Pharmacological Inhibition of Lysine-Specific Demethylase 1A Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice by a Mechanism Involving Decreased Oxidative Stress and Inflammation; Potential Implications in Human Atherosclerosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Harvesting of Human Non-Atherosclerotic and Atherosclerotic Arterial Tissues
2.3. Set-Up of Experimental Atherosclerosis Mouse Model and Treatment Strategy
2.4. Cell Culture Experimental Design
2.5. Histology and Microscopic Examination
2.6. Assessment of Plasma Total Cholesterol and Triglyceride Levels in Mice
2.7. Assessment of Atherosclerotic Lesion Formation in Mice
2.8. Real-Time Polymerase Chain Reaction Assay (Real-Time PCR)
2.9. Western Blot Assay
2.10. Transfection Assay
2.11. Statistical Analysis
3. Results
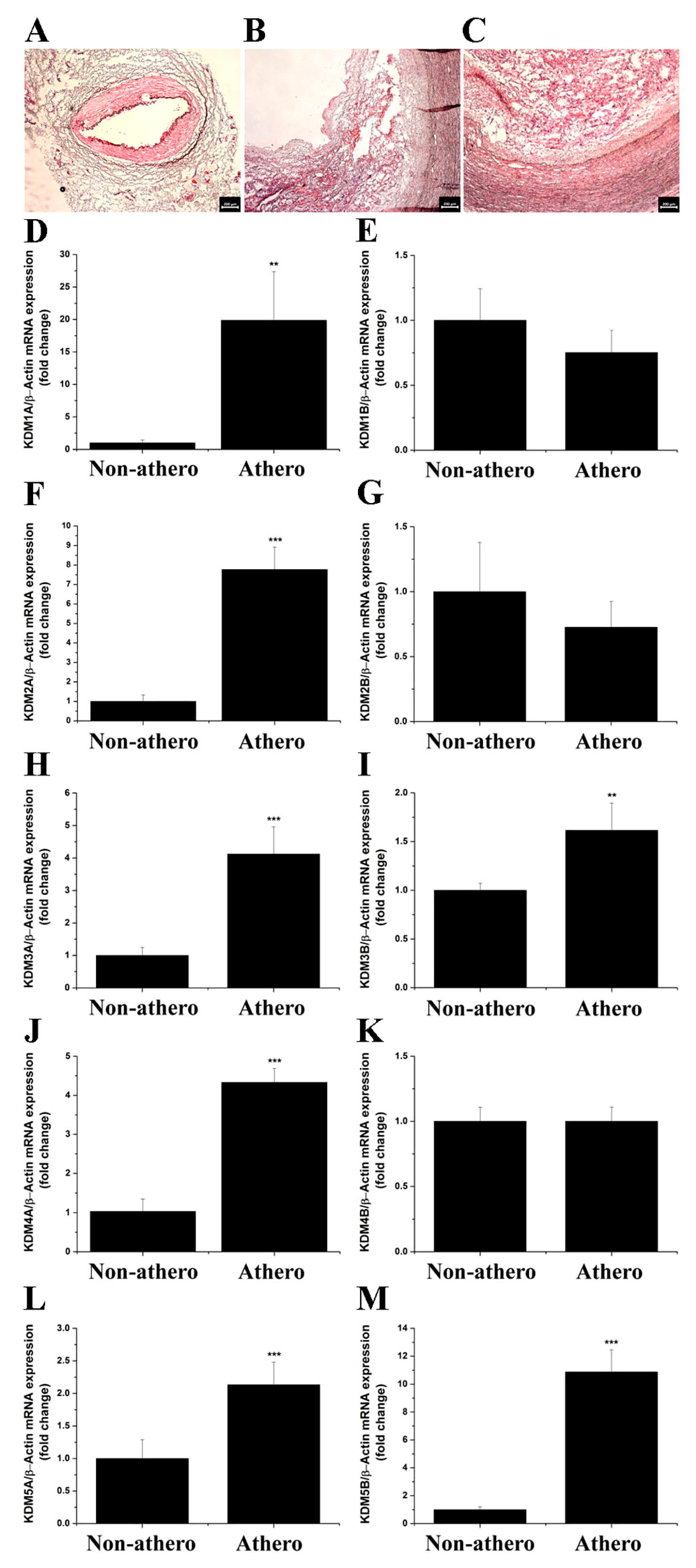
3.1. LSD1 Expression Is Up-Regulated in Atherosclerotic Human Carotid Arteries
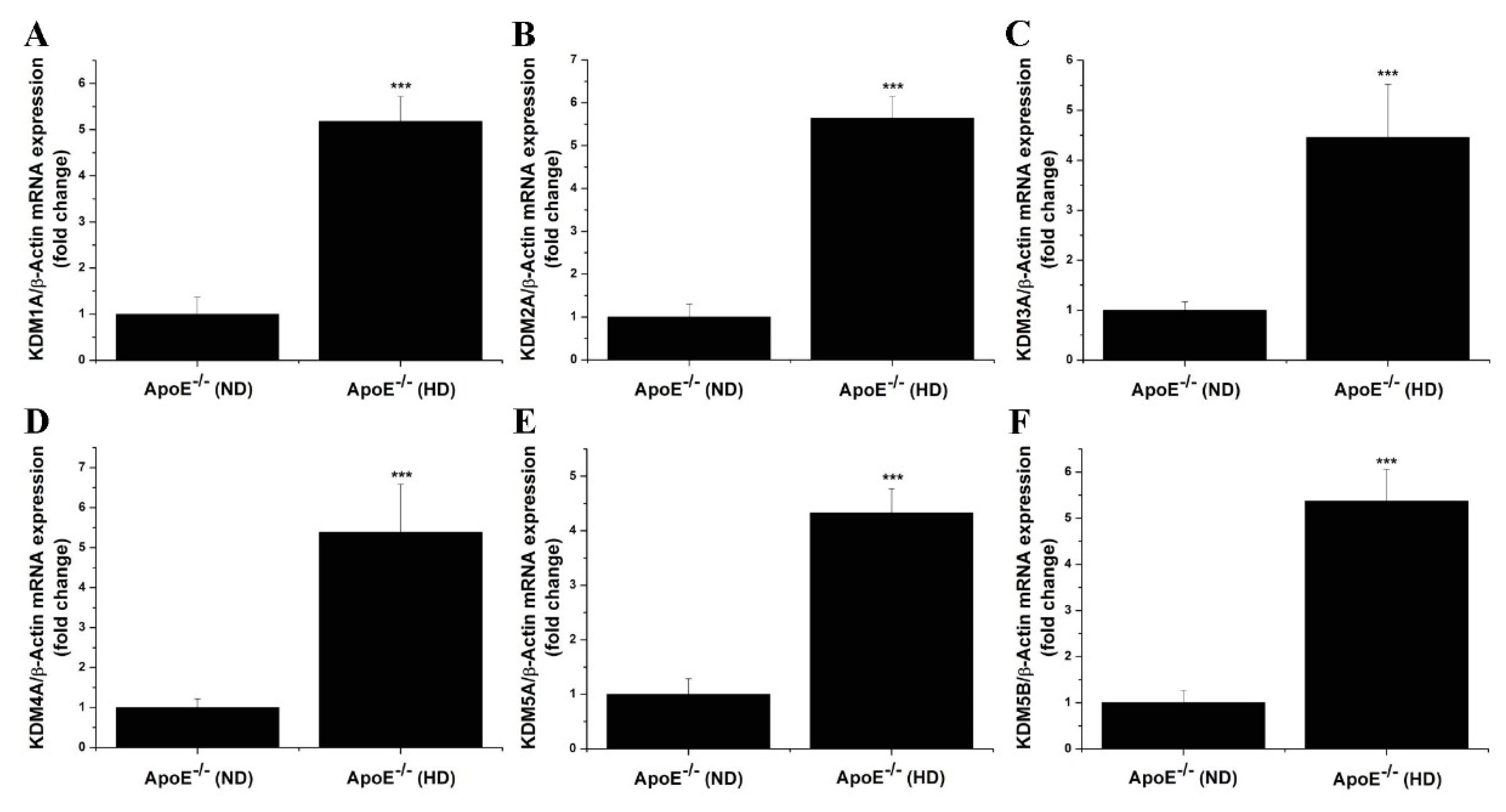
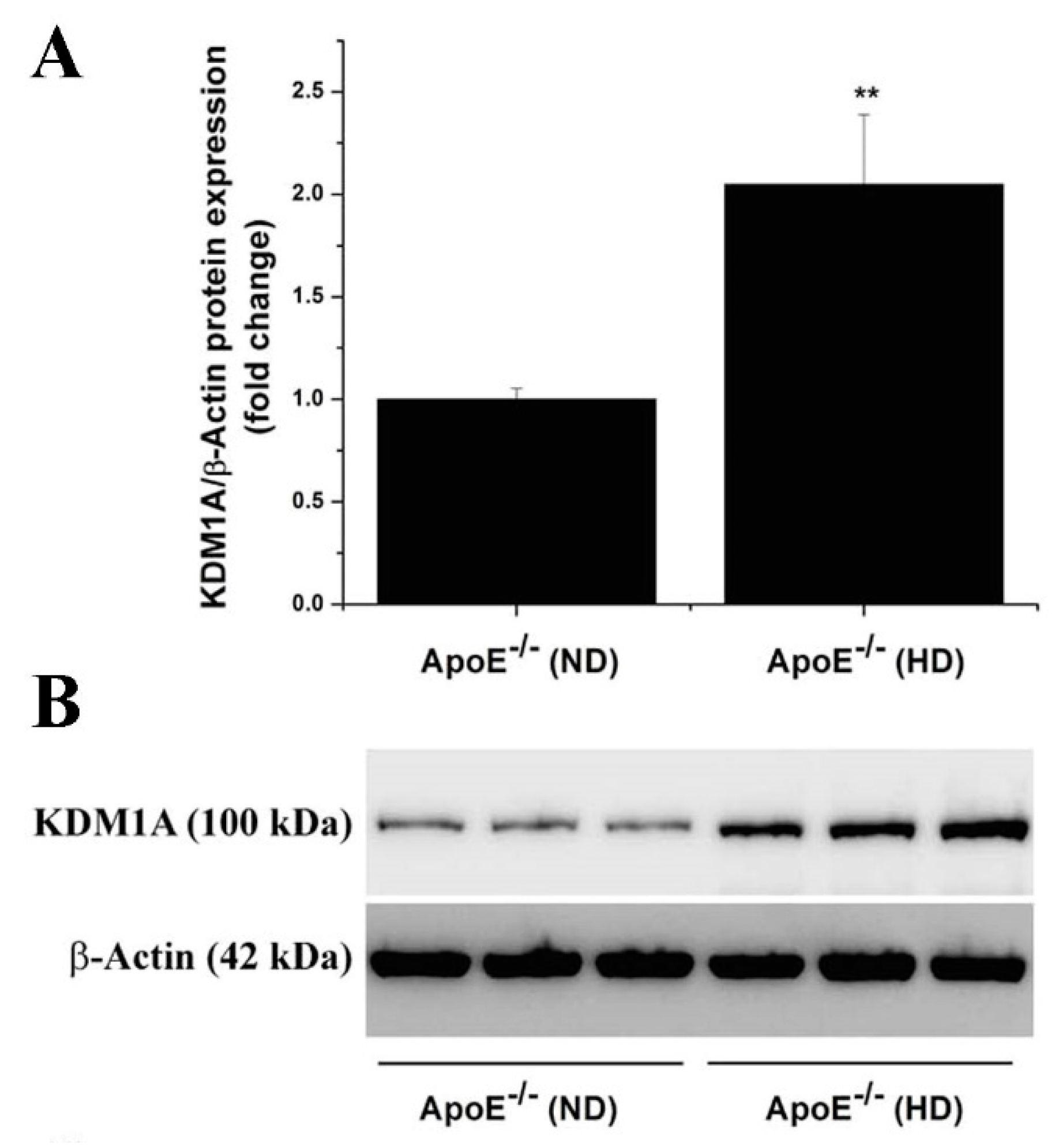
3.2. The Gene and Protein Expression Levels of LSD1 Are Up-Regulated in the Aorta of Atherosclerotic Mice
3.3. Pharmacological Inhibition of LSD1 Activity by GSK2879552 Reduces Atherosclerotic Lesion Formation in the Aorta of Hypercholesterolemic Mice
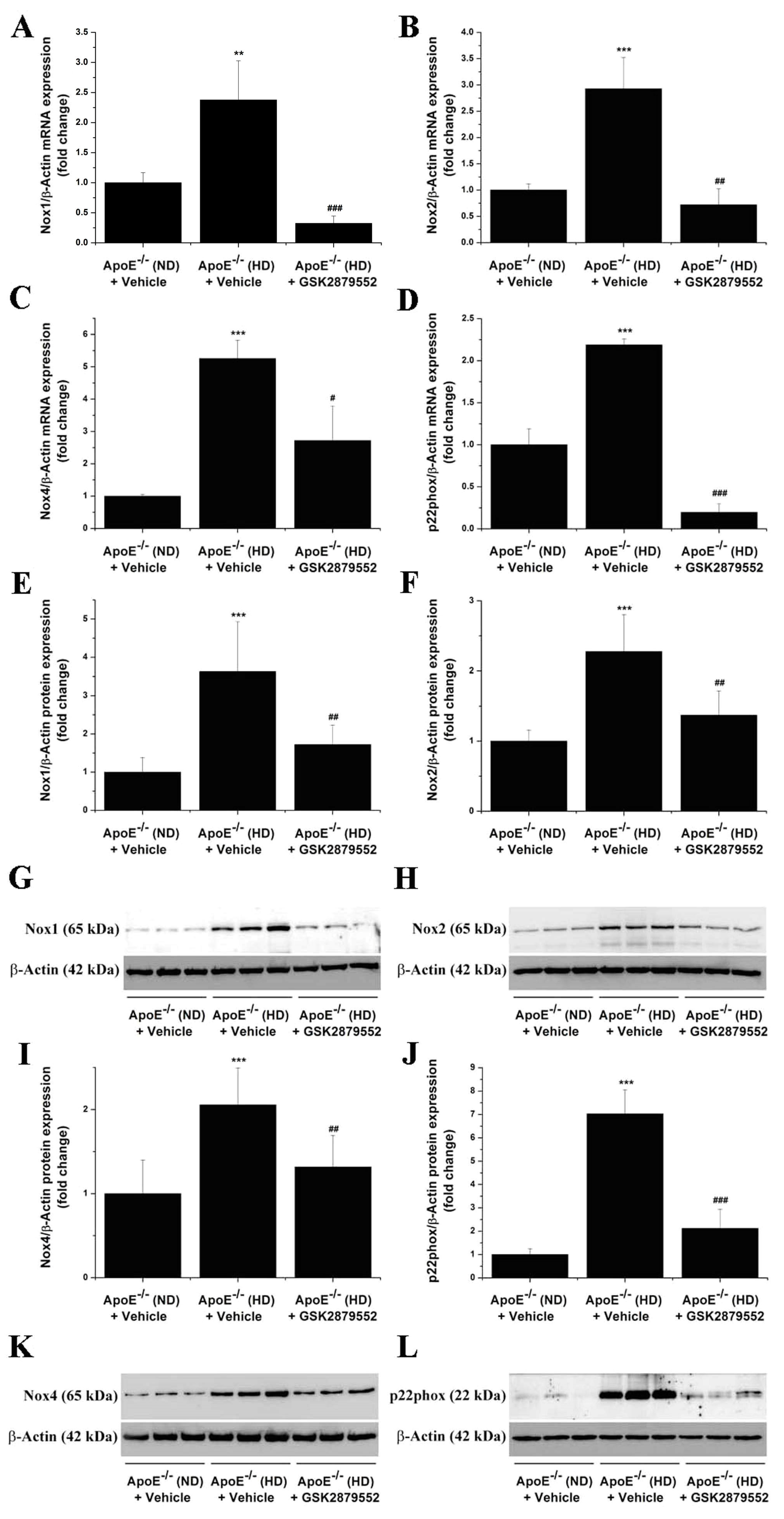
3.4. LSD1-Dependent Signaling Mediates the Up-Regulation of Nox Subunit Expression in the Atherosclerotic Mice Aorta
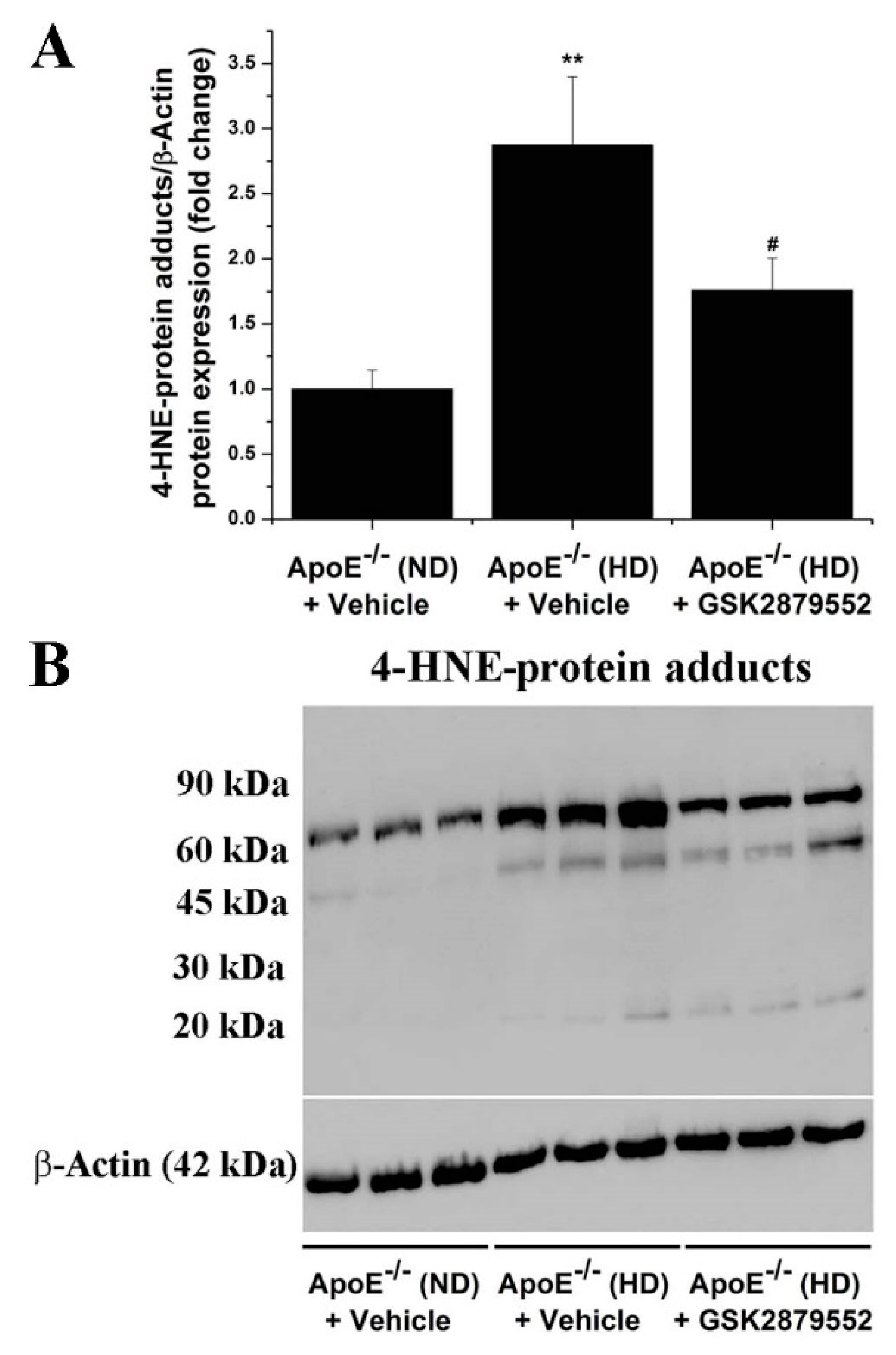
3.5. Inhibition of LSD1 Function Reduces the Formation of 4-HNE-Protein Adducts in the Aorta of Atherosclerotic Mice
