Effects of Processing on Chemical Composition of Extracts from Sour Cherry Fruits, a Neglected Functional Food
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Processing
2.3. Colorimetric CIEL*a*b Analysis
2.4. Hydroalcoholic Extraction
2.5. Solid Phase Extraction (SPE)
2.6. HPLC-DAD Analysis
2.7. DI-ESI-MS Analysis
2.8. DPPH Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Processing and Color Analyses of Only Homogenized Samples
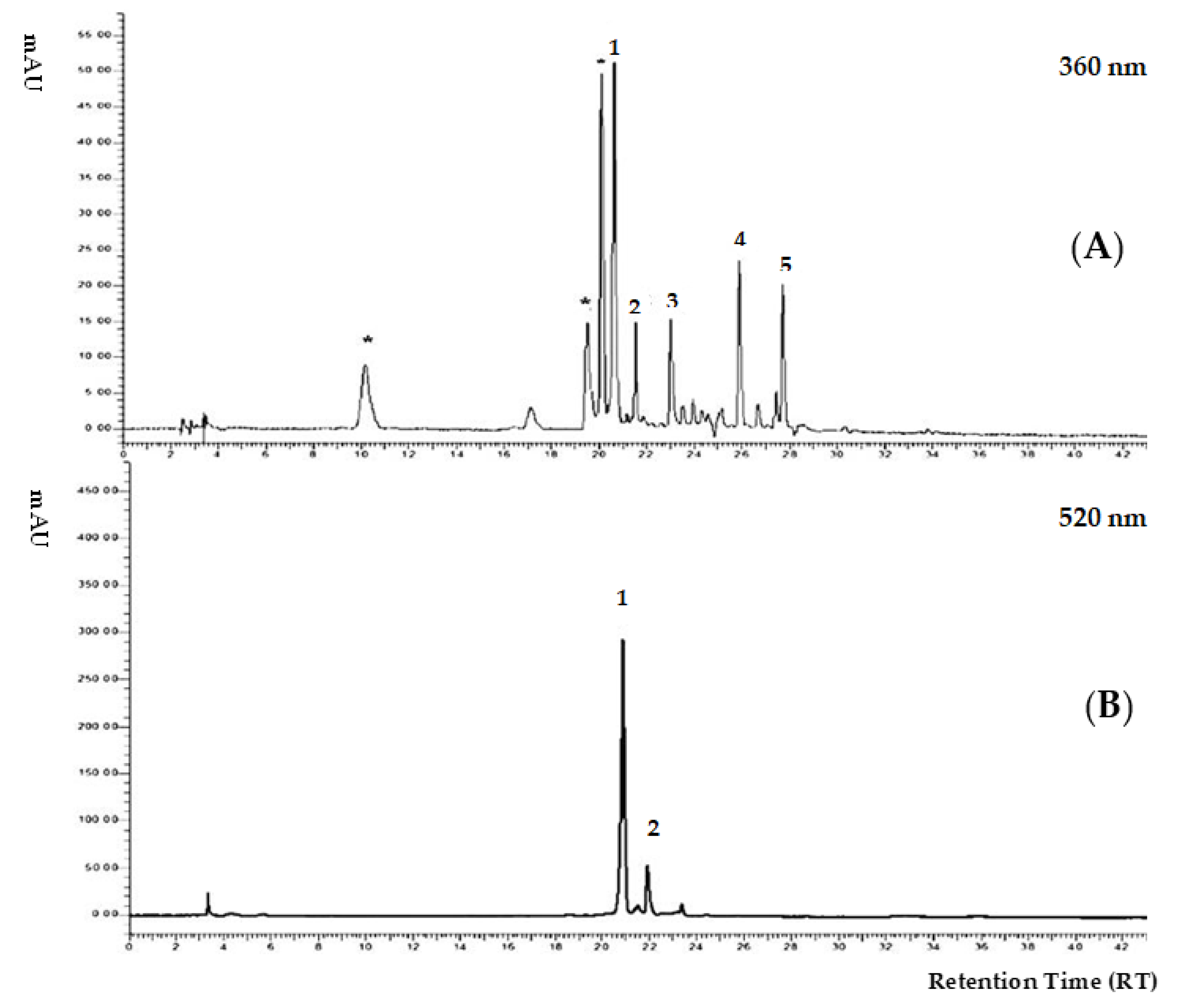
3.2. Extraction Procedure and HPLC-DAD Analysis
3.3. DI-ESI-MS and MS/MS Analyses
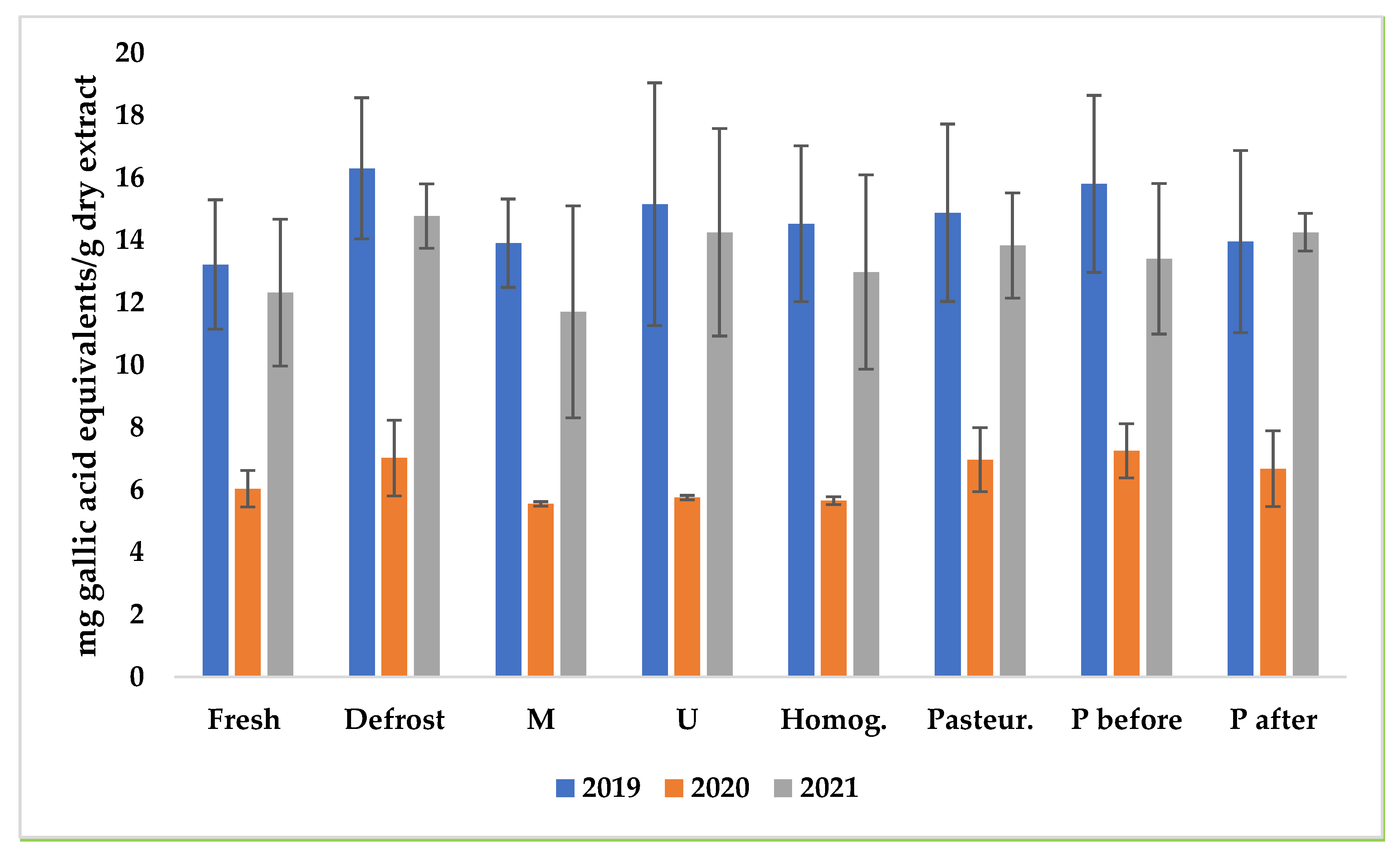
3.4. Anti-Radical Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Blando, F.; Oomah, B.D. Sweet and sour cherries: Origin, distribution, nutritional composition and health benefits. Trends Food Sci. Technol. 2019, 86, 517–529. [Google Scholar] [CrossRef]
- Webster, A.D.; Looney, N.E. Cherries: Crop Physiology, Production and Uses; CAB International: Wallingford, UK, 1996. [Google Scholar]
- Cesa, S.; Carradori, S.; Bellagamba, G.; Locatelli, M.; Casadei, M.A.; Masci, A.; Paolicelli, P. Evaluation of processing effects on anthocyanin content and colour modifications of blueberry (Vaccinium spp.) extracts: Comparison between HPLC-DAD and CIELAB analyses. Food Chem. 2017, 232, 114–123. [Google Scholar] [CrossRef]
- Garzoli, S.; Cairone, F.; Carradori, S.; Mocan, A.; Menghini, L.; Paolicelli, P.; Cesa, S. Effects of Processing on Polyphenolic and Volatile Composition and Fruit Quality of Clery Strawberries. Antioxidants 2020, 9, 632. [Google Scholar] [CrossRef] [PubMed]
- Szalóki-Dorkó, L.; Végvári, G.; Ladányi, M.; Ficzek, G.; Stéger-Máté, M. Degradation of anthocyanin content in sour cherry juice during heat treatment. Food Technol. Biotechnol. 2015, 53, 354. [Google Scholar] [CrossRef]
