Limnospira indica PCC 8005 Supplementation Prevents Pelvic Irradiation-Induced Dysbiosis but Not Acute Inflammation in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Bacteria and Growth Conditions
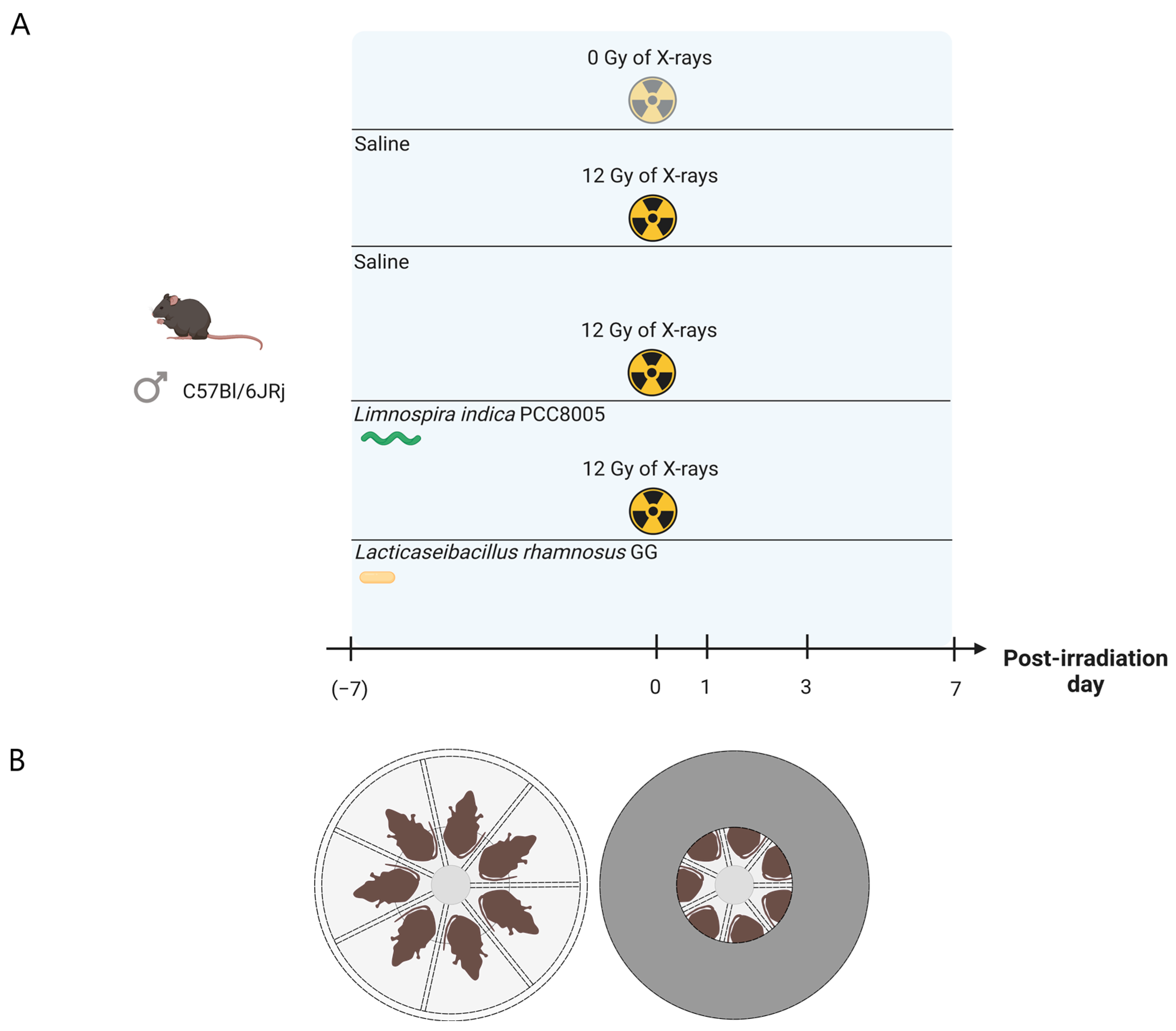
2.3. Experimental Setup
2.4. Irradiation Protocol
2.5. Ileal histology and Histochemistry
2.6. Ileal Myeloperoxidase Activity Assay
2.7. Cytokine Analyses
2.8. Western Blot Analysis of Claudin 5
2.9. Fecal DNA Extraction and 16S rRNA Gene Sequencing
2.10. Sequencing Data Processing and Analyses
2.11. Statistical Analyses
3. Results
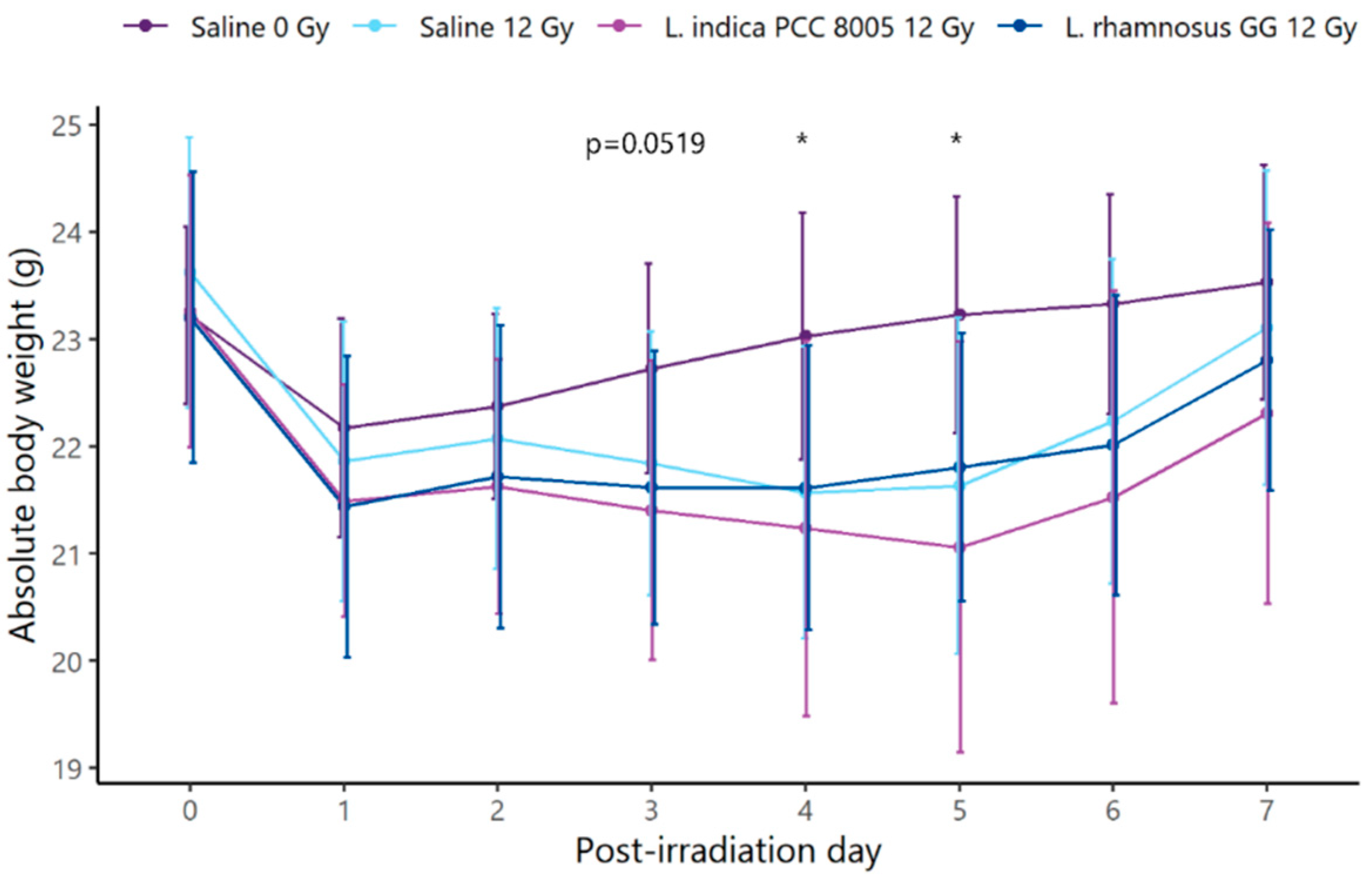
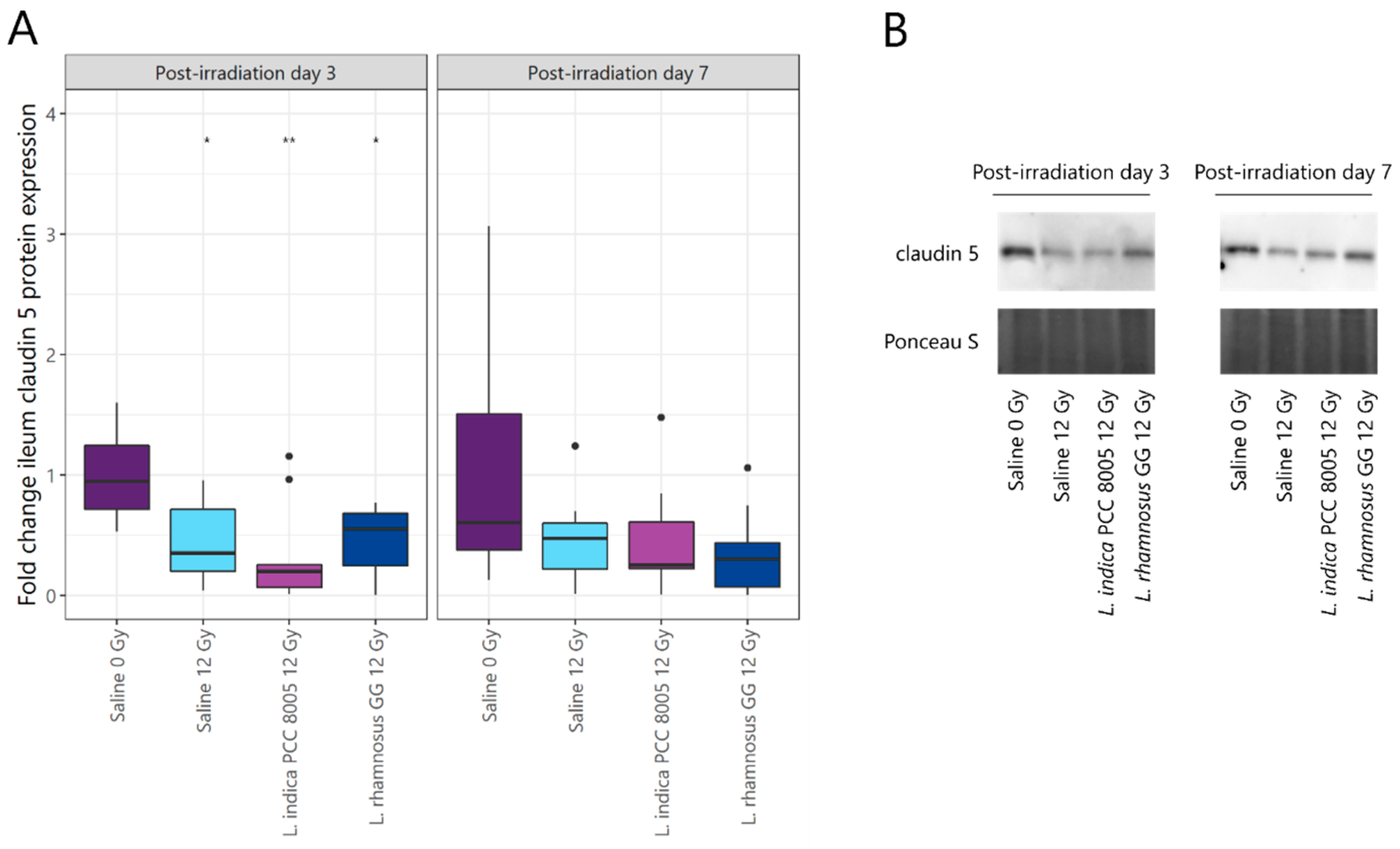
3.1. L. indica PCC 8005 and L. rhamnosus GG Are Unsuccessful in Protecting the Ileal Barrier Damaged by Pelvic Irradiation
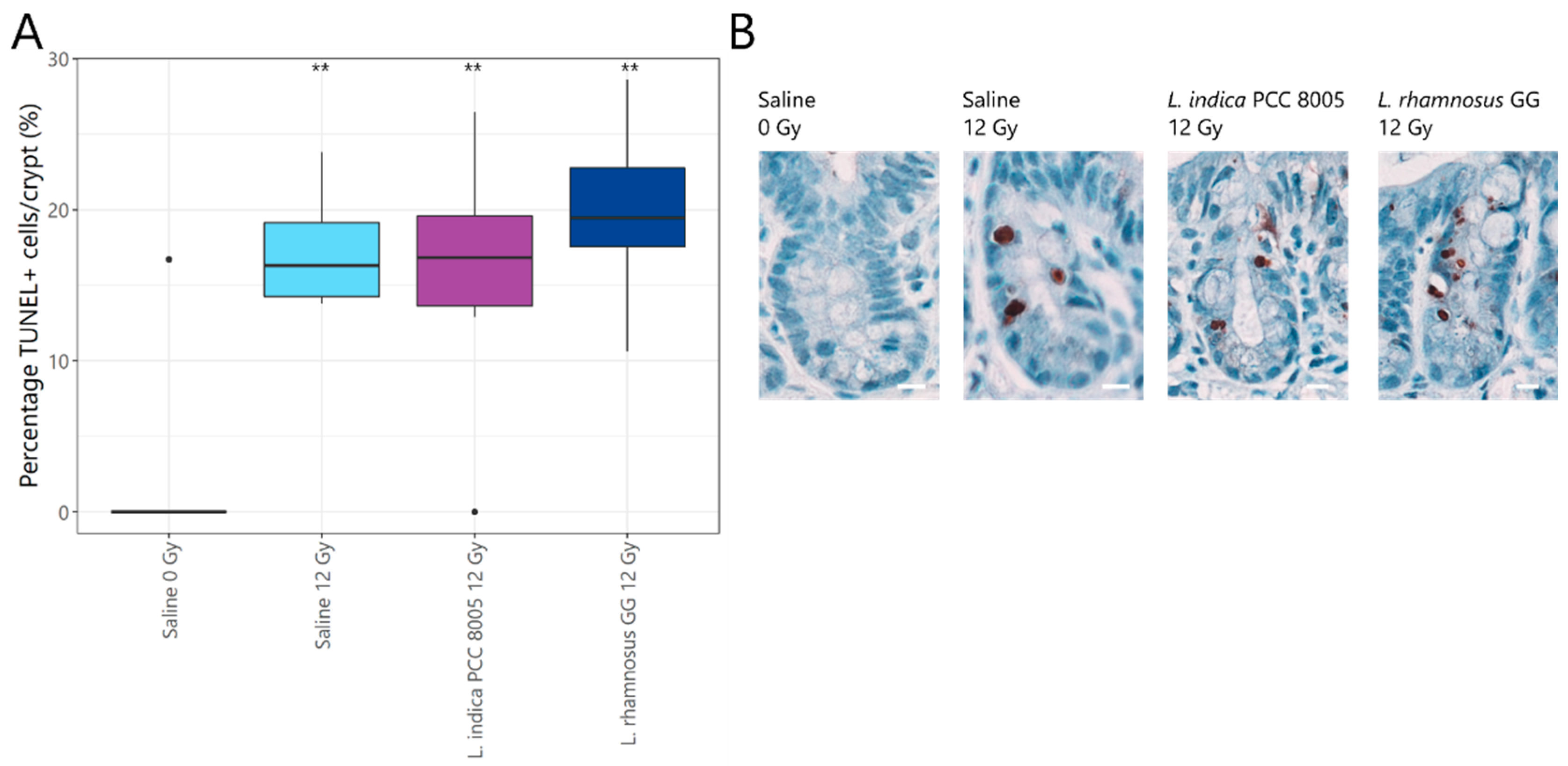
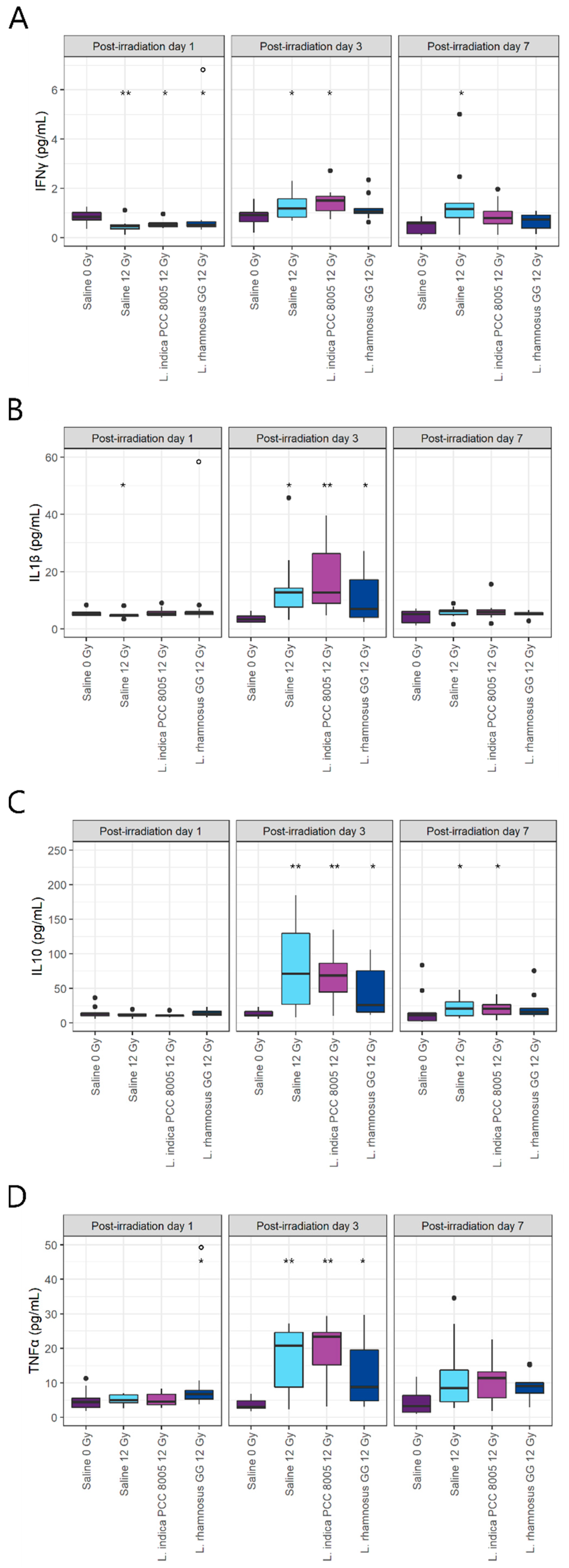
3.2. L. rhamnosus GG and L. indica PCC 8005 Supplementation Are Not Able to Prevent Acute Ileal Inflammation Induced by Pelvic Irradiation
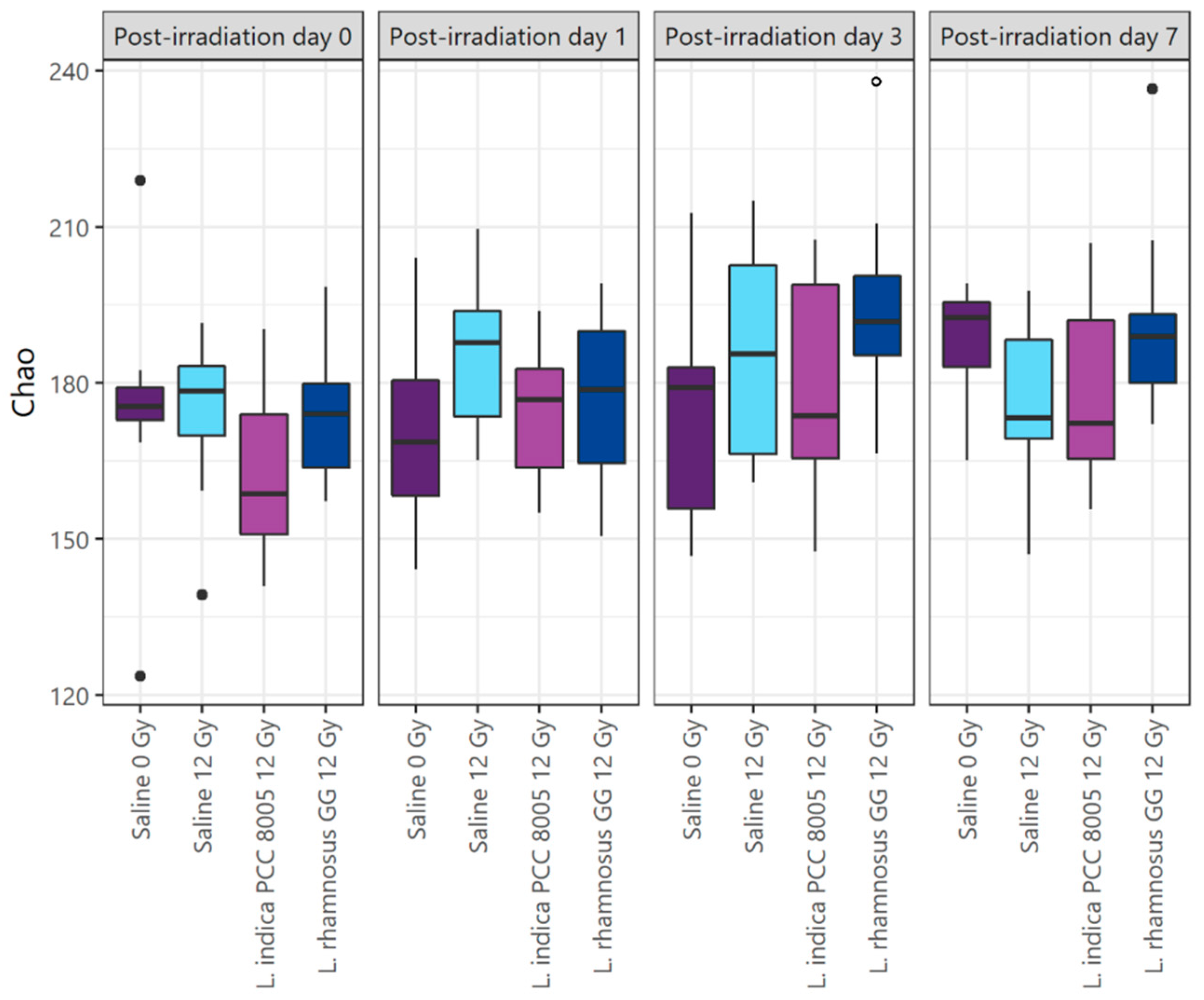
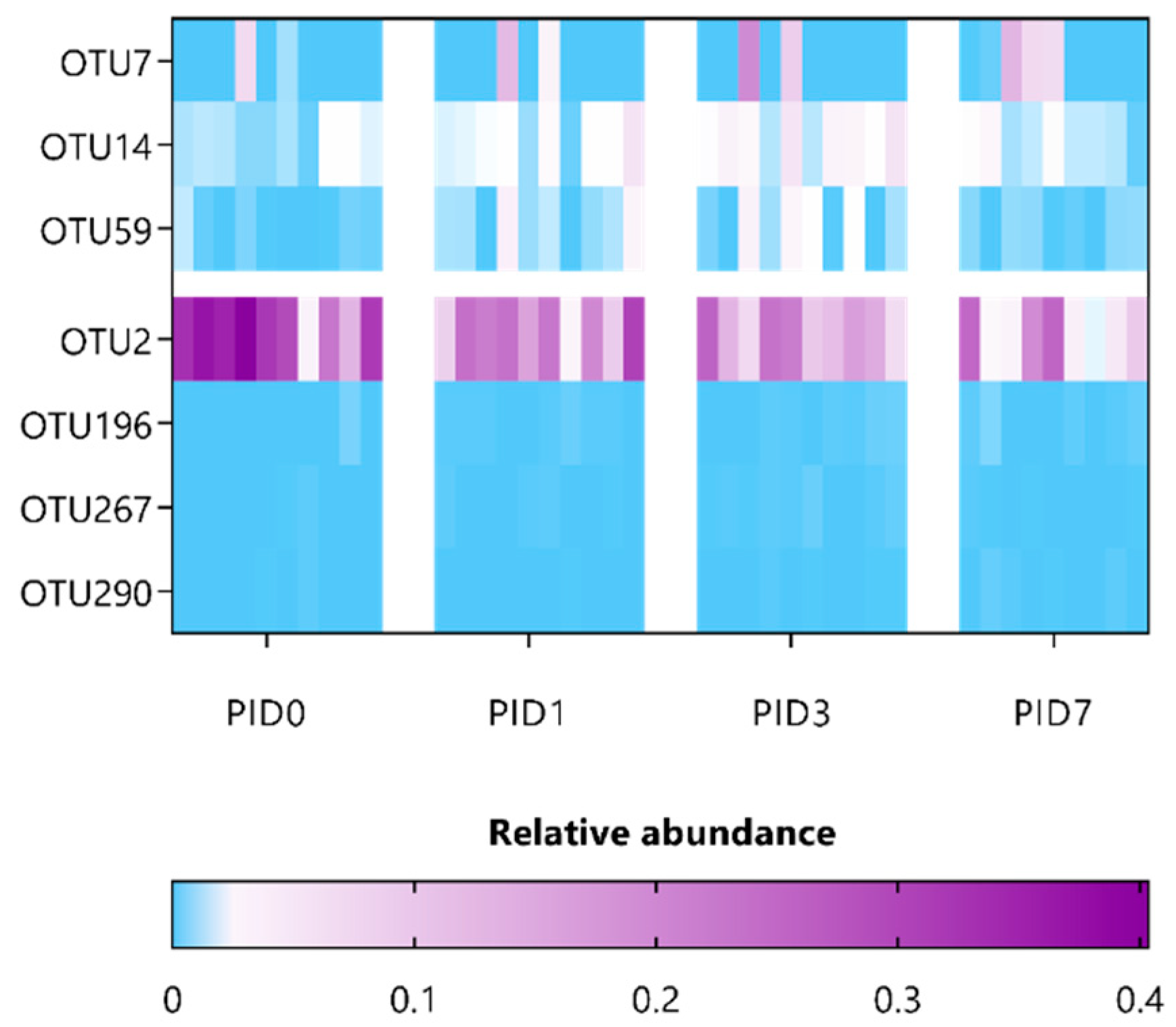
3.3. L. indica PCC 8005 and L. rhamnosus GG Prevent Pelvic Irradiation-Induced Dysbiosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J.N. Radiation Enteropathy--Pathogenesis, Treatment and Prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Segers, C.; Verslegers, M.; Baatout, S.; Leys, N.; Lebeer, S.; Mastroleo, F. Food Supplements to Mitigate Detrimental Effects of Pelvic Radiotherapy. Microorganisms 2019, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Segers, C.; Mysara, M.; Claesen, J.; Baatout, S.; Leys, N.; Lebeer, S.; Verslegers, M.; Mastroleo, F. Intestinal Mucositis Precedes Dysbiosis in a Mouse Model for Pelvic Irradiation. ISME Commun. 2021, 1, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome Definition Re-Visited: Old Concepts and New Challenges. Microbiome 2020, 8, 1–22. [Google Scholar]
- Gerassy-Vainberg, S.; Blatt, A.; Danin-Poleg, Y.; Gershovich, K.; Sabo, E.; Nevelsky, A.; Daniel, S.; Dahan, A.; Ziv, O.; Dheer, R.; et al. Radiation Induces Proinflammatory Dysbiosis: Transmission of Inflammatory Susceptibility by Host Cytokine Induction. Gut 2018, 67, 97–107. [Google Scholar] [CrossRef]
