Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches
Abstract
1. Introduction
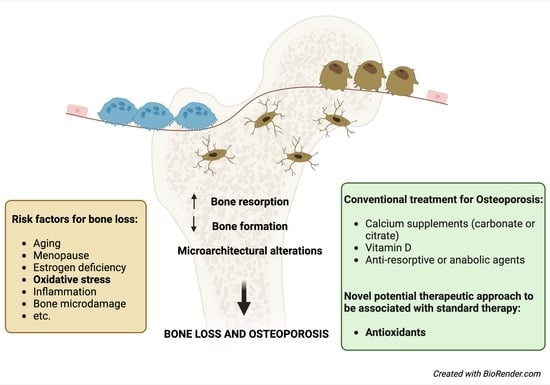
2. Oxidative Stress and Proinflammatory Factors in Bone Remodeling and Mineralization Process
2.1. Oxidative Stress in Bone Remodeling and Mineralization Process
2.2. Role of Osteocytes in Bone Remodeling in the Presence of OS and Estrogen Deficiency
2.3. Pathways and Molecular Factors Involved in Redox Regulation of Bone Remodeling
2.4. Relationship among RANKL/OPG Axis, Wnt/β-Catenin Signaling Pathway, and OS
3. Natural Antioxidants and Derivatives in Bone Remodeling, the Mineralization Process, and the Treatment and Prevention of Osteoporosis
3.1. Natural Antioxidants and Derivatives on Bone Metabolism
3.2. Polyphenols
3.3. Flavonoids
3.4. Nonflavonoid Polyphenols
3.5. Non-Polyphenolic Antioxidants
3.5.1. Carotenoids
3.5.2. Vitamins
3.5.3. Thiol Antioxidants
4. New Potential Therapeutic Approaches for Osteoporosis: Role of Antioxidants
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Huang, X.; Huang, L.; Cheng, Y.; Li, F.; Zhou, Q.; Wu, C.; Shi, Q.; Guan, Z.; Liao, J.; Hong, W. Zoledronic Acid Inhibits Osteoclast Differentiation and Function through the Regulation of NF-ΚB and JNK Signalling Pathways. Int. J. Mol. Med. 2019, 44, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Clarke, B.L.; Harris, S.T.; Hurley, D.L.; Kleerekoper, M.; Lewiecki, E.M.; Miller, P.D.; Narula, H.S.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis–2016. Endocr. Pract. 2016, 22, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Clynes, M.A.; Harvey, N.C.; Curtis, E.M.; Fuggle, N.R.; Dennison, E.M.; Cooper, C. The Epidemiology of Osteoporosis. Br. Med. Bull. 2020, 133, ldaa005. [Google Scholar] [CrossRef] [PubMed]
- Kimball, J.S.; Johnson, J.P.; Carlson, D.A. Oxidative Stress and Osteoporosis. J. Bone Jt. Surg. 2021, 103, 1451–1461. [Google Scholar] [CrossRef]
- Savasky, B.M.; Mascotti, D.P.; Patel, N.; Rodriguez-Collazo, E. Nutritional and Pharmacological Effects on Oxidative Stress in Soft Tissue and Bone Remodeling. J. Nutr. Metab. 2018, 2018, 4183407. [Google Scholar] [CrossRef]
- Ohyama, Y.; Ito, J.; Kitano, V.J.; Shimada, J.; Hakeda, Y. The Polymethoxy Flavonoid Sudachitin Suppresses Inflammatory Bone Destruction by Directly Inhibiting Osteoclastogenesis Due to Reduced ROS Production and MAPK Activation in Osteoclast Precursors. PLoS ONE 2018, 13, e0191192. [Google Scholar] [CrossRef]
- Domazetovic, V. Oxidative Stress in Bone Remodeling: Role of Antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef]
- Callaway, D.A.; Jiang, J.X. Reactive Oxygen Species and Oxidative Stress in Osteoclastogenesis, Skeletal Aging and Bone Diseases. J. Bone Miner. Metab. 2015, 33, 359–370. [Google Scholar] [CrossRef]
- Mohamad, N.-V.; Ima-Nirwana, S.; Chin, K.-Y. Are Oxidative Stress and Inflammation Mediators of Bone Loss Due to Estrogen Deficiency? A Review of Current Evidence. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 1478–1487. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox Homeostasis: The Golden Mean of Healthy Living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking Aging to Chronic Disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- Feng, Y.-L.; Tang, X.-L. Effect of Glucocorticoid-Induced Oxidative Stress on the Expression of Cbfa1. Chem. Biol. Interact. 2014, 207, 26–31. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Biswas, S.K.; Rahman, I. Environmental Toxicity, Redox Signaling and Lung Inflammation: The Role of Glutathione. Mol. Asp. Med. 2009, 30, 60–76. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Jones, D.P. [11] Redox Potential of GSH/GSSG Couple: Assay and Biological Significance. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2002; Volume 348, pp. 93–112. ISBN 978-0-12-182251-4. [Google Scholar]
- Romagnoli, C.; Marcucci, G.; Favilli, F.; Zonefrati, R.; Mavilia, C.; Galli, G.; Tanini, A.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Role of GSH/GSSG Redox Couple in Osteogenic Activity and Osteoclastogenic Markers of Human Osteoblast-like SaOS-2 Cells. FEBS J. 2012, 280, 867–879. [Google Scholar] [CrossRef]
- Romagnoli, C.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Role of N-Acetylcysteine and GSH Redox System on Total and Active MMP-2 in Intestinal Myofibroblasts of Crohn’s Disease Patients. Int. J. Color. Dis. 2013, 28, 915–924. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of Apoptosis Signalling Pathways by Reactive Oxygen Species. Biochim. Biophys. Acta (BBA)–Mol. Cell Res. 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Tew, K.D.; Townsend, D.M. Glutathione-S-Transferases As Determinants of Cell Survival and Death. Antioxid. Redox Signal. 2012, 17, 1728–1737. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R.; Kaser, A.; Pines, A.; Dotan, I. Gut, Inflammation and Osteoporosis: Basic and Clinical Concepts. Gut 2008, 57, 684–694. [Google Scholar] [CrossRef]
- Effendy, N.; Shuid, A. Time and Dose-Dependent Effects of Labisia Pumila on Bone Oxidative Status of Postmenopausal Osteoporosis Rat Model. Nutrients 2014, 6, 3288–3302. [Google Scholar] [CrossRef]
- Muthusami, S.; Gopalakrishnan, V.; Stanley, J.A.; Krishnamoorthy, S.; Ilangovan, R.; Gopalakrishnan, V.K.; Srinivasan, N. Cissus Quadrangularis Prevented the Ovariectomy Induced Oxidative Stress in the Femur of Adult Albino Rats. Biomed. Pharmacother. 2016, 81, 416–423. [Google Scholar] [CrossRef]
- Vural, P.; Akgül, C.; Canbaz, M. Effects of Menopause and Tibolone on Antioxidants in Postmenopausal Women. Ann. Clin. Biochem. 2005, 42, 220–223. [Google Scholar] [CrossRef]
- Bellanti, F.; Matteo, M.; Rollo, T.; De Rosario, F.; Greco, P.; Vendemiale, G.; Serviddio, G. Sex Hormones Modulate Circulating Antioxidant Enzymes: Impact of Estrogen Therapy. Redox Biol. 2013, 1, 340–346. [Google Scholar] [CrossRef]
- Cervellati, C.; Bergamini, C.M. Oxidative Damage and the Pathogenesis of Menopause Related Disturbances and Diseases. Clin. Chem. Lab. Med. (CCLM) 2016, 54, 739–753. [Google Scholar] [CrossRef]
- Wauquier, F.; Leotoing, L.; Coxam, V.; Guicheux, J.; Wittrant, Y. Oxidative Stress in Bone Remodelling and Disease. Trends Mol. Med. 2009, 15, 468–477. [Google Scholar] [CrossRef]
- Zhou, M.; Li, S.; Pathak, J.L. Pro-Inflammatory Cytokines and Osteocytes. Curr. Osteoporos. Rep. 2019, 17, 97–104. [Google Scholar] [CrossRef]
- Bonaccorsi, G.; Piva, I.; Greco, P.; Cervellati, C. Oxidative Stress as a Possible Pathogenic Cofactor of Post-Menopausal Osteoporosis: Existing Evidence in Support of the Axis Oestrogen Deficiency-Redox Imbalance-Bone Loss. Indian J. Med. Res. 2018, 147, 341. [Google Scholar] [CrossRef]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative Effects of Nutritive Polyphenols on Bone Metabolism In Vivo—Evidence from Human Studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef]