- Homoki, J.R.; Nemes, A.; Fazekas, E.; Gyémánt, G.; Balogh, P.; Gál, F.; Al-Asri, J.; Mortier, J.; Wolber, G.; Babinszky, L.; et al. Anthocyanin composition, antioxidant efficiency, and α-amylase inhibitor activity of different Hungarian sour cherry varieties (Prunus cerasus L.). Food Chem. 2016, 194, 222–229. [Google Scholar] [CrossRef]
- Borowy, A.; Chrzanowska, E.; Kapłan, M. Comparison of three sour cherry cultivars grown in central-eastern Poland. Acta Sci. Pol. Hortorum Cultus 2018, 17, 63–73. [Google Scholar] [CrossRef]
- Aslantas, R.; Angin, I.; Gokalp Boydas, M.; Ozkan, G.; Kara, M. Fruit characteristics and detachment parameters of sour cherry (Prunus cerasus L. cv. ‘Kütahya’) as affected by various maturity stages. Erwerbs-Obstbau 2016, 58, 127–134. [Google Scholar] [CrossRef]
- Damar, İ.; Ekşi, A. Antioxidant capacity and anthocyanin profile of sour cherry (Prunus cerasus L.) juice. Food Chem. 2012, 135, 2910–2914. [Google Scholar] [CrossRef] [PubMed]
- Chaovanalikit, A.; Wrolstad, R.E. Anthocyanin and polyphenolic composition of fresh and processed cherries. J. Food Sci. 2004, 69, FCT73. [Google Scholar] [CrossRef]
- Fischer, R.R.; Von Elbe, J.H. The Identification of Cyanidin 3-2G Glucosyl-Rutinoside in Montmorency Cherries. J. Milk Food Technol. 1970, 33, 481–483. [Google Scholar] [CrossRef]
- Seeram, N.P.; Momin, R.A.; Nair, M.G.; Bourquin, L.D. Cyclooxygenase inhibitory and antioxidant cyanidin glycosides in cherries and berries. Phytomedicine 2001, 8, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Blando, F.; Gerardi, C.; Nicoletti, I. Sour cherry (Prunus cerasus L) anthocyanins as ingredients for functional foods. J. Biomed. Biotechnol. 2004, 2004, 253. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, H.B.; Negi, P.S. Phytochemical constituents and anthelmintic potential of Surinam cherry (Eugenia uniflora L.) at different fruit developmental stages. S. Afr. J. Bot. 2022, 145, 512–521. [Google Scholar] [CrossRef]
