Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy
Abstract
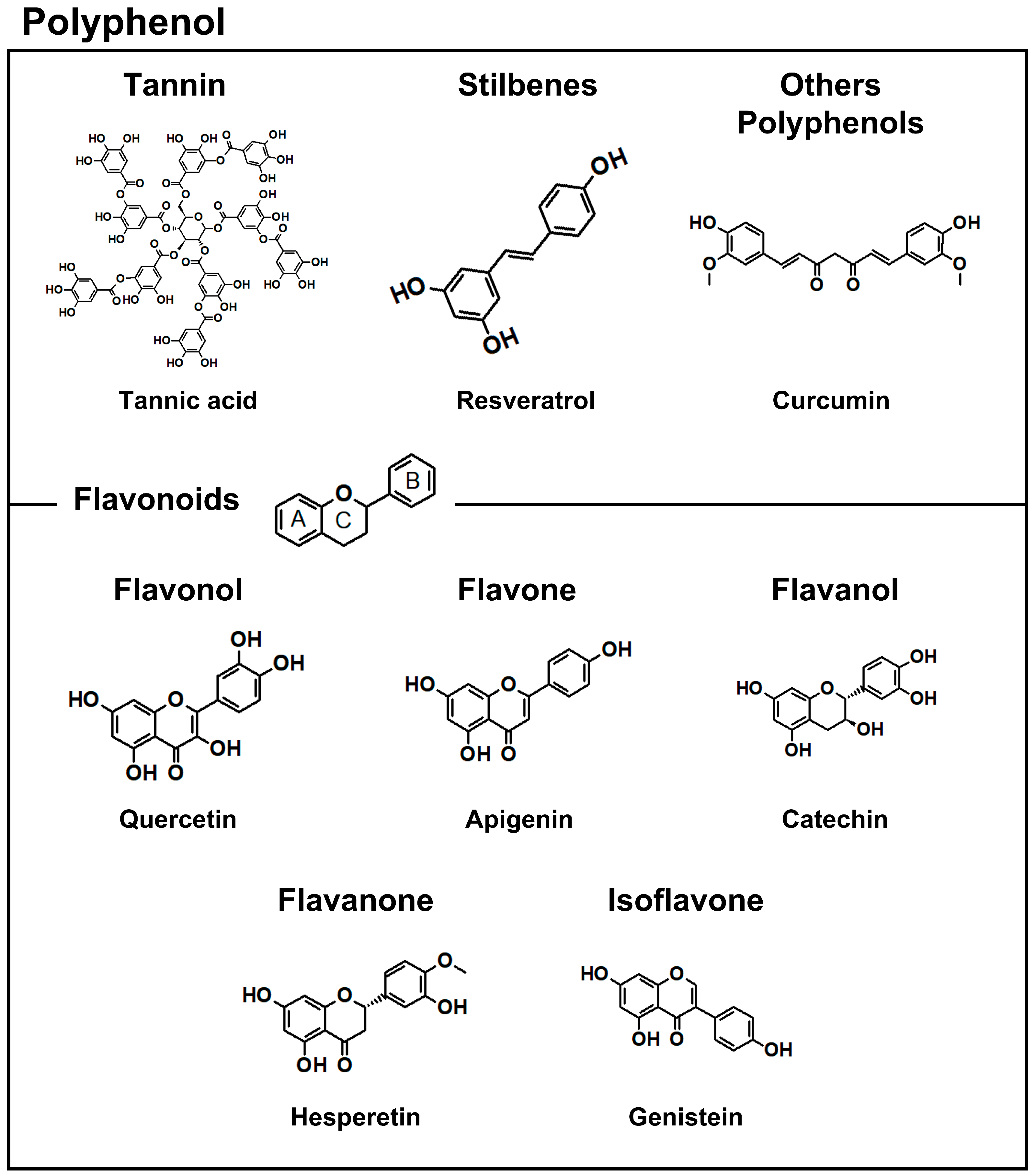
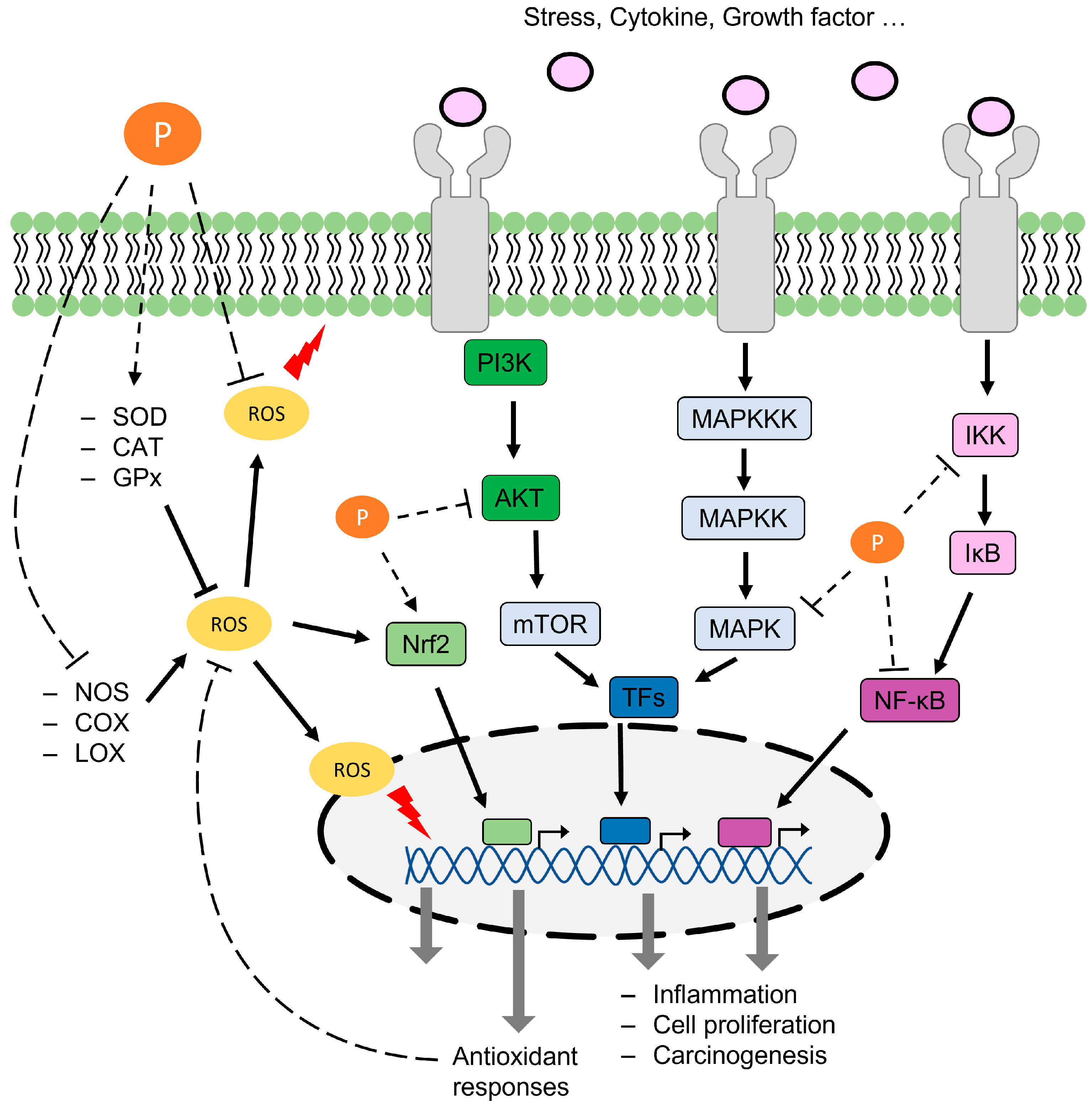
1. Introduction
2. Enhancement of Polyphenol Anticancer Effects Using DDSs
2.1. Lipid-Based System
2.1.1. Liposomes and Phytosomes
2.1.2. Nanostructured Lipid Carrier
2.2. Polymer-Based System
2.3. Protein Formulation
2.4. Other Materials
3. Consideration for Polyphenol Delivery System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Davatgaran-Taghipour, Y.; Masoomzadeh, S.; Farzaei, M.H.; Bahramsoltani, R.; Karimi-Soureh, Z.; Rahimi, R.; Abdollahi, M. Polyphenol nanoformulations for cancer therapy: Experimental evidence and clinical perspective. Int. J. Nanomed. 2017, 12, 2689–2702. [Google Scholar] [CrossRef]
- Visconti, R.; Grieco, D. New insights on oxidative stress in cancer. Curr. Opin. Drug Discov. Dev. 2009, 12, 240–245. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed]
- El-Kenawi, A.; Ruffell, B. Inflammation, ROS, and Mutagenesis. Cancer Cell 2017, 32, 727–729. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, X.; Zuo, X.; Wang, M. Chemopreventive effects of some popular phytochemicals on human colon cancer: A review. Food Funct. 2018, 9, 4548–4568. [Google Scholar] [CrossRef]
- Chen, Z.; Farag, M.A.; Zhong, Z.; Zhang, C.; Yang, Y.; Wang, S.; Wang, Y. Multifaceted role of phyto-derived polyphenols in nanodrug delivery systems. Adv. Drug Deliv. Rev. 2021, 176, 113870. [Google Scholar] [CrossRef]
- de Araujo, F.F.; de Paulo Farias, D.; Neri-Numa, I.A.; Pastore, G.M. Polyphenols and their applications: An approach in food chemistry and innovation potential. Food Chem. 2021, 338, 127535. [Google Scholar] [CrossRef]
- Dufour, C.; Loonis, M.; Delosiere, M.; Buffiere, C.; Hafnaoui, N.; Sante-Lhoutellier, V.; Remond, D. The matrix of fruit & vegetables modulates the gastrointestinal bioaccessibility of polyphenols and their impact on dietary protein digestibility. Food Chem. 2018, 240, 314–322. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal chelation of polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Cheon, B.S.; Kim, Y.H.; Son, K.S.; Chang, H.W.; Kang, S.S.; Kim, H.P. Effects of prenylated flavonoids and biflavonoids on lipopolysaccharide-induced nitric oxide production from the mouse macrophage cell line RAW 264.7. Planta Med. 2000, 66, 596–600. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Chen, P.X.; Zhang, H.; Marcone, M.F.; Pauls, K.P.; Liu, R.; Tang, Y.; Zhang, B.; Renaud, J.B.; Tsao, R. Anti-inflammatory effects of phenolic-rich cranberry bean (Phaseolus vulgaris L.) extracts and enhanced cellular antioxidant enzyme activities in Caco-2 cells. J. Funct. Foods 2017, 38, 675–685. [Google Scholar] [CrossRef]
- Salzano, S.; Checconi, P.; Hanschmann, E.M.; Lillig, C.H.; Bowler, L.D.; Chan, P.; Vaudry, D.; Mengozzi, M.; Coppo, L.; Sacre, S.; et al. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc. Natl. Acad. Sci. USA 2014, 111, 12157–12162. [Google Scholar] [CrossRef]
- Guo, W.; Kong, E.; Meydani, M. Dietary polyphenols, inflammation, and cancer. Nutr. Cancer 2009, 61, 807–810. [Google Scholar] [CrossRef]
- Petersen, K.S.; Smith, C. Ageing-Associated Oxidative Stress and Inflammation Are Alleviated by Products from Grapes. Oxid. Med. Cell. Longev. 2016, 2016, 6236309. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Holvoet, S.; Mercenier, A. Dietary polyphenols in the prevention and treatment of allergic diseases. Clin. Exp. Allergy 2011, 41, 1346–1359. [Google Scholar] [CrossRef]
- Shabbir, U.; Tyagi, A.; Elahi, F.; Aloo, S.O.; Oh, D.H. The Potential Role of Polyphenols in Oxidative Stress and Inflammation Induced by Gut Microbiota in Alzheimer’s Disease. Antioxidants 2021, 10, 1370. [Google Scholar] [CrossRef]
- Dias, A.S.; Porawski, M.; Alonso, M.; Marroni, N.; Collado, P.S.; Gonzalez-Gallego, J. Quercetin decreases oxidative stress, NF-kappaB activation, and iNOS overexpression in liver of streptozotocin-induced diabetic rats. J. Nutr. 2005, 135, 2299–2304. [Google Scholar] [CrossRef] [PubMed]
- Guan, C.; Zhou, X.; Li, H.; Ma, X.; Zhuang, J. NF-kappaB inhibitors gifted by nature: The anticancer promise of polyphenol compounds. Biomed. Pharmacother. 2022, 156, 113951. [Google Scholar] [CrossRef]
