The Influence of Exercise on Oxidative Stress after Spinal Cord Injury: A Narrative Review
Abstract
1. Introduction
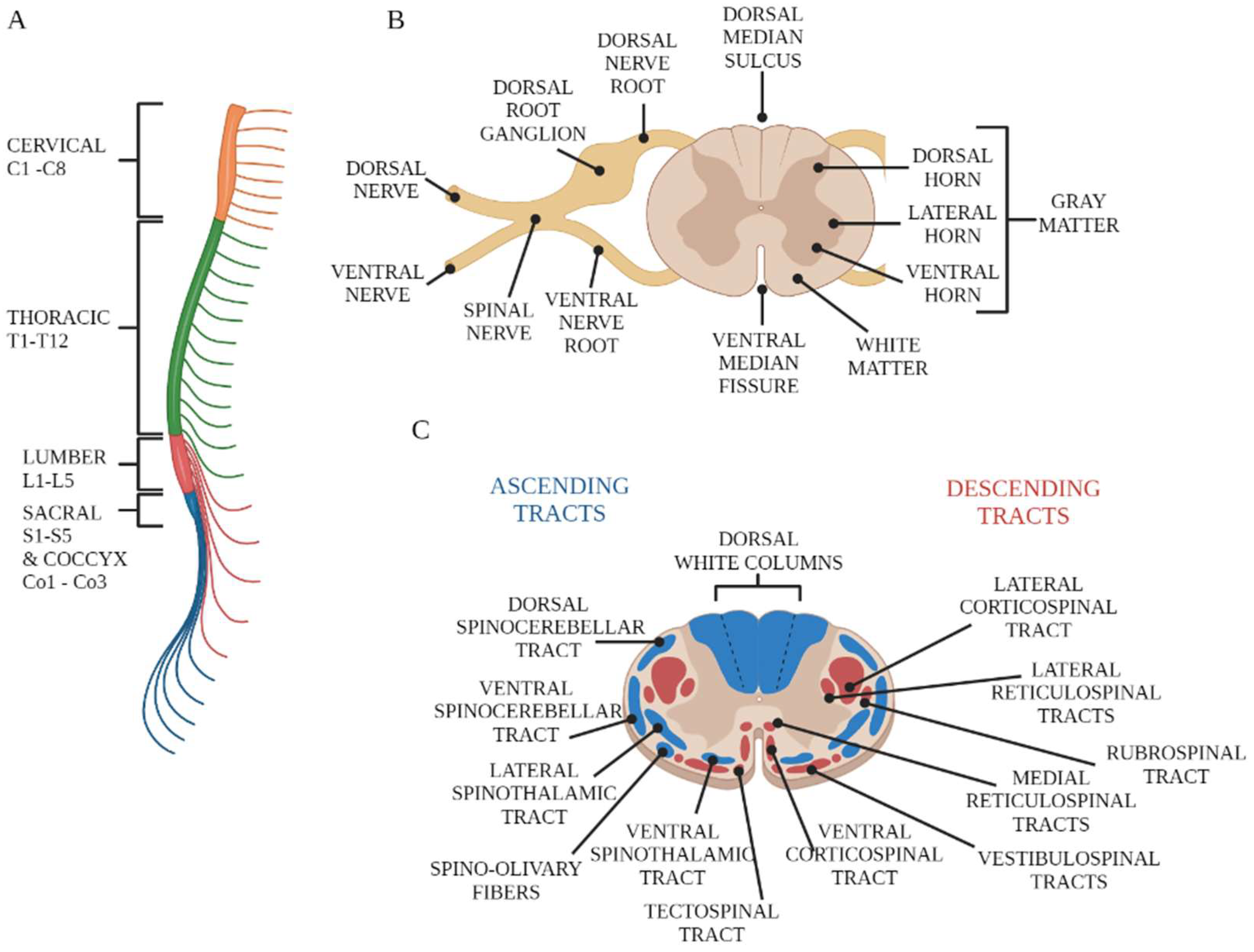
2. Overview of Spinal Cord Anatomy
3. Spinal Cord Injuries
4. Oxidative Stress in SCI
5. The Influence of Exercise on Oxidative Stress in Individuals with SCI
| Authors | Groups | Training Protocols | Training Session Duration | Training Period | Training Intensity | Fitness Level Assessment | Oxidative Stress Markers | Antioxidative Stress Markers | Techniques | Results |
|---|---|---|---|---|---|---|---|---|---|---|
| Van Duijnhoven et al. [80] | AB SCI | FES during leg cycle ergometer exercise | 30′ | 20 sessions | ~6.1 W at 50 rpm | Incremental maximal exercise | MDA | SOD GPx | MDA-TBA: fluorescence detection SOD: photometry GPx: spectroscopy | Correlation between fitness level and MDA. No changes in MDA, SOD, or GPx. |
| Goldhardt et al. [81] | SCI | FES + treadmill walking session FES + floor walker session | 30′ + 60′ 30′ + 60′ | Single session | 4.5 km/h, as fast as they could | NS | TBARSs AOPPs Nox | CAT GSH | TBARSs: spectrofluorimetry Nitrite: spectrophotometry AOPPs: spectrophotometry CAT: plasma samples were incubated with ethanol and triton GSH: plasma incubated with DTNB, b-NADPH, and NaHCO3 as stabilizing agents and M phosphate–potassium buffer | Treadmill session: increases in AOPPs, NOx, and TBARSs without changes in antioxidant mediators. Walker training: elevations in AOPPs and NOx but also in CAT and GSH levels. |
| Ordonez et al. [83] | SCI | Arm-cranking | 30′ | 36 sessions | 50% to 65% of HRmax | Continuous incremental workload test to exhaustion | MDA carbonyl groups | TAS GPx | TAS: spectrophotometry MDA: fluorimetric detection Plasma carbonyl: hemolysis GPX: supernatant of erythrocyte hemolysates | TAS and GPX activity were significantly increased, with reductions in MDA and carbonyl groups. |
| Mitsui et al. [84] | SCI AB | Arm-cranking | 2 h | Single session | 60% Vo2 | Progressive VO2 max test | oxLDL | Adrenaline | oxLDL: enzyme-linked immunosorbent assay Adrenaline: high-performance liquid chromatography | Increased plasma adrenaline levels in both groups but less so with SCI. Plasma oxLDL significantly increased levels only in AB subjects. |
| Wang et al. [90] | AB SCI | Continuous incremental workload test until exhaustion | Until exhaustion | Single session | +10 W every 2′ | Continuous incremental workload test to exhaustion | urinary 8-iso-PGF2_ 11-dehydro- TXB2 | NO | NO: Griess reagent-based colorimetric method 11-dehydro-TXB2: Jaffe alkaline picrate method 8-iso-PGF2_: enzyme-linked immunosorbent assay | Strenuous arm exercise increased levels of urinary 8-iso-PGF2_ and 11-dehydro-TXB2 only in the SCI group. NO increased only in AB subjects. |