- Wang, T.; Yu, X.; He, C. Pro-Inflammatory Cytokines: Cellular and Molecular Drug Targets for Glucocorticoid-Induced-Osteoporosis via Osteocyte. CDT 2018, 20, 1–15. [Google Scholar] [CrossRef]
- Leslie, W.D.; Morin, S.N. New Developments in Fracture Risk Assessment for Current Osteoporosis Reports. Curr. Osteoporos. Rep. 2020, 18, 115–129. [Google Scholar] [CrossRef]
- Stepan, J.J.; Hruskova, H.; Kverka, M. Update on Menopausal Hormone Therapy for Fracture Prevention. Curr. Osteoporos. Rep. 2019, 17, 465–473. [Google Scholar] [CrossRef]
- Kenkre, J.; Bassett, J. The Bone Remodelling Cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Falsetti, I.; Bilia, A.R.; Vincenzini, M.T.; Brandi, M.L.; Iantomasi, T. Blueberry Juice Antioxidants Protect Osteogenic Activity against Oxidative Stress and Improve Long-Term Activation of the Mineralization Process in Human Osteoblast-Like SaOS-2 Cells: Involvement of SIRT1. Antioxidants 2020, 9, 125. [Google Scholar] [CrossRef]
- Cao, X.; Luo, D.; Li, T.; Huang, Z.; Zou, W.; Wang, L.; Lian, K.; Lin, D. MnTBAP Inhibits Bone Loss in Ovariectomized Rats by Reducing Mitochondrial Oxidative Stress in Osteoblasts. J. Bone Miner. Metab. 2020, 38, 27–37. [Google Scholar] [CrossRef]
- Agidigbi, T.S.; Kim, C. Reactive Oxygen Species in Osteoclast Differentiation and Possible Pharmaceutical Targets of ROS-Mediated Osteoclast Diseases. Int. J. Mol. Sci. 2019, 20, 3576. [Google Scholar] [CrossRef]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry Juice Protects Osteocytes and Bone Precursor Cells against Oxidative Stress Partly through SIRT 1. FEBS Open Bio. 2019, 9, 1082–1096. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Ru, J.; Wang, Y. Osteocyte Apoptosis: The Roles and Key Molecular Mechanisms in Resorption-Related Bone Diseases. Cell Death Dis. 2020, 11, 846. [Google Scholar] [CrossRef]
- Parvaneh, K.; Ebrahimi, M.; Sabran, M.R.; Karimi, G.; Hwei, A.N.M.; Abdul-Majeed, S.; Ahmad, Z.; Ibrahim, Z.; Jamaluddin, R. Probiotics (Bifidobacterium Longum) Increase Bone Mass Density and Upregulate Sparc and Bmp-2 Genes in Rats with Bone Loss Resulting from Ovariectomy. BioMed. Res. Int. 2015, 2015, 897639. [Google Scholar] [CrossRef]
- Isomura, H.; Fujie, K.; Shibata, K.; Inoue, N.; Iizuka, T.; Takebe, G.; Takahashi, K.; Nishihira, J.; Izumi, H.; Sakamoto, W. Bone Metabolism and Oxidative Stress in Postmenopausal Rats with Iron Overload. Toxicology 2004, 197, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Wheater, G.; Elshahaly, M.; Tuck, S.P.; Datta, H.K.; van Laar, J.M. The Clinical Utility of Bone Marker Measurements in Osteoporosis. J. Transl. Med. 2013, 11, 201. [Google Scholar] [CrossRef] [PubMed]
- Tresguerres, F.G.F.; Torres, J.; López-Quiles, J.; Hernández, G.; Vega, J.A.; Tresguerres, I.F. The Osteocyte: A Multifunctional Cell within the Bone. Ann. Anat. Anat. Anz. 2020, 227, 151422. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F. The Amazing Osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Chen, H.; Senda, T.; Kubo, K. The Osteocyte Plays Multiple Roles in Bone Remodeling and Mineral Homeostasis. Med. Mol. Morphol. 2015, 48, 61–68. [Google Scholar] [CrossRef]
- Bellido, T. Osteocyte-Driven Bone Remodeling. Calcif. Tissue Int. 2014, 94, 25–34. [Google Scholar] [CrossRef]
- Buenzli, P.R.; Sims, N.A. Quantifying the Osteocyte Network in the Human Skeleton. Bone 2015, 75, 144–150. [Google Scholar] [CrossRef]
- Jilka, R.L.; Noble, B.; Weinstein, R.S. Osteocyte Apoptosis. Bone 2013, 54, 264–271. [Google Scholar] [CrossRef]
- Al-Dujaili, S.A.; Lau, E.; Al-Dujaili, H.; Tsang, K.; Guenther, A.; You, L. Apoptotic Osteocytes Regulate Osteoclast Precursor Recruitment and Differentiation In Vitro. J. Cell Biochem. 2011, 112, 2412–2423. [Google Scholar] [CrossRef]
- Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Glutathione, N-Acetylcysteine and Lipoic Acid Down-Regulate Starvation-Induced Apoptosis, RANKL/OPG Ratio and Sclerostin in Osteocytes: Involvement of JNK and ERK1/2 Signalling. Calcif. Tissue Int. 2015, 96, 335–346. [Google Scholar] [CrossRef]
- Werner, S.L.; Sharma, R.; Woodruff, K.; Horn, D.; Harris, S.E.; Gorin, Y.; Lee, D.; Hua, R.; Gu, S.; Fajardo, R.J.; et al. CSF-1 in Osteocytes Inhibits Nox4-mediated Oxidative Stress and Promotes Normal Bone Homeostasis. JBMR Plus 2020, 4, e10080. [Google Scholar] [CrossRef] [PubMed]
- Jilka, R.L.; O’Brien, C.A. The Role of Osteocytes in Age-Related Bone Loss. Curr. Osteoporos. Rep. 2016, 14, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Bjelaković, G.; Beninati, S.; Pavlović, D.; Kocić, G.; Jevtović, T.; Kamenov, Β.; Šaranac, L.J.; Bjelaković, B.; Stojanović, I.; Bašić, J. Glucocorticoids and Oxidative Stress. J. Basic Clin. Physiol. Pharmacol. 2007, 18, 115–128. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Fontani, F.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Estrogen Inhibits Starvation-Induced Apoptosis in Osteocytes by a Redox-Independent Process Involving Association of JNK and Glutathione S-Transferase P1-1. FEBS Open Bio 2017, 7, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Mazzaferro, S.; Cianciolo, G.; De Pascalis, A.; Guglielmo, C.; Urena Torres, P.A.; Bover, J.; Tartaglione, L.; Pasquali, M.; La Manna, G. Bone, Inflammation and the Bone Marrow Niche in Chronic Kidney Disease: What Do We Know? Nephrol. Dial. Transplant. 2018, 33, 2092–2100. [Google Scholar] [CrossRef]
- Allison, D.J.; Ditor, D.S. Immune Dysfunction and Chronic Inflammation Following Spinal Cord Injury. Spinal. Cord. 2015, 53, 14–18. [Google Scholar] [CrossRef]
- Metzger, C.E.; Narayanan, A.; Zawieja, D.C.; Bloomfield, S.A. Inflammatory Bowel Disease in a Rodent Model Alters Osteocyte Protein Levels Controlling Bone Turnover: IBD in Rodent Model Alters Osteocyte Protein Controlling Bone Turnover. J. Bone Miner. Res. 2017, 32, 802–813. [Google Scholar] [CrossRef]
- Narayanan, S.A.; Metzger, C.E.; Bloomfield, S.A.; Zawieja, D.C. Inflammation-induced Lymphatic Architecture and Bone Turnover Changes Are Ameliorated by Irisin Treatment in Chronic Inflammatory Bowel Disease. FASEB J. 2018, 32, 4848–4861. [Google Scholar] [CrossRef]
- Valacchi, G.; Pecorelli, A.; Cervellati, C.; Hayek, J. 4-Hydroxynonenal Protein Adducts: Key Mediator in Rett Syndrome Oxinflammation. Free Radic. Biol. Med. 2017, 111, 270–280. [Google Scholar] [CrossRef]
- Delgobo, M.; Agnes, J.P.; Gonçalves, R.M.; dos Santos, V.W.; Parisotto, E.B.; Zamoner, A.; Zanotto-Filho, A. N-Acetylcysteine and Alpha-Lipoic Acid Improve Antioxidant Defenses and Decrease Oxidative Stress, Inflammation and Serum Lipid Levels in Ovariectomized Rats via Estrogen-Independent Mechanisms. J. Nutr. Biochem. 2019, 67, 190–200. [Google Scholar] [CrossRef]
- Abu-Taha, M.; Rius, C.; Hermenegildo, C.; Noguera, I.; Cerda-Nicolas, J.-M.; Issekutz, A.C.; Jose, P.J.; Cortijo, J.; Morcillo, E.J.; Sanz, M.-J. Menopause and Ovariectomy Cause a Low Grade of Systemic Inflammation That May Be Prevented by Chronic Treatment with Low Doses of Estrogen or Losartan. J. Immunol. 2009, 183, 1393–1402. [Google Scholar] [CrossRef]