- Min, Y.-D.; Choi, C.-H.; Bark, H.; Son, H.-Y.; Park, H.-H.; Lee, S.; Park, J.-W.; Park, E.-K.; Shin, H.-I.; Kim, S.-H. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-κB and p38 MAPK in HMC-1 human mast cell line. Inflamm. Res. 2007, 56, 210–215. [Google Scholar] [CrossRef]
- Romier, B.; Van De Walle, J.; During, A.; Larondelle, Y.; Schneider, Y.J. Modulation of signalling nuclear factor-kappaB activation pathway by polyphenols in human intestinal Caco-2 cells. Br. J. Nutr. 2008, 100, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264.7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Saitoh, S.I.; Miyake, K.; Kang, K.W.; Choi, Y.J.; Hwang, D.H. Suppression of MyD88-and TRIF-dependent signaling pathways of Toll-like receptor by (−)-epigallocatechin-3-gallate, a polyphenol component of green tea. Biochem. Pharmacol. 2006, 72, 850–859. [Google Scholar] [CrossRef]
- Cavet, M.E.; Harrington, K.L.; Vollmer, T.R.; Ward, K.W.; Zhang, J.Z. Anti-inflammatory and anti-oxidative effects of the green tea polyphenol epigallocatechin gallate in human corneal epithelial cells. Mol. Vis. 2011, 17, 533–542. [Google Scholar] [PubMed]
- Garcia-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sanchez-Campos, S.; Tunon, M.J.; Gonzalez-Gallego, J. The anti-inflammatory flavones quercetin and kaempferol cause inhibition of inducible nitric oxide synthase, cyclooxygenase-2 and reactive C-protein, and down-regulation of the nuclear factor kappaB pathway in Chang Liver cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.S.; Pan, M.H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Hong, J.; Smith, T.J.; Ho, C.T.; August, D.A.; Yang, C.S. Effects of purified green and black tea polyphenols on cyclooxygenase- and lipoxygenase-dependent metabolism of arachidonic acid in human colon mucosa and colon tumor tissues. Biochem. Pharmacol. 2001, 62, 1175–1183. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, S.X.; Zhou, Z.Q.; Wang, Z.; Zhang, Y.G.; Zhang, Y.; Zhao, P. Apoptotic effect of genistein on human colon cancer cells via inhibiting the nuclear factor-kappa B (NF-kappaB) pathway. Tumour Biol. 2014, 35, 11483–11488. [Google Scholar] [CrossRef]
- Goh, Y.X.; Jalil, J.; Lam, K.W.; Husain, K.; Premakumar, C.M. Genistein: A Review on its Anti-Inflammatory Properties. Front. Pharmacol. 2022, 13, 820969. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Granato, M.; Rizzello, C.; Gilardini Montani, M.S.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef]
- Maurya, A.K.; Vinayak, M. Quercetin Attenuates Cell Survival, Inflammation, and Angiogenesis via Modulation of AKT Signaling in Murine T-Cell Lymphoma. Nutr. Cancer 2017, 69, 470–480. [Google Scholar] [CrossRef]
- Priyadarsini, R.V.; Murugan, R.S.; Maitreyi, S.; Ramalingam, K.; Karunagaran, D.; Nagini, S. The flavonoid quercetin induces cell cycle arrest and mitochondria-mediated apoptosis in human cervical cancer (HeLa) cells through p53 induction and NF-κB inhibition. Eur. J. Pharmacol. 2010, 649, 84–91. [Google Scholar] [CrossRef]
- Lee, J.; Im, Y.H.; Jung, H.H.; Kim, J.H.; Park, J.O.; Kim, K.; Kim, W.S.; Ahn, J.S.; Jung, C.W.; Park, Y.S.; et al. Curcumin inhibits interferon-alpha induced NF-kappaB and COX-2 in human A549 non-small cell lung cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 313–318. [Google Scholar] [CrossRef]
- Ghasemi, F.; Shafiee, M.; Banikazemi, Z.; Pourhanifeh, M.H.; Khanbabaei, H.; Shamshirian, A.; Amiri Moghadam, S.; ArefNezhad, R.; Sahebkar, A.; Avan, A.; et al. Curcumin inhibits NF-kB and Wnt/beta-catenin pathways in cervical cancer cells. Pathol. Res. Pract. 2019, 215, 152556. [Google Scholar] [CrossRef]
- Yu, L.L.; Wu, J.G.; Dai, N.; Yu, H.G.; Si, J.M. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-kappaB transcription factor. Oncol. Rep. 2011, 26, 1197–1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhang, Y.; Wang, Y.; Rao, J.; Jiang, X.; Xu, Z. Curcumin inhibits proliferation of breast cancer cells through Nrf2-mediated down-regulation of Fen1 expression. J. Steroid Biochem. Mol. Biol. 2014, 143, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, A.; Fathi, M.; Jafari, S.M. Nanoencapsulation of hydrophobic and low-soluble food bioactive compounds within different nanocarriers. Food Hydrocoll. 2019, 88, 146–162. [Google Scholar] [CrossRef]
