Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Preparation
2.2. Pasting Properties
2.3. Proximate Composition
2.4. Total Phenolics Content (TPC) with Use of Folin–Ciocalteu Method
2.5. DPPH Method Free Radical Scavenging Activity
2.6. Water Absorption Index (WAI)
2.7. Water Solubility Index (WSI)
2.8. Cutting Force Measurements
2.9. Color Profile of Snack Pellets
2.10. Statistical Analysis
3. Results and Discussion
3.1. Pasting Characteristics of Raw Materials Blends
3.2. The Basic Composition and Fatty Acids Profile of Snack Pellets
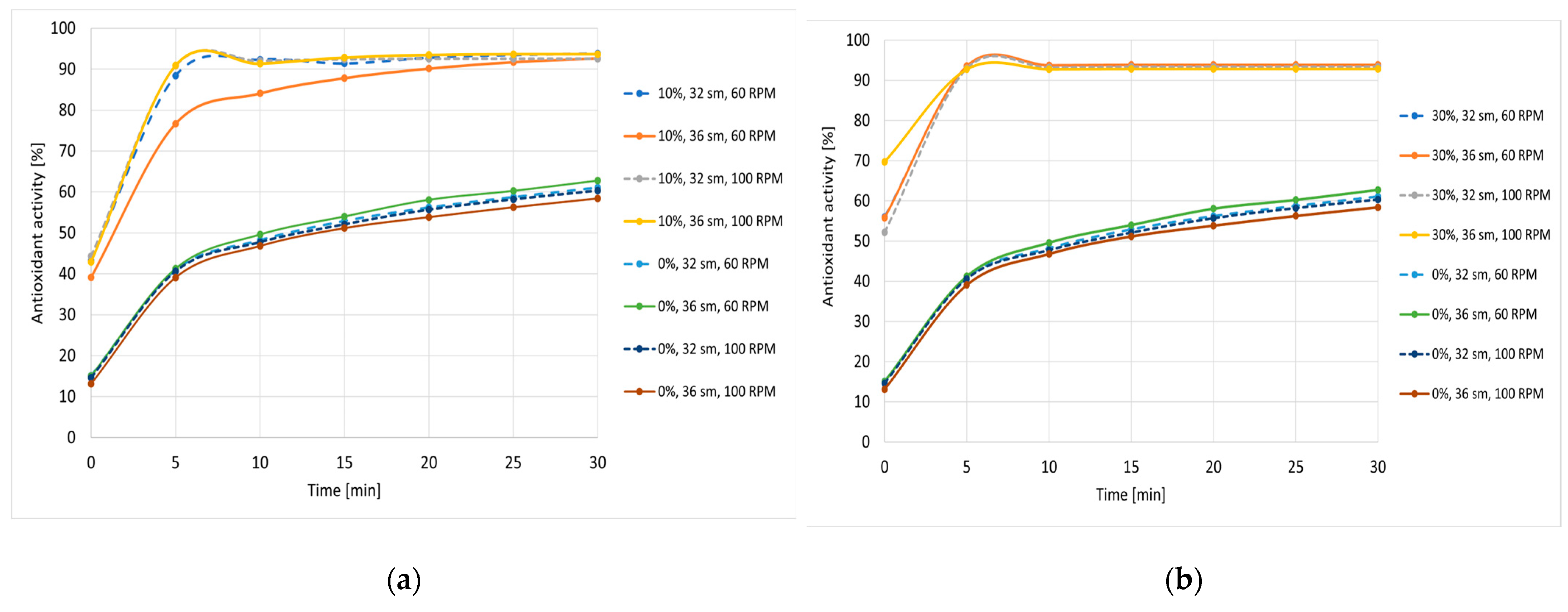
3.3. Free Radical Scavenging Activity and Phenolic Compounds Content of Snack Pellets Enriched with Cricket Flour
3.4. WAI and WSI of Snack Pellets Enriched with Cricket Flour
3.5. Texture of Snack Pellets
3.6. Results of Snack Pellets Color
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belluco, S.; Losasso, C.; Maggioletti, M.; Alonzi, C.C.; Paoletti, M.G.; Ricci, A. Edible insects in a food safety and nutritional perspective: A critical review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 296–313. [Google Scholar] [CrossRef]
- Yates-Doerr, E. The world in a box? Food security, edible insects, and “One World, One Health” collaboration. Soc. Sci. Med. 2015, 129, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Prather, C.M.; Laws, A.N. Insects as a piece of the puzzle to mitigate global problems: An opportunity for ecologists. Basic Appl. Ecol. 2018, 26, 71–81. [Google Scholar] [CrossRef]
- Liceaga, A.M.; Aguilar-Toalá, J.E.; Vallejo-Cordoba, B.; González-Córdova, A.F.; Hernández-Mendoza, A. Insects as an Alternative Protein Source. Annu. Rev. Food Sci. Technol. 2022, 13, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Pinckaers, P.J.M.; van Loon, J.J.A.; van Loon, L.J.C. Consideration of insects as a source of dietary protein for human consumption. Nutr. Rev. 2017, 75, 1035–1045. [Google Scholar] [CrossRef]
- Akhtar, Y.; Isman, M.B. Insects as an Alternative Protein Source. In Proteins in Food Processing, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 128–139. [Google Scholar]
- Rumpold, B.A.; Schlüter, O. Insect-based protein sources and their potential for human consumption: Nutritional composition and processing. Anim. Front. 2015, 5, 20–22.4. [Google Scholar]
- Weru, J.; Chege, P.; Kinyuru, J. Nutritional potential of edible insects: A systematic review of published data. Int. J. Trop. Insect Sci. 2021, 41, 2015–2037. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Dossey, A.T.; Berhow, M. Self-selection of food ingredients and agricultural by-products by the house cricket, Acheta domesticus (Orthoptera: Gryllidae): A holistic approach to develop optimized diets. PLoS ONE 2020, 15, e0227400. [Google Scholar] [CrossRef]
