Abstract
Oxidative stress driven by several environmental and local airway factors associated with chronic obstructive bronchiolitis, a hallmark feature of COPD, plays a crucial role in disease pathomechanisms. Unbalance between oxidants and antioxidant defense mechanisms amplifies the local inflammatory processes, worsens cardiovascular health, and contributes to COPD-related cardiovascular dysfunctions and mortality. The current review summarizes recent developments in our understanding of different mechanisms contributing to oxidative stress and its countermeasures, with special attention to those that link local and systemic processes. Major regulatory mechanisms orchestrating these pathways are also introduced, with some suggestions for further research in the field.
1. Introduction
Oxidative stress driven by several environmental and local airway factors associated with chronic obstructive bronchiolitis, a hallmark feature of chronic obstructive pulmonary disease (COPD), plays a crucial role in the disease pathomechanisms [1,2]. Unbalance between oxidants and antioxidant defense mechanisms amplifies the local inflammatory processes, has systemic effects, contributes to developing COPD–related comorbidities, and worsens cardiovascular health. COPD often coexists with cardiovascular diseases (CVDs). CVDs are not only the most common comorbidities perceived in COP, but also account for an increased risk of death in COPD patients [3,4,5]. Approximately 30% of COPD patients are reported to die due to CVD. COPD and CVD share common pathophysiological mechanisms strongly related to oxidative stress [6]. This review summarizes our current understanding of the local and systemic processes that link COPD and various CVDs via oxidative stress. We focus on some relevant mechanisms that orchestrate the systemic responses leading to the parallel development of respiratory and cardiovascular dysfunctions. We aim to update and extend previous reviews related to the field by describing biomarkers, discussing the relationship between COPD and CVDs in a broader sense instead of focusing on certain specific CVDs and highlighting novel, less investigated mechanisms connecting the two disease entities via oxidative stress [4,7,8,9,10,11].
2. Pathways of Oxidative Stress
Oxidative stress is when the oxidative burden imposed by exposure to exogenous and endogenous free radicals exceeds the antioxidant defense capacities. This may occur due to excessive oxidant production, exhaustion, or the defective functioning of antioxidant mechanisms (Figure 1). Reactive oxygen species (ROS), such as hydroxyl radical and superoxide anion, are produced by each cell in the body during mitochondrial respiration and cell signaling processes. ROS production by the immune, mainly phagocytic cells, is also essential in the immune defense against pathogens [1,2]. To protect the physiological function of cells from the harmful effects of exogenous and endogenous radicals, the body maintains powerful antioxidant mechanisms.
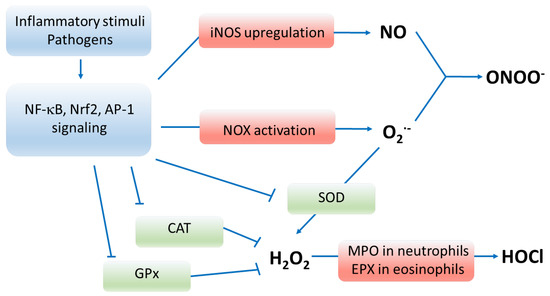
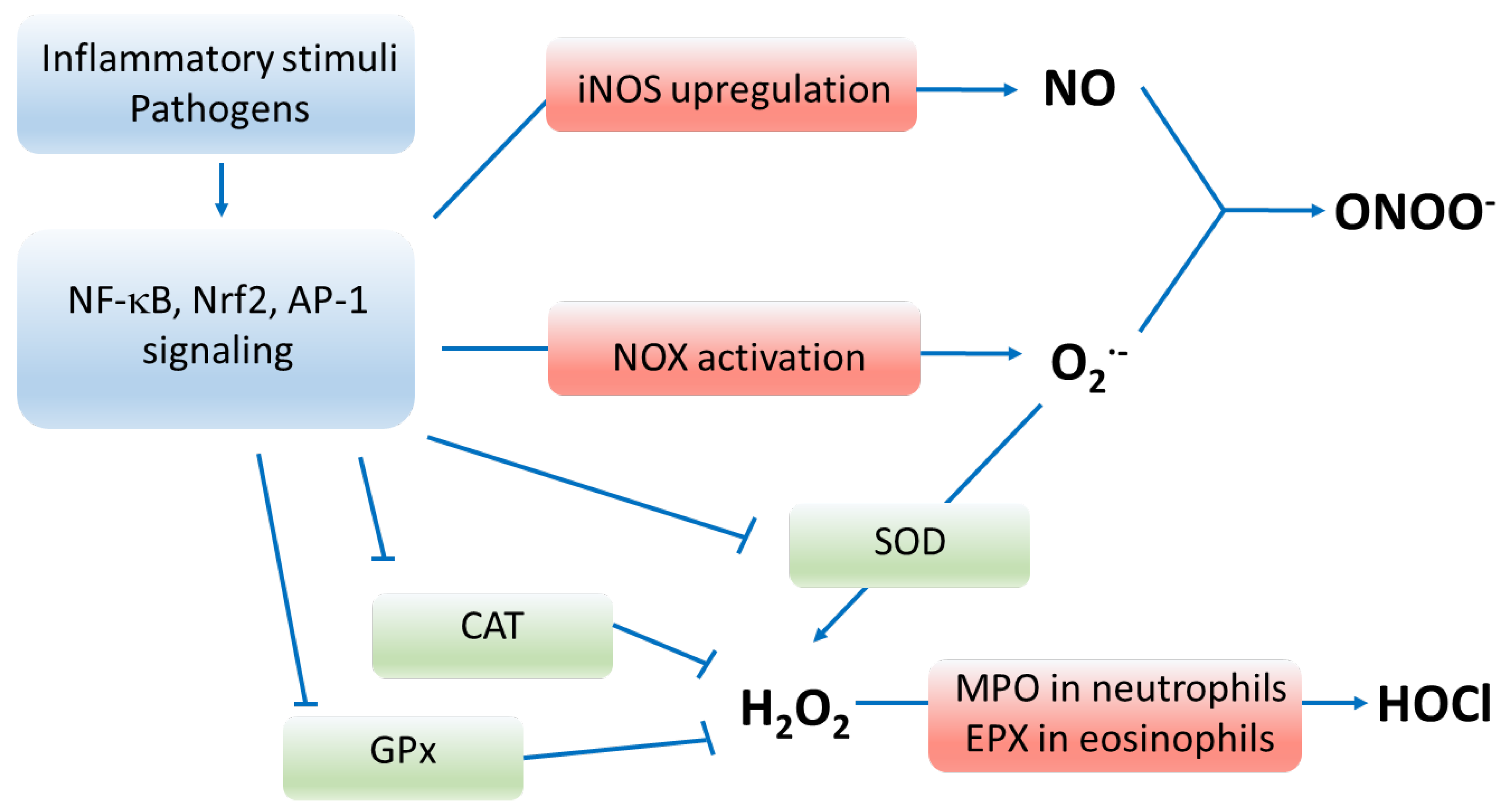
Figure 1.
Pathways of oxidative stress. Enzymes marked in red participate in producing oxygen radicals, whereas enzymes marked in green deactivate reactive oxygen species. Inflammatory stimuli, pathogens and oxidants upregulate and activate signaling via NF-κB, Nrf2 and AP-1 transcription factors that result in enhanced production of reactive species and depressed functioning of antioxidant enzymes. Abbreviations: NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf2—nuclear factor erythroid 2-related factor 2; AP-1—activator protein 1; iNOS—inducible nitric oxide synthase; NO—nitric oxide; NOX—nicotinamide adenine dinucleotide phosphate oxidase; O2•−—superoxide anion, ONOO−—peroxynitrite; SOD—superoxide dismutase; CAT—catalase; GPx—glutathione peroxidase; H2O2—hydrogen peroxide; MPO—myeloperoxidase; EPX—eosinophil peroxidase; HOCl—hypochlorous acid; RNS—reactive nitrogen species; RCS—reactive carbonyl species.
2.1. Production of Oxygen Radicals
Phagocyte ROS generation relies on the operation of nicotinamide adenine dinucleotide phosphate (NADPH)-oxidase (NOX) enzymes which produce superoxide anion (O2•−) by transferring an electron from NADPH to O2 as a result of activation of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) signaling. NOX enzymes are localized to the membrane, and their different isoforms are expressed in numerous tissues and cell types in the body [12,13]. The O2•− anion is unstable and is rapidly dismutated to hydrogen peroxide (H2O2) by the enzyme superoxide dismutase (SOD) [14]. Phagocyte lysosomes also contain the enzyme myeloperoxidase, which catalyzes the conversion of H2O2 to hypochlorous acid (HOCl), a highly oxidizing agent [15]. H2O2 can also be converted to reactive nitrogen and carbonyl species (RNS and RCS) in the Haber–Weiss and Fenton reactions [16,17].
A further essential source of ROS is excessive nitrogen monoxide (NO) production by inducible nitric oxide synthase in phagocytes and various cell types as part of the inflammatory responses [18]. When NO and O2•− are present at increased concentrations, as seen in inflammation, they readily combine to form peroxynitrite (ONOO−). Peroxynitrite is a highly reactive oxidant with enhanced stability [2,18].
Reactive species can oxidize thiols, amines, and amino acid residues of proteins such as cysteine, methionine, and tyrosine. This may alter the tertiary structure and function of the protein. In addition, ROS can also be harmful to lipids and DNA, which may cause membrane dysfunction and transcriptional errors [1,2,19,20,21].
Nuclear factor-κB (NF-κB) signaling connects ROS production to local and systemic inflammation in various diseases. While certain NF-κB regulated genes control ROS generation by the cell, ROS also have complex inhibitory and stimulatory effects on NF-κB signaling, mediating mainly proinflammatory responses [22]. Another transcription factor that is relevant in exacerbating ROS production in inflammatory responses is activator protein 1 (AP-1). AP-1 activity is redox-sensitive and induced by many physiological, pathophysiological, and environmental stimuli, including various cytokines and bacterial and viral infections [23]. The products of AP-1-induced genes participate in inflammatory processes and ROS production and have been shown to contribute to the etiology of disease conditions in the respiratory and cardiovascular systems [23,24,25].
2.2. Antioxidative Defense
The action of ROS is kept under control by enzymatic and non-enzymatic defense mechanisms [1,2]. Antioxidant molecules, metal-binding proteins, and unsaturated lipids acting as electron donors or recipients can scavenge non-enzymatic radicals. In the lung, the antioxidants vitamin C (ascorbate) and vitamin E (tocopherol) are found in abundance in the airway surface liquid [26,27]. In addition, albumin, mucin in extracellular body fluids and glutathione within cells are relevant scavengers as they offer methionine and cysteine residues for radicals [28,29,30].
Enzymatic ROS antioxidation is carried out by three significant enzymes, superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx). Superoxide dismutase (SOD1, SOD2 and SOD3) quickly remove O2− by converting it to H2O2 to prevent it from causing damage or producing extremely damaging peroxyl radicals [14]. However, this process produces H2O2, which can be the precursor of further hydroxyl radical generation. Catalase and GPx eliminate H2O2 by splitting it into H2O and O2 [31,32]. In the GPx-catalyzed reaction, glutathione (GSH) acts as a hydrogen ion donor, becoming glutathione disulphide (GSSG). Expression of antioxidant enzymes is highly regulated by the transcription factor ‘nuclear factor erythroid 2-related factor 2 (Nrf2)’. Decreased activation of Nrf2 due to inflammatory cytokines and depression of anti-aging mechanisms participates in the downregulation and loss of antioxidant defense in COPD and CVDs [33,34,35,36]. Moreover, Nrf2 is downregulated by oxidative stress itself, initiating a vicious circle [37,38,39].
2.3. Sources of Oxidative Stress in COPD
In COPD development, exogenous radicals from cigarette and biomass smoke, air pollution, and occupational exposure contribute substantially to the oxidative stress of small molecules [2,40,41]. In addition, cigarette smoke can enhance NOX activity in lung tissue and stimulate leukocyte migration [42,43]. Compared with non-smokers, the neutrophil count in COPD patients is higher in BAL fluid and sputum and enhanced NOX activity can be detected in circulating neutrophils [12,43]. Moreover, NOX4 was upregulated in COPD patients’ airway smooth muscle cells, correlated with disease severity, and was associated with pulmonary hypertension [43,44,45,46].
Furthermore, the increased oxidant burden causes the upregulation of antioxidant genes that play protective roles. For example, the induction of the GSH gene increases the accumulation of GSH in the epithelial lining fluid in the airspaces, which is important for preventing oxidative injury [47,48]. Similarly, increased SOD and catalase activity have been observed in the sputum of COPD patients during acute exacerbation [49]. On the other hand, cigarette smoke exposure and long-term inflammation have been shown to reduce the activity of antioxidant enzymes, such as catalase and superoxide dismutase, contributing to the severe perturbation of oxidative balance in the lung tissue [50,51] (see in details later).
3. Oxidative Stress—A Link between COPD and Cardiovascular Comorbidities
COPD and CVDs share common pathophysiological mechanisms that involve systemic inflammation, endothelial dysfunction, vascular inflammation and remodeling, alteration in heart rate variability, and clotting abnormalities [6]. These underlying mechanisms (at least in part) participate in the development of pulmonary arterial hypertension (PAH), hypertension, accelerated atherosclerosis and its consequences, such as stroke, ischemic heart disease and, in the long run, cardiac failure (Figure 2).
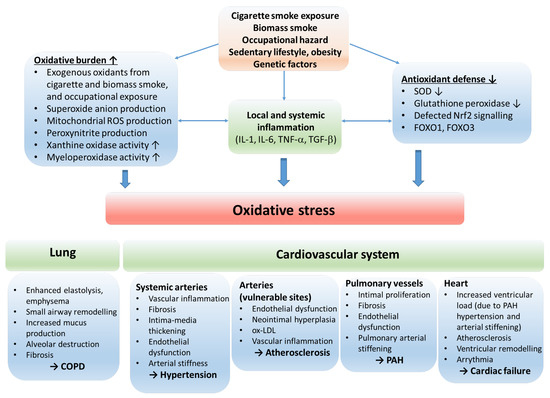
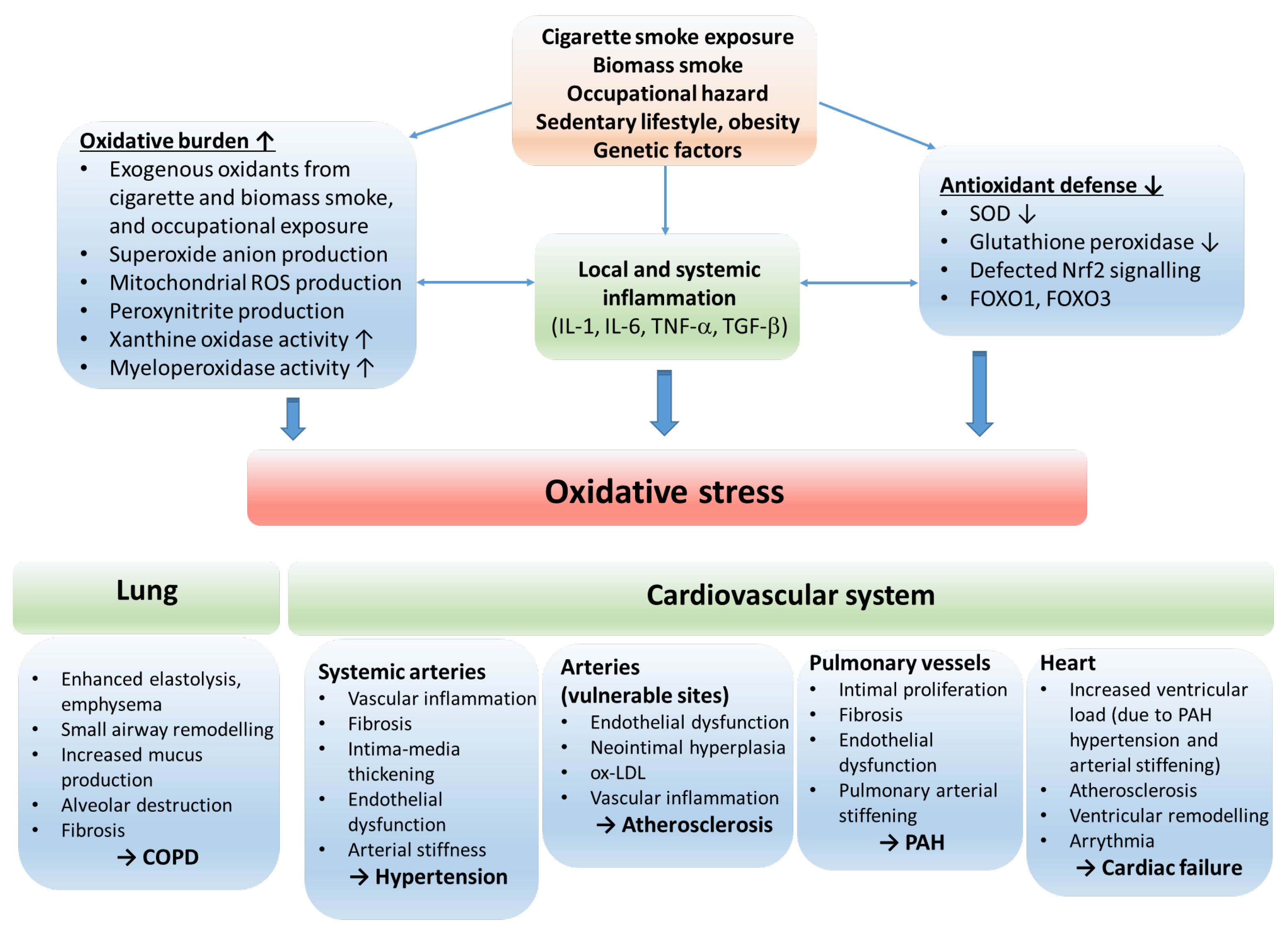
Figure 2.
The role of oxidative stress in the etiology of COPD and cardiovascular comorbidities. The oxidative balance of the body is disturbed by risk factors resulting in inflammation, increased oxidative burden and production of reactive oxygen radicals, and reduction in antioxidant defense mechanisms. The consequential oxidative stress stimulates processes that lead to COPD and cardiovascular disorders. Abbreviations: ROS—reactive oxygen species; IL—interleukin; TNF—tumor necrosis factor; SOD—superoxide dismutase; Nrf2—nuclear factor erythroid 2-related factor 2; FOXO1, FOXO3—forkhead box O1 and O3; COPD—chronic obstructive pulmonary disease; ox-LDL—oxidized low-density lipoprotein; PAH—pulmonary arterial hypertension.
