, E.; Jacob, H. Suppression of malaria infection by oxidant- sensitive host ery- hrocytes. Nature 1976, 264, 758–760. [Google Scholar]
- Idoniye, O.; Festus, O.; Okhiai, O.; Akpamu, U. Comparative Study of the Status of a biomarker of lipid peroxidation (malondialdehyde) in patients with Plasmodium falciparum and Plasmodium vivax malaria infection. Asian J. Biol. Sci. 2011, 4, 506–513. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd: London, UK, 2008. [Google Scholar]
- Forman, H.J.; Augusto, O.; Brigelius-Flohe, R.; Dennery, P.A.; Kalyanaraman, B.; Ischiropoulos, H.; Mann, G.E.; Radi, R.; Roberts, L.J., 2nd; Vina, J.; et al. Even free radicals should follow some rules: A guide to free radical research terminology and methodology. Free Radic. Biol. Med. 2015, 78, 233–235. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Mahittikorn, A.; Mala, W.; Masangkay, F.R.; Kotepui, K.U.; Wilairatana, P.; Kotepui, M. Increased interferon-gamma levels and risk of severe malaria: A meta-analysis. Sci. Rep. 2022, 12, 18917. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials revisited. Contemp. Clin. Trials 2015, 45, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Lin, L. The trim-and-fill method for publication bias: Practical guidelines and recommendations based on a large database of meta-analyses. Medicine 2019, 98, e15987. [Google Scholar] [CrossRef] [PubMed]
- Willis, B.H.; Riley, R.D. Measuring the statistical validity of summary meta-analysis and meta-regression results for use in clinical practice. Stat. Med. 2017, 36, 3283–3301. [Google Scholar] [CrossRef]
- Awalu, J.C.; Ukibe, N.R.; Onyenekwe, C.C.; Ahaneku, J.E.; Ihim, A.C.; Udeh, T.; Onah, C.E.; Ehiaghe, F.A.; Ukibe, G.E.; Ukibe, B.C. Assessment of oxidative stress and antioxidant status in newly admitted healthy undergraduate students in Nnamdi Azikiwe University Awka, Nigeria. J. Pharm. Negat. Results 2022, 13, 1388–1399. [Google Scholar]
- Balogun, J.B.; Muhammad, S.S.; Dogara, M.M.; Okolugbo, C.B.; Muhammed, H.; Sadiq, A.; Sow, G.J. Effect of malaria infection on biomarker of lipid peroxidation (malondialdehyde) and lipid profile in pregnant women. Sci. World J. 2022, 17, 138–142. [Google Scholar]
- Bilgin, R.; Yalcin, M.S.; Yucebilgic, G.; Koltas, I.S.; Yazar, S. Oxidative stress in vivax malaria. Korean J. Parasitol. 2012, 50, 375–377. [Google Scholar] [CrossRef]
- D’Souza, B.; D’Souza, V.; Swagata, H.; Vijayalaxmi, K.; Namratha, A.S. Erythrocyte antioxidant enzymes and their correlation with malondialdehyde in malaria. Biomed. Res. J. 2009, 20, 25–27. [Google Scholar]
- Das, B.S.; Thurnham, D.I.; Patnaik, J.K.; Das, D.B.; Satpathy, R.; Bose, T.K. Increased plasma lipid peroxidation in riboflavin-deficient, malaria-infected children. Am. J. Clin. Nutr. 1990, 51, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, C.; De Cássia Mascarenhas-Netto, R.; Lalwani, P.; Melo, G.C.; Magalhães, B.M.L.; Alexandre, M.A.A.; Lacerda, M.V.G.; Lima, E.S. Lipid peroxidation and antioxidant enzymes activity in Plasmodium vivax malaria patients evolving with cholestatic jaundice. Malar. J. 2013, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Shyntum, Y.Y.; Emerole, G.O. Influence of chloroquine treatment and Plasmodium falciparum malaria infection on some enzymatic and non-enzymatic antioxidant defense indices in humans. Drug Chem. Toxicol. 2003, 26, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Krishna, A.P.; Chandrika; Kumar, S.; Acharya, M.; Patil, S.L. Variation in common lipid parameters in malaria infected patients. Indian. J. Physiol. Pharmacol. 2009, 53, 271–274. [Google Scholar]