- Magara, H.J.O.; Niassy, S.; Ayieko, M.A.; Mukundamago, M.; Egonyu, J.P.; Tanga, C.M.; Kimathi, E.K.; Ongere, J.O.; Fiaboe, K.K.M.; Hugel, S.; et al. Edible Crickets (Orthoptera) Around the World: Distribution, Nutritional Value, and Other Benefits—A Review. Front. Nutr. 2021, 7, 537915. [Google Scholar] [CrossRef]
- Stone, A.K.; Tanaka, T.; Nickerson, M.T. Protein quality and physicochemical properties of commercial cricket and mealworm powders. J. Food Sci. Technol. 2019, 56, 3355–3363. [Google Scholar] [CrossRef]
- Zielińska, E.; Pankiewicz, U.; Sujka, M. Nutritional, physiochemical, and biological value of muffins enriched with edible insects flour. Antioxidants 2021, 10, 1122. [Google Scholar] [CrossRef] [PubMed]
- Hall, F.; Reddivari, L.; Liceaga, A.M. Identification and characterization of edible cricket peptides on hypertensive and glycemic in vitro inhibition and their anti-inflammatory activity on raw 264.7 macrophage cells. Nutrients 2020, 12, 8450. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, F.; Proksch, P.; Fiedler, K. Flavonoid sequestration by the common blue butterfly Polyommatus icarus: Quantitative intraspecific variation in relation to larval hostplant, sex and body size. Biochem. Syst. Ecol. 2001, 29, 875–889. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, C.; Ono, H.; Meng, Y.; Shimada, T.; Daimon, T. Flavonoids from the cocoon of Rondotia menciana. Phytochemistry 2013, 94, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, M.; Bianchi, E.; Vigani, B.; Sánchez-Espejo, R.; Spano, M.; Totaro Fila, C.; Mannina, L.; Viseras, C.; Rossi, S.; Sandri, G. Nutritional and functional properties of novel Italian spray-dried cricket powder. Antioxidants 2023, 12, 112. [Google Scholar] [CrossRef]
- Ferreres, F.; Valentão, P.; Pereira, J.A.; Bento, A.; Noites, A.; Seabra, R.M.; Andrade, P.B. HPLC-DAD-MS/MS-ESI screening of phenolic compounds in Pieris brassicae L. reared on Brassica rapa var. rapa L. J. Agric. Food Chem. 2008, 56, 844–853. [Google Scholar] [CrossRef]
- Ferreres, F.; Fernandes, F.; Pereira, D.M.; Pereira, J.A.; Valentao, P.; Andrade, P.B. Phenolics metabolism in insects: Pieris brassicae—Brassica oleracea var. costata ecological duo. J. Agric. Food Chem. 2009, 57, 9035–9043. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.; Chidewe, C.; Ngadze, R.; Macheka, L.; Manditsera, F.; Mubaiwa, J.; Masheka, A. Nutritional and bioactive compounds composition of Eulepida mashona, an edible beetle in Zimbabwe. J. Insects Food Feed. 2016, 2, 179–187. [Google Scholar] [CrossRef]
- Musundire, R.; Zvidzai, C.; Chidewe, C.; Samende, B.; Manditsera, F. Nutrient and anti-nutrient composition of Henicus whellani (Orthoptera: Stenopelmatidae), an edible ground cricket, in south-eastern Zimbabwe. Int. J. Trop. Insect Sci. 2014, 34, 223–231. [Google Scholar] [CrossRef]
- Cappelli, A.; Oliva, N.; Bonaccorsi, G.; Lorini, C.; Cini, E. Assessment of the rheological properties and bread characteristics obtained by innovative protein sources (Cicer arietinum, Acheta domesticus, Tenebrio molitor): Novel food or potential improvers for wheat flour? LWT 2020, 118, 108867. [Google Scholar] [CrossRef]
