The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae
Abstract
1. Introduction
2. Biochemistry of Antioxidants and Their Mode of Action
- The presence of the aromatic nucleus of phenol or naphthol, of a secondary or tertiary hydroxyl group which increases the effectiveness (most antioxidants have a phenolic structure).
- The presence of allylic groups in the ortho or para position compared to the hydroxyl groups which have a favorable effect.
- The antioxidant effect increases proportionally with the length of the chain.
- Alkylation in the meta position is less effective.
- The esterification of the hydroxyl groups which causes a total disappearance of the antioxidant activity.
3. Antioxidants as Food Additives
- ➢
- The addition of the antioxidant must be authorized by the legislation of the country where the food products will be consumed.
- ➢
- The action of the antioxidant must not be limited only to finding it as such and must be limited to food products in which the respective fat was later incorporated as an ingredient.
- ➢
- The addition of the antioxidant must be simple, without lengthy or complicated manipulations.
- ➢
- The appearance or taste of the respective product must not be modified in any way by the presence of the antioxidant.
- ➢
- No negative effect on the human body is allowed even after continuous and prolonged incorporation in the daily food ration.
- ➢
- The antioxidant must be effective in very small quantities so that its addition exerts an insignificant influence on the cost price of the respective product.
- ➢
- The presence of the antioxidant in fats or other food products must be able to be determined through simple analysis, preferably both quantitatively and qualitatively.
4. Natural Antioxidants and Their Benefits
4.1. Food and Fruits Antioxidants
4.2. Marine Algae Antioxidants

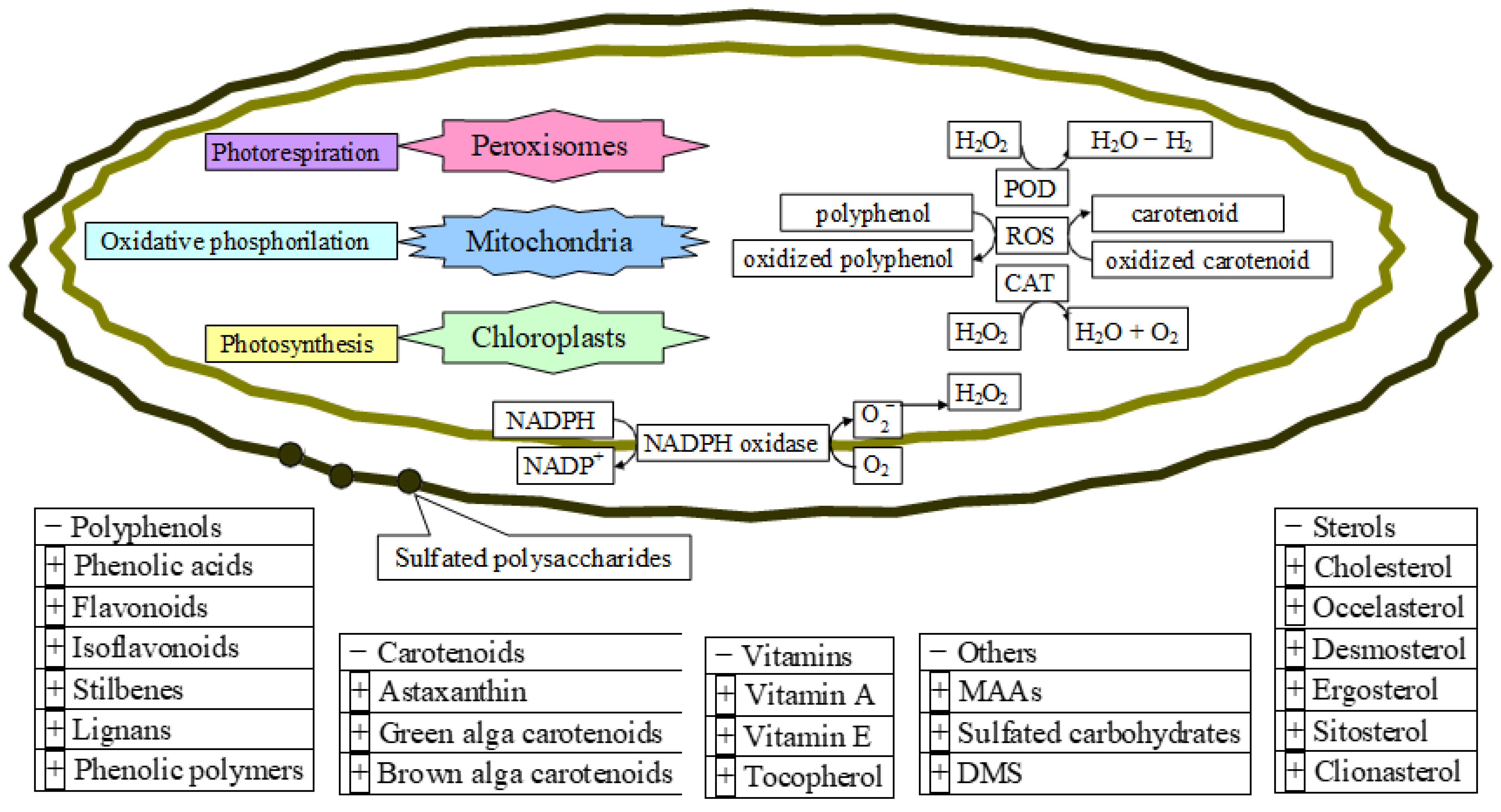
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Benzie, I.F.F.; Choi, S.W. Antioxidants in food: Content measurement, significance, action, cautions, caveats, and research needs (Chapter 1). Adv. Food Nutr. 2014, 71, 1–53. [Google Scholar]
- Lü, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and Molecular Mechanisms of Antioxidants: Experimental Approaches and Model Systems. J. Cell. Mol. Med. 2010, 14, 840–860. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Reactive oxygen species (ROS), oxygen radicals and antioxidants: Where are we now, where is the field going and where should we go? Biochem. Biophys. Res. Commun. 2022, 633, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Larson, R.A.; McCord, J.M. Environmental chemistry of reactive oxygen species. CRC Crit. Rev. Env. Control 1977, 8, 197–246. [Google Scholar] [CrossRef]
- Herlihy, D.; Waegele, M.; Chen, X.; Pemmaraju, C.D.; Prendergast, D.; Cuk, T. Detecting the oxyl radical of photocatalytic water oxidation at an n-SrTiO3/aqueous interface through its subsurface vibration. Nat. Chem. 2016, 8, 549–555. [Google Scholar] [CrossRef]
- Stone, W.L.; Pham, T.; Mohiuddin, S.S. Biochemistry, Antioxidants. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541064/ (accessed on 29 January 2023).
- Weidinger, A.; Kozlov, A.V. Biological Activities of Reactive Oxygen and Nitrogen Species: Oxidative Stress versus Signal Transduction. Biomolecules 2015, 5, 472–484. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Jiang, Y.X.; Yang, Y.C.; Liu, J.Y.; Huo, C.; Ji, X.L.; Qu, Y.Q. Cigarette smoke extract induces pyroptosis in human bronchial epithelial cells through the ROS/NLRP3/caspase-1 pathway. Life Sci. 2021, 269, 119090. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar]
- de Jager, T.L.; Cockrell, A.E.; Du Plessis, S.S. Ultraviolet Light Induced Generation of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 996, 15–23. [Google Scholar]
- Wang, H.; Mu, X.; He, H.; Zhang, X.D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Upadhyayula, S.S.; Bryce, W.Q.T.; Balamurugan, A.V.; Anand, D.J. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar]
- Lee, S.M.; Kim-Ha, J.; Choi, W.Y.; Lee, J.; Kim, D.; Lee, J.; Choi, E.; Kim, Y.-J. Interplay of genetic and epigenetic alterations in hepatocellular carcinoma. Epigenomics 2016, 8, 993–1005. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signaling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, J.; Wang, M.; Naruse, K.; Takahashi, K. Role of the TRPM4 channel in mitochondrial function, calcium release, and ROS generation in oxidative stress. Biochem. Biophys. Res. Commun. 2021, 566, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Karekar, P.; Jensen, H.N.; Russart, K.L.G.; Ponnalagu, D.; Seeley, S.; Sanghvi, S.; Smith, S.A.; Pyter, L.M.; Singh, H.; Gururaja, R.S. Tumor-Induced Cardiac Dysfunction: A Potential Role of ROS. Antioxidants 2021, 10, 1299. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Panich, U. Role of Phytochemicals in Skin Photoprotection via Regulation of Nrf2. Front. Pharmacol. 2022, 13, 823–881. [Google Scholar] [CrossRef]
- Chen, L.; Chen, Y.; Ding, W.; Zhan, T.; Zhu, J.; Zhang, L.; Wang, H.; Shen, B.; Wang, Y. Oxidative Stress-Induced TRPV2 Expression Increase Is Involved in Diabetic Cataracts and Apoptosis of Lens Epithelial Cells in a High-Glucose Environment. Cells 2022, 11, 1196. [Google Scholar] [CrossRef]
- Maandi, S.C.; Maandi, M.T.; Patel, A.; Manville, R.W.; Mabley, J.G. Divergent effects of HIV reverse transcriptase inhibitors on pancreatic beta-cell function and survival: Potential role of oxidative stress and mitochondrial dysfunction. Life Sci. 2022, 294, 120–329. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The Role of Oxidative Stress and Antioxidant Balance in Pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Davies, K.J.A. Oxidative DNA damage & repair: An introduction. Free Radic. Biol. Med. 2017, 107, 2–12. [Google Scholar]
- van der Reest, J.; Nardini Cecchino, G.; Haigis, M.C.; Kordowitzki, P. Mitochondria: Their relevance during oocyte ageing. Ageing Res. Rev. 2021, 70, 101378. [Google Scholar] [CrossRef] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Adwas, A.A.; Elsayed, A.S.I.; Azab, A.E.; Quwaydir, F.A. Oxidative stress and antioxidant mechanisms in human body. J. Appl. Biotechnol. Bioeng. 2019, 6, 43–47. [Google Scholar]
- Yang, C.S.; Ho, C.T.; Zhang, J.; Wan, X.; Zhang, K.; Lim, J. Antioxidants: Differing Meanings in Food Science and Health Science. J. Agric. Food Chem. 2018, 66, 3063–3068. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Miao, L.; Zhu, J.; Yin, Y.; Luo, Q.; Xu, J.; Shen, J.; Liu, J. A supramolecular bifunctional artificial enzyme with superoxid dismutase and glutathione peroxidase activities. Bioorg. Chem. 2010, 38, 159–164. [Google Scholar] [CrossRef]
- Ighodaro, O.M.; Akinloye, O.A. First time defense antioxidants—Superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx): Their functional role in the entire antioxidant defense grid. Alex. J. Med. 2018, 54, 287–293. [Google Scholar]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Lange, M.; Olzmann, J.A. Hydropersulfides are endogenous antioxidants that inhibit ferroptosis. Cell Chem. Biol. 2022, 29, 1661–1663. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Khodade, V.S.; Chauvin, J.R.; Rodriguez, D.; Toscano, J.P.; Pratt, D.A. Hydropersulfides Inhibit Lipid Peroxidation and Protect Cells from Ferroptosis. J. Am. Chem. Soc. 2022, 144, 15825–15837. [Google Scholar] [CrossRef]
- Barayeu, U.; Schilling, D.; Eid, M.; da Silva, T.N.X.; Schlicker, L.; Mitreska, N.; Zapp, C.; Gräter, F.; Miller, A.K.; Kappl, R.; et al. Hydropersulfides inhibit lipid peroxidation and ferroptosis by scavenging radicals. Nat. Chem. Biol. 2023, 19, 28–37. [Google Scholar] [CrossRef]
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, C.; Hu, H.; Yin, S. Food Sources of Selenium and Its Relationship with Chronic Diseases. Nutrients 2021, 13, 1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Cominetti, C.; Seale, L.A. Editorial: Selenium, Human Health and Chronic Disease. Front. Nutr. 2022, 8, 827759. [Google Scholar] [CrossRef] [PubMed]