| Inglés et al. [86] | Active SCI Non-active SCI | MVPA | >180′ | 7 days | >3 METS | GET until volitional exhaustion | MDA protein carbonylation | CAT GPx | MDA: chromatographic technique Protein carbonylation: immunoblot detection of plasma protein carbonyl groups Catalase and GPx mRNA: real-time PCR | Significant increases in plasma MDA and protein carbonyls after the GET, but subjects with active SCI had higher exercise-induced catalase and GPx expression than non-active subjects. |
| Hübner-Woźniak et al. [87] | Sedentary AB Rugby AB Rugby T Sedentary T | Wheelchair rugby | 2 h 2 days/w | 7.1 ± 3.4 years | NS | NS | NS | SOD CAT GPx GR8 TAS | SOD: erythrocyte hemolysates GPX: whole-blood hemolysates GR: erythrocyte hemolysates CAT: erythrocyte hemolysates CAT: standard curve TAS: ABTS | CAT and GPX activities were significantly higher in rugby players than sedentary subjects. TAS was higher in AB groups. |
| Garbeloti et al. [88] | Basketball SCI Sedentary SCI Sedentary AB | Wheelchair basketball | 3 days/w | 7 years | NS | NS | TBARSs | NO | NO: Griess nitrate reductase method TBARSs: OXltek® TBARS assay | Sedentary AB group had higher levels of TBARSs. No differences were found between groups in NO concentrations. |
| Mitsui et al. [89] | C SCI L SCI | Wheelchair half-marathon race | NS | Single session | NS | NS | D-ROMs oxLDL | BAP Adrenaline | oxLDL: enzyme-linked immunosorbent assay Oxidative stress: d-ROM test Antioxidant abilities: BAP test Adrenaline: high-performance liquid chromatography | d-ROMs and oxLDL did not change. BAP and adrenaline increased only in LSCI. |
- FES can be useful in programming the training plans of individuals with SCI when combined with stimuli with appropriate intensities, but the duration, intensity, and timing of administration can affect the effects on oxidative stress management;
- Twelve weeks of aerobic exercise with a three-week frequency and an intensity of about 60% of the maximum heart rate are suitable to train the antioxidant capacities of subjects with SCI;
- Exhaustion exercise seems to be poorly tolerated in subjects with SCI because they fail to have adequate antioxidant responses;
- Active (>180′ per week of MVPA) SCI subjects have greater antioxidative capacities in response to oxidative damage, induced by high-intensity to exhaustive exercise, than inactive subjects with SCI;
- The exercise intensity of wheelchair basketball is adequate and that of a half-marathon race is not excessive for trained SCI subjects because the balance between oxidation and antioxidation is maintained during these two sports activities.
6. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Hamid, R.; Averbeck, M.A.; Chiang, H.; Garcia, A.; Al Mousa, R.T.; Oh, S.J.; Patel, A.; Plata, M.; Del Popolo, G. Epidemiology and pathophysiology of neurogenic bladder after spinal cord injury. World J. Urol. 2018, 36, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L., Jr.; Yarar-Fisher, C. Contributors to Metabolic Disease Risk Following Spinal Cord Injury. Curr. Phys. Med. Rehabil. Rep. 2016, 4, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Barbiellini Amidei, C.; Salmaso, L.; Bellio, S.; Saia, M. Epidemiology of traumatic spinal cord injury: A large population-based study. Spinal Cord 2022, 60, 812–819. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Wilson, J.R.; Forgione, N.; Fehlings, M.G. Emerging therapies for acute traumatic spinal cord injury. CMAJ 2013, 185, 485–492. [Google Scholar] [CrossRef]
- Stahel, P.F.; VanderHeiden, T.; Finn, M.A. Management strategies for acute spinal cord injury: Current options and future perspectives. Curr. Opin. Crit. Care 2012, 18, 651–660. [Google Scholar] [CrossRef]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Lee, I.M.; Skerrett, P.J. Physical activity and all-cause mortality: What is the dose-response relation? Med. Sci. Sports Exerc. 2001, 33, S459–S471; discussion S454–S493. [Google Scholar] [CrossRef]
- Maugeri, G.; Musumeci, G. Adapted Physical Activity to Ensure the Physical and Psychological Well-Being of COVID-19 Patients. J. Funct. Morphol. Kinesiol. 2021, 6, 13. [Google Scholar] [CrossRef]
- Maugeri, G.; D’Agata, V.; Magri, B.; Roggio, F.; Castorina, A.; Ravalli, S.; Di Rosa, M.; Musumeci, G. Neuroprotective Effects of Physical Activity via the Adaptation of Astrocytes. Cells 2021, 10, 1542. [Google Scholar] [CrossRef]
- Lauretta, G.; Ravalli, S.; Maugeri, G.; D’Agata, V.; Rosa, M.D.; Musumeci, G. The Impact of Physical Exercise on the Hippocampus in Physiological Condition and Ageing-Related Decline: Current Evidence from Animal and Human Studies. Curr. Pharm. Biotechnol. 2022, 23, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Udina, E.; Cobianchi, S.; Allodi, I.; Navarro, X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann. Anat. 2011, 193, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Di Liegro, C.M.; Schiera, G.; Proia, P.; Di Liegro, I. Physical Activity and Brain Health. Genes 2019, 10, 720. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.; Kokkinos, P.; Nyelin, E. Physical Activity, Cardiorespiratory Fitness, and the Metabolic Syndrome. Nutrients 2019, 11, 1652. [Google Scholar] [CrossRef] [PubMed]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.J.; Quintanilha, A.T.; Brooks, G.A.; Packer, L. Free radicals and tissue damage produced by exercise. Biochem. Biophys. Res. Commun. 1982, 107, 1198–1205. [Google Scholar] [CrossRef]
- Khan, Y.S.; Lui, F. Neuroanatomy, Spinal Cord. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Adigun, O.O.; Reddy, V.; Varacallo, M. Anatomy, Back, Spinal Cord. In StatPearls; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Purves, D.; Augustine, G.J.; Fitzpatrick, D.; Katz, L.C.; LaMantia, A.-S.; McNamara, J.O.; Williams, S.M. (Eds.) The Internal Anatomy of the Spinal Cord. In Neuroscience, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2001. [Google Scholar]
- Bican, O.; Minagar, A.; Pruitt, A.A. The spinal cord: A review of functional neuroanatomy. Neurol. Clin. 2013, 31, 1–18. [Google Scholar] [CrossRef]
- Hayta, E.; Elden, H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J. Chem. Neuroanat. 2018, 87, 25–31. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef]