- Baiano, A. Edible insects: An overview on nutritional characteristics, safety, farming, production technologies, regulatory framework, and socio-economic and ethical implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Fernandez-Cassi, X.; Supeanu, A.; Jansson, A.; Boqvist, S.; Vagsholm, I. Novel foods: A risk profile for the house cricket (acheta domesticus). EFSA J. 2018, 16, 16082. [Google Scholar] [CrossRef]
- Burt, K.G.; Kotao, T.; Lopez, I.; Koeppel, J.; Goldstein, A.; Samuel, L.; Stopler, M. Acceptance of Using Cricket Flour as a Low Carbohydrate, High Protein, Sustainable Substitute for All-Purpose Flour in Muffins. J. Culin. Sci. Technol. 2020, 18, 201–213. [Google Scholar] [CrossRef]
- Aboge, D.O.; Orinda, M.A.; Konyole, S.O. Effect of partial substitution of soybean flour with cricket flour on the nutritional composition, in vitro-protein digestibility and functional properties of complementary porridge flour. Int. J. Trop. Insect Sci. 2022, 42, 1137–1145. [Google Scholar] [CrossRef]
- Kiiru, S.M.; Kinyuru, J.N.; Kiage, B.N.; Martin, A.; Marel, A.K.; Osen, R. Extrusion texturization of cricket flour and soy protein isolate: Influence of insect content, extrusion temperature, and moisture-level variation on textural properties. Food Sci. Nutr. 2020, 8, 4112–4120. [Google Scholar] [CrossRef]
- Kiiru, S.M.; Kinyuru, J.N.; Kiage, B.N.; Marel, A.K. Partial substitution of soy protein isolates with cricket flour during extrusion affects firmness and in vitro protein digestibility. J. Insects Food Feed. 2020, 6, 169–177. [Google Scholar] [CrossRef]
- Igual, M.; García-Segovia, P.; Martínez-Monzó, J. Effect of Acheta domesticus (house cricket) addition on protein content, colour, texture, and extrusion parameters of extruded products. J. Food Eng. 2020, 282, 110032. [Google Scholar] [CrossRef]
- Ribeiro, L.; Cunha, L.M.; García-Segovia, P.; Martínez-Monzó, J.; Igual, M. Effect of the house cricket (Acheta domesticus) inclusion and process temperature on extrudate snack properties. J. Insects Food Feed. 2021, 7, 117–1129. [Google Scholar] [CrossRef]
- Tao, J.; Davidov-Pardo, G.; Burns-Whitmore, B.; Cullen, E.M.; Li, Y.O. Effects of edible insect ingredients on the physicochemical and sensory properties of extruded rice products. J. Insects Food Feed. 2017, 3, 263–278. [Google Scholar] [CrossRef]
- Mitrus, M.; Wójtowicz, A.; Kocira, S.; Kasprzycka, A.; Szparaga, A.; Oniszczuk, T.; Combrzyński, M.; Kupryaniuk, K.; Matwijczuk, A. Effect of extrusion-cooking conditions on the pasting properties of extruded white and red bean seeds. Int. Agrophys. 2020, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Approved Methods of the American Association of Cereal Chemists, 9th ed.; AACC: St. Paul, MN, USA, 1995.