- Zern, T.L.; Fernandez, M.L. Cardioprotective effects of dietary polyphenols. J. Nutr. 2005, 135, 2291–2294. [Google Scholar] [CrossRef]
- Yang, B.; Dong, Y.; Wang, F.; Zhang, Y. Nanoformulations to Enhance the Bioavailability and Physiological Functions of Polyphenols. Molecules 2020, 25, 4613. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Li, Y.; Xu, Q.; Liu, Z. Mesoporous silica nanoparticles for stimuli-responsive controlled drug delivery: Advances, challenges, and outlook. Int. J. Nanomed. 2017, 12, 87–110. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef]
- Deng, Y.; Zhang, X.; Shen, H.; He, Q.; Wu, Z.; Liao, W.; Yuan, M. Application of the Nano-Drug Delivery System in Treatment of Cardiovascular Diseases. Front. Bioeng. Biotechnol. 2019, 7, 489. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, J.; Hu, Y.; Lin, Z.; Ma, Y.; Richardson, J.J.; Caruso, F. Polyphenol-Based Nanoparticles for Intracellular Protein Delivery via Competing Supramolecular Interactions. ACS Nano 2020, 14, 12972–12981. [Google Scholar] [CrossRef]
- Neethirajan, S.; Jayas, D.S. Nanotechnology for the Food and Bioprocessing Industries. Food Bioprocess Technol. 2011, 4, 39–47. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Chen, Z.G.; Shin, D.M. Advances of cancer therapy by nanotechnology. Cancer Res. Treat. 2009, 41, 1–11. [Google Scholar] [CrossRef]
- Lagoa, R.; Silva, J.; Rodrigues, J.R.; Bishayee, A. Advances in phytochemical delivery systems for improved anticancer activity. Biotechnol. Adv. 2020, 38, 107382. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arraez-Roman, D.; Martinez-Ferez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef]
- Jhaveri, A.; Deshpande, P.; Pattni, B.; Torchilin, V. Transferrin-targeted, resveratrol-loaded liposomes for the treatment of glioblastoma. J. Control. Release 2018, 277, 89–101. [Google Scholar] [CrossRef]
- Zhao, Y.N.; Cao, Y.N.; Sun, J.; Liang, Z.; Wu, Q.; Cui, S.H.; Zhi, D.F.; Guo, S.T.; Zhen, Y.H.; Zhang, S.B. Anti-breast cancer activity of resveratrol encapsulated in liposomes. J. Mater. Chem. B 2020, 8, 27–37. [Google Scholar] [CrossRef]
- Zhou, Q.; Fu, Z. In vitro and in vivo Study of a Novel Liposome-Mediated Dual Drug Delivery for Synergistic Lung Cancer Therapy via Oral Administration. Onco Targets Ther. 2020, 13, 12695–12703. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Babazadeh, A.; Hamishehkar, H. Nano-phytosome as a potential food-grade delivery system. Food Biosci. 2016, 15, 126–135. [Google Scholar]
- Amin, T.; Bhat, S.V. A review on phytosome technology as a novel approach to improve the bioavailability of nutraceuticals. Int. J. Adv. Res. Technol. 2012, 1, 43–57. [Google Scholar]
- Vinod, K.; Sandhya, S.; Chandrashekar, J.; Swetha, R.; Rajeshwar, T.; Banji, D.; Anbuazhagan, S. A review on genesis and characterization of phytosomes. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 69–75. [Google Scholar]
- Komeil, I.A.; El-Refaie, W.M.; Gowayed, M.A.; El-Ganainy, S.O.; El Achy, S.N.; Huttunen, K.M.; Abdallah, O.Y. Oral genistein-loaded phytosomes with enhanced hepatic uptake, residence and improved therapeutic efficacy against hepatocellular carcinoma. Int. J. Pharm. 2021, 601, 120564. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Fahmy, U.A.; Alruwaili, N.K.; Awan, Z.A.; Caruso, G.; Alfaleh, M.A.; Alaofi, A.L.; Arif, F.O.; Ahmed, O.A.A.; et al. Thymoquinone-Loaded Soy-Phospholipid-Based Phytosomes Exhibit Anticancer Potential against Human Lung Cancer Cells. Pharmaceutics 2020, 12, 761. [Google Scholar] [CrossRef]
- Xie, J.; Li, Y.; Song, L.; Pan, Z.; Ye, S.; Hou, Z. Design of a novel curcumin-soybean phosphatidylcholine complex-based targeted drug delivery systems. Drug Deliv. 2017, 24, 707–719. [Google Scholar] [CrossRef]
