Effect of Oil Type on Spatial Partition of Resveratrol in the Aqueous Phase, the Protein Interface and the Oil Phase of O/W Emulsions Stabilized by Whey Protein and Caseinate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Emulsion Preparation
2.3. Particle Size and ζ-Potential
2.4. Interfacial Protein Percentage
2.5. Competitive Replacement of Interfacial Protein
2.6. Partition of Resveratrol in Emulsion
2.7. Quantitation of Resveratrol Using HPLC and UV Method
2.8. Solubility of Resveratrol in Protein Solution and Bulk Oil
2.9. Statistical Analysis
3. Results and Discussion
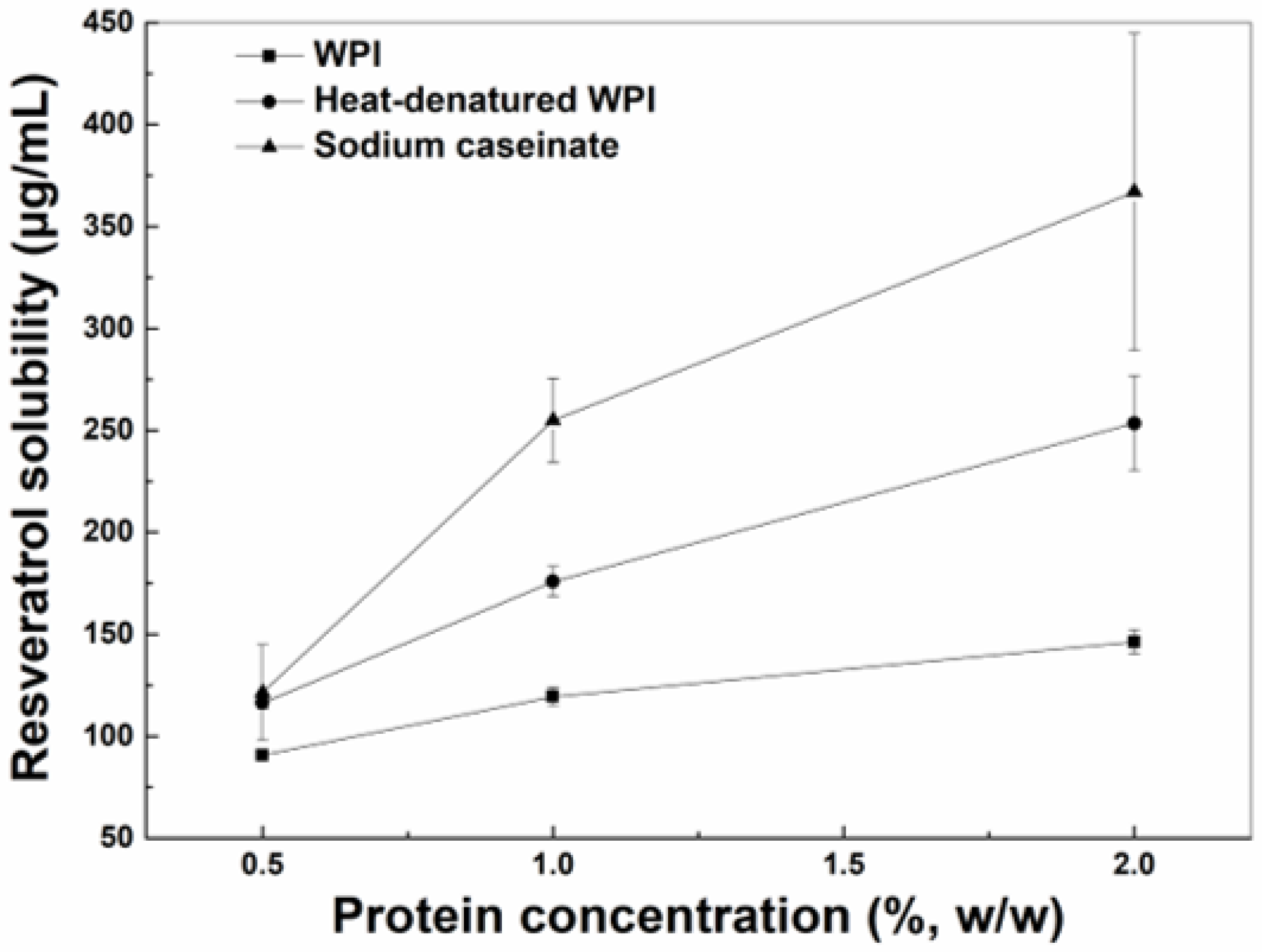
3.1. Solubility of Resveratrol in Bulk Oils and Protein Aqueous Solutions
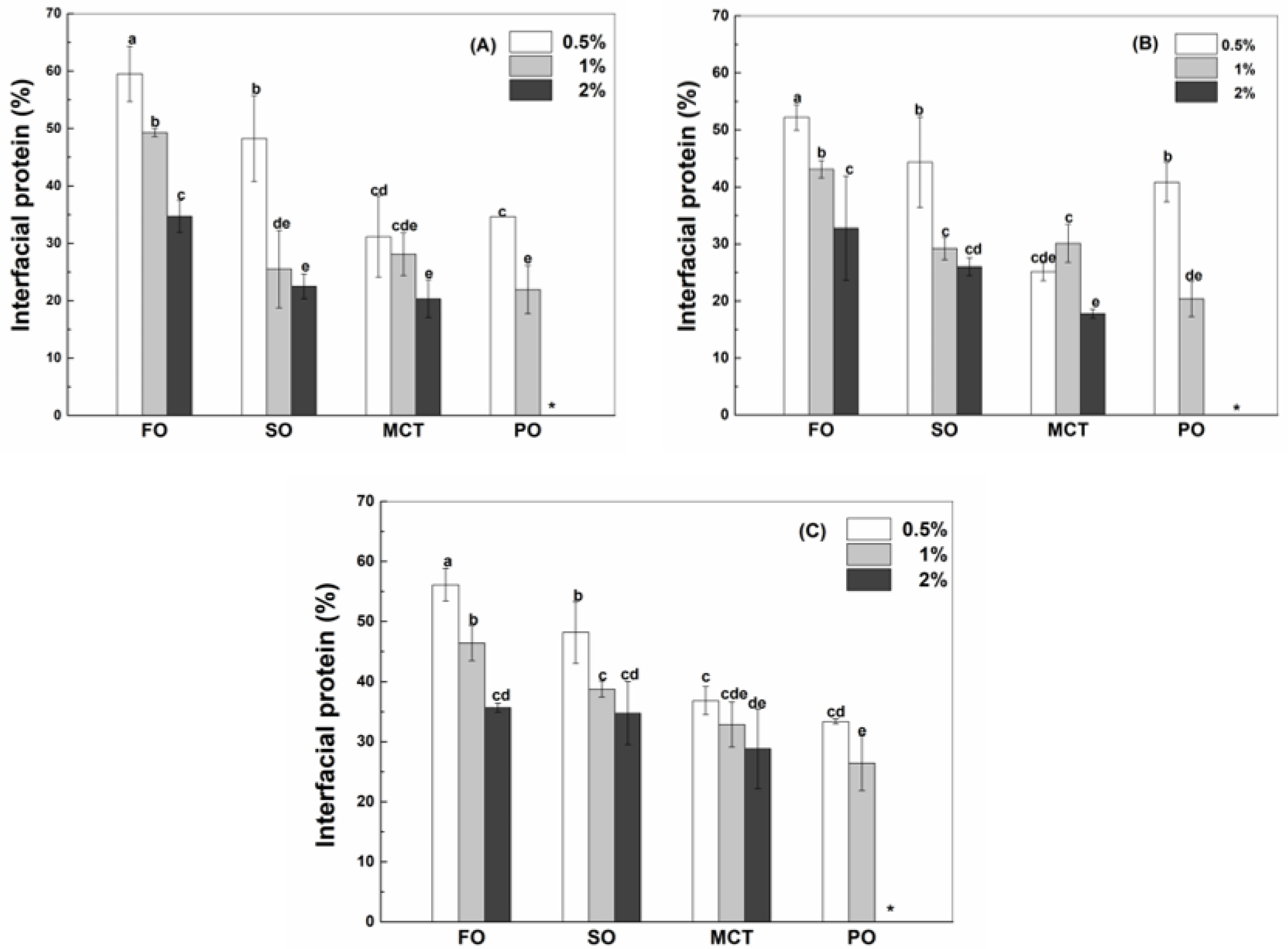
3.2. Interfacial Protein in Emulsions
3.3. Interfacial Protein Replacement
3.4. Spatial Partition of Resveratrol in Emulsions
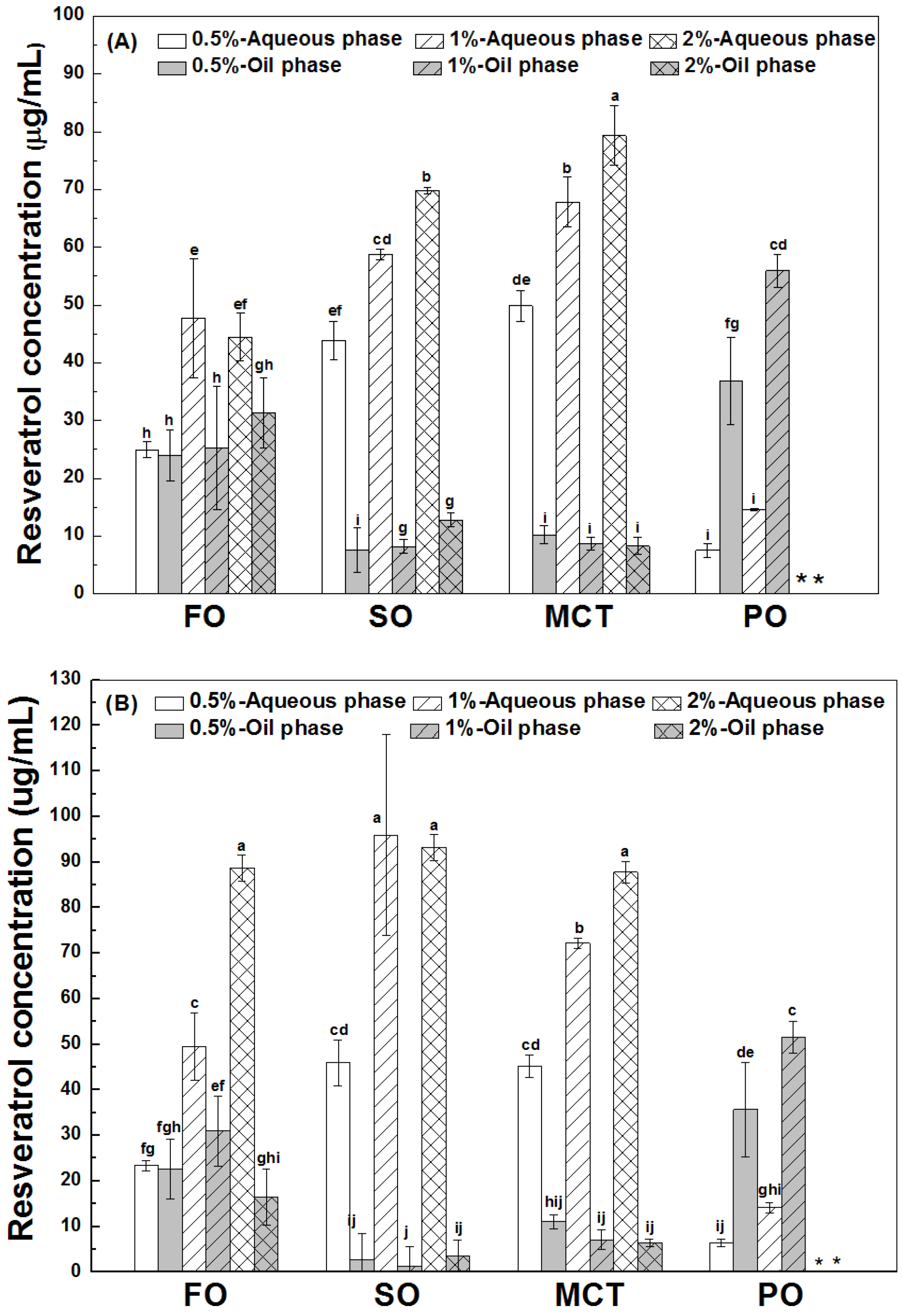
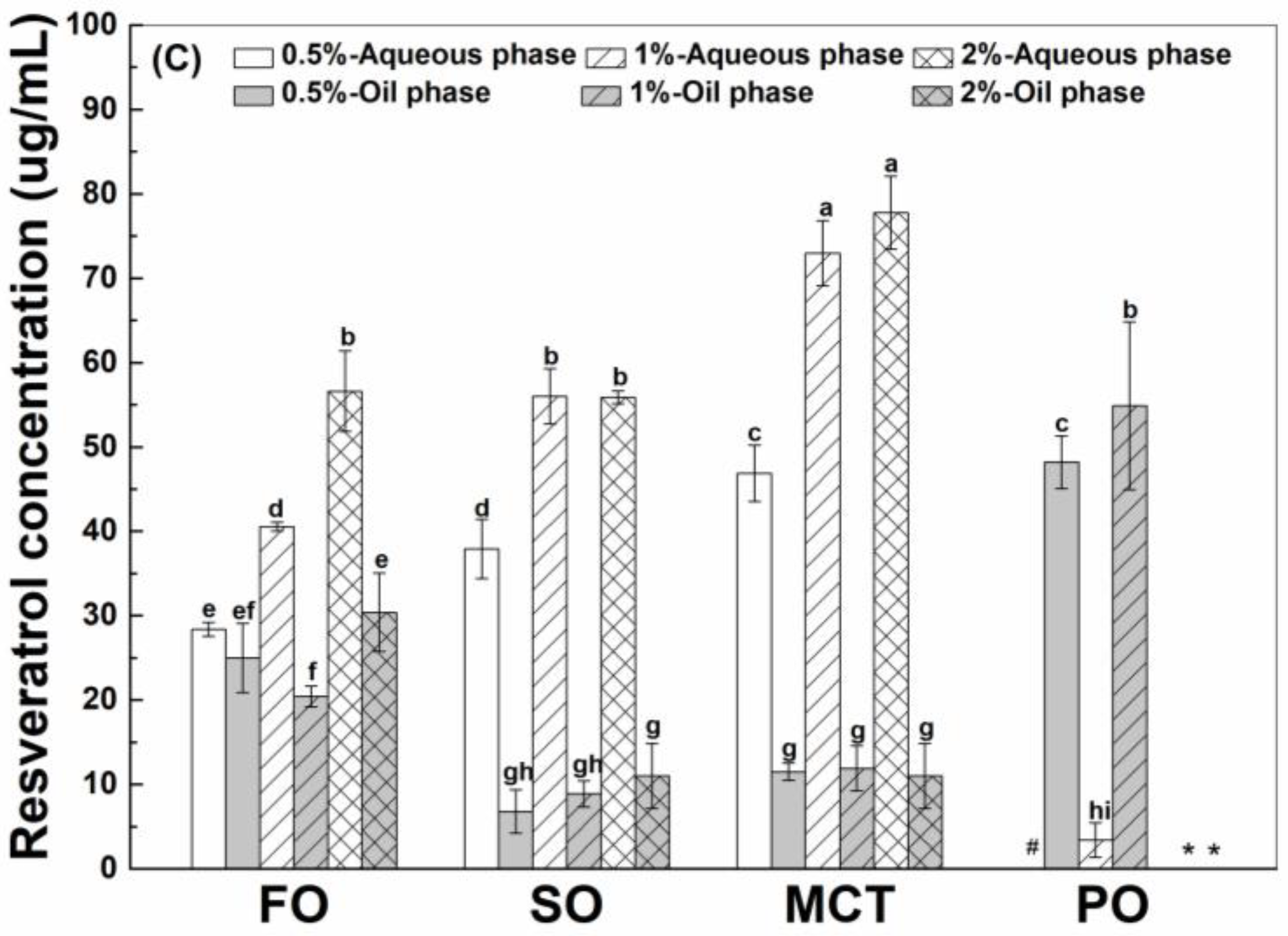
3.4.1. Resveratrol in the Aqueous Phase
3.4.2. Interfacial Resveratrol
3.4.3. Resveratrol in the Oil Phase
3.5. Mechanism of Resveratrol Partition in Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nooshkam, M.; Varidi, M. Maillard conjugate-based delivery systems for the encapsulation, protection, and controlled release of nutraceuticals and food bioactive ingredients: A review. Food Hydrocoll. 2020, 100, 105389. [Google Scholar] [CrossRef]
- Xu, W.; Lv, K.; Mu, W.; Zhou, S.; Yang, Y. Encapsulation of α-tocopherol in whey protein isolate/chitosan particles using oil-in-water emulsion with optimal stability and bioaccessibility. LWT 2021, 148, 111724. [Google Scholar] [CrossRef]
- Wang, L.; Gao, Y.; Li, J.; Subirade, M.; Song, Y.; Liang, L. Effect of resveratrol or ascorbic acid on the stability of α-tocopherol in O/W emulsions stabilized by whey protein isolate: Simultaneous encapsulation of the vitamin and the protective antioxidant. Food Chem. 2016, 196, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Polyphenolic Antioxidants in Lipid Emulsions: Partitioning Effects and Interfacial Phenomena. Foods 2021, 10, 539. [Google Scholar] [CrossRef]