- Mitić, M.N.; Obradović, M.V.; Kostić, D.A.; Micić, R.J.; Paunović, D.Đ. Phenolic profile and antioxidant capacities of dried red currant from Serbia, extracted with different solvent. Food Sci. Biotechnol. 2011, 20, 1625–1631. [Google Scholar] [CrossRef]
- Dudonné, S.; Dubé, P.; Anhê, F.F.; Pilon, G.; Marette, A.; Lemire, M.; Harris, C.; Dewailly, E.; Desjardins, Y. Comprehensive analysis of phenolic compounds and abscisic acid profiles of twelve native Canadian berries. J. Food Compos. Anal. 2015, 44, 214–224. [Google Scholar] [CrossRef]
- Martin, H.; Burgess, E.J.; Smith, W.A.; McGhie, T.K.; Cooney, J.M.; Lunken, R.C.; de Guzman, E.; Trower, T.; Perry, N.B. JAK2 and AMP-kinase inhibition in vitro by food extracts, fractions and purified phytochemicals. Food Funct. 2015, 6, 304–311. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef]
- Blando, F.; Calabriso, N.; Berland, H.; Maiorano, G.; Gerardi, C.; Carluccio, M.A.; Andersen, Ø.M. Radical scavenging and anti-inflammatory activities of representative anthocyanin groupings from pigment-rich fruits and vegetables. Int. J. Mol. Sci. 2018, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Alba C, M.A.; Daya, M.; Franck, C. Tart Cherries and health: Current knowledge and need for a better understanding of the fate of phytochemicals in the human gastrointestinal tract. Crit. Rev. Food Sci. Nutr. 2019, 59, 626–638. [Google Scholar] [CrossRef]
- Official Journal of the Italian Ministry of Agriculture, Food Sovereignty and Forests, n.67 of 21 March 2022, Ordinary Supplement n. 12. Available online: https://www.politicheagricole.it/flex/cm/pages/ServeBLOB.php/L/IT/IDPagina/17979 (accessed on 29 December 2022).
- Recinella, L.; Chiavaroli, A.; Masciulli, F.; Fraschetti, C.; Filippi, A.; Cesa, S.; Cairone, F.; Gorica, E.; De Leo, M.; Braca, A.; et al. Protective effects induced by a hydroalcoholic Allium sativum extract in isolated mouse heart. Nutrients 2021, 13, 2332. [Google Scholar] [CrossRef]
