Molecular Hydrogen: From Molecular Effects to Stem Cells Management and Tissue Regeneration
Abstract
1. Introduction
2. A Brief Outline of the History of the Discovery of Molecular Hydrogen as a Biological Agent and the Formation of Hydrogen Biomedicine
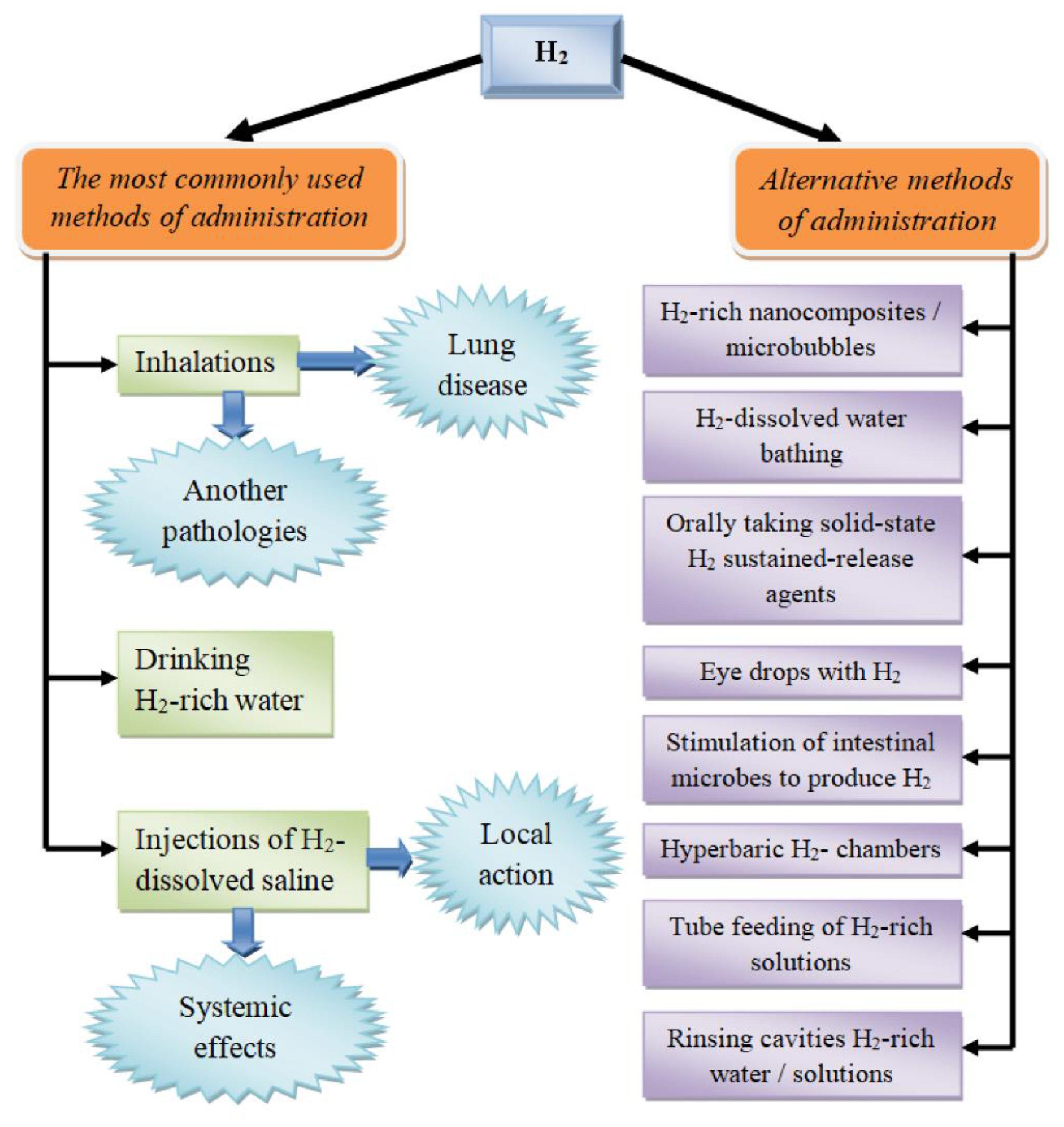
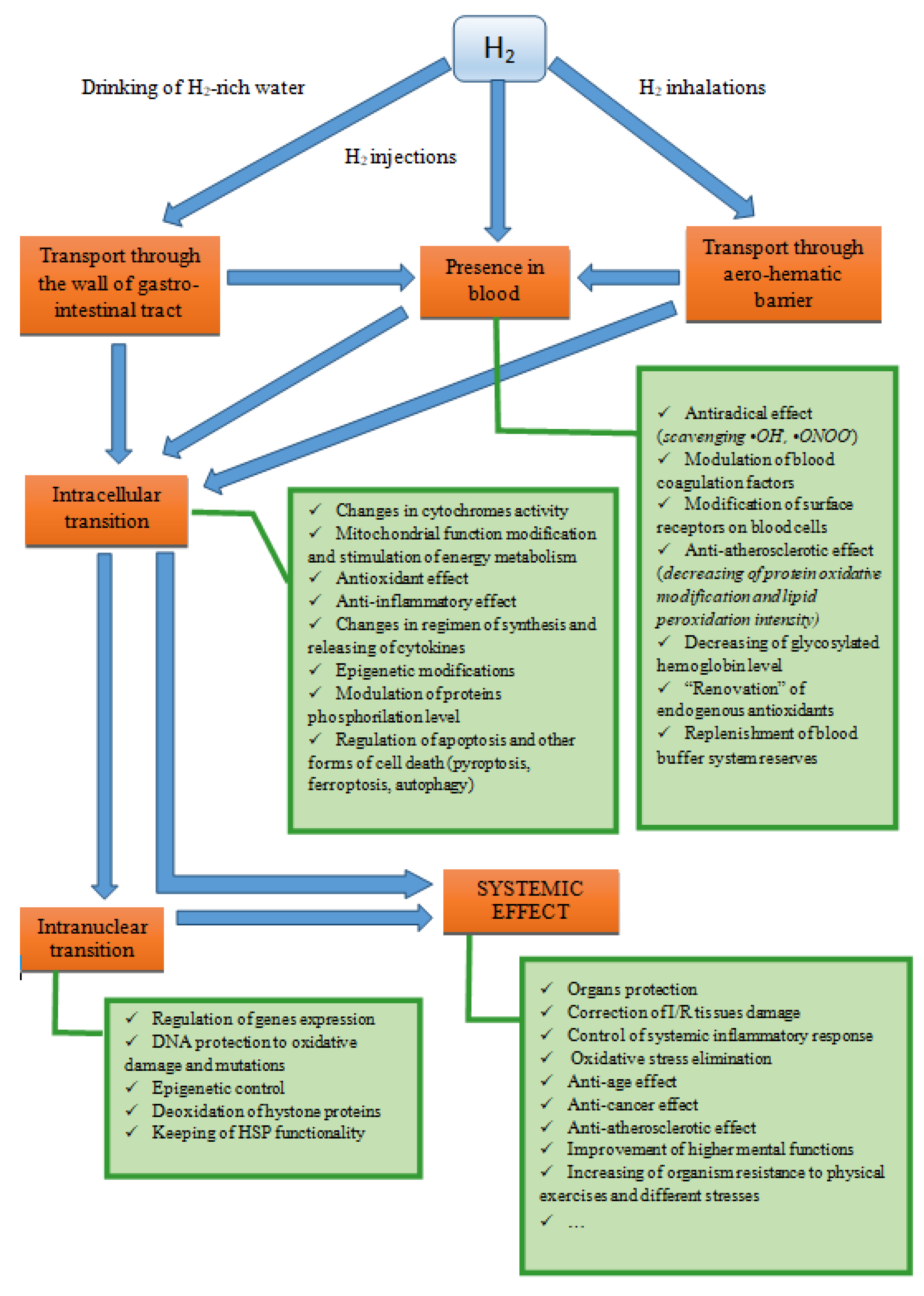
3. Routes of Introducing Molecular Hydrogen into the Body
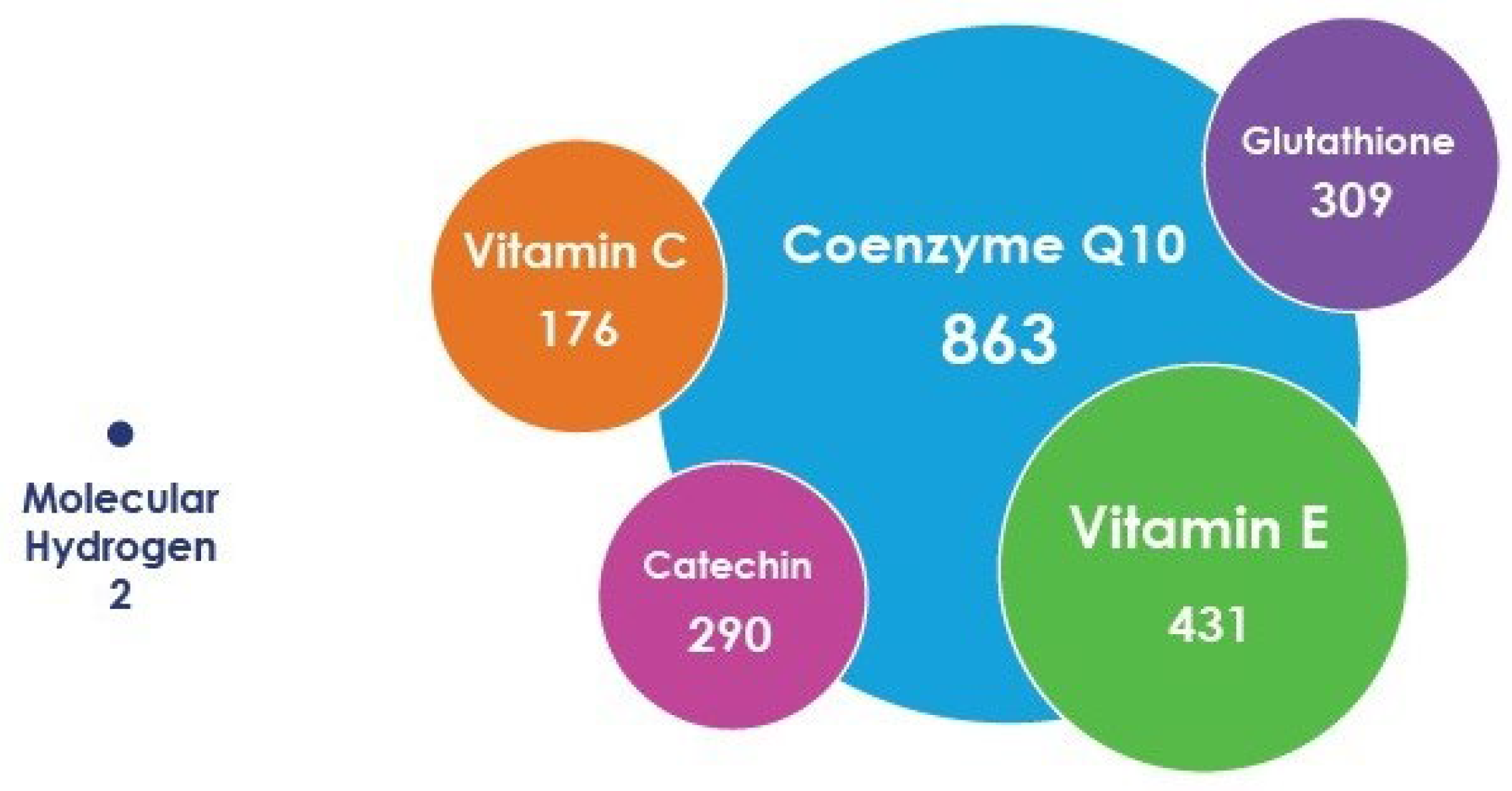
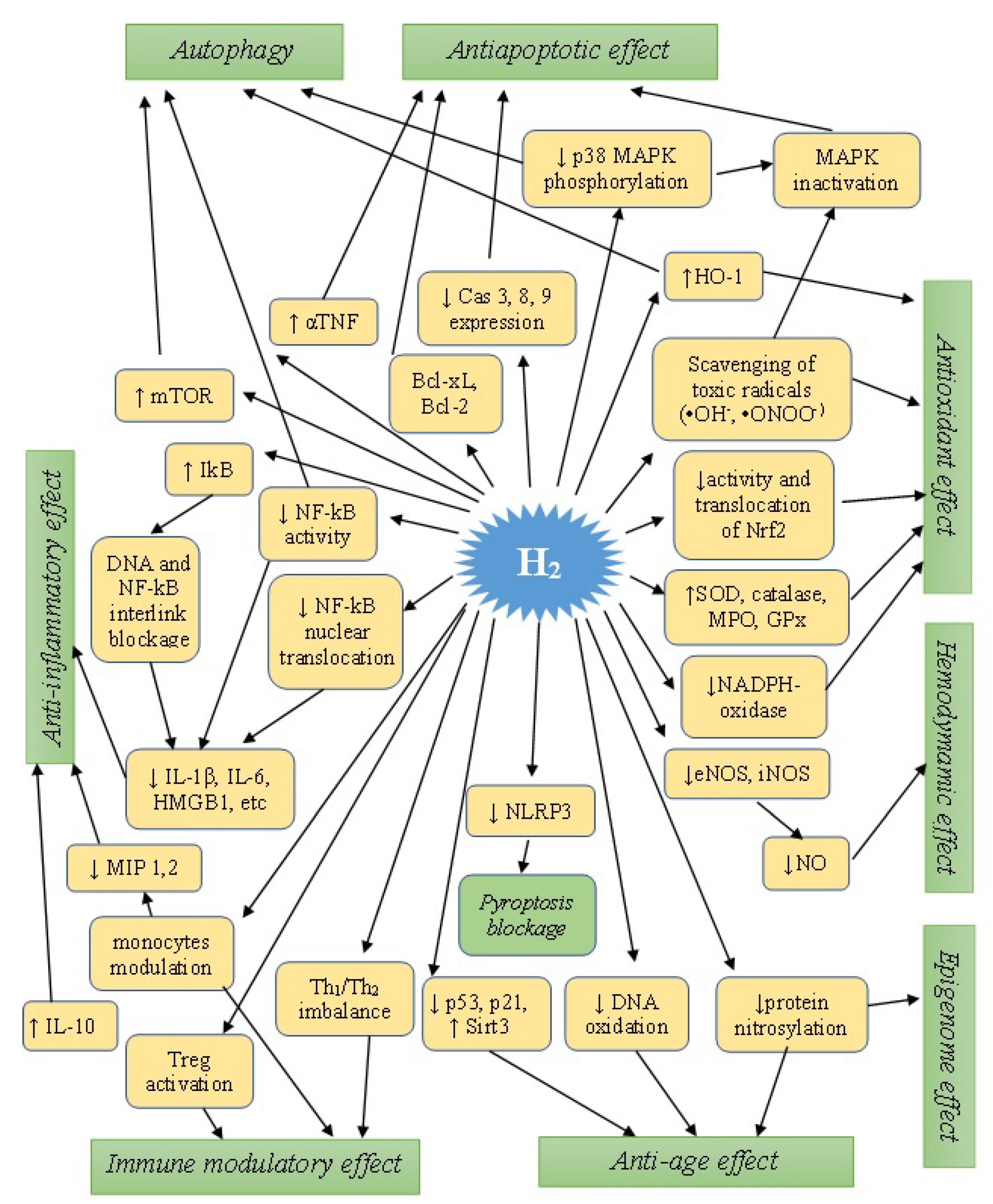
4. Characteristics of Molecular and Cellular Aspects of the Biological Action of H2
4.1. Antioxidant Effects
4.2. Anti-Inflammatory Effects
- -
- inhibition of the activity of NF-kB itself and its associated cytokines [87];
- -
- -
4.3. Anti-Apoptotic Effects
4.4. Regulation of Pyroptosis
4.5. Modulation of Autophagy
4.6. Cellular and Tissue Effects of Molecular Hydrogen
- -
- organoprotective effects;
- -
- minimization of the consequences of ischemic–reperfusion lesions;
- -
- limitation of systemic inflammatory responses;
- -
- antitumor effects;
- -
- anti-aging effects;
- -
- increasing the body’s resistance to stressors of various nature;
- -
- improved exercise tolerance.
