Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Chemical Characterization of the Feedstock
2.3. Experimental Design for Extraction Using Deep Eutectic Solvents (DES)
2.4. Chemical Characterization of Liquid and Solid Fractions after the Extraction
2.5. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.6. Antioxdant Capacity Assays
2.7. Identification and Quantification of Phytochemicals Using HPLC-ESI
2.8. Processing of the Phenolic-Free Avocado Seed to Increase Enzymatic Digestibility: DES-Delignification or Microwave-Asssisted Autohydrolysis
2.9. Enzymatic Susceptiblity of the Spent Solid
2.10. Statiscal Analysis
3. Results
3.1. Feedstock Characterization
3.2. Selection of Solvent
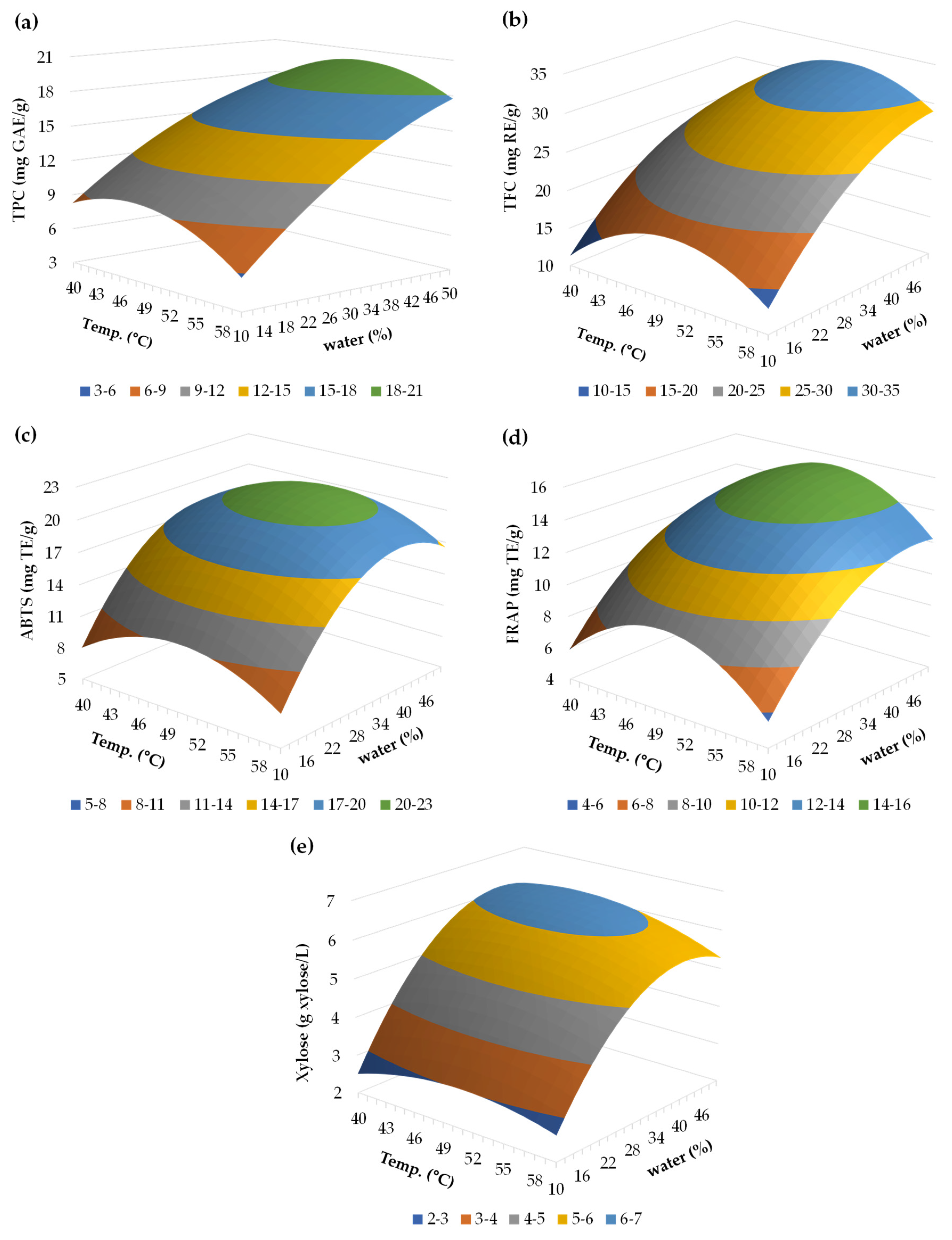
3.3. Optimization of Extraction Conditions with DES
3.3.1. Total Phenolic Content (TPC)
3.3.2. Total Flavonoid Content (TFC)
3.3.3. Antioxidant Capacity (ABTS and FRAP)
3.3.4. Xylose Content
3.4. Optimization of the Conditions and Validation of the Model