- Maccelli, A.; Cesa, S.; Cairone, F.; Secci, D.; Menghini, L.; Chiavarino, B.; Fornarini, S.; Crestoni, M.E.; Locatelli, M. Metabolic profiling of different wild and cultivated Allium species based on high-resolution mass spectrometry, high-performance liquid chromatography-photodiode array detector, and color analysis. J. Mass Spectrom. 2020, 55, e4525. [Google Scholar] [CrossRef]
- Yılmaz, F.M.; Karaaslan, M.; Vardin, H. Optimization of extraction parameters on the isolation of phenolic compounds from sour cherry (Prunus cerasus L.) pomace. J. Food Sci. Technol. 2015, 52, 2851–2859. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Szőllősi, E.; Stündl, L.; Biró, A.; Homoki, J.R.; Szarvas, M.M.; Balogh, P.; Cziáky, Z.; Remenyik, J. Determination of flavonoid and proanthocyanidin profile of hungarian sour cherry. Molecules 2018, 23, 3278. [Google Scholar] [CrossRef]
- Sheikh, A.A.; Wani, Z.A.; Shah, A.M.; Hassan, Q.P.; Mondhe, D.M.; Verma, M.K. Chemopreventive effects of Prunus cerasus L. against human cancer cells & ascites mice models and its phytochemical investigation by LC-Q-TOF-MS/MS. Phytomed. Plus 2022, 2, 100336. [Google Scholar]
- Cairone, F.; Petralito, S.; Scipione, L.; Cesa, S. Study on Extra Virgin Olive Oil: Quality Evaluation by Anti-Radical Activity, Color Analysis, and Polyphenolic HPLC-DAD Analysis. Foods 2021, 10, 1808. [Google Scholar] [CrossRef]
- Horuz, E.; Bozkurt, H.; Karataş, H.; Maskan, M. Effects of hybrid (microwave-convectional) and convectional drying on drying kinetics, total phenolics, antioxidant capacity, vitamin C, color and rehydration capacity of sour cherries. Food Chem. 2017, 230, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Pedisić, S.; Dragović-Uzelac, V.; Levaj, B.; Škevin, D. Effect of maturity and geographical region on anthocyanin content of sour cherries (Prunus cerasus var. marasca). Food Technol. Biotechnol. 2010, 48, 86–93. [Google Scholar]