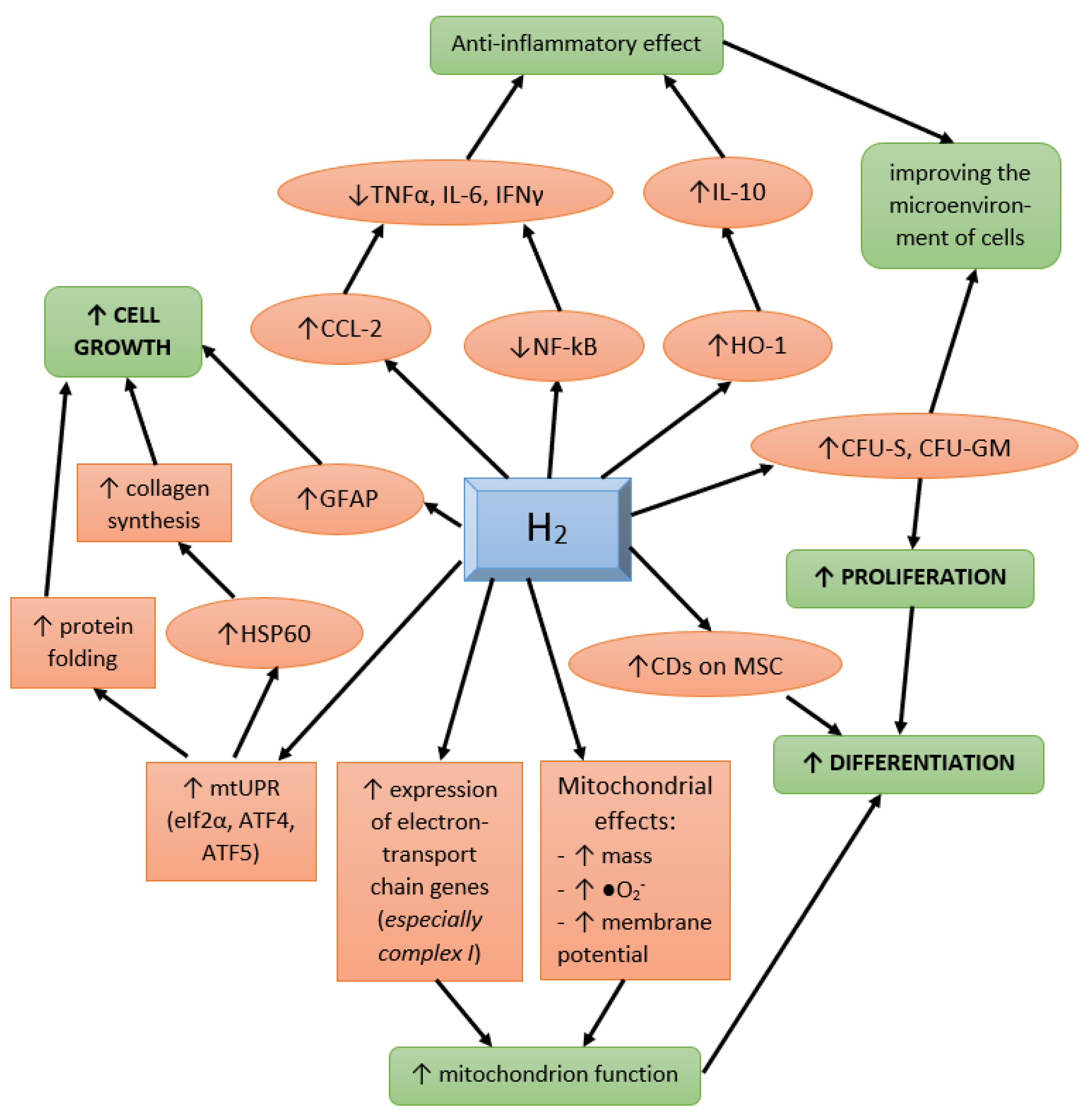
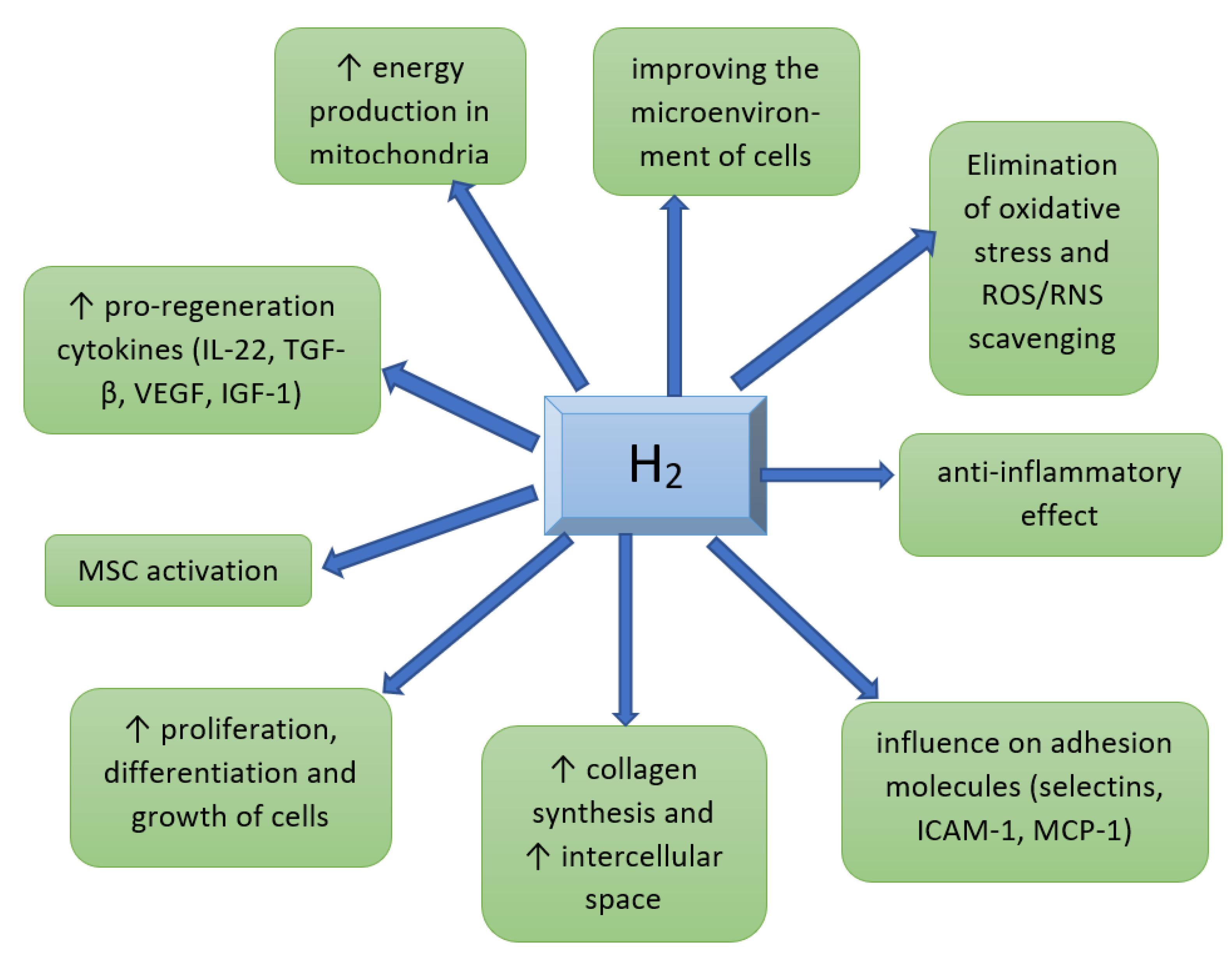
5. The Effect of Molecular Hydrogen on Various Cell Pools and Regeneration Processes
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zafonte, R.S.; Wang, L.; Arbelaez, C.A.; Dennison, R.; Teng, Y.D. Medical Gas Therapy for Tissue, Organ, and CNS Protection: A Systematic Review of Effects, Mechanisms, and Challenges. Adv. Sci. 2022, 9, 2104136. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.D.; Wang, L.; Kabatas, S.; Ulrich, H.; Zafonte, R.D. Cancer Stem Cells or Tumor Survival Cells? Stem Cells Dev. 2018, 27, 1466–1478. [Google Scholar] [CrossRef] [PubMed]
- Nazarov, E.I.; Khlusov, I.A.; Noda, M. Homeostatic and endocrine responses as the basis for systemic therapy with medical gases: Ozone, xenon and molecular hydrogen. Med. Gas Res. 2021, 11, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Vanin, A.F. Physico-Chemistry of Dinitrosyl Iron Complexes as a Determinant of Their Biological Activity. Int. J. Mol. Sci. 2021, 22, 10356. [Google Scholar] [CrossRef]
- Olas, B. Gasomediators (·NO, CO, and H₂S) and their role in hemostasis and thrombosis. Clin. Chim. Acta 2015, 445, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.; Franks, N.P. Bench-to-bedside review: Molecular pharmacology and clinical use of inert gases in anesthesia and neuroprotection. Crit. Care 2010, 14, 229. [Google Scholar] [CrossRef]
- Deng, R.M.; Li, H.Y.; Li, X.; Shen, H.T.; Wu, D.G.; Wang, Z.; Chen, G. Neuroprotective effect of helium after neonatal hypoxic ischemia: A narrative review. Med. Gas Res. 2021, 11, 121–123. [Google Scholar] [CrossRef]
- Winkler, D.A.; Thornton, A.; Farjot, G.; Katz, I. The diverse biological properties of the chemically inert noble gases. Pharmacol. Ther. 2016, 160, 44–64. [Google Scholar] [CrossRef]
- Martusevich, A.; Surovegina, A.; Popovicheva, A.; Didenko, N.; Artamonov, M.; Nazarov, V. Some Beneficial Effects of Inert Gases on Blood Oxidative Metabolism: In Vivo Study. BioMed Res. Int. 2022, 2022, 5857979. [Google Scholar] [CrossRef]
- Ichihara, M.; Sobue, S.; Ito, M.; Ito, M.; Hirayama, M.; Ohno, K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen–comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 1–21. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, H.; Yu, C.; Wang, F.; Sun, X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med. Gas Res. 2014, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.I.; Ichikawa, Y.; Kurokawa, R.; Takefuji, Y.; Satoh, F. A “philosophical molecule,” hydrogen may overcome senescence and intractable diseases. Med. Gas Res. 2020, 10, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Tao, G.; Song, G.; Qin, S. Molecular hydrogen: Current knowledge on mechanism in alleviating free radical damage and diseases. Acta Biochim. Biophys. Sin. 2019, 51, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, C.; Wu, S.; Xiao, G.; Zhuge, X.; Lei, P.; Xie, K. Hydrogen gas treatment improves the neurological outcome after traumatic brain injury via increasing miR-21 expression. Shock 2018, 50, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, R.; Yang, D.; Tang, W.; Chen, Z.; Sun, Q.; Liu, L.; Zang, R. Hydrogen postconditioning promotes survival of rat retinal ganglion cells against ischemia/reperfusion injury through the PI3K. Akt pathway. Biochem. Biophys. Res. Commun. 2018, 495, 2462–2468. [Google Scholar] [CrossRef]
- Hirano, S.-I.; Ichikawa, Y.; Sato, B.; Yamamoto, H.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Potential Clinically Applicable Radioprotective Agent. Int. J. Mol. Sci. 2021, 22, 4566. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, Y.; Wu, S.; Wu, W.; Deng, Y.; Shao, A. Molecular hydrogen: A potential radioprotective agent. Biomed. Pharmacother. 2020, 130, 110589. [Google Scholar] [CrossRef]
- Runtuwene, J.; Amitani, H.; Amitani, M.; Asakawa, A.; Cheng, K.C.; Inui, K. Hydrogen-water enhances 5-fluorouracil-induced inhibition of colon cancer. PeerJ 2015, 3, e859. [Google Scholar] [CrossRef]
- Hirano, S.-I.; Yamamoto, H.; Ichikawa, Y.; Sato, B.; Takefuji, Y.; Satoh, F. Molecular Hydrogen as a Novel Antitumor Agent: Possible Mechanisms Underlying Gene Expression. Int. J. Mol. Sci. 2021, 22, 8724. [Google Scholar] [CrossRef]
- Wang, D.; Wang, L.; Zhang, Y.; Zhao, Y.; Chen, G. Hydrogen gas inhibits lung cancer progression through targeting SMC. Biomed. Pharmacol. 2018, 104, 788–797. [Google Scholar] [CrossRef]
- Boyle, R. Tracts written by the honourable Robert Boyle Containing New Experiments Touching the Relation Betwixt Flame And Air: And About Explosions: An Hydrostatical Discourse Occasion’d by Some Objections of Dr. Henry More Against Some Explications of New Experiments Made by the Author of these Tracts: To Which is Annex’t, an Hydrostatical Letter, Dilucidating an Experiment about a Way of Weighing Water in Water; Printed for Richard Davis, Book-Seller in Oxon. 1672. Available online: https://quod.lib.umich.edu/e/eebo2/A29057.0001.001/1:21.1?rgn=div2;view=fulltext (accessed on 22 January 2023).