- Yezierski, R.P. Pain following spinal cord injury: Pathophysiology and central mechanisms. Prog. Brain Res. 2000, 129, 429–449. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Li, J.; Wang, X.; Misra, H.; Li, Y. Oxidative stress in spinal cord injury and antioxidant-based intervention. Spinal Cord 2012, 50, 264–274. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Garcia, E.; Aguilar-Cevallos, J.; Silva-Garcia, R.; Ibarra, A. Cytokine and Growth Factor Activation In Vivo and In Vitro after Spinal Cord Injury. Mediat. Inflamm. 2016, 2016, 9476020. [Google Scholar] [CrossRef]
- Fitch, M.T.; Doller, C.; Combs, C.K.; Landreth, G.E.; Silver, J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: In vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J. Neurosci. 1999, 19, 8182–8198. [Google Scholar] [CrossRef]
- Shao, C.; Roberts, K.N.; Markesbery, W.R.; Scheff, S.W.; Lovell, M.A. Oxidative stress in head trauma in aging. Free Radic. Biol. Med. 2006, 41, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.A.; Al Shehabi, T.S.; Eid, A.H. Inflammogenesis of Secondary Spinal Cord Injury. Front. Cell. Neurosci. 2016, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Wingrave, J.M.; Schaecher, K.E.; Sribnick, E.A.; Wilford, G.G.; Ray, S.K.; Hazen-Martin, D.J.; Hogan, E.L.; Banik, N.L. Early induction of secondary injury factors causing activation of calpain and mitochondria-mediated neuronal apoptosis following spinal cord injury in rats. J. Neurosci. Res. 2003, 73, 95–104. [Google Scholar] [CrossRef] [PubMed]
- D’Autreaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell. Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef]
- Vieira, H.L.; Alves, P.M.; Vercelli, A. Modulation of neuronal stem cell differentiation by hypoxia and reactive oxygen species. Prog. Neurobiol. 2011, 93, 444–455. [Google Scholar] [CrossRef]
- Kennedy, K.A.; Sandiford, S.D.; Skerjanc, I.S.; Li, S.S. Reactive oxygen species and the neuronal fate. Cell Mol. Life Sci. 2012, 69, 215–221. [Google Scholar] [CrossRef]
- Allen, R.G.; Tresini, M. Oxidative stress and gene regulation. Free Radic. Biol. Med. 2000, 28, 463–499. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, G.; Piazza, S.; Carlesi, C.; Del Corona, A.; Franzini, M.; Pompella, A.; Malvaldi, G.; Mancuso, M.; Paolicchi, A.; Murri, L. Antioxidant capacity and protein oxidation in cerebrospinal fluid of amyotrophic lateral sclerosis. J. Neurol. 2007, 254, 575–580. [Google Scholar] [CrossRef]
- Yan, Y.; Wei, C.L.; Zhang, W.R.; Cheng, H.P.; Liu, J. Cross-talk between calcium and reactive oxygen species signaling. Acta Pharmacol. Sin. 2006, 27, 821–826. [Google Scholar] [CrossRef]
- Feissner, R.F.; Skalska, J.; Gaum, W.E.; Sheu, S.S. Crosstalk signaling between mitochondrial Ca2+ and ROS. Front. Biosci. 2009, 14, 1197–1218. [Google Scholar] [CrossRef] [PubMed]
- Azbill, R.D.; Mu, X.; Bruce-Keller, A.J.; Mattson, M.P.; Springer, J.E. Impaired mitochondrial function, oxidative stress and altered antioxidant enzyme activities following traumatic spinal cord injury. Brain Res. 1997, 765, 283–290. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Wen, J. Elevation of hydrogen peroxide after spinal cord injury detected by using the Fenton reaction. Free Radic. Biol. Med. 1999, 27, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Lewen, A.; Gasche, Y.; Yu, F.; Chan, P.H. Overexpression of SOD1 protects vulnerable motor neurons after spinal cord injury by attenuating mitochondrial cytochrome c release. FASEB J. 2002, 16, 1997–1999. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ling, X.; Wen, J.; Liu, J. The role of reactive nitrogen species in secondary spinal cord injury: Formation of nitric oxide, peroxynitrite, and nitrated protein. J. Neurochem. 2000, 75, 2144–2154. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Sun, D.; Wen, J. The time course of hydroxyl radical formation following spinal cord injury: The possible role of the iron-catalyzed Haber-Weiss reaction. J. Neurotrauma 2004, 21, 805–816. [Google Scholar] [CrossRef]