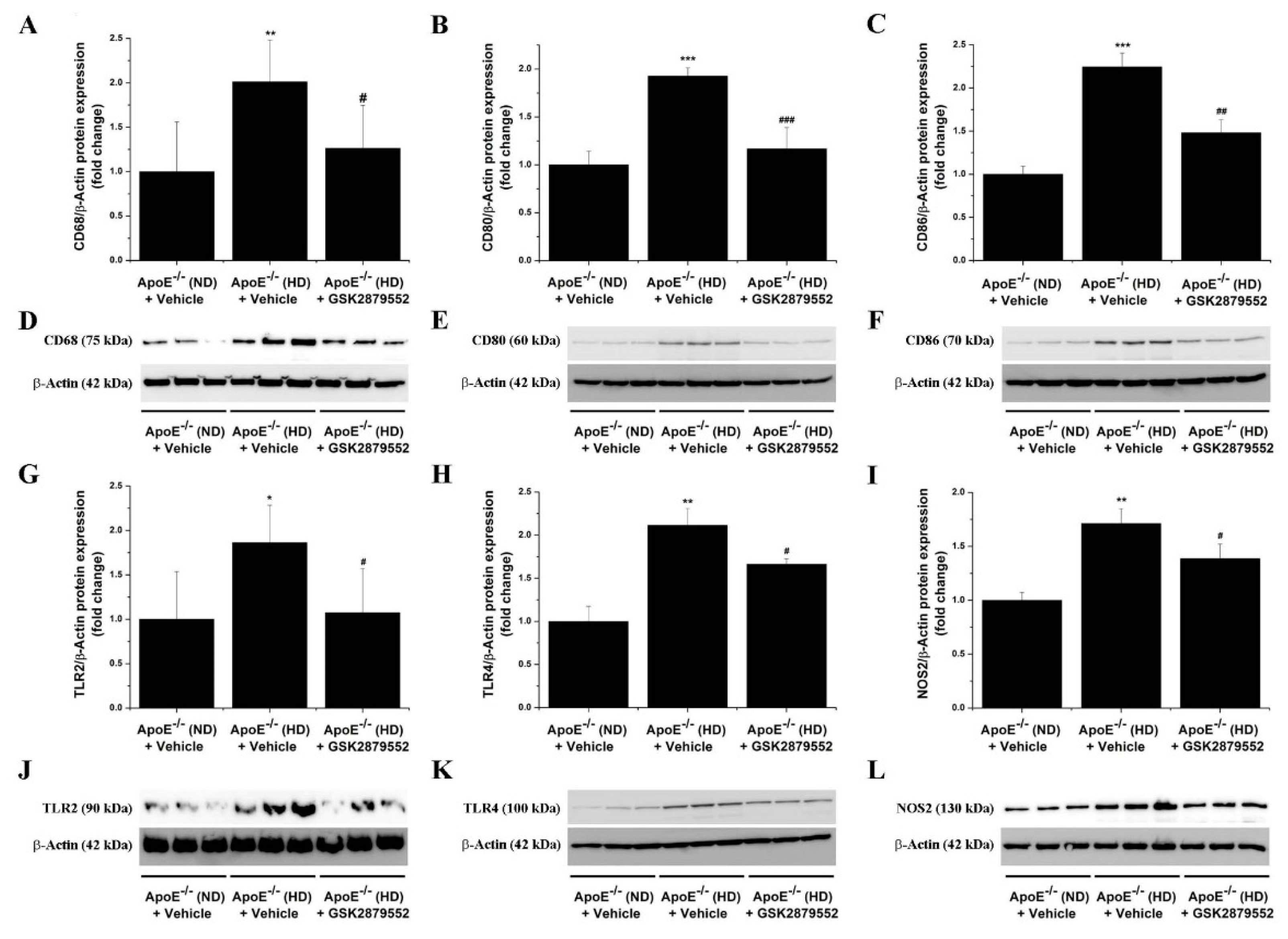
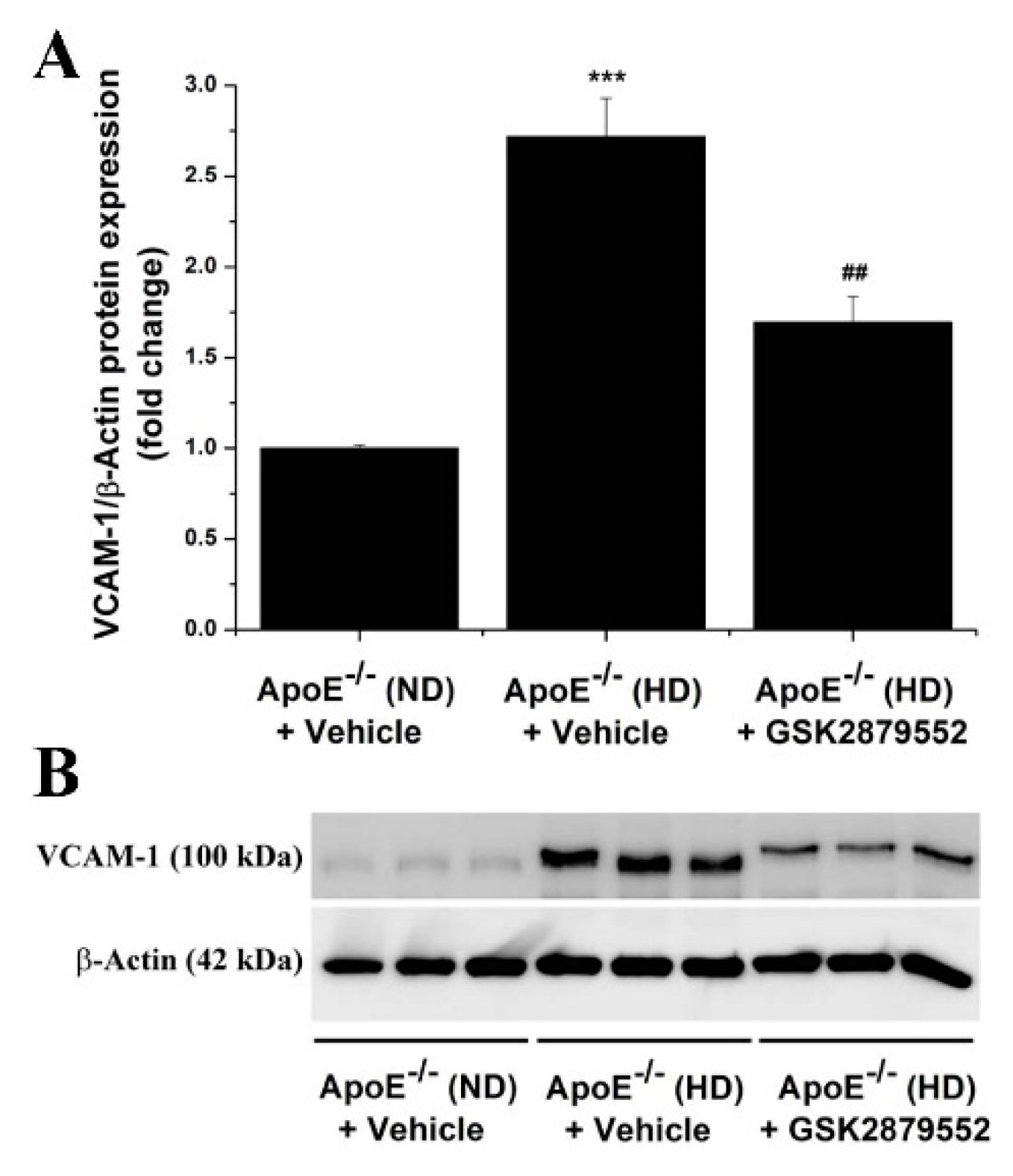
3.6. Pharmacological Inhibition of LSD1 Down-Regulates the Aortic Expression of Markers of Immune Cells, Inflammation, and Vascular Remodeling in Atherosclerotic ApoE-/- Mice
3.7. Increased Expression of LSD1 Is Associated with a Pro-Inflammatory Mac Phenotype
3.8. Inhibition of LSD1 Down-Regulates the Expression of Oxidative Stress and Pro-Inflammatory Genes Associated with M1-Mac Phenotype
3.9. Overexpression of LSD1 Induces the Up-Regulation of Nox Subunit Transcript Levels in HEK293 Reporter Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, W.; Song, M.; Qu, J.; Liu, G.H. Epigenetic modifications in cardiovascular aging and diseases. Circ. Res. 2018, 123, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Miao, X.; Liu, Y.; Li, F.; Liu, Q.; Sun, J.; Cai, L. Dysregulation of histone acetyltransferases and deacetylases in cardiovascular diseases. Oxidative Med. Cell. Longev. 2014, 2014, 641979. [Google Scholar] [CrossRef]
- Herman, A.B.; Occean, J.R.; Sen, P. Epigenetic dysregulation in cardiovascular aging and disease. J. Cardiovasc. Aging. 2021, 1, 10. [Google Scholar] [CrossRef]
- Costantino, S.; Libby, P.; Kishore, R.; Tardif, J.C.; El-Osta, A.; Paneni, F. Epigenetics and precision medicine in cardiovascular patients: From basic concepts to the clinical arena. Eur. Heart J. 2018, 39, 4150–4158. [Google Scholar] [CrossRef] [PubMed]