- Horwitz, W.; Latimer, G.W., Jr. (Eds.) AOAC Official Methods of Analysis of AOAC International, 18th ed.; Revision 4; AOAC International: Gaithersburg, MD, USA, 2011. [Google Scholar]
- Helrich, K. (Ed.) AOAC Official Methods of Analysis of AOAC, 13th ed.; AOAC International: Rockville, MD, USA, 1990. [Google Scholar]
- Kasprzak-Drozd, K.; Oniszczuk, T.; Kowalska, I.; Mołdoch, J.; Combrzyński, M.; Gancarz, M.; Dobrzański, B., Jr.; Kondracka, A.; Oniszczuk, A. Effect of the production parameters and in vitro digestion on the content of polyphenolic compounds, phenolic acids, and antiradical properties of innovative snacks enriched with wild garlic (Allium ursinum L.) leaves. Int. J. Mol. Sci. 2022, 23, 14458. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W. Antioxidant and antiradical activities of flavonoids. Agric. Food Chem. 2001, 49, 2774–2779. [Google Scholar] [CrossRef]
- Wójtowicz, A.; Mościcki, L. Influence of legume type and addition level on quality characteristics, texture and microstructure of enriched precooked pasta. LWT-Food Sci. Technol. 2014, 59, 1175–1185. [Google Scholar] [CrossRef]
- Jin, Z.; Hsiehl, F.; Huff, H.E. Effects of soy fiber, salt, sugar and screw speed on physical properties and microstructure of corn meal extrudate. J. Cereal Sci. 1995, 22, 185–194. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Gancarz, M. Characteristics of newly developed extruded products supplemented with plants in a form of microwave-expanded snacks. Materials 2021, 14, 2791. [Google Scholar] [CrossRef]
- Kinyuru, J.N.; Mogendi, J.B.; Riwa, C.A.; Ndung’u, N.W. Edible insects—A novel source of essential nutrients for human diet: Learning from traditional knowledge. Anim. Front. 2015, 5, 14–19. [Google Scholar]
- Liceaga, A.M. Edible insects, a valuable protein source from ancient to modern times. Adv. Food Nutr. Res. 2022, 101, 129–152. [Google Scholar] [PubMed]
- Paulin, I.G.; Purwanto, M.G. Nutritional characteristics of teak grasshopper (Valanga nigricornis Burmeister), cricket (Brachytrupes portentosus L.), and meal-worm (Tenebrio molitor) as alternative food sources in Indonesia. Indones. J. Biotechnol Biodivers. 2020, 4, 52–61. [Google Scholar] [CrossRef]
- Hirsch, A.; Cho, Y.H.; Kim, Y.H.B.; Jones, O.G. Contributions of protein and milled chitin extracted from domestic cricket powder to emulsion stabilization. Curr. Res. Food Sci. 2019, 1, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rivera, E.J.; Hernández-Santos, B.; Juárez-Barrientos, J.M.; Torruco-Uco, J.G.; Ramírez-Figueroa, E.; Rodríguez-Miranda, J. Effects of formulation and process conditions on chemical composition, color parameters, and acceptability of extruded insect-rich snack. J. Food Process. Preserv. 2021, 45, e15499. [Google Scholar] [CrossRef]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of insects and pea powder as alternative protein and mineral sources in extruded snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Zia-ur-Rehman, Z.; Islam, M.; Shah, W.H. Effect of microwave and conventional cooking on insoluble dietary fibre components of vegetables. Food Chem. 2003, 80, 237–240. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S. The effect of extrusion processing on the physiochemical properties of extruded orange pomace. Food Chem. 2016, 192, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Altan, A.; McCarthy, K.L.; Maskan, M. Effect of extrusion process on antioxidant activity, total phenolics and β-glucan content of extrudates developed from barley-fruit and vegetable by-products. Int. J. Food Sci. Technol. 2009, 44, 1263–1271. [Google Scholar] [CrossRef]