- Ciorba, M.A.; Riehl, T.E.; Rao, M.S.; Moon, C.; Ee, X.; Nava, G.M.; Walker, M.R.; Marinshaw, J.M.; Stappenbeck, T.S.; Stenson, W.F. Lactobacillus Probiotic Protects Intestinal Epithelium from Radiation Injury in a TLR-2/Cyclo-Oxygenase-2-Dependent Manner. Gut 2012, 61, 829–838. [Google Scholar] [CrossRef]
- Riehl, T.E.; Alvarado, D.; Ee, X.; Zuckerman, A.; Foster, L.; Kapoor, V.; Thotala, D.; Ciorba, M.A.; Stenson, W.F. Lactobacillus Rhamnosus GG Protects the Intestinal Epithelium from Radiation Injury through Release of Lipoteichoic Acid, Macrophage Activation and the Migration of Mesenchymal Stem Cells. Gut 2019, 68, 1003–1013. [Google Scholar] [CrossRef]
- Baù, M.; Moretti, A.; Bertoni, E.; Vazzoler, V.; Luini, C.; Agosti, M.; Salvatore, S. Risk and Protective Factors for Gastrointestinal Symptoms Associated with Antibiotic Treatment in Children: A Population Study. Pediatr. Gastroenterol. Hepatol. Nutr. 2020, 23, 35–48. [Google Scholar] [CrossRef]
- Esposito, C.; Roberti, A.; Turrà, F.; Cerulo, M.; Severino, G.; Settimi, A.; Escolino, M. Frequency of Antibiotic-Associated Diarrhea and Related Complications in Pediatric Patients Who Underwent Hypospadias Repair: A Comparative Study Using Probiotics vs Placebo. Probiotics Antimicrob. Proteins 2018, 10, 323–328. [Google Scholar] [CrossRef]
- Gamallat, Y.; Meyiah, A.; Kuugbee, E.D.; Hago, A.M.; Chiwala, G.; Awadasseid, A.; Bamba, D.; Zhang, X.; Shang, X.; Luo, F.; et al. Lactobacillus Rhamnosus Induced Epithelial Cell Apoptosis, Ameliorates Inflammation and Prevents Colon Cancer Development in an Animal Model. Biomed. Pharmacother. 2016, 83, 536–541. [Google Scholar] [CrossRef]
- Escamilla, J.; Lane, M.A.; Maitin, V. Cell-Free Supernatants from Probiotic Lactobacillus Casei and Lactobacillus Rhamnosus GG Decrease Colon Cancer Cell Invasion in Vitro. Nutr. Cancer 2012, 64, 871–878. [Google Scholar] [CrossRef]
- Yoda, K.; Miyazawa, K.; Hosoda, M.; Hiramatsu, M.; Yan, F.; He, F. Lactobacillus GG-Fermented Milk Prevents DSS-Induced Colitis and Regulates Intestinal Epithelial Homeostasis through Activation of Epidermal Growth Factor Receptor. Eur. J. Nutr. 2014, 53, 105–115. [Google Scholar] [CrossRef]