- Nanda, R.; Mishra, P.K.; Das, U.K.; Rout, S.B.; Mohapatra, P.C.; Panda, A. Evaluating role of oxidative stress in determining the pathogenesis of falciparum malaria induced acute renal failure. Indian. J. Clin. Biochem. 2004, 19, 93–96. [Google Scholar] [CrossRef]
- Narsaria, N.; Mohanty, C.; Das, B.K.; Mishra, S.P.; Prasad, R. Oxidative stress in children with severe malaria. J. Trop. Pediatr. 2012, 58, 147–150. [Google Scholar] [CrossRef]
- Narsaria, N.; Singh, A.K.; Mishra, P.K.; Luthra, M. Pro-oxidants in pathogenesis of severe malaria in children. Indian. J. Community Health 2015, 27, 346–350. [Google Scholar]
- Nsonwu-Anyanwu, A.C.; Osuoha, U.O.; Nsonwu, M.C.; Usoro, C.A.O. Antimalaria therapy and changes in oxidative stress indices in falciparum malaria infection in Calabar metropolis, Nigeria. Trop. J. Pharm. Res. 2019, 18, 2431–2437. [Google Scholar]
- Nwagha, U.I.; Okeke, T.C.; Nwagha, T.U.; Ejezie, F.E.; Ogbodo, S.O.; Dim, C.C.; Anyaehie, B.U. Asymptomatic malaria parasitemia does not induce additional oxidative stress in pregnant women of South East Nigeria. Asian Pac. J. Trop. Med. 2011, 4, 229–233. [Google Scholar] [CrossRef] [PubMed]
- Polat, G.; Aslan, G.; Eskandari, G.H.; Delialioglu, N.; Bagdatoglu, O.; Atik, U. The role of nitric oxide and lipid peroxidation in patients with Plasmodium vivax malaria. Parasite 2002, 9, 371–374. [Google Scholar] [PubMed]
- Sakyi, S.A.; Ephraim, R.K.D.; Antoh, E.O.; Obirikorang, C.; Berchie, G.O. Lipid peroxidation and catalase levels among children presenting with severe falciparum malaria in the Sefwi Wiawso Municipality, Ghana. J. Med. Sci. 2012, 12, 141–147. [Google Scholar] [CrossRef][Green Version]
- Tiyong Ifoue, S.H.; Teugwa Mofor, C.; Gouado, I.; Teto, G.; Asonganyi, T.; Amvam Zollo, P.H. Evaluation of oxidative stress and antioxidant status of pregnant women suffering from malaria in Cameroon. Indian. J. Clin. Biochem. 2009, 24, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, D.N.; Vyas, R.K.; Sharma, M.L.; Soni, Y.; Rajnee. Comparison in serum profile of peroxidants (MDA) and non enzymatic antioxidants (Vitamins E and C) among patients suffering from Plasmodium falciparum and vivax malaria. J. Postgrad. Med. Inst. 2011, 25, 96–100. [Google Scholar]
- Villaverde, C.; Namazzi, R.; Shabani, E.; Park, G.S.; Datta, D.; Hanisch, B.; Opoka, R.O.; John, C.C. Retinopathy-positive cerebral malaria is associated with greater inflammation, blood-brain barrier breakdown, and neuronal damage than retinopathy-negative cerebral malaria. J. Pediatr. Infect. Dis. Soc. 2020, 9, 580–586. [Google Scholar] [CrossRef]
- Akanbi, O.M.; Badaki, J.A.; Adeniran, O.Y.; Olotu, O.O. Effect of blood group and demographic characteristics on malaria infection, oxidative stress and haemoglobin levels in south western Nigeria. Afr. J. Microbiol. Res. 2010, 4, 877–880. [Google Scholar]
- Erel, O.; Kocyigit, A.; Avci, S.; Aktepe, N.; Bulut, V. Oxidative stress and antioxidative status of plasma and erythrocytes in patients with vivax malaria. Clin. Biochem. 1997, 30, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Varshney, S.K.; Shahid, M.; Khan, H.M.; Malik, M.A.; Mahdi, A.A.; Shujatullah, F. Lipid peroxidation in cerebral malaria and role of antioxidants. IOSR J. Pharm. 2013, 3, 15–18. [Google Scholar]