3.1. COPD and Vascular Aging, Hypertension
Though widely debated, many experts view the development of COPD as a manifestation of accelerated aging [52]. Indeed, a strong association between vascular aging and COPD is well-established in the literature. COPD manifests as small airway obstruction (chronic obstructive bronchiolitis) and emphysema. Pathologically, chronic inflammation and fibrosis of peripheral airways, increased mucus secretion, luminal accumulation, and destruction of lung parenchyma and alveoli are typical alterations. These overlapping phenotypes may manifest with varying severity and might dominate the clinical picture of individual patients [52,53]. The aging vasculature is characterized by fibrotic remodeling and thickening of the arterial wall, intima-media hyperplasia, and endothelial dysfunction [39,54,55,56]. The aging arteries stiffen, and the consequential alteration in their biomechanical properties is a critical factor in developing hypertension, one of the significant CV comorbidities in COPD [54,57]. Early vascular aging is best detected by measuring pulse wave velocity (PWV), as pulse propagation is typically faster in stiffer, aged arteries. Numerous studies have found that PWV is abnormally high in COPD patients [58,59]. Arterial stiffness, as measured by PWV, was independently associated with the severity of emphysema [60] and airway obstruction [59,61,62,63,64]. Furthermore, it was established by studying twins that the link between lung function and arterial stiffness is not genetically determined. However, there is a phenotypic association between spirometric parameters used to assess airway obstruction, such as forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1), and augmentation index, a marker of pulse wave reflection pointing towards shared pathways of their co-development in COPD [65]. The observations that arterial stiffness seems more severe in frequently exacerbating COPD patients and to intensify acutely during exacerbation suggest a dynamic, reversible component of this relationship that is not fully characterized [66]. COPD rehabilitation programs have been shown to benefit arterial stiffness in a subpopulation of patients significantly but not in general [67]. This observation is similar to those demonstrating that lung function values or even regulatory molecules known as part of antioxidant defense cannot be improved much by these programs despite their well-documented positive effects on the overall health status of involved patients [68]. It is also worth mentioning that COPD often associates with obstructive sleep apnea (OSA) [69]. OSA is widely recognized as a significant risk factor for developing arterial hypertension and its complications [70]. Among the underlying mechanisms, the contribution of hypoxic periods during sleep in OSA to oxidative stress has the utmost relevance [71].
Among the common underlying mechanisms of vascular aging and COPD, persistent systemic low-grade inflammation, oxidative stress (i.e., overproduction of reactive oxygen species and decreased antioxidant capacity) and deterioration of anti-aging mechanisms have critical relevance.
3.1.1. Oxidative Stress in COPD and Vascular Aging
Enhanced oxidative stress plays a significant role in COPD and vascular aging pathogenesis. It is attributable to various pathophysiological mechanisms involving mitochondrial senescence, NADPH oxidase (NOX) overactivation, endothelial dysfunction, overactivation of the tissue renin-angiotensin-aldosterone system (RAAS), and also to COPD-related hypoxia [1,2,39,54,55,56,57,72].
The sources of oxidative stress are manifold in both conditions. Cigarette smoke exposure, a significant risk factor in COPD and vascular aging, is a direct source of inhaled oxidants and irritants that generate inflammation. In COPD, dysfunctional mitochondria of structural cells (airway epithelium, fibroblasts), NADPH oxidases (NOX) of airway epithelial cells, and myeloperoxidase enzymes of neutrophils and macrophages produce a substantial amount of reactive oxygen species [1,2,26,52]. An increased ROS production by mitochondria and NOX enzymes is also typical in the aging vasculature [54,73]. Inflammatory cytokines, adipokines, activation of endothelin1 and angiotensin1 receptors, and dysfunctional NO synthase operation further aggravate oxidative stress by activating NOX enzymes both in the vasculature and the lung [2,26,36,55,57,74,75].
Oxidative stress is further amplified by the decreased antioxidant capacity of the lung and vascular tissue [39,55,56,75,76,77]. Reduced superoxide dismutase (SOD) activity has been observed in relation to vascular aging [57,75,78]. Though acute exacerbations in COPD are associated with increased extracellular SOD activity [49], altered SOD function due to SOD2 and SOD3 gene polymorphism has been implicated in the etiology of COPD [79,80]. Lower antioxidant capacity is also reflected by lower circulating and cellular glutathione concentrations in COPD and during vascular aging [39,81]. However, glutathione concentrations measured in BAL fluid and sputum are elevated in COPD [82]. In addition, the transcription factor Nrf2 is downregulated and exhibits impaired activation in response to oxidative stress [37,38,39]. This results in decreased expression of several antioxidant enzymes in the lung and vascular tissue [33,34,35,36] and ROS production by NOX in the vasculature [37]. Catalase activity is reduced in COPD patients [83,84], and a decreased expression was found in the bronchial epithelium [50].
In contrast, during acute exacerbation, enhanced catalase activity can be observed in the sputum [49]. Decreased catalase activity has been linked to several age-related diseases, including cardiovascular disorders [32]. Concerning GPx activity, a decrease was observed in erythrocytes [83,85,86,87], and blood and plasma samples [88,89,90] of COPD sufferers. Aging and vascular abnormalities have also been related to depressed GPx functioning by several studies [91,92,93]. Nitrative stress is also well-documented in COPD and is further aggravated during exacerbations [94]. Defected heme-oxygenase-1 (HO-1) signaling also contributes to decreased antioxidant and anti-inflammatory defense in lung and cardiovascular diseases. HO-1 is an inducible stress protein implicated in chronic airway inflammation [95]. The major activity of HO-1 is to eliminate the high oxidant-free heme by converting it to biliverdin, ferrous iron and carbon monoxide. Its expression is strongly influenced by Nrf2 [95].
3.1.2. The Consequences and Aggravators of Oxidative Stress
The consequences of oxidative stress include inflammation, disruption of anti-aging processes and endothelial injury, which typically manifest in a systemic form in COPD. Though oxidative burden is a key factor in igniting these processes, they also fuel and aggravate oxidative stress by activating signaling pathways that induce ROS production and/or downregulate antioxidant defense mechanisms.
Systemic inflammation. Oxidative stress induces redox-sensitive proinflammatory signaling in various cell types. Increased generation of ROS species is associated with the activation of proinflammatory transcription factors and proteins such as NF-κB, activator protein 1, transforming growth factor-β (TGF-β), different isoforms of matrix metalloproteinases, p38MAPK both in the lung and vascular tissue. Activation of these pathways results in the upregulation and release of inflammatory cytokines (i.e., TGF-β, TNF-α, IL-1, IL-6), chemokines and adhesion molecules that perpetuate inflammation locally and systemically [2,35,36,52,55,57,73,74,75,96]. In addition, local inflammation triggers maladaptive remodeling. Activation of MMPs breaks down elastic fibers, and profibrotic processes (activation of local RAAS and fibroblasts) operate to give rise to small airway fibrosis and emphysema in the lung, and intima-media thickening and calcification in the arterial wall [39,52,54,57,58,72,97].
Endothelial abnormalities. Endothelial injury and dysfunction are also obligate consequences of long-term oxidative stress in the lung and vasculature and common features of COPD and arterial aging [96]. The normal endothelium releases. NO, is a gaseous signaling molecule which has beneficial effects on systemic and pulmonary vasculature. It decreases vascular tone, has an antiproliferative impact on smooth muscle cells, and inhibits platelet aggregation and the release of inflammatory mediators. In oxidative stress, superoxide species react with NO to form peroxynitrite, a short-lived, highly potent oxidant that induces cell injury and mediates proinflammatory processes [18]. In addition, in oxidative stress, tetrahydrobiopterin, a cofactor of NO synthase (NOS), gets oxidized leading to NOS uncoupling. The uncoupled NOS produces superoxide instead of NO, further exacerbating oxidative stress. As a result, the bioavailability of NO decreases and its beneficial effects deteriorate [98,99].
Furthermore, NOS activity is reduced due to the accumulation of its endogenous inhibitor, asymmetric dimethylarginine (ADMA) [100]. Elevated plasma ADMA levels have been associated with endothelial dysfunctions and cardiovascular diseases, including ischemic stroke, pulmonary hypertension, and heart failure [101]. In addition, the bioavailability of NO is further aggravated by the upregulation of arginase, the enzyme that cleaves l-arginine, the precursor of NO [102]. Elevation of arginase activity reduces the availability of l-arginine to NOS, which can reduce NO formation, uncouple NOS, and increase peroxynitrite production contributing to airway hypercontractility and vascular remodeling [100,103,104,105]. Moreover, NOS expression and activity are directly reduced by cigarette smoke exposure, oxidative stress, and inflammatory processes [99,106,107]. Besides uncoupled eNOS, activation of xanthine oxidase and NADH/NADPH oxidase pathways by ROS and RNS contained in cigarette smoke and generated by inflammatory cells makes endothelial cells an important source of further ROS production [18].
Oxidative stress contributes to endothelial dysfunction also by inducing increases in lipid peroxidation [108,109] and AGE-RAGE activation [110]. In addition, decreased antioxidant capacity in the lung tissue (Nrf2 downregulation in epithelial cells [111,112]), the direct toxic effect of cigarette smoke exposure (by stimulating endothelial cell apoptosis) [113,114], and endothelial cell senescence induced by oxidative stress and smoking also may play a role in the pathogenesis of endothelial dysfunction [96].
As a result of endothelial derangement, proliferative and fibrotic processes dominate vascular homeostasis and vascular contractility increases. Endothelial injury has been reported to affect the etiology of various COPD-related vascular disorders, such as pulmonary arterial hypertension, hypertension, renal dysfunction, and venous thromboembolism [96,99]. The damaged endothelium is a critical factor in developing CVD complications and promotes the progression of emphysema. Several human and animal model studies provided evidence for a link between endothelial damage and emphysema [96,115]. Moreover, a model study with rats showed that treatment with vascular endothelial growth factor (VEGF—a trophic factor promoting endothelial cell survival) inhibitors initiated emphysema development without inflammation [116]. However, stimulators of soluble guanylate cyclase (a target enzyme of NO in smooth muscle cells) in a rodent model exposed to cigarette smoke were beneficial for pulmonary vascular remodeling and prevented emphysema progression [117]. Another potential link between emphysema and endothelial dysfunction in COPD might be the aberrant purinergic signaling and elevated pulmonary ATP levels with plausible interactions with ongoing oxidative stress [118,119,120].
Accelerated aging. Oxidative stress contributes to the development of COPD and related CV disorders by weakening and disrupting certain anti-aging processes, such as sirtuin activity and balance of the Klotho protein—fibroblast growth factor (FGF) 23 system, and also by aggravating processes that stimulate cellular senescence, such as telomere shortening and adverse epigenetic modifications. Sirtuins (SIRTs) are enzymes of the silent information regulator 2 (Sir2) class III deacetylase family. As their activity is regulated by NAD+, they are highly redox-sensitive. They participate in biological processes, which include cellular response mechanisms against a wide range of stressors. SIRTs modulate transcription, cell growth, oxidative stress-tolerance and metabolism and thereby help to alleviate aging-related mitochondrial dysfunction, genomic instability, and inflammation [121,122]. Among the seven mammalian sirtuins, SIRT1 and SIRT6 have been implicated to have protective effects against COPD. SIRT1 and SIRT6 are downregulated by cigarette smoke exposure and in the lungs of COPD patients [123,124,125]. SIRT1 is known to deactivate redox-sensitive transcription factor NF-κB by deacetylating its RelA/p65 subunit [126]. NF-κB stimulates the transcription of proinflammatory genes (e.g., IL-8, IL6, TNFα) [126]. Therefore, reduced levels of SIRT1 enhance the proinflammatory effects of oxidative stress and contribute to the pathogenesis of COPD. Lower SIRT1 activity may participate in COPD development by promoting senescence in different cell types of the lung tissue, as SIRT1 is also known to deacetylate p53 and negatively regulate the forkhead box O3 (FOXO3) pathway that is involved in the transcription of genes responsible for cellular senescence [127,128]. SIRT6 has also been shown to have effects which may be protective against COPD by antagonizing the senescence of human bronchial epithelial cells [129].
Impaired sirtuin activity also plays a crucial role in aging-associated vascular remodeling [39,55,56,130]. SIRT1 is highly expressed in endothelial cells, and it directly activates eNOS in the cytoplasm and increases eNOS expression. By inhibiting p53, forkhead box O1 (FOXO1) [131], and plasminogen activator inhibitor-1 pathways [132], it protects against endothelial senescence. Acting in vascular smooth muscle cells inhibits migration and proliferation, tunica media remodeling, and protects against DNA damage, neointima formation and atherosclerosis [133,134,135]. SIRT6 inhibits proprotein convertase subtilisin/kexin type 9 (PCSK9) and insulin-like growth factor (IGF)-Akt signaling in the vasculature, thereby reducing senescence and protecting against vascular aging [39,130].
The FGF23—α Klotho (KL) system has emerged as an endocrine axis essential for maintaining phosphate homeostasis. FGF23 is a bone-derived hormone, and its binding to its FGF receptor in the kidney and parathyroid gland requires KL as an obligate co-receptor [136]. KL is a transmembrane protein, but it also occurs in a soluble form in the blood produced by either alternative splicing or proteolytic cleavage [137,138]. KL has been attributed to anti-inflammatory and anti-senescence effects [136]. In addition, the Klotho protein protects cells and tissues from oxidative stress. The mechanisms include activating FOXO transcription factors and the NF-κB and Nrf2 pathways [139,140,141]. Transgenic mice are deficient in Klotho exhibit phosphate retention, accelerated aging, and lung emphysema [142]. Therefore, it has been postulated that Klotho is protective against COPD development. Despite this, studies investigating the association between KL and COPD are scarce in the literature, and the findings are controversial. Gao et al. found that KL expression was decreased in the lungs of smokers and further reduced in patients with COPD [139].
Moreover, they found that KL depletion increased cell sensitivity to cigarette smoke-induced inflammation and oxidative stress-induced cell damage in a mouse model. In the blood, a slightly lower KL level was measured by Patel et al. in COPD patients [143], while Pako et al. detected decreased KL levels in OSA [144]. However, other studies found that plasma KL levels did not correlate with clinical parameters in stable COPD patients [145], and their levels were not affected by pulmonary rehabilitation [68].
The FGF 23—KL axis has also been shown to be associated with cardiovascular health [146]. Several studies have found an inverse relationship between KL concentrations and the likelihood of having CVD [147,148]. Arking et al. identified a KL gene variant (KL-VS) which conferred cardioprotective advantages on heterozygous subjects concerning high-density lipoprotein cholesterol levels, systolic blood pressure, stroke, and longevity. Interestingly, they found that homozygosity for KL-VS is disadvantageous compared to wild-type genetic background [149]. Using mouse models, Hu et al. proved an association between KL levels and vascular calcification. They found that overexpression was protective, whereas KL deficiency promoted calcium deposition in the vessel wall [150]. KL deficiency was also found to participate in the development of salt-sensitive hypertension through vascular non-canonical Wnt5a/RhoA activation [151]. The significant cardioprotective effect of KL may be the suppression of inflammation and oxidative stress in vascular smooth muscle (VSMC) and endothelial cells. KL inhibits phosphate entry in VSMCs through the PiT1 carrier, which is known to stimulate the production of ROS. In addition, KL inhibits sodium overload-induced ROS production in endothelial cells [152].
Telomere shortening and epigenetic modifications of the DNA are hallmarks of aging, and both are accelerated by oxidative stress [153,154]. Oxidative stress and inflammation influence the cell’s epigenetic machinery, from DNA and histones to histone modifiers resulting in adverse modifications, such as hydroxylation of pyrimidines and impaired DNA demethylation [154]. Enhanced tissue and leukocyte telomere shortening and various epigenetic modifications be associated with the development of COPD and vascular remodeling [56,155,156,157].
Alfa-1 antitrypsin deficiency. Alfa-1 antitrypsin deficiency (A1ATD) is a hereditary disease that is the consequence of the genetic mutations of the SERPINA1 gene and predisposes homozygous and heterozygous subjects to the development of emphysema and liver disease. Although it is considered a rare disease, several authors have proposed that it might not be rare but severely underdiagnosed [158]. The genetic disorder leads to the accumulation of misfolded proteins in α1-antitrypsin producing cells, mainly in hepatocytes and, to a lesser extent, in lung epithelial cells. The main function of alfa-1 antitrypsin is to antagonize neutrophil elastase activity, but it also operates as an acute phase protein with anti-inflammatory effects. In its absence, the degradation of elastin fibers and extracellular tissue matrix in the lung overactivates upon activation of neutrophil cells and promotes the development and progression of emphysema [159,160]. In addition, the additive effect of cigarette smoke exposure multiplies the risk of emphysema.
The effect of A1ATD on the cardiovascular system is also manifold but controversial. The degradation of elastic elements in the vessel wall impairs its physiological distensibility. As a result, arterial compliance increases and the Windkessel function gets compromised. A recent study of 91,353 subjects has shown that this decreases systolic and diastolic blood pressure values [161]. Losing elastic properties can also lead to aorta distension and aneurysms [162,163]. In addition, the absence or lower level of alfa-1 antitrypsin is associated with inflammatory vascular diseases such as fibromuscular dysplasia and ANCA-positive vasculitis [164,165].
Several studies in animal models and humans indicate that AA1TD is associated with enhanced oxidative stress and decreased antioxidant defense even at early stages of disease progression [166,167,168,169].
3.2. COPD and Pulmonary Arterial Hypertension (PAH)
Pulmonary arterial hypertension and consequential right heart failure are common cardiovascular complications in COPD. The prevalence of PAH is 5% in moderate (GOLD stage II), 27% in severe (GOLD stage III), and 53% in very severe (GOLD stage IV) COPD [170]. As the diagnostic criterion for PAH is mean pulmonary arterial pressure ≥25 mmHg at rest, these statistics reflect an advanced stage of pulmonary circulation abnormality. Several studies on animal models as well as human studies, however, have shown that pulmonary vascular changes occur in mild COP, or even before the development of lung emphysema [171,172,173]. Moreover, right ventricular dysfunction and remodeling have been observed in COPD patients without PAH [174,175].
Vascular changes in COPD are characterized by remodeling the pulmonary vessels and endothelial dysfunction [176,177]. In addition, vascular derangement in emphysema may also contribute to the pathogenesis of PAH [176]. Pulmonary arterial remodeling affects mainly the intimal layer. Intimal hyperplasia develops due to the proliferation of poorly differentiated smooth muscle cells and extracellular matrix deposition [9,178]. In addition, pulmonary arterial stiffening increases right ventricular afterload and the pulsatile load on the pulmonary microcirculation [177]. The latter induces endothelial dysfunction and inflammation in the distal pulmonary vasculature [179,180].
Pulmonary endothelial dysfunction is an early injury in PAH development and has similar mechanisms and consequences as in systemic circulation (see above). It is characterized by reduced expression of eNOS, diminished production of NO and prostacyclin, increased secretion of endothelin, and expression of TGFβ receptors. These alterations promote vasoconstriction and contribute to pulmonary vascular remodeling.