- Ferreira, I.; Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C. Modulating the interfacial concentration of gallates to improve the oxidative stability of fish oil-in-water emulsions. Food Res. Int. 2018, 112, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zheng, B.; McClements, D.J. In Vitro Gastrointestinal Stability of Lipophilic Polyphenols is Dependent on their Oil–Water Partitioning in Emulsions: Studies on Curcumin, Resveratrol, and Quercetin. J. Agric. Food Chem. 2021, 69, 3340–3350. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, S.L.; Bravo-Díaz, C.; Paiva-Martins, F.; Romsted, L.S. Maxima in Antioxidant Distributions and Efficiencies with Increasing Hydrophobicity of Gallic Acid and Its Alkyl Esters. The Pseudophase Model Interpretation of the “Cutoff Effect”. J. Agric. Food Chem. 2013, 61, 6533–6543. [Google Scholar] [CrossRef]
- Costa, M.; Losada-Barreiro, S.; Paiva-Martins, F.; Bravo-Díaz, C.; Romsted, L.S. A direct correlation between the antioxidant efficiencies of caffeic acid and its alkyl esters and their concentrations in the interfacial region of olive oil emulsions. The pseudophase model interpretation of the “cut-off” effect. Food Chem. 2015, 175, 233–242. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Costa, M.; Bravo-Díaz, C.; Paiva-Martins, F. Distribution and Antioxidant Efficiency of Resveratrol in Stripped Corn Oil Emulsions. Antioxidants 2014, 3, 212–228. [Google Scholar] [CrossRef]
- Fan, Q.; Wang, L.; Song, Y.; Fang, Z.; Subirade, M.; Liang, L. Partition and stability of resveratrol in whey protein isolate oil-in-water emulsion: Impact of protein and calcium concentrations. Int. Dairy J. 2017, 73, 128–135. [Google Scholar] [CrossRef]