- Peacock, M. Phosphate Metabolism in Health and Disease. Calcif. Tissue Int. 2021, 108, 3–15. [Google Scholar] [CrossRef]
- Cohen, A. Premenopausal Osteoporosis. Endocrinol. Metab. Clin. N. A. 2017, 46, 117–133. [Google Scholar] [CrossRef]
- Kitaura, H.; Marahleh, A.; Ohori, F.; Noguchi, T.; Shen, W.-R.; Qi, J.; Nara, Y.; Pramusita, A.; Kinjo, R.; Mizoguchi, I. Osteocyte-Related Cytokines Regulate Osteoclast Formation and Bone Resorption. Int. J. Mol. Sci. 2020, 21, 5169. [Google Scholar] [CrossRef]
- Dussold, C.; Gerber, C.; White, S.; Wang, X.; Qi, L.; Francis, C.; Capella, M.; Courbon, G.; Wang, J.; Li, C.; et al. DMP1 Prevents Osteocyte Alterations, FGF23 Elevation and Left Ventricular Hypertrophy in Mice with Chronic Kidney Disease. Bone Res. 2019, 7, 12. [Google Scholar] [CrossRef]
- Zheng, S. Protective Effect of Polygonatum Sibiricum Polysaccharide on D-Galactose-Induced Aging Rats Model. Sci. Rep. 2020, 10, 2246. [Google Scholar] [CrossRef]
- Domazetovic, V.; Falsetti, I.; Ciuffi, S.; Iantomasi, T.; Marcucci, G.; Vincenzini, M.T.; Brandi, M.L. Effect of Oxidative Stress-Induced Apoptosis on Active FGF23 Levels in MLO-Y4 Cells: The Protective Role of 17-β-Estradiol. Int. J. Mol. Sci. 2022, 23, 2103. [Google Scholar] [CrossRef]
- Ryan, Z.C.; Ketha, H.; McNulty, M.S.; McGee-Lawrence, M.; Craig, T.A.; Grande, J.P.; Westendorf, J.J.; Singh, R.J.; Kumar, R. Sclerostin Alters Serum Vitamin D Metabolite and Fibroblast Growth Factor 23 Concentrations and the Urinary Excretion of Calcium. Proc. Natl. Acad. Sci. USA 2013, 110, 6199–6204. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Osteocyte Control of Bone Remodeling: Is Sclerostin a Key Molecular Coordinator of the Balanced Bone Resorption–Formation Cycles? Osteoporos Int. 2014, 25, 2685–2700. [Google Scholar] [CrossRef]
- Ramli, F.F.; Chin, K.-Y. A Review of the Potential Application of Osteocyte-Related Biomarkers, Fibroblast Growth Factor-23, Sclerostin, and Dickkopf-1 in Predicting Osteoporosis and Fractures. Diagnostics 2020, 10, 145. [Google Scholar] [CrossRef]
- Francis, C.; David, V. Inflammation Regulates Fibroblast Growth Factor 23 Production. Curr. Opin. Nephrol. Hypertens. 2016, 25, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Aguirre, J.I.; Kousteni, S.; Manolagas, S.C.; Bellido, T. Bisphosphonates and Estrogens Inhibit Osteocyte Apoptosis via Distinct Molecular Mechanisms Downstream of Extracellular Signal-Regulated Kinase Activation. J. Biol. Chem. 2005, 280, 7317–7325. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Johnson, M.L. Osteocytes, Mechanosensing and Wnt Signaling. Bone 2008, 42, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Karim, M.A.; Che, H.; Geng, Q.; Miao, D. Deletion of P16 Prevents Estrogen Deficiency-Induced Osteoporosis by Inhibiting Oxidative Stress and Osteocyte Senescence. Am. J. Transl. Res. 2020, 12, 672–683. [Google Scholar] [PubMed]
- Kalajzic, I.; Matthews, B.G.; Torreggiani, E.; Harris, M.A.; Divieti Pajevic, P.; Harris, S.E. In Vitro and in Vivo Approaches to Study Osteocyte Biology. Bone 2013, 54, 296–306. [Google Scholar] [CrossRef]
- McCutcheon, S.; Majeska, R.J.; Spray, D.C.; Schaffler, M.B.; Vazquez, M. Apoptotic Osteocytes Induce RANKL Production in Bystanders via Purinergic Signaling and Activation of Pannexin Channels. J. Bone Miner. Res. 2020, 35, 966–977. [Google Scholar] [CrossRef]
- Cheung, W.-Y.; Simmons, C.A.; You, L. Osteocyte Apoptosis Regulates Osteoclast Precursor Adhesion via Osteocytic IL-6 Secretion and Endothelial ICAM-1 Expression. Bone 2012, 50, 104–110. [Google Scholar] [CrossRef]
- He, F.; Bai, J.; Wang, J.; Zhai, J.; Tong, L.; Zhu, G. Irradiation-induced Osteocyte Damage Promotes HMGB1-mediated Osteoclastogenesis In Vitro. J. Cell. Physiol. 2019, 234, 17314–17325. [Google Scholar] [CrossRef]
- Xiong, J.; Piemontese, M.; Onal, M.; Campbell, J.; Goellner, J.J.; Dusevich, V.; Bonewald, L.; Manolagas, S.C.; O’Brien, C.A. Osteocytes, Not Osteoblasts or Lining Cells, Are the Main Source of the RANKL Required for Osteoclast Formation in Remodeling Bone. PLoS ONE 2015, 10, e0138189. [Google Scholar] [CrossRef]
- Davies, J.M.S.; Cillard, J.; Friguet, B.; Cadenas, E.; Cadet, J.; Cayce, R.; Fishmann, A.; Liao, D.; Bulteau, A.-L.; Derbré, F.; et al. The Oxygen Paradox, the French Paradox, and Age-Related Diseases. GeroScience 2017, 39, 499–550. [Google Scholar] [CrossRef]
- Nahian, A.; AlEssa, A.M. Histology, Osteocytes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Mazière, C.; Salle, V.; Gomila, C.; Mazière, J.-C. Oxidized Low Density Lipoprotein Enhanced RANKL Expression in Human Osteoblast-like Cells. Involvement of ERK, NFkappaB and NFAT. Biochim. Biophys. Acta (BBA)–Mol. Basis Dis. 2013, 1832, 1756–1764. [Google Scholar] [CrossRef]
- Compton, J.T.; Lee, F.Y. A Review of Osteocyte Function and the Emerging Importance of Sclerostin. J. Bone Jt. Surg. 2014, 96, 1659–1668. [Google Scholar] [CrossRef]
- Notsu, M.; Kanazawa, I.; Takeno, A.; Yokomoto-Umakoshi, M.; Tanaka, K.; Yamaguchi, T.; Sugimoto, T. Advanced Glycation End Product 3 (AGE3) Increases Apoptosis and the Expression of Sclerostin by Stimulating TGF-β Expression and Secretion in Osteocyte-Like MLO-Y4-A2 Cells. Calcif. Tissue Int. 2017, 100, 402–411. [Google Scholar] [CrossRef]
- Artsi, H.; Cohen-Kfir, E.; Gurt, I.; Shahar, R.; Bajayo, A.; Kalish, N.; Bellido, T.M.; Gabet, Y.; Dresner-Pollak, R. The Sirtuin1 Activator SRT3025 Down-Regulates Sclerostin and Rescues Ovariectomy-Induced Bone Loss and Biomechanical Deterioration in Female Mice. Endocrinology 2014, 155, 3508–3515. [Google Scholar] [CrossRef]
- Gurt, I.; Artsi, H.; Cohen-Kfir, E.; Hamdani, G.; Ben-Shalom, G.; Feinstein, B.; El-Haj, M.; Dresner-Pollak, R. The Sirt1 Activators SRT2183 and SRT3025 Inhibit RANKL-Induced Osteoclastogenesis in Bone Marrow-Derived Macrophages and Down-Regulate Sirt3 in Sirt1 Null Cells. PLoS ONE 2015, 10, e0134391. [Google Scholar] [CrossRef]
- Manolagas, S.C.; O’Brien, C.A.; Almeida, M. The Role of Estrogen and Androgen Receptors in Bone Health and Disease. Nat. Rev. Endocrinol. 2013, 9, 699–712. [Google Scholar] [CrossRef]
- Mödder, U.I.; Clowes, J.A.; Hoey, K.; Peterson, J.M.; McCready, L.; Oursler, M.J.; Riggs, B.L.; Khosla, S. Regulation of Circulating Sclerostin Levels by Sex Steroids in Women and in Men. J. Bone Miner. Res. 2011, 26, 27–34. [Google Scholar] [CrossRef]
- Cervellati, C.; Romani, A.; Cremonini, E.; Bergamini, C.M.; Fila, E.; Squerzanti, M.; Greco, P.; Massari, L.; Bonaccorsi, G. Higher Urinary Levels of 8-Hydroxy-2′-Deoxyguanosine Are Associated with a Worse RANKL/OPG Ratio in Postmenopausal Women with Osteopenia. Oxidative Med. Cell. Longev. 2016, 2016, 6038798. [Google Scholar] [CrossRef]