- Woźniak, Ł.; Marszałek, K.; Skąpska, S. Extraction of phenolic compounds from sour cherry pomace with supercritical carbon dioxide: Impact of process parameters on the composition and antioxidant properties of extracts. Sep. Sci. Technol. 2016, 51, 1472–1479. [Google Scholar] [CrossRef]
- Mitić, M.N.; Obradović, M.V.; Kostić, D.A.; Micić, R.J.; Pecev, E.T. Polyphenol content and antioxidant activity of sour cherries from Serbia. Chem. Ind. Chem. Eng. Q. CICEQ 2012, 18, 53–62. [Google Scholar] [CrossRef]
- Kim, D.O.; Heo, H.J.; Kim, Y.J.; Yang, H.S.; Lee, C.Y. Sweet and sour cherry phenolics and their protective effects on neuronal cells. J. Agric. Food Chem. 2005, 53, 9921–9927. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Laskowski, P.; Oszmianski, J. Evaluation of sour cherry (Prunus cerasus L.) fruits for their polyphenol content, antioxidant properties, and nutritional components. J. Agric. Food Chem. 2014, 62, 12332–12345. [Google Scholar] [CrossRef] [PubMed]
- Obón, J.M.; Díaz-García, M.C.; Castellar, M.R. Red fruit juice quality and authenticity control by HPLC. J. Food Compos. Anal. 2011, 24, 760–771. [Google Scholar] [CrossRef]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC–MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Bonerz, D.; Würth, K.; Dietrich, H.; Will, F. Analytical characterization and the impact of ageing on anthocyanin composition and degradation in juices from five sour cherry cultivars. Eur. Food Res. Technol. 2007, 224, 355–364. [Google Scholar] [CrossRef]
- Šimunić, V.; Kovač, S.; Gašo-Sokač, D.; Pfannhauser, W.; Murkovic, M. Determination of anthocyanins in four Croatian cultivars of sour cherries (Prunus cerasus). Eur. Food Res. Technol. 2005, 220, 575–578. [Google Scholar] [CrossRef]