- Claes, I.J.J.; Lebeer, S.; Shen, C.; Verhoeven, T.L.A.; Dilissen, E.; De Hertogh, G.; Bullens, D.M.A.; Ceuppens, J.L.; Van Assche, G.; Vermeire, S.; et al. Impact of Lipoteichoic Acid Modification on the Performance of the Probiotic Lactobacillus Rhamnosus GG in Experimental Colitis. Clin. Exp. Immunol. 2010, 162, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Segers, C.; Mysara, M.; Coolkens, A.; Baatout, S.; Leys, N.; Lebeer, S.; Verslegers, M.; Mastroleo, F. Limnospira Indica PCC 8005 or Lacticaseibacillus Rhamnosus GG Dietary Supplementation Modulate the Gut Microbiome in Mice. Appl. Microbiol. 2022, 2, 636–650. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Farouk, S.M.; Madkour, F.F.; Azab, S.S. Anti-Inflammatory and Immunomodulatory Effects of Spirulina Platensis in Comparison to Dunaliella Salina in Acetic Acid-Induced Rat Experimental Colitis. Immunopharmacol. Immunotoxicol. 2015, 37, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Morsy, M.A.; Gupta, S.; Nair, A.B.; Venugopala, K.N.; Greish, K.; El-Daly, M. Protective Effect of Spirulina Platensis Extract against Dextran-Sulfate-Sodium-Induced Ulcerative Colitis in Rats. Nutrients 2019, 11, 2309. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Y.; Chen, X.; Xiong, W.; Tang, Y.; Lin, L. Spirulina Platensis Alleviates Chronic Inflammation with Modulation of Gut Microbiota and Intestinal Permeability in Rats Fed a High-Fat Diet. J. Cell. Mol. Med. 2020, 24, 8603–8613. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Li, Y.; Pakpour, S.; Wang, S.; Pan, Z.; Liu, J.; Wei, Q.; She, J.; Cang, H.; Zhang, R.X. Dose Effects of Orally Administered Spirulina Suspension on Colonic Microbiota in Healthy Mice. Front. Cell. Infect. Microbiol. 2019, 9, 1–13. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and Antifungal Activities of Selected Microalgae and Cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Alwaleed, E.A.; El-Sheekh, M.; Abdel-Daim, M.M.; Saber, H. Effects of Spirulina Platensis and Amphora Coffeaeformis as Dietary Supplements on Blood Biochemical Parameters, Intestinal Microbial Population, and Productive Performance in Broiler Chickens. Environ. Sci. Pollut. Res. 2020, 28, 1801–1811. [Google Scholar] [CrossRef]