- Wankasi, M.M.; Waibode, A.P.; Agoro, E.Y.S. The effects of Plasmodium falciparum parasitaemia on liver synthetic fidelity and oxidative stress markers. Eur. J. Med. Sci. 2020, 2, 1–5. [Google Scholar]
- Browning, J.D.; Horton, J.D. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Investig. 2004, 114, 147–152. [Google Scholar] [CrossRef]
- Clemens, M.R.; Waller, H.D. Lipid peroxidation in erythrocytes. Chem. Phys. Lipids 1987, 45, 251–268. [Google Scholar]
- Florens, L.; Washburn, M.P.; Raine, J.D.; Anthony, R.M.; Graingerk, M.; Haynes, J.D.; Moch, J.K.; Muster, N.; Sacci, J.B.; Tabb, D.L.; et al. A proteomic view of the Plasmodium falciparum life cycle. Nature 2002, 419, 520–526. [Google Scholar]
- Nsiah, K.; Bahaah, B.; Oppong Afranie, B.; Koffie, S.; Akowuah, E.; Donkor, S. Oxidative stress and hemoglobin level of complicated and uncomplicated malaria cases among children: A cross-sectional study in Kumasi Metropolis, Ghana. J. Trop. Med. 2019, 2019, 8479076. [Google Scholar] [CrossRef]
- Pabón, A.; Carmona, J.; Burgos, L.C.; Blair, S. Oxidative stress in patients with non-complicated malaria. Clin. Biochem. 2003, 36, 71–78. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2020, 11, 552969. [Google Scholar] [CrossRef]
- Kotepui, K.U.; Mueangson, O.; Mala, W.; Mahittikorn, A.; Wangdi, K.; Kotepui, M. Status of blood levels of superoxide dismutase in patients with malaria: A systematic review and meta-analysis. Antioxid. Redox Signal 2023. [Google Scholar] [CrossRef]
- Blatt, D.B.; Hanisch, B.; Co, K.; Datta, D.; Bond, C.; Opoka, R.O.; Cusick, S.E.; Michelow, I.C.; John, C.C. Impact of oxidative stress on risk of death and readmission in african children with severe malaria: A prospective observational study. J. Infect. Dis. 2022, 226, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Skorokhod, O.A.; Khoo, S.K.; Aguilar, R.; Wiertsema, S.; Nhabomba, A.J.; Marrocco, T.; McNamara-Smith, M.; Manaca, M.N.; Barbosa, A.; et al. Plasma advanced oxidative protein products are associated with anti-oxidative stress pathway genes and malaria in a longitudinal cohort. Malar. J. 2014, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Javeed, T.; Mustafa, G.; Khan, I.; Khan, M.K. Secondary defense antioxidant status of vitamin C, vitamin E and GSH in malaria, caused by Plasmodium falciparum and Plasmodium vivax. Pak. J. Pharm. Sci. 2011, 24, 103–107. [Google Scholar] [PubMed]
- Sarin, K.; Kumar, A.; Prakash, A.; Sharma, A. Oxidative stress and antioxidant defence mechanism in Plasmodium vivax malaria before and after chloroquine treatment. Indian. J. Malariol. 1993, 30, 127–133. [Google Scholar]

| Characteristics | N (23 Studies) | % |
|---|---|---|
| Publication year | ||
| 2010–2022 | 15 | 65.2 |
| 2000–2009 | 6 | 26.1 |
| Before 2000 | 2 | 8.70 |
| Study design | ||
| Cross-sectional | 21 | 91.2 |
| Case–control | 1 | 4.35 |
| Cohort | 1 | 4.35 |
| Study area | ||
| Africa | 11 | 41.8 |
| Nigeria | 7 | 63.6 |
| Cameroon | 2 | 18.2 |
| Uganda | 1 | 9.10 |
| Ghana | 1 | 9.10 |
| Asia | 11 | 41.8 |
| India | 8 | 72.7 |
| Turkey | 3 | 27.3 |
| South America | 1 | 4.35 |
| Brazil | 1 | |
| Plasmodium species | ||
| P. falciparum | 11 | 47.8 |
| P. vivax | 4 | 17.4 |
| Plasmodium spp. | 8 | 34.8 |
| Participants | ||
| Adults | 9 | 39.1 |