- Nicolin, V.; De Tommasi, N.; Nori, S.L.; Costantinides, F.; Berton, F.; Di Lenarda, R. Modulatory Effects of Plant Polyphenols on Bone Remodeling: A Prospective View From the Bench to Bedside. Front. Endocrinol. 2019, 10, 494. [Google Scholar] [CrossRef]
- Higgs, J.; Derbyshire, E.; Styles, K. Nutrition and Osteoporosis Prevention for the Orthopaedic Surgeon: A Wholefoods Approach. EFORT Open Rev. 2017, 2, 300–308. [Google Scholar] [CrossRef]
- Shen, C.-L.; von Bergen, V.; Chyu, M.-C.; Jenkins, M.R.; Mo, H.; Chen, C.-H.; Kwun, I.-S. Fruits and Dietary Phytochemicals in Bone Protection. Nutr. Res. 2012, 32, 897–910. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Sandhu, A.; Edirisinghe, I.; Burton-Freeman, B. Characterization of Wild Blueberry Polyphenols Bioavailability and Kinetic Profile in Plasma over 24-h Period in Human Subjects. Mol. Nutr. Food Res. 2017, 61, 1700405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lazarenko, O.P.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J.J.; Chen, J.-R. Feeding Blueberry Diets in Early Life Prevent Senescence of Osteoblasts and Bone Loss in Ovariectomized Adult Female Rats. PLoS ONE 2011, 6, e24486. [Google Scholar] [CrossRef] [PubMed]
- Nash, L.A.; Sullivan, P.J.; Peters, S.J.; Ward, W.E. Rooibos Flavonoids, Orientin and Luteolin, Stimulate Mineralization in Human Osteoblasts through the Wnt Pathway. Mol. Nutr. Food Res. 2015, 59, 443–453. [Google Scholar] [CrossRef]
- Weaver, C.M.; Alekel, D.L.; Ward, W.E.; Ronis, M.J. Flavonoid Intake and Bone Health. J. Nutr. Gerontol. Geriatr. 2012, 31, 239–253. [Google Scholar] [CrossRef]
- Li, T.; Wu, S.-M.; Xu, Z.-Y.; Ou-Yang, S. Rabbiteye Blueberry Prevents Osteoporosis in Ovariectomized Rats. J. Orthop. Surg. Res. 2014, 9, 56. [Google Scholar] [CrossRef]
- Islam, M.A.; Alam, F.; Solayman, M.; Khalil, M.I.; Kamal, M.A.; Gan, S.H. Dietary Phytochemicals: Natural Swords Combating Inflammation and Oxidation-Mediated Degenerative Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 5137431. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and Biological Activities of Natural Polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Yagi, H.; Tan, J.; Tuan, R.S. Polyphenols Suppress Hydrogen Peroxide-Induced Oxidative Stress in Human Bone-Marrow Derived Mesenchymal Stem Cells. J. Cell Biochem. 2013, 114, 1163–1173. [Google Scholar] [CrossRef]
- Chen, J.-R.; Lazarenko, O.P.; Wu, X.; Kang, J.; Blackburn, M.L.; Shankar, K.; Badger, T.M.; Ronis, M.J. Dietary-Induced Serum Phenolic Acids Promote Bone Growth via P38 MAPK/β-Catenin Canonical Wnt Signaling: Effects of Blueberry Diet on Bone. J. Bone Miner. Res. 2010, 25, 2399–2411. [Google Scholar] [CrossRef]
- Novotny, R.; Daida, Y.G.; Grove, J.S.; Acharya, S.; Vogt, T.M.; Paperny, D. Adolescent Dairy Consumption and Physical Activity Associated with Bone Mass. Prev. Med. 2004, 39, 355–360. [Google Scholar] [CrossRef]
- Gilsanz, V.; Wren, T. Assessment of Bone Acquisition in Childhood and Adolescence. Pediatrics 2007, 119, S145–S149. [Google Scholar] [CrossRef]
- Benetou, V.; Orfanos, P.; Pettersson-Kymmer, U.; Bergström, U.; Svensson, O.; Johansson, I.; Berrino, F.; Tumino, R.; Borch, K.B.; Lund, E.; et al. Mediterranean Diet and Incidence of Hip Fractures in a European Cohort. Osteoporos. Int. 2013, 24, 1587–1598. [Google Scholar] [CrossRef]
- Seiquer, I.; Mesías, M.; Muñoz Hoyos, A.; Galdó, G.; Navarro, M.P. A Mediterranean Dietary Style Improves Calcium Utilization in Healthy Male Adolescents. J. Am. Coll. Nutr. 2008, 27, 454–462. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. Olives and Bone: A Green Osteoporosis Prevention Option. IJERPH 2016, 13, 755. [Google Scholar] [CrossRef]
- Shen, C.-L.; Chyu, M.-C.; Yeh, J.K.; Zhang, Y.; Pence, B.C.; Felton, C.K.; Brismée, J.-M.; Arjmandi, B.H.; Doctolero, S.; Wang, J.-S. Effect of Green Tea and Tai Chi on Bone Health in Postmenopausal Osteopenic Women: A 6-Month Randomized Placebo-Controlled Trial. Osteoporos. Int. 2012, 23, 1541–1552. [Google Scholar] [CrossRef]
- Arcoraci, V.; Atteritano, M.; Squadrito, F.; D’Anna, R.; Marini, H.; Santoro, D.; Minutoli, L.; Messina, S.; Altavilla, D.; Bitto, A. Antiosteoporotic Activity of Genistein Aglycone in Postmenopausal Women: Evidence from a Post-Hoc Analysis of a Multicenter Randomized Controlled Trial. Nutrients 2017, 9, 179. [Google Scholar] [CrossRef]
- Hooshmand, S.; Chai, S.C.; Saadat, R.L.; Payton, M.E.; Brummel-Smith, K.; Arjmandi, B.H. Comparative Effects of Dried Plum and Dried Apple on Bone in Postmenopausal Women. Br. J. Nutr. 2011, 106, 923–930. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kern, M.; Metti, D.; Shamloufard, P.; Chai, S.C.; Johnson, S.A.; Payton, M.E.; Arjmandi, B.H. The Effect of Two Doses of Dried Plum on Bone Density and Bone Biomarkers in Osteopenic Postmenopausal Women: A Randomized, Controlled Trial. Osteoporos. Int. 2016, 27, 2271–2279. [Google Scholar] [CrossRef]
- Law, Y.-Y.; Chiu, H.-F.; Lee, H.-H.; Shen, Y.-C.; Venkatakrishnan, K.; Wang, C.-K. Consumption of Onion Juice Modulates Oxidative Stress and Attenuates the Risk of Bone Disorders in Middle-Aged and Post-Menopausal Healthy Subjects. Food Funct. 2016, 7, 902–912. [Google Scholar] [CrossRef]
- Martin, B.R.; McCabe, G.P.; McCabe, L.; Jackson, G.S.; Horcajada, M.N.; Offord-Cavin, E.; Peacock, M.; Weaver, C.M. Effect of Hesperidin with and without a Calcium (Calcilock) Supplement on Bone Health in Postmenopausal Women. J. Clin. Endocrinol. Metab. 2016, 101, 923–927. [Google Scholar] [CrossRef] [PubMed]
- Brink, E.; Coxam, V.; Robins, S.; Wahala, K.; Cassidy, A.; Branca, F. PHYTOS Investigators Long-Term Consumption of Isoflavone-Enriched Foods Does Not Affect Bone Mineral Density, Bone Metabolism, or Hormonal Status in Early Postmenopausal Women: A Randomized, Double-Blind, Placebo Controlled Study. Am. J. Clin. Nutr. 2008, 87, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Kenny, A.M.; Mangano, K.M.; Abourizk, R.H.; Bruno, R.S.; Anamani, D.E.; Kleppinger, A.; Walsh, S.J.; Prestwood, K.M.; Kerstetter, J.E. Soy Proteins and Isoflavones Affect Bone Mineral Density in Older Women: A Randomized Controlled Trial. Am. J. Clin. Nutr. 2009, 90, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. Aoac. Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Díaz-García, M.C.; Obón, J.M.; Castellar, M.R.; Collado, J.; Alacid, M. Quantification by UHPLC of Total Individual Polyphenols in Fruit Juices. Food Chem. 2013, 138, 938–949. [Google Scholar] [CrossRef]
- Lohachoompol, V.; Srzednicki, G.; Craske, J. The Change of Total Anthocyanins in Blueberries and Their Antioxidant Effect After Drying and Freezing. J. Biomed. Biotechnol. 2004, 2004, 248–252. [Google Scholar] [CrossRef]
- Michalska, A.; Łysiak, G. Bioactive Compounds of Blueberries: Post-Harvest Factors Influencing the Nutritional Value of Products. Int. J. Mol. Sci. 2015, 16, 18642–18663. [Google Scholar] [CrossRef]
