Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material
2.3. Preparation of Extracts
2.4. Antioxidant Activity
- (a)
- DPPH free radical antioxidant activity assay
- (b)
- ABTS free radical antioxidant activity assay
2.5. Dilution of Standard Compounds
2.6. LC/Q-TOF/HRMS Conditions
2.7. Quantification of the Identified Compounds: Single Point External Standard Method
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robson, N.K.B. Studies in the genus Hypericum L. (Hypericaceae) 5(1). Sections 10. Olympia to 15/16 Crossophyllum. Phytotaxa 2010, 4, 5–126. [Google Scholar] [CrossRef]
- Crockett, S.L.; Robson, N.K. Taxonomy and Chemotaxonomy of the Genus Hypericum. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 1–13. [Google Scholar]
- Nürk, N.M.; Crockett, S.L. Morphological and Phytochemical Diversity among Hypericum Species of the Mediterranean Basin. Med. Aromat. Plant Sci. Biotechnol. 2011, 5, 14–28. [Google Scholar]
- Committee on Herbal Medicinal Products (HMPC). Community Herbal Monograph on Hypericum perforatum L., Herba (Traditional Use); EMA/HMPC/45508/2017; European Medicines Agency: London, UK, 2017. [Google Scholar]
- Rusalepp, L.; Raal, A.; Püssa, T.; Mäeorg, U. Comparison of chemical composition of Hypericum perforatum and H. maculatum in Estonia. Biochem. Syst. Ecol. 2017, 73, 41–46. [Google Scholar] [CrossRef]
- Barnes, J.; Arnason, J.T.; Roufogalis, B.D. St John’s wort (Hypericum perforatum L.): Botanical, chemical, pharmacological and clinical advances. J. Pharm. Pharmacol. 2019, 71, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Wurglics, M.; Schubert-Zsilavecz, M. Hypericum perforatum: A ‘modern’ herbal antidepressant: Pharmacokinetics of active ingredients. Clin. Pharmacokinet. 2006, 45, 449–468. [Google Scholar] [CrossRef] [PubMed]
- Wölfle, U.; Seelinger, G.; Schempp, C. Topical Application of St. Johnʼs Wort (Hypericum perforatum). Planta Med. 2013, 80, 109–120. [Google Scholar] [CrossRef]
- Oliveira, A.I.; Pinho, C.; Sarmento, B.; Dias, A.C. Neuroprotective Activity of Hypericum perforatum and Its Major Components. Front. Plant Sci. 2016, 7, 1004. [Google Scholar] [CrossRef] [PubMed]
- The American Herbal Pharmacopoeia and Therapeutic Compendium. St John’s Wort Hypericum perforatum Quality Control, Analytical and Therapeutic Monograph; The American Herbal Pharmacopoeia: Scotts Valley, CA, USA, 1997. [Google Scholar]
- Robson, N.K.B. Studies in the genus Hypericum L. (guttiferae). 2. Characters of the genus. Bull. Brit. Mus. Bot. 1981, 8, 55–226. [Google Scholar]
- Southwell, I.A.; Bourke, C.A. Seasonal variation in hypericin content of Hypericum perforatum L. (St. John’s wort). Phytochemistry 2001, 56, 437–441. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chou, Y.C.; Liao, K.C.; Shieh, C.J.; Deng, T.S. Optimization of Light Intensity, Temperature, and Nutrients to Enhance the Bioactive Content of Hyperforin and Rutin in St. John’s Wort. Molecules 2020, 25, 4256. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Hryb, A.B.; Cunha, M.P.; Kaster, M.P.; Rodrigues, A.L.S. Natural Polyphenols and Terpenoids for Depression Treatment: Current Status. Stud. Nat. Prod. Chem. 2018, 55, 181–221. [Google Scholar]
- Avato, P. A survey on the Hypericum genus: Secondary metabolites and bioactivity. Stud. Nat. Prod. Chem. 2005, 30, 603–634. [Google Scholar]