- Aziz, M.M.; Eid, N.I.; Nada, A.S.; Amin, N.E.D.; Ain-Shoka, A.A. Possible Protective Effect of the Algae Spirulina against Nephrotoxicity Induced by Cyclosporine A and/or Gamma Radiation in Rats. Environ. Sci. Pollut. Res. 2018, 25, 9060–9070. [Google Scholar] [CrossRef]
- Qishen, P.; Guo, B.J.; Kolman, A. Radioprotective Effect of Extract from Spirulina Platensis in Mouse Bone Marrow Cells Studied by Using the Micronucleus Test. Toxicol. Lett. 1989, 48, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lu, L.; Liu, B.; Qin, S. Effects of Phycocyanin on Pulmonary and Gut Microbiota in a Radiation-Induced Pulmonary Fibrosis Model. Biomed. Pharmacother. 2020, 132, 110826. [Google Scholar] [CrossRef]
- Williams, J.P.; Brown, S.L.; Georges, G.E.; Hauer-Jensen, M.; Hill, R.P.; Huser, A.K.; Kirsch, D.G.; Macvittie, T.J.; Mason, K.A.; Medhora, M.M.; et al. Animal Models for Medical Countermeasures to Radiation Exposure. Radiat. Res. 2010, 173, 557–578. [Google Scholar] [CrossRef] [PubMed]
- Cogne, G.; Lehmann, B.; Dussap, C.-G.; Gros, J.-B. Uptake of Macrominerals and Trace Elements by the Cyanobacterium Spirulina Platensis (Arthrospira Platensis PCC 8005) under Photoautotrophic Conditions: Culture Medium Optimization. Biotechnol. Bioeng. 2003, 81, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Doron, S.; Snydman, D.R.; Gorbach, S.L. Lactobacillus GG: Bacteriology and Clinical Applications. Gastroenterol. Clin. North Am. 2005, 34, 483–498. [Google Scholar] [CrossRef]
- Gorbach, S.L. The Discovery of Lactobacillus GG. Nutr. Today 1996, 31, 2S–4S. [Google Scholar] [CrossRef]
- de Man, J.C.; Rogosa, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Gamallat, Y.; Ren, X.; Walana, W.; Meyiah, A.; Xinxiu, R.; Zhu, Y.; Li, M.; Song, S.; Xie, L.; Jamalat, Y.; et al. Probiotic Lactobacillus Rhamnosus Modulates the Gut Microbiome Composition Attenuates Preneoplastic Colorectal Aberrant Crypt Foci. J. Funct. Foods 2019, 53, 146–156. [Google Scholar] [CrossRef]