- Kuntz, S.; Rudloff, S.; Asseburg, H.; Borsch, C.; Fröhling, B.; Unger, F.; Dold, S.; Spengler, B.; Römpp, A.; Kunz, C. Uptake and Bioavailability of Anthocyanins and Phenolic Acids from Grape/Blueberry Juice and Smoothie in Vitro and in Vivo. Br. J. Nutr. 2015, 113, 1044–1055. [Google Scholar] [CrossRef]
- Amic, D.; Davidovic-Amic, D.; Be\vslo, D.; Trinajstic, N. Structure-Radical Scavenging Activity Relationships of Flavonoids. Croat. Chem. Acta 2003, 76, 55–61. [Google Scholar]
- Hardcastle, A.C.; Aucott, L.; Reid, D.M.; Macdonald, H.M. Associations between Dietary Flavonoid Intakes and Bone Health in a Scottish Population. J. Bone Miner. Res. 2011, 26, 941–947. [Google Scholar] [CrossRef]
- Léotoing, L.; Davicco, M.-J.; Lebecque, P.; Wittrant, Y.; Coxam, V. The Flavonoid Fisetin Promotes Osteoblasts Differentiation through Runx2 Transcriptional Activity. Mol. Nutr. Food Res. 2014, 58, 1239–1248. [Google Scholar] [CrossRef]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual Flavonoid Intakes Are Positively Associated with Bone Mineral Density in Women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef]
- Zhang, Z.-Q.; He, L.-P.; Liu, Y.-H.; Liu, J.; Su, Y.-X.; Chen, Y.-M. Association between Dietary Intake of Flavonoid and Bone Mineral Density in Middle Aged and Elderly Chinese Women and Men. Osteoporos. Int. 2014, 25, 2417–2425. [Google Scholar] [CrossRef]
- Zheng, X.; Mun, S.; Lee, S.G.; Vance, T.M.; Hubert, P.; Koo, S.I.; Lee, S.-K.; Chun, O.K. Anthocyanin-Rich Blackcurrant Extract Attenuates Ovariectomy-Induced Bone Loss in Mice. J. Med. Food 2016, 19, 390–397. [Google Scholar] [CrossRef]
- Ancillotti, C.; Ciofi, L.; Pucci, D.; Sagona, E.; Giordani, E.; Biricolti, S.; Gori, M.; Petrucci, W.A.; Giardi, F.; Bartoletti, R.; et al. Polyphenolic Profiles and Antioxidant and Antiradical Activity of Italian Berries from Vaccinium myrtillus L. and Vaccinium uliginosum L. Subsp. Gaultherioides (Bigelow) S.B. Young. Food Chem. 2016, 204, 176–184. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry Prevents Bone Loss in Ovariectomized Rat Model of Postmenopausal Osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef]
- Song, S.; Gao, Z.; Lei, X.; Niu, Y.; Zhang, Y.; Li, C.; Lu, Y.; Wang, Z.; Shang, P. Total Flavonoids of Drynariae Rhizoma Prevent Bone Loss Induced by Hindlimb Unloading in Rats. Molecules 2017, 22, 1033. [Google Scholar] [CrossRef]
- Hong, G.; Chen, Z.; Han, X.; Zhou, L.; Pang, F.; Wu, R.; Shen, Y.; He, X.; Hong, Z.; Li, Z.; et al. A Novel RANKL-targeted Flavonoid Glycoside Prevents Osteoporosis through Inhibiting NFATc1 and Reactive Oxygen Species. Clin. Transl. Med. 2021, 11, e392. [Google Scholar] [CrossRef]
- Ren, T.; Huang, C.; Cheng, M. Dietary Blueberry and Bifidobacteria Attenuate Nonalcoholic Fatty Liver Disease in Rats by Affecting SIRT1-Mediated Signaling Pathway. Oxidative Med. Cell. Longev. 2014, 2014, 469059. [Google Scholar] [CrossRef]
- Shimizu, S.; Matsushita, H.; Morii, Y.; Ohyama, Y.; Morita, N.; Tachibana, R.; Watanabe, K.; Wakatsuki, A. Effect of Anthocyanin-Rich Bilberry Extract on Bone Metabolism in Ovariectomized Rats. Biom. Rep. 2018, 8, 198–204. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Park, Y.-J.; Hwang, S.-C.; Kim, K.-D.; Moon, D.-K.; Kim, D.-H. Cytotoxic Effects of Delphinidin in Human Osteosarcoma Cells. Acta Orthop. Traumatol. Turc. 2018, 52, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Moriwaki, S.; Suzuki, K.; Muramatsu, M.; Nomura, A.; Inoue, F.; Into, T.; Yoshiko, Y.; Niida, S. Delphinidin, One of the Major Anthocyanidins, Prevents Bone Loss through the Inhibition of Excessive Osteoclastogenesis in Osteoporosis Model Mice. PLoS ONE 2014, 9, e97177. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Gu, D.R.; So, H.S.; Kim, K.J.; Lee, S.H. Dual Role of Cyanidin-3-Glucoside on the Differentiation of Bone Cells. J. Dent. Res. 2015, 94, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Imangali, N.; Phan, Q.T.; Mahady, G.; Winkler, C. The Dietary Anthocyanin Delphinidin Prevents Bone Resorption by Inhibiting Rankl-induced Differentiation of Osteoclasts in a Medaka (Oryzias latipes) Model of Osteoporosis. J. Fish Biol. 2021, 98, 1018–1030. [Google Scholar] [CrossRef] [PubMed]
- Puel, C.; Quintin, A.; Mathey, J.; Obled, C.; Davicco, M.J.; Lebecque, P.; Kati-Coulibaly, S.; Horcajada, M.N.; Coxam, V. Prevention of Bone Loss by Phloridzin, an Apple Polyphenol, in Ovariectomized Rats under Inflammation Conditions. Calcif. Tissue Int. 2005, 77, 311–318. [Google Scholar] [CrossRef]
- Lee, E.-J.; Kim, J.-L.; Kim, Y.-H.; Kang, M.-K.; Gong, J.-H.; Kang, Y.-H. Phloretin Promotes Osteoclast Apoptosis in Murine Macrophages and Inhibits Estrogen Deficiency-Induced Osteoporosis in Mice. Phytomedicine 2014, 21, 1208–1215. [Google Scholar] [CrossRef]
- Antika, L.D.; Lee, E.-J.; Kim, Y.-H.; Kang, M.-K.; Park, S.-H.; Kim, D.Y.; Oh, H.; Choi, Y.-J.; Kang, Y.-H. Dietary Phlorizin Enhances Osteoblastogenic Bone Formation through Enhancing β-Catenin Activity via GSK-3β Inhibition in a Model of Senile Osteoporosis. J. Nutr. Biochem. 2017, 49, 42–52. [Google Scholar] [CrossRef]
- Nanjo, F.; Mori, M.; Goto, K.; Hara, Y. Radical Scavenging Activity of Tea Catechins and Their Related Compounds. Biosci. Biotechnol. Biochem. 1999, 63, 1621–1623. [Google Scholar] [CrossRef]
- Huang, H.-T.; Cheng, T.-L.; Lin, S.-Y.; Ho, C.-J.; Chyu, J.Y.; Yang, R.-S.; Chen, C.-H.; Shen, C.-L. Osteoprotective Roles of Green Tea Catechins. Antioxidants 2020, 9, 1136. [Google Scholar] [CrossRef]
- Wu, X.; Xie, C.; Zhu, Q.; Wang, M.; Sun, B.; Huang, Y.; Shen, C.; An, M.; Zhao, Y.; Wang, X.; et al. Green Tea (Camellia sinensis) Aqueous Extract Alleviates Postmenopausal Osteoporosis in Ovariectomized Rats and Prevents RANKL-Induced Osteoclastogenesis In Vitro. Food Nutr. Res. 2018, 62, 1478. [Google Scholar] [CrossRef]
- Xu, H.; Liu, T.; Li, J.; Xu, J.; Chen, F.; Hu, L.; Zhang, B.; Zi, C.; Wang, X.; Sheng, J. Oxidation Derivative of (-)-Epigallocatechin-3-Gallate (EGCG) Inhibits RANKL-Induced Osteoclastogenesis by Suppressing RANK Signaling Pathways in RAW 264.7 Cells. Biomed. Pharmacother. 2019, 118, 109237. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Kan, J.Y.; Lu, C.-C.; Huang, H.H.; Cheng, T.-L.; Huang, H.-T.; Ho, C.-J.; Lee, T.-C.; Chuang, S.-C.; Lin, Y.-S.; et al. Green Tea Catechin (-)-Epigallocatechin-3-Gallate (EGCG) Facilitates Fracture Healing. Biomolecules 2020, 10, 620. [Google Scholar] [CrossRef]
- Lin, S.-Y.; Kang, L.; Chen, J.-C.; Wang, C.-Z.; Huang, H.H.; Lee, M.-J.; Cheng, T.-L.; Chang, C.-F.; Lin, Y.-S.; Chen, C.-H. (−)-Epigallocatechin-3-Gallate (EGCG) Enhances Healing of Femoral Bone Defect. Phytomedicine 2019, 55, 165–171. [Google Scholar] [CrossRef]