- Wanjiru, J.; Gathirwa, J.; Sauli, E.; Swai, H.S. Formulation, Optimization, and Evaluation of Moringa oleifera Leaf Polyphenol-Loaded Phytosome Delivery System against Breast Cancer Cell Lines. Molecules 2022, 27, 4430. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Kanwal, T.; Saifullah, S.; ur Rehman, J.; Kawish, M.; Razzak, A.; Maharjan, R.; Imran, M.; Ali, I.; Roome, T.; Simjee, S.U. Design of absorption enhancer containing self-nanoemulsifying drug delivery system (SNEDDS) for curcumin improved anti-cancer activity and oral bioavailability. J. Mol. Liq. 2021, 324, 114774. [Google Scholar] [CrossRef]
- Radbeh, Z.; Asefi, N.; Hamishehkar, H.; Roufegarinejad, L.; Pezeshki, A. Novel carriers ensuring enhanced anti-cancer activity of Cornus mas (cornelian cherry) bioactive compounds. Biomed. Pharmacother. 2020, 125, 109906. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Nazari Soltan Ahmad, S.; Barghi, S.; Maroufi, N.F.; Taheri, R.A. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2018, 46, 283–292. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Targeted hyaluronic acid-based lipid nanoparticle for apigenin delivery to induce Nrf2-dependent apoptosis in lung cancer cells. J. Drug Deliv. Sci. Technol. 2019, 49, 268–276. [Google Scholar] [CrossRef]
- Wang, X.; Fan, Y.; Yan, J.; Yang, M. Engineering polyphenol-based polymeric nanoparticles for drug delivery and bioimaging. Chem. Eng. J. 2022, 439, 135661. [Google Scholar] [CrossRef]
- Tagde, P.; Kulkarni, G.T.; Mishra, D.K.; Kesharwani, P. Recent advances in folic acid engineered nanocarriers for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 2020, 56, 101613. [Google Scholar] [CrossRef]
- Mary Lazer, L.; Sadhasivam, B.; Palaniyandi, K.; Muthuswamy, T.; Ramachandran, I.; Balakrishnan, A.; Pathak, S.; Narayan, S.; Ramalingam, S. Chitosan-based nano-formulation enhances the anticancer efficacy of hesperetin. Int. J. Biol. Macromol. 2018, 107, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Sunoqrot, S.; Abujamous, L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J. Drug Deliv. Sci. Technol. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Thongnopkoon, T.; Chittasupho, C. Curcumin composite particles prepared by spray drying and in vitro anti-cancer activity on lung cancer cell line. J. Drug Deliv. Sci. Technol. 2018, 45, 397–407. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, F.; Zhang, K.; Wang, Q.; Chen, Y.; Luo, X. pH-Responsive reversibly cross-linked micelles by phenol–yne click via curcumin as a drug delivery system in cancer chemotherapy. J. Mater. Chem. B 2019, 7, 3884–3893. [Google Scholar] [CrossRef]
- Shitole, A.A.; Sharma, N.; Giram, P.; Khandwekar, A.; Baruah, M.; Garnaik, B.; Koratkar, S. LHRH-conjugated, PEGylated, poly-lactide-co-glycolide nanocapsules for targeted delivery of combinational chemotherapeutic drugs Docetaxel and Quercetin for prostate cancer. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111035. [Google Scholar] [CrossRef]
- Ma, W.; Guo, Q.; Li, Y.; Wang, X.; Wang, J.; Tu, P. Co-assembly of doxorubicin and curcumin targeted micelles for synergistic delivery and improving anti-tumor efficacy. Eur. J. Pharm. Biopharm. 2017, 112, 209–223. [Google Scholar] [CrossRef]
- Ben-Zichri, S.; Meltzer, M.; Lacham-Hartman, S.; Kolusheva, S.; Hadad, U.; Papo, N.; Jelinek, R. Synergistic Activity of Anticancer Polyphenols Embedded in Amphiphilic Dendrimer Nanoparticles. ACS Appl. Polym. Mater. 2022, 4, 8913–8925. [Google Scholar] [CrossRef]
- Kashyap, D.; Tuli, H.S.; Yerer, M.B.; Sharma, A.; Sak, K.; Srivastava, S.; Pandey, A.; Garg, V.K.; Sethi, G.; Bishayee, A. Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 2021, 69, 5–23. [Google Scholar] [CrossRef]
- Jain, A.; Singh, S.K.; Arya, S.K.; Kundu, S.C.; Kapoor, S. Protein Nanoparticles: Promising Platforms for Drug Delivery Applications. ACS Biomater. Sci. Eng. 2018, 4, 3939–3961. [Google Scholar] [CrossRef]
- Suktham, K.; Koobkokkruad, T.; Wutikhun, T.; Surassmo, S. Efficiency of resveratrol-loaded sericin nanoparticles: Promising bionanocarriers for drug delivery. Int. J. Pharm. 2018, 537, 48–56. [Google Scholar] [CrossRef]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood–brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Han, Z.; Song, B.; Yang, J.; Wang, B.; Ma, Z.; Yu, L.; Li, Y.; Xu, H.; Qiao, M. Curcumin-Encapsulated Fusion Protein-Based Nanocarrier Demonstrated Highly Efficient Epidermal Growth Factor Receptor-Targeted Treatment of Colorectal Cancer. J. Agric. Food Chem. 2022, 70, 15464–15473. [Google Scholar] [CrossRef]