- Cavendish, H. XIX. Three papers, containing experiments on factitious air. Phil. Trans. R. Soc. 1766, 56, 141–184. [Google Scholar] [CrossRef]
- Beddoes, T. A Letter to Erasmus Darwin, M.D. On A New Method of Treating Pulmonary Consumption, and Some Other Diseases Hitherto Found Incurable; Bulgin & Rosser: Bristol, UK, 1793. [Google Scholar]
- Beddoes, T. Considerations on the Medicinal Use, and on the Production of Factitious Airs. Ann. Med. 1796, 1, 245–265. [Google Scholar]
- Levitt, M.D. Production and excretion of hydrogen gas in man. N. Engl. J. Med. 1969, 281, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Dole, M.; Wilson, F.R.; Fife, W.P. Hyperbaric hydrogen therapy: A possible treatment for cancer. Science 1975, 190, 152–154. [Google Scholar] [CrossRef]
- Lanphier, E.H. Human respiration under increased pressures. Symp. Soc. Exp. Biol. 1972, 26, 379–394. [Google Scholar] [PubMed]
- van Haaster, D.J.; Hagedoorn, P.L.; Jongejan, J.A.; Hagen, W.R. On the relationship between affinity for molecular hydrogen and the physiological directionality of hydrogenases. Biochem. Soc. Trans. 2005, 33 Pt 1, 12–14. [Google Scholar] [CrossRef]
- Yanagihara, T.; Arai, K.; Miyamae, K.; Sato, B.; Shudo, T.; Yamada, M.; Aoyama, M. Electrolyzed Hydrogen-Saturated Water for Drinking Use Elicits an Antioxidative Effect: A Feeding Test with Rats. Biosci. Biotechnol. Biochem. 2005, 69, 1985–1987. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef]
- Guan, W.J.; Chen, R.C.; Zhong, N.S. Strategies for the prevention and management of coronavirus disease 2019. Eur. Respir. J. 2020, 55, 2000597. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, Y.; Wang, Y.; Chen, Y.; Fan, W.; Zhou, J.; Qiao, J. Hydrogen, a Novel Therapeutic Molecule, Regulates Oxidative Stress, Inflammation, and Apoptosis. Front. Physiol. 2021, 12, 789507. [Google Scholar] [CrossRef]
- Fu, Z.; Zhang, J. Molecular hydrogen is a promising therapeutic agent for pulmonary disease. J. Zhejiang Univ. Sci. B 2022, 23, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Wei, C.H.; Chen, A.L.; Sun, X.C.; Guo, G.Y.; Zou, X.; Shi, J.D.; Lai, P.Z.; Zheng, Z.G.; Zhong, N.S. Hydrogen/oxygen mixed gas inhalation improves disease severity and dyspnea in patients with Coronavirus disease 2019 in a recent multicenter, open-label clinical trial. J. Thorac. Dis. 2020, 12, 3448–3452. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, C.; Wang, X.; Wang, W.; Li, Z.; Wang, X.; Wang, P.; Sun, W.; Xue, B. Hydrogen coadministration slows the development of COPD-like lung disease in a cigarette smoke-induced rat model. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1309–1324. [Google Scholar] [CrossRef]
- Zheng, Z.G.; Sun, W.Z.; Hu, J.Y.; Jie, Z.J.; Xu, J.F.; Cao, J.; Song, Y.L.; Wang, C.H.; Wang, J.; Zhao, H.; et al. Hydrogen/oxygen therapy for the treatment of an acute exacerbation of chronic obstructive pulmonary disease: Results of a multicenter, randomized, double-blind, parallel-group controlled trial. Respir. Res. 2021, 22, 149. [Google Scholar] [CrossRef]
- Huang, P.; Wei, S.; Huang, W.; Wu, P.; Chen, S.; Tao, A.; Wang, H.; Liang, Z.; Chen, R.; Yan, J.; et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int. Immunopharmacol. 2019, 74, 105646. [Google Scholar] [CrossRef] [PubMed]
- LeBaron, T.W.; Kura, B.; Kalocayova, B.; Tribulova, N.; Slezak, J. A new approach for the prevention and treatment of cardiovascular disorders. Molecular hydrogen significantly reduces the effects of oxidative stress. Molecules 2019, 24, 2076. [Google Scholar] [CrossRef]
- Cole, A.R.; Sperotto, F.; DiNardo, J.A.; Carlisle, S.; Rivkin, M.J.; Sleeper, L.A.; Kheir, J.N. Safety of prolonged inhalation of hydrogen gas in air in healthy adults. Crit. Care Explor. 2021, 3, e543. [Google Scholar] [CrossRef] [PubMed]
- Asada, R.; Tazawa, K.; Sato, S.; Miwa, N. Effects of hydrogen-rich water prepared by alternating-current-electrolysis on antioxidant activity, DNA oxidative injuries, and diabetes related markers. Med. Gas Res. 2020, 10, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Shimouchi, A.; Nose, K.; Shirai, M.; Kondo, T. Estimation of molecular hydrogen consumption in the human whole body after the ingestion of hydrogen-rich water. Adv. Exp. Med. Biol. 2012, 737, 245–250. [Google Scholar] [CrossRef]
- Liu, C.; Kurokawa, R.; Fujino, M.; Hirano, S.; Sato, B.; Li, X.K. Estimation of the hydrogen concentration in rat tissue using an airtight tube following the administration of hydrogen via various routes. Sci. Rep. 2014, 4, 5485. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, M.; Imamura, R.; Kobayashi, Y.; Taniguchi, A.; Nakazawa, S.; Kato, T.; Namba-Hamano, T.; Abe, T.; Uemura, M.; Kobayashi, H.; et al. Oral administration of Si-based agent attenuates oxidative stress and ischemia-reperfusion injury in a rat model: A novel hydrogen administration method. Front. Med. 2020, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.H.; Jin, Z.K.; Chen, Q.; Meng, J.; Lu, X.; He, Q. Local generation of hydrogen for enhanced photothermal therapy. Nat. Commun. 2018, 9, 4241. [Google Scholar] [CrossRef] [PubMed]
- Kou, Z.; Zhao, P.H.; Wang, Z.H.; Jin, Z.; Chen, L.; Su, B.L.; He, Q. Acid-responsive H2-releasing Fe nanoparticles for safe and effective cancer therapy. J. Mater. Chem. B 2019, 7, 2759–2765. [Google Scholar] [CrossRef]
- He, Y.; Zhang, B.; Chen, Y.; Jin, Q.; Wu, J.; Yan, F. Image-guided hydrogen gas delivery for protection from myocardial ischemia-reperfusion injury via microbubbles. ACS Appl. Mater. Interfaces 2017, 9, 21190–21199. [Google Scholar] [CrossRef] [PubMed]
- Katiukhin, L.N. Influence of the course of treatment by injections of ozonized saline on rheological properties of erythrocytes in patients with complex pathology. Hum. Physiol. 2016, 42, 672–677. [Google Scholar] [CrossRef]