- Morera, L.; Lübbert, M.; Jung, M. Targeting histone methyltransferases and demethylases in clinical trials for cancer therapy. Clin. Epigenetics 2016, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Agrawal, D.K.; Boosani, C.S. Cell specific histone modifications in atherosclerosis. Mol. Med. Rep. 2018, 18, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Yi, X.; Zhu, X.H.; Jiang, D.S. Histone methylation and vascular biology. Clin. Epigenetics 2020, 12, 30. [Google Scholar] [CrossRef]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of histone post-translational modifications in inflammatory diseases. Front. Immunol. 2022, 13, 852272. [Google Scholar] [CrossRef] [PubMed]
- Yun, M.; Wu, J.; Workman, J.L.; Li, B. Readers of histone modifications. Cell. Res. 2011, 21, 564–578. [Google Scholar] [CrossRef]
- Kim, D.; Kim, K.I.; Baek, S.H. Roles of lysine-specific demethylase 1 (LSD1) in homeostasis and diseases. J. Biomed. Sci. 2021, 28, 41. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.; Lin, Y.; Wang, Z.; Wu, X.; Ye, Z.; Wang, Y.; Lan, H. Biological roles of LSD1 beyond its demethylase activity. Cell. Mol. Life Sci. 2020, 77, 3341–3350. [Google Scholar] [CrossRef] [PubMed]
- Lan, H.; Tan, M.; Zhang, Q.; Yang, F.; Wang, S.; Li, H.; Xiong, X.; Sun, Y. LSD1 destabilizes FBXW7 and abrogates FBXW7 functions independent of its demethylase activity. Proc. Natl. Acad. Sci. USA 2019, 116, 12311–12320. [Google Scholar] [CrossRef]