Several underlying factors have been identified that precipitate vascular changes in COPD-related PAH, such as hypoxia, activation of sympathetic nerves, cigarette smoking, biomass smoke exposure, and epithelial cell injury [176]. Hypoxia is a well-established cause of pulmonary vascular remodeling and PAH. However, its role in COPD-related PAH is debated, as vascular abnormalities are present even in patients with mild COPD and without hypoxemia [176]. Acting on smooth muscle cells, endothelial cells and fibroblasts, hypoxia can induce cell proliferation by inhibiting antimitogenic and stimulating mitogenic stimuli and increasing the production of inflammatory mediators. [181] A key factor linking hypoxia to the activation of these pathways and oxidative stress is the hypoxia-inducible factor 1 (HIF-1) [182], the serum level of which is elevated in COPD patients [183,184]. COPD is also associated with increased sympathetic tone and activation of the renin-angiotensin-aldosterone system. This neurohormonal imbalance favors increased oxidative stress and activation of inflammatory and fibrogenic responses, which lead to adverse remodeling in the heart and vasculature [185]. Cigarette smoke and biomass smoke stimulate vascular remodeling by direct toxic effects on the endothelial cells by enhancing gene expression and release of inflammatory cytokines locally and systemically [186,187], downregulating eNOS [188] and inducing oxidative and nitrative stress [2,96,176]. In addition, injured bronchial epithelial cells in COPD are considered to orchestrate many immune and inflammatory processes in COPD pathogenesis, also contributing to vascular remodeling [189,190].
3.3. COPD and Accelerated Atherosclerosis
Atherosclerosis is the leading cause of stroke, coronary heart disease and peripheral arterial disease, which are responsible for a high percentage of mortality in COPD patients. COPD and atherosclerosis share several common risk factors and underlying mechanisms, such as cigarette smoking, sedentary lifestyle, oxidative stress, endothelial dysfunction, high blood pressure and adverse platelet activation [4,11]. In addition, several studies indicate that the severity of COPD and airflow limitation correlate with the severity of atherosclerotic disease [191,192].
Indeed, several pathophysiological mechanisms observed in COPD participate in the progression of atherosclerosis [10]. The impaired endothelial function has relevance at the early stages of plaque formation, as the inflammatory profile of the injured endothelium enhances the secretion of adhesion molecules, increases the permeability of the endothelial barrier, and aids the recruitment of inflammatory immune cells to the lesion [193]. In addition, systemic inflammation and increased oxidative stress can fuel plaque development by aggravating local inflammatory processes in vulnerable sites of the arterial tree and promoting the oxidization of low-density lipoprotein particles [10,194,195,196].
3.4. COPD and Cardiac Diseases
COPD often associates with various abnormalities of cardiac function that lead to heart failure (HF). The prevalence of HF in COPD ranges from 7–42% [8]. The effect of PAH on right ventricular function is well documented. The increased afterload of the right heart initiates maladaptive remodeling processes, and right heart failure develops [177,197]. The early signs of right ventricular dysfunction begin to develop at the early stages of PAH progression, even when pulmonary arterial pressures are in the normal range, but signs of pulmonary vascular derangement are already present [174,175,198]. COPD exacerbations impose an additional load on the heart due to hypoxic pulmonary vasoconstriction and hyperinflation of the lung [199,200]. Maladaptive alteration in the right heart also led to dilatation and electrical remodeling of the right atrium and ventricle, which increases the risk of cardiac arrhythmias [197,201].
Abnormal lung function in COPD also affects the function of the left heart. Emphysema-related hyperinflation of the lung and depressed right ventricular function impairs left ventricular filling and reduces cardiac output [197,202]. Hypoxemia observed in more severe COPD and during exacerbations can increase the risk of cardiac ischemia, and due to altered repolarization, the risk of ventricular arrhythmias and sudden cardiac death [199,201,203]. In addition, cardiac ischemia exposes the heart to oxidative stress that causes derangements in cardiomyocyte homeostasis, such as disturbed calcium handling and lipid signaling [204,205,206]. Cardiac dysfunction further aggravates tissue hypoxia that perpetuates systemic oxidative stress.
COPD-related systemic inflammation, oxidative stress and accelerated cardiovascular aging can directly act on the ventricular muscle and activate signaling pathways leading to maladaptive remodeling and HF [197,207]. In addition, arterial stiffness and hypertension developing in COPD increases left ventricular load and impairs ventriculo-arterial coupling, which also contributes to the development of HF [208]. Accelerated atherosclerosis and endothelial dysfunction increase the occurrence of coronary heart disease (CHD), too. Indeed, approximately 15% of COPD patients also suffer from concomitant CHD [209,210].
4. Biomarkers of Oxidative Stress in COPD and Cardiovascular Diseases
4.1. Biological Biomarkers
A multitude of studies is available in the literature that addressed characterize systemic and local oxidative stress in association with COPD and various forms of cardiovascular diseases [1,2,211,212,213]. In addition, several biomarkers of oxidative stress are available in the blood, tissues, and other biological samples, such as exhaled breath condensate and sputum [1,211,214]. However, the direct measurement of ROS production is challenging because of the short half-life of reactive oxidants. Therefore, it is more feasible to assess oxidative stress by measuring oxidation target products, such as lipid peroxidation end products and oxidized proteins, as well as the activities of enzymes of the oxidant and antioxidant pathways [215].
Regarding COPD, circulating biomarkers have been widely assessed to correlate with disease and disease severity. These studies relate the systemic manifestation of oxidative stress to COPD rather than local oxidative stress of the lungs. However, samples obtained directly from the respiratory system, such as exhaled breath condensate and sputum, are more informative about the local oxidative burden [1,211]. Table 1 summarizes the biological samples and biomarkers used for evaluating oxidative stress in COPD. Among these, the measurement of a lipid peroxidation product, malondialdehyde (MDA) level, and its reaction with thiobutyric acid to obtain thiobutyric acid reactive substances (TBARS) is the most frequently applied approach to assess oxidative damage. The elevation of MDA in COPD is the most consistent finding among studies which relate oxidative stress to COPD [76,83,86,89,90,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230]. Measurement of protein and non-protein thiols in various biological samples is also an comprehensive tool to evaluate ROS activity. Thiols undergo oxidation in the presence of ROS, constituting an essential component of the intra- and extracellular antioxidant defense system. Therefore, the level and ratio of reduced and oxidized thiols can characterize the oxidative state of the body. In COPD, glutathione (GSH) and its oxidized products are widely used markers of oxidative stress (Table 1) [1,213]. Assessment of antioxidant pathways in COPD has been undertaken by measuring the total antioxidant capacity and enzymatic antioxidant activity of SOD, CAT and GPx. Most studies found decreased antioxidant activity, especially when circulating markers were measured [83,86,88,89,90,216,228,230,231,232,233]. However, higher CAT and SOD activity in sputum was found in exacerbated COPD, most probably due to compensatory response during infectious inflammation [49]. In addition, protein oxidation products, lipid peroxidation products of membrane lipids and phospholipids (hexanal, heptanal, nonanal, acrolein, 8-isoprostane), as well as markers of inflammatory processes induced by oxidative stress, such as leukotrienes can also be used to characterize oxidative burden in COPD (for selected studies see Table 1) [1,213].
Oxidative stress in cardiovascular diseases can also be assessed by measurement of circulating blood biomarkers similar to COPD. In addition, the measurement of fluorescent oxidation products (FlOPs), as a stable biomarker of global oxidative damage reflecting oxidation of lipids, proteins, DNA, and carbohydrates, has been used to assess oxidative stress in various CVDs [234,235,236,237], and may also be of growing interest in respiratory disorders [238,239]. However, the evaluation of local oxidative stress in the heart and vasculature has limited relevance due to the limited availability of tissue samples. The wide literature on oxidative stress in cardiovascular diseases (including reports on human and animal studies) also shows increased oxidant and decreased antioxidant activity in various disease conditions, including hypertension, atherosclerosis, vascular aging, ischemic heart, and cerebral diseases [39,55,57,74,75,77,212]. Interestingly, in atherosclerotic conditions, several studies have shown increased antioxidant activity using blood markers, which may show the compensatory upregulation of antioxidant defense mechanisms in this condition [240,241,242]. The findings of selected representative studies are summarized in Table 2.
4.2. Heart Rate Variability—A Potential Non-Conventional Biomarker of Oxidative Stress in COPD and CVD
Impaired autonomic control is a shared characteristic of COPD and cardiovascular diseases and is also associated with inflammation and oxidative stress [243,244,245]. The strong association between bronchial and cardiac vagal tone is also established in the literature [246]. Autonomic dysfunction can be detected by alterations in heart rate variability (HRV). HRV describes the fluctuation in the time interval between heartbeats brought about by oscillating regulatory mechanisms which affect heart rate mainly by modifying the balance of sympathetic and parasympathetic effects on the heart. Numerous parameters—time-domain, frequency-domain and non-linear HRV indices, can be used to characterize the HRV complexly. These parameters are calculated by defining interbeat intervals from continuous ECG recordings obtained over a specified period (2 min to 24 h). High HRV generally represents better body resilience to physiological and pathological challenges and is associated with better health and cardiovascular status [247,248].
In COPD, decreased HRV has been detected in several studies. Moreover, depressed HRV is related to the risk of exacerbations [249,250,251]. Although cardiovascular diseases are also associated with decreased HRV, alterations of certain HRV indices have been proposed to be applicable for assessing prognosis in post-infarction patients and in patients with congestive heart failure [252,253,254,255]. Not surprisingly, several studies also found a correlation between HRV depression and oxidative stress [256,257,258]. These observations suggest that HRV parameters could be used as a non-invasive biomarker of oxidative stress in COPD and CVDs. However, this requires further extensive research. The rationale for the idea is that parameters similar to HRV indices can be obtained from peripheral arterial pulse wave recordings, which are extensively available for analysis, as a wide variety of smart wearable accessories are equipped with photoplethysmographic detectors capable of capturing pulse wave signals [259].

Table 1.
Biomarkers of systemic and local oxidative stress in COPD. Representative studies reporting the association of oxidative stress biomarkers in various biological samples with COPD. Abbreviations: GSH—reduced glutathione, SOD—superoxide dismutase, CAT—catalase, GPx—glutathion peroxidase, MDA—malondialdehyde, AOPP—advanced oxidation protein products, LTB4—leukotriene B4. ↓: decrease in level/activity; →: unchanged level; ↑: increased level/activity.
Table 1.
Biomarkers of systemic and local oxidative stress in COPD. Representative studies reporting the association of oxidative stress biomarkers in various biological samples with COPD. Abbreviations: GSH—reduced glutathione, SOD—superoxide dismutase, CAT—catalase, GPx—glutathion peroxidase, MDA—malondialdehyde, AOPP—advanced oxidation protein products, LTB4—leukotriene B4. ↓: decrease in level/activity; →: unchanged level; ↑: increased level/activity.
| Sample | Biomarker | Finding | Reference |
|---|---|---|---|
| Blood (systemic oxidative stress) | |||
| erythrocytes | reduced GSH | ↓ in COPD patients (n = 236) vs controls (n = 150) and correlates with disease severity—all patients are smokers or ex-smokers | [216] |
| ↓ in stable COPD patients (n = 41) vs. controls (n = 30); and further decreased in exacerbated COPD (n = 21)—varying smoking status | [218] | ||
| SOD activity | ↓ in COPD patients (n = 140) vs. healthy controls (n = 75)—varying smoking status | [83] | |
| ↓ in COPD patients (n = 234) vs. healthy controls (n = 182)—varying smoking status | [233] | ||
| ↓ in COPD patients (n = 82) vs. non-smoking healthy controls (n = 22) | [86] | ||
| ↓ in stable COPD patients (n = 21) vs. non-smoking healthy controls (n = 24) | [88] | ||
| CAT activity | ↓ in COPD patients (n = 236) vs controls (n = 150) and correlates with disease severity—all patients are smokers or ex-smokers | [216] | |
| ↓ in COPD patients (n = 140) vs. healthy controls (n = 75)—varying smoking status | [83] | ||
| → comparable in COPD patients (n = 82) and non-smoking healthy controls (n = 22) | [86] | ||
| GPx activity | ↓ in COPD patients (n = 236) vs. controls (n = 150)—all patients are smokers or ex-smokers | [216] | |
| ↓ in COPD patients (n = 140) vs. healthy controls (n = 75)—varying smoking status | [83] | ||
| ↓ in COPD patients (n = 82) vs. non-smoking healthy controls (n = 22) | [86] | ||
| ↓ in COPD patients (n = 20) vs. healthy controls (n = 50)—varying smoking status | [232] | ||
| plasma | MDA | ↑ in COPD patients (n = 236) vs. controls (n = 150)—and correlates with disease severity. All patients are smokers or ex-smokers | [216] |
| ↑ in stable COPD patients (n = 41) vs. controls (n = 30); and further decreased in exacerbated COPD (n = 21)—varying smoking status | [218] | ||
| ↑ in COPD patients (n = 140) vs. healthy controls (n = 75)—varying smoking status | [83] | ||
| ↑ in COPD patients (n = 82) vs. non-smoking healthy controls (n = 22) | [86] | ||
| ↑ in COPD patients (n = 20) vs. healthy controls (n = 50)—varying smoking status | [232] | ||
| ↑ in COPD patients (n = 100) vs. controls (n = 100)—varying smoking status | [221] | ||
| ↑ in COPD patients (n = 100) vs. controls (n = 100)—varying smoking status | [222] | ||
| ↑ in healthy smokers (n = 30) and in patients with stable (n = 7) and exacerbated COPD (n = 31) than in healthy non-smokers (n = 30) | [223] | ||
| ↑ in COPD patients (n = 106) vs. controls (n = 45)—varying smoking status | [225] | ||
| ↑ in COPD patients exposed to wood smoke (n = 30) and tobacco smoking (n = 30) vs. healthy controls (n = 30) | [226] | ||
| ↑ in COPD patients (n = 815) vs. controls (n = 530)—varying smoking status—meta-analysis | [227] | ||
| ↑ in severe COPD patients (n = 74) vs. controls (n = 41)—varying smoking status | [228] | ||
| ↑ in COPD patients (n = 26) vs. controls (n = 28) –smoking status n.a. | [229] | ||
| ↑ in smoker COPD patients (n = 202) vs. smoker controls without COPD (n = 136) | [89,230] | ||
| ↑ in patients with exacerbated (n = 43) and stable (n = 35), and in healthy smokers (n = 14) vs. healthy non-smokers (n = 14) | [90] | ||
| → comparable in ex-smoker COPD patients (n = 11) and non-smoking healthy controls (n = 12), exercise induces increase only in COPD | [260] | ||
| AOPP | ↑ in severe COPD patients (n = 74) vs. controls (n = 41)—varying smoking status | [228] | |
| reduced GSH | ↓ in COPD patients (n = 20) vs. healthy controls (n = 50)—varying smoking status | [232] | |
| ↓ in chronic smokers with stable COPD (n = 20) and without COPD (n = 20) vs. healthy non-smokers (n = 20) | [261] | ||
| ↓ in smoker COPD patients (n = 202) vs. smoker controls without COPD (n = 136) | [89,230] | ||
| ↓ in patients with exacerbated (n = 43) and stable (n = 35), and in healthy smokers (n = 14) vs. healthy non-smokers (n = 14) | [90] | ||
| SOD activity | ↓ in severe COPD patients (n = 74) vs. controls (n = 41)—varying smoking status | [228] | |
| ↓ in patients with exacerbated (n = 43) and stable (n = 35), and in healthy smokers (n = 14) vs. healthy non-smokers (n = 14) | [90] | ||
| ↓ in patients with stable COPD (n = 96) vs. controls without COPD (n = 96)—varying smoking status | [231] | ||
| CAT activity | ↓ in smoker COPD patients (n = 202) vs. smoker controls without COPD (n = 136) | [89,230] | |
| → comparable in patients with stable COPD (n = 96) and without COPD (n = 96)—varying smoking status | [231] | ||
| GPx activity | ↓ in smoker COPD patients (n = 202) vs. smoker controls without COPD (n = 136) | [89,230] | |
| ↓ in patients with exacerbated (n = 43) and stable (n = 35), and in healthy smokers (n = 14) vs. healthy non-smokers (n = 14) | [90] | ||
| ↓ in COPD patients (n = 82) vs. non-smoking healthy controls (n = 22) | [86] | ||
| whole blood | total glutathione | ↑ in COPD patients (n = 140) vs. healthy controls (n = 75)—varying smoking status | [83,86] |
| ↑ in COPD patients (n = 82) vs. non-smoking healthy controls (n = 22) | [86] | ||
| GPx activity | ↓ in stable COPD patients (n = 21) vs. non-smoking healthy controls (n = 24) | [88] | |
| Exhaled air (systemic/local oxidative stress) | |||
| CO | ↑ in ex-smokers with COPD (n = 15) and in smokers with COPD (n = 15) vs. non-smoking healthy controls (n = 10) | [262] | |
| ethane | ↑ COPD (n = 12) vs. healthy (n = 14) (all ex-smokers) | [263] | |
| Exhaled breath condensate (systemic/local oxidative stress) | |||
| hexanal, heptanal, nonanal | ↑ in patients with stable COPD (n = 20) vs. non-smoking healthy subjects (n = 20), but not vs. smoking controls (n = 12) | [220] | |
| ↑ in patients with COPD (n = 11; smokers and ex-smokers) vs. non-smoking controls (n = 9) | [219] | ||
| MDA | ↑ in patients with stable COPD (n = 20) vs. non-smoking healthy subjects (n = 20), and also vs. smoking controls (n = 12) | [220] | |
| ↑ in patients with COPD (n = 11; smokers and ex-smokers) vs. non-smoking controls (n = 9) | [219] | ||
| ↑ in patients with COPD (n = 73) vs. healthy non-smokers (n = 14); an inverse correlation between MDA concentrations and FEV1(%) was found | [217] | ||
| → comparable values in patients with exacerbated COPD (n = 34), stable COPD (n = 21) and healthy controls (n = 20)—all ex-smokers | [76] | ||
| ↑ in patients with COPD (n = 53) vs. healthy (n = 10); MDA correlates with disease severity—all patients were retired coal miners with varying smoking status | [224] | ||
| H2O2 | ↑ in patients with COPD (n = 30) vs. healthy (n = 10) and increases with disease severity—all smokers | [264] | |
| ↑ in patients with stable COPD (n = 12) and with exacerbated COPD (n = 19) (smokers and ex-smokers) vs. healthy never-smokers (n = 10) | [265] | ||
| pH | ↓ in COPD exacerbation vs. recovery (n = 29)—current and ex-smokers | [266] | |
| Condensate pH remained unchanged during COPD exacerbation, both in smokers (n = 21) and ex-smokers (n = 17) | [267] | ||
| nitrotyrosine | ↑ in patients with COPD (n = 53) vs. healthy (n = 10)—patients were retired coalminers with varying smoking status | [224] | |
| 8-isoprotane | ↑ in exacerbating COPD patients (n = 21) and fell after treatment with antibiotics | [268] | |
| ↑ in patients with COPD (n = 30) vs. healthy (n = 10)—all smokers | [264] | ||
| LTB4 | ↑ in exacerbating COPD patients (n = 21) and fell after treatment with antibiotics | [268] | |
| ↑ in steroid naïve (n = 20) and steroid treated patients with COPD (n = 25) compared to control subjects (n = 15)—all ex-smokers | [269] | ||
| Sputum (local oxidative stress) | |||
| hexanal, heptanal, nonanal | ↑ in patients with COPD (n = 11; smokers and ex-smokers) vs. non-smoking controls (n = 9) | [219] | |
| MDA | ↑ in patients with stable COPD (n = 21) vs. healthy controls (n = 20); increased further iv exacerbated COPD patients and decreased during recovery (n = 34)—all ex-smokers | [76] | |
| ↑ in patients with COPD (n = 11; smokers and ex-smokers) vs. non-smoking controls (n = 9) | [219] | ||
| SOD | SOD activity was comparable between stable COPD patients and (n = 24) and healthy controls (n = 23); but it increased in COPD exacerbation (n = 36)—all patients were ex-smokers | [49] | |
| CAT | CAT activity was comparable between stable COPD patients and (n = 24) and healthy controls (n = 23); but it increased in COPD exacerbation (n = 36)—all patients were ex-smokers | [49] | |

Table 2.