- Zengin, G.; Locatelli, M.; Stefanucci, A.; Macedonio, G.; Novellino, E.; Mirzaie, S.; Dvorácskó, S.; Carradori, S.; Brunetti, L.; Orlando, G.; et al. Chemical characterization, antioxidant properties, anti-inflammatory activity, and enzyme inhibition of Ipomoea batatas L. leaf extracts. Int. J. Food Prop. 2017, 20, 1907–1919. [Google Scholar] [CrossRef]
- Chiavaroli, A.; Recinella, L.; Ferrante, C.; Locatelli, M.; Macchione, N.; Zengin, G.; Leporini, L.; Leone, S.; Martinotti, S.; Brunetti, L.; et al. Crocus sativus, Serenoa repens and Pinus massoniana extracts modulate inflammatory response in isolated rat prostate challenged with LPS. J. Boil. Regul. Homeost. Agents 2017, 31, 531–541. [Google Scholar]



| Harvest Year | Type of Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | dM | U | dU | MP | dMP | UP | dUP | PM | dPM | PU | dPU | ||
| 2019 | L* | 30.99 | 31.10 | 31.53 | 34.94 | 31.09 | 31.86 | 33.38 | 32.94 | 32.02 | 30.66 | 33.34 | 31.43 |
| 2020 | 26.84 | 27.72 | 26.38 | 30.26 | 28.84 | 30.33 | 29.35 | 28.86 | 31.75 | 27.72 | 32.60 | 31.38 | |
| 2021 | 27.77 | 30.17 | 27.97 | 31.78 | 29.19 | 31.13 | 28.71 | 30.55 | 27.68 | 31.22 | 30.63 | 33.95 | |
| 2019 | a* | 14.46 | 17.07 | 16.37 | 23.18 | 15.70 | 16.58 | 17.85 | 19.89 | 16.78 | 16.51 | 18.72 | 18.78 |
| 2020 | 11.95 | 9.33 | 11.59 | 14.85 | 9.86 | 1015 | 10.22 | 10.41 | 12.79 | 10.32 | 15.63 | 17.19 | |
| 2021 | 9.70 | 15.54 | 12.73 | 18.69 | 13.58 | 17.31 | 13.07 | 16.33 | 9.74 | 18.30 | 17.92 | 22.71 | |
| 2019 | b* | 4.95 | 6.13 | 5.76 | 8.89 | 5.61 | 6.27 | 6.61 | 7.65 | 5.70 | 5.63 | 6.38 | 6.39 |
| 2020 | 2.54 | 3.08 | 2.19 | 5.59 | 3.55 | 3.48 | 3.79 | 3.36 | 4.37 | 3.24 | 5.62 | 5.97 | |
| 2021 | 3.39 | 5.59 | 4.64 | 6.81 | 4.93 | 6.53 | 4.77 | 5.94 | 3.44 | 6.75 | 6.52 | 8.51 | |
| 2019 | C*ab | 15.29 | 18.14 | 17.35 | 24.83 | 16.68 | 17.73 | 19.04 | 21.31 | 17.72 | 17.44 | 19.77 | 19.84 |