- Napoli, E.; Siracusa, L.; Ruberto, G.; Carrubba, A.; Lazzara, S.; Speciale, A.; Cimino, F.; Saija, A.; Cristani, M. Phytochemical profiles, phototoxic and antioxidant properties of eleven Hypericum species—A comparative study. Phytochemistry 2018, 152, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Alahmad, A.; Alghoraibi, I.; Zein, R.; Kraft, S.; Dräger, G.; Walter, J.G.; Scheper, T. Identification of Major Constituents of Hypericum perforatum L. Extracts in Syria by Development of a Rapid, Simple, and Reproducible HPLC-ESI-Q-TOF MS Analysis and Their Antioxidant Activities. ACS Omega 2022, 7, 13475–13493. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Nahrstedt, A.; Butterweck, V. Lessons learned from herbal medicinal products: The example of St. John’s Wort (perpendicular). J. Nat. Prod. 2010, 73, 1015–1021. [Google Scholar] [CrossRef]
- Çirak, C.; Ivanauskas, L.; Janulis, V.; Radušienė, J. Chemical constituents of Hypericum adenotrichum Spach, an endemic Turkish species. Nat. Prod. Res. 2009, 23, 1189–1195. [Google Scholar] [CrossRef]
- Daskalaki, A.; Grafakou, M.E.; Barda, C.; Kypriotakis, Z.; Heilmann, J.; Skaltsa, E. Secondary metabolites from Hypericum trichocaulon Boiss. & Heldr., growing wild in the island of Crete. Biochem. Syst. Ecol. 2021, 97, 104294. [Google Scholar]
- Trigas, P. A new Hypericum (sect. Drosocarpium, Hypericaceae) from the Cyclades Islands (Greece). Nord. J. Bot. 2018, 36, e02205. [Google Scholar] [CrossRef]
- Kakouri, E.; Kanakis, C.; Trigas, P.; Tarantilis, P.A. Characterization of the chemical composition of Drimia numidica plant parts using high-resolution mass spectrometry: Study of their total phenolic content and antioxidant activity. Anal. Bioanal. Chem. 2019, 411, 3135–3150. [Google Scholar] [CrossRef]
- Liu, F.; Pan, C.; Drumm, P.; Ang, C.Y. Liquid chromatography-mass spectrometry studies of St. John’s wort methanol extraction: Active constituents and their transformation. J. Pharm. Biomed. Anal. 2005, 37, 303–312. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Petrakis, E.A.; Kimbaris, A.C.; Pappas, C.; Tarantilis, P.A.; Polissiou, M.G. Classification of Greek Mentha pulegium L. (Pennyroyal) samples, according to geographical location by Fourier transform infrared spectroscopy. Phytochem. Anal. 2012, 23, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Linde, K.; Ramirez, G.; Mulrow, C.D.; Pauls, A.; Weidenhammer, W.; Melchart, D. St John’s wort for depression—An overview and meta-analysis of randomized clinical trials. BMJ 1996, 313, 253–258. [Google Scholar] [CrossRef]
- Shelton, R.C.; Keller, M.B.; Gelenberg, A.; Dunner, D.L.; Hirschfeld, R.; Thase, M.E.; Russell, J.; Lydiard, R.B.; Crits-Cristoph, P.; Gallop, R.; et al. Effectiveness of St John’s wort in major depression: A randomized controlled trial. JAMA 2001, 285, 1978–1986. [Google Scholar] [CrossRef]
- Vorbach, E.U.; Arnoldt, K.H.; Hubner, W.D. Efficacy and tolerability of St. John’s wort extract LI 160 versus imipramine in patients with severe depressive episodes according to ICD-10. Pharmacopsychiatry 1997, 30, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.L. Vitamin C causes cancer! St. John’s wort useless for depression! Altern. Med. Rev. 2001, 6, 353–354. [Google Scholar] [PubMed]
- European Pharmacopoeia. 10.0 European Directorate for the Quality of Medicines; European Pharmacopoeia: Strasburg, Germany, 2020. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2004; Volume 2, Herba Hyperici.