- Perillo, B.; Tramontano, A.; Pezone, A.; Migliaccio, A. LSD1: More than demethylation of histone lysine residues. Exp. Mol. Med. 2020, 52, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Greißel, A.; Culmes, M.; Burgkart, R.; Zimmermann, A.; Eckstein, H.H.; Zernecke, A.; Pelisek, J. Histone acetylation and methylation significantly change with severity of atherosclerosis in human carotid plaques. Cardiovasc. Pathol. 2016, 25, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Harman, J.L.; Dobnikar, L.; Chappell, J.; Stokell, B.G.; Dalby, A.; Foote, K.; Finigan, A.; Freire-Pritchett, P.; Taylor, A.L.; Worssam, M.D.; et al. Epigenetic regulation of vascular smooth muscle cells by histone H3 lysine 9 dimethylation attenuates target gene-induction by inflammatory Signaling. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2289–2302. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Luan, Y.; Yuan, R.X.; Luan, Y. Histone methylation related therapeutic challenge in cardiovascular diseases. Front. Cardiovasc. Med. 2021, 8, 710053. [Google Scholar] [CrossRef]
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Görlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1533–1554. [Google Scholar] [CrossRef]
- Yi, X.; Zhu, Q.X.; Wu, X.L.; Tan, T.T.; Jiang, X.J. Histone methylation and oxidative stress in cardiovascular diseases. Oxid. Med. Cell. Longev. 2022, 2022, 6023710. [Google Scholar] [CrossRef]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef]
- Mohammad, H.P.; Smitheman, K.N.; Kamat, C.D.; Soong, D.; Federowicz, K.E.; Van Aller, G.S.; Schneck, J.L.; Carson, J.D.; Liu, Y.; Butticello, M.; et al. A DNA hypomethylation signature predicts antitumor activity of LSD1 inhibitors in SCLC. Cancer Cell. 2015, 28, 57–69. [Google Scholar] [CrossRef]