Circulating biomarkers in cardiovascular diseases. Selected studies show the association between blood biomarkers of oxidative stress and various cardiovascular disease conditions. Abbreviations: CV—cardiovascular, GSH—reduced glutathione, CAD—coronary artery disease, SOD—superoxide dismutase, BMI—body mass index, IHD—ischemic heart disease, CAT—catalase, GPx—glutathione peroxidase, ox-LDL—oxidized low-density lipoprotein, TIA—transient ischemic attack, FlOPs—fluorescent oxidation products, CHD—coronary heart disease. ↓: decrease in level/activity; ↑: increased level/activity.
Table 2.
Circulating biomarkers in cardiovascular diseases. Selected studies show the association between blood biomarkers of oxidative stress and various cardiovascular disease conditions. Abbreviations: CV—cardiovascular, GSH—reduced glutathione, CAD—coronary artery disease, SOD—superoxide dismutase, BMI—body mass index, IHD—ischemic heart disease, CAT—catalase, GPx—glutathione peroxidase, ox-LDL—oxidized low-density lipoprotein, TIA—transient ischemic attack, FlOPs—fluorescent oxidation products, CHD—coronary heart disease. ↓: decrease in level/activity; ↑: increased level/activity.
| Biomarker | CV Disease | Finding | Reference |
|---|---|---|---|
| Reduced GSH | Atherosclerosis, arterial aging | lower GSH is a predictor of intima/media thickness | [270,271] |
| Hypertension | ↑ GSH increased glutathione-related antioxidant defense in treated hypertensives | [272] | |
| CAD | ↓ in angiographically proven CAD | [240] | |
| SOD activity | Arterial aging | negatively correlated with systolic and diastolic blood pressure, low serum SOD activity is an independent predictor of carotid intima/media thickening | [273] |
| Hypertension | ↓ in hypertensive patients regardless of BMI | [274] | |
| IHD, CAD | ↑ in angiographically proven CAD and IHD | [240,241,242] | |
| CAT activity | Hypertension | ↓ in hypertensive patients regardless of BMI | [274] |
| IHD | ↑ in men with IHD | [242] | |
| GPx activity | Atherosclerosis | ↓ in prevalent atherosclerosis and lower values are associated with an increased risk of future cardiovascular events | [275] |
| Hypertension | lower levels associated with high blood pressure in black women | [276] | |
| IHD | ↓ in men with IHD | [242] | |
| any cardiovascular events | lower GPx is associated with a higher risk of CV events | [277] | |
| MDA | Atherosclerosis, arterial aging | ↑ with carotid intima/media thickening | [271] |
| Hypertension | ↑ in untreated hypertension | [278,279] | |
| CAD | ↑ in angiographically proven CAD | [240] | |
| ox-LDL | Atherosclerosis, arterial aging | ↑ associated with carotid intima/media thickening, and higher arterial stiffness | [271,280] |
| Hypertension | ↑ in hypertensive men and prehypertensive subjects of both genders | [281,282] | |
| CAD | ↑ ox-LDL associated with CAD, with the severity of CAD and was found to be prognostic for CAD events | [283,284,285,286] | |
| Stroke | higher values are associated with cerebrovascular events and increased risk of recurrent stroke in TIA patients | [287,288,289] | |
| FlOPS | CHD | an independent predictor of CHD events in men | [236] |
| higher levels associated with the risk of CHD in women | [235] |
5. Conclusions and Future Perspectives
The pathogenesis of COPD and its most frequent cardiovascular comorbidities is linked via shared genetic, environmental and lifestyle risk factors and numerous pathophysiological processes, including systemic inflammation, endothelial dysfunction, and accelerated aging. Many of these are strongly related to oxidative stress in a complex manner. On the one hand, they are activated by exogenous and endogenous oxidative radicals. On the other, they impose a further oxidative burden on the body by inducing ROS production and weakening antioxidant defense mechanisms. As oxidative stress is a common mechanism driving and perpetuating COPD and coexisting CVD progression that can be monitored successfully by several biological and other potential physiological biomarkers, therapeutic approaches to restore oxidative balance have been the focus of extensive research in the last few decades. Strategies to influence oxidative balance with dietary supplementation and drugs targeted at different oxidative stress pathways have been extensively reviewed recently [2,290]. Though there are promising observations with dietary supplementation of antioxidants such as vitamin C, vitamin E, resveratrol and flavonoids and with the application of thiol-based antioxidants, such as N-acetylcysteine and carbocysteine, the exact place of these treatments in COPD and CVD prevention and therapy is still not established [2,291]. There are also attempts to normalize oxidative balance with antioxidant mimetics (SOD, catalase, GPx), NOX and MPO inhibitors, and Nrf2 activators, but their application is in the phase of preclinical and clinical studies [2]. The antioxidant capacity of the body can also be influenced positively by supporting anti-aging processes. Indeed, activation of SIRTs with NAD+ precursor supplementation has been shown to benefit the respiratory and cardiovascular systems [292,293,294,295,296]. Also, there is evidence to show the potential benefit of Klotho treatment/supplementation [297,298]. For completeness, physical activity and pulmonary rehabilitation should not be excluded from the possible therapeutic approaches to restore redox status. Exercise enhances antioxidant response, decreases age-related oxidative stress, improves endothelial function, and reduces inflammatory and oxidative signaling, thereby protecting cardiovascular health [299,300]. Pulmonary rehabilitation has also benefited redox responses in COPD patients [301,302,303]. As restoration of oxidative balance is a preventive/therapeutic approach which could favorably influence the underlying processes driving COPD and CVD development, studies to understand better signaling pathways that orchestrate the derangement of oxidative-antioxidative balance are essential to establish antioxidant therapy in COPD patients.
Author Contributions
Conceptualization, I.H. and Z.M.; writing—original draft preparation, I.H. and Z.M.; writing—review and editing, I.H. and Z.M.; supervision, I.H.; funding acquisition, I.H. All authors have read and agreed to the published version of the manuscript.
Funding
This review is related to original research funded by the Hungarian National Research, Development and Innovation Office, Budapest, Hungary grant number OTKA 128666 and OTKA 124343 for the National Koranyi Institute for Pulmonology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Antus, B.; Kardos, Z. Oxidative Stress in COPD: Molecular Background and Clinical Monitoring. Curr. Med. Chem. 2015, 22, 627–650. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef]
- Divo, M.; Cote, C.; de Torres, J.P.; Casanova, C.; Marin, J.M.; Pinto-Plata, V.; Zulueta, J.; Cabrera, C.; Zagaceta, J.; Hunninghake, G.; et al. Comorbidities and Risk of Mortality in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2012, 186, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Morgan, A.D.; Zakeri, R.; Quint, J.K. Defining the relationship between COPD and CVD: What are the implications for clinical practice? Ther. Adv. Respir. Dis. 2018, 12, 175346581775052. [Google Scholar] [CrossRef] [PubMed]
- Axson, E.L.; Ragutheeswaran, K.; Sundaram, V.; Bloom, C.I.; Bottle, A.; Cowie, M.R.; Quint, J.K. Hospitalisation and mortality in patients with comorbid COPD and heart failure: A systematic review and meta-analysis. Respir. Res. 2020, 21, 54. [Google Scholar] [CrossRef]
- Cavaillès, A.; Brinchault-Rabin, G.; Dixmier, A.; Goupil, F.; Gut-Gobert, C.; Marchand-Adam, S.; Meurice, J.-C.; Morel, H.; Person-Tacnet, C.; Leroyer, C.; et al. Comorbidities of COPD. Eur. Respir. Rev. 2013, 22, 454–475. [Google Scholar] [CrossRef]
- Austin, V.; Crack, P.; Bozinovski, S.; Miller, A.A.; Vlahos, R. COPD and stroke: Are systemic inflammation and oxidative stress the missing links? Clin. Sci. 2016, 130, 1039–1050. [Google Scholar] [CrossRef]
- Balbirsingh, V.; Mohammed, A.S.; Turner, A.M.; Newnham, M. Cardiovascular disease in chronic obstructive pulmonary disease: A narrative review. Thorax 2022, 77, 939–945. [Google Scholar] [CrossRef]
- Barberà, J.A. Mechanisms of Development of Chronic Obstructive Pulmonary Disease-Associated Pulmonary Hypertension. Pulm. Circ. 2013, 3, 160–164. [Google Scholar] [CrossRef]
- Brassington, K.; Selemidis, S.; Bozinovski, S.; Vlahos, R. Chronic obstructive pulmonary disease and atherosclerosis: Common mechanisms and novel therapeutics. Clin. Sci. 2022, 136, 405–423. [Google Scholar] [CrossRef]
- Maclay, J.D.; MacNee, W. Cardiovascular Disease in COPD: Mechanisms. Chest 2013, 143, 798–807. [Google Scholar] [CrossRef] [PubMed]
- Panday, A.; Sahoo, M.K.; Osorio, D.; Batra, S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015, 12, 5–23. [Google Scholar] [CrossRef]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef]
- Azadmanesh, J.; Borgstahl, G.E.O. A Review of the Catalytic Mechanism of Human Manganese Superoxide Dismutase. Antioxidants 2018, 7, 25. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Biswas, S.; Mano, J. Lipid Peroxide-Derived Reactive Carbonyl Species as Mediators of Oxidative Stress and Signaling. Front. Plant Sci. 2021, 12, 720867. [Google Scholar] [CrossRef] [PubMed]
- Cinelli, M.A.; Do, H.T.; Miley, G.P.; Silverman, R.B. Inducible nitric oxide synthase: Regulation, structure, and inhibition. Med. Res. Rev. 2020, 40, 158–189. [Google Scholar] [CrossRef]
- Hawkins, C.L.; Davies, M.J. Hypochlorite-induced oxidation of proteins in plasma: Formation of chloramines and nitrogen-centred radicals and their role in protein fragmentation. Biochem. J. 1999, 340, 539–548. [Google Scholar] [CrossRef]
- Prütz, W.A. Hypochlorous Acid Interactions with Thiols, Nucleotides, DNA, and Other Biological Substrates. Arch. Biochem. Biophys. 1996, 332, 110–120. [Google Scholar] [CrossRef]
- Van Der Vliet, A.; Eiserich, J.; Oneill, C.; Halliwell, B.; Cross, C. Tyrosine Modification by Reactive Nitrogen Species: A Closer Look. Arch. Biochem. Biophys. 1995, 319, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.J.; Liu, Z.-G. Crosstalk of reactive oxygen species and NF-kappaκB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Ding, Y.; Wild, C.; Shen, Q.; Zhou, J. Small Molecule Inhibitors Targeting Activator Protein 1 (AP-1). J. Med. Chem. 2014, 57, 6930–6948. [Google Scholar] [CrossRef]
- Barnes, P.J. Transcription factors in airway diseases. Lab. Investig. 2006, 86, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Manea, S.A.; Gafencu, A.V.; Raicu, M.; Simionescu, M. AP-1–Dependent Transcriptional Regulation of NADPH Oxidase in Human Aortic Smooth Muscle Cells: Role of p22phox Subunit. Arter. Thromb. Vasc. Biol. 2008, 28, 878–885. [Google Scholar] [CrossRef]
- McGuinness, A.J.A.; Sapey, E. Oxidative Stress in COPD: Sources, Markers, and Potential Mechanisms. J. Clin. Med. 2017, 6, 21. [Google Scholar] [CrossRef]
- Abdala-Valencia, H.; Berdnikovs, S.; Cook-Mills, J.M. Vitamin E Isoforms as Modulators of Lung Inflammation. Nutrients 2013, 5, 4347–4363. [Google Scholar] [CrossRef]
- Iwao, Y.; Ishima, Y.; Yamada, J.; Noguchi, T.; Kragh-Hansen, U.; Mera, K.; Honda, D.; Suenaga, A.; Maruyama, T.; Otagiri, M. Quantitative evaluation of the role of cysteine and methionine residues in the antioxidant activity of human serum albumin using recombinant mutants. IUBMB Life 2012, 64, 450–454. [Google Scholar] [CrossRef]
- Kim, H.-J.; Ha, S.; Lee, H.Y.; Lee, K.-J. ROSics: Chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom. Rev. 2015, 34, 184–208. [Google Scholar] [CrossRef]
- Levine, R.L.; Mosoni, L.; Berlett, B.S.; Stadtman, E.R. Methionine residues as endogenous antioxidants in proteins. Proc. Natl. Acad. Sci. USA 1996, 93, 15036–15040. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef] [PubMed]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Gao, Y.; Ci, X. Role of Nrf2 and Its Activators in Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 7090534. [Google Scholar] [CrossRef] [PubMed]
- Mercado, N.; Thimmulappa, R.; Thomas, C.M.; Fenwick, P.S.; Chana, K.K.; Donnelly, L.E.; Biswal, S.; Ito, K.; Barnes, P.J. Decreased histone deacetylase 2 impairs Nrf2 activation by oxidative stress. Biochem. Biophys. Res. Commun. 2011, 406, 292–298. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Jimenez, R.; Losonczy, G.; Zhang, C.; Ballabh, P.; Recchia, F.A.; Wilkerson, D.C.; Sonntag, W.E.; et al. Adaptive induction of NF-E2-related factor-2-driven antioxidant genes in endothelial cells in response to hyperglycemia. Am. J. Physiol. Circ. Physiol. 2011, 300, H1133–H1140. [Google Scholar] [CrossRef]
- Ungvari, Z.; Bailey-Downs, L.; Gautam, T.; Sosnowska, D.; Wang, M.; Monticone, R.E.; Telljohann, R.; Pinto, J.T.; de Cabo, R.; Sonntag, W.E.; et al. Age-Associated Vascular Oxidative Stress, Nrf2 Dysfunction, and NF- B Activation in the Nonhuman Primate Macaca mulatta. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 866–875. [Google Scholar] [CrossRef]
- Alves-Lopes, R.; Neves, K.B.; Montezano, A.C.; Harvey, A.; Carneiro, F.S.; Touyz, R.M.; Tostes, R.C. Internal Pudental Artery Dysfunction in Diabetes Mellitus Is Mediated by NOX1-Derived ROS-, Nrf2-, and Rho Kinase–Dependent Mechanisms. Hypertension 2016, 68, 1056–1064. [Google Scholar] [CrossRef]
- Lopes, R.A.; Neves, K.B.; Tostes, R.C.; Montezano, A.C.; Touyz, R.M. Downregulation of Nuclear Factor Erythroid 2–Related Factor and Associated Antioxidant Genes Contributes to Redox-Sensitive Vascular Dysfunction in Hypertension. Hypertension 2015, 66, 1240–1250. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Donato, A.J.; Galvan, V.; Csiszar, A. Mechanisms of Vascular Aging. Circ. Res. 2018, 123, 849–867. [Google Scholar] [CrossRef]
- Church, D.F.; Pryor, W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ. Health Perspect. 1985, 64, 111–126. [Google Scholar] [CrossRef]
- Yang, I.A.; Jenkins, C.R.; Salvi, S.S. Chronic obstructive pulmonary disease in never-smokers: Risk factors, pathogenesis, and implications for prevention and treatment. Lancet Respir. Med. 2022, 10, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Overbeek, S.A.; Braber, S.; Henricks, P.A.J.; Kleinjan, M.; Kamp, V.M.; Georgiou, N.A.; Garssen, J.; Kraneveld, A.D.; Folkerts, G. Cigarette smoke induces β2-integrin-dependent neutrophil migration across human endothelium. Respir. Res. 2011, 12, 75. [Google Scholar] [CrossRef] [PubMed]
- Schiffers, C.; Reynaert, N.L.; Wouters, E.F.M.; van der Vliet, A. Redox Dysregulation in Aging and COPD: Role of NOX Enzymes and Implications for Antioxidant Strategies. Antioxidants 2021, 10, 1799. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fan, Y.; Cui, J.; Hao, B.; Zhu, L.; Sun, X.; He, J.; Yang, J.; Dong, J.; Wang, Y.; et al. NOX4 expression and distal arteriolar remodeling correlate with pulmonary hypertension in COPD. BMC Pulm. Med. 2018, 18, 111. [Google Scholar] [CrossRef] [PubMed]
- Hollins, F.; Sutcliffe, A.; Gomez, E.; Berair, R.; Russell, R.; Szyndralewiez, C.; Saunders, R.; Brightling, C. Airway smooth muscle NOX4 is upregulated and modulates ROS generation in COPD. Respir. Res. 2016, 17, 84. [Google Scholar] [CrossRef]
- Liu, X.; Hao, B.; Ma, A.; He, J.; Liu, X.; Chen, J. The Expression of NOX4 in Smooth Muscles of Small Airway Correlates with the Disease Severity of COPD. BioMed Res. Int. 2016, 2016, 2891810. [Google Scholar] [CrossRef]
- Gould, N.S.; Min, E.; Gauthier, S.; Martin, R.J.; Day, B.J. Lung glutathione adaptive responses to cigarette smoke exposure. Respir. Res. 2011, 12, 133. [Google Scholar] [CrossRef]