- Sirichocktanasap, P.; Chanput, W.P. Phenolic compounds from cricket powder and its protein extract after passing through the simulated in vitro digestion. J. Food Sci. Agric. Technol. 2022, 6, 62–66. [Google Scholar]
- Andersen, S.O. Insect cuticular sclerotization: A review. Insect Biochem. Mol. Biol. 2010, 40, 166–178. [Google Scholar] [CrossRef]
- Nino, M.C.; Reddivari, L.; Osorio, C.; Kaplan, I.; Liceaga, A.M. Insects as a source of phenolic compounds and potential health benefits. J. Insects Food Feed. 2021, 7, 1077–1087. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Gumienna, M.; Rybicka, I.; Górna, B.; Sarbak, P.; Dziedzic, K.; Kmiecik, D. Nutritional value and biological activity of gluten-free bread enriched with cricket powder. Molecules 2021, 26, 1184. [Google Scholar] [CrossRef]
- Suh, H.-J.; Kim, S.-R.; Lee, K.-S.; Park, S.; Kang, S.C. Antioxidant activity of various solvent extracts from Allomyrina dichotoma (Arthropoda: Insecta) larvae. J. Photochem. Photobiol. B Biol. 2010, 99, 67–73. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Yang, Q. Antioxidant activity and phenolic compounds of Holotrichia parallela Motschulsky extracts. Food Chem. 2012, 134, 1885–1891. [Google Scholar] [CrossRef]
- Del Hierro, J.N.; Gutiérrez-Docio, A.; Otero, P.; Reglero, G.; Martin, D. Characterization, antioxidant activity, and inhibitory effect on pancreatic lipase of extracts from the edible insects Acheta domesticus and Tenebrio molitor. Food Chem. 2020, 309, 125742. [Google Scholar] [CrossRef]
- Kasprzak, K.; Oniszczuk, T.; Wojtowicz, A.; Waksmundzka-Hajnos, M.; Olech, M.; Nowak, R.; Polak, R.; Oniszczuk, A. Phenolic acid content and antioxidant properties of extruded corn snacks enriched with kale. J. Anal. Met. Chem. 2018, 2018, 7830546. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Baraniak, B.; Karaś, M. Antioxidant and anti-inflammatory activities of hydrolysates and peptide fractions obtained by enzymatic hydrolysis of selected heat-treated edible insects. Nutrients 2017, 9, 970. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M. Identification of antioxidant and anti-inflammatory peptides obtained by simulated gastrointestinal digestion of three edible insects species (Gryllodes sigillatus, Tenebrio molitor, Schistocerca gragaria). Int. J. Food Sci. Technol. 2018, 53, 2542–2551. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Lisiecka, K.; Wójtowicz, A.; Samborska, K.; Mitrus, M.; Oniszczuk, T.; Combrzyński, M.; Soja, J.; Lewko, P.; Kasprzak Drozd, K.; Oniszczuk, A. Structure and texture characteristics of novel snacks expanded by various methods. Materials 2023, 16, 1541. [Google Scholar] [CrossRef]
- Téllez-Morales, J.; Hernández-Santos, B.; Navarro-Cortez, R.; Rodríguez-Miranda, J. Impact of the addition of cricket flour (Sphenarium purpurascens) on the physicochemical properties, optimization and extrusion conditions of extruded nixtamalized corn flour. Appl. Food Res. 2022, 2, 100149. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A.; Mitrus, M.; Oniszczuk, T.; Combrzyński, M. New type of potato-based snack-pellets supplemented with fresh vegetables from the Allium genus and its selected properties. LWT-Food Sci. Technol. 2021, 145, 111233. [Google Scholar] [CrossRef]