- Visavadiya, N.P.; Patel, S.P.; VanRooyen, J.L.; Sullivan, P.G.; Rabchevsky, A.G. Cellular and subcellular oxidative stress parameters following severe spinal cord injury. Redox Biol. 2016, 8, 59–67. [Google Scholar] [CrossRef]
- Carrico, K.M.; Vaishnav, R.; Hall, E.D. Temporal and spatial dynamics of peroxynitrite-induced oxidative damage after spinal cord contusion injury. J. Neurotrauma 2009, 26, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Hakan, T.; Toklu, H.Z.; Biber, N.; Celik, H.; Erzik, C.; Ogunc, A.V.; Cetinel, S.; Sener, G. Meloxicam exerts neuroprotection on spinal cord trauma in rats. Int. J. Neurosci. 2011, 121, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Wang, Z.; Zheng, J.; Wang, R. Protective effects of gallic acid against spinal cord injury-induced oxidative stress. Mol. Med. Rep. 2015, 12, 3017–3024. [Google Scholar] [CrossRef]
- Zhou, L.; Ouyang, L.; Lin, S.; Chen, S.; Liu, Y.; Zhou, W.; Wang, X. Protective role of beta-carotene against oxidative stress and neuroinflammation in a rat model of spinal cord injury. Int. Immunopharmacol. 2018, 61, 92–99. [Google Scholar] [CrossRef]
- Chen, A.; McEwen, M.L.; Sun, S.; Ravikumar, R.; Springer, J.E. Proteomic and phosphoproteomic analyses of the soluble fraction following acute spinal cord contusion in rats. J. Neurotrauma 2010, 27, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Rabchevsky, A.G.; Hall, E.D. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J. Neurochem. 2007, 100, 639–649. [Google Scholar] [CrossRef]
- Wozniak, B.; Wozniak, A.; Mila-Kierzenkowska, C.; Kasprzak, H.A. Correlation of Oxidative and Antioxidative Processes in the Blood of Patients with Cervical Spinal Cord Injury. Oxid. Med. Cell. Longev. 2016, 2016, 6094631. [Google Scholar] [CrossRef]
- Bastani, N.E.; Kostovski, E.; Sakhi, A.K.; Karlsen, A.; Carlsen, M.H.; Hjeltnes, N.; Blomhoff, R.; Iversen, P.O. Reduced antioxidant defense and increased oxidative stress in spinal cord injured patients. Arch. Phys. Med. Rehabil. 2012, 93, 2223–2228.e2222. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wang, H.D.; Wang, X.L.; Tian, L.; Xu, J.Y. Disruption of Nrf2 exacerbated the damage after spinal cord injury in mice. J. Trauma Acute Care Surg. 2012, 72, 189–198. [Google Scholar] [CrossRef]
- Toklu, H.Z.; Hakan, T.; Celik, H.; Biber, N.; Erzik, C.; Ogunc, A.V.; Akakin, D.; Cikler, E.; Cetinel, S.; Ersahin, M.; et al. Neuroprotective effects of alpha-lipoic acid in experimental spinal cord injury in rats. J. Spinal Cord Med. 2010, 33, 401–409. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H.; Deng, L.; Li, J. Exercise Training Promotes Functional Recovery after Spinal Cord Injury. Neural Plast. 2016, 2016, 4039580. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, K.J.; Linderman, J.K.; Basso, D.M. Skeletal muscle adaptations following spinal cord contusion injury in rat and the relationship to locomotor function: A time course study. J. Neurotrauma 2001, 18, 1075–1089. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Pedroni, A.; Bertuzzi, M.; Kizil, C.; Simon, A.; Ampatzis, K. Locomotion dependent neuron-glia interactions control neurogenesis and regeneration in the adult zebrafish spinal cord. Nat. Commun. 2021, 12, 4857. [Google Scholar] [CrossRef]