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. St John’s wort (Hypericum perforatum L.): A review of its chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2001, 53, 583–600. [Google Scholar] [CrossRef]
- Butterweck, V.; Schmidt, M. St. John’s wort: Role of active compounds for its mechanism of action and efficacy. Wien. Med. Wochenschr. 2007, 157, 356–361. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, A.S.; Lobo, J.M.; Ree, R.; Beerling, D.J.; Sanmartín, I. Integrating Fossils, Phylogenies, and Niche Models into Biogeography to Reveal Ancient Evolutionary History: The Case of Hypericum (Hypericaceae). Syst. Biol. 2015, 64, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Fuzzati, N.B.; Gabetta, I.; Villa, S.F. High-performance liquid chromatography–electrospray ionization mass spectrometry and multiple mass spectrometry studies of hyperforin degradation products. J. Chromatogr. A 2001, 926, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, A.; Bordignon, S.; Mans, D.R.A.; Schmitt, A.; Ravazzolo, A.P.; von Poser, G.L. Screening for the Presence of Hypericins in Southern Brazilian Species of Hypericum. Pharm. Biol. 2002, 40, 294–297. [Google Scholar] [CrossRef]
- Fico, G.; Vitalini, S.; Colombo, N.; Tomè, F. Hypericum Perforatum L., H. Maculatum Crantz., H. Calycinum L. and H. pulchrum L.: Phytochemical and Morphological Studies. Nat. Prod. Commun. 2006, 1, 1129–1132. [Google Scholar] [CrossRef]
- Momekov, G.; Ferdinandov, D.; Zheleva-Dimitrova, D.; Nedialkov, P.; Girreser, U.; Kitanov, G. Cytotoxic effects of hyperatomarin, a prenylated phloroglucinol from Hypericum annulatum Moris subsp. annulatum, in a panel of malignant cell lines. Phytomedicine 2008, 15, 1010–1015. [Google Scholar] [CrossRef]
- Shiu, W.K.; Rahman, M.M.; Curry, J.; Stapleton, P.; Zloh, M.; Malkinson, J.P.; Gibbons, S. Antibacterial acylphloroglucinols from Hypericum olympicum. J. Nat. Prod. 2012, 75, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Karioti, A.; Bilia, A.R. Hypericins as potential leads for new therapeutics. Int. J. Mol. Sci. 2010, 11, 562–594. [Google Scholar] [CrossRef]
- Kitanov, G.M. Hypericin and pseudohypericin in some Hypericum species. Biochem. Syst. Ecol. 2001, 29, 171–178. [Google Scholar] [CrossRef]
- Lazzara, S.; Carrubba, A.; Napoli, E. Variability of hypericins and hyperforin in Hypericum species from the Sicilian flora. Chem. Biodivers. 2020, 17, e1900596. [Google Scholar] [CrossRef]
- Smelcerovic, A.; Verma, V.; Spiteller, M.; Ahmad, S.M.; Puri, S.C.; Qazi, G.N. Phytochemical analysis and genetic characterization of six Hypericum species from Serbia. Phytochemistry 2006, 67, 171–177. [Google Scholar] [CrossRef]