| Children | 6 | 26.1 |
| Pregnant women | 3 | 13.0 |
| Patients with malaria (unspecified age) | 3 | 13.0 |
| Pregnant and nonpregnant women | 2 | 8.70 |
| Methods of Plasmodium detection | ||
| Microscopy | 12 | 52.2 |
| Microscopy with other methods | 3 | 13.0 |
| PCR | 1 | 4.35 |
| RDT | 1 | 4.35 |
| Unspecified | 6 | 26.1 |
| No. | Author, Year | Study Location | Age Range (Years) | Study Findings |
|---|---|---|---|---|
| 1 | Akanbi et al., 2010 [52] | Nigeria | Not specified | MDA levels showed a significant positive correlation with parasite density (r = 0.53, p < 0.05). |
| 2 | Awalu et al., 2022 [34] | Nigeria | 17–21 years | There were significantly increased levels of MDA in malaria, typhoid, malaria/typhoid, and peptic ulcer groups compared with healthy participants (p < 0.05). |
| 3 | Babalola et al., 2022 [35] | Nigeria | 15–40 years | Differences in MDA levels between malaria-positive pregnant women and controls were not statistically significant (p > 0.05). The mean MDA level was significantly higher (p < 0.05) in malaria-positive primigravidae and secundigravidae than in multigravidae. The difference across the groups (control, mild, moderate, and severe) was not statistically significant (p > 0.05). |
| 4 | Bilgin et al., 2012 [36] | Turkey | 15–46 years | MDA levels were significantly higher in patients with P. vivax malaria than in healthy controls (p < 0.05). |
| 5 | D’Souza et al., 2009 [37] | India | 18–60 years | MDA levels were highly and significantly increased in both P. vivax and P. falciparum malaria patients (p < 0.001) compared with controls. The increase in MDA levels in P. falciparum malaria patients was much more than in P. vivax malaria patients. |
| 6 | Das et al., 1990 [38] | India | 1–12 years | Plasma MDA levels were significantly higher in malaria patients than in control subjects (p < 0.001). |
| 7 | Erel et al., 1997 [53] | Turkey | 15–35 years | MDA levels were significantly higher in malaria patients than in controls (p < 0.05). |
| 8 | Fabbri et al., 2013 [39] | Brazil | Not specified | Plasma MDA levels were significantly increased in malaria patients with and without jaundice compared with controls. |
| 9 | Farombi et al., 2003 [40] | Nigeria | 18–35 years | MDA levels were significantly increased in malaria patients, uninfected patients treated with chloroquine, and malaria patients treated with chloroquine compared with controls. |
| 10 | Krishna et al., 2009 [41] | India | 15–55 years | MDA levels were significantly increased in malaria patients compared with healthy controls (p < 0.05). MDA levels were significantly higher in patients with P. vivax malaria than in those with P. falciparum malaria (p < 0.05). |
| 11 | Megnekou et al., 2015 [7] | Cameroon | 16–39 years | MDA levels were significantly higher in women with malaria than in uninfected women (p = 0.0047). The MDA levels also correlated positively with parasitemia (p = 0.0024). |
| 12 | Nanda et al., 2004 [42] | India | Not specified | MDA levels were significantly elevated in cases where P. falciparum malaria induced acute renal failure compared with uncomplicated P. falciparum malaria (p < 0.001) and healthy controls (p < 0.001). Serum MDA levels were positively correlated with urea (r = 0.62, p < 0.025), creatinine (r = 0.65, p < 0.05), and bilirubin (r = 0.72, p < 0.001) levels. |