- Xi, J.; Li, Q.; Luo, X.; Li, J.; Guo, L.; Xue, H.; Wu, G. Epigallocatechin-3-gallate Protects against Secondary Osteoporosis in a Mouse Model via the Wnt/Β-catenin Signaling Pathway. Mol. Med. Rep. 2018, 18, 4555–4562. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Xu, S.; Wang, F.; Wang, B.; Han, K.; Sun, D.; Li, L. Epigallocatechin-3-Gallate Protects against Hydrogen Peroxide-Induced Inhibition of Osteogenic Differentiation of Human Bone Marrow-Derived Mesenchymal Stem Cells. Stem. Cells Int. 2016, 2016, 7532798. [Google Scholar] [CrossRef]
- Vester, H.; Holzer, N.; Neumaier, M.; Lilianna, S.; Nüssler, A.K.; Seeliger, C. Green Tea Extract (GTE) Improves Differentiation in Human Osteoblasts during Oxidative Stress. J. Inflamm. 2014, 11, 15. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Q.; Tjhioe, W.; Zhao, J.; Lu, A.; Zhang, G.; Tan, R.X.; Zhou, M.; Xu, J.; Feng, H.T. Therapeutic Potential and Outlook of Alternative Medicine for Osteoporosis. CDT 2017, 18, 1051–1068. [Google Scholar] [CrossRef]
- Ai, Z.; Wu, Y.; Yu, M.; Li, J.; Li, S. Theaflavin-3, 3′-Digallate Suppresses RANKL-Induced Osteoclastogenesis and Attenuates Ovariectomy-Induced Bone Loss in Mice. Front. Pharmacol. 2020, 11, 803. [Google Scholar] [CrossRef]
- Ge, G.; Yang, S.; Hou, Z.; Gan, M.; Tao, H.; Zhang, W.; Li, W.; Wang, Z.; Hao, Y.; Gu, Y.; et al. Theaflavin-3,3′-Digallate Promotes the Formation of Osteoblasts Under Inflammatory Environment and Increases the Bone Mass of Ovariectomized Mice. Front. Pharmacol. 2021, 12, 648969. [Google Scholar] [CrossRef]
- Sun, K.; Wang, L.; Ma, Q.; Cui, Q.; Lv, Q.; Zhang, W.; Li, X. Association between Tea Consumption and Osteoporosis: A Meta-Analysis. Medicine 2017, 96, e9034. [Google Scholar] [CrossRef]
- Panahande, S.B.; Maghbooli, Z.; Hossein-nezhad, A.; Qorbani, M.; Moeini-Nodeh, S.; Haghi-Aminjan, H.; Hosseini, S. Effects of French Maritime Pine Bark Extract (Oligopin®) Supplementation on Bone Remodeling Markers in Postmenopausal Osteopenic Women: A Randomized Clinical Trial. Phytother. Res. 2019, 33, 1233–1240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, Y.; Xu, Q.; Zhang, Y.; Liu, L.; Li, H.; Li, F.; Liu, Z.; Yang, X.; Yu, X.; et al. The Effect of Soy Isoflavone Combined with Calcium on Bone Mineral Density in Perimenopausal Chinese Women: A 6-Month Randomised Double-Blind Placebo-Controlled Study. Int. J. Food Sci. Nutr. 2020, 71, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xue, J.; Shen, T.; Ba, G.; Yu, D.; Fu, Q. Curcumin Alleviates Glucocorticoid-Induced Osteoporosis by Protecting Osteoblasts from Apoptosis In Vivo and In Vitro. Clin. Exp. Pharmacol. Physiol. 2016, 43, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Xue, Y.; Wang, T.; Xie, Q.; Lin, J.; Wang, Y. Curcumin Inhibits the Migration of Osteoclast Precursors and Osteoclastogenesis by Repressing CCL3 Production. BMC Complement. Med. Ther. 2020, 20, 234. [Google Scholar] [CrossRef]
- Khanizadeh, F.; Rahmani, A.; Asadollahi, K.; Ahmadi, M.R.H. Combination Therapy of Curcumin and Alendronate Modulates Bone Turnover Markers and Enhances Bone Mineral Density in Postmenopausal Women with Osteoporosis. Arch. Endocrinol. Metab. 2018, 62, 438–445. [Google Scholar] [CrossRef]
- Ke, D.; Wang, Y.; Yu, Y.; Wang, Y.; Zheng, W.; Fu, X.; Han, J.; Zhang, G.; Xu, J. Curcumin-Activated Autophagy Plays a Negative Role in Its Anti-Osteoclastogenic Effect. Mol. Cell. Endocrinol. 2020, 500, 110637. [Google Scholar] [CrossRef]
- Zhao, K.; Jia, Y.; Peng, J.; Pang, C.; Zhang, T.; Han, W.; Jiang, J.; Lu, X.; Zhu, J.; Qian, Y. Anacardic Acid Inhibits RANKL-induced Osteoclastogenesis In Vitro and Prevents Ovariectomy-induced Bone Loss In Vivo. FASEB J. 2019, 33, 9100–9115. [Google Scholar] [CrossRef]
- Ornstrup, M.J.; Harsløf, T.; Kjær, T.N.; Langdahl, B.L.; Pedersen, S.B. Resveratrol Increases Bone Mineral Density and Bone Alkaline Phosphatase in Obese Men: A Randomized Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 2014, 99, 4720–4729. [Google Scholar] [CrossRef]
- Tou, J.C. Evaluating Resveratrol as a Therapeutic Bone Agent: Preclinical Evidence from Rat Models of Osteoporosis: Resveratrol and Rat Models of Osteoporosis. Ann. N. Y. Acad. Sci. 2015, 1348, 75–85. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol Promotes Osteogenesis via Activating SIRT1/FoxO1 Pathway in Osteoporosis Mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol Mediated Modulation of Sirt-1/Runx2 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells: Potential Role of Runx2 Deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.-M.; Guo, C.; Han, J.-F. Resveratrol Promotes Osteoblastic Differentiation in a Rat Model of Postmenopausal Osteoporosis by Regulating Autophagy. Nutr. Metab. 2020, 17, 29. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.; He, Y.; Deng, N.; Chen, Y.; Huang, J.; Xie, W. Protective Effect of Resveratrol on Estrogen Deficiency-Induced Osteoporosis Though Attenuating NADPH Oxidase 4/Nuclear Factor Kappa B Pathway by Increasing MiR-92b-3p Expression. Int. J. Immunopathol. Pharmacol. 2020, 34, 205873842094176. [Google Scholar] [CrossRef]
- Csaki, C.; Mobasheri, A.; Shakibaei, M. Synergistic Chondroprotective Effects of Curcumin and Resveratrol in Human Articular Chondrocytes: Inhibition of IL-1β-Induced NF-ΚB-Mediated Inflammation and Apoptosis. Arthritis Res Ther. 2009, 11, R165. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, T.; Wang, Y.; Guo, L. The Role and Mechanism of SIRT1 in Resveratrol-Regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019, 9, 18424. [Google Scholar] [CrossRef]
- Wong, R.H.; Thaung Zaw, J.J.; Xian, C.J.; Howe, P.R. Regular Supplementation with Resveratrol Improves Bone Mineral Density in Postmenopausal Women: A Randomized, Placebo-Controlled Trial. J. Bone Miner. Res. 2020, 35, 2121–2131. [Google Scholar] [CrossRef]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites—Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef]
- Domazetovic, V.; Fontani, F.; Tanini, D.; D’Esopo, V.; Viglianisi, C.; Marcucci, G.; Panzella, L.; Napolitano, A.; Brandi, M.L.; Capperucci, A.; et al. Protective Role of Benzoselenophene Derivatives of Resveratrol on the Induced Oxidative Stress in Intestinal Myofibroblasts and Osteocytes. Chem. Biol. Interact. 2017, 275, 13–21. [Google Scholar] [CrossRef]
- Tenkumo, T.; Aobulikasimu, A.; Asou, Y.; Shirato, M.; Shishido, S.; Kanno, T.; Niwano, Y.; Sasaki, K.; Nakamura, K. Proanthocyanidin-Rich Grape Seed Extract Improves Bone Loss, Bone Healing, and Implant Osseointegration in Ovariectomized Animals. Sci. Rep. 2020, 10, 8812. [Google Scholar] [CrossRef]
- Oršolić, N.; Nemrava, J.; Jeleč, Ž.; Kukolj, M.; Odeh, D.; Terzić, S.; Fureš, R.; Bagatin, T.; Bagatin, D. The Beneficial Effect of Proanthocyanidins and Icariin on Biochemical Markers of Bone Turnover in Rats. Int. J. Mol. Sci. 2018, 19, 2746. [Google Scholar] [CrossRef]