- Kuršvietienė, L.; Mongirdienė, A.; Bernatonienė, J.; Šulinskienė, J.; Stanevičienė, I. Selenium Anticancer Properties and Impact on Cellular Redox Status. Antioxidants 2020, 9, 80. [Google Scholar] [CrossRef]
- Silva, M.M.; Lidon, F.C. An overview on applications and side effects of antioxidant food additives. Emir. J. Food Agric. 2016, 28, 823–832. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Antioxidants versus Food Antioxidant Additives and Food Preservatives. Antioxidants 2019, 8, 542. [Google Scholar] [CrossRef] [PubMed]
- Adams, A.K.; Best, T.M. The role of antioxidants in exercise and disease prevention. Physician Sportsmed. 2015, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Baranowski, B.J.; Marko, D.M.; Fenech, R.K.; Yang, A.J.T.; MacPherson, R.E.K. Healthy brain, healthy life: A review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl. Physiol. Nutr. Metab. 2020, 45, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Wahlqvist, M.L. Antioxidant relevance to human health. Asia Pac. J. Clin. Nutr. 2013, 22, 171–176. [Google Scholar]
- Zeb, A. Concept, mechanism, and applications of phenolic antioxidants in foods. J. Food Biochem. 2020, 44, e13394. [Google Scholar] [CrossRef]
- Shaw, K.A.; Zello, G.A.; Rodgers, C.D.; Warkentin, T.D.; Baerwald, A.R.; Chilibeck, P.D. Benefits of a plant-based diet and considerations for the athlete. Eur. J. Appl. Physiol. 2022, 122, 1163–1178. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
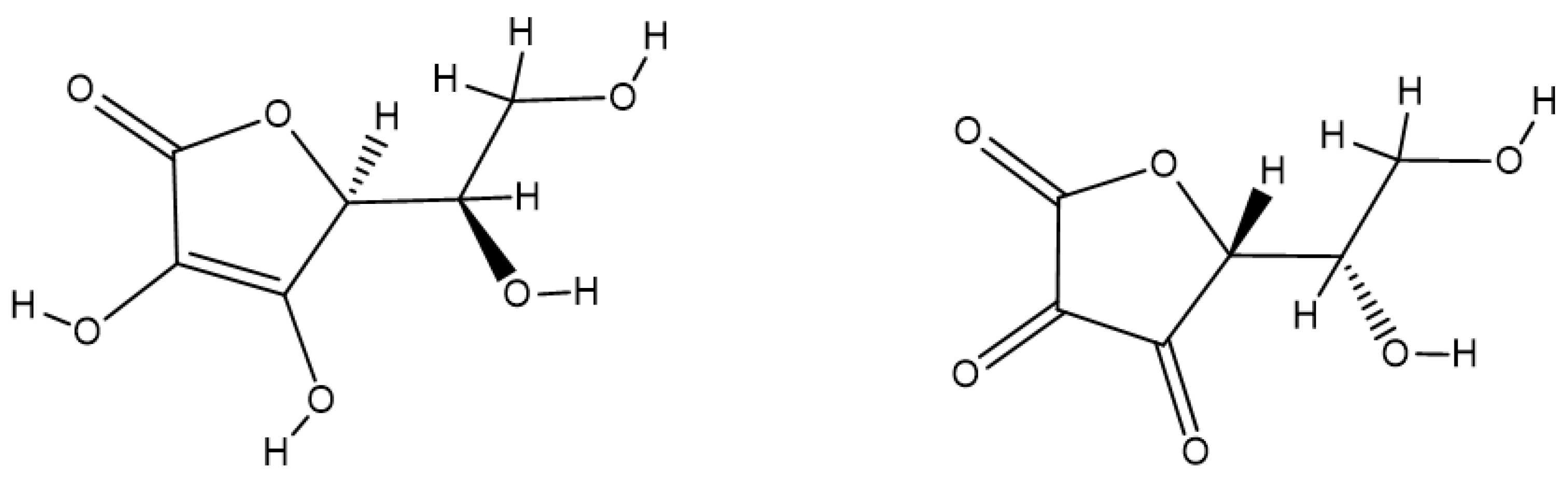
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in Plants: From Functions to Biofortification. Antioxidants 2019, 8, 519. [Google Scholar] [CrossRef]
- Smirnoff, N. Ascorbic acid metabolism and functions: A comparison of plants and mammals. Free Radic. Biol. Med. 2018, 122, 116–129. [Google Scholar] [CrossRef]
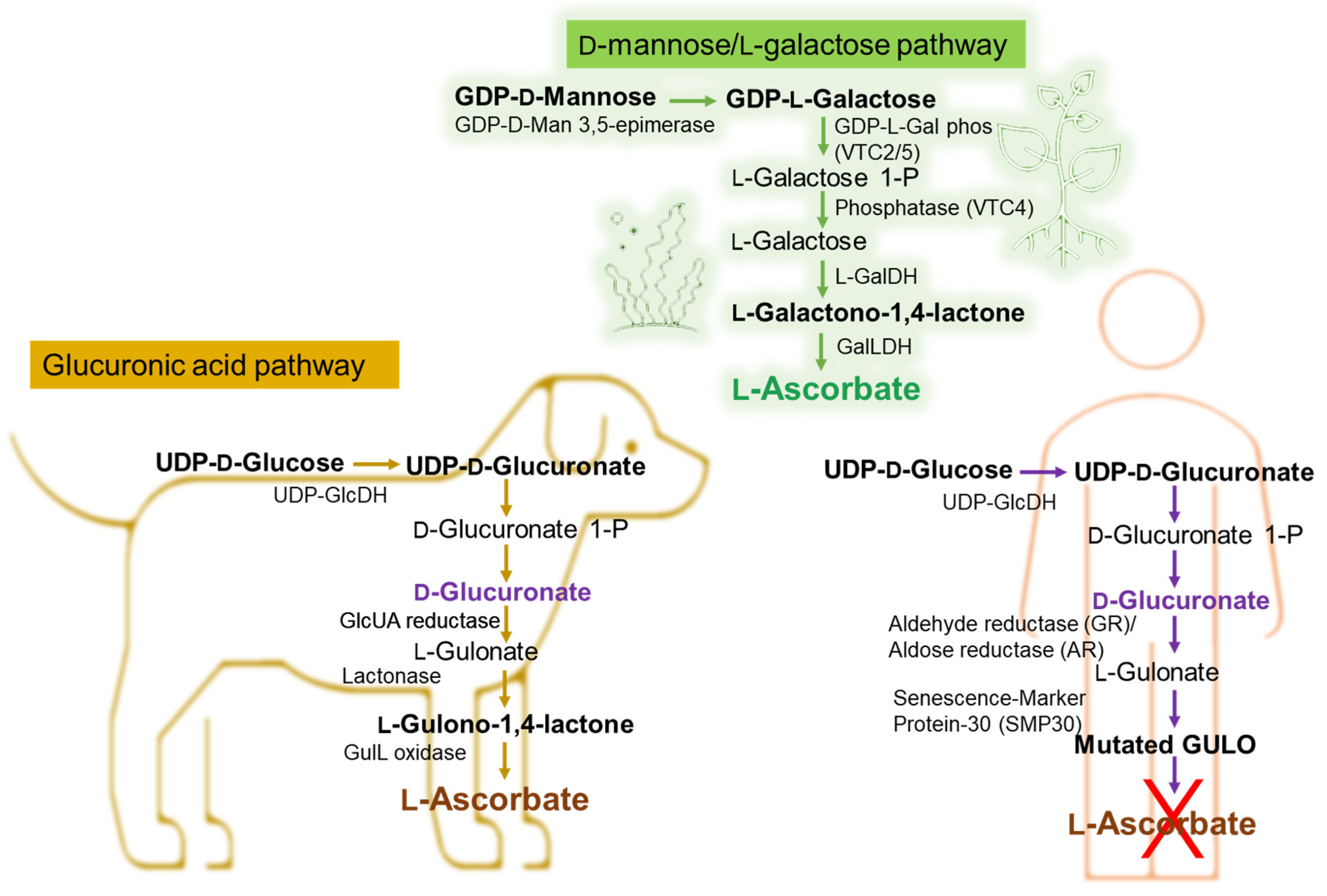
- Duan, W.; Ren, J.; Li, Y.; Liu, T.; Song, X.; Chen, Z.; Huang, Z.; Hou, X.; Li, Y. Conservation and Expression Patterns Divergence of Ascorbic Acid d-mannose/l-galactose Pathway Genes in Brassica rapa. Front. Plant Sci. 2016, 7, 778. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Gong, M.; Zhang, Q.; Tan, H.; Li, L.; Tang, Y.; Li, Z.; Peng, M.; Deng, W. Metabolism and Regulation of Ascorbic Acid in Fruits. Plants 2022, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Broad, R.C.; Bonneau, J.P.; Hellens, R.P.; Johnson, A.A.T. Manipulation of Ascorbate Biosynthetic, Recycling, and Regulatory Pathways for Improved Abiotic Stress Tolerance in Plants. Int. J. Mol. Sci. 2020, 21, 1790. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J. Ascorbate is a multifunctional micronutrient whose synthesis is lacking in primates. J. Clin. Biochem. Nutr. 2021, 69, 1–15. [Google Scholar] [CrossRef]
- Hishiyama, N.; Kayanuma, H.; Matsui, T.; Yano, H.; Suganuma, T.; Funaba, M.; Fujise, H. Plasma concentration of vitamin C in dogs with a portosystemic shunt. Can. J. Vet. Res. 2006, 70, 305–307. [Google Scholar] [PubMed]
- Gordon, D.S.; Rudinsky, A.J.; Guillaumin, J.; Parker, V.J.; Creighton, K.J. Vitamin C in Health and Disease: A Companion Animal Focus. Top. Companion Anim. Med. 2020, 39, 100432. [Google Scholar] [CrossRef]
- Drouin, G.; Godin, J.R.; Pagé, B. The genetics of vitamin C loss in vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef]
- Sharma, Y.; Popescu, A.; Horwood, C.; Hakendorf, P.; Thompson, C. Relationship between vitamin C deficiency and cognitive impairment in older hospitalised patients: A cross—Sectional study. Antioxidants 2022, 11, 463. [Google Scholar] [CrossRef]
- DePhillipo, N.N.; Aman, Z.S.; Kennedy, M.I.; Begley, J.P.; Moatshe, G.; LaPrade, R.F. Efficacy of vitamin c supplementation on collagen synthesis and oxidative stress after musculoskeletal injuries: A systematic review. Orthop. J. Sports Med. 2018, 6, 2325967118804544. [Google Scholar] [CrossRef]
- Linster, C.L.; Van Schaftingen, E. Vitamin C biosynthesis, recycling and degradation in mammals. FEBS J. 2007, 274, 1–22. [Google Scholar] [CrossRef]
- Baek, S.-M.; Lee, S.-W.; Kim, T.-U.; Choi, S.-K.; Yun, S.; Lee, W.-J.; Han, S.-H.; Hong, I.-H.; Park, S.-J.; Kim, T.-H.; et al. Senescence Marker Protein 30 (SMP30): A Novel Pan-Species Diagnostic Marker for the Histopathological Diagnosis of Breast Cancer in Humans and Animals. Int. J. Mol. Sci. 2021, 22, 2340. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Hao, Z.; Huang, C. Molecular evolution of GDP-L-galactose phosphorylase, a key regulatory gene in plant ascorbate biosynthesis. AoB Plants 2020, 12, plaa055. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, K.H.; Bohren, K.M.; Morello, R.; Bertin, T.; Liu, J.; Vogel, P. Ascorbate synthesis pathway: Dual role of ascorbate in bone homeostasis. J. Biol. Chem. 2010, 285, 19510–19520. [Google Scholar] [CrossRef]
- Yang, H. Conserved or lost: Molecular evolution of the key gene GULO in vertebrate vitamin C biosynthesis. Biochem. Genet. 2013, 51, 413–425. [Google Scholar] [CrossRef]
- Meščić Macan, A.; Gazivoda Kraljević, T.; Raić-Malić, S. Therapeutic Perspective of Vitamin C and Its Derivatives. Antioxidants 2019, 8, 247. [Google Scholar] [CrossRef]
- Nandi, A.; Mukhopadhyay, C.K.; Ghosh, M.K.; Chattopadhyay, D.J.; Chatterjee, I.B. Evolutionary significance of vitamin C biosynthesis in terrestrial vertebrates. Free Radic. Biol. Med. 1997, 22, 1047–1054. [Google Scholar] [CrossRef]
- Schröder, G.C.; Meilleur, F. Metalloprotein catalysis: Structural and mechanistic insights into oxidoreductases from neutron protein crystallography. Acta Crystallogr. Sect. D Struct. Biol. 2021, 77, 1251–1269. [Google Scholar] [CrossRef]
- Fain, O. Musculoskeletal manifestations of scurvy. Jt. Bone Spine 2005, 72, 124–128. [Google Scholar] [CrossRef] [PubMed]
- El Basuini, M.F.; Shahin, S.A.; Teiba, I.I.; Zaki, M.A.A.; El Hais, A.M.; Sewilam, H.; Almeer, R.; Abdelkhalek, N.; Dawood, M.A.O. The influence of dietary coenzyme Q10 and vitamin C on the growth rate, immunity, oxidative-related genes, and the resistance against Streptococcus agalactiae of Nile tilapia (Oreochromis niloticus). Aquaculture 2021, 531, 735862. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 2007, 137, 2171–2184. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Kang, R.; Klionsky, D.J.; Tang, D. Ferroptosis: Machinery and regulation. Autophagy 2021, 17, 2054–2081. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S. Membrane lipid peroxidation and its conflict of interest: The two faces of oxidative stress. Curr. Sci. 2014, 107, 1811–1823. [Google Scholar]
- Hawkins, C.L.; Davies, M.J. Detection, identification, and quantification of oxidative protein modifications. J. Biol. Chem. 2019, 20, 19683–19708. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Xiao, J. Ferroptosis: A Double-Edged Sword in Gastrointestinal Disease. Int. J. Mol. Sci. 2021, 22, 12403. [Google Scholar] [CrossRef]
- Nguyen-Powanda, P.; Robaire, B. Aging and oxidative stress alter DNA repair mechanisms in male germ cells of superoxide dismutase-1 null mice. Biol. Reprod. 2021, 105, 944–957. [Google Scholar] [CrossRef]
- Bárcena, B.; Salamanca, A.; Pintado, C.; Mazuecos, L.; Villar, M.; Moltó, E.; Bonzón-Kulichenko, E.; Vázquez, J.; Andrés, A.; Gallardo, N. Aging Induces Hepatic Oxidative Stress and Nuclear Proteomic Remodeling in Liver from Wistar Rats. Antioxidants 2021, 10, 1535. [Google Scholar] [CrossRef]
- Menon, A.V.; Liu, J.; Tsai, H.P.; Zeng, L.; Yang, S.; Asnani, A.; Kim, J. Excess heme upregulates heme oxygenase 1 and promotes cardiac ferroptosis in mice with sickle cell disease. Blood 2022, 139, 936–941. [Google Scholar] [CrossRef]
- Jaganjac, M.; Cindric, M.; Jakovcevic, A.; Žarkovic, K.; Žarkovic, N. Lipid peroxidation in brain tumors. Neurochem. Int. 2021, 149, 105–118. [Google Scholar] [CrossRef]
- Satish, B.N.; Dilipkumar, P. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Prenzler, P.D.; Ryan, D.; Robards, K. Introduction to Basic Principles of Antioxidant Activity. In Handbook of Antioxidant Methodology: Approaches to Activity Determination; Royal Society of Chemistry: London, UK, 2021; pp. 1–62. [Google Scholar]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [PubMed]