- Maisenbacher, P.; Kovar, K.A. Adhyperforin: A Homologue of Hyperforin from Hypericum perforatum. Planta Med. 1992, 58, 291–293. [Google Scholar] [CrossRef]
- Nedialkov, P.T.; Kitanov, G.M.; Zheleva-Dimitrova, D.Z.; Girreser, U. Flavonoids and a xanthone from Hypericum umbellatum (Guttiferae). Biochem. Syst. Ecol. 2007, 35, 118–120. [Google Scholar] [CrossRef]
- Camas, N.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Kayikci, S.; Cirak, C. Chemical composition of Hypericum species from the Taeniocarpium and Drosanthe sections. Plant Syst. Evol. 2014, 300, 953–960. [Google Scholar] [CrossRef]
- Del Monte, D.; De Martino, L.; Marandino, A.; Fratianni, F.; Nazzaro, F.; De Feo, V. Phenolic content, antimicrobial and antioxidant activities of Hypericum perfoliatum L. Ind. Crops Prod. 2015, 74, 342–347. [Google Scholar] [CrossRef]
- Zeliou, K.; Vogiatzoglou, A.P.; Kalachanis, D.; Iatrou, G.; Lamari, F.N. Anatomical Characterization and UHPLC-MS Analysis of Hypericum vesiculosum. Rev. Bras. Farmacogn. 2020, 30, 416–422. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed]
- De Lima Cherubim, D.J.; Buzanello Martins, C.V.; Oliveira Fariña, L.; da Silva de Lucca, R.A. Polyphenols as natural antioxidants in cosmetics applications. J. Cosmet. Dermatol. 2020, 19, 33–37. [Google Scholar] [CrossRef]
- Öztürk, N.; Tunçel, M.; Potoğlu-Erkara, İ. Phenolic compounds and antioxidant activities of some Hypericum species: A comparative study with H. perforatum. Pharm. Biol. 2009, 47, 120–127. [Google Scholar] [CrossRef]
- Orčić, D.Z.; Mimica-Dukić, N.M.; Francišković, M.M.; Petrović, S.S.; Jovin, E.Đ. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem. Cent. J. 2011, 5, 34. [Google Scholar] [CrossRef]
- Radulović, N.; Stankov-Jovanović, V.; Stojanović, G.; Šmelcerović, A.; Spiteller, M.; Asakawa, Y. Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem. 2007, 103, 15–21. [Google Scholar] [CrossRef]
- Zdunic, G.; Godjevac, D.; Savikin, K.; Petrovic, S. Comparative analysis of phenolic compounds in seven Hypericum species and their antioxidant properties. Nat. Prod. Commun. 2017, 12, 1805–1811. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacoghmadn. Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Chen, X.; Niu, S.Q.; Zhou, H.Y.; Li, Q.S. Protective Antioxidant Effects of Amentoflavone and Total Flavonoids from Hedyotis diffusa on H2 O2 -Induced HL-O2 Cells through ASK1/p38 MAPK Pathway. Chem. Biodivers. 2020, 17, e2000251. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Máthé, A.; Máthé, I. Quality assurance of cultivated and gathered medicinal plants. Acta Hortic. 2008, 765, 67–76. [Google Scholar] [CrossRef]
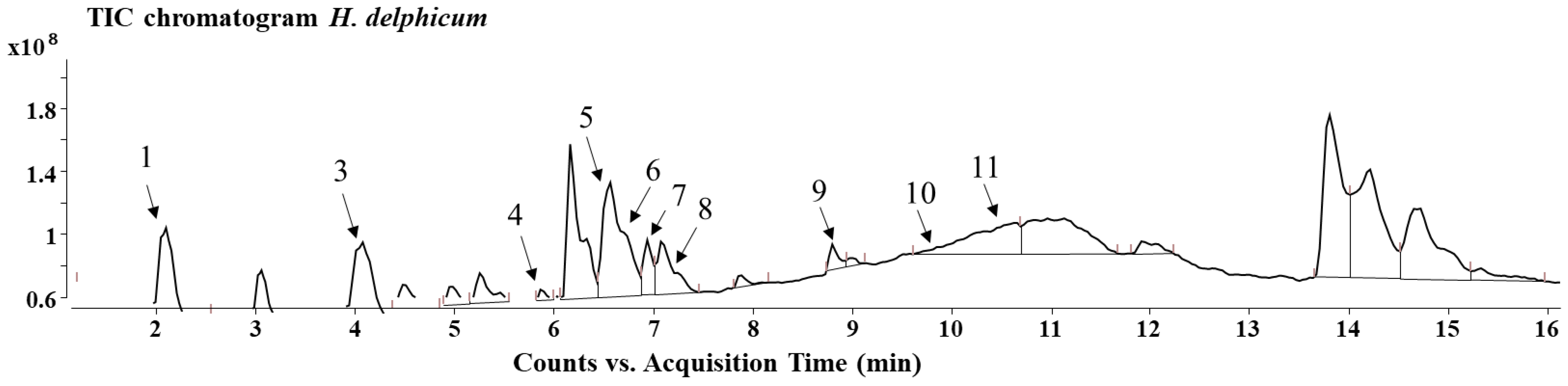
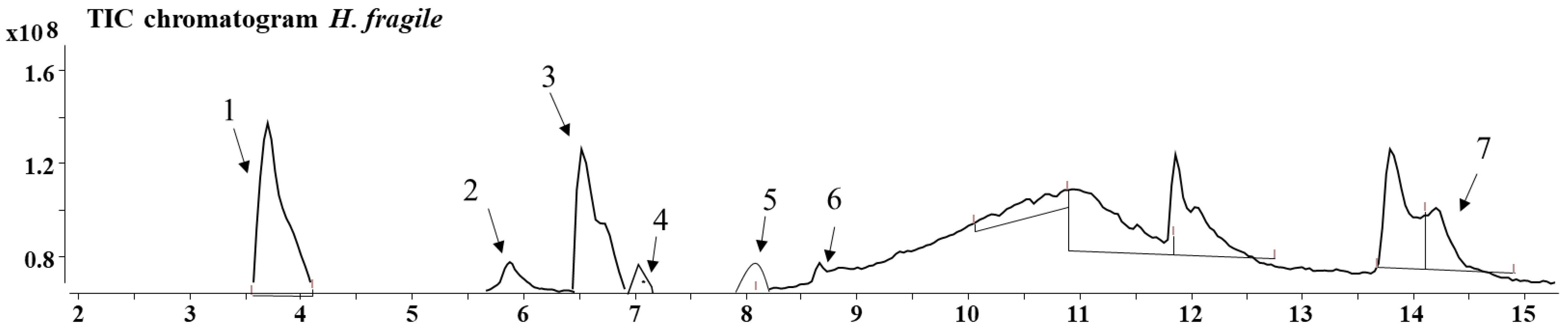