3.5. Identification of Phytochemicals
3.6. Processing of the Phenolic-Free Avocado Seed to Increase Enzymatic Digestibility: DES-Delignification or Microwave-Asssisted Autohydrolysis
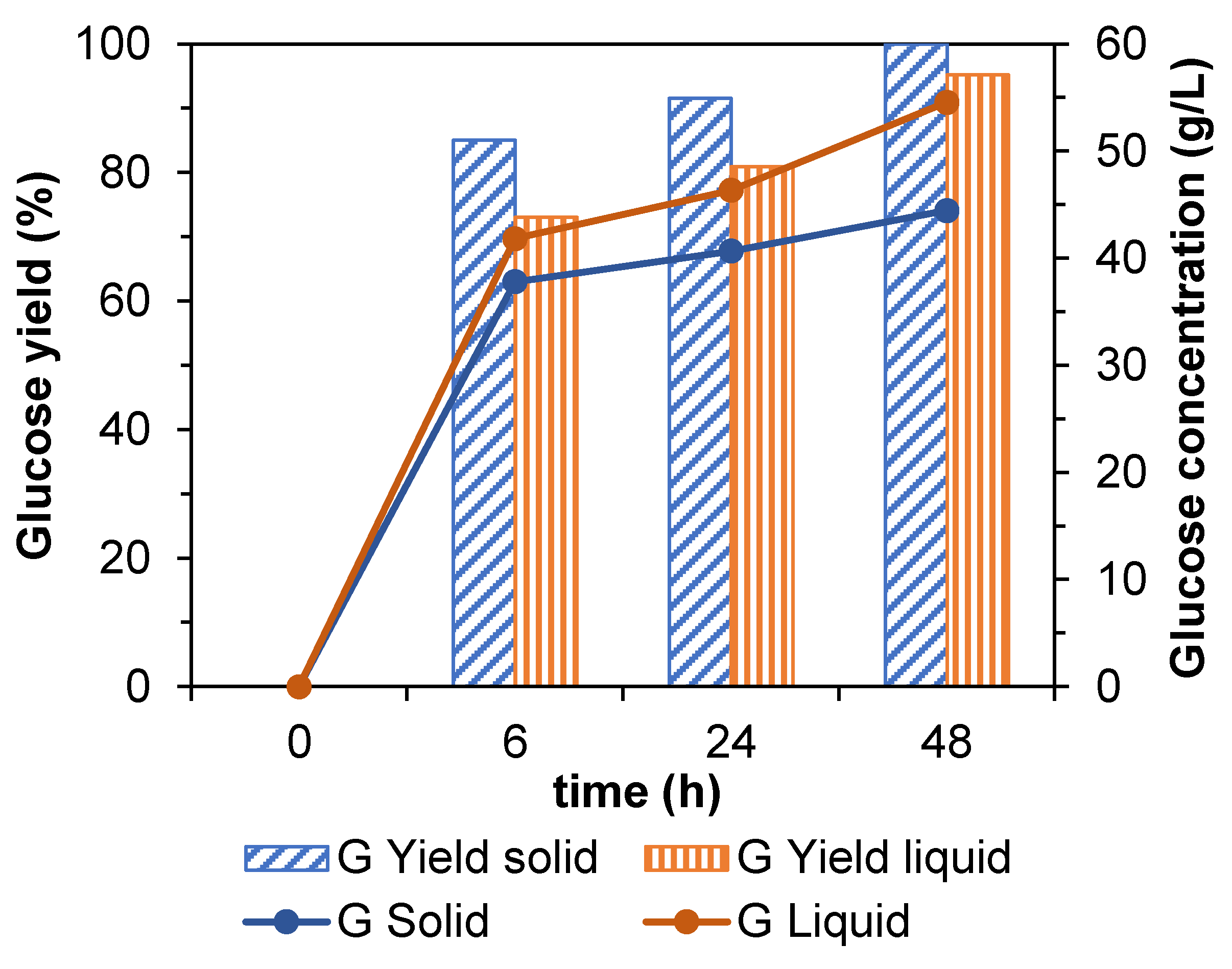
3.7. Enzymatic Hydrolysis of the Spent Solids of Avocado Seed after Processing
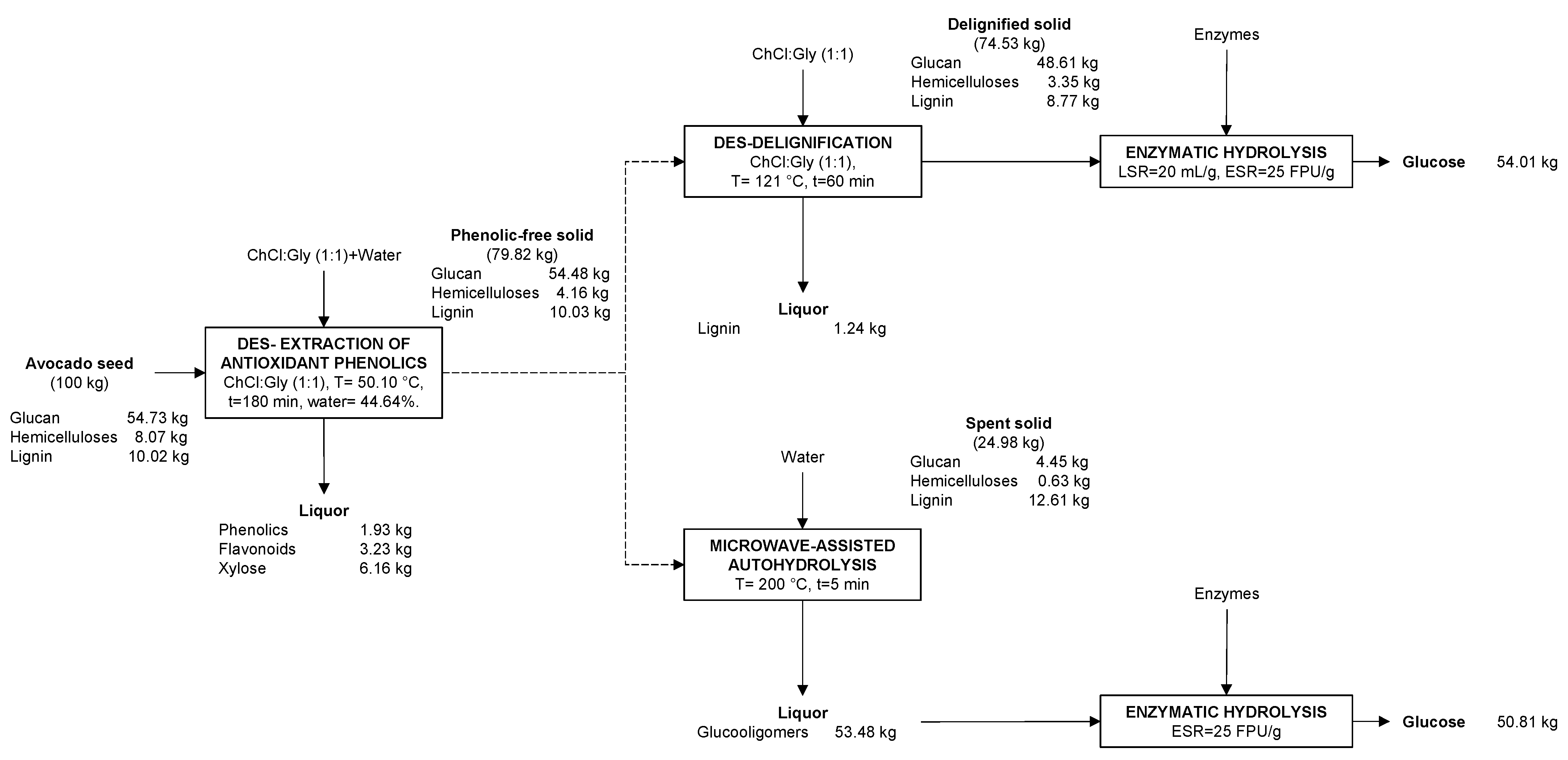
3.8. Mass Balance of the Overall Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and Recovery of Valuable Bioactive Compounds from Potato Peels: A Comprehensive Review. Antioxidants 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Pintado, M.M.E.; Aguilar, C.N. Avocado By-Products: Nutritional and Functional Properties. Trends Food Sci. Technol. 2018, 80, 51–60. [Google Scholar] [CrossRef]
- Figueroa, J.G.; Borrás-Linares, I.; Lozano-Sánchez, J.; Quirantes-Piné, R.; Segura-Carretero, A. Optimization of Drying Process and Pressurized Liquid Extraction for Recovery of Bioactive Compounds from Avocado Peel By-Product. Electrophoresis 2018, 39, 1908–1916. [Google Scholar] [CrossRef]
- Del Castillo-Llamosas, A.; del Río, P.G.; Pérez-Pérez, A.; Yáñez, R.; Garrote, G.; Gullón, B. Recent Advances to Recover Value-Added Compounds from Avocado by-Products Following a Biorefinery Approach. Curr. Opin. Green Sustain. Chem. 2021, 28, 100433. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Romaní, A.; Eibes, G.; Garrote, G.; Gullón, B.; del Río, P.G. Potential and Prospects for Utilization of Avocado By-Products in Integrated Biorefineries. Bioresour. Technol. 2022, 364, 128034. [Google Scholar] [CrossRef]
- Tamayo-Ramos, D.I.; Salazar-González, J.A.; Casson, S.A.; Urrea-López, R. Old and New Horizons on Persea Americana Transformation Techniques and Applications. Plant Cell Tissue Organ Cult. 2022, 150, 253–266. [Google Scholar] [CrossRef]
- FAO. FAOSTAT-Producción Agrícola. Available online: https://www.fao.org/faostat/en/#data (accessed on 27 December 2022).