- Musakhanian, J.; Rodier, J.D.; Dave, M. Oxidative Stability in Lipid Formulations: A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 2022, 23, 151. [Google Scholar] [CrossRef] [PubMed]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Flieger, J.; Flieger, W.; Baj, J.; Maciejewski, R. Antioxidants: Classification, Natural Sources, Activity/Capacity Measurements, and Usefulness for the Synthesis of Nanoparticles. Materials 2021, 14, 4135. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]
- Lushchak, V.I. Glutathione homeostasis and functions: Potential targets for medical interventions. J. Amino Acids 2012, 2012, 736837. [Google Scholar] [CrossRef]
- Kesarwani, P.; Murali, A.K.; Al-Khami, A.A.; Mehrotra, S. Redox regulation of T-cell function: From molecular mechanisms to significance in human health and disease. Antioxid. Redox Signal. 2013, 18, 1497–1534. [Google Scholar] [CrossRef]
- Galano, A.; Alvarez-Idaboy, J.R. Glutathione: Mechanism and kinetics of its non-enzymatic defense action against free radicals. RSC Adv. 2011, 1, 1763–1771. [Google Scholar] [CrossRef]
- Du, X.; Yu, J.; Sun, X.; Qu, S.; Zhang, H.; Hu, M.; Yang, S.; Zhou, P. Impact of epigallocatechin-3-gallate on expression of nuclear factor erythroid 2-related factor 2 and γ-glutamyl cysteine synthetase genes in oxidative stress-induced mouse renal tubular epithelial cells. Mol. Med. Rep. 2018, 17, 7952–7958. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Fierro-Fernández, M.; Sánchez-Gómez, F.; Rodríguez-Pascual, F.; Alique, M.; Ruiz-Ortega, M.; Beraza, N.; Martínez-Chantar, M.L.; Fernández-Hernando, C.; Lamas, S. Targeting of Gamma-Glutamyl-Cysteine Ligase by miR-433 Reduces Glutathione Biosynthesis and Promotes TGF-β-Dependent Fibrogenesis. Antioxid. Redox Signal. 2015, 23, 1092–1105. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Regulation of glutathione synthesis. Curr. Top. Cell. Regul. 2001, 36, 95–116. [Google Scholar]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, N.; Krance, S.M.; Notenboom, S.; Shi, S.; Tieu, K.; Hammond, C.L. Glutathione dysregulation and the etiology and progression of human diseases. Biol. Chem. 2009, 390, 191–214. [Google Scholar] [CrossRef]
- Sitohang, I.B.S.; Anwar, A.I.; Jusuf, N.K.; Arimuko, A.; Norawati, L.; Veronica, S. Evaluating Oral Glutathione Plus Ascorbic Acid, Alpha-lipoic Acid, and Zinc Aspartate as a Skin-lightening Agent: An Indonesian Multicenter, Randomized, Controlled Trial. J. Clin. Aesthetic Dermatol. 2021, 14, 53–58. [Google Scholar]
- Minich, D.M.; Brown, B.I. A Review of Dietary (Phyto)Nutrients for Glutathione Support. Nutrients 2019, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Hossain, A.; Khatun, M.A.; Islam, M.; Huque, R. Enhancement of Antioxidant Quality of Green Leafy Vegetables upon Different Cooking Method. Prev. Nutr. Food Sci. 2017, 22, 216–222. [Google Scholar]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef]
- Rubio-Riquelme, N.; Huerta-Retamal, N.; Gómez-Torres, M.J.; Martínez-Espinosa, R.M. Catalase as a Molecular Target for Male Infertility Diagnosis and Monitoring: An Overview. Antioxidants 2020, 9, 78. [Google Scholar] [CrossRef]
- Sheng, Y.; Abreu, I.A.; Cabelli, D.E.; Maroney, M.J.; Miller, A.; Teixeira, M.; Valentine, J.S. Superoxide Dismutases and Superoxide Reductases. Chem. Rev. 2014, 114, 3854–3918. [Google Scholar] [CrossRef] [PubMed]
- Mondola, P.; Damiano, S.; Sasso, A.; Santillo, M. The Cu, Zn Superoxide Dismutase: Not Only a Dismutase Enzyme. Front. Physiol. 2016, 7, 594. [Google Scholar] [CrossRef] [PubMed]
- Stephenie, S.; Chang, Y.P.; Gnanasekaran, A.; Esa, N.M.; Gnanaraj, C. An insight on superoxide dismutase (SOD) from plants for mammalian health enhancement. J. Funct. Foods 2020, 68, 103917. [Google Scholar] [CrossRef]
- Zehiroglu, C.; Ozturk Sarikaya, S.B. The importance of antioxidants and place in today’s scientific and technological studies. J. Food Sci. Technol. 2019, 56, 4757–4774. [Google Scholar] [CrossRef]
- López-Pedrouso, M.; Lorenzo, J.M.; Franco, D. Advances in Natural Antioxidants for Food Improvement. Antioxidants 2022, 11, 1825. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C.D.S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef]
- Kurek, M.; Benaida-Debbache, N.; Elez Garofulić, I.; Galić, K.; Avallone, S.; Voilley, A.; Waché, Y. Antioxidants and Bioactive Compounds in Food: Critical Review of Issues and Prospects. Antioxidants 2022, 11, 742. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Li, J.; Lin, Y.; He, L.; Ou, R.; Chen, T.; Zhang, X.; Li, Q.; Zeng, Z.; Long, Q. Two New Isoprenoid Flavonoids from Sophora flavescens with Antioxidant and Cytotoxic Activities. Molecules 2021, 26, 7228. [Google Scholar] [CrossRef]
- Naheed, N.; Maher, S.; Saleem, F.; Khan, A.; Wadood, A.; Rasheed, S.; Choudhary, M.I.; Froeyen, M.; Abdullah, I.; Mirza, M.U.; et al. New isolate from Salvinia molesta with antioxidant and urease inhibitory activity. Drug Dev. Res. 2021, 82, 1169–1181. [Google Scholar] [CrossRef]
- Arab, Z.; Jafarian, S.; Karimi-Maleh, H.; Roozbeh Nasiraie, L.; Ahmadi, M. Monitoring of Butylated Hydroxyanisole in Food and Wastewater Samples Using Electroanalytical Two-Fold Amplified Sensor. Sustainability 2022, 14, 2169. [Google Scholar] [CrossRef]
- Delanghe, T.; Huyghe, J.; Lee, S.; Priem, D.; Van Coillie, S.; Gilbert, B.; Choi, S.M.; Vandenabeele, P.; Degterev, A.; Cuny, G.D.; et al. Antioxidant and food additive BHA prevents TNF cytotoxicity by acting as a direct RIPK1 inhibitor. Cell Death Dis. 2021, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Liu, A.; Hu, S.; Ares, I.; Martínez-Larrañaga, M.-R.; Wang, X.; Martínez, M.; Anadón, A.; Martínez, M.-A. Synthetic phenolic antioxidants: Metabolism, hazards and mechanism of action. Food Chem. 2021, 353, 129488. [Google Scholar] [CrossRef]
- Felter, S.P.; Zhang, X.; Thompson, C. Butylated hydroxyanisole: Carcinogenic food additive to be avoided or harmless antioxidant important to protect food supply? Regul. Toxicol. Pharmacol. 2021, 121, 104887. [Google Scholar] [CrossRef] [PubMed]
- Shamsadin-Azad, Z.; Taher, M.A.; Cheraghi, S.; Karimi-Maleh, H. A nanostructure voltammetric platform amplified with ionic liquid for determination of tert-butylhydroxyanisole in the presence kojic acid. J. Food Meas. Charact. 2019, 13, 1781–1787. [Google Scholar] [CrossRef]
- Zhang, R.; Li, J.; Cui, X. Tissue distribution, excretion, and metabolism of 2,6-di-tert-butyl-hydroxytoluene in mice. Sci. Total Environ. 2020, 739, 139862. [Google Scholar] [CrossRef]
- Sun, Z.; Gao, R.; Chen, X.; Liu, X.; Ding, Y.; Geng, Y.; Mu, X.; Liu, T.; Li, F.; Wang, Y.; et al. Exposure to butylated hydroxytoluene compromises endometrial decidualization during early pregnancy. Environ. Sci. Pollut. Res. Int. 2021, 28, 42024–42036. [Google Scholar] [CrossRef]
- Mizobuchi, M.; Ishidoh, K.; Kamemura, N. A comparison of cell death mechanisms of antioxidants, butylated hydroxyanisole and butylated hydroxytoluene. Drug Chem. Toxicol. 2022, 45, 1899–1906. [Google Scholar] [CrossRef]
- Abou-Hadeed, A.H.; Mohamed, A.T.; Hegab, D.Y.; Ghoneim, M.H. Ethoxyquin and Butylated Hydroxy Toluene Distrub the Hematological Parameters and Induce Structural and Functional Alterations in Liver of Rats. Arch. Razi Inst. 2021, 76, 1765–1776. [Google Scholar]
- Nieva-Echevarría, B.; Manzanos, M.J.; Goicoechea, E.; Guillén, M.D. 2,6-Di-Tert-Butyl-Hydroxytoluene and Its Metabolites in Foods. Compr. Rev. Food Sci. Food Saf. 2015, 14, 67–80. [Google Scholar] [CrossRef]
- Zhao, M.T.; Liu, Z.Y.; Li, A.; Zhao, G.H.; Xie, H.K.; Zhou, D.Y.; Wang, T. Gallic acid and its alkyl esters emerge as effective antioxidants against lipid oxidation during hot air drying process of Ostrea talienwhanensis. LWT 2021, 139, 110551. [Google Scholar] [CrossRef]
- Choubey, S.; Varughese, L.R.; Kumar, V.; Beniwal, V. Medicinal importance of gallic acid and its ester derivatives: A patent review. Pharm. Pat. Anal. 2015, 4, 305–315. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and diseases: A mechanistic review. Iran J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Keramat, M.; Niakousari, M.; Golmakani, M.T. Comparing the antioxidant activity of gallic acid and its alkyl esters in emulsion gel and non-gelled emulsion. Food Chem. 2023, 407, 135078. [Google Scholar] [CrossRef]