- Chen, L.; Hu, S.-L.; Xie, J.; Yan, D.-Y.; Weng, S.-J.; Tang, J.-H.; Wang, B.-Z.; Xie, Z.-J.; Wu, Z.-Y.; Yang, L. Proanthocyanidins-Mediated Nrf2 Activation Ameliorates Glucocorticoid-Induced Oxidative Stress and Mitochondrial Dysfunction in Osteoblasts. Oxidative Med. Cell. Longev. 2020, 2020, 9102012. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yin, Z.; Zhang, Q.; Guo, S.; Shen, Y.; Liu, T.; Liu, B.; Wan, L.; Li, S.; Chen, X.; et al. Proanthocyanidins Inhibit Osteoclast Formation and Function by Inhibiting the NF-ΚB and JNK Signaling Pathways during Osteoporosis Treatment. Biochem. Biophys. Res. Commun. 2019, 509, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Zhang, L.; Yang, X. Ferulic Acid, a Natural Polyphenol, Protects against Osteoporosis by Activating SIRT1 and NF-ΚB in Neonatal Rats with Glucocorticoid-Induced Osteoporosis. Biomed. Pharmacother. 2019, 120, 109205. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, O.; De Luna-Bertos, E.; Ramos-Torrecillas, J.; Ruiz, C.; Milia, E.; Lorenzo, M.L.; Jimenez, B.; Sánchez-Ortiz, A.; Rivas, A. Phenolic Compounds in Extra Virgin Olive Oil Stimulate Human Osteoblastic Cell Proliferation. PLoS ONE 2016, 11, e0150045. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health Effects of Phenolic Compounds Found in Extra-Virgin Olive Oil, By-Products, and Leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea Europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Puel, C.; Mardon, J.; Agalias, A.; Davicco, M.-J.; Lebecque, P.; Mazur, A.; Horcajada, M.-N.; Skaltsounis, A.-L.; Coxam, V. Major Phenolic Compounds in Olive Oil Modulate Bone Loss in an Ovariectomy/Inflammation Experimental Model. J. Agric. Food Chem. 2008, 56, 9417–9422. [Google Scholar] [CrossRef]
- Hagiwara, K.; Goto, T.; Araki, M.; Miyazaki, H.; Hagiwara, H. Olive Polyphenol Hydroxytyrosol Prevents Bone Loss. Eur. J. Pharmacol. 2011, 662, 78–84. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Ghorbel, H.; Feki, I.; Bouallagui, Z.; Guermazi, F.; Ayadi, L.; Sayadi, S. Oleuropein and Hydroxytyrosol Protect Rats’ Pups against Bisphenol A Induced Hypothyroidism. Biomed. Pharmacother. 2018, 103, 1115–1126. [Google Scholar] [CrossRef]
- Saleh, N.K.; Saleh, H.A. Olive Oil Effectively Mitigates Ovariectomy-Induced Osteoporosis in Rats. BMC Complement. Altern. Med. 2011, 11, 10. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; Illescas-Montes, R.; Ramos-Torrecillas, J.; de Luna-Bertos, E.; Ruiz, C.; García-Martínez, O. Bone Protective Effect of Extra-Virgin Olive Oil Phenolic Compounds by Modulating Osteoblast Gene Expression. Nutrients 2019, 11, 1722. [Google Scholar] [CrossRef]
- Melguizo-Rodríguez, L.; Manzano-Moreno, F.J.; De Luna-Bertos, E.; Rivas, A.; Ramos-Torrecillas, J.; Ruiz, C.; García-Martínez, O. Effect of Olive Oil Phenolic Compounds on Osteoblast Differentiation. Eur. J. Clin. Investig. 2018, 48, e12904. [Google Scholar] [CrossRef]
- Fernández-Real, J.M.; Bulló, M.; Moreno-Navarrete, J.M.; Ricart, W.; Ros, E.; Estruch, R.; Salas-Salvadó, J. A Mediterranean Diet Enriched with Olive Oil Is Associated with Higher Serum Total Osteocalcin Levels in Elderly Men at High Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2012, 97, 3792–3798. [Google Scholar] [CrossRef]
- Filip, R.; Possemiers, S.; Heyerick, A.; Pinheiro, I.; Raszewski, G.; Davicco, M.-J.; Coxam, V. Twelve-Month Consumption of a Polyphenol Extract from Olive (Olea europaea) in a Double Blind, Randomized Trial Increases Serum Total Osteocalcin Levels and Improves Serum Lipid Profiles in Postmenopausal Women with Osteopenia. J. Nutr. Health Aging 2015, 19, 77–86. [Google Scholar] [CrossRef]
- Khachik, F.; Carvalho, L.; Bernstein, P.S.; Muir, G.J.; Zhao, D.-Y.; Katz, N.B. Chemistry, Distribution, and Metabolism of Tomato Carotenoids and Their Impact on Human Health. Exp. Biol. Med. 2002, 227, 845–851. [Google Scholar] [CrossRef]
- Mackinnon, E.S.; Rao, A.V.; Josse, R.G.; Rao, L.G. Supplementation with the Antioxidant Lycopene Significantly Decreases Oxidative Stress Parameters and the Bone Resorption Marker N-Telopeptide of Type I Collagen in Postmenopausal Women. Osteoporos. Int. 2011, 22, 1091–1101. [Google Scholar] [CrossRef]
- Costa-Rodrigues, J.; Fernandes, M.H.; Pinho, O.; Monteiro, P.R.R. Modulation of Human Osteoclastogenesis and Osteoblastogenesis by Lycopene. J. Nutr. Biochem. 2018, 57, 26–34. [Google Scholar] [CrossRef]
- Oliveira, G.R.; Vargas-Sanchez, P.K.; Fernandes, R.R.; Ricoldi, M.S.T.; Semeghini, M.S.; Pitol, D.L.; de Sousa, L.G.; Siessere, S.; Bombonato-Prado, K.F. Lycopene Influences Osteoblast Functional Activity and Prevents Femur Bone Loss in Female Rats Submitted to an Experimental Model of Osteoporosis. J. Bone Min. Metab. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Mackinnon, E.S.; venket Rao, A.; Rao, L.G. Dietary Restriction of Lycopene for a Period of One Month Resulted in Significantly Increased Biomarkers of Oxidative Stress and Bone Resorption in Postmenopausal Women. J. Nutr. Health Aging 2011, 15, 133–138. [Google Scholar] [CrossRef]
- Russo, C.; Ferro, Y.; Maurotti, S.; Salvati, M.A.; Mazza, E.; Pujia, R.; Terracciano, R.; Maggisano, G.; Mare, R.; Giannini, S.; et al. Lycopene and Bone: An In Vitro Investigation and a Pilot Prospective Clinical Study. J. Transl. Med. 2020, 18, 43. [Google Scholar] [CrossRef]
- Hayhoe, R.P.G.; Lentjes, M.A.H.; Mulligan, A.A.; Luben, R.N.; Khaw, K.-T.; Welch, A.A. Carotenoid Dietary Intakes and Plasma Concentrations Are Associated with Heel Bone Ultrasound Attenuation and Osteoporotic Fracture Risk in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk Cohort. Br. J. Nutr. 2017, 117, 1439–1453. [Google Scholar] [CrossRef] [PubMed]
- Brzezińska, O.; Łukasik, Z.; Makowska, J.; Walczak, K. Role of Vitamin C in Osteoporosis Development and Treatment—A Literature Review. Nutrients 2020, 12, 2394. [Google Scholar] [CrossRef] [PubMed]
- Nazrun Shuid, A.; Das, S.; Mohamed, I.N. Therapeutic Effect of Vitamin E in Preventing Bone Loss: An Evidence-Based Review. Int. J. Vitam. Nutr. Res. 2019, 89, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.; Mohamad, N.-V.; Ibrahim, N.; Chin, K.-Y.; Shuid, A.; Ima-Nirwana, S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019, 20, 1453. [Google Scholar] [CrossRef] [PubMed]
- Odai, T.; Terauchi, M.; Hirose, A.; Kato, K.; Miyasaka, N. Bone Mineral Density in Premenopausal Women Is Associated with the Dietary Intake of α-Tocopherol: A Cross-Sectional Study. Nutrients 2019, 11, 2474. [Google Scholar] [CrossRef]
- Shen, C.-L.; Yang, S.; Tomison, M.D.; Romero, A.W.; Felton, C.K.; Mo, H. Tocotrienol Supplementation Suppressed Bone Resorption and Oxidative Stress in Postmenopausal Osteopenic Women: A 12-Week Randomized Double-Blinded Placebo-Controlled Trial. Osteoporos. Int. 2018, 29, 881–891. [Google Scholar] [CrossRef]
- Östman, B.; Michaëlsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative Stress and Bone Mineral Density in Elderly Men: Antioxidant Activity of Alpha-Tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef]