- FAO. Major Tropical Fruits—Preliminary Results 2020; FAO Statistical Compendium: Rome, Italy, 2021; Volume 1, p. 18. [Google Scholar]
- Chel-Guerrero, L.; Barbosa-Martín, E.; Martínez-Antonio, A.; González-Mondragón, E.; Betancur-Ancona, D. Some Physicochemical and Rheological Properties of Starch Isolated from Avocado Seeds. Int. J. Biol. Macromol. 2016, 86, 302–308. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Pasini, F.; Caboni, M.F.; Segura-Carretero, A.; Fernández-Gutiérrez, A. HPLC-DAD-ESI-QTOF-MS and HPLC-FLD-MS as Valuable Tools for the Determination of Phenolic and Other Polar Compounds in the Edible Part and by-Products of Avocado. LWT 2016, 73, 505–513. [Google Scholar] [CrossRef]
- Mora-Sandí, A.; Ramírez-González, A.; Castillo-Henríquez, L.; Lopretti-Correa, M.; Vega-Baudrit, J.R. Persea Americana Agro-Industrial Waste Biorefinery for Sustainable High-Value-Added Products. Polymers 2021, 13, 1727. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruíz, H.A.; Govea-Salas, M.; Pintado, M.; Aguilar, C.N. Recovery of Bioactive Components from Avocado Peels Using Microwave-Assisted Extraction. Food Bioprod. Process. 2021, 127, 152–161. [Google Scholar] [CrossRef]
- Páramos, P.R.S.; Granjo, J.F.O.; Corazza, M.L.; Matos, H.A. Extraction of High Value Products from Avocado Waste Biomass. J. Supercrit. Fluids 2020, 165, 104988. [Google Scholar] [CrossRef]
- Gao, M.Z.; Cui, Q.; Wang, L.T.; Meng, Y.; Yu, L.; Li, Y.Y.; Fu, Y.J. A Green and Integrated Strategy for Enhanced Phenolic Compounds Extraction from Mulberry (Morus alba L.) Leaves by Deep Eutectic Solvent. Microchem. J. 2020, 154, 104598. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Zhao, R.; Wang, D.; Ma, Y.; Ai, L. Plant Natural Products: Promising Resources For. Molecules 2021, 26, 933. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Ferreira-Santos, P.; Alfonso, I.M.; Martínez, S.; Genisheva, Z.; Gullón, B. Deep Eutectic Solvents as a Green Tool for the Extraction of Bioactive Phenolic Compounds from Avocado Peels. Molecules 2022, 27, 6646. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Ayumi Shiwaku, I.; Maximo, G.J.; Caldas Batista, E.A. Choline Chloride-Based Deep Eutectic Solvents as Potential Solvent for Extraction of Phenolic Compounds from Olive Leaves: Extraction Optimization and Solvent Characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and Green Extraction of Bioactive Compounds from Lippia Citriodora by Tailor-Made Natural Deep Eutectic Solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Benvenutti, L.; Zielinski, A.A.F.; Ferreira, S.R.S. Pressurized Aqueous Solutions of Deep Eutectic Solvent (DES): A Green Emergent Extraction of Anthocyanins from a Brazilian Berry Processing by-Product. Food Chem. X 2022, 13, 100236. [Google Scholar] [CrossRef]
- Pontes, P.V.d.A.; Czaikoski, A.; Almeida, N.A.; Fraga, S.; Rocha, L.d.O.; Cunha, R.L.; Maximo, G.J.; Batista, E.A.C. Extraction Optimization, Biological Activities, and Application in O/W Emulsion of Deep Eutectic Solvents-Based Phenolic Extracts from Olive Pomace. Food Res. Int. 2022, 161, 111753. [Google Scholar] [CrossRef] [PubMed]
- Rashid, R.; Mohd Wani, S.; Manzoor, S.; Masoodi, F.A.; Masarat Dar, M. Green Extraction of Bioactive Compounds from Apple Pomace by Ultrasound Assisted Natural Deep Eutectic Solvent Extraction: Optimisation, Comparison and Bioactivity. Food Chem. 2022, 398, 133871. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Huang, M.; Tang, W.; Ma, C. Improving Enzymatic Hydrolysis of Sunflower Straw Pretreated by Deep Eutectic Solvent with Different Carboxylic Acids as Hydrogen Bond Donors. Ind. Crops Prod. 2023, 193, 116157. [Google Scholar] [CrossRef]