- Perazzoli, M.R.; Perondi, C.K.; Baratto, C.M.; Winter, E.; Creczynski-Pasa, T.B.; Locatelli, C. Gallic Acid and Dodecyl Gallate Prevents Carbon Tetrachloride-Induced Acute and Chronic Hepatotoxicity by Enhancing Hepatic Antioxidant Status and Increasing p53 Expression. Biol. Pharm. Bull. 2017, 40, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Delgado, A.; Al-Hamimi, S.; Ramadan, M.F.; De Wit, M.; Durazzo, A.; Nyam, K.L.; Issaoui, M. Contribution of Tocols to Food Sensorial Properties, Stability, and Overall Quality. J. Food Qual. 2020, 2020, 8885865. [Google Scholar] [CrossRef]
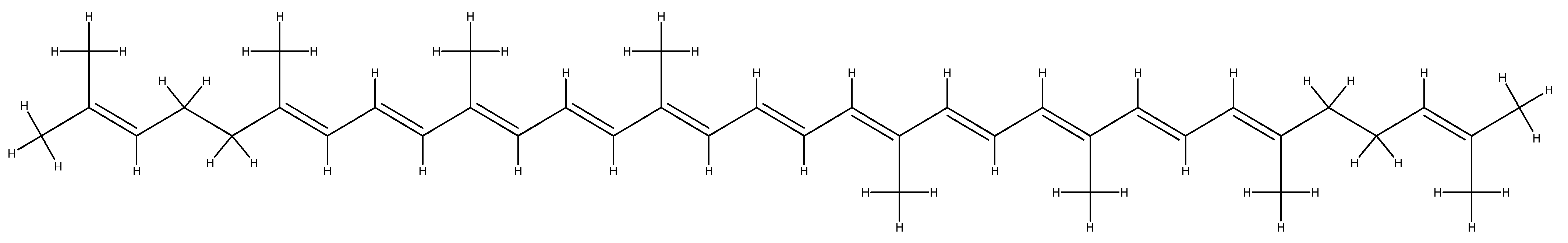
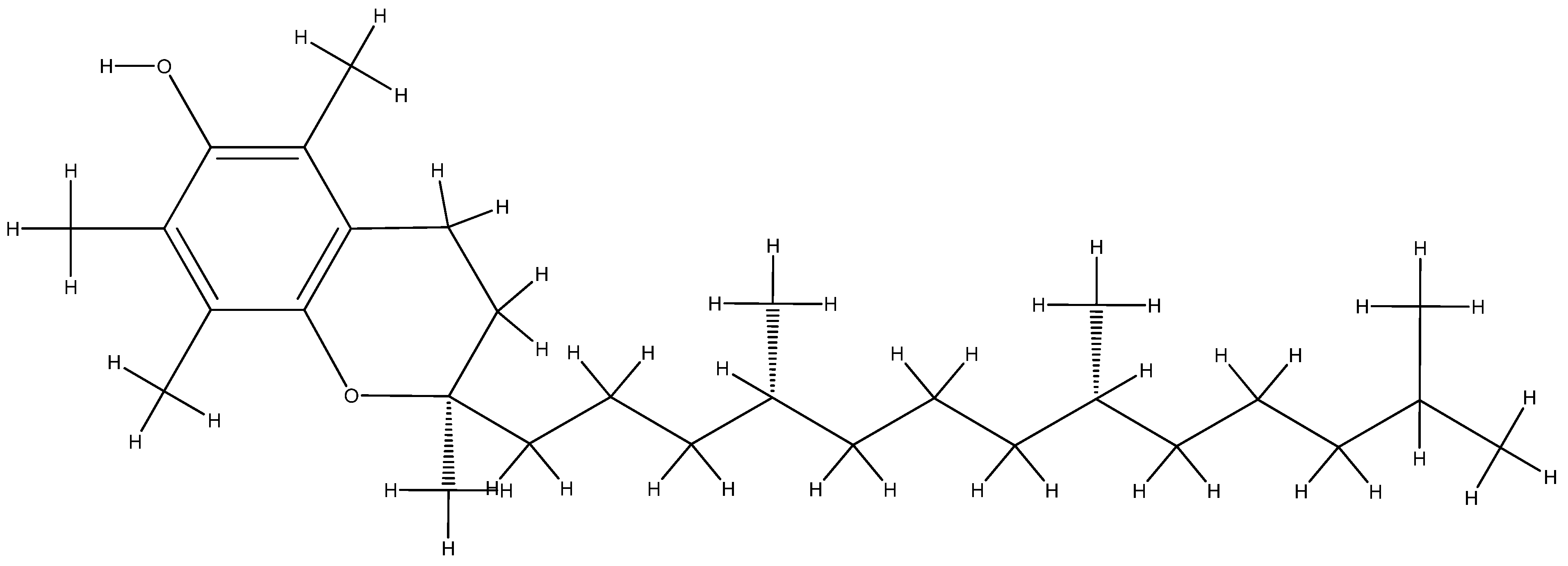
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Li, G.X.; Lee, M.J.; Liu, A.B.; Yang, Z.; Lin, Y.; Shih, W.J.; Yang, C.S. δ-Tocopherol is more active than α- or γ-tocopherol in inhibiting lung tumorigenesis in vivo. Cancer Prev. Res. 2011, 4, 404–413. [Google Scholar] [CrossRef]
- Jiang, Q. Natural Forms of Vitamin E as Effective Agents for Cancer Prevention and Therapy. Adv. Nutr. 2017, 8, 850–867. [Google Scholar] [CrossRef]
- Tufiño, C.; Bernal, C.; Ottone, C.; Romero, O.; Illanes, A.; Wilson, L. Synthesis with Immobilized Lipases and Downstream Processing of Ascorbyl Palmitate. Molecules 2019, 24, 3227. [Google Scholar] [CrossRef]
- Ledinski, M.; Marić, I.; Peharec Štefanić, P.; Ladan, I.; Caput Mihalić, K.; Jurkin, T.; Gotić, M.; Urlić, I. Synthesis and In Vitro Characterization of Ascorbyl Palmitate-Loaded Solid Lipid Nanoparticles. Polymers 2022, 14, 1751. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.A.; Seo, J.K.; Parvin, R.; Ko, J.; Yang, H.S. Comparison of Butylated Hydroxytoluene, Ascorbic Acid, and Clove Extract as Antioxidants in Fresh Beef Patties at Refrigerated Storage. Food Sci. Anim. Resour. 2019, 39, 768–779. [Google Scholar] [CrossRef]
- Boroujeni, L.S.; Hojjatoleslamy, M. Using Thymus carmanicus and Myrtus communis essential oils to enhance the physicochemical properties of potato chips. Food Sci. Nutr. 2018, 6, 1006–1014. [Google Scholar] [CrossRef]
- Pei, X.C.; Yin, F.W.; Zhong, X.; Liu, H.L.; Song, L.; Zhao, G.H.; Wang, Y.F.; Zhou, D.Y. Effects of different antioxidants and their combinations on the oxidative stability of DHA algae oil and walnut oil. Food Sci. Nutr. 2022, 10, 2804–2812. [Google Scholar] [CrossRef]
- Badea, G.A.; Antoce, A.O. Glutathione as a possible replacement of sulfur dioxide in winemaking technologies: A review. Sci. Pap. Ser. B Hortic. 2015, LIX, 123–140. [Google Scholar]
- Zhang, D.; Wang, X.; Tian, X.; Zhang, L.; Yang, G.; Tao, Y.; Liang, C.; Li, K.; Yu, X.; Tang, X.; et al. The Increased Endogenous Sulfur Dioxide Acts as a Compensatory Mechanism for the Downregulated Endogenous Hydrogen Sulfide Pathway in the Endothelial Cell Inflammation. Front. Immunol. 2018, 9, 882. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Saraiva, J.A.; Coimbra, M.A. Chemical and physical methodologies for the replacement/reduction of sulfur dioxide use during winemaking: Review of their potentialities and limitations. Eur. Food Res. Technol. 2012, 234, 1–12. [Google Scholar] [CrossRef]
- Padayatty, S.J.; Levine, M. Vitamin C: The known and the unknown and Goldilocks. Oral Dis. 2016, 22, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Chen, K.; Cheng, H.; Feng, S.; Song, Y.; Liang, L. Chemical Stability of Ascorbic Acid Integrated into Commercial Products: A Review on Bioactivity and Delivery Technology. Antioxidants 2022, 11, 153. [Google Scholar] [CrossRef]
- Bradshaw, M.P.; Barril, C.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Ascorbic acid: A review of its chemistry and reactivity in relation to a wine environment. Crit. Rev. Food Sci. Nutr. 2012, 51, 479–498. [Google Scholar] [CrossRef]
- Mousavi, A.; Pourakbar, L.; Moghaddam, S.S. Effects of malic acid and EDTA on oxidative stress and antioxidant enzymes of okra (Abelmoschus esculentus L.) exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2022, 248, 114320. [Google Scholar] [CrossRef]
- Li, J.; Li, F.; Xu, Y.; Yang, W.; Qu, L.; Xiang, Q.; Liu, C.; Li, D. Chemical composition and synergistic antioxidant activities of essential oils from Atractylodes macrocephala and Astragalus membranaceus. Nat. Prod. Commun. 2013, 8, 1321–1324. [Google Scholar] [CrossRef]
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural antioxidants in meat and poultry products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Poljsak, B.; Kovač, V.; Milisav, I. Antioxidants, Food Processing and Health. Antioxidants 2021, 10, 433. [Google Scholar] [CrossRef]
- Rather, S.A.; Masoodi, F.A.; Akhter, R.; Rather, J.A.; Shiekh, K.A. Advances in use of Natural Antioxidants as Food Additives for improving the Oxidative Stability of Meat Products. Madr. J. Food Technol. 2016, 1, 10–17. [Google Scholar]
- Tajik, H.; Farhangfar, A.; Moradi, M.; Rohani, S.M.R. Effectiveness of clove essential oil and grape seed extract combination on microbial and lipid oxidation characteristics of raw buffalo patty during storage at abuse refrigeration temperature. J. Food Process. Preserv. 2014, 38, 31–38. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Verma, A.K.; Swarup, D. Moringa oleifera leaves extract: A natural antioxidant for retarding lipid peroxidation in cooked goat meat patties. Int. J. Food Sci. Technol. 2012, 47, 585–591. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Del-río, I.; López-Ibáñez, S.; Magadán-Corpas, P.; Fernández-Calleja, L.; Pérez-Valero, Á.; Tuñón-Granda, M.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Terpenoids and polyphenols as natural antioxidant agents in food preservation. Antioxidants 2021, 10, 1264. [Google Scholar] [CrossRef] [PubMed]
- Poliński, S.; Topka, P.; Tańska, M.; Kowalska, S.; Czaplicki, S.; Szydłowska-Czerniak, A. Impact of Bioactive Compounds of Plant Leaf Powders in White Chocolate Production: Changes in Antioxidant Properties during the Technological Processes. Antioxidants 2022, 11, 752. [Google Scholar] [CrossRef]
- Faheem, F.; Liu, Z.W.; Rabail, R.; Haq, I.U.; Gul, M.; Bryła, M.; Roszko, M.; Kieliszek, M.; Din, A.; Aadil, R.M. Uncovering the Industrial Potentials of Lemongrass Essential Oil as a Food Preservative: A Review. Antioxidants 2022, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Thahira Banu, A.; Sri Ramani, P.; Aswini, M. Effect of seaweed coating on quality characteristics and shelf life of tomato (Lycopersicon esculentum mill). Food Sci. Hum. Wellness 2020, 9, 176–183. [Google Scholar] [CrossRef]
- Sanches-Silva, A.; Costa, D.; Albuquerque, T.G.; Buonocore, G.G.; Ramos, F.; Castilho, M.C.; Machado, A.V.; Costa, H.S. Trends in the use of natural antioxidants in active food packaging: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2014, 31, 374–395. [Google Scholar] [CrossRef] [PubMed]
- Rangaraj, V.; Rambabu, K.; Banat, F.; Mittal, V. Natural antioxidants-based edible active food packaging: An overview of current advancements. Food Biosci. 2021, 43, 101251. [Google Scholar] [CrossRef]
- Vieira, I.R.S.; de Carvalho, A.P.A.; Conte-Junior, C.A. Recent advances in biobased and biodegradable polymer nanocomposites, nanoparticles, and natural antioxidants for antibacterial and antioxidant food packaging applications. CRFSFS 2022, 21, 3673–3716. [Google Scholar] [CrossRef]
- Crespo, Y.A.; Bravo Sánchez, L.R.; Quintana, Y.G.; Cabrera, A.S.T.; Bermúdez Del Sol, A.; Mayancha, D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon 2019, 5, e01942. [Google Scholar] [CrossRef]
- Pruteanu, L.L.; Kopanitsa, L.; Módos, D.; Kletnieks, E.; Samarova, E.; Bender, A.; Gomez, L.D.; Bailey, D.S. Transcriptomics predicts compound synergy in drug and natural product treated glioblastoma cells. PLoS ONE 2020, 15, e0239551. [Google Scholar] [CrossRef]