- Gong, T.; Chen, B.; Hu, C.Y.; Guo, Y.R.; Shen, Y.H.; Meng, Y.H. Resveratrol inhibits lipid and protein co-oxidation in sodium caseinate-walnut oil emulsions by reinforcing oil-water interface. Food Res. Int. 2022, 158, 111541. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, H.; Li, D.; Duan, H.; Liang, L. Impact of oil type on the location, partition and chemical stability of resveratrol in oil-in-water emulsions stabilized by whey protein isolate plus gum Arabic. Food Hydrocoll. 2020, 109, 106119. [Google Scholar] [CrossRef]
- Courthaudon, J.-L.; Dickinson, E.; Matsumura, Y.; Clark, D.C. Competitive adsorption of β-lactoglobulin + tween 20 at the oil-water interface. Colloids Surf. 1991, 56, 293–300. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, J.; Zhao, X.; Xu, X. An optimized approach to recovering O/W interfacial myofibrillar protein: Emphasizing on interface-induced structural changes. Food Hydrocoll. 2022, 124, 107194. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhao, C.; Yi, J.; Liu, N.; Cao, Y.; Decker, E.A.; McClements, D.J. Impact of Interfacial Composition on Lipid and Protein Co-Oxidation in Oil-in-Water Emulsions Containing Mixed Emulisifers. J. Agric. Food Chem. 2018, 66, 4458–4468. [Google Scholar] [CrossRef]
- Liang, L.; Line, V.L.S.; Remondetto, G.E.; Subirade, M. In vitro release of α-tocopherol from emulsion-loaded β-lactoglobulin gels. Int. Dairy J. 2010, 20, 176–181. [Google Scholar] [CrossRef]
- Liang, L.; Tremblay-Hébert, V.; Subirade, M. Characterisation of the β-lactoglobulin/α-tocopherol complex and its impact on α-tocopherol stability. Food Chem. 2011, 126, 821–826. [Google Scholar] [CrossRef]
- Hung, C.-F.; Chen, J.-K.; Liao, M.-H.; Lo, H.-M.; Fang, J.-Y. Development and Evaluation of Emulsion-Liposome Blends for Resveratrol Delivery. J. Nanosci. Nanotechnol. 2006, 6, 2950–2958. [Google Scholar] [CrossRef]