| Sect. Drosocarpium | Sect. Hypericum | Sect. Taeniocarpium | Sect. Olympia | Sect. Adenosepalum | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | Molecular Formula | H. perfoliatum | H. rumeliacum subsp. apollinis | H. vesiculosum | H. cycladicum | H. perforatum | H. tetrapterum | H. fragile | H. olympicum | H. delphicum |
| Flavan-3-olsand Proanthocyanidins | ||||||||||
| Procyanidin B type | C30H26O14 | 0.01 | - | - | - | - | - | - | - | - |
| Catechin * | C15H14O6 | 0.65 | - | - | - | 2.33 | - | - | - | - |
| Procyanidin B1 * | C30H26O12 | 0.61 | - | - | 1.26 | 2.06 | - | - | - | - |
| Procyanidin B type | C30H26O12 | 2.20 | - | 0.05 | 1.41 | - | - | - | - | - |
| B-type trimer procyanidin (C1) | C45H38O18 | 0.42 | - | - | - | - | - | - | - | - |
| B-type trimer procyanidin (C2) | C45H38O18 | 1.65 | - | - | 1.93 | 2.02 | - | - | - | 2.68 |
| Epicatechin * | C15H14O6 | 3.83 | - | - | - | 9.21 | - | - | - | 10.02 |
| Quinic acid derivates | ||||||||||
| Neo-chlorogenic acid | C16H18O9 | - | 13.51 | 4.94 | 6.61 | - | - | - | 15.83 | 17.49 |
| Chlorogenic acid * | C16H18O9 | - | 46.22 | 0.05 | 50.13 | 0.20 | 36.97 | 41.6 | 28.56 | 0.46 |
| p-coumaroylquinic acid | C16H18O8 | - | 1.04 | - | - | - | - | - | - | - |
| Benzoic acid derivates | ||||||||||
| Vanillic acid hexoside | C14H8O9 | - | - | n.q1 | - | - | - | - | - | - |
| Flavones | ||||||||||
| Apigenin hexoside | C21H20O10 | 4.73 | - | - | 3.74 | - | - | - | - | - |
| Luteolin malonylhexoside | C24H22O14 | 4.03 | - | 5.82 | 3.44 | - | - | - | - | - |
| Luteolin glucuronide | C21H18O12 | 0.13 | - | - | - | - | - | - | - | - |
| Luteolin glucoside * | C21H20O11 | - | - | - | 32.34 | - | - | - | - | - |
| Myricetin glucoside * | C21H20O13 | - | 55.89 | 1.38 | - | 13.02 | - | - | 315.52 | 68.98 |
| Myricetin arabinoside | C20H18O12 | - | 24.78 | - | - | - | - | 64.99 | - | |
| Myricitrin * | C21H20O12 | - | 304.75 | - | - | - | - | - | - | - |
| Flavonols | ||||||||||
| Hyperoside | C21H20O12 | 0.011 | 0.02 | 1.17 | 3.59 | - | - | 0.008 | 1.48 | 0.01 |
| Isoquercitrin | C21H20O12 | 1.39 | 0.02 | 0.01 | 3.98 | 4.26 | 1.67 | 1.13 | 0.01 | 2.55 |
| Kaempferol glucoside * | C21H20O11 | 6.60 | 108.93 | - | 33.24 | - | - | - | - | - |
| Quercitrin * | C21H20O11 | 0.011 | 4.94 | 0.36 | 1.51 | - | - | - | - | 0.90 |
| Kaempferol malonylhexoside | C24H22O14 | 0.88 | - | 1.61 | 1.03 | - | - | - | - | - |
| Miquelianin | C21H18O13 | - | - | - | - | 0.65 | - | - | - | - |
| Quercetin arabinofuranoside | C20H18O11 | - | 0.93 | - | - | - | - | - | - | 2.78 |
| Quercetin 3.4-di-O-glucoside * | - | - | - | - | - | 0.06 | 3.61 | - | - | |
| Quercetin glucoside acetate | C23H22O13 | - | - | - | - | 0.28 | - | - | - | - |