| 2020 | 12.22 | 9.83 | 11.79 | 15.86 | 10.52 | 10.73 | 10.91 | 10.94 | 13.51 | 10.81 | 16.53 | 18.20 | |
| 2021 | 10.28 | 16.51 | 13.55 | 19.89 | 14.45 | 18.50 | 13.92 | 17.37 | 10.33 | 19.50 | 19.06 | 24.25 | |
| 2019 | hab | 18.78 | 19.72 | 19.36 | 20.99 | 19.57 | 20.66 | 20.25 | 20.99 | 18.81 | 18.83 | 18.78 | 18.68 |
| 2020 | 20.94 | 18.22 | 20.31 | 20.63 | 19.83 | 18.91 | 21.12 | 17.91 | 18.83 | 17.42 | 20.09 | 19.13 | |
| 2021 | 19.24 | 19.76 | 20.14 | 20.02 | 19.90 | 20.64 | 20.12 | 20.00 | 19.43 | 20.27 | 19.09 | 20.54 | |
| Harvest Year | Type of Sample | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | dM | U | dU | MP | dMP | UP | dUP | PM | dPM | PU | dPU | ||
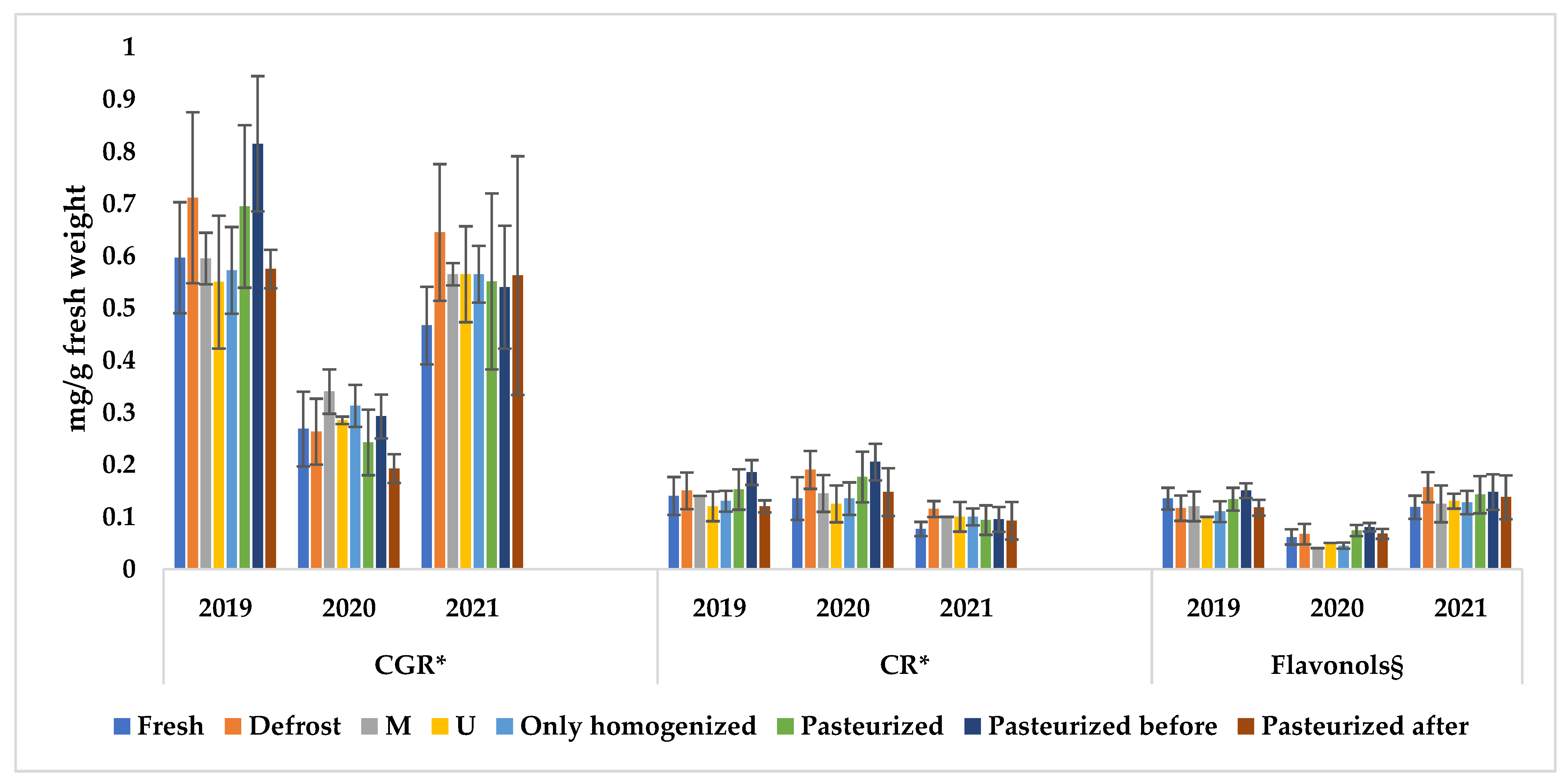
| 2019 | CGR * | 0.56 | 0.63 | 0.46 | 0.64 | 0.56 | 0.63 | 0.55 | 0.56 | 0.73 | 0.81 | 0.72 | 1.00 |
| 2020 | 0.37 | 0.31 | 0.29 | 0.28 | 0.22 | 0.21 | 0.16 | 0.18 | 0.29 | 0.35 | 0.28 | 0.25 | |
| 2021 | 0.58 | 0.55 | 0.50 | 0.63 | 0.48 | 0.52 | 0.36 | 0.89 | 0.42 | 0.62 | 0.46 | 0.66 | |
| 2019 | CR * | 0.14 | 0.14 | 0.10 | 0.14 | 0.13 | 0.13 | 0.11 | 0.11 | 0.20 | 0.17 | 0.16 | 0.21 |
| 2020 | 0.12 | 0.17 | 0.10 | 0.15 | 0.16 | 0.18 | 0.08 | 0.17 | 0.18 | 0.24 | 0.17 | 0.23 | |
| 2021 | 0.10 | 0.10 | 0.08 | 0.12 | 0.07 | 0.10 | 0.06 | 0.14 | 0.07 | 0.12 | 0.08 | 0.11 | |
| 2019 | Flavonols § | 0.14 | 0.10 | 0.10 | 0.10 | 0.13 | 0.11 | 0.13 | 0.10 | 0.15 | 0.13 | 0.16 | 0.16 |
| 2020 | 0.04 | 0.04 | 0.05 | 0.05 | 0.06 | 0.08 | 0.07 | 0.06 | 0.07 | 0.08 | 0.08 | 0.09 | |
| 2021 | 0.10 | 0.15 | 0.12 | 0.14 | 0.11 | 0.20 | 0.12 | 0.12 | 0.10 | 0.15 | 0.16 | 0.18 | |
| Mass (m/z) | Peak Area % | ||||
|---|---|---|---|---|---|
| Compound | [M + H]+ | dM | dMP | dU | dUP |
| Cyanidin-3-O-glu | 449.3 | 3 | 4 | 2 | 3 |
| CR | 595.3 | 12 | 12 | 11 | 12 |
| Peonidin-3-O-rut | 609.3 | 7 | 7 | 5 | 4 |
| Rutin | 611.2 | 5 | 5 | 4 | 5 |