- Lopez-Maldonado, A.; Pastoriza, S.; Rufián-Henares, J.Á. Assessing the antioxidant and metabolic effect of an alpha-lipoic acid and acetyl-L-carnitine nutraceutical. Curr. Res. Food Sci. 2021, 4, 336–344. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Bin-Jumah, M.N.; Nadeem, M.S.; Gilani, S.J.; Mubeen, B.; Ullah, I.; Alzarea, S.I.; Ghoneim, M.M.; Alshehri, S.; Al-Abbasi, F.A.; Kazmi, I. Lycopene: A Natural Arsenal in the War against Oxidative Stress and Cardiovascular Diseases. Antioxidants 2022, 11, 232. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- George, J.; Edwards, D.; Pun, S.; Williams, D. Evaluation of Antioxidant Capacity (ABTS and CUPRAC) and Total Phenolic Content (Folin-Ciocalteu) Assays of Selected Fruit, Vegetables, and Spices. Int. J. Food Sci. 2022, 2022, 2581470. [Google Scholar] [CrossRef]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef]
- Caturla, N.; Funes, L.; Pérez-Fons, L.; Micol, V. A randomized, double-blinded, placebo-controlled study of the effect of a combination of lemon verbena extract and fish oil omega-3 fatty acid on joint management. J. Altern. Complement Med. 2011, 17, 1051–1063. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bøhn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J.; Duthie, G.G.; Russell, W.R.M.; Kyle, J.A.; Macdiarmid, J.I.; Rungapamestry, V.; Stephen, S.; Megias-Baeza, C.; Kaniewska, J.J.; Shaw, L.; et al. Effect of increasing fruit and vegetable intake by dietary intervention on nutritional biomarkers and attitudes to dietary change: A randomised trial. Eur. J. Nutr. 2018, 57, 1855–1872. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, 511. [Google Scholar] [CrossRef]
- Srivastava, K.K.; Kumar, R. Stress, oxidative injury and disease. Indian J. Clin. Biochem. 2015, 30, 3–10. [Google Scholar] [CrossRef]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative stress: Role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 2018, 9, 17181–17198. [Google Scholar] [CrossRef] [PubMed]
- Sayin, V.I.; Ibrahim, M.X.; Larsson, E.; Nilsson, J.A.; Lindahl, P.; Bergo, M.O. Antioxidants accelerate lung cancer progression in mice. Sci. Transl. Med. 2014, 6, 221ra15. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Lu, W.; Li, J.; Yu, S.; Brown, E.J.; Stanger, B.Z.; Rabinowitz, J.D.; Yang, X. G6PD-mediated increase in de novo NADP+ biosynthesis promotes antioxidant defense and tumor metastasis. Sci. Adv. 2022, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef]
- Babich, O.; Larina, V.; Ivanova, S.; Tarasov, A.; Povydysh, M.; Orlova, A.; Strugar, J.; Sukhikh, S. Phytotherapeutic Approaches to the Prevention of Age-Related Changes and the Extension of Active Longevity. Molecules 2022, 27, 2276. [Google Scholar] [CrossRef]
- Jacob, K.; Periago, M.J.; Böhm, V.; Berruezo, G.R. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br. J. Nutr. 2008, 99, 137–146. [Google Scholar] [CrossRef]
- Metha, D.; Belemkar, S. Pharmacological activity of Spinacia oleracea Linn.—A complete overview. Asian J. Pharm. 2014, 2, 83–93. [Google Scholar]
- Banel, D.K.; Hu, F.B. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: A meta-analysis and systematic review. Am. J. Clin. Nutr. 2009, 90, 56–63. [Google Scholar] [CrossRef]
- Ayadi, J.; Debouba, M.; Rahmani, R.; Bouajila, J. Brassica Genus Seeds: A Review on Phytochemical Screening and Pharmacological Properties. Molecules 2022, 27, 6008. [Google Scholar] [CrossRef]
- Ke, Y.Y.; Shyu, Y.T.; Wu, S.J. Evaluating the Anti-Inflammatory and Antioxidant Effects of Broccoli Treated with High Hydrostatic Pressure in Cell Models. Foods 2021, 10, 167. [Google Scholar] [CrossRef]
- Kalt, W.; Cassidy, A.; Howard, L.R.; Krikorian, R.; Stull, A.J.; Tremblay, F.; Zamora-Ros, R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020, 11, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Ribera-Fonseca, A.; Jiménez, D.; Leal, P.; Riquelme, I.; Roa, J.C.; Alberdi, M.; Peek, R.M.; Reyes-Díaz, M. The Anti-Proliferative and Anti-Invasive Effect of Leaf Extracts of Blueberry Plants Treated with Methyl Jasmonate on Human Gastric Cancer In Vitro Is Related to Their Antioxidant Properties. Antioxidants 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M. Green tea and prevention of esophageal and lung cancers. Mol. Nutr. Food Res. 2011, 55, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, H.; Zhou, L.; Li, G.; Yi, D.; Zhang, Y.; Wu, Y.; Liu, X.; Wu, X.; Song, Q.; et al. Association between green tea intake and risk of gastric cancer: A systematic review and dose–response meta-analysis of observational studies. Public Health Nutr. 2017, 20, 3183–3192. [Google Scholar] [CrossRef] [PubMed]
- Fujiki, H.; Watanabe, T.; Sueoka, E.; Rawangkan, A.; Suganuma, M. Cancer Prevention with Green Tea and Its Principal Constituent, EGCG: From Early Investigations to Current Focus on Human Cancer Stem Cells. Mol. Cells 2018, 41, 73–82. [Google Scholar]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Halvorsen, B.L.; Holte, K.; Myhrstad, M.C.W.; Barikmo, I.; Hvattum, E.; Remberg, S.F.; Wold, A.B.; Haffner, K.; Baugerod, H.; Andersen, L.F.; et al. A systematic screening of total antioxidants in dietary plants. J. Nutr. 2002, 132, 461–471. [Google Scholar] [CrossRef]
- Dragland, S.; Senoo, H.; Wake, K.; Holte, K.; Blomhoff, R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J. Nutr. 2003, 133, 1286–1290. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Nunes, A.R.; Falcão, A.; Alves, G.; Silva, L.R. Dietary Effects of Anthocyanins in Human Health: A Comprehensive Review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Salehi, B.; Cappellini, F.; Reiner, Ž.; Zorzan, D.; Imran, M.; Sener, B.; Kilic, M.; Fahmy, N.M.; Martorell, M.; Tonelli, C.; et al. The Therapeutic Potential of Anthocyanins: Current Approaches Based on Their Molecular Mechanism of Action. Front. Pharmacol. 2020, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.A.; Khan, R.A. Anthocyanins: Traditional Uses, Structural and Functional Variations, Approaches to Increase Yields and Products’ Quality, Hepatoprotection, Liver Longevity, and Commercial Products. Int. J. Mol. Sci. 2022, 23, 2149. [Google Scholar] [CrossRef]
- Pennington, J.A.T.; Fisher, R.A. Classification of fruits and vegetables. J. Food Compos. Anal. 2009, 22, S23–S31. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Mukai, R.; Fukuda, T.; Ohnishi, A.; Nikawa, T.; Furusawa, M.; Terao, J. Chocolate as a food matrix reduces the bioavailability of galloylated catechins from green tea in healthy women. Food Funct. 2021, 12, 408–416. [Google Scholar] [CrossRef]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Burri, B.J.; La Frano, M.R.; Zhu, C. Absorption, metabolism, and functions of β-cryptoxanthin. Nutr. Rev. 2016, 74, 69–82. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, M.A.; Ortega-Regules, A.E.; Anaya de Parrodi, C.; Lozada-Ramírez, J.D. Chemistry, Occurrence, Properties, Applications, and Encapsulation of Carotenoids—A Review. Plants 2023, 12, 313. [Google Scholar] [CrossRef]
- Mutalipassi, M.; Esposito, R.; Ruocco, N.; Viel, T.; Costantini, M.; Zupo, V. Bioactive Compounds of Nutraceutical Value from Fishery and Aquaculture Discards. Foods 2021, 10, 1495. [Google Scholar] [CrossRef]
- Liang, H.; Ji, K.; Ge, X.; Mi, H.; Xi, B.; Ren, M. Effects of dietary copper on growth, antioxidant capacity and immune responses of juvenile blunt snout bream (Megalobrama amblycephala) as evidenced by pathological examination. Aquac. Rep. 2020, 17, 100296. [Google Scholar] [CrossRef]
- Ramesh, P.; Jagadeesan, R.; Sekaran, S.; Dhanasekaran, A.; Vimalraj, S. Flavonoids: Classification, Function, and Molecular Mechanisms Involved in Bone Remodelling. Front. Endocrinol. 2021, 12, 779638. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and bioactives in citrus fruits: Different citrus varieties, fruit parts, and growth stages. Crit. Rev. Food Sci. Nutr. 2021, 1–24. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.J.; Chen, J.B.; Cao, J.P.; Li, X.; Sun, C.D. Citrus flavonoids and their antioxidant evaluation. Crit. Rev. Food Sci. Nutr. 2022, 62, 3833–3854. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Singh, N.P.; Singh, U.P.; Rouse, M.; Zhang, J.; Chatterjee, S.; Nagarkatti, P.S.; Nagarkatti, M. Dietary Indoles Suppress Delayed-Type Hypersensitivity by Inducing a Switch from Proinflammatory Th17 Cells to Anti-Inflammatory Regulatory T Cells through Regulation of MicroRNA. J. Immunol. 2016, 196, 1108–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, R.W.; Huang, H.; Fletcher, A.; Yu, L.; Pham, Q.; Yu, L.; He, Q.; Wang, T.T.Y. Inhibition of Tumor Growth by Dietary Indole-3-Carbinol in a Prostate Cancer Xenograft Model May Be Associated with Disrupted Gut Microbial Interactions. Nutrients 2019, 11, 467. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef] [PubMed]