- Ki, Y.; Kim, W.; Cho, H.; Ahn, K.; Choi, Y.; Kim, D. The Effect of Probiotics for Preventing Radiation-Induced Morphological Changes in Intestinal Mucosa of Rats. J. Korean Med. Sci. 2014, 29, 1372–1378. [Google Scholar] [CrossRef]
- Li, X.; Hu, D.; Tian, Y.; Song, Y.; Hou, Y.; Sun, L.; Zhang, Y.; Man, C.; Zhang, W.; Jiang, Y. Protective Effects of a Novel Lactobacillus Rhamnosus Strain with Probiotic Characteristics against Lipopolysaccharide-Induced Intestinal Inflammation in Vitro and in Vivo. Food Funct. 2020, 11, 5799–5814. [Google Scholar] [CrossRef]
- Mittal, A.; Suresh Kumar, P.V.; Banerjee, S.; Rao, A.R.; Kumar, A. Modulatory Potential of Spirulina Fusiformis on Carcinogen Metabolizing Enzymes in Swiss Albino Mice. Phyther. Res. 1999, 13, 111–114. [Google Scholar] [CrossRef]
- Chamorro-Cevallos, G.; Garduño-Siciliano, L.; Barrón, B.L.; Madrigal-Bujaidar, E.; Cruz-Vega, D.E.; Pages, N. Chemoprotective Effect of Spirulina (Arthrospira) against Cyclophosphamide-Induced Mutagenicity in Mice. Food Chem. Toxicol. 2008, 46, 567–574. [Google Scholar] [CrossRef]
- El Bialy, B.E.; El-Boraey, N.G.; Hamouda, R.A.; Abdel-Daim, M.M. Comparative Protective Effects of Spirulina and Spirulina Supplemented with Thiamine against Sub-Acute Carbon Tetrachloride Toxicity in Rats. Biomed. Pharmacol. J. 2019, 12, 511–525. [Google Scholar] [CrossRef]
- Sharma, M.K.; Sharma, A.; Kumar, A.; Kumar, M. Spirulina Fusiformis Provides Protection against Mercuric Chloride Induced Oxidative Stress in Swiss Albino Mice. Food Chem. Toxicol. 2007, 45, 2412–2419. [Google Scholar] [CrossRef]
- Gutiérrez-Rebolledo, G.A.; Galar-Martínez, M.; García-Rodríguez, R.V.; Chamorro-Cevallos, G.A.; Hernández-Reyes, A.G.; Martínez-Galero, E. Antioxidant Effect of Spirulina (Arthrospira) Maxima on Chronic Inflammation Induced by Freund’s Complete Adjuvant in Rats. J. Med. Food 2015, 18, 865–871. [Google Scholar] [CrossRef]
- Hornung, B.V.H.; Zwittink, R.D.; Kuijper, E.J. Issues and Current Standards of Controls in Microbiome Research. FEMS Microbiol. Ecol. 2019, 95, 1–7. [Google Scholar] [CrossRef]