- Perez-Vich, B.; Velasco, L.; Fernández-Martínez, J.M. Determination of seed oil content and fatty acid composition in sunflower through the analysis of intact seeds, husked seeds, meal and oil by near-infrared reflectance spectroscopy. J. Am. Oil Chem. Soc. 1998, 75, 547–555. [Google Scholar] [CrossRef]
- Riachi, L.G.; De Maria, C.A. Peppermint antioxidants revisited. Food Chem. 2015, 176, 72–81. [Google Scholar] [CrossRef]
- Ahmed, K.; Li, Y.; McClements, D.J.; Xiao, H. Nanoemulsion- and emulsion-based delivery systems for curcumin: Encapsulation and release properties. Food Chem. 2012, 132, 799–807. [Google Scholar] [CrossRef]
- Hemar, Y.; Gerbeaud, M.; Oliver, C.M.; Augustin, M.A. Investigation into the interaction between resveratrol and whey proteins using fluorescence spectroscopy. Int. J. Food Sci. Technol. 2011, 46, 2137–2144. [Google Scholar] [CrossRef]
- Acharya, D.P.; Sanguansri, L.; Augustin, M.A. Binding of resveratrol with sodium caseinate in aqueous solutions. Food Chem. 2013, 141, 1050–1054. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Cheng, H.; Wusigale; Dong, H.; Huang, W.; Liang, L. Resveratrol Stabilization and Loss by Sodium Caseinate, Whey and Soy Protein Isolates: Loading, Antioxidant Activity, Oxidability. Antioxidants 2022, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Subirade, M. Study of the acid and thermal stability of β-lactoglobulin–ligand complexes using fluorescence quenching. Food Chem. 2012, 132, 2023–2029. [Google Scholar] [CrossRef]
- Hunt, J.A.; Dalgleish, D.G. Adsorption behaviour of whey protein isolate and caseinate in soya oil-in-water emulsions. Food Hydrocoll. 1994, 8, 175–187. [Google Scholar] [CrossRef]
- Zhang, M.; Fan, L.; Liu, Y.; Huang, S.; Li, J. Effects of proteins on emulsion stability: The role of proteins at the oil–water interface. Food Chem. 2022, 397, 133726. [Google Scholar] [CrossRef]