- Sharma, V.; Nargotra, P.; Sharma, S.; Sawhney, D.; Vaid, S.; Bangotra, R.; Dutt, H.C.; Bajaj, B.K. Microwave Irradiation-Assisted Ionic Liquid or Deep Eutectic Solvent Pretreatment for Effective Bioconversion of Sugarcane Bagasse to Bioethanol. Energy, Ecol. Environ. 2023, 8, 141–156. [Google Scholar] [CrossRef]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective Delignification of Lignocellulosic Biomass by Microwave Assisted Deep Eutectic Solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Ji, Q.; Yu, X.; Li, M.; Abiola Fakayode, O.; Yagoub, A.E.G.A.; Chen, L.; Zhou, C. Multimode-Ultrasound and Microwave Assisted Natural Ternary Deep Eutectic Solvent Sequential Pretreatments for Corn Straw Biomass Deconstruction under Mild Conditions. Ultrason. Sonochem. 2021, 72, 105414. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Kłosowski, G. High-Pressure Microwave-Assisted Pretreatment of Softwood, Hardwood and Non-Wood Biomass Using Different Solvents in the Production of Cellulosic Ethanol. Biotechnol. Biofuels Bioprod. 2023, 16, 19. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, J.W. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Nrel, J.W. Determination of Ash in Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008; Volume 36, pp. 302–305. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP); National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- del Río, P.G.; Gullón, B.; Romaní, A.; Garrote, G. Fast-Growing Paulownia Wood Fractionation by Microwave-Assisted Hydrothermal Treatment: A Kinetic Assessment. Bioresour. Technol. 2021, 338, 125535. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New Method for Quantitative Determination of Uronic Acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Blasa, M.; Candiracci, M.; Accorsi, A.; Piacentini, M.P.; Albertini, M.C.; Piatti, E. Raw Millefiori Honey Is Packed Full of Antioxidants. Food Chem. 2006, 97, 217–222. [Google Scholar] [CrossRef]
- Gullón, B.; Eibes, G.; Moreira, M.T.; Dávila, I.; Labidi, J.; Gullón, P. Antioxidant and Antimicrobial Activities of Extracts Obtained from the Refining of Autohydrolysis Liquors of Vine Shoots. Ind. Crops Prod. 2017, 107, 105–113. [Google Scholar] [CrossRef]
- Ghose, T.K. Measurement of Cellulase Activities. Int. J. Sel. Assess. 1987, 3, 245–247. [Google Scholar] [CrossRef]
- Paquot, P.M.; Thonart, P. Hydrolyse Enzymatique de La Ceilulose Régénérée. Holzforschung 1982, 36, 177–181. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodriguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Pintado, M.E.; Aguilar, C.N. Process Optimization of Microwave-Assisted Extraction of Bioactive Molecules from Avocado Seeds. Ind. Crops Prod. 2020, 154, 112623. [Google Scholar] [CrossRef]
- Oubannin, S.; Bijla, L.; Gagour, J.; Hajir, J.; Aabd, N.A.; Sakar, E.H.; Salama, M.A.; Gharby, S. A Comparative Evaluation of Proximate Composition, Elemental Profiling and Oil Physicochemical Properties of Black Cumin (Nigella sativa L.) Seeds and Argan (Argania spinosa L. Skeels) Kernels. Chem. Data Collect. 2022, 41, 100920. [Google Scholar] [CrossRef]
- Hernández, V.; Romero-García, J.M.; Dávila, J.A.; Castro, E.; Cardona, C.A. Techno-Economic and Environmental Assessment of an Olive Stone Based Biorefinery. Resour. Conserv. Recycl. 2014, 92, 145–150. [Google Scholar] [CrossRef]
- Liang, S.; McDonald, A.G. Chemical and Thermal Characterization of Potato Peel Waste and Its Fermentation Residue as Potential Resources for Biofuel and Bioproducts Production. J. Agric. Food Chem. 2014, 62, 8421–8429. [Google Scholar] [CrossRef]
- Shang, X.; Zhang, M.; Hu, J.; Zhang, Y.; Yang, L.; Hou, X. Chemical Compositions, Extraction Optimizations, and In Vitro Bioactivities of Flavonoids from Perilla Leaves (Perillae Folium) by Microwave-Assisted Natural Deep Eutectic Solvents. Antioxidants 2023, 12, 104. [Google Scholar] [CrossRef]