- Macheleidt, I.F.; Dalvi, P.S.; Lim, S.Y.; Meemboor, S.; Meder, L.; Käsgen, O.; Müller, M.; Kleemann, K.; Wang, L.; Nürnberg, P.; et al. Preclinical studies reveal that LSD1 inhibition results in tumor growth arrest in lung adenocarcinoma independently of driver mutations. Mol. Oncol. 2018, 12, 1965–1979. [Google Scholar] [CrossRef]
- Manea, S.A.; Vlad, M.L.; Fenyo, I.M.; Lazar, A.G.; Raicu, M.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological inhibition of histone deacetylase reduces NADPH oxidase expression, oxidative stress and the progression of atherosclerotic lesions in hypercholesterolemic apolipoprotein E-deficient mice; potential implications for human atherosclerosis. Redox Biol. 2020, 28, 101338. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Buchmann, G.K.; Schürmann, C.; Warwick, T.; Schulz, M.H.; Spaeth, M.; Müller, O.J.; Schröder, K.; Jo, H.; Weissmann, N.; Brandes, R.P. Deletion of NoxO1 limits atherosclerosis development in female mice. Redox Biol. 2020, 37, 101713. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Di Marco, E.; Okabe, J.; Szyndralewiez, C.; Heitz, F.; Montezano, A.C.; de Haan, J.B.; Koulis, C.; El-Osta, A.; Andrews, K.L.; et al. NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 2013, 127, 1888–1902. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.P.; Jha, J.C.; Kennedy, K.; van Bommel, E.; Chew, P.; Szyndralewiez, C.; Touyz, R.M.; Schmidt, H.H.H.W.; Cooper, M.E.; Jandeleit-Dahm, K.A.M. Combined NOX1/4 inhibition with GKT137831 in mice provides dose-dependent reno- and atheroprotection even in established micro- and macrovascular disease. Diabetologia 2017, 60, 927–937. [Google Scholar] [CrossRef]