- Kieserling, H.; Pankow, A.; Keppler, J.K.; Wagemans, A.M.; Drusch, S. Conformational state and charge determine the interfacial film formation and film stability of β-lactoglobulin. Food Hydrocoll. 2021, 114, 106561. [Google Scholar] [CrossRef]
- Bergfreund, J.; Bertsch, P.; Kuster, S.; Fischer, P. Effect of Oil Hydrophobicity on the Adsorption and Rheology of β-Lactoglobulin at Oil–Water Interfaces. Langmuir 2018, 34, 4929–4936. [Google Scholar] [CrossRef]
- Wang, X.; Huang, H.; Chu, X.; Han, Y.; Li, M.; Li, G.; Liu, X. Encapsulation and binding properties of curcumin in zein particles stabilized by Tween 20. Colloids Sur. A Physicochem. Eng. Asp. 2019, 577, 274–280. [Google Scholar] [CrossRef]
- Summerlin, N.; Soo, E.; Thakur, S.; Qu, Z.; Jambhrunkar, S.; Popat, A. Resveratrol nanoformulations: Challenges and opportunities. Int. J. Pharm. 2015, 479, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Fan, Q.; Liu, T.; Wusigale; Liang, L. Co-encapsulation of α-tocopherol and resveratrol in oil-in-water emulsion stabilized by sodium caseinate: Impact of polysaccharide on the stability and bioaccessibility. J. Food Eng. 2020, 264, 109685. [Google Scholar] [CrossRef]
- Wan, Z.-L.; Wang, J.-M.; Wang, L.-Y.; Yuan, Y.; Yang, X.-Q. Complexation of resveratrol with soy protein and its improvement on oxidative stability of corn oil/water emulsions. Food Chem. 2014, 161, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Landy, P.; Voilley, A.; Wedzicha, B.L. Interphase Transport of Benzoic Acid in Emulsions. J. Colloid Interface Sci. 1998, 205, 503–509. [Google Scholar] [CrossRef]
- Bravo-Díaz, C.; Romsted, L.S.; Liu, C.; Losada-Barreiro, S.; Pastoriza-Gallego, M.J.; Gao, X.; Gu, Q.; Krishnan, G.; Sánchez-Paz, V.; Zhang, Y.; et al. To Model Chemical Reactivity in Heterogeneous Emulsions, Think Homogeneous Microemulsions. Langmuir 2015, 31, 8961–8979. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sánchez-Paz, V.; Bravo-Díaz, C. Transfer of antioxidants at the interfaces of model food emulsions: Distributions and thermodynamic parameters. Org. Biomol. Chem. 2015, 13, 876–885. [Google Scholar] [CrossRef]