| 13 | Narsaria et al., 2012 [43] | India | 0–15 years | Plasma MDA levels were significantly raised in malaria cases compared with controls (p < 0.001). |
| 14 | Narsaria et al., 2015 [44] | India | 0–16 years | Mean plasma MDA levels were significantly higher in patients with severe malaria compared with controls (p < 0.05). |
| 15 | Nsonwu-Anyanwu et al., 2019 [45] | Nigeria | 18–60 years | MDA levels were higher in malaria patients with or without antimalarial therapy compared with controls (p < 0.05). Parasitemia and MDA levels were positively correlated (r = 0.399, p = 0.029) in malaria patients without antimalarial therapy. |
| 16 | Nwagha et al., 2011 [46] | Nigeria | 21–30 years | Differences in serum MDA levels between malaria-negative and malaria-positive subjects were not statistically significant (1st trimester: p = 0.69, 2nd trimester: p = 0.68, 3rd trimester: p = 0.57; and control: p = 0.59). |
| 17 | Polat et al., 2002 [47] | Turkey | Not specified | MDA levels in malaria patients were higher than in controls (p < 0.001). |
| 18 | Raza et al., 2013 [54] | India | 0.5–5 years | MDA levels in malaria patients were significantly higher compared with controls (p < 0.05). |
| 19 | Sakyi et al., 2012 [48] | Ghana | Children 10 years of age and below | MDA levels were higher in severe malaria patients compared with the controls and patients with uncomplicated malaria. MDA levels and malaria parasite density were positively correlated (r = 0.936, p < 0.05). |
| 20 | Tiyong Ifoue et al., 2009 [49] | Cameroon | 16–44 years | MDA levels were significantly higher in patients compared with controls (p < 0.001). MDA levels were higher in primigravidae and correlated well with parasite density (p < 0.001). |
| 21 | Upadhyay et al., 2011 [50] | India | 20–52 years | Serum MDA levels were significantly increased in malaria patients (p < 0.001). MDA levels were significantly increased in patients with P. falciparum compared with P. vivax malaria (p < 0.001). |
| 22 | Villaverde et al., 2020 [51] | Ugandan | 1.5–11.7 years | Differences in MDA levels between malaria cases and controls were not statistically significant. |
| 23 | Wankasi et al., 2020 [55] | Nigeria | 18–65 years | MDA levels were significantly increased (p < 0.05) in malaria patients compared with the control group. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mueangson, O.; Mahittikorn, A.; Anabire, N.G.; Mala, W.; Kotepui, M. Increased Blood Concentrations of Malondialdehyde in Plasmodium Infection: A Systematic Review and Meta-Analysis. Antioxidants 2023, 12, 1502. https://doi.org/10.3390/antiox12081502
Mueangson O, Mahittikorn A, Anabire NG, Mala W, Kotepui M. Increased Blood Concentrations of Malondialdehyde in Plasmodium Infection: A Systematic Review and Meta-Analysis. Antioxidants. 2023; 12(8):1502. https://doi.org/10.3390/antiox12081502
Chicago/Turabian StyleMueangson, Onchuma, Aongart Mahittikorn, Nsoh Godwin Anabire, Wanida Mala, and Manas Kotepui. 2023. "Increased Blood Concentrations of Malondialdehyde in Plasmodium Infection: A Systematic Review and Meta-Analysis" Antioxidants 12, no. 8: 1502. https://doi.org/10.3390/antiox12081502
APA StyleMueangson, O., Mahittikorn, A., Anabire, N. G., Mala, W., & Kotepui, M. (2023). Increased Blood Concentrations of Malondialdehyde in Plasmodium Infection: A Systematic Review and Meta-Analysis. Antioxidants, 12(8), 1502. https://doi.org/10.3390/antiox12081502