- Cantin, A.M.; North, S.L.; Hubbard, R.C.; Crystal, R.G. Normal alveolar epithelial lining fluid contains high levels of glutathione. J. Appl. Physiol. 1987, 63, 152–157. [Google Scholar] [CrossRef]
- Antus, B.; Paska, C.; Simon, B.; Barta, I. Monitoring Antioxidant Enzyme Activity during Exacerbations of Chronic Obstructive Pulmonary Disease. COPD J. Chronic Obstr. Pulm. Dis. 2018, 15, 496–502. [Google Scholar] [CrossRef]
- Betsuyaku, T.; Fuke, S.; Inomata, T.; Kaga, K.; Morikawa, T.; Odajima, N.; Adair-Kirk, T.; Nishimura, M. Bronchiolar epithelial catalase is diminished in smokers with mild COPD. Eur. Respir. J. 2013, 42, 42–53. [Google Scholar] [CrossRef]
- Padmavathi, P.; Raghu, P.S.; Reddy, V.D.; Bulle, S.; Marthadu, S.B.; Maturu, P.; Varadacharyulu, N. Chronic cigarette smoking-induced oxidative/nitrosative stress in human erythrocytes and platelets. Mol. Cell. Toxicol. 2018, 14, 27–34. [Google Scholar] [CrossRef]
- Barnes, P.J.; Burney, P.G.J.; Silverman, E.K.; Celli, B.R.; Vestbo, J.; Wedzicha, J.A.; Wouters, E.F.M. Chronic obstructive pulmonary disease. Nat. Rev. Dis. Prim. 2015, 1, 15076. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C.; Timens, W. The Pathology of Chronic Obstructive Pulmonary Disease. Annu. Rev. Pathol. Mech. Dis. 2009, 4, 435–459. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.; Montezano, A.C.; Touyz, R.M. Vascular biology of ageing—Implications in hypertension. J. Mol. Cell. Cardiol. 2015, 83, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Kaley, G.; De Cabo, R.; Sonntag, W.E.; Csiszar, A. Mechanisms of Vascular Aging: New Perspectives. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2010, 65, 1028–1041. [Google Scholar] [CrossRef]
- Ungvari, Z.; Tarantini, S.; Sorond, F.; Merkely, B.; Csiszar, A. Mechanisms of Vascular Aging, A Geroscience Perspective: JACC Focus Seminar. J. Am. Coll. Cardiol. 2020, 75, 931–941. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Vivodtzev, I.; Tamisier, R.; Baguet, J.-P.; Borel, J.C.; Lévy, P.; Pépin, J.-L. Arterial Stiffness in COPD. Chest 2014, 145, 861–875. [Google Scholar] [CrossRef]
- Qvist, L.; Nilsson, U.; Johansson, V.; Larsson, K.; Rönmark, E.; Langrish, J.; Blomberg, A.; Lindberg, A. Central arterial stiffness is increased among subjects with severe and very severe COPD: Report from a population-based cohort study. Eur. Clin. Respir. J. 2015, 2, 27023. [Google Scholar] [CrossRef]
- McAllister, D.A.; Maclay, J.D.; Mills, N.L.; Mair, G.; Miller, J.; Anderson, D.; Newby, D.E.; Murchison, J.T.; MacNee, W. Arterial Stiffness Is Independently Associated with Emphysema Severity in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2007, 176, 1208–1214. [Google Scholar] [CrossRef]
- Albu, A.; Fodor, D.; Bondor, C.; Suciu, O. Carotid arterial stiffness in patients with chronic obstructive pulmonary disease. Acta Physiol. Hung. 2011, 98, 117–127. [Google Scholar] [CrossRef]
- Castagna, O.; Boussuges, A.; Vallier, J.-M.; Prefaut, C.; Brisswalter, J. Is impairment similar between arm and leg cranking exercise in COPD patients? Respir. Med. 2007, 101, 547–553. [Google Scholar] [CrossRef]
- Luehrs, R.E.; Newell, J.D., Jr.; Comellas, A.P.; Hoffman, E.A.; Warner, K.; Croghan, A.; DuBose, L.E.; Nopoulos, P.; Magnotta, V.; Arndt, S.; et al. CT-measured lung air-trapping is associated with higher carotid artery stiffness in individuals with chronic obstructive pulmonary disease. J. Appl. Physiol. 2018, 125, 1760–1766. [Google Scholar] [CrossRef]
- Cinarka, H.; Kayhan, S.; Gumus, A.; Durakoglugil, M.E.; Erdogan, T.; Ezberci, I.; Yavuz, A.; Ozkaya, S.; Sahin, U. Arterial Stiffness Measured Via Carotid Femoral Pulse Wave Velocity Is Associated With Disease Severity in COPD. Respir. Care 2014, 59, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Tarnoki, D.L.; Tarnoki, A.D.; Lazar, Z.; Medda, E.; Littvay, L.; Cotichini, R.; Fagnani, C.; Stazi, M.A.; Nisticó, L.; Lucatelli, P.; et al. Genetic and environmental factors on the relation of lung function and arterial stiffness. Respir. Med. 2013, 107, 927–935. [Google Scholar] [CrossRef]
- Patel, A.R.C.; Kowlessar, B.S.; Donaldson, G.C.; Mackay, A.J.; Singh, R.; George, S.N.; Garcha, D.S.; Wedzicha, J.A.; Hurst, J.R. Cardiovascular Risk, Myocardial Injury, and Exacerbations of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2013, 188, 1091–1099. [Google Scholar] [CrossRef]
- Aldabayan, Y.S.; Ridsdale, H.A.; Alrajeh, A.M.; Aldhahir, A.M.; Lemson, A.; Alqahtani, J.S.; Brown, J.S.; Hurst, J.R. Pulmonary rehabilitation, physical activity and aortic stiffness in COPD. Respir. Res. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Pako, J.; Barta, I.; Balogh, Z.; Kerti, M.; Drozdovszky, O.; Bikov, A.; Antus, B.; Horvath, I.; Varga, J. Assessment of the Anti-Aging Klotho Protein in Patients with COPD Undergoing Pulmonary Rehabilitation. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Flenley, D. Sleep in Chronic Obstructive Lung Disease. Clin. Chest Med. 1985, 6, 651–661. [Google Scholar] [CrossRef]
- Kapa, S.; Kuniyoshi, F.H.S.; Somers, V.K. Sleep Apnea and Hypertension: Interactions and Implications for Management. Hypertension 2008, 51, 605–608. [Google Scholar] [CrossRef]
- Lavie, L. Oxidative stress in obstructive sleep apnea and intermittent hypoxia–Revisited–The bad ugly and good: Implications to the heart and brain. Sleep Med. Rev. 2015, 20, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, S.; Mao, Y. The mechanisms of vascular aging. Aging Med. 2021, 4, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.-H. Aging: A revisited theory based on free radicals generated by NOX family NADPH oxidases. Exp. Gerontol. 2007, 42, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Puca, A.A.; Carrizzo, A.; Villa, F.; Ferrario, A.; Casaburo, M.; Maciag, A.; Vecchione, C. Vascular ageing: The role of oxidative stress. Int. J. Biochem. Cell Biol. 2013, 45, 556–559. [Google Scholar] [CrossRef]
- Antus, B.; Harnasi, G.; Drozdovszky, O.; Barta, I. Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde. Respirology 2014, 19, 74–79. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Oxidative stress and vascular inflammation in aging. Free. Radic. Biol. Med. 2013, 65, 380–401. [Google Scholar] [CrossRef]
- Rybka, J.; Kupczyk, D.; Kędziora-Kornatowska, K.; Pawluk, H.; Czuczejko, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Antonioli, M.; Carvalho, L.A.; Kędziora, J. Age-related changes in an antioxidant defense system in elderly patients with essential hypertension compared with healthy controls. Redox Rep. 2011, 16, 71–77. [Google Scholar] [CrossRef]
- Siedlinski, M.; van Diemen, C.C.; Postma, D.S.; Vonk, J.M.; Boezen, H.M. Superoxide dismutases, lung function and bronchial responsiveness in a general population. Eur. Respir. J. 2009, 33, 986–992. [Google Scholar] [CrossRef]
- Dahl, M.; Bowler, R.P.; Juul, K.; Crapo, J.D.; Levy, S.; Nordestgaard, B.G. Superoxide Dismutase 3 Polymorphism Associated with Reduced Lung Function in Two Large Populations. Am. J. Respir. Crit. Care Med. 2008, 178, 906–912. [Google Scholar] [CrossRef]
- Sotgia, S.; Paliogiannis, P.; Sotgiu, E.; Mellino, S.; Zinellu, E.; Fois, A.G.; Pirina, P.; Carru, C.; Mangoni, A.A.; Zinellu, A. Systematic Review and Meta-Analysis of the Blood Glutathione Redox State in Chronic Obstructive Pulmonary Disease. Antioxidants 2020, 9, 1146. [Google Scholar] [CrossRef]
- Beeh, K.M.; Beier, J.; Koppenhoefer, N.; Buhl, R. Increased Glutathione Disulfide and Nitrosothiols in Sputum Supernatant of Patients With Stable COPD. Chest 2004, 126, 1116–1122. [Google Scholar] [CrossRef]
- Ahmad, A.; Shameem, M.; Husain, Q. Altered oxidant-antioxidant levels in the disease prognosis of chronic obstructive pulmonary disease. Int. J. Tuberc. Lung Dis. 2013, 17, 1104–1109. [Google Scholar] [CrossRef]
- Tavilani, H.; Nadi, E.; Karimi, J.; Goodarzi, M.T. Oxidative Stress in COPD Patients, Smokers, and Non-smokers. Respir. Care 2012, 57, 2090–2094. [Google Scholar] [CrossRef] [PubMed]
- Joppa, P.; Petrášová, D.; Stančák, B.; Dorková, Z.; Tkáčová, R. Oxidative stress in patients with COPD and pulmonary hypertension. Wien. Klin. Wochenschr. 2007, 119, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.; Raj, H.G.; Chhabra, S.K. Increased Oxidative Stress and Altered Levels of Antioxidants in Chronic Obstructive Pulmonary Disease. Inflammation 2005, 29, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, A.; Górecki, D.; Szpinda, M.; Mila-Kierzenkowska, C.; Woźniak, B. Oxidant-Antioxidant Balance in the Blood of Patients with Chronic Obstructive Pulmonary Disease After Smoking Cessation. Oxidative Med. Cell. Longev. 2013, 2013, 897075. [Google Scholar] [CrossRef]
- Santos, M.C.; Oliveira, A.L.; Viegas-Crespo, A.M.; Vicente, L.; Barreiros, M.A.; Monteiro, P.; Pinheiro, T.; De Almeida, A.B. Systemic markers of the redox balance in chronic obstructive pulmonary disease. Biomarkers 2004, 9, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Vibhuti, A.; Arif, E.; Mishra, A.; Deepak, D.; Singh, B.; Rahman, I.; Mohammad, G.; Pasha, M.A.Q. CYP1A1, CYP1A2 and CYBA gene polymorphisms associated with oxidative stress in COPD. Clin. Chim. Acta 2010, 411, 474–480. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y.; Jiang, Y.; Lu, G.; Huang, X.; Guan, K. Local and systemic oxidative stress and glucocorticoid receptor levels in chronic obstructive pulmonary disease patients. Can. Respir. J. 2013, 20, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Oelze, M.; Kröller-Schön, S.; Steven, S.; Lubos, E.; Doppler, C.; Hausding, M.; Tobias, S.; Brochhausen, C.; Li, H.; Torzewski, M.; et al. Glutathione Peroxidase-1 Deficiency Potentiates Dysregulatory Modifications of Endothelial Nitric Oxide Synthase and Vascular Dysfunction in Aging. Hypertension 2014, 63, 390–396. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Guo, H.; Fedarko, N.; DeZern, A.; Fried, L.P.; Xue, Q.-L.; Leng, S.; Beamer, B.; Walston, J.D. Glutathione peroxidase enzyme activity in aging. J. Gerontol. A Biol. Sci. Med. Sci. 2008, 63, 505–509. [Google Scholar] [CrossRef]
- He, T.; Joyner, M.J.; Katusic, Z.S. Aging decreases expression and activity of glutathione peroxidase-1 in human endothelial progenitor cells. Microvasc. Res. 2009, 78, 447–452. [Google Scholar] [CrossRef]
- Lázár, Z.; Kelemen, Á.; Gálffy, G.; Losonczy, G.; Horváth, I.; Bikov, A. Central and peripheral airway nitric oxide in patients with stable and exacerbated chronic obstructive pulmonary disease. J. Breath Res. 2018, 12, 036017. [Google Scholar] [CrossRef]
- Ryter, S.W. Heme Oxygenase-1: An Anti-Inflammatory Effector in Cardiovascular, Lung, and Related Metabolic Disorders. Antioxidants 2022, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Polverino, F.; Celli, B.R.; Owen, C.A. COPD as an endothelial disorder: Endothelial injury linking lesions in the lungs and other organs? (2017 Grover Conference Series). Pulm. Circ. 2018, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mei, D.; Tan, W.D.; Liao, W.; Heng, C.M.; Wong, W.F. Activation of angiotensin II type-2 receptor protects against cigarette smoke-induced COPD. Pharmacol. Res. 2020, 161, 105223. [Google Scholar] [CrossRef] [PubMed]
- Pober, J.S.; Sessa, W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007, 7, 803–815. [Google Scholar] [CrossRef]
- Donato, A.J.; Machin, D.R.; Lesniewski, L.A. Mechanisms of Dysfunction in the Aging Vasculature and Role in Age-Related Disease. Circ. Res. 2018, 123, 825–848. [Google Scholar] [CrossRef]
- Ruzsics, I.; Nagy, L.; Keki, S.; Sarosi, V.; Illes, B.; Illes, Z.; Horvath, I.; Bogar, L.; Molnar, T. L-Arginine Pathway in COPD Patients with Acute Exacerbation: A New Potential Biomarker. COPD J. Chronic Obstr. Pulm. Dis. 2016, 13, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Vallance, P.; Leiper, J. Cardiovascular Biology of the Asymmetric Dimethylarginine:Dimethylarginine Dimethylaminohydrolase Pathway. Arter. Thromb. Vasc. Biol. 2004, 24, 1023–1030. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, R.W.; Rodriguez, P.C.; Toque, H.A.; Narayanan, S.P.; Caldwell, R.B. Arginase: A Multifaceted Enzyme Important in Health and Disease. Physiol. Rev. 2018, 98, 641–665. [Google Scholar] [CrossRef]
- Durante, W. Role of Arginase in Vessel Wall Remodeling. Front. Immunol. 2013, 4, 111. [Google Scholar] [CrossRef] [PubMed]
- Maarsingh, H.; Zaagsma, J.; Meurs, H. Arginase: A key enzyme in the pathophysiology of allergic asthma opening novel therapeutic perspectives. Br. J. Pharmacol. 2009, 158, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Guzmán, M.J.; Romero, G.P.-B.; Rial, S.P.; del Castillo, C.S.; Rodríguez, M.P.; Mahillo-Fernandez, I.; Villar-Álvarez, F. Elevated levels of arginase activity are related to inflammation in patients with COPD exacerbation. BMC Pulm. Med. 2021, 21, 271. [Google Scholar] [CrossRef]
- Abdelghany, T.M.; Ismail, R.S.; Mansoor, F.A.; Zweier, J.R.; Lowe, F.; Zweier, J.L. Cigarette smoke constituents cause endothelial nitric oxide synthase dysfunction and uncoupling due to depletion of tetrahydrobiopterin with degradation of GTP cyclohydrolase. Nitric Oxide 2018, 76, 113–121. [Google Scholar] [CrossRef]
- He, Z.; Chen, Y.; Hou, C.; He, W.; Chen, P. Cigarette Smoke Extract Changes Expression of Endothelial Nitric Oxide Synthase (eNOS) and p16(INK4a) and is Related to Endothelial Progenitor Cell Dysfunction. Med. Sci. Monit. 2017, 23, 3224–3231. [Google Scholar] [CrossRef]
- Stanisavljevic, N.; Stojanovich, L.; Marisavljevic, D.; Djokovic, A.; Dopsaj, V.; Kotur-Stevuljevic, J.; Martinovic, J.; Memon, L.; Radovanovic, S.; Todic, B.; et al. Lipid peroxidation as risk factor for endothelial dysfunction in antiphospholipid syndrome patients. Clin. Rheumatol. 2016, 35, 2485–2493. [Google Scholar] [CrossRef]
- Tejovathi, B.; Suchitra, M.M.; Suresh, V.; Reddy, V.S.; Sachan, A.; Rao, P.N.S.; Bitla, A.R. Association of Lipid Peroxidation with Endothelial Dysfunction in Patients with Overt Hypothyroidism. Exp. Clin. Endocrinol. Diabetes 2013, 121, 306–309. [Google Scholar] [CrossRef]
- Watson, A.M.D.; Soro-Paavonen, A.; Jandeleit-Dahm, K.A. AGE-RAGE signalling in endothelial dysfunction and atherosclerosis in diabetes. In Endothelial Dysfunction and Inflammation; Dauphinee, S., Karsan, A., Eds.; Springer Basel: Basel, Switzerland, 2010; pp. 161–174. [Google Scholar] [CrossRef]
- Goven, D.; Boutten, A.; Lecon-Malas, V.; Marchal-Somme, J.; Amara, N.; Crestani, B.; Fournier, M.; Leseche, G.; Soler, P.; Boczkowski, J.; et al. Altered Nrf2/Keap1-Bach1 equilibrium in pulmonary emphysema. Thorax 2008, 63, 916–924. [Google Scholar] [CrossRef]
- Yamada, K.; Asai, K.; Nagayasu, F.; Sato, K.; Ijiri, N.; Yoshii, N.; Imahashi, Y.; Watanabe, T.; Tochino, Y.; Kanazawa, H.; et al. Impaired nuclear factor erythroid 2-related factor 2 expression increases apoptosis of airway epithelial cells in patients with chronic obstructive pulmonary disease due to cigarette smoking. BMC Pulm. Med. 2016, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Nana-Sinkam, S.P.; Lee, J.D.; Sotto-Santiago, S.; Stearman, R.S.; Keith, R.L.; Choudhury, Q.; Cool, C.; Parr, J.; Moore, M.D.; Bull, T.M.; et al. Prostacyclin Prevents Pulmonary Endothelial Cell Apoptosis Induced by Cigarette Smoke. Am. J. Respir. Crit. Care Med. 2007, 175, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Voelkel, N.F. Cigarette Smoke Is an Endothelial Cell Toxin. Am. J. Respir. Crit. Care Med. 2018, 197, 274. [Google Scholar] [CrossRef] [PubMed]
- Green, C.E.; Turner, A.M. The role of the endothelium in asthma and chronic obstructive pulmonary disease (COPD). Respir. Res. 2017, 18, 20. [Google Scholar] [CrossRef]