- Martusevich, A.K.; Peretyagin, S.P.; Ruchin, M.V.; Struchkov, A.A. Ozone Therapy in Patients with Burn Disease. J. Biomed. Sci. Eng. 2018, 11, 27–35. [Google Scholar] [CrossRef]
- Martínez-Sánchez, G.; Schwartz, A.; Di Donna, V. Potential Cytoprotective Activity of Ozone Therapy in SARS-CoV-2/COVID-19. Antioxidants 2020, 9, 389. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.; Li, Y.; Chen, Z.; Wang, L.; Xiong, H.; Dai, E.; Wu, J.; Fan, B.; Ping, L.; et al. Positive effects of hydrogen-water bathing in patients of psoriasis and parapsoriasis en plaques. Sci. Rep. 2018, 8, 8051. [Google Scholar] [CrossRef]
- Asada, R.; Saitoh, Y.; Miwa, N. Effects of hydrogen-rich water bath on visceral fat and skin blotch, with boiling-resistant hydrogen bubbles. Med. Gas Res. 2019, 9, 68–73. [Google Scholar] [CrossRef]
- Oharazawa, H.; Igarashi, T.; Yokota, T.; Fujii, H.; Suzuki, H.; Machide, M. Protection of the retina by rapid diffusion of hydrogen: Administration of hydrogen-loaded eye drops in retinal ischemia-reperfusion injury. Invest. Ophthalmol. Vis. Sci. 2010, 51, 487–492. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, X.; Shi, J.; Shi, D.; Ye, Z.; Liu, W.; Li, M.; Wang, Q.; Kang, Z.; Bi, H.; et al. Lactulose ameliorates cerebral ischemia-reperfusion injury in rats by inducing hydrogen by activating Nrf2 expression. Free Radic. Biol. Med. 2013, 65, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xu, Y.; Zhang, J.; Sun, Z.; Ban, Y.; Wang, B.; Hou, X.; Cai, Y.; Li, J.; Wang, M.; et al. Application of methane and hydrogen-based breath test in the study of gestational diabetes mellitus and intestinal microbes. Diabetes Res. Clin. Pract. 2021, 176, 108818. [Google Scholar] [CrossRef] [PubMed]
- Jahng, J.; Jung, I.S.; Choi, E.J.; Conklin, J.L.; Park, H. The effects of methane and hydrogen gases produced by enteric bacteria on ileal motility and colonic transit time. Neurogastroenterol. Motil. 2012, 24, 185-e92. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Qi, J.; Shao, B.; Ruan, Z.; Ren, Y.; Sui, S.; Wu, X.; Sun, X.; Liu, S.; Li, S.; et al. Microbial hydrogen economy alleviates colitis by reprogramming colonocyte metabolism and reinforcing intestinal barrier. Gut Microbes 2022, 14, 2013764. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kato, S.; Matsuoka, D.; Tanaka, H.; Miwa, N. Hydrogen water intake via tube- feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med. Gas Res. 2013, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, H.; Ji, M.; Jia, M.; Chen, H.; Yang, J.; Duan, M. Hydrogen-rich saline attenuates neuronal ischemia-reperfusion injury by protecting mitochondrial function in rats. J. Surg. Res. 2014, 192, 564–572. [Google Scholar] [CrossRef]
- Ostojic, S.M. Molecular Hydrogen in Sports Medicine: New Therapeutic Perspectives. Int. J. Sports Med. 2015, 36, 273–279. [Google Scholar] [CrossRef]
- Noda, K.; Shigemura, N.; Tanaka, Y.; Kawamura, T.; Hyun Lim, S.; Kokubo, K. A novel method of preserving cardiac grafts using a hydrogen-rich water bath. J. Heart Lung Transpl. 2013, 32, 241–250. [Google Scholar] [CrossRef]
- Sano, M.; Ichihara, G.; Katsumata, Y.; Hiraide, T.; Hirai, A.; Momoi, M. Pharmacokinetics of a single inhalation of hydrogen gas in pigs. PLoS ONE 2020, 15, e0234626. [Google Scholar] [CrossRef]
- Sobue, S.; Yamai, K.; Ito, M.; Ohno, K.; Iwamoto, T. Simultaneous oral and inhalational intake of molecular hydrogen additively suppresses signaling pathways in rodents. Mol. Cell Biochem. 2015, 403, 231–241. [Google Scholar] [CrossRef]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: Oxford, UK, 2015. [Google Scholar] [CrossRef]
- Grassi, D.; Desideri, G.; Ferri, L.; Aggio, A.; Tiberti, S.; Ferri, C. Oxidative stress and endothelial dysfunction: Say no to cigarette smoking! Curr. Pharm. Des. 2010, 16, 2539–2550. [Google Scholar] [CrossRef]
- Harma, M.I.; Harma, M.; Erel, O. Measuring plasma oxidative stress biomarkers in sport medicine. Eur. J. Appl. Physiol. 2006, 97, 505–508. [Google Scholar] [CrossRef]
- Kim, Y.W.; Byzova, T.V. Oxidative stress in angiogenesis and vascular disease. Blood 2014, 123, 625–631. [Google Scholar] [CrossRef]
- Tanriverdi, H.; Evrengul, H.; Kuru, O.; Tanriverdi, S.; Seleci, D.; Enli, Y.; Kaftan, A.H.; Kilic, M. Cigarette smoking induced oxidative stress may impair endothelial function and coronary blood flow in angiographically normal coronary arteries. Circ. J. 2006, 70, 593–599. [Google Scholar] [CrossRef]
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef]
- Setsukinai, K.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170–3175. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a preventive and therapeutic medical gas: Initiation, development and potential of hydrogen medicine. Pharmacol. Ther. 2014, 144, 1–11. [Google Scholar] [CrossRef]
- Ohta, S. Molecular hydrogen as a novel antioxidant: Overview of the advantages of hydrogen for medical applications. Methods Enzymol. 2015, 555, 289–317. [Google Scholar] [CrossRef]
- Gharib, B.; Hanna, S.; Abdallahi, O.M.; Lepidi, H.; Gardette, B.; De Reggi, M. Anti-inflammatory properties of molecular hydrogen: Investigation on parasite-induced liver inflammation. Comptes Rendus Acad. Sci. III 2001, 324, 719–724. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef]
- Xie, K.L.; Zhang, Y.; Wang, Y.Q.; Meng, X.; Wang, Y.; Yu, Y.; Chen, H. Hydrogen attenuates sepsis-associated encephalopathy by NRF2 mediated NLRP3 pathway inactivation. Inflamm. Res. 2020, 69, 697–710. [Google Scholar] [CrossRef]
- Yu, Y.; Yang, Y.Y.; Yang, M.; Wang, C.; Xie, K.; Yu, Y. Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/ HO-1-dependent pathway. Int. Immunopharmacol. 2019, 69, 11–18. [Google Scholar] [CrossRef]