- Sirker, A.; Zhang, M.; Shah, A.M. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011, 106, 735–747. [Google Scholar] [CrossRef]
- Manea, A.; Manea, S.A.; Todirita, A.; Albulescu, I.C.; Raicu, M.; Sasson, S.; Simionescu, M. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res. 2015, 361, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.; Prange, K.H.M.; Glass, C.K.; de Winther, M.P.J. Transcriptional and epigenetic regulation of macrophages in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 216–228. [Google Scholar] [CrossRef]
- Hoeksema, M.A.; de Winther, M.P. Epigenetic regulation of monocyte and macrophage function. Antioxid. Redox Signal. 2016, 25, 758–774. [Google Scholar] [CrossRef]
- Chinetti-Gbaguidi, G.; Colin, S.; Staels, B. Macrophage subsets in atherosclerosis. Nat. Rev. Cardiol. 2015, 12, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Ritsick, D.; Cheng, G.; Lambeth, J.D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005, 280, 31859–31869. [Google Scholar] [CrossRef] [PubMed]
- Sweeny, E.A.; Schlanger, S.; Stuehr, D.J. Dynamic regulation of NADPH oxidase 5 by intracellular heme levels and cellular chaperones. Redox Biol. 2020, 36, 101656. [Google Scholar] [CrossRef]
- Han, J.; Jin, C.; Zhong, Y.; Zhu, J.; Liu, Q.; Sun, D.; Feng, J.; Xia, X.; Peng, X. Involvement of NADPH oxidase in patulin-induced oxidative damage and cytotoxicity in HEK293 cells. Food Chem. Toxicol. 2021, 150, 112055. [Google Scholar] [CrossRef] [PubMed]