- Lu, M.; Wu, M.; Huang, Y.; Yao, J.; Shao, Z.; Chen, X. Animal protein-plant protein composite nanospheres for dual-drug loading and synergistic cancer therapy. J. Mater. Chem. B 2022, 10, 3798–3807. [Google Scholar] [CrossRef]
- Razi, M.A.; Wakabayashi, R.; Tahara, Y.; Goto, M.; Kamiya, N. Genipin-stabilized caseinate-chitosan nanoparticles for enhanced stability and anti-cancer activity of curcumin. Colloids Surf. B Biointerfaces 2018, 164, 308–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, L.; Ju, Y.; Dai, Y. Recent Advances in Metal-Phenolic Networks for Cancer Theranostics. Small 2021, 17, e2100314. [Google Scholar] [CrossRef]
- Zhan, K.; Li, Z.; Chen, J.; Hou, Y.; Zhang, J.; Sun, R.; Bu, Z.; Wang, L.; Wang, M.; Chen, X. Tannic acid modified single nanopore with multivalent metal ions recognition and ultra-trace level detection. Nano Today 2020, 33, 100868. [Google Scholar] [CrossRef]
- Fan, G.; Cottet, J.; Rodriguez-Otero, M.R.; Wasuwanich, P.; Furst, A.L. Metal–phenolic networks as versatile coating materials for biomedical applications. ACS Appl. Bio Mater. 2022, 5, 4687–4695. [Google Scholar] [CrossRef]
- Liu, P.; Shi, X.; Zhong, S.; Peng, Y.; Qi, Y.; Ding, J.; Zhou, W. Metal-phenolic networks for cancer theranostics. Biomater. Sci. 2021, 9, 2825–2849. [Google Scholar] [CrossRef]
- Chen, Y.; Jia, D.; Wang, Q.; Sun, Y.; Rao, Z.; Lei, X.; Zhao, J.; Zeng, K.; Xu, Z.; Ming, J. Promotion of the anticancer activity of curcumin based on a metal–polyphenol networks delivery system. Int. J. Pharm. 2021, 602, 120650. [Google Scholar] [CrossRef]
- Linko, V.; Ora, A.; Kostiainen, M.A. DNA nanostructures as smart drug-delivery vehicles and molecular devices. Trends Biotechnol. 2015, 33, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wang, L.; Li, W.; Xu, X.; Jiang, W. DNA nanostructure-based drug delivery nanosystems in cancer therapy. Int. J. Pharm. 2017, 533, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cui, Y.; Gu, Z.; Yang, D. Controllable assembly/disassembly of polyphenol-DNA nanocomplex for cascade-responsive drug release in cancer cells. Biomaterials 2021, 273, 120846. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Cui, Y.; Li, F.; Gu, Z.; Yang, D. Responsive disassembly of nucleic acid nanocomplex in cells for precision medicine. Nano Today 2021, 39, 101160. [Google Scholar] [CrossRef]
- Maleki Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Housman, G.; Byler, S.; Heerboth, S.; Lapinska, K.; Longacre, M.; Snyder, N.; Sarkar, S. Drug resistance in cancer: An overview. Cancers 2014, 6, 1769–1792. [Google Scholar] [CrossRef]
- Hu, Q.; Sun, W.; Wang, C.; Gu, Z. Recent advances of cocktail chemotherapy by combination drug delivery systems. Adv. Drug Deliv. Rev. 2016, 98, 19–34. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Hazafa, A.; Rehman, K.U.; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Abbaszadeh, H.; Keikhaei, B.; Mottaghi, S. A review of molecular mechanisms involved in anticancer and antiangiogenic effects of natural polyphenolic compounds. Phytother. Res. 2019, 33, 2002–2014. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Jodoin, J.; Demeule, M.; Beliveau, R. Inhibition of the multidrug resistance P-glycoprotein activity by green tea polyphenols. Biochim. Biophys. Acta 2002, 1542, 149–159. [Google Scholar] [CrossRef]
- Alvarez, A.I.; Real, R.; Perez, M.; Mendoza, G.; Prieto, J.G.; Merino, G. Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 2010, 99, 598–617. [Google Scholar] [CrossRef]
- Lopes-Rodrigues, V.; Sousa, E.; Vasconcelos, M.H. Curcumin as a modulator of P-glycoprotein in cancer: Challenges and perspectives. Pharmaceuticals 2016, 9, 71. [Google Scholar] [CrossRef]
- Hasima, N.; Ozpolat, B. Regulation of autophagy by polyphenolic compounds as a potential therapeutic strategy for cancer. Cell Death Dis. 2014, 5, e1509. [Google Scholar] [CrossRef]