- Chew, C.; Sengelaub, D.R. Exercise promotes recovery after motoneuron injury via hormonal mechanisms. Neural Regen. Res. 2020, 15, 1373–1376. [Google Scholar] [CrossRef]
- Davaa, G.; Hong, J.Y.; Kim, T.U.; Lee, S.J.; Kim, S.Y.; Hong, K.; Hyun, J.K. Exercise Ameliorates Spinal Cord Injury by Changing DNA Methylation. Cells 2021, 10, 143. [Google Scholar] [CrossRef]
- Jung, S.Y.; Seo, T.B.; Kim, D.Y. Treadmill exercise facilitates recovery of locomotor function through axonal regeneration following spinal cord injury in rats. J. Exerc. Rehabil. 2016, 12, 284–292. [Google Scholar] [CrossRef]
- Hwang, D.H.; Shin, H.Y.; Kwon, M.J.; Choi, J.Y.; Ryu, B.Y.; Kim, B.G. Survival of neural stem cell grafts in the lesioned spinal cord is enhanced by a combination of treadmill locomotor training via insulin-like growth factor-1 signaling. J. Neurosci. 2014, 34, 12788–12800. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Kressler, J.; Cowan, R.E.; Bigford, G.E.; Nash, M.S. Reducing cardiometabolic disease in spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2014, 25, 573–604. [Google Scholar] [CrossRef]
- Sandrow-Feinberg, H.R.; Houle, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef]
- West, C.R.; Crawford, M.A.; Poormasjedi-Meibod, M.S.; Currie, K.D.; Fallavollita, A.; Yuen, V.; McNeill, J.H.; Krassioukov, A.V. Passive hind-limb cycling improves cardiac function and reduces cardiovascular disease risk in experimental spinal cord injury. J. Physiol. 2014, 592, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Alessio, H.M. Exercise-induced oxidative stress. Med. Sci. Sports Exerc. 1993, 25, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L. Antioxidants and oxidative stress in exercise. Proc. Soc. Exp. Biol. Med. 1999, 222, 283–292. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef]
- Douris, P.C.; Elokda, A.S.; Handrakis, J.P.; Principal, S.; Rondo, E.; Bovell, J.; Coughlin, W.P.; Mastroianni, C.N.; Wong, M.J.; Zimmerman, T. Martial art training enhances the glutathione antioxidant system in middle-aged adults. J. Strength Cond. Res. 2009, 23, 1518–1523. [Google Scholar] [CrossRef]
- Knez, W.L.; Jenkins, D.G.; Coombes, J.S. Oxidative stress in half and full Ironman triathletes. Med. Sci. Sports Exerc. 2007, 39, 283–288. [Google Scholar] [CrossRef]
- Teixeira, V.; Valente, H.; Casal, S.; Marques, F.; Moreira, P. Antioxidant status, oxidative stress, and damage in elite trained kayakers and canoeists and sedentary controls. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 443–456. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef]
- Mancini, D.M.; Walter, G.; Reichek, N.; Lenkinski, R.; McCully, K.K.; Mullen, J.L.; Wilson, J.R. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation 1992, 85, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Bloomer, R.J.; Fisher-Wellman, K.H. Blood oxidative stress biomarkers: Influence of sex, exercise training status, and dietary intake. Gend. Med. 2008, 5, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, L.T.; Hubinger, L.; Lepre, F. Effects of physical activity and diet on lipoprotein(a). Med. Sci. Sports Exerc. 1997, 29, 1429–1436. [Google Scholar] [CrossRef]
- Shern-Brewer, R.; Santanam, N.; Wetzstein, C.; White-Welkley, J.; Parthasarathy, S. Exercise and cardiovascular disease: A new perspective. Arter. Thromb. Vasc. Biol. 1998, 18, 1181–1187. [Google Scholar] [CrossRef]