- Turner, A.H.; Holbrey, J.D. Investigation of Glycerol Hydrogen-Bonding Networks in Choline Chloride/Glycerol Eutectic-Forming Liquids Using Neutron Diffraction. Phys. Chem. Chem. Phys. 2019, 21, 21782–21789. [Google Scholar] [CrossRef]
- El Kantar, S.; Rajha, H.N.; Boussetta, N.; Vorobiev, E.; Maroun, R.G.; Louka, N. Green Extraction of Polyphenols from Grapefruit Peels Using High Voltage Electrical Discharges, Deep Eutectic Solvents and Aqueous Glycerol. Food Chem. 2019, 295, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula Pedunculata Subsp. Lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Zannou, O.; Koca, I. Optimization and Stabilization of the Antioxidant Properties from Alkanet (Alkanna Tinctoria) with Natural Deep Eutectic Solvents. Arab. J. Chem. 2020, 13, 6437–6450. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Choline Chloride Derivative-Based Deep Eutectic Liquids as Novel Green Alternative Solvents for Extraction of Phenolic Compounds from Olive Leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Permal, R.; Chia, T.; Arena, G.; Fleming, C.; Chen, J.; Chen, T.; Chang, W.L.; Seale, B.; Hamid, N.; Kam, R. Converting Avocado Seeds into a Ready to Eat Snack and Analysing for Persin and Amygdalin. Food Chem. 2023, 399, 134011. [Google Scholar] [CrossRef]
- Đorđević, B.S.; Todorović, Z.B.; Troter, D.Z.; Stanojević, L.P.; Stojanović, G.S.; Đalović, I.G.; Mitrović, P.M.; Veljković, V.B. Extraction of Phenolic Compounds from Black Mustard (Brassica nigra L.) Seed by Deep Eutectic Solvents. J. Food Meas. Charact. 2021, 15, 1931–1938. [Google Scholar] [CrossRef]
- Dabetić, N.; Todorović, V.; Panić, M.; Redovniković, I.R.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Z.; Li, S.; Wang, L.; Zhang, J. Eco-Friendly and High-Efficient Extraction of Natural Antioxidants from Polygonum Aviculare Leaves Using Tailor-Made Deep Eutectic Solvents as Extractants. Sep. Purif. Technol. 2021, 262, 118339. [Google Scholar] [CrossRef]
- Chen, X.Q.; Li, Z.H.; Liu, L.L.; Wang, H.; Yang, S.H.; Zhang, J.S.; Zhang, Y. Green Extraction Using Deep Eutectic Solvents and Antioxidant Activities of Flavonoids from Two Fruits of Rubia Species. LWT 2021, 148, 111708. [Google Scholar] [CrossRef]
- del Río, P.G.; Gullón, B.; Wu, J.; Saddler, J.; Garrote, G.; Romaní, A. Current Breakthroughs in the Hardwood Biorefineries: Hydrothermal Processing for the Co-Production of Xylooligosaccharides and Bioethanol. Bioresour. Technol. 2022, 343, 126100. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Llamosas, A.; Rodríguez-Martínez, B.; del Río, P.G.; Eibes, G.; Garrote, G.; Gullón, B. Hydrothermal Treatment of Avocado Peel Waste for the Simultaneous Recovery of Oligosaccharides and Antioxidant Phenolics. Bioresour. Technol. 2021, 342, 125981. [Google Scholar] [CrossRef]
- Saavedra, J.; Córdova, A.; Navarro, R.; Díaz-Calderón, P.; Fuentealba, C.; Astudillo-Castro, C.; Toledo, L.; Enrione, J.; Galvez, L. Industrial Avocado Waste: Functional Compounds Preservation by Convective Drying Process. J. Food Eng. 2017, 198, 81–90. [Google Scholar] [CrossRef]
- Pahua-Ramos, M.E.; Ortiz-Moreno, A.; Chamorro-Cevallos, G.; Hernández-Navarro, M.D.; Garduño-Siciliano, L.; Necoechea-Mondragón, H.; Hernández-Ortega, M. Hypolipidemic Effect of Avocado (Persea Americana Mill) Seed in a Hypercholesterolemic Mouse Model. Plant Foods Hum. Nutr. 2012, 67, 10–16. [Google Scholar] [CrossRef]
- Wong, X.; Carrasco-Pozo, C.; Escobar, E.; Navarrete, P.; Blachier, F.; Andriamihaja, M.; Lan, A.; Tomé, D.; Cires, M.J.; Pastene, E.; et al. Deleterious Effect of P-Cresol on Human Colonic Epithelial Cells Prevented by Proanthocyanidin-Containing Polyphenol Extracts from Fruits and Proanthocyanidin Bacterial Metabolites. J. Agric. Food Chem. 2016, 64, 3574–3583. [Google Scholar] [CrossRef]