- Sanchez-Alvarez, M.; Strippoli, R.; Donadelli, M.; Bazhin, A.V.; Cordani, M. Sestrins as a Therapeutic Bridge between ROS and Autophagy in Cancer. Cancers 2019, 11, 1415. [Google Scholar] [CrossRef]
- Dong, L.; He, J.; Luo, L.; Wang, K. Targeting the Interplay of Autophagy and ROS for Cancer Therapy: An Updated Overview on Phytochemicals. Pharmaceuticals 2023, 16, 92. [Google Scholar] [CrossRef]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef]
- Kiruthiga, C.; Devi, K.P.; Nabavi, S.M.; Bishayee, A. Autophagy: A Potential Therapeutic Target of Polyphenols in Hepatocellular Carcinoma. Cancers 2020, 12, 562. [Google Scholar] [CrossRef]
- Asadollahi, L.; Mahoutforoush, A.; Dorreyatim, S.S.; Soltanfam, T.; Paiva-Santos, A.C.; Peixoto, D.; Veiga, F.; Hamishehkar, H.; Zeinali, M.; Abbaspour-Ravasjani, S. Co-Delivery of erlotinib and resveratrol via nanostructured lipid Carriers: A synergistically promising approach for cell proliferation prevention and ROS-Mediated apoptosis activation. Int. J. Pharm. 2022, 624, 122027. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, Z.; Chen, Y.; Gao, D.; Wang, P.; Lin, Y.; Wang, Y.; Wang, F.; Han, Y.; Yuan, H. Co-delivery of Docetaxel and Resveratrol by liposomes synergistically boosts antitumor efficiency against prostate cancer. Eur. J. Pharm. Sci. 2022, 174, 106199. [Google Scholar] [CrossRef] [PubMed]
- Karami, P.; Othman, G.; Housein, Z.; Salihi, A.; Hosseinpour Feizi, M.A.; Azeez, H.J.; Babaei, E. Nanoformulation of Polyphenol Curcumin Enhances Cisplatin-Induced Apoptosis in Drug-Resistant MDA-MB-231 Breast Cancer Cells. Molecules 2022, 27, 2917. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Liu, C.; Chen, C.; Yu, X.; Chen, G.; Shi, Y.; Qin, F.; Ou, J.; Qiu, K.; Li, G. Quercetin and doxorubicin co-encapsulated biotin receptor-targeting nanoparticles for minimizing drug resistance in breast cancer. Oncotarget 2016, 7, 32184–32199. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T. Possible Side Effects of Polyphenols and Their Interactions with Medicines. Molecules 2023, 28, 2536. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Chen, L. Polyphenols and bioavailability: An update. Crit. Rev. Food Sci. Nutr. 2019, 59, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Patisaul, H.B.; Jefferson, W. The pros and cons of phytoestrogens. Front. Neuroendocrinol. 2010, 31, 400–419. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef]
- Nassiri Koopaei, N.; Abdollahi, M. Opportunities and obstacles to the development of nanopharmaceuticals for human use. Daru 2016, 24, 23. [Google Scholar] [CrossRef]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Zhang, M.; Merlin, D. Nanoparticle-Based Oral Drug Delivery Systems Targeting the Colon for Treatment of Ulcerative Colitis. Inflamm. Bowel Dis. 2018, 24, 1401–1415. [Google Scholar] [CrossRef]
- Ladaycia, A.; Passirani, C.; Lepeltier, E. Microbiota and nanoparticles: Description and interactions. Eur. J. Pharm. Biopharm. 2021, 169, 220–240. [Google Scholar] [CrossRef]
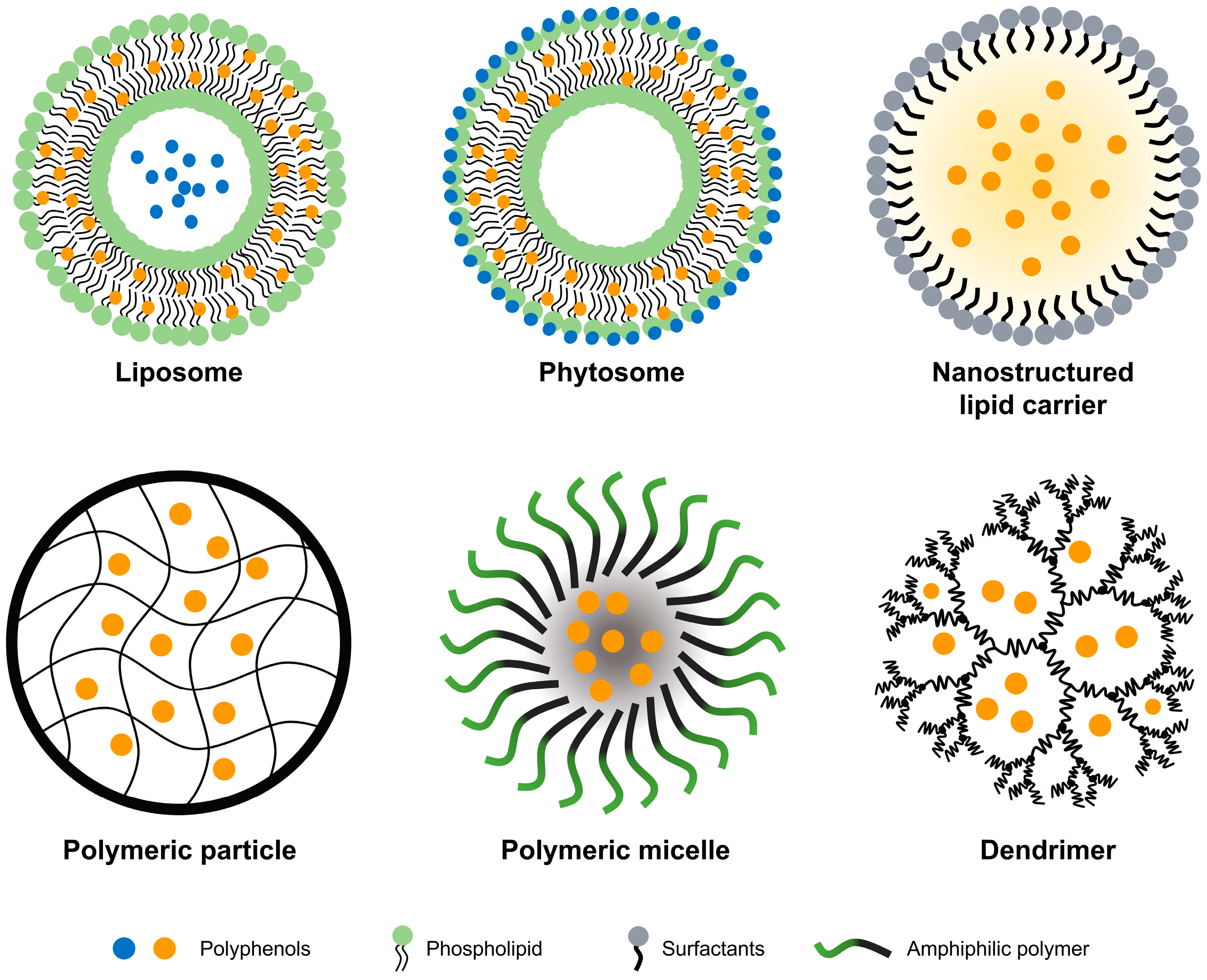

| Materials | Polyphenols | Methods and Functionalization | EE * and LC ** | Particle Size | Ref. |
|---|---|---|---|---|---|
| Liposome | Resveratrol | PEGylation TF moiety | EE ~75% | 211.2 nm | [55] |
| Peptide liposome | EE > 90% | 140 nm | [56] | ||
| Curcumin | Co delivery with gefitinib PEGylation | EE > 80% | 110–130 nm | [57] | |
| Phytosome | Genistein | With MCT or LCT Solvent evaporation method | EE > 95% | 150–300 nm | [61] |
| Thymoquinone | Soy-phospholipid | No data | 45.6 nm | [62] | |
| Curcumin | PEGylation Folate modification | LC 24.3% | 185.3 nm | [63] | |
| Moringa oleifera leaf polyphenols | Soy-phosphatidylcholine Thin-layer hydration method | EE 90.23% | 296 nm | [64] | |
| Nanostructured lipid particle | Curcumin | Cinnamon oil, Tween 80 PEG 200, Absorption enhancer | No data | 106 nm | [66] |
| Cornus mas extract (CME) | Cubosome Glyceryl monooleate Poloxamer® 407 Eudragit® S100 | EE 95.14% LC 9.51% | 22.7 nm | [67] | |
| EGCG | Arginyl-glycyl-aspartic acid (RGD) modification | EE 80% LC 75% | 85 nm | [68] | |
| Apigenin | Hyaluronic acid modification Co-treatment with docetaxel | EE 70% LC 3.5% | 88 nm | [69] |
| Materials | Polyphenols | Drug Release | Target | Improvement | Ref. |