| Quercetin malonylhexoside | C24H22O15 | 0.14 | - | 0.23 | 0.15 | - | - | - | - | - |
| Kaempferol rutinoside | C27H30O15 | - | - | 11.39 | 4.70 | - | 2.63 | 5.30 | - | - |
| Quercetin * | C15H10O7 | - | 0.06 | 0.25 | - | 0.31 | - | 0.11 | 015 | - |
| Kaempferol * | C15H10O6 | - | - | - | 0.003 | - | - | - | - | - |
| Kaempferol rhamnoside | C21H20O10 | - | - | - | - | 4.92 | - | - | - | 10.64 |
| Rutin * | C27H30O16 | 0.13 | 0.30 | 3.89 | 0.14 | 0.13 | 0.24 | 22.21 | 0.18 | 0.55 |
| Bi-flavonoids | ||||||||||
| I3.II8 biapigenin | C30H18O10 | 4.18 | 270.79 | 181.11 | 371.12 | 176.92 | 130.75 | 54.73 | 48.73 | 158.90 |
| Amentoflavone * | C30H18O10 | - | - | 23.95 | 102.94 | 14.39 | 11.83 | 9.44 | 6.23 | 19.54 |
| Naphthodianthrones | ||||||||||
| Pseudohypericin | C30H16O9 | 0.99 | 5.44 | 1.40 | 8.52 | 0.007 | - | 4.57 | 0.10 | 20.87 |
| Hypericin * | C30H16O8 | - | - | - | - | 7.18 | - | - | - | 2.34 |
| Protohypericin | C30H18O8 | - | 2.65 | 0.31 | - | - | - | - | - | - |
| Acylphloroglucinols | ||||||||||
| Hyperforin * | C35H52O4 | 0.38 | - | - | - | 608.39 | - | 0.05 | - | - |
| Adhyperforin | C36H54O4 | - | - | - | - | 35.53 | - | - | - | - |
| Species | DPPH Assay | ABTS Assay |
|---|---|---|
| IC50 (μg/mL) * | IC50 (μg/mL) * | |
| H. perfoliatum | 13.32 ± 2.03 | 4.71 ± 1.63 |
| H. rumeliacum subsp. apollinis | 16.14 ± 0.68 | 5.07 ± 107 |
| H. vesiculosum | 28.39 ± 1.41 | - |
| H. cycladicum | 16.8 ± 1.02 | 6.80 ± 1.46 |
| H. perforatum | 10.45 ± 0.61 | 6.06 ± 0.93 |
| H. tetrapterum | 17.85 ± 1.57 | 8.64 ± 1.43 |
| H. fragile | 23.99 ± 1.24 | 7.86 ± 0.91 |
| H. olympicum | 14.1 ± 0.94 | 3.92 ± 0.73 |
| H. delphicum | 12.98 ± 1.09 | 5.84 ± 1.28 |
| Trolox | 4.84 ± 1.15 | 2.84 ± 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakouri, E.; Trigas, P.; Daferera, D.; Skotti, E.; Tarantilis, P.A.; Kanakis, C. Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece. Antioxidants 2023, 12, 899. https://doi.org/10.3390/antiox12040899
Kakouri E, Trigas P, Daferera D, Skotti E, Tarantilis PA, Kanakis C. Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece. Antioxidants. 2023; 12(4):899. https://doi.org/10.3390/antiox12040899
Chicago/Turabian StyleKakouri, Eleni, Panayiotis Trigas, Dimitra Daferera, Efstathia Skotti, Petros A. Tarantilis, and Charalabos Kanakis. 2023. "Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece" Antioxidants 12, no. 4: 899. https://doi.org/10.3390/antiox12040899
APA StyleKakouri, E., Trigas, P., Daferera, D., Skotti, E., Tarantilis, P. A., & Kanakis, C. (2023). Chemical Characterization and Antioxidant Activity of Nine Hypericum Species from Greece. Antioxidants, 12(4), 899. https://doi.org/10.3390/antiox12040899