- Wierda, R.J.; Geutskens, S.B.; Jukema, J.W.; Quax, P.H.; van den Elsen, P.J. Epigenetics in atherosclerosis and inflammation. J. Cell. Mol. Med. 2010, 14, 1225–1240. [Google Scholar] [CrossRef]
- Villeneuve, L.M.; Reddy, M.A.; Lanting, L.L.; Wang, M.; Meng, L.; Natarajan, R. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc. Natl. Acad. Sci. USA 2008, 105, 9047–9052. [Google Scholar] [CrossRef]
- Reddy, M.A.; Natarajan, R. Epigenetic mechanisms in diabetic vascular complications. Cardiovasc. Res. 2011, 90, 421–429. [Google Scholar] [CrossRef]
- Gomez, D.; Swiatlowska, P.; Owens, G.K. Epigenetic control of smooth muscle cell identity and lineage memory. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 2508–2516. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, P.; Seeger, W.; Pullamsetti, S.S. Epigenetic mechanisms in pulmonary arterial hypertension: The need for global perspectives. Eur. Respir. Rev. 2016, 25, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Edgar, L.; Akbar, N.; Braithwaite, A.T.; Krausgruber, T.; Gallart-Ayala, H.; Bailey, J.; Corbin, A.L.; Khoyratty, T.E.; Chai, J.T.; Alkhalil, M.; et al. Hyperglycemia induces trained immunity in macrophages and their precursors and promotes atherosclerosis. Circulation 2021, 144, 961–982. [Google Scholar] [CrossRef] [PubMed]