- Chao, A.; Jost, L. Coverage-Based Rarefaction and Extrapolation: Standardizing Samples by Completeness Rather than Size. Ecology 2012, 93, 2533–2547. [Google Scholar] [CrossRef]
- Edgar, R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Banerjee, S.; Fu, Q.; Shah, S.K.; Melnyk, S.B.; Sterneck, E.; Hauer-Jensen, M.; Pawar, S.A. C/EBPδ Protects from Radiation-Induced Intestinal Injury and Sepsis by Suppression of Inflammatory and Nitrosative Stress. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Mohammady, E.Y.; Soaudy, M.R.; Sabae, S.A.; Mahmoud, A.M.A.; El-Haroun, E.R. Comparative Study on the Effect of Dietary β-Carotene and Phycocyanin Extracted from Spirulina Platensis on Immune-Oxidative Stress Biomarkers, Genes Expression and Intestinal Enzymes, Serum Biochemical in Nile Tilapia, Oreochromis Niloticus. Fish Shellfish Immunol. 2021, 108, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, W.; Sun, C.; Kang, S.; Qin, S. Phycocyanin Ameliorates Radiation-Induced Acute Intestinal Toxicity by Regulating the Effect of the Gut Microbiota on the TLR4 / Myd88/NF- κ B Pathway. J. Parenter. Enter. Nutr. 2019, 44, 1308–1317. [Google Scholar] [CrossRef]
- Han, X.; Lee, A.; Huang, S.; Gao, J.; Spence, J.R.; Owyang, C. Lactobacillus Rhamnosus GG Prevents Epithelial Barrier Dysfunction Induced by Interferon-Gamma and Fecal Supernatants from Irritable Bowel Syndrome Patients in Human Intestinal Enteroids and Colonoids. Gut Microbes 2019, 10, 59–76. [Google Scholar] [CrossRef]
- de Oliveira Garcia, F.A.; Sales-Campos, H.; Yuen, V.G.; Machado, J.R.; de Barros Viana, G.S.; Oliveira, C.J.F.; McNeill, J.H. Arthrospira (Spirulina) Platensis Attenuates Dextran Sulfate Sodium-Induced Colitis in Mice by Suppressing Key Pro-Inflammatory Cytokines. Korean J. Gastroenterol. 2020, 76, 150–158. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus Alistipes: Gut Bacteria With Emerging Implications to Inflammation, Cancer, and Mental Health. Front. Immunol. 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Lebeer, S.; Claes, I.J.J.; Verhoeven, T.L.A.; Shen, C.; Lambrichts, I.; Ceuppens, J.L.; Vanderleyden, J.; De Keersmaecker, S.C.J. Impact of LuxS and Suppressor Mutations on the Gastrointestinal Transit of Lactobacillus Rhamnosus GG. Appl. Environ. Microbiol. 2008, 74, 4711–4718. [Google Scholar] [CrossRef]