- van Duijnhoven, N.; Hesse, E.; Janssen, T.; Wodzig, W.; Scheffer, P.; Hopman, M. Impact of exercise training on oxidative stress in individuals with a spinal cord injury. Eur. J. Appl. Physiol. 2010, 109, 1059–1066. [Google Scholar] [CrossRef]
- Goldhardt, M.G.; Andreia, A.; Dorneles, G.P.; da Silva, I.R.; Pochmann, D.; Peres, A.; Rostirola Elsner, V. Does a single bout of exercise impacts BDNF, oxidative stress and epigenetic markers in spinal cord injury patients? Funct. Neurol. 2019, 34, 158–166. [Google Scholar]
- Holme, E.; Mohr, T.; Kjaer, M.; Nielsen, B. Temperature responses to electrically induced cycling in spinal cord injured persons. Med. Sci. Sports Exerc. 2001, 33, 431–435. [Google Scholar] [CrossRef]
- Ordonez, F.J.; Rosety, M.A.; Camacho, A.; Rosety, I.; Diaz, A.J.; Fornieles, G.; Bernardi, M.; Rosety-Rodriguez, M. Arm-cranking exercise reduced oxidative damage in adults with chronic spinal cord injury. Arch. Phys. Med. Rehabil. 2013, 94, 2336–2341. [Google Scholar] [CrossRef]
- Mitsui, T.; Nakamura, T.; Ito, T.; Umemoto, Y.; Sakamoto, K.; Kinoshita, T.; Nakagawa, M.; Tajima, F. Exercise significantly increases plasma adrenaline and oxidized low-density lipoprotein in normal healthy subjects but not in persons with spinal cord injury. Arch. Phys. Med. Rehabil. 2012, 93, 725–727. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant activity of L-adrenaline: A structure-activity insight. Chem. Biol. Interact. 2009, 179, 71–80. [Google Scholar] [CrossRef]
- Inglés, M.; Serra-Añó, P.; Gambini, J.; Abu-Sharif, F.; Dromant, M.; Garcia-Valles, R.; Pareja-Galeano, H.; Garcia-Lucerga, C.; Gomez-Cabrera, M.C. Active paraplegics are protected against exercise-induced oxidative damage through the induction of antioxidant enzymes. Spinal Cord 2016, 54, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Hübner-Woźniak, E.; Morgulec-Adamowicz, N.; Malara, M.; Lewandowski, P.; Okęcka-Szymańska, J. Effect of rugby training on blood antioxidant defenses in able-bodied and spinal cord injured players. Spinal Cord 2012, 50, 253–256. [Google Scholar] [CrossRef]
- Garbeloti, E.J.R.; Paiva, R.C.A.; Restini, C.B.A.; Durand, M.T.; Miranda, C.E.S.; Teixeira, V.E. Biochemical biomarkers are not dependent on physical exercise in patients with spinal cord injury. BBA Clin. 2016, 6, 5–11. [Google Scholar] [CrossRef]
- Mitsui, T.; Ito, T.; Sasaki, Y.; Kawasaki, T.; Nakamura, T.; Nishimura, Y.; Ibusuki, T.; Higuchi, Y.; Hosoe, S.; Ito, F.; et al. Changes in oxidized LDL during a half marathon in athletes with spinal cord injuries. Spinal Cord Ser. Cases 2017, 3, 17015. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.S.; Yang, C.F.; Wong, M.K.; Chow, S.E.; Chen, J.K. Effect of strenuous arm exercise on oxidized-LDL-potentiated platelet activation in individuals with spinal cord injury. Thromb. Haemost. 2000, 84, 118–123. [Google Scholar] [PubMed]
- Petrie, M.A.; Kimball, A.L.; Shields, R.K. Acute Low Force Electrically Induced Exercise Modulates Post Prandial Glycemic Markers in People with Spinal Cord Injury. J. Funct. Morphol. Kinesiol. 2022, 7, 89. [Google Scholar] [CrossRef]
- Nightingale, T.E.; Walhin, J.P.; Thompson, D.; Bilzon, J.L.J. Impact of Exercise on Cardiometabolic Component Risks in Spinal Cord-injured Humans. Med. Sci. Sports Exerc. 2017, 49, 2469–2477. [Google Scholar] [CrossRef]
- Bergmann, M.; Zahharova, A.; Reinvee, M.; Asser, T.; Gapeyeva, H.; Vahtrik, D. The Effect of Functional Electrical Stimulation and Therapeutic Exercises on Trunk Muscle Tone and Dynamic Sitting Balance in Persons with Chronic Spinal Cord Injury: A Crossover Trial. Medicina 2019, 55, 619. [Google Scholar] [CrossRef]