- Stanisławska, I.J.; Figat, R.; Kiss, A.K.; Bobrowska-Korczak, B. Essential Elements and Isoflavonoids in the Prevention of Prostate Cancer. Nutrients 2022, 14, 1225. [Google Scholar] [CrossRef] [PubMed]
- Osmakov, D.I.; Kalinovskii, A.P.; Belozerova, O.A.; Andreev, Y.A.; Kozlov, S.A. Lignans as Pharmacological Agents in Disorders Related to Oxidative Stress and Inflammation: Chemical Synthesis Approaches and Biological Activities. Int. J. Mol. Sci. 2022, 23, 6031. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Toledo, E.; Delgado-Rodríguez, M.; Gaforio, J.J. Naturally Lignan-Rich Foods: A Dietary Tool for Health Promotion? Molecules 2019, 24, 917. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Lee, J.C.; Leung, H.H.; Lam, W.C.; Fu, Z.; Lo, A.C.Y. Lutein Supplementation for Eye Diseases. Nutrients 2020, 12, 1721. [Google Scholar] [CrossRef]
- Manzoor, S.; Rashid, R.; Prasad Panda, B.; Sharma, V.; Azhar, M. Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil. Ultrason. Sonochem. 2022, 85, 105994. [Google Scholar] [CrossRef] [PubMed]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; et al. Lycopene: Food Sources, Biological Activities, and Human Health Benefits. Oxidative Med. Cell. Longev. 2021, 2021, 2713511. [Google Scholar] [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J.O.; Atanasov, A.G. Lycopene and Vascular Health. Front. Pharmacol. 2018, 9, 521. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef] [PubMed]
- Goñi, I.; Hernández-Galiot, A. Intake of Nutrient and Non-Nutrient Dietary Antioxidants. Contribution of Macromolecular Antioxidant Polyphenols in an Elderly Mediterranean Population. Nutrients 2019, 11, 2165. [Google Scholar] [CrossRef] [PubMed]
- Nani, A.; Murtaza, B.; Sayed Khan, A.; Khan, N.A.; Hichami, A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules 2021, 26, 985. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, K.; Yan, C.; Yin, Y.; He, S.; Qiu, L.; Li, G. Natural Polyphenols for Treatment of Colorectal Cancer. Molecules 2022, 27, 8810. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Qamar, M.; Yuan, Y.; Nazir, Y.; Wilairatana, P.; Mubarak, M.S. Dietary Polyphenols: Extraction, Identification, Bioavailability, and Role for Prevention and Treatment of Colorectal and Prostate Cancers. Molecules 2022, 27, 2831. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Das, S.; Patra, S.K.; Efferth, T.; Jena, M.; Bhutia, S.K. Dietary polyphenols in chemoprevention and synergistic effect in cancer: Clinical evidences and molecular mechanisms of action. Phytomedicine 2021, 90, 153554. [Google Scholar] [CrossRef]
- Intra, J.; Kuo, S.M. Physiological levels of tea catechins increase cellular lipid antioxidant activity of vitamin C and vitamin E in human intestinal caco-2 cells. Chem. Biol. Interact. 2007, 169, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Gebicki, J.M.; Nauser, T. Fast Antioxidant Reaction of Polyphenols and Their Metabolites. Antioxidants 2021, 10, 1297. [Google Scholar] [CrossRef] [PubMed]
- Khatoon, S.; Kalam, N.; Shaikh, M.F.; Hasnain, M.S.; Hafiz, A.K.; Ansari, M.T. Nanoencapsulation of Polyphenols as Drugs and Supplements for Enhancing Therapeutic Profile—A Review. Curr. Mol. Pharmacol. 2022, 15, 77–107. [Google Scholar] [PubMed]
- Hossen, M.S.; Ali, M.Y.; Jahurul, M.H.A.; Abdel-Daim, M.M.; Gan, S.H.; Khalil, M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: A review. Pharmacol. Rep. 2017, 69, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kitts, D.; Giovannucci, E.L.; Sahye-Pudaruth, S.; Paquette, M.; Blanco Mejia, S.; Patel, D.; Kavanagh, M.; Tsirakis, T.; Kendall, C.W.C.; et al. Selenium, antioxidants, cardiovascular disease, and all-cause mortality: A systematic review and meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2020, 112, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Xie, T.; Li, S.; Wang, W.; Wang, Y.; Cao, Z.; Yang, H. Effects of Selenium as a Dietary Source on Performance, Inflammation, Cell Damage, and Reproduction of Livestock Induced by Heat Stress: A Review. Front. Immunol. 2022, 12, 820853. [Google Scholar] [CrossRef]
- Flohé, L. The glutathione peroxidase reaction: Molecular basis of the antioxidant function of selenium in mammals. Curr. Top. Cell. Regul. 1985, 27, 473–478. [Google Scholar] [PubMed]
- Tsuji, P.A.; Santesmasses, D.; Lee, B.J.; Gladyshev, V.N.; Hatfield, D.L. Historical Roles of Selenium and Selenoproteins in Health and Development: The Good, the Bad and the Ugly. Int. J. Mol. Sci. 2021, 23, 5. [Google Scholar] [CrossRef]
- Labunskyy, V.M.; Hatfield, D.L.; Gladyshev, V.N. Selenoproteins: Molecular pathways and physiological roles. Physiol. Rev. 2014, 94, 739–777. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–Fascinating Microelement, Properties and Sources in Food. Molecules 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Kronzucker, H.J.; Shi, W. Selenium Biofortification and Interaction With Other Elements in Plants: A Review. Front. Plant Sci. 2020, 11, 586421. [Google Scholar] [CrossRef] [PubMed]
- Connelly-Frost, A.; Poole, C.; Satia, J.A.; Kupper, L.L.; Millikan, R.; Sandler, R.S. Selenium, folate, and colon cancer. Nutr. Cancer 2009, 61, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Alfieri, M.L.; Panzella, L.; Amorati, R.; Cariola, A.; Valgimigli, L.; Napolitano, A. Role of Sulphur and Heavier Chalcogens on the Antioxidant Power and Bioactivity of Natural Phenolic Compounds. Biomolecules 2022, 12, 90. [Google Scholar] [CrossRef]
- Mukwevho, E.; Ferreira, Z.; Ayeleso, A. Potential role of sulfur-containing antioxidant systems in highly oxidative environments. Molecules 2014, 19, 19376–19389. [Google Scholar] [CrossRef] [PubMed]
- Beydoun, M.A.; Canas, J.A.; Fanelli-Kuczmarski, M.T.; Maldonado, A.I.; Shaked, D.; Kivimaki, M.; Evans, M.K.; Zonderman, A.B. Association of Antioxidant Vitamins A, C, E and Carotenoids with Cognitive Performance over Time: A Cohort Study of Middle-Aged Adults. Nutrients 2020, 12, 3558. [Google Scholar] [CrossRef]
- Różanowska, M.B.; Czuba-Pełech, B.; Różanowski, B. Is There an Optimal Combination of AREDS2 Antioxidants Zeaxanthin, Vitamin E and Vitamin C on Light-Induced Toxicity of Vitamin A Aldehyde to the Retina? Antioxidants 2022, 11, 1132. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Reider, C.A.; Chung, R.Y.; Devarshi, P.P.; Grant, R.W.; Hazels Mitmesser, S. Inadequacy of Immune Health Nutrients: Intakes in US Adults, the 2005–2016 NHANES. Nutrients 2020, 12, 1735. [Google Scholar] [CrossRef]
- Hornung, T.C.; Biesalski, H. Glut-1 explains the evolutionary advantage of the loss of endogenous vitamin C-synthesis. The electron transfer hypothesis. Evol. Med. Public Health 2019, 1, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Cisternas, P.; Silva-Alvarez, C.; Martínez, F.; Fernández, E.; Ferrada, L.; Oyarce, K.; Salazar, K.; Bolanos, J.P.; Nualart, F. The oxidized form of vitamin C, dehydroascorbic acid, regulates neuronal energy metabolism. J. Neurochem. 2014, 129, 663–671. [Google Scholar] [CrossRef]
- Doostabadi, M.R.; Hassanzadeh-Taheri, M.; Asgharzadeh, M.; Mohammadzadeh, M. Protective effect of vitamin E on sperm parameters, chromatin quality, and DNA fragmentation in mice treated with different doses of ethanol: An experimental study. Int. J. Reprod. Biomed. 2021, 19, 525–536. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.; Appathurai, A.; Yeoh, H.-L.; Driscoll, K.; Faisal, W. Vitamin E in Cancer Treatment: A Review of Clinical Applications in Randomized Control Trials. Nutrients 2022, 14, 4329. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Im, S.; Wagner, J.G.; Hernandez, M.L.; Peden, D.B. Gamma-tocopherol, a major form of vitamin E in diets: Insights into antioxidant and anti-inflammatory effects, mechanisms, and roles in disease management. Free Radic. Biol. Med. 2022, 178, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, L.; Xue, C.; Jiang, X.; Wei, Z. Assessing the Impact of Oil Types and Grades on Tocopherol and Tocotrienol Contents in Vegetable Oils with Chemometric Methods. Molecules 2020, 25, 5076. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Bobrowska-Korczak, B. Role of Zinc in Immune System and Anti-Cancer Defense Mechanisms. Nutrients 2019, 11, 2273. [Google Scholar] [CrossRef] [PubMed]