| Cyanidin-3-O-(2G-xyl)-rut | 727.2 | 3 | 4 | 2 | 3 |
| Pelargonidin-3-O-(2G-glu)-rut | 741.2 | 3 | 3 | 2 | 2 |
| CGR | 757.2 | 66 | 64 | 74 | 72 |
| [M − H]− | dM | dMP | dU | dUP | |
| Coumaroylquinic acid isomer a | 337.2 | + | + | + | + |
| Harvest Year | Type of Sample | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | dM | U | dU | MP | dMP | UP | dUP | PM | dPM | PU | dPU | |
| 2019 | 12.9 | 14.9 | 12.4 | 17.9 | 15.0 | 15.4 | 9.6 | 15.8 | 14.9 | 13.8 | 14.5 | 20.0 |
| 2020 | 5.6 | 5.5 | 5.8 | 5.7 | 5.8 | 8.2 | 5.6 | 7.1 | 7.1 | 7.2 | 6.3 | 8.4 |
| 2021 | 9.3 | 14.1 | 11.9 | 16.6 | 13.7 | 14.0 | 15.1 | 14.2 | 9.9 | 14.3 | 14.0 | 15.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cairone, F.; Fraschetti, C.; Menghini, L.; Zengin, G.; Filippi, A.; Casadei, M.A.; Cesa, S. Effects of Processing on Chemical Composition of Extracts from Sour Cherry Fruits, a Neglected Functional Food. Antioxidants 2023, 12, 445. https://doi.org/10.3390/antiox12020445
Cairone F, Fraschetti C, Menghini L, Zengin G, Filippi A, Casadei MA, Cesa S. Effects of Processing on Chemical Composition of Extracts from Sour Cherry Fruits, a Neglected Functional Food. Antioxidants. 2023; 12(2):445. https://doi.org/10.3390/antiox12020445
Chicago/Turabian StyleCairone, Francesco, Caterina Fraschetti, Luigi Menghini, Gokhan Zengin, Antonello Filippi, Maria Antonietta Casadei, and Stefania Cesa. 2023. "Effects of Processing on Chemical Composition of Extracts from Sour Cherry Fruits, a Neglected Functional Food" Antioxidants 12, no. 2: 445. https://doi.org/10.3390/antiox12020445
APA StyleCairone, F., Fraschetti, C., Menghini, L., Zengin, G., Filippi, A., Casadei, M. A., & Cesa, S. (2023). Effects of Processing on Chemical Composition of Extracts from Sour Cherry Fruits, a Neglected Functional Food. Antioxidants, 12(2), 445. https://doi.org/10.3390/antiox12020445