- Kasahara, Y.; Tuder, R.M.; Taraseviciene-Stewart, L.; Le Cras, T.D.; Abman, S.; Hirth, P.K.; Waltenberger, J.; Voelkel, N.F. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J. Clin. Investig. 2000, 106, 1311–1319. [Google Scholar] [CrossRef]
- Weissmann, N.; Lobo, B.; Pichl, A.; Parajuli, N.; Seimetz, M.; Puig-Pey, R.; Ferrer, E.; Peinado, V.I.; Domínguez-Fandos, D.; Fysikopoulos, A.; et al. Stimulation of Soluble Guanylate Cyclase Prevents Cigarette Smoke–induced Pulmonary Hypertension and Emphysema. Am. J. Respir. Crit. Care Med. 2014, 189, 1359–1373. [Google Scholar] [CrossRef]
- Chellappan, D.K.; Paudel, K.R.; Tan, N.W.; Cheong, K.S.; Khoo, S.S.Q.; Seow, S.M.; Chellian, J.; Candasamy, M.; Patel, V.K.; Arora, P.; et al. Targeting the mitochondria in chronic respiratory diseases. Mitochondrion 2022, 67, 15–37. [Google Scholar] [CrossRef]
- Lázár, Z.; Huszár, E.; Kullmann, T.; Barta, I.; Antus, B.; Bikov, A.; Kollai, M.; Horvath, I. Adenosine triphosphate in exhaled breath condensate of healthy subjects and patients with chronic obstructive pulmonary disease. Inflamm. Res. 2008, 57, 367–373. [Google Scholar] [CrossRef]
- Lazar, Z.; Müllner, N.; Lucattelli, M.; Ayata, C.K.; Cicko, S.; Yegutkin, G.G.; De Cunto, G.; Müller, T.; Meyer, A.; Hossfeld, M.; et al. NTPDase1/CD39 and aberrant purinergic signalling in the pathogenesis of COPD. Eur. Respir. J. 2016, 47, 254–263. [Google Scholar] [CrossRef]
- Chun, P. Role of sirtuins in chronic obstructive pulmonary disease. Arch. Pharmacal Res. 2015, 38, 1–10. [Google Scholar] [CrossRef]
- Wu, Q.-J.; Zhang, T.-N.; Chen, H.-H.; Yu, X.-F.; Lv, J.-L.; Liu, Y.-Y.; Liu, Y.-S.; Zheng, G.; Zhao, J.-Q.; Wei, Y.-F.; et al. The sirtuin family in health and disease. Signal Transduct. Target. Ther. 2022, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Rajendrasozhan, S.; Yang, S.-R.; Kinnula, V.L.; Rahman, I. SIRT1, an Antiinflammatory and Antiaging Protein, Is Decreased in Lungs of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2008, 177, 861–870. [Google Scholar] [CrossRef]
- Takasaka, N.; Araya, J.; Hara, H.; Ito, S.; Kobayashi, K.; Kurita, Y.; Wakui, H.; Yoshii, Y.; Yumino, Y.; Fujii, S.; et al. Autophagy Induction by SIRT6 through Attenuation of Insulin-like Growth Factor Signaling Is Involved in the Regulation of Human Bronchial Epithelial Cell Senescence. J. Immunol. 2014, 192, 958–968. [Google Scholar] [CrossRef]
- Yang, S.-R.; Wright, J.; Bauter, M.; Seweryniak, K.; Kode, A.; Rahman, I. Sirtuin regulates cigarette smoke-induced proinflammatory mediator release via RelA/p65 NF-κB in macrophages in vitro and in rat lungs in vivo: Implications for chronic inflammation and aging. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 292, L567–L576. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Yao, H.; Chung, S.; Hwang, J.-W.; Rajendrasozhan, S.; Sundar, I.K.; Dean, D.A.; McBurney, M.W.; Guarente, L.; Gu, W.; Rönty, M.; et al. SIRT1 protects against emphysema via FOXO3-mediated reduction of premature senescence in mice. J. Clin. Investig. 2012, 122, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Li, Y.; Liu, J.; Ying, X.; Liu, Y.; Yan, J.; Chen, C.; Zhou, H.; Cao, L.; Ma, Y. LncRNA-mediated SIRT1/FoxO3a and SIRT1/p53 signaling pathways regulate type II alveolar epithelial cell senescence in patients with chronic obstructive pulmonary disease. Mol. Med. Rep. 2017, 15, 3129–3134. [Google Scholar] [CrossRef]
- Minagawa, S.; Araya, J.; Numata, T.; Nojiri, S.; Hara, H.; Yumino, Y.; Kawaishi, M.; Odaka, M.; Morikawa, T.; Nishimura, S.L.; et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-β-induced senescence of human bronchial epithelial cells. Am. J. Physiol. Cell. Mol. Physiol. 2011, 300, L391–L401. [Google Scholar] [CrossRef]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef]
- Potente, M.; Ghaeni, L.; Baldessari, D.; Mostoslavsky, R.; Rossig, L.; Dequiedt, F.; Haendeler, J.; Mione, M.; Dejana, E.; Alt, F.W.; et al. SIRT1 controls endothelial angiogenic functions during vascular growth. Genes Dev. 2007, 21, 2644–2658. [Google Scholar] [CrossRef]
- Wan, Y.; Gao, P.; Zhou, S.; Zhang, Z.; Hao, D.; Lian, L.; Li, Y.; Chen, H.-Z.; Liu, D.-P. SIRT 1-mediated epigenetic downregulation of plasminogen activator inhibitor-1 prevents vascular endothelial replicative senescence. Aging Cell 2014, 13, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, H.-N.; Chen, H.-Z.; Gao, P.; Zhu, L.-H.; Li, H.-L.; Lv, X.; Zhang, Q.-J.; Zhang, R.; Wang, Z.; et al. SIRT1 Acts as a Modulator of Neointima Formation Following Vascular Injury in Mice. Circ. Res. 2011, 108, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Gorenne, I.; Kumar, S.; Gray, K.; Figg, N.; Yu, H.; Mercer, J.; Bennett, M. Vascular Smooth Muscle Cell Sirtuin 1 Protects Against DNA Damage and Inhibits Atherosclerosis. Circulation 2013, 127, 386–396. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xu, T.-T.; Lu, J.; Li, L.; Xu, J.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. Overexpression of SIRT1 in vascular smooth muscle cells attenuates angiotensin II-induced vascular remodeling and hypertension in mice. J. Mol. Med. 2014, 92, 347–357. [Google Scholar] [CrossRef]
- Kuro-O, M. The FGF23 and Klotho system beyond mineral metabolism. Clin. Exp. Nephrol. 2017, 21, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-D.; Podvin, S.; Gillespie, E.; Leeman, S.E.; Abraham, C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. USA 2007, 104, 19796–19801. [Google Scholar] [CrossRef]
- Shiraki-Iida, T.; Aizawa, H.; Matsumura, Y.; Sekine, S.; Iida, A.; Anazawa, H.; Nagai, R.; Kuro-O, M.; Nabeshima, Y.-I. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998, 424, 6–10. [Google Scholar] [CrossRef]
- Gao, W.; Yuan, C.; Zhang, J.; Li, L.; Yu, L.; Wiegman, C.H.; Barnes, P.J.; Adcock, I.M.; Huang, M.; Yao, X. Klotho expression is reduced in COPD airway epithelial cells: Effects on inflammation and oxidant injury. Clin. Sci. 2015, 129, 1011–1023. [Google Scholar] [CrossRef]
- Kuro-O, M. Klotho as a regulator of oxidative stress and senescence. Biol. Chem. 2008, 389, 233–241. [Google Scholar] [CrossRef]
- Yamamoto, M.; Clark, J.D.; Pastor, J.V.; Gurnani, P.; Nandi, A.; Kurosu, H.; Miyoshi, M.; Ogawa, Y.; Castrillon, D.H.; Rosenblatt, K.P.; et al. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005, 280, 38029–38034. [Google Scholar] [CrossRef]
- Kuro-O, M.; Matsumura, Y.; Aizawa, H.; Kawaguchi, H.; Suga, T.; Utsugi, T.; Ohyama, Y.; Kurabayashi, M.; Kaname, T.; Kume, E.; et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997, 390, 45–51. [Google Scholar] [CrossRef]
- Patel, M.S.; Lewis, A.; Natanek, S.A.; Martolini, D.; Bruijnzeel, P.; Anikin, V.; McGonigle, N.; Jordan, S.; Hopkinson, N.S.; Man, W.D.-C.; et al. Klotho expression is reduced in COPD. Eur. Respir. J. 2013, 42, P3708. [Google Scholar]
- Pákó, J.; Kunos, L.; Mészáros, M.; Tárnoki, D.L.; Tárnoki, D.; Horváth, I.; Bikov, A. Decreased Levels of Anti-Aging Klotho in Obstructive Sleep Apnea. Rejuvenation Res. 2020, 23, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Pákó, J.; Barta, I.; Kerti, M.; Balogh, Z.; Drozdovszky, O.; Antus, B.; Varga, J.; Horvath, I. Assessment of plasma klotho concentration in stable COPD. Eur. Respir. J. 2016, 48, PA1001. [Google Scholar] [CrossRef]
- Lim, K.; Halim, A.; Lu, T.-S.; Ashworth, A.; Chong, I. Klotho: A Major Shareholder in Vascular Aging Enterprises. Int. J. Mol. Sci. 2019, 20, 4637. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Bs, C.C.; Guralnik, J.M.; Ferrucci, L. Plasma Klotho and Cardiovascular Disease in Adults. J. Am. Geriatr. Soc. 2011, 59, 1596–1601. [Google Scholar] [CrossRef]
- Semba, R.D.; Cappola, A.R.; Sun, K.; Bandinelli, S.; Dalal, M.; Crasto, C.; Guralnik, J.M.; Ferrucci, L. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 794–800. [Google Scholar] [CrossRef]
- Arking, D.E.; Atzmon, G.; Arking, A.; Barzilai, N.; Dietz, H.C. Association Between a Functional Variant of the KLOTHO Gene and High-Density Lipoprotein Cholesterol, Blood Pressure, Stroke, and Longevity. Circ. Res. 2005, 96, 412–418. [Google Scholar] [CrossRef]
- Hu, M.C.; Shi, M.; Zhang, J.; Quiñones, H.; Griffith, C.; Kuro-O, M.; Moe, O.W. Klotho Deficiency Causes Vascular Calcification in Chronic Kidney Disease. J. Am. Soc. Nephrol. 2011, 22, 124–136. [Google Scholar] [CrossRef]
- Kawarazaki, W.; Mizuno, R.; Nishimoto, M.; Ayuzawa, N.; Hirohama, D.; Ueda, K.; Kawakami-Mori, F.; Oba, S.; Marumo, T.; Fujita, T. Salt causes aging-associated hypertension via vascular Wnt5a under Klotho deficiency. J. Clin. Investig. 2020, 130, 4152–4166. [Google Scholar] [CrossRef]
- Lanzani, C.; Citterio, L.; Vezzoli, G. Klotho: A link between cardiovascular and non-cardiovascular mortality. Clin. Kidney J. 2020, 13, 926–932. [Google Scholar] [CrossRef]
- Barnes, R.P.; Fouquerel, E.; Opresko, P.L. The impact of oxidative DNA damage and stress on telomere homeostasis. Mech. Ageing Dev. 2019, 177, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Guillaumet-Adkins, A.; Yañez, Y.; Peris-Diaz, M.D.; Calabria, I.; Palanca-Ballester, C.; Sandoval, J. Epigenetics and Oxidative Stress in Aging. Oxidative Med. Cell. Longev. 2017, 2017, 9175806. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, E.; Sillanpää, E.; Karrasch, S.; Alves, A.C.; Codd, V.; Hovatta, I.; Buxton, J.L.; Nelson, C.P.; Broer, L.; Hägg, S.; et al. Telomere length in circulating leukocytes is associated with lung function and disease. Eur. Respir. J. 2014, 43, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.G.; Donato, A.J.; Walker, A.E. Telomere uncapping and vascular aging. Am. J. Physiol. Circ. Physiol. 2018, 315, H1–H5. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.-D.; Ouyang, R.-Y.; Chen, P. Epigenetic mechanisms in chronic obstructive pulmonary disease. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 844–856. [Google Scholar] [PubMed]
- Horváth, I.; Canotilho, M.; Chlumský, J.; Chorostowska-Wynimko, J.; Corda, L.; Derom, E.; Ficker, J.H.; Kneussl, M.; Miravitlles, M.; Sucena, M.; et al. Diagnosis and management of α1-antitrypsin deficiency in Europe: An expert survey. ERJ Open Res. 2019, 5, 00171–02018. [Google Scholar] [CrossRef]
- Greene, C.M.; Marciniak, S.J.; Teckman, J.; Ferrarotti, I.; Brantly, M.L.; Lomas, D.A.; Stoller, J.K.; McElvaney, N.G. α1-Antitrypsin deficiency. Nat. Rev. Dis. Prim. 2016, 2, 16051. [Google Scholar] [CrossRef]
- Strnad, P.; McElvaney, N.G.; Lomas, D.A. Alpha1-Antitrypsin Deficiency. N. Engl. J. Med. 2020, 382, 1443–1455. [Google Scholar] [CrossRef]
- Winther, S.V.; Ahmed, D.; Al-Shuweli, S.; Landt, E.M.; Nordestgaard, B.G.; Seersholm, N.; Dahl, M. Severe α1-antitrypsin deficiency associated with lower blood pressure and reduced risk of ischemic heart disease: A cohort study of 91,540 individuals and a meta-analysis. Respir. Res. 2022, 23, 55. [Google Scholar] [CrossRef]
- Pini, L.; Peroni, M.; Zanotti, C.; Pini, A.; Bossoni, E.; Giordani, J.; Bargagli, E.; Perger, E.; Ferrarotti, I.; Vizzardi, E.; et al. Investigating the Link between Alpha-1 Antitrypsin Deficiency and Abdominal Aortic Aneurysms. Ann. Vasc. Surg. 2021, 77, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Dako, F.; Zhao, H.; Mulvenna, A.; Gupta, S.; Simpson, S.; Kueppers, F. Relationship between alpha-1-antitrypsin deficiency and ascending aortic distention. Eur. Respir. J. 2019, 54, PA5391. [Google Scholar] [CrossRef]
- Schievink, W.I.; Bjöurnsson, J.; Parisi, J.E.; Prakash, U.B. Arterial Fibromuscular Dysplasia Associated With Severe α1-Antitrypsin Deficiency. Mayo Clin. Proc. 1994, 69, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Bofinger, A.; Hawley, C.; Fisher, P.; Daunt, N.; Stowasser, M.; Gordon, R. Alpha-1-antitrypsin phenotypes in patients with renal arterial fibromuscular dysplasia. J. Hum. Hypertens. 2000, 14, 91–94. [Google Scholar] [CrossRef]
- Dasí, F.; Amor, M.; Sanz, F.; Codoñer-Franch, P.; Navarro-García, M.M.; Escribano, A. Oxidative stress in serum of patients with alpha-1 antitrypsin deficiency. Eur. Respir. J. 2013, 42, 1488. [Google Scholar]
- Escribano, A.; Amor, M.; Pastor, S.; Castillo, S.; Sanz, F.; Codoñer-Franch, P.; Dasí, F. Decreased glutathione and low catalase activity contribute to oxidative stress in children with α-1 antitrypsin deficiency. Thorax 2015, 70, 82–83. [Google Scholar] [CrossRef]
- Marcus, N.Y.; Blomenkamp, K.; Ahmad, M.; Teckman, J.H. Oxidative stress contributes to liver damage in a murine model of alpha-1-antitrypsin deficiency. Exp. Biol. Med. 2012, 237, 1163–1172. [Google Scholar] [CrossRef]
- Rubio, M.L.; Martin-Mosquero, M.C.; Ortega, M.; Peces-Barba, G.; González-Mangado, N. Oral N-Acetylcysteine Attenuates Elastase-Induced Pulmonary Emphysema in Rats. Chest 2004, 125, 1500–1506. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjørten, I.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Haemodynamic responses to exercise in patients with COPD. Eur. Respir. J. 2013, 41, 1031–1041. [Google Scholar] [CrossRef]
- Ferrer, E.; Peinado, V.I.; Castaneda, J.; Prieto-Lloret, J.; Olea, E.; Gonzalez-Martin, M.C.; Vega-Agapito, M.V.; Diez, M.; Dominguez-Fandos, D.; Obeso, A.; et al. Effects of cigarette smoke and hypoxia on pulmonary circulation in the guinea pig. Eur. Respir. J. 2011, 38, 617–627. [Google Scholar] [CrossRef]
- Peinado, V.I.; Barberá, J.A.; Abate, P.; Ramírez, J.; Roca, J.; Santos, S.; Rodriguez-Roisin, R. Inflammatory Reaction in Pulmonary Muscular Arteries of Patients with Mild Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 1999, 159, 1605–1611. [Google Scholar] [CrossRef]
- Seimetz, M.; Parajuli, N.; Pichl, A.; Veit, F.; Kwapiszewska, G.; Weisel, F.C.; Milger, K.; Egemnazarov, B.; Turowska, A.; Fuchs, B.; et al. Inducible NOS Inhibition Reverses Tobacco-Smoke-Induced Emphysema and Pulmonary Hypertension in Mice. Cell 2011, 147, 293–305. [Google Scholar] [CrossRef]
- Gao, Y.; Du, X.; Qin, W.; Li, K. Assessment of the right ventricular function in patients with chronic obstructive pulmonary disease using MRI. Acta Radiol. 2011, 52, 711–715. [Google Scholar] [CrossRef]
- Vonk-Noordegraaf, A.; Marcus, J.T.; Holverda, S.; Roseboom, B.; Postmus, P. Early Changes of Cardiac Structure and Function in COPD Patients With Mild Hypoxemia. Chest 2005, 127, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Blanco, I.; Piccari, L.; Barberà, J.A. Pulmonary vasculature in COPD: The silent component. Respirology 2016, 21, 984–994. [Google Scholar] [CrossRef] [PubMed]
- Weir-McCall, J.R.; Struthers, A.D.; Lipworth, B.J.; Houston, J.G. The role of pulmonary arterial stiffness in COPD. Respir. Med. 2015, 109, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Peinado, V.; Ramírez, J.; Melgosa, T.; Roca, J.; Rodriguez-Roisin, R.; Barberà, J. Characterization of pulmonary vascular remodelling in smokers and patients with mild COPD. Eur. Respir. J. 2002, 19, 632–638. [Google Scholar] [CrossRef]
- Li, M.; Scott, D.E.; Shandas, R.; Stenmark, K.R.; Tan, W. High Pulsatility Flow Induces Adhesion Molecule and Cytokine mRNA Expression in Distal Pulmonary Artery Endothelial Cells. Ann. Biomed. Eng. 2009, 37, 1082–1092. [Google Scholar] [CrossRef]
- Scott, D.; Tan, Y.; Shandas, R.; Stenmark, K.R.; Tan, W. High pulsatility flow stimulates smooth muscle cell hypertrophy and contractile protein expression. Am. J. Physiol. Cell. Mol. Physiol. 2013, 304, L70–L81. [Google Scholar] [CrossRef]
- Pak, O.; Aldashev, A.; Welsh, D.; Peacock, A. The effects of hypoxia on the cells of the pulmonary vasculature. Eur. Respir. J. 2007, 30, 364–372. [Google Scholar] [CrossRef]
- Zepeda, A.B.; Pessoa, A., Jr.; Castillo, R.L.; Figueroa, C.A.; Pulgar, V.; Farías, J.G. Cellular and molecular mechanisms in the hypoxic tissue: Role of HIF-1 and ROS. Cell Biochem. Funct. 2013, 31, 451–459. [Google Scholar] [CrossRef]