|---|---|---|---|---|---|
| Liposome | Resveratrol | Slow release (No data) | U-87 MG human glioblastoma cells | Apoptosis 2.5-fold ↑ In vivo tumor volume 2-fold ↓ | [55] |
| 98% at pH 5.5 30% at pH 6.8 (~48 h) | MCF-7 breast cancer cell | Cytotoxicity 26% ↑ Apoptosis induced p53, Bax, Bcl-2, and caspase | [56] | ||
| Curcumin | At pH 7.4 (~48 h) >90% (alone) >70% (with gefitinib) | PC-9 Gefitinib-resistant H1975 | Cytotoxicity 10–15% ↑ In vivo tumor growth inhibition 45% ↑ | [57] | |
| Phytosome | Genistein | Unstable in pH 4.5 | Hepatocellular carcinoma | Liver toxicity reduction (AST 30%, ALT 10%) Caspase 8 ↑ Angiogenesis and invasion↓ | [61] |
| Thymoquinone | >95% (45%↑) (~12 h) | A549 Human lung cancer | IC50 < 5 μM (4-fold ↓) Caspase 3 expression ↑ | [62] | |
| Curcumin | 50% at pH 5.5 20% at pH 7.4 (~48 h) | HeLa cell | IC50 11.9 μg/mL (3-fold ↓) Selective accumulation in cancer 40% ↑ | [63] | |
| Moringa oleifera leaf polyphenols | At pH 7.4 43% (~8 h) 53% (~72 h) | 4T1 cancer cell | Stability in gastric condition 2-fold ↑ Cytotoxicity 4-fold ↑ Proliferation inhibition (more than DOX at 100 μg/mL) | [64] | |
| Nanostructured lipid particle | Curcumin | 80% at pH 1.2 >95% at pH 4.6 (~24 h) | Caco-2 Human colon cancer | Cytotoxicity 57% ↑ Cmax 5-fold ↑ AUC 4.5-fold ↑ | [66] |
| Cornus mas extract (CME) | Sustained (~80 h) >90% intestinal >70% gastric | Caco-2 Human colon cancer | DPPH scavenging 27%↑ Cytotoxicity DNA fragmentation↑ Cell cycle arrest (G0-G1 phase) ↑ | [67] | |
| EGCG | No data | MDA-MB-231 human breast cancer | IC50 38.8 μM (22.5% ↓) Cell cycle arrest Apoptosis ↑ | [68] | |
| Apigenin | Sustained (~80 h) 90% at pH 5.5 85% at pH 7.4 | A549 Human lung cancer | IC50 23.51μM (3-fold ↓) Apoptosis 15% ↑ | [69] |
| Materials | Polyphenols | Methods and Functionalization | EE * and LC ** | Particle Size | Ref. |
|---|---|---|---|---|---|
| Polymer | Hesperestin | Chitosan-folate conjugate Sodium tripolyphosphate (cross-linking) | EE 98% | 457 nm | [72] |
| Quercetin | Eudragit S100 | EE 41.8% LC 2.2% | 66.8 nm | [73] | |
| Curcumin | Spray drying HPMC15LV or PVP-K30 Lactose monohydrate | EE 13~78% | 4.8–8.3 nm | [74] | |
| Phenol–yne click reaction mPEG-b-PHEMA-5HA | EE 43.4% LC 17.8% | 104 nm | [75] | ||
| Quercetin | Luteinizing-hormone-releasing hormone (LHRH) ligand PLGA, PEG Co-delivery with docetaxel | EE 75.8% | 120–140 nm | [76] | |
| Curcumin | Co-delivery with doxorubicin Polymeric micelle Hyaluronic acid–vitamin E succinate | EE 72% LC 8.3% | 223 nm | [77] | |
| Resveratrol and curcumin | Bolton W3000 dendritic polymer solvent displacement method | No data | 130 nm | [78] | |
| Protein | Resveratrol | Sericin silk protein | EE 71–75% | 200–350 nm | [81] |
| Curcumin | Zein protein Dodecamer peptide (G23) Polydopamine | EE 81.7% LC 8.11% | 106.3 nm | [82] | |
| Hydrophobin (HGFI) GE11 peptide | EE 88% LC 47% | 80 nm | [83] | ||
| Zein protein Silk protein Co-formulation with Paclitaxel | EE 93% LC 12% | 265 nm | [84] | ||
| Caseinate–chitosan Genipin cross-linking | EE 88.6% LC 4.2% | 272 nm | [85] | ||
| Iron ion | Curcumin | Metal–phenolic network between EGCG and iron, coating | EE 90% LC 40% | 184.8 nm | [90] |
| Nucleic acid | Tannic acid | Branched DNA, Aptamer antisense of C-raf mRNA | EE 85% | 150 nm | [93] |
| Branched DNA, siPLK1 RNA A549 membrane | No data | 164.2 nm | [94] |
| Materials | Polyphenols | Drug Release | Target | Improvement | Ref. |
|---|---|---|---|---|---|
| Polymer | Hesperestin | ~45% at pH 7.4 <20% at pH 3 (~72 h) | HCT15 Human colon cancer cell | IC50 28 μM (6.7-fold↓) Apoptosis 2.4-fold↑ Bax and Bad mRNA 35%↑ | [72] |
| Quercetin | No release at < pH 4.5 (~2 h) 90% at pH 7.4 (~20 h) | CT26 colon cancer cell | IC50 0.8 μM (81-fold↓) Cell viability 40%↓ | [73] | |
| Curcumin | ~28% at pH 7.4 (~90 min) | A549 Human lung cancer | Solubility 14-fold↑ IC50 44 µM (3-fold↓) | [74] | |
| ~25% at pH 5 ~72% at pH 7.4 (~30 h) | 4T1 cancer cell HeLa cell | IC50 4–9 µg/mL Half-life 6.16 h (14-fold↑) Tumor growth 27%↓ | [75] | ||
| Quercetin | 95% at pH 7.4 (with serum, ~48 h) | PCa cell lines (PC-3 and LNCaP) | IC50 36–82%↓ Caspase 3 activation↑ in vivo tumor growth 40%↓ | [76] | |