- Tupe, R.S.; Tupe, S.G.; Tarwadi, K.V.; Agte, V.V. Effect of different dietary zinc levels on hepatic antioxidant and micronutrients indices under oxidative stress conditions. Metabolism 2010, 59, 1603–1611. [Google Scholar] [CrossRef]
- Pelton, R. Coenzyme Q10: A Miracle Nutrient Advances in Understanding. Integr. Med. 2020, 19, 16–20. [Google Scholar]
- Saini, R. Coenzyme Q10: The essential nutrient. J. Pharm. Bioallied Sci. 2011, 3, 466–467. [Google Scholar] [CrossRef]
- Sifuentes-Franco, S.; Sánchez-Macías, D.C.; Carrillo-Ibarra, S.; Rivera-Valdés, J.J.; Zuñiga, L.Y.; Sánchez-López, V.A. Antioxidant and Anti-Inflammatory Effects of Coenzyme Q10 Supplementation on Infectious Diseases. Healthcare 2022, 10, 487. [Google Scholar] [CrossRef]
- Ayunin, Q.; Miatmoko, A.; Soeratri, W.; Erawati, T.; Susanto, J.; Legowo, D. Improving the anti-ageing activity of coenzyme Q10 through protransfersome-loaded emulgel. Sci. Rep. 2022, 12, 906. [Google Scholar] [CrossRef] [PubMed]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Mariscal, F.M.; de la Cruz-Ares, S.; Torres-Peña, J.D.; Alcalá-Diaz, J.F.; Yubero-Serrano, E.M.; López-Miranda, J. Coenzyme Q10 and Cardiovascular Diseases. Antioxidants 2021, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, S.; Cheng, T.; Li, Y.; Mai, X.; Jiang, G.; Yang, Y.; Zhang, Q.; Li, J.; Zheng, L.; et al. Decylubiquinone Inhibits Colorectal Cancer Growth Through Upregulating Sirtuin2. Front. Pharmacol. 2022, 12, 804265. [Google Scholar] [CrossRef]
- Thapa, M.; Dallmann, G. Role of coenzymes in cancer metabolism. Semin. Cell Dev. Biol. 2020, 98, 44–53. [Google Scholar] [CrossRef]
- Shidal, C.; Yoon, H.S.; Zheng, W.; Wu, J.; Franke, A.A.; Blot, W.J.; Shu, X.; Cai, Q. Prospective study of plasma levels of coenzyme Q10 and lung cancer risk in a low-income population in the Southeastern United States. Cancer Med. 2021, 10, 1439–1447. [Google Scholar] [CrossRef]
- Kiremitli, T.; Kiremitli, S.; Akselim, B.; Yilmaz, B.; Mammadov, R.; Tor, I.H.; Yazici, G.N.; Gulaboglu, M. Protective effect of Coenzyme Q10 on oxidative ovarian and uterine damage induced by methotrexate in rats. Hum. Exp. Toxicol. 2021, 40, 1537–1544. [Google Scholar] [CrossRef]
- Alimohammadi, M.; Rahimi, A.; Faramarzi, F.; Golpour, M.; Jafari-Shakib, R.; Alizadeh-Navaei, R.; Rafiei, A. Effects of coenzyme Q10 supplementation on inflammation, angiogenesis, and oxidative stress in breast cancer patients: A systematic review and meta-analysis of randomized controlled-trials. Inflammopharmacology 2021, 29, 579–593. [Google Scholar] [CrossRef]
- Alcázar-Fabra, M.; Navas, P.; Brea-Calvo, G. Coenzyme Q biosynthesis and its role in the respiratory chain structure. Biochim. Biophys. Acta 2016, 1857, 1073–1078. [Google Scholar] [CrossRef]
- Fehér, A.; Gazdecki, M.; Véha, M.; Szakály, M.; Szakály, Z. A Comprehensive Review of the Benefits of and the Barriers to the Switch to a Plant-Based Diet. Sustainability 2020, 12, 4136. [Google Scholar] [CrossRef]
- Dressler, J.; Storz, M.A.; Müller, C.; Kandil, F.I.; Kessler, C.S.; Michalsen, A.; Jeitler, M. Does a Plant-Based Diet Stand Out for Its Favorable Composition for Heart Health? Dietary Intake Data from a Randomized Controlled Trial. Nutrients 2022, 14, 4597. [Google Scholar] [CrossRef] [PubMed]
- Giromini, C.; Givens, D.I. Benefits and Risks Associated with Meat Consumption during Key Life Processes and in Relation to the Risk of Chronic Diseases. Foods 2022, 11, 2063. [Google Scholar] [CrossRef]
- Barthelmie, R.J. Impact of Dietary Meat and Animal Products on GHG Footprints: The UK and the US. Climate 2022, 10, 43. [Google Scholar] [CrossRef]
- Mathur, M.B.; Peacock, J.; Reichling, D.B.; Nadler, J.; Bain, P.A.; Gardner, C.D.; Robinson, T.N. Interventions to reduce meat consumption by appealing to animal welfare: Meta-analysis and evidence-based recommendations. Appetite 2021, 164, 105277. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.J. Plant-based animal product alternatives are healthier and more environmentally sustainable than animal products. Future Foods 2022, 6, 100174. [Google Scholar] [CrossRef]
- Sansone, C.; Brunet, C. Marine Algal Antioxidants. Antioxidants 2020, 2, 206. [Google Scholar] [CrossRef]
- Šimat, V.; Elabed, N.; Kulawik, P.; Ceylan, Z.; Jamroz, E.; Yazgan, H.; Čagalj, M.; Regenstein, J.M.; Özogul, F. Recent Advances in Marine-Based Nutraceuticals and Their Health Benefits. Mar. Drugs 2020, 18, 627. [Google Scholar] [CrossRef]
- Vladkova, T.; Georgieva, N.; Staneva, A.; Gospodinova, D. Recent Progress in Antioxidant Active Substances from Marine Biota. Antioxidants 2022, 11, 439. [Google Scholar] [CrossRef]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Oxidative Damage and Antioxidative System in Algae. Toxicol. Rep. 2019, 6, 1309–1313. [Google Scholar] [CrossRef]
- Nedumaran, T.; Arulbalachandran, D. Seaweeds: A promising source for sustainable development. In Environmental Sustainability: Role of Green Technologies; Springer: New Delhi, India, 2015; pp. 65–88. [Google Scholar]
- Tziveleka, L.A.; Tammam, M.A.; Tzakou, O.; Roussis, V.; Ioannou, E. Metabolites with Antioxidant Activity from Marine Macroalgae. Antioxidants 2021, 10, 1431. [Google Scholar] [CrossRef]
- Harb, T.B.; Pereira, M.S.; Cavalcanti, M.I.L.G.; Fujii, M.T.; Chow, F. Antioxidant Activity and Related Chemical Composition of Extracts from Brazilian Beach-Cast Marine Algae: Opportunities of Turning a Waste into a Resource. J. Appl. Phycol. 2021, 33, 3383–3395. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxidative Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef] [PubMed]
- Kosanić, M.; Ranković, B.; Stanojković, T. Biological Activities of Two Macroalgae from Adriatic Coast of Montenegro. Saudi J. Biol. Sci. 2015, 22, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, O.; Karim, A.-; Farouk Gheda, S.; Ismail, G.A.; Abo-Shady, A.M.; Assistant Of Phycology, T. Phytochemical Screening and Antioxidant Activity of Chlorella Vulgaris. Delta J. Sci. 2020, 41, 81–91. [Google Scholar]
- Haq, S.H.; Al-Ruwaished, G.; Al-Mutlaq, M.A.; Naji, S.A.; Al-Mogren, M.; Al-Rashed, S.; Ain, Q.T.; Al-Amro, A.A.; Al-Mussallam, A. Antioxidant, Anticancer Activity and Phytochemical Analysis of Green Algae, Chaetomorpha Collected from the Arabian Gulf. Sci. Rep. 2019, 9, 18906. [Google Scholar] [CrossRef]
- Sonani, R.R.; Rastogi, R.P.; Madamwar, D. Chapter 5—Natural Antioxidants From Algae: A Therapeutic Perspective. In Algal Green Chemistry; Rastogi, R.P., Madamwar, D., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 91–120. [Google Scholar]
- Galasso, C.; Corinaldesi, C.; Sansone, C. Carotenoids from Marine Organisms: Biological Functions and Industrial Applications. Antioxidants 2017, 6, 96. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Ferdous, U.T.; Yusof, Z.N.B. Medicinal Prospects of Antioxidants From Algal Sources in Cancer Therapy. Front. Pharmacol. 2021, 12, 593116. [Google Scholar] [CrossRef]
- Wang, Q.; Decker, E.A.; Rao, J.; Chen, B. A combination of monoacylglycerol crystalline network and hydrophilic antioxidants synergistically enhances the oxidative stability of gelled algae oil. Food Funct. 2019, 1, 315–324. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Pulz, O.; Gross, W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004, 65, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of Carotenoids. Mar. Drugs 2011, 9, 625. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, B.; Nayak, R.; Patra, S.; Jit, B.P.; Ragusa, A.; Jena, M. Bioactive Metabolites from Marine Algae as Potent Pharmacophores against Oxidative Stress-Associated Human Diseases: A Comprehensive Review. Molecules 2020, 26, 37. [Google Scholar] [CrossRef] [PubMed]