- Lisiecka, K.; Wójtowicz, A. Effect of fresh beetroot application and processing conditions on some quality features of new type of potato-based snacks. LWT-Food Sci. Technol. 2021, 141, 110919. [Google Scholar] [CrossRef]
- Ruiz-Armenta, X.A.; Zazueta-Morales, J.D.J.; Delgado-Nieblas, C.I.; Carrillo-López, A.; Aguilar-Palazuelos, E.; Camacho-Hernández, I.L. Effect of the extrusion process and expansion by microwave heating on physicochemical, phytochemical, and antioxidant properties during the production of indirectly expanded snack foods. J. Food Process. Preserv. 2019, 43, e14261. [Google Scholar] [CrossRef]

| Recipe | Gelatinization Temperature [°C] | PV [mPas] | HPV [mPas] | CPV [mPas] | BD [mPas] | SB [mPas] |
|---|---|---|---|---|---|---|
| Control blend | 73.0 ± 0.9 a | 472 ± 2 c | 354 ± 4 c | 666 ± 2 c | 118 ± 2 c | 312 ± 2 c |
| 10% cricket flour | 74.7 ± 1.2 a | 369 ± 3 b | 284 ± 5 b | 582 ± 3 b | 85 ± 2 b | 298 ± 2 b |
| 30% cricket flour | 80.7 ± 0.7 b | 197 ± 1 a | 156 ± 2 a | 303 ± 4 a | 41 ± 3 a | 147 ± 4 a |
| Cricket Flour Content [%] | Screw Speed [rpm] | Moisture Content [%] | Component [g/100 g] | ||||
|---|---|---|---|---|---|---|---|
| Crude Protein | Crude Ash | Crude Fat | Crude Fiber | Carbohydrates | |||
| 0 | 60 | 32 | 9.33 ± 0.87 a | 2.90 ± 0.84 a | 0.42 ± 0.06 a | 0.00 ± 0.00 a | 77.20 |
| 36 | 9.18 ± 0.90 a | 2.88 ± 0.72 a | 0.33 ± 0.04 a | 0.00 ± 0.00 a | 77.39 | ||
| 100 | 32 | 9.21 ± 0.88 a | 2.91 ± 0.68 a | 0.41 ± 0.04 a | 0.00 ± 0.00 a | 77.38 | |
| 36 | 9.31 ± 0.92 a | 2.85 ± 0.76 a | 0.80 ± 0.06 b | 0.00 ± 0.00 a | 76.99 | ||
| 10 | 60 | 32 | 13.53 ± 1.12 b | 3.04 ± 0.78 a | 2.54 ± 0.42 b | 0.64 ± 0.06 b | 70.47 |
| 36 | 14.56 ± 1.16 b | 2.99 ± 0.76 a | 1.95 ± 0.46 b | 0.95 ± 0.08 c | 69.92 | ||
| 100 | 32 | 14.44 ± 1.18 b | 3.04 ± 0.72 a | 3.33 ± 0.58 b | 0.98 ± 0.12 c | 68.53 | |
| 36 | 14.72 ± 1.08 b | 3.05 ± 0.82 a | 1.26 ± 0.28 b | 0.93 ± 0.14 c | 70.24 | ||
| 30 | 60 | 32 | 24.07 ± 1.66 c | 3.29 ± 0.84 a | 8.28 ± 0.96 c | 3.24 ± 0.36 d | 52.16 |
| 36 | 24.54 ± 1.62 c | 3.10 ± 0.76 a | 6.01 ± 0.68 c | 3.30 ± 0.42 d | 54.18 | ||
| 100 | 32 | 24.12 ± 1.54 c | 3.23 ± 0.84 a | 7.77 ± 0.82 c | 3.02 ± 0.38 d | 53.09 | |
| 36 | 24.60 ± 1.62 c | 3.22 ± 0.92 a | 7.76 ± 0.78 c | 3.27 ± 0.44 d | 52.40 | ||
| Fatty Acids | Extrudates | |||||||
|---|---|---|---|---|---|---|---|---|
| 10% Cricket Flour | 30% Cricket Flour | |||||||
| 32% m.c. | 36% m.c. | 32% m.c. | 36% m.c. | |||||
| 60 rpm | 100 rpm | 60 rpm | 100 rpm | 60 rpm | 100 rpm | 60 rpm | 100 rpm | |
| Butyric acid C 4:0 | nd | 0.049 ± 0.008 a | 0.052 ± 0.008 a | nd | nd | nd | nd | 0.045 ± 0.006 a |
| Caproic acid C 6:0 | nd | nd | nd | nd | nd | nd | nd | 0.026 ± 0.004 a |
| Caprylic acid C 8:0 | nd | nd | nd | nd | nd | nd | 0.044 ± 0.008 a | 0.057 ± 0.008 a |
| Lauric acid C 12:0 | nd | nd | nd | 0.063 ± 0.010 a | nd | 0.053 ± 0.008 a | 0.058 ± 0.006 a | 0.059 ± 0.006 a |
| Myrystic acid C 14:0 | 0.328 ± 0.022 a | 0.370 ± 0.030 a | 0.330 ± 0.028 a | 0.394 ± 0.034 a | 0.472 ± 0.036 b | 0.494 ± 0.038 b | 0.480 ± 0.034 b | 0.471 ± 0.036 b |
| Pentadecylic acid C 15:0 | 0.069 ± 0.006 a | 0.066 ± 0.004 a | 0.086 ± 0.006 b | 0.112 ± 0.008 c | 0.085 ± 0.008 b | 0.073 ± 0.006 a | 0.113 ± 0.008 c | 0.065 ± 0.004 a |