| Curcumin | ~69% at pH 4.5 ~37% at pH 7.4 (~24 h) | MCF-7 cell MCF-7/Adr cell | Reduction of drug efflux IC50 14.8-fold↓ Apoptosis 4.6-fold↑ Tumor inhibition 55.2% (2-fold↑) | [77] | |
| Resveratrol and curcumin | Earlier in pH 5.4 than pH 7 | SH-SY5Y cancer cell | Cytotoxicity 37%↑ Intracellular calcium release 20%↑ Cytochrome C oxidase activation↓ Mitochondrial depolarization | [78] |
| Materials | Polyphenols | Drug Release | Target | Improvement | Ref. |
|---|---|---|---|---|---|
| Protein | Resveratrol | 65% at pH 7.4 (~72 h, high concentration) | Caco-2 cancer cell | 97% cellular uptake after 24 h IC50 < 6% wt No toxicity to normal cell (100% wt) | [81] |
| Curcumin | 80% at pH 5 45% at pH 7.4 (~48 h) | C6 glioma cell | Blood brain barrier penetration↑ (2-fold↑) Cancer proliferation and migration > 50%↓ | [82] | |
| At pH 7.4 ~53% (~8 h) ~94% (~72 h) | HCT 116 cancer cell | Cellular uptake 2-fold↑ IC50 3.7 µg/mL (free curcumin is not toxic to 20 µg/mL) | [83] | ||
| Sustained~180 h ~66% at pH 5 ~49% at pH 7.4 | MCF-7 breast cancer cell | IC50 0.72 µg/mL (6-fold↓) Synergistic effect (CI > 1) | [84] | ||
| 77% at pH 5.5 60% at pH 7.4 (~6 h) | HeLa cell A549 cell | IC50 6.5 µg/mL (35%↓) Cell surface attachment and localization | [85] | ||
| Iron ion | Curcumin | 91% at pH 5 34% at pH 7.4 (~24 h) | MCF-7 human breast cancer cell | Uptake efficiency 93% after 3 h Bcl-2 down-regulation (2.6-fold↓) Bax and caspase 3 up-regulation (40%↑) | [90] |
| Nucleic acid | Tannic acid | Disassembly in pH 5.5 | A549 human lung cancer cell | Cellular uptake 7-fold↑ Apoptosis 40%↑ by tannic acid and 20%↑ by therapeutic genes | [93] |
| In acidic pH (when fused with endosomal/lysosomal vesicle) | A549 human lung cancer cell | Homotypic targeting (3-fold↑) Macrophage uptake 30%↓ Half-life 2.13 h (26-fold↑) Tumor growth 5-fold↓ | [94] |
| Combination | DDS Method | Target/Treated Conc. | Results | Ref. |
|---|---|---|---|---|
| Erlotinib and resveratrol | Nanostructured lipid particle Hot homogenization method Miglyol and Precirol Poloxamer 470 Drug ratio 1:8 | A549 human lung cancer cell /E 5 µg/mL and R 40 µg/mL | Cell viability 12.6% (75%↓) Apoptosis 85.5% (20–30%↑) (Bax and p53 expression↑, Caspase 3, 8, and 9 activation↑) Cell cycle arrest 40% in G2/M phase | [110] |
| Docetaxel and resveratrol | PEGylated nano-liposomes Thin-film hydration method Soy lecithin, Cholesterol, and DSPE-MPEG2000 Drug ratio 1:2 | PC3 and DU145 Human prostate cancer cell lines/D 32.3 µg/mL and R 18.3 µg/mL PC3-bearing mice/ D 10 mg/kg and R 5.65 µg/mL | IC50 (docetaxel) 1.49 µg/mL (10-fold↓) Apoptosis induction 69% (2-fold↑) Cellular uptake 3.5–5.5-fold↑ In vivo tumor growth inhibition (Volume 3-fold↓) Survival day ~42 day, (5-fold↑ than free drugs) In vivo toxicity reduction | [111] |
| Cisplatin and curcumin | mPEG urethane gemini surfactant nanoparticle | MDA-MB-231 breast cancer cells/ Ci 13 µM and Cu 20 µM | Sub G1 cell ratio 20% (2–4-fold↑) Late apoptosis 59% (39–290%↑) Bax/Bcl-2 ratio 50–200%↑ | [112] |
| Doxorubicin and quercetin | Polymeric nanoparticle Biotin-PEG2k-PCL5k | MCF-7 and MCF-7/ADR breast cancer cells /D 5 μg/mL and Q 11.75 μg/mL MCF-7/ADR-bearing nude mice/D 5 mg/kg and Q 11.75 mg/kg | Drug resistance↓(136-fold↓) Drug efflux 15–48%↓ P-glycoprotein activity 1.8–2.5-fold↓ In vivo tumor volume 3-fold↓ | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.H.; Ki, M.-R.; Min, K.H.; Pack, S.P. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants 2023, 12, 1048. https://doi.org/10.3390/antiox12051048
Kim KH, Ki M-R, Min KH, Pack SP. Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants. 2023; 12(5):1048. https://doi.org/10.3390/antiox12051048
Chicago/Turabian StyleKim, Koung Hee, Mi-Ran Ki, Ki Ha Min, and Seung Pil Pack. 2023. "Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy" Antioxidants 12, no. 5: 1048. https://doi.org/10.3390/antiox12051048
APA StyleKim, K. H., Ki, M.-R., Min, K. H., & Pack, S. P. (2023). Advanced Delivery System of Polyphenols for Effective Cancer Prevention and Therapy. Antioxidants, 12(5), 1048. https://doi.org/10.3390/antiox12051048