- Alander, M.; Satokari, R.; Korpela, R.; Saxelin, M.; Vilpponen-Salmela, T.; Mattila-Sandholm, T.; Von Wright, A. Persistence of Colonization of Human Colonic Mucosa by a Probiotic Strain, Lactobacillus Rhamnosus GG, after Oral Consumption. VTT Publ. 2001, 65, 79–82. [Google Scholar] [CrossRef]
- Chandrarathna, H.P.S.U.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S. Marine Microaglae, Spirulina Maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef]
- Lebeer, S.; Vanderleyden, J.; De Keersmaecker, S.C.J. Genes and Molecules of Lactobacilli Supporting Probiotic Action. Microbiol. Mol. Biol. Rev. 2008, 72, 728–764. [Google Scholar] [CrossRef]
- Ma, H.; Xiong, H.; Zhu, X.; Ji, C.; Xue, J.; Li, R.; Ge, B.; Cui, H. Polysaccharide from Spirulina Platensis Ameliorates Diphenoxylate-Induced Constipation Symptoms in Mice. Int. J. Biol. Macromol. 2019, 133, 1090–1101. [Google Scholar] [CrossRef]
- Cirstea, M.; Radisavljevic, N.; Finlay, B.B. Good Bug, Bad Bug: Breaking through Microbial Stereotypes. Cell Host Microbe 2018, 23, 10–13. [Google Scholar] [CrossRef] [PubMed]


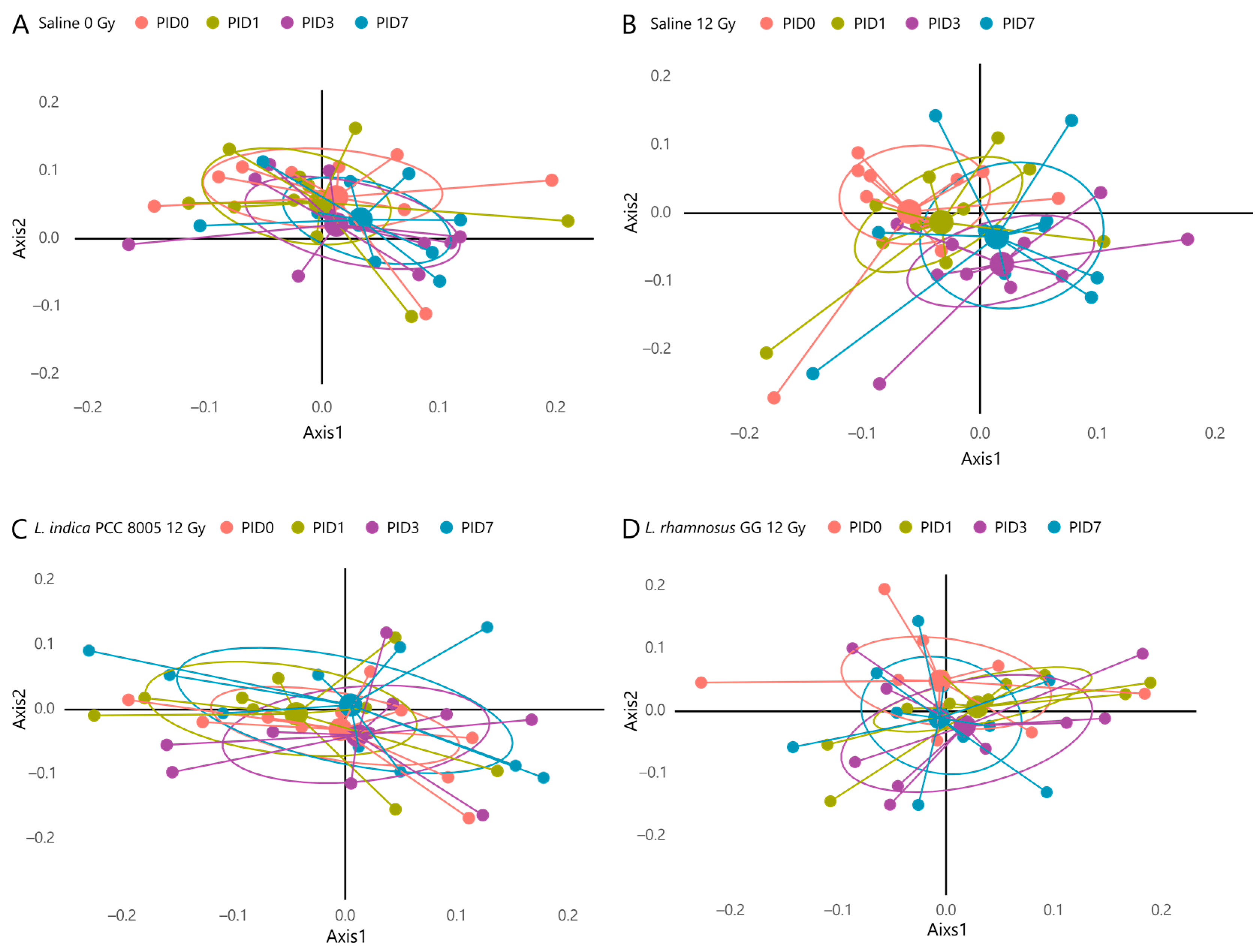

| Unweighted UniFrac Beta Diversity | |
|---|---|
| Saline 0 Gy | Overall p value: 0.453 |
| Saline 12 Gy | Overall p value: 0.006 ** Post-hoc p values:
|
| L. indica PCC 8005 12 Gy | Overall p value: 0.124 |
| L. rhamnosus GG 12 Gy | Overall p value: 0.422 |
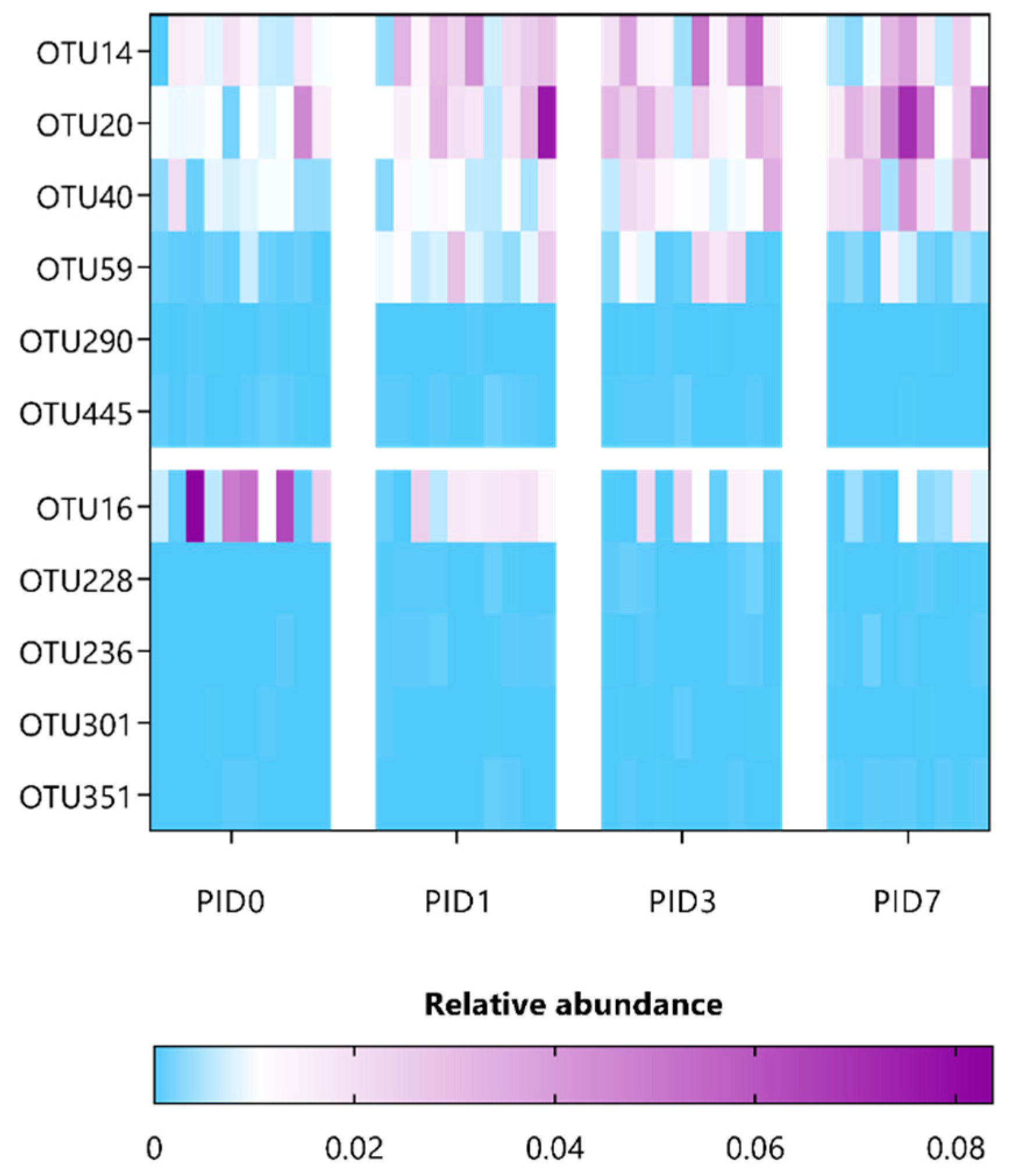
| Taxonomic Classification (Following Ribosomal Database Project) | ANCOM Biomarkers’ Effect Size and W-Statistic | Highest NCBI Blast Hit (% Identity) |
|---|---|---|
| Akkermansia_OTU7 | 1.03; W = 0.9 (PID7) | Akkermansia muciniphila (~100% identity) |
| Bacteroidales_OTU14 | 1.58; W = 0.9 (PID3) | |
| Ruminococcus_OTU59 | 1.57; W= 0.7 (PID3) | |
| Porphyromonadaceae_OTU2 | −1.21; W = 0.9 (PID7) | |
| Ruminococcaceae_OTU196 | −1.21; W = 0.9 (PID1) | |
| Ruminococcaceae_OTU267 | −1.10; W = 0.9 (PID3) | |
| Ruminococcaceae_OTU290 | −1.16; W = 0.9 (PID7) |