- Jun, J.H.; Lee, S.-H.; Kwak, H.B.; Lee, Z.H.; Seo, S.-B.; Woo, K.M.; Ryoo, H.-M.; Kim, G.-S.; Baek, J.-H. N-Acetylcysteine Stimulates Osteoblastic Differentiation of Mouse Calvarial Cells. J. Cell Biochem. 2008, 103, 1246–1255. [Google Scholar] [CrossRef]
- Ueno, T.; Yamada, M.; Igarashi, Y.; Ogawa, T. N-Acetyl Cysteine Protects Osteoblastic Function from Oxidative Stress. J. Biomed. Mater. Res. 2011, 99A, 523–531. [Google Scholar] [CrossRef]
- Han, B.; Geng, H.; Liu, L.; Wu, Z.; Wang, Y. GSH Attenuates RANKL-Induced Osteoclast Formation in Vitro and LPS-Induced Bone Loss In Vivo. Biomed. Pharmacother. 2020, 128, 110305. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Z.; Ni, Y.; Yu, Y.; Wang, G.; Chen, L. Suppression Effect of N-Acetylcysteine on Bone Loss in Ovariectomized Mice. Am. J. Transl. Res. 2020, 12, 731–742. [Google Scholar]
- Polat, B.; Halici, Z.; Cadirci, E.; Albayrak, A.; Karakus, E.; Bayir, Y.; Bilen, H.; Sahin, A.; Yuksel, T.N. The Effect of Alpha-Lipoic Acid in Ovariectomy and Inflammation-Mediated Osteoporosis on the Skeletal Status of Rat Bone. Eur. J. Pharmacol. 2013, 718, 469–474. [Google Scholar] [CrossRef]
- Lu, S.-Y.; Wang, C.-Y.; Jin, Y.; Meng, Q.; Liu, Q.; Liu, Z.; Liu, K.-X.; Sun, H.-J.; Liu, M.-Z. The Osteogenesis-Promoting Effects of Alpha-Lipoic Acid against Glucocorticoid-Induced Osteoporosis through the NOX4, NF-KappaB, JNK and PI3K/AKT Pathways. Sci. Rep. 2017, 7, 3331. [Google Scholar] [CrossRef]
- Byun, C.-H.; Koh, J.-M.; Kim, D.K.; Park, S.-I.; Lee, K.-U.; Kim, G.S. α-Lipoic Acid Inhibits TNF-α-Induced Apoptosis in Human Bone Marrow Stromal Cells. J. Bone Miner. Res. 2005, 20, 1125–1135. [Google Scholar] [CrossRef]
- Nardone, V.; D’Asta, F.; Brandi, M.L. Pharmacological Management of Osteogenesis. Clinics 2014, 69, 438–446. [Google Scholar] [CrossRef]
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Brown, J.P.; Morin, S.; Leslie, W.; Papaioannou, A.; Cheung, A.M.; Davison, K.S.; Goltzman, D.; Hanley, D.A.; Hodsman, A.; Josse, R.; et al. Bisphosphonates for Treatment of Osteoporosis: Expected Benefits, Potential Harms, and Drug Holidays. Can. Fam. Physician 2014, 60, 324–333. [Google Scholar]
- Zaheer, S.; LeBoff, M.; Lewiecki, E.M. Denosumab for the Treatment of Osteoporosis. Expert Opin. Drug Metab. Toxicol. 2015, 11, 461–470. [Google Scholar] [CrossRef]
- Lindsay, R.; Krege, J.H.; Marin, F.; Jin, L.; Stepan, J.J. Teriparatide for Osteoporosis: Importance of the Full Course. Osteoporos. Int. 2016, 27, 2395–2410. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- McClung, M.R.; Brown, J.P.; Diez-Perez, A.; Resch, H.; Caminis, J.; Meisner, P.; Bolognese, M.A.; Goemaere, S.; Bone, H.G.; Zanchetta, J.R.; et al. Effects of 24 Months of Treatment with Romosozumab Followed by 12 Months of Denosumab or Placebo in Postmenopausal Women with Low Bone Mineral Density: A Randomized, Double-Blind, Phase 2, Parallel Group Study: SEQUENTIAL THERAPY AFTER ROMOSOZUMAB. J. Bone Min. Res. 2018, 33, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the Future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Ng, K.W. Implications of Osteoblast-Osteoclast Interactions in the Management of Osteoporosis by Antiresorptive Agents Denosumab and Odanacatib. Curr. Osteoporos. Rep. 2014, 12, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Mirza, F.; Canalis, E. MANAGEMENT OF ENDOCRINE DISEASE: Secondary Osteoporosis: Pathophysiology and Management. Eur. J. Endocrinol. 2015, 173, R131–R151. [Google Scholar] [CrossRef]

| Natural Antioxidants | |||
|---|---|---|---|
| Polyphenols | Flavonoids | Flavonols Flavones Isoflavones Anthocyanidins Anthocyanins Flavanols Phlorizidin Epigallocatechin gallate Theaflavin-3,3′-digallate | |
| Nonflavonoids | Phenolic acids | Curcumin Anacardic acid | |
| Xanthones | |||
| Tannins | |||
| Stilbenes | Resveratrol | ||
| Ferulic acid | |||
| Hydroxytyrosol | |||
| Oleuropein | |||
| Tyrosol | |||
| Non-polyphenolic antioxidants | Carotenoids | Lycopene | |
| Vitamins | Vitamin C Vitamin E | ||
| Thiol antioxidants | Glutathione Alpha lipoic acid | ||
| Natural Compound | Source | Molecular Structure | Bone Effects | Ref. |
|---|---|---|---|---|
| Anthocyanins | Blueberries, bilberries, vegetables, cereals, dry legumes, chocolate, tea, coffee, wine | 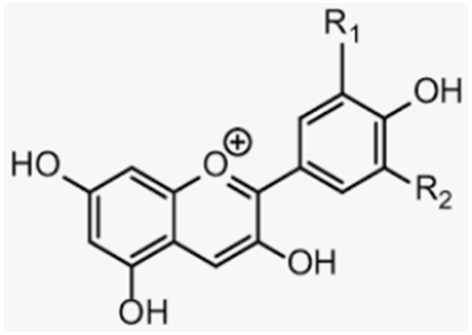 |
| [35,38,91,97,98,101,127] [35,95,97,100,126] [98,126,128,129,130,132] [134,135,136] [5,97,98,102,128] |
| Phlorizidin | Apple | 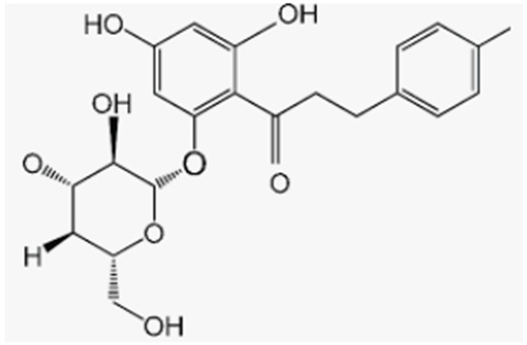 |
| [137] |
| Phloretin | Apple | 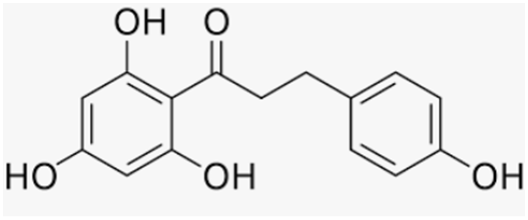 |
| [138] [139] |
| Epigallocatechin gallate | Green tea | 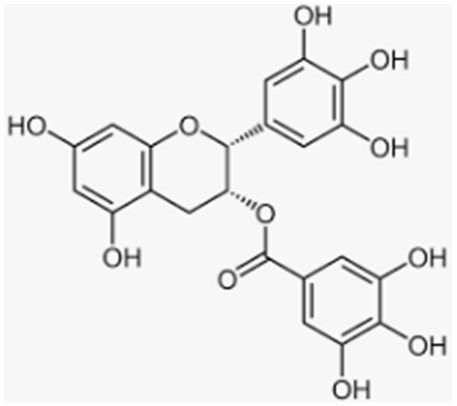 |
| [142,143,146,147,148] [144] [148] [146] |
| Theaflavin-3,3′-digallate | Black tea | 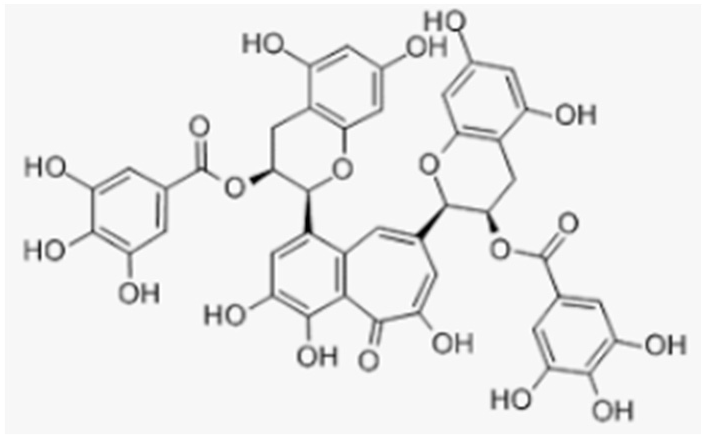 |
| [149,151] [150,151] |
| Genistein | Soy | 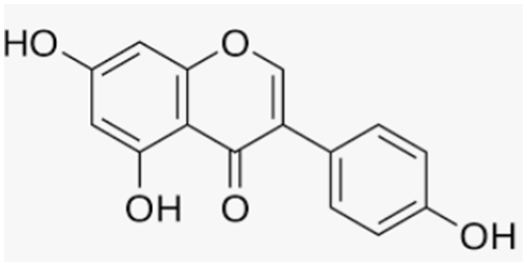 |
| [109,154] |
| Curcumin | Turmeric plant | 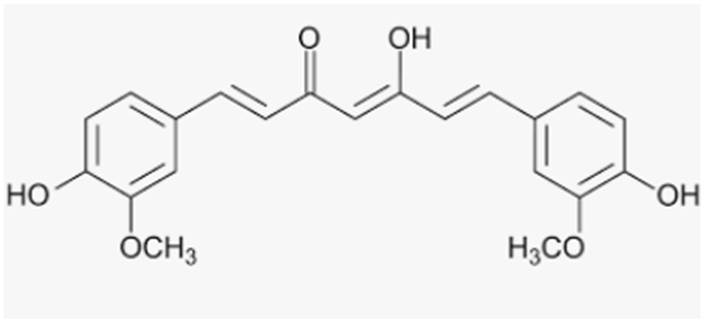 |
| [156,157,158] [157] [155] |
| Anacardic acid | Cashew nut |  |
| [159] |
| Resveratrol | Grapes, wine, grape juice, Itadori tea, peanuts, cocoa, blueberries, bilberries, cranberries | 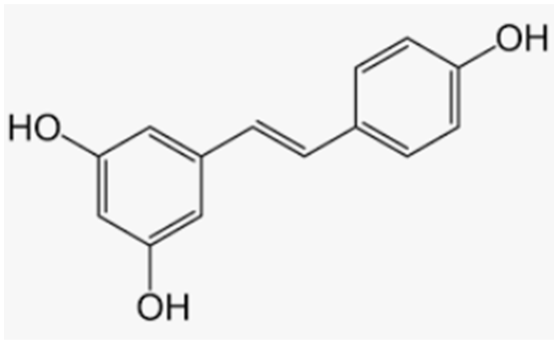 |
| [162,163,164] [160] [165,166,167] [168] |
| Tannins | Grape seeds | 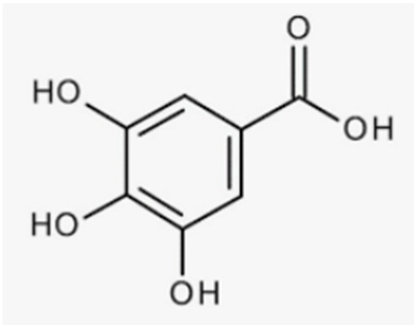 |
| [173] [174] [171,172] |
| Ferulic acid | Fruits | 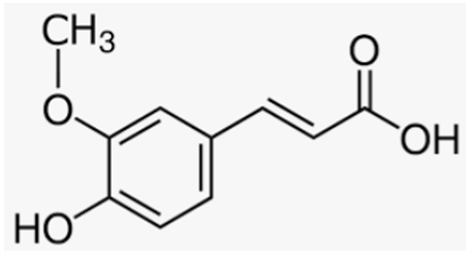 |
| [175] |
| Oleuropein, hydroxytyrosol, tyrosol | Olive oil | 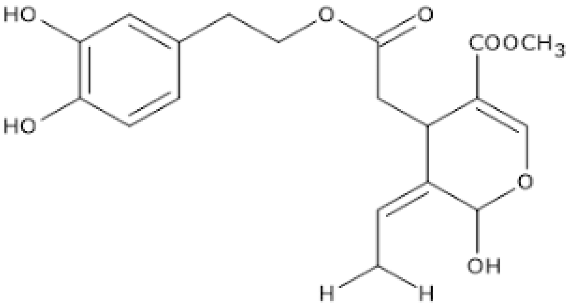 |
| [179,180,181] [181,182] |
| Lycopene | Tomatoes |  |
| [187,188,189,190] [191] [192,193] |
| Vitamin C | Fruits and vegetables | 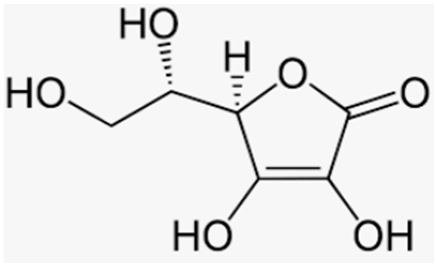 |
| [194] |
| Vitamin E | Fruits, vegetables, seeds | 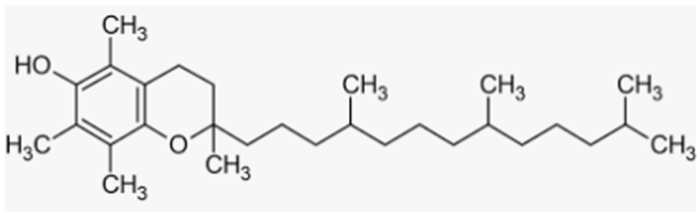 |
| [195,198,199] [196,197,198,199] [197] |
| Glutathione | Intracellular |  |
| [13,14,15,17,18] [17,202] [51] |
| Alpha lipoic acid | Intracellular, vegetables, red meat | 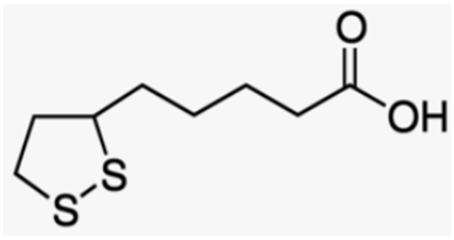 |
| [51,205,206] [51] [204,205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcucci, G.; Domazetovic, V.; Nediani, C.; Ruzzolini, J.; Favre, C.; Brandi, M.L. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants 2023, 12, 373. https://doi.org/10.3390/antiox12020373
Marcucci G, Domazetovic V, Nediani C, Ruzzolini J, Favre C, Brandi ML. Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants. 2023; 12(2):373. https://doi.org/10.3390/antiox12020373
Chicago/Turabian StyleMarcucci, Gemma, Vladana Domazetovic, Chiara Nediani, Jessica Ruzzolini, Claudio Favre, and Maria Luisa Brandi. 2023. "Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches" Antioxidants 12, no. 2: 373. https://doi.org/10.3390/antiox12020373
APA StyleMarcucci, G., Domazetovic, V., Nediani, C., Ruzzolini, J., Favre, C., & Brandi, M. L. (2023). Oxidative Stress and Natural Antioxidants in Osteoporosis: Novel Preventive and Therapeutic Approaches. Antioxidants, 12(2), 373. https://doi.org/10.3390/antiox12020373