- Fu, X.; Zhang, F. Role of the HIF-1 signaling pathway in chronic obstructive pulmonary disease. Exp. Ther. Med. 2018, 16, 4553–4561. [Google Scholar] [CrossRef]
- Rong, B.; Liu, Y.; Li, M.; Fu, T.; Gao, W.; Liu, H. Correlation of serum levels of HIF-1α and IL-19 with the disease progression of COPD: A retrospective study. Int. J. Chronic Obstr. Pulm. Dis. 2018, 13, 3791–3803. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Leopold, J.A.; Hemnes, A.R. Metabolic syndrome, neurohumoral modulation, and pulmonary arterial hypertension. Br. J. Pharmacol. 2020, 177, 1457–1471. [Google Scholar] [CrossRef] [PubMed]
- Elisia, I.; Lam, V.; Cho, B.; Hay, M.; Li, M.Y.; Yeung, M.; Bu, L.; Jia, W.; Norton, N.; Lam, S.; et al. The effect of smoking on chronic inflammation, immune function and blood cell composition. Sci. Rep. 2020, 10, 19480. [Google Scholar] [CrossRef] [PubMed]
- Llinàs, L.; Peinado, V.I.; Goñi, J.R.; Rabinovich, R.; Pizarro, S.; Rodriguez-Roisin, R.; Barberà, J.A.; Bastos, R. Similar gene expression profiles in smokers and patients with moderate COPD. Pulm. Pharmacol. Ther. 2011, 24, 32–41. [Google Scholar] [CrossRef]
- Melgosa, M.; Peinado, V.; Santos, S.; Morales, J.; Ramirez, J.; Roca, J.; Rodriguez-Roisin, R.; Barbera, J. Expression of endothelial nitric oxide synthase (eNOS) and endothelin-1 (ET-1) in pulmonary arteries of patients with severe COPD. Eur. Respir. J. 2003, 22, 20s. [Google Scholar]
- Wang, Y.; Li, X.; Niu, W.; Chen, J.; Zhang, B.; Zhang, X.; Wang, Y.; Dang, S.; Li, Z. The alveolar epithelial cells are involved in pulmonary vascular remodeling and constriction of hypoxic pulmonary hypertension. Respir. Res. 2021, 22, 134. [Google Scholar] [CrossRef]
- Gao, W.; Li, L.; Wang, Y.; Zhang, S.; Adcock, I.M.; Barnes, P.J.; Huang, M.; Yao, X. Bronchial epithelial cells: The key effector cells in the pathogenesis of chronic obstructive pulmonary disease? Respirology 2015, 20, 722–729. [Google Scholar] [CrossRef]
- Chandra, D.; Gupta, A.; Strollo, P.J., Jr.; Fuhrman, C.R.; Leader, J.K.; Bon, J.; Slivka, W.A.; Shoushtari, A.H.; Avolio, J.; Kip, K.E.; et al. Airflow Limitation and Endothelial Dysfunction. Unrelated and Independent Predictors of Atherosclerosis. Am. J. Respir. Crit. Care Med. 2016, 194, 38–47. [Google Scholar] [CrossRef]
- Topsakal, R.; Kalay, N.; Ozdogru, I.; Cetinkaya, Y.; Oymak, S.; Kaya, M.G.; Dogan, A.; Inanc, M.T.; Ergin, A. Effects of chronic obstructive pulmonary disease on coronary atherosclerosis. Heart Vessel. 2009, 24, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Gimbrone, M.A., Jr.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed]
- Arnold, N.; Lechner, K.; Waldeyer, C.; Shapiro, M.D.; Koenig, W. Inflammation and Cardiovascular Disease: The Future. Eur. Cardiol. Rev. 2021, 16, e20. [Google Scholar] [CrossRef] [PubMed]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip. Top. Gerontol. 2015, 40, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Freixa, X.; Portillo, K.; Paré, C.; Garcia-Aymerich, J.; Gomez, F.P.; Benet, M.; Roca, J.; Farrero, E.; Ferrer, J.; Fernandez-Palomeque, C.; et al. Echocardiographic abnormalities in patients with COPD at their first hospital admission. Eur. Respir. J. 2013, 41, 784–791. [Google Scholar] [CrossRef]
- Hilde, J.M.; Skjørten, I.; Grøtta, O.J.; Hansteen, V.; Melsom, M.N.; Hisdal, J.; Humerfelt, S.; Steine, K. Right Ventricular Dysfunction and Remodeling in Chronic Obstructive Pulmonary Disease Without Pulmonary Hypertension. J. Am. Coll. Cardiol. 2013, 62, 1103–1111. [Google Scholar] [CrossRef]
- Rabe, K.F.; Hurst, J.R.; Suissa, S. Cardiovascular disease and COPD: Dangerous liaisons? Eur. Respir. Rev. 2018, 27, 180057. [Google Scholar] [CrossRef]
- Kunisaki, K.M.; Dransfield, M.T.; Anderson, J.A.; Brook, R.D.; Calverley, P.M.A.; Celli, B.R.; Crim, C.; Hartley, B.F.; Martinez, F.J.; Newby, D.E.; et al. Exacerbations of Chronic Obstructive Pulmonary Disease and Cardiac Events. A Post Hoc Cohort Analysis from the SUMMIT Randomized Clinical Trial. Am. J. Respir. Crit. Care Med. 2018, 198, 51–57. [Google Scholar] [CrossRef]
- Park, J.F.; Liang, J.; Umar, S. Electrical Remodeling in Right Ventricular Failure Due to Pulmonary Hypertension: Unraveling Novel Therapeutic Targets. Int. J. Mol. Sci. 2023, 24, 4633. [Google Scholar] [CrossRef]
- Boussuges, A.; Pinet, C.; Molenat, F.; Burnet, H.; Ambrosi, P.; Badier, M.; Sainty, J.-M.; Orehek, J. Left Atrial and Ventricular Filling in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 162, 670–675. [Google Scholar] [CrossRef]
- Sievi, N.A.; Clarenbach, C.F.; Camen, G.; Rossi, V.A.; Van Gestel, A.J.R.; Kohler, M. High prevalence of altered cardiac repolarization in patients with COPD. BMC Pulm. Med. 2014, 14, 55. [Google Scholar] [CrossRef]
- Ivanics, T.; Miklós, Z.; Dézsi, L.; Ikrényi, K.; Tóth, A.; Roemen, T.H.; Van Der Vusse, G.J.; Geti, L.L. Concomitant accumulation of intracellular free calcium and arachidonic acid in the ischemic-reperfused rat heart. Mol. Cell. Biochem. 2001, 226, 119–128. [Google Scholar] [CrossRef]
- Miklós, Z.; Ivanics, T.; Roemen, T.H.; Van Der Vusse, G.J.; Dézsi, L.; Szekeres, M.; Kemecsei, P.; Tóth, A.; Kollai, M.; Ligeti, L. Time related changes in calcium handling in the isolated ischemic and reperfused rat heart. Mol. Cell. Biochem. 2003, 250, 115–124. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Shah, A.K.; Adameova, A.; Bartekova, M. Role of Oxidative Stress in Cardiac Dysfunction and Subcellular Defects Due to Ischemia-Reperfusion Injury. Biomedicines 2022, 10, 1473. [Google Scholar] [CrossRef]
- Pelà, G.; Calzi, M.L.; Pinelli, S.; Andreoli, R.; Sverzellati, N.; Bertorelli, G.; Goldoni, M.; Chetta, A.A. Left ventricular structure and remodeling in patients with COPD. Int. J. Chronic Obstr. Pulm. Dis. 2016, 11, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Chantler, P.D.; Lakatta, E.G. Arterial–Ventricular Coupling with Aging and Disease. Front. Physiol. 2012, 3, 90. [Google Scholar] [CrossRef] [PubMed]
- Karch, A.; Vogelmeier, C.; Welte, T.; Bals, R.; Kauczor, H.-U.; Biederer, J.; Heinrich, J.; Schulz, H.; Gläser, S.; Holle, R.; et al. The German COPD cohort COSYCONET: Aims, methods and descriptive analysis of the study population at baseline. Respir. Med. 2016, 114, 27–37. [Google Scholar] [CrossRef]
- Trinkmann, F.; Saur, J.; Borggrefe, M.; Akin, I. Cardiovascular Comorbidities in Chronic Obstructive Pulmonary Disease (COPD)—Current Considerations for Clinical Practice. J. Clin. Med. 2019, 8, 69. [Google Scholar] [CrossRef] [PubMed]
- Antus, B. Oxidative Stress Markers in Sputum. Oxidative Med. Cell. Longev. 2016, 2016, 2930434. [Google Scholar] [CrossRef]
- Gopcevic, K.R.; Gkaliagkousi, E.; Nemcsik, J.; Acet, O.; Bernal-Lopez, M.R.; Bruno, R.M.; Climie, R.E.; Fountoulakis, N.; Fraenkel, E.; Lazaridis, A.; et al. Pathophysiology of Circulating Biomarkers and Relationship With Vascular Aging: A Review of the Literature From VascAgeNet Group on Circulating Biomarkers, European Cooperation in Science and Technology Action 18216. Front. Physiol. 2021, 12, 789690. [Google Scholar] [CrossRef]
- Zinellu, E.; Zinellu, A.; Fois, A.G.; Carru, C.; Pirina, P. Circulating biomarkers of oxidative stress in chronic obstructive pulmonary disease: A systematic review. Respir. Res. 2016, 17, 150. [Google Scholar] [CrossRef]
- Kiss, H.; Örlős, Z.; Gellért, Á.; Megyesfalvi, Z.; Mikáczó, A.; Sárközi, A.; Vaskó, A.; Miklós, Z.; Horváth, I. Exhaled Biomarkers for Point-of-Care Diagnosis: Recent Advances and New Challenges in Breathomics. Micromachines 2023, 14, 391. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Rossi, R.; Colombo, R.; Giustarini, D.; Milzani, A. Biomarkers of Oxidative Damage in Human Disease. Clin. Chem. 2006, 52, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Arja, C.; Surapaneni, K.M.; Raya, P.; Adimoolam, C.; Balisetty, B.; Kanala, K.R. Oxidative stress and antioxidant enzyme activity in South Indian male smokers with Chronic Obstructive Pulmonary Disease. Respirology 2013, 18, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, M.L.; Novelli, F.; Costa, F.; Malagrinò, L.; Melosini, L.; Bacci, E.; Cianchetti, S.; Dente, F.L.; Di Franco, A.; Vagaggini, B.; et al. Malondialdehyde in Exhaled Breath Condensate as a Marker of Oxidative Stress in Different Pulmonary Diseases. Mediat. Inflamm. 2011, 2011, 891752. [Google Scholar] [CrossRef]
- Çalikoğlu, M.; Ünlü, A.; Tamer, L.; Ercan, B.; Buğdayci, R.; Atik, U. The Levels of Serum Vitamin C, Malonyldialdehyde and Erythrocyte Reduced Glutathione in Chronic Obstructive Pulmonary Disease and in Healthy Smokers. Clin. Chem. Lab. Med. 2002, 40, 1028–1031. [Google Scholar] [CrossRef] [PubMed]
- Corradi, M.; Pignatti, P.; Manini, P.; Andreoli, R.; Goldoni, M.; Poppa, M.; Moscato, G.; Balbi, B.; Mutti, A. Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation. Eur. Respir. J. 2004, 24, 1011–1017. [Google Scholar] [CrossRef]
- Corradi, M.; Rubinstein, I.; Andreoli, R.; Manini, P.; Caglieri, A.; Poli, D.; Alinovi, R.; Mutti, A. Aldehydes in Exhaled Breath Condensate of Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2003, 167, 1380–1386. [Google Scholar] [CrossRef]
- Dhakal, N.; Lamsal, M.; Baral, N.; Shrestha, S.; Dhakal, S.S.; Bhatta, N.; Dubey, R.K. Oxidative Stress and Nutritional Status in Chronic Obstructive Pulmonary Disease. J. Clin. Diagn. Res. 2015, 9, BC01–BC04. [Google Scholar] [CrossRef]
- Dos Santos, C.F.; Braz, M.G.; de Arruda, N.M.; Caram, L.; Nogueira, D.L.; Tanni, S.E.; de Godoy, I.; Ferrari, R. DNA damage and antioxidant capacity in COPD patients with and without lung cancer. PLoS ONE 2022, 17, e0275873. [Google Scholar] [CrossRef] [PubMed]
- Hanta, I.; Kocabas, A.; Canacankatan, N.; Kuleci, S.; Seydaoglu, G. Oxidant–Antioxidant Balance in Patients with COPD. Lung 2006, 184, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, J.H.; Hwang, J.-H.; Baek, J.E.; Choi, B.-S. Malondialdehyde and 3-Nitrotyrosine in Exhaled Breath Condensate in Retired Elderly Coal Miners with Chronic Obstructive Pulmonary Disease. Saf. Health Work. 2014, 5, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Kopčinović, L.M.; Domijan, A.-M.; Posavac, K.; Čepelak, I.; Grubišić, T.; Rumora, L. Systemic redox imbalance in stable chronic obstructive pulmonary disease. Biomarkers 2016, 21, 692–698. [Google Scholar] [CrossRef]
- Montaño, M.; Cisneros, J.; Ramírez-Venegas, A.; Pedraza-Chaverri, J.; Mercado, D.; Ramos, C.; Sansores, R.H. Malondialdehyde and superoxide dismutase correlate with FEV1in patients with COPD associated with wood smoke exposure and tobacco smoking. Inhal. Toxicol. 2010, 22, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Paliogiannis, P.; Fois, A.G.; Sotgia, S.; Mangoni, A.A.; Zinellu, E.; Pirina, P.; Carru, C.; Zinellu, A. Circulating malondialdehyde concentrations in patients with stable chronic obstructive pulmonary disease: A systematic review and meta-analysis. Biomarkers Med. 2018, 12, 771–781. [Google Scholar] [CrossRef]
- Stanojkovic, I.; Kotur-Stevuljevic, J.; Milenkovic, B.; Spasic, S.; Vujic, T.; Stefanovic, A.; Llic, A.; Ivanisevic, J. Pulmonary function, oxidative stress and inflammatory markers in severe COPD exacerbation. Respir. Med. 2011, 105, S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Sunnetcioglu, A.; Alp, H.H.; Ndan, B.S.; Balaharoglu, R.; Gunbatar, H. Evaluation of Oxidative Damage and Antioxidant Mechanisms in COPD, Lung Cancer, and Obstructive Sleep Apnea Syndrome. Respir. Care 2016, 61, 205–211. [Google Scholar] [CrossRef]
- Vibhuti, A.; Arif, E.; Deepak, D.; Singh, B.; Pasha, M.Q. Correlation of oxidative status with BMI and lung function in COPD. Clin. Biochem. 2007, 40, 958–963. [Google Scholar] [CrossRef]
- Ambade, V.N.; Sontakke, A.N.; Barthwal; Tyagi, R.; Basannar, D.R. Diagnostic Utility of Biomarkers in COPD. Respir. Care 2015, 60, 1729–1742. [Google Scholar] [CrossRef]
- Cristovao, C.; Cristovao, L.; Nogueira, F.; Bicho, M. Evaluation of the oxidant and antioxidant balance in the pathogenesis of chronic obstructive pulmonary disease. Rev. Port. Pneumol. 2013, 19, 70–75. [Google Scholar] [CrossRef]
- Lakhdar, R.; Denden, S.; Mouhamed, M.H.; Chalgoum, A.; Leban, N.; Knani, J.; Lefranc, G.; Miled, A.; Ben Chibani, J.; Khelil, A.H. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp. Lung Res. 2011, 37, 195–204. [Google Scholar] [CrossRef]
- Dillard, C.J.; Tappel, A.L. [41] Fluorescent damage products of lipid peroxidation. Methods Enzym. 1984, 105, 337–341. [Google Scholar] [CrossRef]
- Jensen, M.K.; Wang, Y.; Rimm, E.B.; Townsend, M.K.; Willett, W.; Wu, T. Fluorescent Oxidation Products and Risk of Coronary Heart Disease: A Prospective Study in Women. J. Am. Heart Assoc. 2013, 2, e000195. [Google Scholar] [CrossRef]
- Wu, T.; Rifai, N.; Willett, W.C.; Rimm, E.B. Plasma Fluorescent Oxidation Products: Independent Predictors of Coronary Heart Disease in Men. Am. J. Epidemiol. 2007, 166, 544–551. [Google Scholar] [CrossRef]
- Wu, T.; Willett, W.C.; Rifai, N.; Rimm, E.B. Plasma Fluorescent Oxidation Products as Potential Markers of Oxidative Stress for Epidemiologic Studies. Am. J. Epidemiol. 2007, 166, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Havet, A.; Li, Z.; Zerimech, F.; Sanchez, M.; Siroux, V.; Le Moual, N.; Brunekreef, B.; Künzli, N.; Jacquemin, B.; Varraso, R.; et al. Does the oxidative stress play a role in the associations between outdoor air pollution and persistent asthma in adults? Findings from the EGEA study. Environ. Health 2019, 18, 90. [Google Scholar] [CrossRef]
- Orsi, L.; Margaritte-Jeannin, P.; Andrianjafimasy, M.; Dumas, O.; Mohamdi, H.; Bouzigon, E.; Demenais, F.; Matran, R.; Zerimech, F.; Nadif, R.; et al. Genome-Wide Association Study of Fluorescent Oxidation Products Accounting for Tobacco Smoking Status in Adults from the French EGEA Study. Antioxidants 2022, 11, 802. [Google Scholar] [CrossRef] [PubMed]
- Bridges, A.B.; Scott, N.A.; Belch, J.J.F.; Pringle, T.H.; Mcneill, G.P. Relationship between the extent of coronary artery disease and indicators of free radical activity. Clin. Cardiol. 1992, 15, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-R.; Lu, T.-T.; Chang, H.-T.; Ge, X.; Huang, B.; Li, W.-M. Elevated Levels of Plasma Superoxide Dismutases 1 and 2 in Patients with Coronary Artery Disease. BioMed Res. Int. 2016, 2016, 3708905. [Google Scholar] [CrossRef]
- Shramko, V.S.; Striukova, E.V.; Polonskaya, Y.V.; Stakhneva, E.M.; Volkova, M.V.; Kurguzov, A.V.; Kashtanova, E.V.; Ragino, Y.I. Associations of Antioxidant Enzymes with the Concentration of Fatty Acids in the Blood of Men with Coronary Artery Atherosclerosis. J. Pers. Med. 2021, 11, 1281. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, J.; Nijs, J.; Ickmans, K.; Godderis, L.; Ghosh, M.; Polli, A. The Interplay between Oxidative Stress, Exercise, and Pain in Health and Disease: Potential Role of Autonomic Regulation and Epigenetic Mechanisms. Antioxidants 2020, 9, 1166. [Google Scholar] [CrossRef]
- Lenard, Z.; Studinger, P.; Mersich, B.; Kocsis, L.; Kollai, M. Maturation of Cardiovagal Autonomic Function From Childhood to Young Adult Age. Circulation 2004, 110, 2307–2312. [Google Scholar] [CrossRef] [PubMed]
- Promsrisuk, T.; Boonla, O.; Kongsui, R.; Sriraksa, N.; Thongrong, S.; Srithawong, A. Oxidative stress associated with impaired autonomic control and severity of lung function in chronic obstructive pulmonary disease patients. J. Exerc. Rehabil. 2023, 19, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Horváth, I.; Argay, K.; Herjavecz, I.; Rollai, M. Relation Between Bronchial and Cardiac Vagal Tone in Healthy Humans. Chest 1995, 108, 701–705. [Google Scholar] [CrossRef]
- Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation 1996, 93, 1043–1065.