| Palmitic acid C 16:0 | 19.135 ± 1.624 a | 19.596 ± 1.686 a | 18.964 ± 1.652 a | 20.051 ± 1.722 a | 23.888 ± 1.746 b | 24.630 ± 1.786 b | 24.197 ± 1.742 b | 24.669 ± 1.766 b |
| Palmitoleic acid C 16:1 n-9 | 0.401 ± 0.032 a | 0.429 ± 0.038 a | 0.426 ± 0.042 a | 0.412 ± 0.040 a | 0.529 ± 0.042 a | 0.604 ± 0.048 a | 0.539 ± 0.038 a | 0.547 ± 0.042 a |
| Palmitoleic acid C 16:1 n-7 | nd | nd | nd | 0.089 ± 0.008 a | nd | nd | nd | nd |
| Heptadecanoic acid C 17:0 | 0.170 ± 0.016 a | 0.157 ± 0.014 a | 0.154 ± 0.008 a | 0.143 ± 0.012 a | 0.167 ± 0.018 a | 0.198 ± 0.022 a | 0.182 ± 0.016 a | 0.188 ± 0.014 a |
| Stearic acid C 18:0 | 5.984 ± 0.522 a | 6.261 ± 0.584 a | 6.076 ± 0.592 a | 6.441 ± 0.576 a | 8.238 ± 0.722 b | 8.359 ± 0.728 b | 8.216 ± 0.742 b | 8.571 ± 0.718 b |
| Oleic acid C 18:1 n-9 | 36.174 ± 3.080 b | 33.852 ± 3.120 b | 35.657 ± 2.982 b | 32.356 ± 3.128 b | 31.330 ± 3.068 b | 30.325 ± 3.144 b | 32.368 ± 2.824 b | 32.773 ± 3.146 b |
| Linoleic acid C 18:2 n-6 | 34.405 ± 3.222 a | 35.954 ± 3.256 a | 34.891 ± 3.842 a | 37.182 ± 3.628 a | 33.137 ± 3.422 a | 32.740 ± 2.980 a | 31.668 ± 2.864 a | 30.607 ± 3.022 a |
| α-Linolenic acid C 18:3 n-3 | 2.286 ± 0.249 b | 2.314 ± 0.264 b | 2.277 ± 0.212 b | 2.024 ± 0.196 b | 1.582 ± 0.125 a | 1.986 ± 0.167 b | 1.503± 0.159 a | 1.367 ± 0.122 a |
| Arachidic acid C 20:0 | 0.440 ± 0.044 a | 0.400 ± 0.036 a | 0.420 ± 0.038 a | 0.380 ± 0.032 a | 0.345 ± 0.040 | 0.334 ± 0.036 a | 0.374 ± 0.033 a | 0.356 ± 0.035 a |
| Gondoic acid C 20:1 n-9 | 0.424 ± 0.042 b | 0.401 ± 0.038 b | 0.422± 0.044 b | 0.353 ± 0.036 b | 0.227 ± 0.028 a | nd | 0.258 ± 0.018 a | 0.200 ± 0.022 a |
| Eicosadienoic acid C 20:2 n-6 | nd | nd | nd | nd | nd | 0.079 ± 0.008 a | nd | nd |
| Behenic acid C 22:0 | 0.185 ± 0.012 c | 0.152 ± 0.014 b | 0.245 ± 0.018 d | nd | nd | 0.125 ± 0.008 a | nd | nd |
| Total [%] | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| ΣSFA [% in total] | 26.311 | 27.051 | 26.327 | 27.584 | 33.195 | 34.265 | 33.664 | 34.507 |
| ΣMUFA [% in total] | 36.999 | 34.682 | 36.505 | 33.121 | 32.085 | 30.929 | 33.165 | 33.520 |
| ΣPUFA [% in total] | 36.690 | 38.267 | 37.168 | 39.205 | 34.719 | 34.805 | 33.171 | 31.973 |
| Cricket Flour Content [%] | Screw Speed [rpm] | Moisture Content [%] | TPC [μg GAE/g Dry Weight] | DPPH Scavenging Activity [%] | TEAC Value [µg/g Product] |
|---|---|---|---|---|---|
| 0 | 60 | 32 | 62.1 ± 1.2 a | 50.11 ± 0.18 a | 111.45 ± 4.38 a |
| 36 | 60.6 ± 1.9 a | 51.02 ± 0.23 b | 113.08 ± 5.12 a | ||
| 100 | 32 | 78.1 ± 2.1 b | 50.28 ± 0.22 a | 106.54 ± 6.11 a | |
| 36 | 60.8 ± 1.8 a | 51.01 ± 0.35 b | 103.50 ± 4.98 a | ||
| 10 | 60 | 32 | 86.3 ± 3.2 c | 93.83 ± 0.11 d | 160.63 ± 7.56 b |
| 36 | 93.0 ± 4.3 d | 92.65 ± 0.23 c | 156.43 ± 6.98 b | ||
| 100 | 32 | 84.9 ± 1.2 c | 92.57 ± 0.34 c | 162.97 ± 8.02 b | |
| 36 | 119.8 ± 6.4 e | 93.67 ± 0.17 d | 163.43 ± 7.32 b | ||
| 30 | 60 | 32 | 152.0 ± 4.4 g | 93.75 ± 0.28 d | 164.14 ± 9.02 b |
| 36 | 137.2 ± 2.8 f | 93.85 ± 0.21 d | 164.25 ± 7.78 b | ||
| 100 | 32 | 146.5 ± 5.4 g | 93.38 ± 0.62 d | 163.79 ± 8.99 b | |
| 36 | 114.5 ± 3.3 e | 92.85 ± 0.29 c | 163.08 ± 8.09 b |
| Sample Parameters | TPC | DPPH | TEAC |
|---|---|---|---|
| 60 rpm, 32% mc | 0.997 | 0.788 | 0.794 |
| 60 rpm, 36% mc | 0.995 | 0.790 | 0.764 |