- Alkaltham, M.S.; Uslu, N.; Özcan, M.M.; Salamatullah, A.M.; Mohamed Ahmed, I.A.; Hayat, K. Effect of Drying Process on Oil, Phenolic Composition and Antioxidant Activity of Avocado (Cv. Hass) Fruits Harvested at Two Different Maturity Stages. LWT 2021, 148, 111716. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass Valorization by Integrating Ultrasonication and Deep Eutectic Solvents: Delignification, Cellulose Digestibility and Solvent Reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, B.; Liang, X.; Luo, J.; Wan, Y. Comparison of Corn Stover Pretreatments with Lewis Acid Catalyzed Choline Chloride, Glycerol and Choline Chloride-Glycerol Deep Eutectic Solvent. Polymers 2021, 13, 1170. [Google Scholar] [CrossRef]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal-Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- Ying, W.; Li, X.; Lian, Z.; Xu, Y.; Zhang, J. An Integrated Process Using Acetic Acid Hydrolysis and Deep Eutectic Solvent Pretreatment for Xylooligosaccharides and Monosaccharides Production from Wheat Bran. Bioresour. Technol. 2022, 363, 127966. [Google Scholar] [CrossRef] [PubMed]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. New Natural and Renewable Low Transition Temperature Mixtures (LTTMs): Screening as Solvents for Lignocellulosic Biomass Processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Umai, R.D.; Jacob, S.; Kumar, V. Deep Eutectic Solvent Pretreatment of Water Hyacinth for Improved Holocellulosic Saccharification and Fermentative Co-Production of Xylitol and Lipids Using Rhodosporidium Toruloides NCIM 3547. Fermentation 2022, 8, 591. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Wilpiszewska, K.; Spychaj, T. Deep Eutectic Solvents for Polysaccharides Processing. A Review. Carbohydr. Polym. 2018, 200, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Zhou, Y.; Zhao, L.; Wang, W.; Yang, Y.; Zhao, X.; Chen, Y.; Yao, X. Recycling of Deep Eutectic Solvent for Sustainable and Efficient Pretreatment of Corncob. Ind. Crops Prod. 2022, 183, 115005. [Google Scholar] [CrossRef]
- Lynam, J.G.; Kumar, N.; Wong, M.J. Bioresource Technology Deep Eutectic Solvents’ Ability to Solubilize Lignin, Cellulose, and Hemicellulose; Thermal Stability; and Density. Bioresour. Technol. 2017, 238, 684–689. [Google Scholar] [CrossRef]
- Zhang, C.; Xia, S.; Ma, P. Facile Pretreatment of Lignocellulosic Biomass Using Deep Eutectic Solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef]
- del Río, P.G.; Alba, P.; Garrote, G.; Gull, B. Manufacturing of Hemicellulosic Oligosaccharides from Fast-Growing Paulownia Wood via Autohydrolysis: Microwave versus Conventional Heating. Ind. Crops Prod. 2022, 187, 115313. [Google Scholar] [CrossRef]
- Amini, N.; Haritos, V.S.; Tanksale, A. Microwave Assisted Pretreatment of Eucalyptus Sawdust Enhances Enzymatic Saccharification and Maximizes Fermentable Sugar Yield. Renew. Energy 2018, 127, 653–660. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Comparison of Microwave and Conduction-Convection Heating Autohydrolysis Pretreatment for Bioethanol Production. Bioresour. Technol. 2017, 243, 273–283. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Coelho, E.; Gullón, B.; Yáñez, R.; Domingues, L. Potato Peels Waste as a Sustainable Source for Biotechnological Production of Biofuels: Process Optimization. Waste Manag. 2023, 155, 320–328. [Google Scholar] [CrossRef] [PubMed]
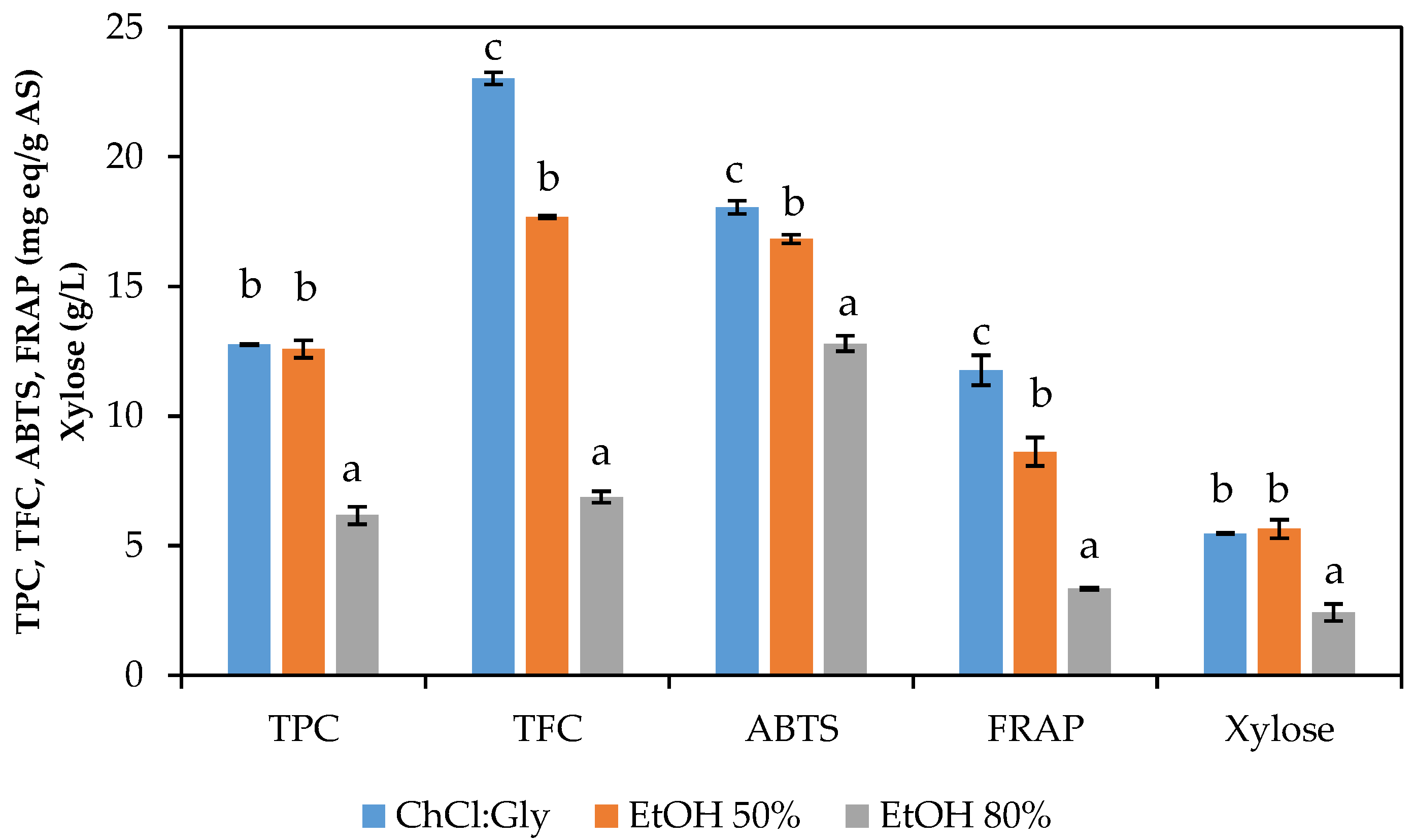
| Exp. | X1-T (°C) | x2-t (min) | x3-Water (%) | y1-TPC | y2-TFC | y3-ABTS | Y4-FRAP | Y5-Xylose |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 (60) | 1 (180) | 1 (50) | 18.02 | 30.18 | 17.22 | 12.12 | 5.64 |