- Sangeetha, R.K.; Bhaskar, N.; Baskaran, V. Comparative effects of beta carotene and fucoxanthin on retinol deficiency induced oxidative stress in rats. Mol. Cell. Biochem. 2009, 331, 59–67. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, S.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef]
- Getachew, A.T.; Saravana, P.S.; Cho, Y.J.; Woo, H.C.; Chun, B.S. Concurrent extraction of oil from roasted coffee (Coffea arabica) and fucoxanthin from brown seaweed (Saccharina japonica) using supercritical carbon dioxide. J. CO2 Util. 2018, 25, 137–146. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, Extraction, Stability, Biological Activities and Its Commercial Applications—A Review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Pashkow, F.J.; Watumull, D.G.; Campbell, C.L. Astaxanthin: A novel potential treatment for oxidative stress and inflammation in cardiovascular disease. Am. J. Cardiol. 2008, 101, 58D–68D. [Google Scholar] [CrossRef]
- Sarada, R.; Tripathi, U.; Ravishankar, G.A. Influence of stress on astaxanthin production in Haematococcus pluvialis grown under different culture conditions. Process Biochem. 2002, 37, 623–627. [Google Scholar] [CrossRef]
- Ranga Rao, A. Production of Astaxanthin from Cultured Green Alga Haematococcus pluvialis and Its Biological Activities. Ph.D. Thesis, University of Mysore, Mysore, India, 15 May 2011. [Google Scholar]
- Sarada, R.; Ranga Rao, A.; Sandesh, B.K.; Dayananda, C.; Anila, N.; Chauhan, V.S.; Ravishankar, G.A. Influence of different culture conditions on yield of biomass and value added products in microalgae. Dyn. Biochem. Proc. Biotechnol. Mol. Biol. 2012, 6, 77–85. [Google Scholar]
- Stachowiak, B.; Szulc, P. Astaxanthin for the Food Industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Gasmi, A.; Lenchyk, L.; Shanaida, M.; Zafar, S.; Mujawdiya, P.K.; Lysiuk, R.; Antonyak, H.; Noor, S.; Akram, M.; et al. The Role of Astaxanthin as a Nutraceutical in Health and Age-Related Conditions. Molecules 2022, 27, 7167. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.E.; Lin, X. Bromophenols in marine algae and their bioactivities. Mar. Drugs 2011, 9, 1273–1292. [Google Scholar] [CrossRef] [PubMed]
- Gross, H.; König, G.M. Terpenoids from marine organisms: Unique structures and their pharmacological potential. Phytochem. Rev. 2006, 5, 115–141. [Google Scholar] [CrossRef]

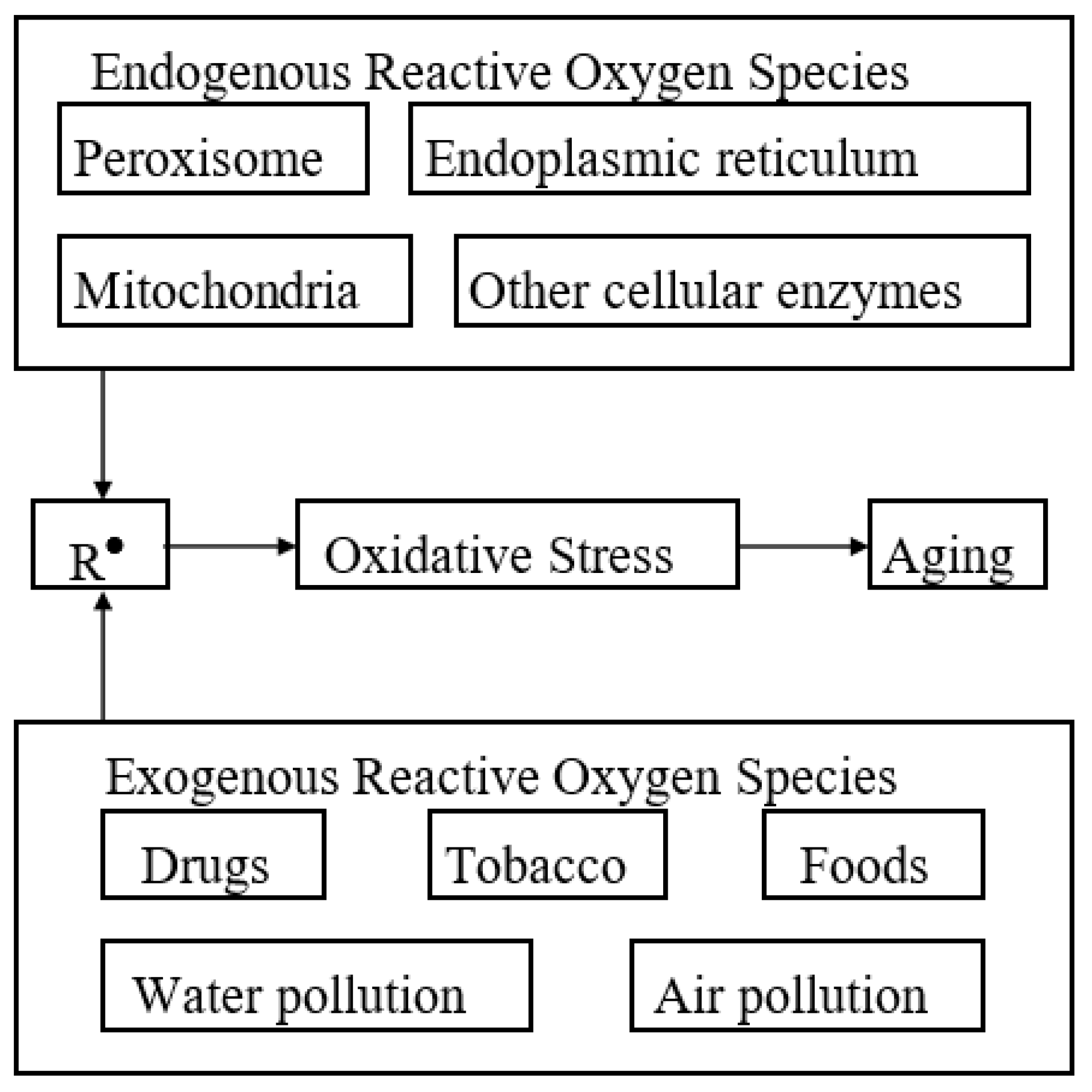
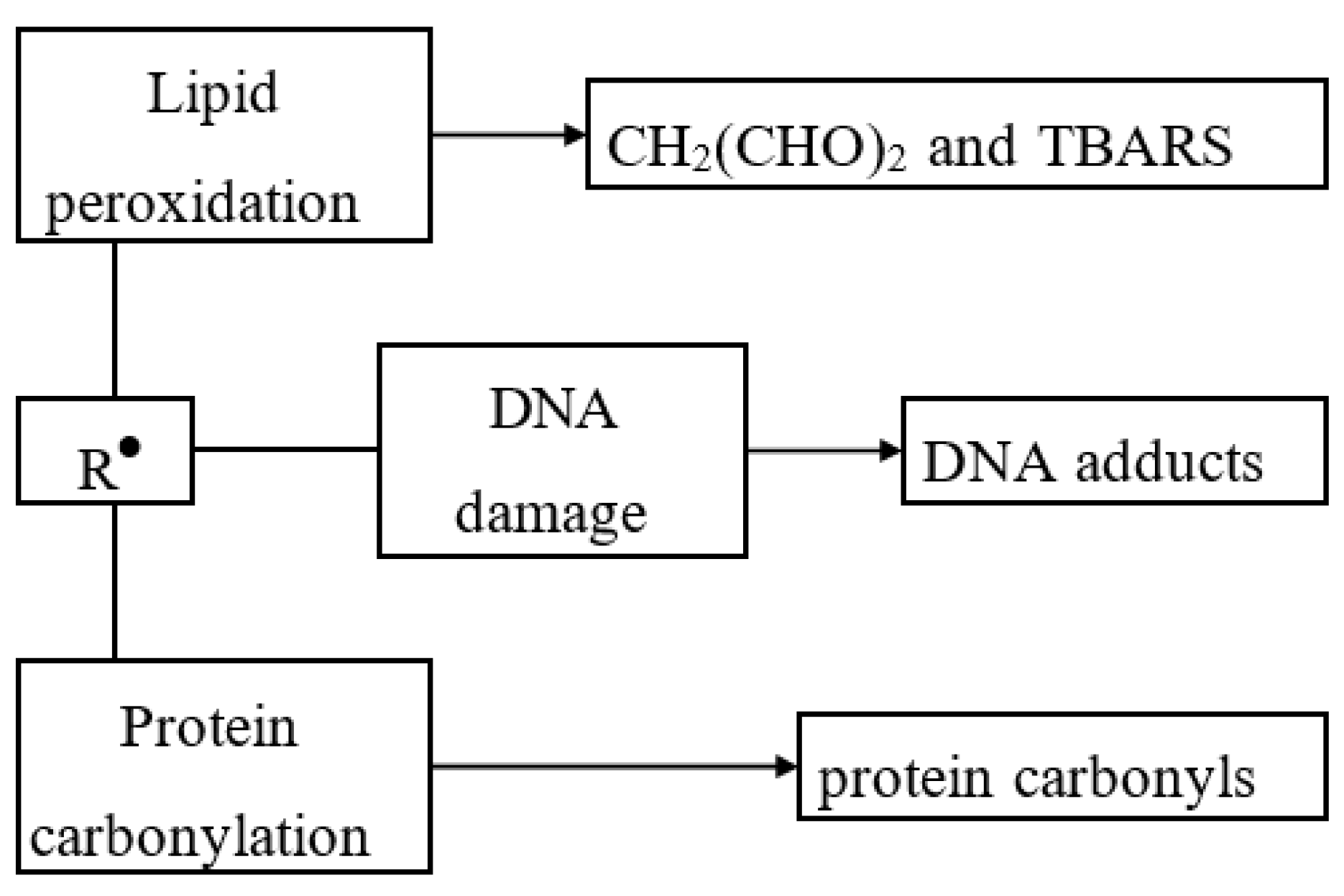
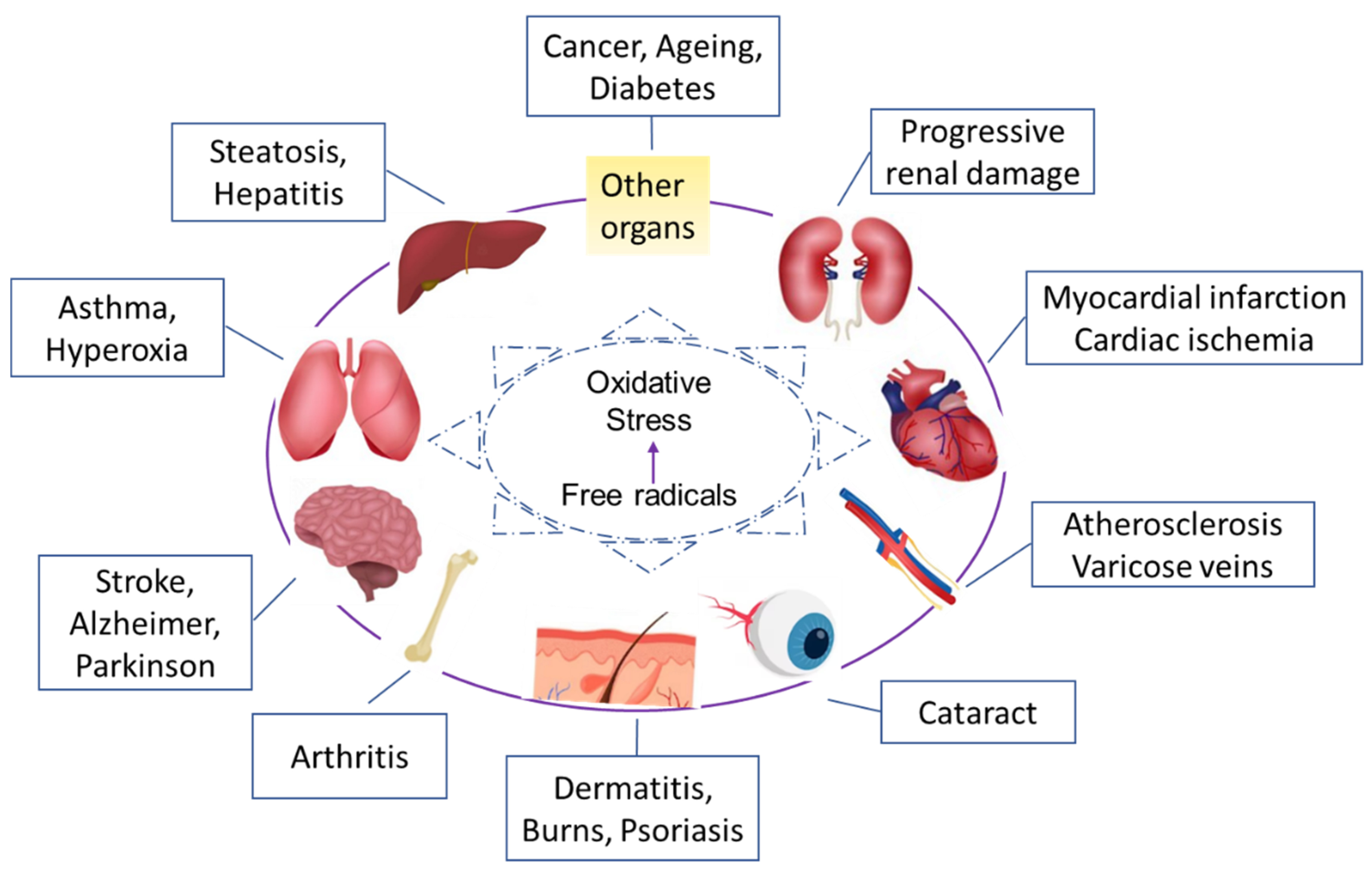


| Type | Mode of Action | Examples |
|---|---|---|
| “Metal scavenger” | Chelates metal ions such as copper and iron, forming inactive complexes | Chelating agents such as EDTA, citric acid, phospholipids, polyphosphates |
| “Oxygen scavenger” | Reacts with oxygen; reduces oxygen | Ascorbic acid, ascorbyl-palmitate |
| Antioxidant (AH) | Interrupts propagation stages in the case of oxidation reactions; donates a hydrogen atom | Phenolic compounds such as BHA, BHT, TBHQ, PG, tocopherols, hydroxytyrosol, caffeic acid, carnosol, etc. |
| Reducing agents (RSH) | Regenerates phenols (synergism) | Ascorbic acid |
| Enzymatic antioxidant | Removes dissolved oxygen or oxidative species | Superoxide dismutase, glutathione peroxidase, glucose-oxidase-catalase |
| Antioxidants with multiple functions | Regenerates primary antioxidants chelated with metals; reduces hydroperoxides | Phospholipids (phosphatidyl-ethanol amine- fish oil), products of the Maillard reaction |
| Methyl-silicone and ethylidene phytosterols | They prevent oxidative polymerization in heated oils | Polydimethylsiloxane, citrostadienol |
| Annotation | Antioxidant | Annotation | Antioxidant |
|---|---|---|---|
| E300 | Ascorbic acid | E310 | Propyl gallate |
| E301 | Sodium ascorbate | E315 | Erythorbic acid |
| E302 | Calcium ascorbate | E316 | Sodium erythorbate |
| E304 | Fatty acid esters of ascorbic acid | E319 | Tertiary-butyl hydroquinone |
| (TBHQ) | |||
| E306 | Tocopherols | E320 | Butylated hydroxyanisole |
| (BHA) | |||
| E307 | α-tocopherol | E321 | Butylated hydroxytoluene |
| (BHT) | |||
| E308 | γ-tocopherol | E392 | Extracts of rosemary |
| E309 | δ-tocopherol | E586 | 4-Hexylresorcinol |
| Anthocyanins | β-Carotene | Catechins | Cryptoxanthines | Copper | Flavonoids | Indoles | Isoflavonoides | Lignans | Lutein | Lycopene | Manganese | Polyphenols | Selenium | Sulphur | Vit. A | Vit. C | Vit. E | Zinc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acai berries | ✔ | ||||||||||||||||||
| Almonds | ✔ | ||||||||||||||||||
| Apricots | ✔ | ||||||||||||||||||
| Artichokes | ✔ | ||||||||||||||||||
| Avocados | ✔ | ✔ | |||||||||||||||||
| Barley | ✔ | ||||||||||||||||||
| Black currants | ✔ | ||||||||||||||||||
| Black rice | ✔ | ||||||||||||||||||
| Blackberries | ✔ | ||||||||||||||||||
| Blueberries | ✔ | ||||||||||||||||||
| Brazil nuts | ✔ | ||||||||||||||||||
| Broccoli | ✔ | ✔ | |||||||||||||||||
| Brown rice | ✔ | ||||||||||||||||||
| Brussels sprouts | ✔ | ||||||||||||||||||
| Butter | ✔ | ||||||||||||||||||
| Cabbage | ✔ | ||||||||||||||||||
| Carrots | ✔ | ✔ | |||||||||||||||||
| Cauliflower | ✔ | ✔ | |||||||||||||||||
| Cherries | ✔ | ||||||||||||||||||
| Chicken | ✔ | ||||||||||||||||||
| Chickpeas | ✔ | ||||||||||||||||||
| Chives | ✔ | ||||||||||||||||||
| Citrus | ✔ | ✔ | ✔ | ||||||||||||||||
| Cocoa | ✔ | ||||||||||||||||||
| Coffee | ✔ | ||||||||||||||||||
| Cottage cheese | ✔ | ||||||||||||||||||
| Dark chocolate | ✔ | ||||||||||||||||||
| Egg yolk | ✔ | ✔ | |||||||||||||||||
| Eggplant | ✔ | ||||||||||||||||||
| Eggs | ✔ | ||||||||||||||||||
| Extra virgin olive oil | ✔ | ||||||||||||||||||
| Flaxseed | ✔ | ||||||||||||||||||
| Garlic | ✔ | ||||||||||||||||||
| Grapefruit | ✔ | ||||||||||||||||||
| Grapes | ✔ | ||||||||||||||||||
| Green tea | ✔ | ||||||||||||||||||
| Kale | ✔ | ✔ | ✔ | ||||||||||||||||
| Kiwi | ✔ | ||||||||||||||||||
| Leeks | ✔ | ||||||||||||||||||
| Lentils | ✔ | ||||||||||||||||||
| Liver | ✔ | ✔ | |||||||||||||||||
| Lobster | ✔ | ||||||||||||||||||
| Mangoes | ✔ | ✔ | |||||||||||||||||
| Mushrooms | ✔ | ||||||||||||||||||
| Mustard seed | ✔ | ||||||||||||||||||
| Oatmeal | ✔ | ||||||||||||||||||
| Onions | ✔ | ✔ | |||||||||||||||||
| Oregano | ✔ | ||||||||||||||||||
| Oysters | ✔ | ||||||||||||||||||
| Papaya | ✔ | ✔ | |||||||||||||||||
| Parsley | ✔ | ✔ | ✔ | ||||||||||||||||
| Peanuts | ✔ | ||||||||||||||||||
| Peas | ✔ | ||||||||||||||||||
| Pecans | ✔ | ||||||||||||||||||
| Pineapples | ✔ | ||||||||||||||||||
| Pinto beans | ✔ | ||||||||||||||||||
| Pistachios | ✔ | ||||||||||||||||||
| Pork | ✔ | ✔ | |||||||||||||||||
| Pumpkins | ✔ | ||||||||||||||||||
| Raspberries | ✔ | ||||||||||||||||||
| Red bell peppers | ✔ | ||||||||||||||||||
| Red grapes | ✔ | ||||||||||||||||||
| Rye | ✔ | ||||||||||||||||||
| Sardines | ✔ | ||||||||||||||||||
| Sesame seeds | ✔ | ||||||||||||||||||
| Shiitake mushrooms | ✔ | ✔ | |||||||||||||||||
| Shrimp | ✔ | ||||||||||||||||||
| Soybeans * | ✔ | ||||||||||||||||||
| Spinach | ✔ | ✔ | |||||||||||||||||
| Spirulina | ✔ | ||||||||||||||||||
| Squash | ✔ | ||||||||||||||||||
| Strawberries | ✔ | ||||||||||||||||||
| Sweet potatoes | ✔ | ✔ | |||||||||||||||||
| Tea | ✔ | ||||||||||||||||||
| Thyme | ✔ | ||||||||||||||||||
| Tomatoes | ✔ | ||||||||||||||||||
| Turnips | ✔ | ||||||||||||||||||
| Watermelon | ✔ | ||||||||||||||||||
| Whole wheat bread | ✔ | ||||||||||||||||||
| Wine | ✔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pruteanu, L.L.; Bailey, D.S.; Grădinaru, A.C.; Jäntschi, L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants 2023, 12, 860. https://doi.org/10.3390/antiox12040860
Pruteanu LL, Bailey DS, Grădinaru AC, Jäntschi L. The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants. 2023; 12(4):860. https://doi.org/10.3390/antiox12040860
Chicago/Turabian StylePruteanu, Lavinia Lorena, David Stanley Bailey, Andrei Cristian Grădinaru, and Lorentz Jäntschi. 2023. "The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae" Antioxidants 12, no. 4: 860. https://doi.org/10.3390/antiox12040860
APA StylePruteanu, L. L., Bailey, D. S., Grădinaru, A. C., & Jäntschi, L. (2023). The Biochemistry and Effectiveness of Antioxidants in Food, Fruits, and Marine Algae. Antioxidants, 12(4), 860. https://doi.org/10.3390/antiox12040860