- Martínez-Aranda, N.; Losada-Barreiro, S.; Bravo-Díaz, C.; Romsted, L.S. Influence of Temperature on the Distribution of Catechin in Corn Oil-in-Water Emulsions and Some Relevant Thermodynamic Parameters. Food Biophys. 2014, 9, 380–388. [Google Scholar] [CrossRef]
- Narkiewicz-Michalek, J.; Szymula, M.; Losada-Barreiro, S.; Bravo-Diaz, C. Concentration of resveratrol at the oil–water interface of corn oil-in-water emulsions. Adsorption 2019, 25, 903–911. [Google Scholar] [CrossRef]
- Elizalde, B.; Pilosof, A.; Bartholomai, G. Prediction of Emulsion Instability from Emulsion Composition and Physicochemical Properties of Proteins. J. Food Sci. 1991, 56, 116–120. [Google Scholar] [CrossRef]
- Bergfreund, J.; Bertsch, P.; Fischer, P. Adsorption of proteins to fluid interfaces: Role of the hydrophobic subphase. J. Colloid Interface Sci. 2021, 584, 411–417. [Google Scholar] [CrossRef]
- Mekhloufi, G.; Vilamosa, N.; Agnely, F. Nanoemulsion stabilized by β-lactoglobulin: A promising strategy to encapsulate curcumin for topical delivery. Mater. Today Proc. 2022, 53, 168–173. [Google Scholar] [CrossRef]
- Zembyla, M.; Murray, B.S.; Radford, S.J.; Sarkar, A. Water-in-oil Pickering emulsions stabilized by an interfacial complex of water-insoluble polyphenol crystals and protein. J. Colloid Interface Sci. 2019, 548, 88–99. [Google Scholar] [CrossRef]
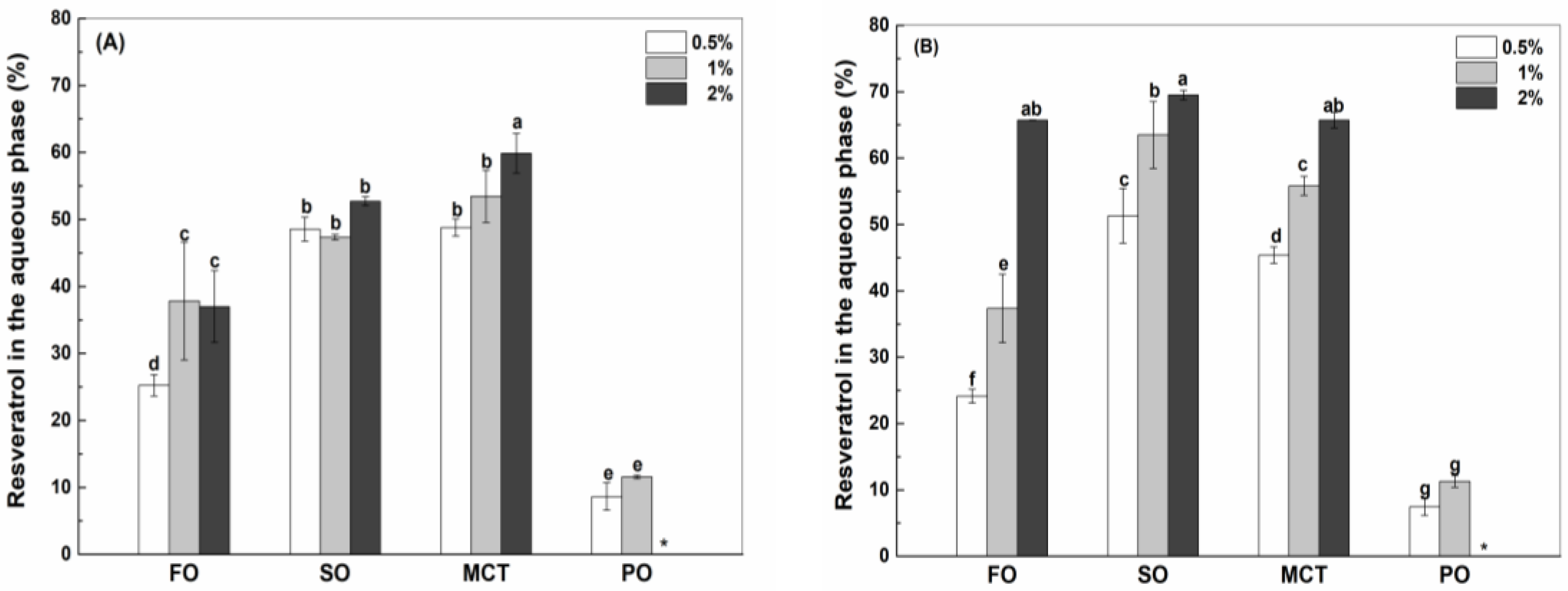
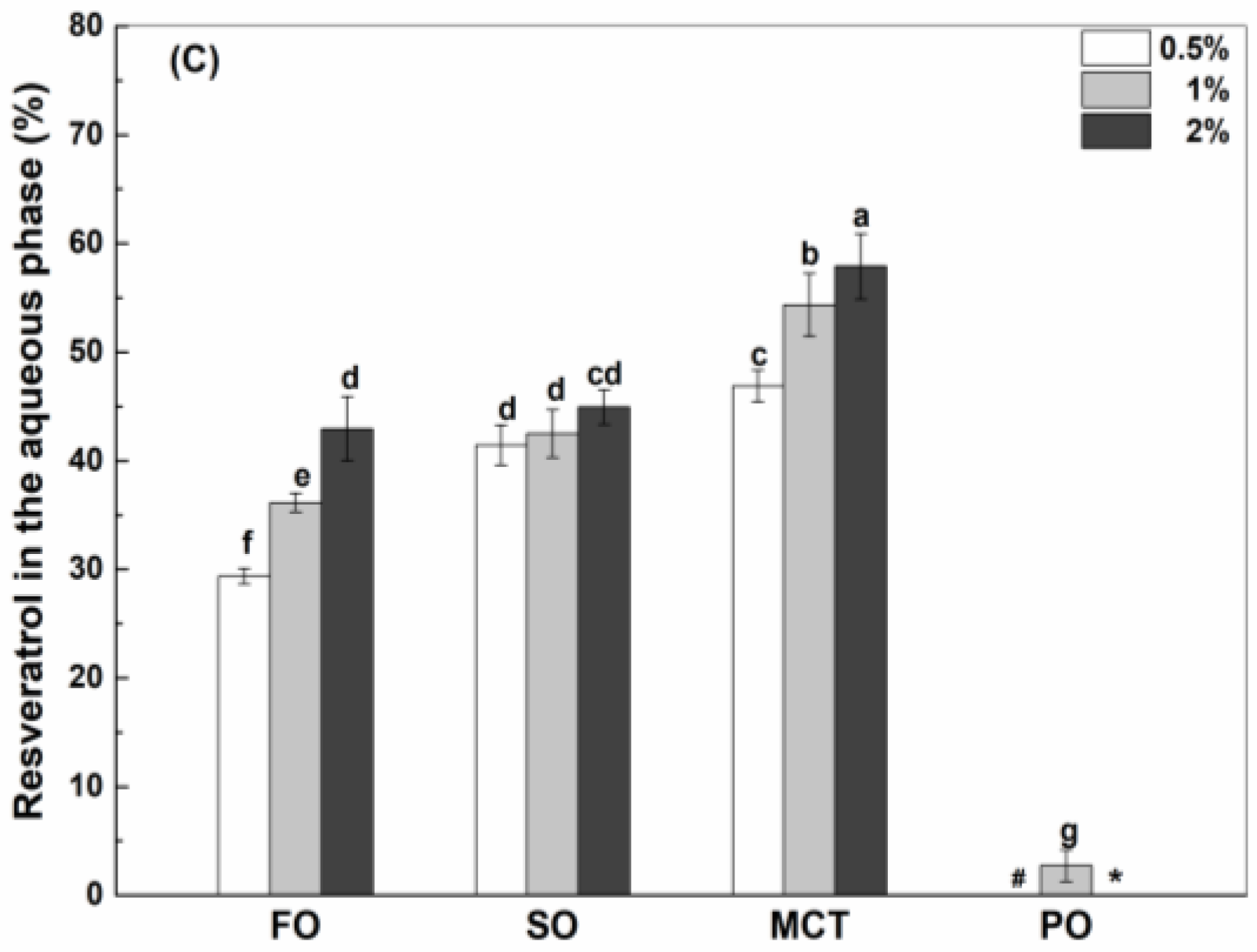


| Oil Type | Fish Oil | Sunflower Oil | MCT | Peppermint Oil |
|---|---|---|---|---|
| Solubility (mg/g) | 0.25 ± 0.05 c | 0.10 ± 0.01 c | 4.20 ± 0.30 b | 15.85 ± 3.90 a |
| Dielectric constant | 3.34 ± 0.02 c | 3.27 ± 0.10 cd | 3.77 ± 0.00 b | 6.80 ± 0.05 a |
| Variable Description | R2 | Regression Coefficient | p | β |
|---|---|---|---|---|
| Constant | 0.806 | 93.237 | 0.000 | |