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, J.S.; Aldhahir, A.M.; Alghamdi, S.M.; Al Ghamdi, S.S.; Al Draiwiesh, I.A.; Alsulayyim, A.S.; Alqahtani, A.S.; Alobaidi, N.Y.; Al Saikhan, L.; Al Rabeeah, S.M.; et al. A systematic review and meta-analysis of heart rate variability in COPD. Front. Cardiovasc. Med. 2023, 10, 1070327. [Google Scholar] [CrossRef]
- Incalzi, R.A.; Corsonello, A.; Trojano, L.; Pedone, C.; Acanfora, D.; Spada, A.; D’addio, G.; Maestri, R.; Rengo, F.; Rengo, G. Heart rate variability and drawing impairment in hypoxemic COPD. Brain Cogn. 2009, 70, 163–170. [Google Scholar] [CrossRef]
- MacDonald, D.M.; Mkorombindo, T.; Ling, S.X.; Adabag, S.; Casaburi, R.; Connett, J.E.; Helgeson, E.S.; Porszasz, J.; Rossiter, H.B.; Stringer, W.W.; et al. Heart Rate Variability on 10-Second Electrocardiogram and Risk of Acute Exacerbation of COPD: A Secondary Analysis of the BLOCK COPD Trial. Chronic Obstr. Pulm. Dis. 2022, 9, 226–236. [Google Scholar] [CrossRef]
- Coviello, I.; Pinnacchio, G.; Laurito, M.; Stazi, A.; Battipaglia, I.; Barone, L.; Mollo, R.; Russo, G.; Villano, A.; Sestito, A.; et al. Prognostic Role of Heart Rate Variability in Patients with ST-Segment Elevation Acute Myocardial Infarction Treated by Primary Angioplasty. Cardiology 2013, 124, 63–70. [Google Scholar] [CrossRef]
- Karp, E.; Shiyovich, A.; Zahger, D.; Gilutz, H.; Grosbard, A.; Katz, A. Ultra-Short-Term Heart Rate Variability for Early Risk Stratification following Acute ST-Elevation Myocardial Infarction. Cardiology 2009, 114, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Kienzle, M.G.; Ferguson, D.W.; Birkett, C.L.; Myers, G.A.; Berg, W.J.; Mariano, D. Clinical, hemodynamic and sympathetic neural correlates of heart rate variability in congestive heart failure. Am. J. Cardiol. 1992, 69, 761–767. [Google Scholar] [CrossRef]
- Stein, P.K.; Domitrovich, P.P.; Huikuri, H.V.; Kleiger, R.E. Cast Investigators Traditional and Nonlinear Heart Rate Variability Are Each Independently Associated with Mortality after Myocardial Infarction. J. Cardiovasc. Electrophysiol. 2005, 16, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Chahine, T.; Baccarelli, A.; Litonjua, A.; Wright, R.O.; Suh, H.; Gold, D.R.; Sparrow, D.; Vokonas, P.; Schwartz, J. Particulate Air Pollution, Oxidative Stress Genes, and Heart Rate Variability in an Elderly Cohort. Environ. Health Perspect. 2007, 115, 1617–1622. [Google Scholar] [CrossRef]
- Chan, C.-C.; Lin, L.-Y.; Lai, C.-H.; Chuang, K.-J.; Wu, M.-T.; Pan, C.-H. Association of Particulate Matter from Cooking Oil Fumes with Heart Rate Variability and Oxidative Stress. Antioxidants 2021, 10, 1323. [Google Scholar] [CrossRef]
- Wang, C.; Lin, J.; Niu, Y.; Wang, W.; Wen, J.; Lv, L.; Liu, C.; Du, X.; Zhang, Q.; Chen, B.; et al. Impact of ozone exposure on heart rate variability and stress hormones: A randomized-crossover study. J. Hazard. Mater. 2022, 421, 126750. [Google Scholar] [CrossRef] [PubMed]
- Antali, F.; Kulin, D.; Lucz, K.I.; Szabó, B.; Szűcs, L.; Kulin, S.; Miklós, Z. Multimodal Assessment of the Pulse Rate Variability Analysis Module of a Photoplethysmography-Based Telemedicine System. Sensors 2021, 21, 5544. [Google Scholar] [CrossRef]
- Couillard, A.; Koechlin-Ramonatxo, C.; Cristol, J.; Varray, A.; Prefaut, C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur. Respir. J. 2002, 20, 1123–1129. [Google Scholar] [CrossRef]
- Premanand, R.; Kumar, S.; Mohan, A. Study of thiobarbituric reactive substances and total reduced glutathione as indices of oxidative stress in chronic smokers with and without chronic obstructive pulmonary disease. Indian J. Chest Dis. Allied Sci. 2007, 49, 9–12. [Google Scholar]
- Montuschi, P.; Kharitonov, S.A.; Barnes, P.J. Exhaled Carbon Monoxide and Nitric Oxide in COPD. Chest 2001, 120, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Paredi, P.; Kharitonov, S.A.; Leak, D.; Ward, S.; Cramer, D.; Barnes, P.J. Exhaled Ethane, a Marker of Lipid Peroxidation, Is Elevated in Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2000, 162, 369–373. [Google Scholar] [CrossRef]
- Kostikas, K.; Papatheodorou, G.; Psathakis, K.; Panagou, P.; Loukides, S. Oxidative Stress in Expired Breath Condensate of Patients with COPD. Chest 2003, 124, 1373–1380. [Google Scholar] [CrossRef]
- Dekhuijzen, P.N.; Aben, K.; Dekker, I.; Aarts, L.; Wielders, P.; Van Herwaarden, C.; Bast, A. Increased exhalation of hydrogen peroxide in patients with stable and unstable chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 1996, 154, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Warwick, G.; Thomas, P.S.; Yates, D.H. Non-invasive biomarkers in exacerbations of obstructive lung disease. Respirology 2013, 18, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Antus, B.; Barta, I.; Kullmann, T.; Lazar, Z.; Valyon, M.; Horváth, I.; Csiszér, E. Assessment of Exhaled Breath Condensate pH in Exacerbations of Asthma and Chronic Obstructive Pulmonary Disease: A longitudinal study. Am. J. Respir. Crit. Care Med. 2010, 182, 1492–1497. [Google Scholar] [CrossRef]
- Biernacki, W.A.; Kharitonov, S.A.; Barnes, P.J. Increased leukotriene B4 and 8-isoprostane in exhaled breath condensate of patients with exacerbations of COPD. Thorax 2003, 58, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Montuschi, P.; Kharitonov, S.A.; Ciabattoni, G.; Barnes, P.J. Exhaled leukotrienes and prostaglandins in COPD. Thorax 2003, 58, 585–588. [Google Scholar] [CrossRef]
- Ashfaq, S.; Abramson, J.L.; Jones, D.P.; Rhodes, S.D.; Weintraub, W.S.; Hooper, W.C.; Vaccarino, V.; Harrison, D.G.; Quyyumi, A.A. The Relationship Between Plasma Levels of Oxidized and Reduced Thiols and Early Atherosclerosis in Healthy Adults. J. Am. Coll. Cardiol. 2006, 47, 1005–1011. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Wang, L.-X.; Sun, L.; Wu, Y.; Lu, J.-M.; Zhao, S.-C.; Dai, F.-M.; Xu, B.-S.; Wang, S.-R. Elevated peroxidative glutathione redox status in atherosclerotic patients with increased thickness of carotid intima media. Chin. Med. J. 2009, 122, 2827–2832. [Google Scholar]
- Rybka, J.; Kupczyk, D.; Kędziora-Kornatowska, K.; Motyl, J.; Czuczejko, J.; Szewczyk-Golec, K.; Kozakiewicz, M.; Pawluk, H.; Carvalho, L.A.; Kędziora, J. Glutathione-Related Antioxidant Defense System in Elderly Patients Treated for Hypertension. Cardiovasc. Toxicol. 2011, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Isogawa, A.; Yamakado, M.; Yano, M.; Shiba, T. Serum superoxide dismutase activity correlates with the components of metabolic syndrome or carotid artery intima-media thickness. Diabetes Res. Clin. Pract. 2009, 86, 213–218. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Gómez-Hermosillo, L.; Casillas-Moreno, J.; Pacheco-Moisés, F.; Campos-Bayardo, T.I.; Román-Rojas, D.; Miranda-Díaz, A.G. Prevalence of Hypertension and Obesity: Profile of Mitochondrial Function and Markers of Inflammation and Oxidative Stress. Antioxidants 2023, 12, 165. [Google Scholar] [CrossRef] [PubMed]
- Espinola-Klein, C.; Rupprecht, H.J.; Bickel, C.; Schnabel, R.; Genth-Zotz, S.; Torzewski, M.; Lackner, K.; Munzel, T.; Blankenberg, S. Glutathione Peroxidase-1 Activity, Atherosclerotic Burden, and Cardiovascular Prognosis. Am. J. Cardiol. 2007, 99, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Van Zyl, C.; Huisman, H.W.; Mels, C.M. Antioxidant enzyme activity is associated with blood pressure and carotid intima media thickness in black men and women: The SABPA study. Atherosclerosis 2016, 248, 91–96. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J. Glutathione Peroxidase 1 Activity and Cardiovascular Events in Patients with Coronary Artery Disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef]
- Armas-Padilla, M.C.; Armas-Hernández, M.J.; Sosa-Canache, B.; Cammarata, R.; Pacheco, B.; Guerrero, J.; Carvajal, A.R.; Hernández-Hernández, R.; Israili, Z.H.; Valasco, M. Nitric Oxide and Malondialdehyde in Human Hypertension. Am. J. Ther. 2007, 14, 172–176. [Google Scholar] [CrossRef]
- Zuin, M.; Capatti, E.; Borghi, C.; Zuliani, G. Serum Malondialdehyde Levels in Hypertensive Patients: A Non-invasive Marker of Oxidative Stress. A Systematic Review and Meta-analysis. High Blood Press. Cardiovasc. Prev. 2022, 29, 263–273. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Nicklas, B.J.; Kanaya, A.M.; Satterfield, S.; Lakatta, E.; Simonsick, E.M.; Sutton-Tyrrell, K.; Kritchevsky, S. Plasma Oxidized Low-Density Lipoprotein Levels and Arterial Stiffness in Older Adults: The health, aging, and body composition study. Hypertension 2009, 53, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, J.; Economou, M.; Papadimitriou, L.; Stefanadis, C. The association between pre-hypertension status and oxidative stress markers related to atherosclerotic disease: The ATTICA study. Atherosclerosis 2007, 192, 169–176. [Google Scholar] [CrossRef]
- Frostegård, J.; Wu, R.; Lemne, C.; Thulin, T.; Witztum, J.L.; De Faire, U. Circulating oxidized low-density lipoprotein is increased in hypertension. Clin. Sci. 2003, 105, 615–620. [Google Scholar] [CrossRef]
- Holvoet, P.; Vanhaecke, J.; Janssens, S.; Van De Werf, F.; Collen, D. Oxidized LDL and Malondialdehyde-Modified LDL in Patients with Acute Coronary Syndromes and Stable Coronary Artery Disease. Circulation 1998, 98, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Ueda, M.; Naruko, T.; Haze, K.; Itoh, A.; Otsuka, M.; Komatsu, R.; Matsuo, T.; Itabe, H.; Takano, T.; et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation 2001, 103, 1955–1960. [Google Scholar] [CrossRef] [PubMed]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma Oxidized Low-Density Lipoprotein, a Strong Predictor for Acute Coronary Heart Disease Events in Apparently Healthy, Middle-Aged Men From the General Population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Holvoet, P.; Mertens, A.; Verhamme, P.; Bogaerts, K.; Beyens, G.; Verhaeghe, R.; Collen, D.; Muls, E.; Van de Werf, F. Circulating Oxidized LDL Is a Useful Marker for Identifying Patients With Coronary Artery Disease. Arter. Thromb. Vasc. Biol. 2001, 21, 844–848. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, B.; He, D.; Zhang, Y.; Liu, J.; Zhang, X. The relationship between oxidized low-density lipoprotein and related ratio and acute cerebral infarction. Medicine 2018, 97, e12642. [Google Scholar] [CrossRef] [PubMed]
- Uno, M.; Kitazato, K.T.; Nishi, K.; Itabe, H.; Nagahiro, S. Raised plasma oxidised LDL in acute cerebral infarction. J. Neurol. Neurosurg. Psychiatry 2003, 74, 312–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Dai, L.; Zhang, N.; Lin, J.; Chen, G.; Zuo, Y.; Li, H.; Wang, Y.; Meng, X.; Wang, Y. Oxidized low-density lipoprotein (LDL) and LDL cholesterol are associated with outcomes of minor stroke and TIA. Atherosclerosis 2020, 297, 74–80. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Wang, W.; Kang, P.M. Oxidative Stress and Antioxidant Treatments in Cardiovascular Diseases. Antioxidants 2020, 9, 1292. [Google Scholar] [CrossRef]
- Fang, T.; Yang, J.; Liu, L.; Xiao, H.; Wei, X. Nicotinamide mononucleotide ameliorates senescence in alveolar epithelial cells. Medcomm 2021, 2, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Kiss, T.; Balasubramanian, P.; Valcarcel-Ares, M.N.; Tarantini, S.; Yabluchanskiy, A.; Csipo, T.; Lipecz, A.; Reglodi, D.; Zhang, X.A.; Bari, F.; et al. Nicotinamide mononucleotide (NMN) treatment attenuates oxidative stress and rescues angiogenic capacity in aged cerebromicrovascular endothelial cells: A potential mechanism for the prevention of vascular cognitive impairment. Geroscience 2019, 41, 619–630. [Google Scholar] [CrossRef]
- Tarantini, S.; Valcarcel-Ares, M.N.; Toth, P.; Yabluchanskiy, A.; Tucsek, Z.; Kiss, T.; Hertelendy, P.; Kinter, M.; Ballabh, P.; Süle, Z.; et al. Nicotinamide mononucleotide (NMN) supplementation rescues cerebromicrovascular endothelial function and neurovascular coupling responses and improves cognitive function in aged mice. Redox Biol. 2019, 24, 101192. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, C.-L.; Li, P.; Li, H.-R.; Liu, Q.; Deng, X.-M.; Wang, J.-F. Nicotinamide Mononucleotide Attenuates LPS-Induced Acute Lung Injury With Anti-Inflammatory, Anti-Oxidative and Anti-Apoptotic Effects. J. Surg. Res. 2023, 283, 9–18. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Sun, A.; Ge, J. The effects of nicotinamide adenine dinucleotide in cardiovascular diseases: Molecular mechanisms, roles and therapeutic potential. Genes Dis. 2022, 9, 959–972. [Google Scholar] [CrossRef]
- Batlahally, S.; Franklin, A.; Damianos, A.; Huang, J.; Chen, P.; Sharma, M.; Duara, J.; Keerthy, D.; Zambrano, R.; Shehadeh, L.A.; et al. Soluble Klotho, a biomarker and therapeutic strategy to reduce bronchopulmonary dysplasia and pulmonary hypertension in preterm infants. Sci. Rep. 2020, 10, 12368. [Google Scholar] [CrossRef]
- Takenaka, T.; Kobori, H.; Miyazaki, T.; Suzuki, H.; Nishiyama, A.; Ishii, N.; Yamashita, M.; Hayashi, M. Klotho protein supplementation reduces blood pressure and renal hypertrophy in db/db mice, a model of type 2 diabetes. Acta Physiol. 2018, 225, e13190. [Google Scholar] [CrossRef]
- El Assar, M.; Álvarez-Bustos, A.; Sosa, P.; Angulo, J.; Rodríguez-Mañas, L. Effect of Physical Activity/Exercise on Oxidative Stress and Inflammation in Muscle and Vascular Aging. Int. J. Mol. Sci. 2022, 23, 8713. [Google Scholar] [CrossRef]
- Ye, Y.; Lin, H.; Wan, M.; Qiu, P.; Xia, R.; He, J.; Tao, J.; Chen, L.; Zheng, G. The Effects of Aerobic Exercise on Oxidative Stress in Older Adults: A Systematic Review and Meta-Analysis. Front. Physiol. 2021, 12, 701151. [Google Scholar] [CrossRef]
- Kelemen, Z.; Drozdovszky, O.; Kerti, M.; Balogh, Z.; Barta, I.; Antus, B.; Varga, J.T. The effect of pulmonary rehabilitation on oxidative stress after acute exacerbation in COPD. Eur. Respir. J. 2016, 48, PA3786. [Google Scholar] [CrossRef]
- Pinho, R.; Chiesa, D.; Mezzomo, K.; Andrades, M.; Bonatto, F.; Gelain, D.; Pizzol, F.D.; Knorst, M.; Moreira, J. Oxidative stress in chronic obstructive pulmonary disease patients submitted to a rehabilitation program. Respir. Med. 2007, 101, 1830–1835. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.; Wilkinson, T.M.A.; Freeman, A. Evidence Around the Impact of Pulmonary Rehabilitation and Exercise on Redox Status in COPD: A Systematic Review. Front. Sports Act. Living 2021, 3, 782590. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).