- Cai, W.W.; Zhang, M.H.; Yu, Y.S.; Cai, J.H. Treatment with hydrogen molecule alleviates TNFα-induced cell injury in osteoblast. Mol. Cell. Biochem. 2013, 373, 1–9. [Google Scholar] [CrossRef]
- Shinbo, T.; Kokubo, K.; Sato, Y.; Hagiri, S.; Hataishi, R.; Hirose, M.; Kobayashi, H. Breathing nitric oxide plus hydrogen gas reduces ischemia-reperfusion injury and nitrotyrosine production in murine heart. Am. J. Physiol. Circ. Physiol. 2013, 305, 542–550. [Google Scholar] [CrossRef]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Rimessi, A.; Previati, M.; Nigro, F.; Wieckowski, M.R.; Pinton, P. Mitochondrial reactive oxygen species and inflammation: Molecular mechanisms, diseases and promising therapies. Int. J. Biochem. Cell Biol. 2016, 81 Pt B, 281–293. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Chen, Y.; Qiu, Y.; Luo, Z.; Zhao, S.; Du, L.; Tian, D. Hydrogen protects lung from hypoxia/re-oxygenation injury by reducing hydroxyl radical production and inhibiting inflammatory responses. Sci. Rep. 2018, 8, 8004. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Mei, K.; Qian, L.; Yang, Y.; Liu, W.; Huang, Y.; Zhang, C.; Sun, X.; Liu, C.; Li, B.; et al. Therapeutic effects of hydrogen-rich solution on aplastic anemia in vivo. Cell. Physiol. Biochem. 2013, 32, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, P.; Yang, Y.; Liu, X.; Jiang, J.; Liu, D.; Xue, G. Hydrogen-rich saline resuscitation alleviates inflammation induced by severe burn with delayed resuscitation. Burns 2015, 41, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Los, M.; Baeuerle, P.A. Redox signalling by transcription factors NF-κB and AP-1 in lymphocytes. Biochem. Pharmacol. 1995, 50, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.; Wu, H.; Hong, Yu.; Tu, S.; Sun, X.; Wu, Q.; Zhao, Q.; Zhang, J.; Sheng, J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: Possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol. Neurobiol. 2016, 53, 3462–3476. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Meng, C.; Kang, J.; Zhang, M.; Ma, L.; Zhou, H. The anti-inflammatory effect of hydrogen on lung transplantation model of pulmonary microvascular endothelial cells during cold storage period. Transplantation 2018, 102, 1253–1261. [Google Scholar] [CrossRef]
- Radyuk, S.N. Mechanisms Underlying the Biological Effects of Molecular Hydrogen. Curr. Pharm. Des. 2021, 27, 626–735. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef]
- Shalini, S.; Dorstyn, L.; Dawar, S.; Kumar, S. Old, new and emerging functions of caspases. Cell Death Differ. 2015, 22, 526–539. [Google Scholar] [CrossRef]
- Westphal, D.; Kluck, R.M.; Dewson, G. Building blocks of the apoptotic pore: How Bax and Bak are activated and oligomerize during apoptosis. Cell Death Differ. 2014, 21, 196–205. [Google Scholar] [CrossRef]
- Chen, K.; Wang, N.; Diao, Y.; Dong, W.; Sun, Y.; Liu, L.; Wu, X. Hydrogen-rich saline attenuates brain injury induced by cardiopulmonary bypass and inhibits microvascular endothelial cell apoptosis via the PI3K/Akt/GSK3β signaling pathway in rats. Cell. Physiol. Biochem. 2017, 43, 1634–1647. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Liu, Y.F.; Ma, X.M.; Xiao, Y.D.; Wang, Y.B.; Zhang, M.Z.; Cheng, A.X.; Wang, T.T.; Li, J.L.; Zhao, P.X.; et al. Hydrogen-rich saline attenuates skin ischemia/reperfusion induced apoptosis via regulating Bax/Bcl-2 ratio and ASK-1/JNK pathway. J. Plast. Reconstr. Aesthetic Surg. 2015, 68, 147–156. [Google Scholar] [CrossRef]
- Mo, X.Y.; Li, X.M.; She, C.S.; Lu, X.Q.; Xiao, C.G.; Wang, S.H.; Huang, G.Q. Hydrogen-rich saline protects rat from oxygen glucose deprivation and reperfusion-induced apoptosis through VDAC1 via Bcl-2. Brain Res. 2019, 1706, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hong, Z.J.; Liu, H.; Zhou, J.; Cui, L.; Yuan, S.; Chu, X.; Yu, P. Hydrogen-rich saline promotes the recovery of renal function after ischemia/ reperfusion injury in rats via anti-apoptosis and anti-inflammation. Front. Pharmacol. 2016, 7, 106. [Google Scholar] [CrossRef]
- Jiao, Y.; Yu, Y.; Li, B.; Gu, X.; Xie, K.; Wang, G.; Yu, Y. Protective effects of hydrogen-rich saline against experimental diabetic peripheral neuro- pathy via activation of the mitochondrial ATP-sensitive potassium channel channels in rats. Mol. Med. Rep. 2020, 21, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, P.Y.; Bao, W.; Chen, S.J.; Wu, F.S.; Zhu, P.Y. Hydrogen inhibits endometrial cancer growth via a ROS/NLRP3/caspase-1/GSDMD-mediated pyroptotic pathway. BMC Cancer 2020, 20, 28. [Google Scholar] [CrossRef]
- Shi, J.J.; Gao, W.Q.; Shao, F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Zha, Q.B.; Wei, H.X.; Li, C.G.; Liang, Y.D.; Xu, L.H.; Bai, W.J.; Pan, H.; He, X.H.; Ouyang, D.Y. ATP-induced inflammasome activation and pyroptosis is regulated by AMP-activated protein kinase in macrophages. Front. Immunol. 2016, 7, 597. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Ding, X.A.R.; Zheng, M.; Li, Z.; Pan, S.; Yang, W. Hydrogen gas inhalation alleviates myocardial ischemia-reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyrop- tosis in rats. Life Sci. 2021, 272, 119248. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.F.; Klionsky, D.J. Mammalian autophagy: Core molecular machinery and signaling regulation. Curr. Opin. Cell Biol. 2010, 22, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apop-tosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Mao, X.; Meng, X.; Li, Y.; Feng, J.; Zhang, L.; Zhang, Y.; Wang, Y.; Yu, Y.; Xie, K. Hydrogen alleviates mitochondrial dysfunction and organ damage via autophagy-mediated NLRP3 inflammasome inactivation in sepsis. Int. J. Mol. Med. 2019, 44, 1309–1324. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Hu, T.; Wang, F.; Han, Z.; Yin, Z.; Ge, X.; Xie, K.; Lei, P. Hydrogen improves cell viability partly through inhibition of autophagy and activation of PI3K/Akt/GSK3β signal pathway in a micro- vascular endothelial cell model of traumatic brain injury. Neurol. Res. 2020, 42, 487–496. [Google Scholar] [CrossRef]