| Resveratrol solubility in bulk oil | −0.003 | 0.000 | −0.977 | |
| Resveratrol solubility in protein solution | 0.012 | 0.634 | 0.050 | |
| Protein concentration in emulsion | −3.272 | 0.453 | −0.101 | |
| Interface protein percentage | −1.110 | 0.000 | −0.606 | |
| Constant | 0.803 | 88.295 | 0.000 | |
| Resveratrol solubility in bulk oil | −0.003 | 0.000 | −0.944 | |
| Interface protein percentage | −1.018 | 0.000 | −0.556 |
| Variable Description | R2 | Regression Coefficient | p | β |
|---|---|---|---|---|
| Constant | 0.786 | −30.692 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.003 | 0.000 | 1.094 | |
| Resveratrol solubility in protein solution | −0.022 | 0.282 | −0.120 | |
| Protein concentration in emulsion | 10.514 | 0.004 | 0.429 | |
| Interface protein percentage | 0.837 | 0.000 | 0.607 | |
| Constant | 0.779 | −30.637 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.003 | 0.000 | 1.081 | |
| Protein concentration in emulsion | 7.994 | 0.003 | 0.327 | |
| Interface protein percentage | 0.808 | 0.000 | 0.586 |
| Variable Description | R2 | Regression Coefficient | p | β |
|---|---|---|---|---|
| Constant | 0.918 | −6.606 | 0.401 | |
| Resveratrol solubility in bulk oil | 0.003 | 0.000 | 0.994 | |
| Resveratrol solubility in protein solution | −0.007 | 0.685 | −0.035 | |
| Protein concentration in emulsion | 2.220 | 0.522 | 0.077 | |
| Interface protein percentage | 0.220 | 0.202 | 0.113 | |
| Constant | 0.912 | 2.114 | 0.079 | |
| Resveratrol solubility in bulk oil | 0.002 | 0.000 | 0.955 |
| Variable Description | R2 | Regression Coefficient | p | β |
|---|---|---|---|---|
| Constant | 0.626 | 37.433 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.000 | 0.006 | 0.367 | |
| Resveratrol solubility in protein solution | 0.010 | 0.516 | 0.096 | |
| Protein concentration in emulsion | −7.237 | 0.007 | −0.527 | |
| Interface protein percentage | 0.273 | 0.020 | 0.353 | |