| 2 | 1 (60) | 1 (180) | −1 (10) | 4.59 | 10.40 | 6.64 | 4.50 | 2.08 |
| 3 | 1 (60) | −1 (60) | 1 (50) | 15.00 | 23.40 | 13.54 | 10.10 | 4.71 |
| 4 | 1 (60) | −1 (60) | −1 (10) | 5.51 | 17.50 | 11.60 | 7.75 | 4.24 |
| 5 | −1 (40) | 1 (180) | 1 (50) | 16.82 | 22.01 | 14.65 | 11.00 | 5.53 |
| 6 | −1 (40) | −1 (60) | 1 (50) | 14.07 | 19.30 | 12.41 | 8.70 | 5.21 |
| 7 | −1 (40) | −1 (60) | −1 (10) | 4.01 | 7.98 | 6.29 | 4.60 | 1.93 |
| 8 | −1 (40) | 1 (180) | −1 (10) | 9.45 | 12.30 | 8.91 | 6.20 | 2.98 |
| 9 | 1 (60) | 0 (120) | 0 (30) | 14.70 | 25.70 | 17.72 | 11.40 | 5.71 |
| 10 | −1 (40) | 0 (120) | 0 (30) | 11.70 | 21.00 | 15.64 | 9.10 | 4.93 |
| 11 | 0 (50) | 1 (180) | 0 (30) | 16.56 | 32.10 | 21.92 | 16.20 | 6.05 |
| 12 | 0 (50) | −1 (60) | 0 (30) | 15.08 | 27.00 | 19.70 | 12.90 | 5.64 |
| 13 | 0 (50) | 0 (120) | 1 (50) | 19.90 | 31.00 | 18.28 | 14.10 | 5.73 |
| 14 | 0 (50) | 0 (120) | −1 (10) | 8.53 | 20.20 | 11.58 | 8.70 | 3.22 |
| 15 | 0 (50) | 0 (120) | 0 (30) | 14.28 | 24.10 | 18.00 | 12.00 | 5.57 |
| 16 | 0 (50) | 0 (120) | 0 (30) | 13.60 | 23.60 | 18.40 | 11.60 | 5.62 |
| 17 | 0 (50) | 0 (120) | 0 (30) | 13.00 | 25.20 | 17.74 | 11.70 | 5.48 |
| Coefficient | y1-TPC | y2-TFC | y3-ABTS | Y4-FRAP | Y5-Xylose |
|---|---|---|---|---|---|
| x0 | 14.76 a | 27.01 a | 19.16 a | 12.79 a | 5.67 a |
| x1 | 0.18 | 2.46 b | 0.88 | 0.63 | 0.18 |
| x2 | 1.18 c | 1.18 | 0.58 | 0.60 | 0.06 |
| x3 | 5.17 a | 5.75 a | 3.11 a | 2.43 a | 1.24 a |
| x12 | −0.76 | −0.92 | −0.77 | −0.64 | −0.32 |
| x13 | 0.69 | 0.58 | 0.08 | 0.13 | −0.23 |
| x23 | 0.16 | 1.53 | 1.03 | 0.75 | 0.30 |
| x11 | −2.42 c | −5.69 b | −3.31 b | −3.31 a | −0.43 |
| x22 | 0.20 | 0.51 | 0.82 | 0.99 | 0.09 |
| x33 | −1.40 | −3.44 | −5.06 a | −2.16 b | −1.27 a |
| R2 | 0.942 | 0.889 | 0.942 | 0.918 | 0.928 |
| F-exp | 12.71 | 6.21 | 12.61 | 8.75 | 9.95 |
| Significance level (%) | 99.85 | 98.75 | 99.85 | 99.54 | 99.69 |
| y1-TPC | y2-TFC | y3-ABTS | y4-FRAP | y5-Xylose | |
|---|---|---|---|---|---|
| Predicted value | 19.32 | 32.31 | 20.91 | 15.59 | 6.24 |
| Experimental value 1 | 19.71 ± 0.28 | 33.41 ± 1.76 | 22.57 ± 0.61 | 16.64 ± 0.15 | 5.47 ± 0.03 |
| Compound | Type | Content (mg/g Initial AS) |
|---|---|---|
| p-coumaric acid | Phenolic acid | 0.43 |
| Salicylic acid | Phenolic acid | 0.27 |
| Ferulic acid | Phenolic acid | 0.15 |
| Phthalic acid | Phenolic acid | 0.85 |
| Protocatechuic acid | Phenolic acid | 0.39 |
| Vanillic acid | Phenolic acid | 0.52 |
| Naringenin | Flavonoid | 1.44 |
| Apigenin | Flavonoid | 0.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del-Castillo-Llamosas, A.; Rodríguez-Rebelo, F.; Rodríguez-Martínez, B.; Mallo-Fraga, A.; Del-Río, P.G.; Gullón, B. Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES). Antioxidants 2023, 12, 1156. https://doi.org/10.3390/antiox12061156
Del-Castillo-Llamosas A, Rodríguez-Rebelo F, Rodríguez-Martínez B, Mallo-Fraga A, Del-Río PG, Gullón B. Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES). Antioxidants. 2023; 12(6):1156. https://doi.org/10.3390/antiox12061156
Chicago/Turabian StyleDel-Castillo-Llamosas, Alexandra, Fernando Rodríguez-Rebelo, Beatriz Rodríguez-Martínez, Adrián Mallo-Fraga, Pablo G. Del-Río, and Beatriz Gullón. 2023. "Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES)" Antioxidants 12, no. 6: 1156. https://doi.org/10.3390/antiox12061156
APA StyleDel-Castillo-Llamosas, A., Rodríguez-Rebelo, F., Rodríguez-Martínez, B., Mallo-Fraga, A., Del-Río, P. G., & Gullón, B. (2023). Valorization of Avocado Seed Wastes for Antioxidant Phenolics and Carbohydrates Recovery Using Deep Eutectic Solvents (DES). Antioxidants, 12(6), 1156. https://doi.org/10.3390/antiox12061156