- Adzavon, Y.M.; Xie, F.; Yi, Y.; Jiang, X.; Zhang, X.; He, J.; Zhao, P.; Liu, M.; Ma, S.; Ma, X. Long-term and daily use of molecular hydrogen induces reprogramming of liver metabolism in rats by modulating NADP/NADPH redox pathways. Sci. Rep. 2022, 12, 3904. [Google Scholar] [CrossRef]
- Kawasaki, H.; Guan, J.; Tamama, K. Hydrogen gas treatment prolongs replicative lifespan of bone marrow multipotential stromal cells in vitro while preserving differentiation and paracrine potentials. Biochem. Biophys. Res. Commun. 2010, 397, 608–613. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ito, M.; Hasegawa, S.; Teranishi, M.; Takeda, K.; Negishi, S.; Nishiwaki, H.; Takeda, J.-i.; LeBaron, T.W.; Ohno, K. Molecular Hydrogen Enhances Proliferation of Cancer Cells That Exhibit Potent Mitochondrial Unfolded Protein Response. Int. J. Mol. Sci. 2022, 23, 2888. [Google Scholar] [CrossRef]
- Fiorese, C.J.; Schulz, A.M.; Lin, Y.F.; Rosin, N.; Pellegrino, M.W.; Haynes, C.M. The Transcription Factor ATF5 Mediates a Mammalian Mitochondrial UPR. Curr. Biol. 2016, 26, 2037–2043. [Google Scholar] [CrossRef]
- Wu, Z.; Senchuk, M.M.; Dues, D.J.; Johnson, B.K.; Cooper, J.F.; Lew, L.; Machiela, E.; Schaar, C.E.; DeJonge, H.; Blackwell, T.K.; et al. Mitochondrial unfolded protein response transcription factor ATFS-1 promotes longevity in a long-lived mitochondrial mutant through activation of stress response pathways. BMC Biol. 2018, 16, 147. [Google Scholar] [CrossRef]
- Lin, Y.F.; Haynes, C.M. Metabolism and the UPR(mt). Mol. Cell 2016, 61, 677–682. [Google Scholar] [CrossRef]
- Zhao, Y.S.; An, J.R.; Yang, S.; Guan, P.; Yu, F.Y.; Li, W.; Li, J.R.; Guo, Y.; Sun, Z.M.; Ji, E.S. Hydrogen and Oxygen Mixture to Improve Cardiac Dysfunction and Myocardial Pathological Changes Induced by Intermittent Hypoxia in Rats. Oxidative Med. Cell. Longev. 2019, 2019, 7415212. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Lim, Y.; McCall, M.N.; Huang, K.T.; Haynes, C.M.; Nehrke, K.; Brookes, P.S. Cardioprotection by the mitochondrial unfolded protein response requires ATF. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H472–H478. [Google Scholar] [CrossRef]
- Berger, E.; Rath, E.; Yuan, D.; Waldschmitt, N.; Khaloian, S.; Allgauer, M.; Staszewski, O.; Lobner, E.M.; Schottl, T.; Giesbertz, P.; et al. Mitochondrial function controls intestinal epithelial stemness and proliferation. Nat. Commun. 2016, 7, 13171. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Xie, F.; Zhang, Y.; Wang, T.-T.; Ma, S.-N.; Zhao, P.-X.; Zhang, X.; LeBaron, T.W.; Yan, X.-L.; Ma, X.-M. Molecular hydrogen suppresses glioblastoma growth via inducing the glioma stem-like cell differentiation. Stem Cell Res. Ther. 2019, 10, 145. [Google Scholar] [CrossRef]
- Fang, W.; Wang, G.; Tang, L.; Su, H.; Chen, H.; Liao, W.; Xu, J. Hydrogen gas inhalation protects against cutaneous ischaemia/reperfusion injury in a mouse model of pressure ulcer. J. Cell. Mol. Med. 2018, 22, 4243–4252. [Google Scholar] [CrossRef]
- Buchholz, B.M.; Masutani, K.; Kawamura, T.; Peng, X.; Toyoda, Y.; Billiar, T.R.; Bauer, A.J.; Nakao, A. Hydrogen-enriched preservation protects the isogeneic intestinal graft and amends recipient gastric function during transplantation. Transplantation 2011, 92, 985–992. [Google Scholar] [CrossRef]
- Deng, L.; Du, C.; Song, P.; Chen, T.; Rui, S.; Armstrong, D.G.; Deng, W. The Role of Oxidative Stress and Antioxidants in Diabetic Wound Healing. Oxidative Med. Cell. Longev. 2021, 2021, 8852759. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J.L. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Litwiniuk, M.; Krejner, A.; Speyrer, M.S.; Gauto, A.R.; Grzela, T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds 2016, 28, 78–88. [Google Scholar]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Dohi, K.; Kraemer, B.C.; Erickson, M.A.; McMillan, P.J.; Kovac, A.; Flachbartova, Z.; Hansen, K.M.; Shah, G.N.; Sheibani, N.; Salameh, T.; et al. Molecular hydrogen in drinking water protects against neurodegenerative changes induced by traumatic brain injury. PLoS ONE 2014, 9, e108034. [Google Scholar] [CrossRef]
- Noda, K.; Tanaka, Y.; Shigemura, N.; Kawamura, T.; Wang, Y.; Masutani, K.; Sun, X.; Toyoda, Y.; Bermudez, C.A.; Nakao, A. Hydrogen-supplemented drinking water protects cardiac allografts from inflammation-associated deterioration. Transpl. Int. 2012, 25, 1213–1222. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artamonov, M.Y.; Martusevich, A.K.; Pyatakovich, F.A.; Minenko, I.A.; Dlin, S.V.; LeBaron, T.W. Molecular Hydrogen: From Molecular Effects to Stem Cells Management and Tissue Regeneration. Antioxidants 2023, 12, 636. https://doi.org/10.3390/antiox12030636
Artamonov MY, Martusevich AK, Pyatakovich FA, Minenko IA, Dlin SV, LeBaron TW. Molecular Hydrogen: From Molecular Effects to Stem Cells Management and Tissue Regeneration. Antioxidants. 2023; 12(3):636. https://doi.org/10.3390/antiox12030636
Chicago/Turabian StyleArtamonov, Mikhail Yu., Andrew K. Martusevich, Felix A. Pyatakovich, Inessa A. Minenko, Sergei V. Dlin, and Tyler W. LeBaron. 2023. "Molecular Hydrogen: From Molecular Effects to Stem Cells Management and Tissue Regeneration" Antioxidants 12, no. 3: 636. https://doi.org/10.3390/antiox12030636
APA StyleArtamonov, M. Y., Martusevich, A. K., Pyatakovich, F. A., Minenko, I. A., Dlin, S. V., & LeBaron, T. W. (2023). Molecular Hydrogen: From Molecular Effects to Stem Cells Management and Tissue Regeneration. Antioxidants, 12(3), 636. https://doi.org/10.3390/antiox12030636