| Constant | 0.521 | 21.351 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.001 | 0.000 | 0.597 | |
| Interface protein percentage | 0.533 | 0.000 | 0.690 |
| Variable Description | R2 | Regression Coefficient | p | β |
|---|---|---|---|---|
| Constant | 0.792 | 30.870 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.001 | 0.000 | 0.618 | |
| Resveratrol solubility in protein solution | 0.002 | 0.902 | 0.016 | |
| Protein concentration in emulsion | −5.518 | 0.018 | −0.395 | |
| Interface protein percentage | 0.364 | 0.001 | 0.495 | |
| Constant | 0.715 | 17.146 | 0.000 | |
| Resveratrol solubility in bulk oil | 0.001 | 0.000 | 0.839 | |
| Interface protein percentage | 0.566 | 0.000 | 0.768 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Cheng, H.; Liang, L. Effect of Oil Type on Spatial Partition of Resveratrol in the Aqueous Phase, the Protein Interface and the Oil Phase of O/W Emulsions Stabilized by Whey Protein and Caseinate. Antioxidants 2023, 12, 589. https://doi.org/10.3390/antiox12030589
Chen Y, Cheng H, Liang L. Effect of Oil Type on Spatial Partition of Resveratrol in the Aqueous Phase, the Protein Interface and the Oil Phase of O/W Emulsions Stabilized by Whey Protein and Caseinate. Antioxidants. 2023; 12(3):589. https://doi.org/10.3390/antiox12030589
Chicago/Turabian StyleChen, Yang, Hao Cheng, and Li Liang. 2023. "Effect of Oil Type on Spatial Partition of Resveratrol in the Aqueous Phase, the Protein Interface and the Oil Phase of O/W Emulsions Stabilized by Whey Protein and Caseinate" Antioxidants 12, no. 3: 589. https://doi.org/10.3390/antiox12030589
APA StyleChen, Y., Cheng, H., & Liang, L. (2023). Effect of Oil Type on Spatial Partition of Resveratrol in the Aqueous Phase, the Protein Interface and the Oil Phase of O/W Emulsions Stabilized by Whey Protein and Caseinate. Antioxidants, 12(3), 589. https://doi.org/10.3390/antiox12030589