- Jitca, G.; Osz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Batrinu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- Wiggs, M.P. Can endurance exercise preconditioning prevention disuse muscle atrophy? Front. Physiol. 2015, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Trewin, A.J.; Lundell, L.S.; Perry, B.D.; Patil, K.V.; Chibalin, A.V.; Levinger, I.; McQuade, L.R.; Stepto, N.K. Effect of N-acetylcysteine infusion on exercise-induced modulation of insulin sensitivity and signaling pathways in human skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E388–E397. [Google Scholar] [CrossRef]
- Vincent, H.K.; Powers, S.K.; Stewart, D.J.; Demirel, H.A.; Shanely, R.A.; Naito, H. Short-term exercise training improves diaphragm antioxidant capacity and endurance. Eur. J. Appl. Physiol. 2000, 81, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cabrera, M.C.; Domenech, E.; Romagnoli, M.; Arduini, A.; Borras, C.; Pallardo, F.V.; Sastre, J.; Vina, J. Oral administration of vitamin C decreases muscle mitochondrial biogenesis and hampers training-induced adaptations in endurance performance. Am. J. Clin. Nutr. 2008, 87, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Martin Ginis, K.A.; van der Scheer, J.W.; Latimer-Cheung, A.E.; Barrow, A.; Bourne, C.; Carruthers, P.; Bernardi, M.; Ditor, D.S.; Gaudet, S.; de Groot, S.; et al. Evidence-based scientific exercise guidelines for adults with spinal cord injury: An update and a new guideline. Spinal Cord 2018, 56, 308–321. [Google Scholar] [CrossRef] [PubMed]
| Grade | Type of Injury | Clinical Description |
|---|---|---|
| A | Complete | Complete injury with loss of motor and sensory functions, including sacral roots. |
| B | Incomplete | Incomplete injury with preserved sensory functions but complete loss of motor function below the neurological level, including the sacral segments S4–S5. |
| C | Incomplete | Preserved motor function below the injury level; less than half of the key muscles below the neurological level will have a muscle grade of less than 3. |
| D | Incomplete | Preserved motor function below the injury level; at least half of the muscles below the neurological level will have a muscle grade of 3 or more. |
| E | Normal | Normal motor and sensory functions. |
| Reactive Species | Chemical Structure |
|---|---|
| Superoxide Anions | O2•− |
| Hydroxyl Radicals | •OH |
| Hydrogen Peroxides | H2O2 |
| Nitric Oxide | •NO |
| Peroxynitrites | ONOO− |
| Lipid Peroxyl | LOO− |
| Lipid Alkoxyl | LO• |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maugeri, G.; Amato, A.; Sortino, M.; D′Agata, V.; Musumeci, G. The Influence of Exercise on Oxidative Stress after Spinal Cord Injury: A Narrative Review. Antioxidants 2023, 12, 1401. https://doi.org/10.3390/antiox12071401
Maugeri G, Amato A, Sortino M, D′Agata V, Musumeci G. The Influence of Exercise on Oxidative Stress after Spinal Cord Injury: A Narrative Review. Antioxidants. 2023; 12(7):1401. https://doi.org/10.3390/antiox12071401
Chicago/Turabian StyleMaugeri, Grazia, Alessandra Amato, Martina Sortino, Velia D′Agata, and Giuseppe Musumeci. 2023. "The Influence of Exercise on Oxidative Stress after Spinal Cord Injury: A Narrative Review" Antioxidants 12, no. 7: 1401. https://doi.org/10.3390/antiox12071401
APA StyleMaugeri, G., Amato, A., Sortino, M., D′Agata, V., & Musumeci, G. (2023). The Influence of Exercise on Oxidative Stress after Spinal Cord Injury: A Narrative Review. Antioxidants, 12(7), 1401. https://doi.org/10.3390/antiox12071401