| 100 rpm, 32% mc | 0.907 | 0.874 | 0.841 |
| 100 rpm, 36% mc | 0.779 | 0.756 | 0.752 |
| Cricket Flour Content [%] | Screw Speed [rpm] | Moisture Content [%] | WAI 1 [g/g] | WSI 1 [%] | Cutting Force 2 [N] |
|---|---|---|---|---|---|
| 0 | 60 | 32 | 3.15 ± 0.32 b | 10.34 ± 0.52 b | 33.01 ± 2.89 d |
| 36 | 3.82 ± 0.28 b | 12.47 ± 0.55 c | 25.01 ± 1.52 c | ||
| 100 | 32 | 3.43 ± 0.22 b | 14.14 ± 0.49 d | 30.79 ± 2.94 d | |
| 36 | 4.43 ± 0.18 c | 14.72 ± 0.46 d | 31.32 ± 2.96 d | ||
| 10 | 60 | 32 | 2.46 ± 0.12 a | 8.09 ± 0.28 a | 15.78 ± 2.21 b |
| 36 | 2.35 ± 0.14 a | 8.81 ± 0.32 b | 25.27 ± 1.72 c | ||
| 100 | 32 | 3.75 ± 0.28 b | 8.70 ± 0.36 b | 13.32 ± 4.21 ab | |
| 36 | 3.70 ± 0.30 b | 9.27 ± 0.46 b | 22.63 ± 3.54 c | ||
| 30 | 60 | 32 | 2.65 ± 0.14 a | 18.24 ± 0.62 e | 11.82 ± 2.13 a |
| 36 | 2.96 ± 0.12 b | 28.73 ± 0.86 f | 10.98 ± 1.15 a | ||
| 100 | 32 | 3.55 ± 0.18 b | 27.27 ± 0.76 f | 11.96 ± 1.63 a | |
| 36 | 3.52 ± 0.22 b | 32.04 ± 0.96 g | 14.73 ± 3.30 b |
| Cricket Flour Content [%] | Screw Speed [rpm] | Moisture Content [%] | Color Parameters | |||
|---|---|---|---|---|---|---|
| L* | a* | b* | ΔE | |||
| 0 | 60 | 32 | 84.46 ± 0.98 d | 3.86 ± 0.18 a | 21.51 ± 0.59 d | ref |
| 36 | 83.65 ± 0.56 d | 4.09 ± 0.23 a | 22.10 ± 1.10 d | ref | ||
| 100 | 32 | 81.56 ± 0.43 d | 5.07 ± 0.03 cd | 24.18 ± 0.45 e | ref | |
| 36 | 81.83 ± 0.40 d | 5.09 ± 0.11 cd | 24.38 ± 0.37 e | ref | ||
| 10 | 60 | 32 | 68.35 ± 1.47 c | 4.75 ± 0.35 bc | 13.88 ± 0.89 bc | 17.87 ± 1.49 b |
| 36 | 67.88 ± 0.96 bc | 4.55 ± 0.20 b | 13.85 ± 0.44 bc | 17.49 ± 1.00 b | ||
| 100 | 32 | 68.47 ± 2.72 c | 4.84 ± 0.27 bc | 14.60 ± 0.32 bc | 17.47 ± 2.52 b | |
| 36 | 72.88 ± 1.65 e | 4.73 ± 0.15 bc | 14.88 ± 0.33 c | 13.10 ± 1.22 a | ||
| 30 | 60 | 32 | 64.59 ± 2.23 a | 5.11 ± 0.20 cd | 12.45 ± 0.75 a | 21.88 ± 2.28 c |
| 36 | 65.17 ± 1.01 ab | 5.45 ± 0.12 d | 13.47 ± 0.44 ab | 19.92 ± 1.06 bc | ||
| 100 | 32 | 65.97 ± 1.30 abc | 5.44 ± 0.15 d | 13.48 ± 0.44 ab | 20.22 ± 1.33 bc | |
| 36 | 65.85 ± 1.60 abc | 5.49 ± 0.23 d | 13.49 ± 0.66 ab | 19.36 ± 1.66 bc | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Combrzyński, M.; Oniszczuk, T.; Wójtowicz, A.; Biernacka, B.; Wojtunik-Kulesza, K.; Bąkowski, M.; Różyło, R.; Szponar, J.; Soja, J.; Oniszczuk, A. Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions. Antioxidants 2023, 12, 1253. https://doi.org/10.3390/antiox12061253
Combrzyński M, Oniszczuk T, Wójtowicz A, Biernacka B, Wojtunik-Kulesza K, Bąkowski M, Różyło R, Szponar J, Soja J, Oniszczuk A. Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions. Antioxidants. 2023; 12(6):1253. https://doi.org/10.3390/antiox12061253
Chicago/Turabian StyleCombrzyński, Maciej, Tomasz Oniszczuk, Agnieszka Wójtowicz, Beata Biernacka, Karolina Wojtunik-Kulesza, Maciej Bąkowski, Renata Różyło, Jarosław Szponar, Jakub Soja, and Anna Oniszczuk. 2023. "Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions" Antioxidants 12, no. 6: 1253. https://doi.org/10.3390/antiox12061253
APA StyleCombrzyński, M., Oniszczuk, T., Wójtowicz, A., Biernacka, B., Wojtunik-Kulesza, K., Bąkowski, M., Różyło, R., Szponar, J., Soja, J., & Oniszczuk, A. (2023). Nutritional Characteristics of New Generation Extruded Snack Pellets with Edible Cricket Flour Processed at Various Extrusion Conditions. Antioxidants, 12(6), 1253. https://doi.org/10.3390/antiox12061253