| Taxonomic Classification (Following Ribosomal Database Project) | ANCOM Biomarkers’ Effect Size and W-Statistic | Highest NCBI Blast Hit (% Identity) |
|---|---|---|
| Bacteroidales_OTU14 | 1.14; W = 0.8 (PID3) | |
| Porphyromonadaceae_OTU20 | 1.27; W = 0.9 (PID7) | |
| Porphyromonadaceae_OTU40 | 1.41; W = 0.7 (PID7) | |
| Ruminococcus_OTU59 | 2.15; W = 0.8 (PID1); 1.43; W = 0.8 (PID3) | |
| Ruminococcaceae_OTU290 | 1.01; W = 0.9 (PID1) | |
| Lachnospiraceae_OTU445 | 1.09; W= 0.9 (PID7) | Muricomes intestine (>97% identity) |
| Lachnospiraceae_OTU16 | −1.31; W = 0.9 (PID7) | |
| Firmicutes_OTU228 | −1.06; W = 0.9 (PID1); −1.17; W = 0.9 (PID7) | |
| Firmicutes_OTU236 | −1.20; W = 0.9 (PID1) | |
| Lachnospiraceae_OTU301 | −1.07; W = 0.9 (PID3) | |
| Lachnospiraceae_OTU351 | −1.17; W = 0.9 (PID7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segers, C.; Mysara, M.; Coolkens, A.; Wouters, S.; Baatout, S.; Leys, N.; Lebeer, S.; Verslegers, M.; Mastroleo, F. Limnospira indica PCC 8005 Supplementation Prevents Pelvic Irradiation-Induced Dysbiosis but Not Acute Inflammation in Mice. Antioxidants 2023, 12, 572. https://doi.org/10.3390/antiox12030572
Segers C, Mysara M, Coolkens A, Wouters S, Baatout S, Leys N, Lebeer S, Verslegers M, Mastroleo F. Limnospira indica PCC 8005 Supplementation Prevents Pelvic Irradiation-Induced Dysbiosis but Not Acute Inflammation in Mice. Antioxidants. 2023; 12(3):572. https://doi.org/10.3390/antiox12030572
Chicago/Turabian StyleSegers, Charlotte, Mohamed Mysara, Amelie Coolkens, Shari Wouters, Sarah Baatout, Natalie Leys, Sarah Lebeer, Mieke Verslegers, and Felice Mastroleo. 2023. "Limnospira indica PCC 8005 Supplementation Prevents Pelvic Irradiation-Induced Dysbiosis but Not Acute Inflammation in Mice" Antioxidants 12, no. 3: 572. https://doi.org/10.3390/antiox12030572
APA StyleSegers, C., Mysara, M., Coolkens, A., Wouters, S., Baatout, S., Leys, N., Lebeer, S., Verslegers, M., & Mastroleo, F. (2023). Limnospira indica PCC 8005 Supplementation Prevents Pelvic Irradiation-Induced Dysbiosis but Not Acute Inflammation in Mice. Antioxidants, 12(3), 572. https://doi.org/10.3390/antiox12030572