- Arifuzzaman, S.; Khatun, M.R.; Khatun, R. Emerging of lysine demethylases (KDMs): From pathophysiological insights to novel therapeutic opportunities. Biomed. Pharmacother. 2020, 129, 110392. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, D.; Li, Q.; Du, X.; Liu, Y.; Dai, X.; Hong, L. Upregulation of LSD1 promotes migration and invasion in gastric cancer through facilitating EMT. Cancer Manag. Res. 2019, 11, 4481–4491. [Google Scholar] [CrossRef] [PubMed]
- Karakaidos, P.; Verigos, J.; Magklara, A. LSD1/KDM1A, a gate-keeper of cancer stemness and a promising therapeutic target. Cancers (Basel) 2019, 11, 1821. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Liao, G.; Yu, B. LSD1/KDM1A inhibitors in clinical trials: Advances and prospects. J. Hematol. Oncol. 2019, 12, 129. [Google Scholar] [CrossRef]
- Rezende, F.; Löwe, O.; Helfinger, V.; Prior, K.K.; Walter, M.; Zukunft, S.; Fleming, I.; Weissmann, N.; Brandes, R.P.; Schröder, K. Unchanged NADPH oxidase activity in Nox1-Nox2-Nox4 triple knockout mice: What do NADPH-stimulated chemiluminescence assays really detect? Antioxid. Redox Signal. 2016, 24, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Brandes, R.P.; Rezende, F.; Schröder, K. Redox regulation beyond ROS: Why ROS should not be measured as often. Circ. Res. 2018, 123, 326–328. [Google Scholar] [CrossRef]
- Manea, S.A.; Vlad, M.L.; Rebleanu, D.; Lazar, A.G.; Fenyo, I.M.; Calin, M.; Simionescu, M.; Manea, A. Detection of vascular reactive oxygen species in experimental atherosclerosis by high-resolution near-infrared fluorescence imaging using VCAM-1-targeted liposomes entrapping a fluorogenic redox-sensitive probe. Oxid. Med. Cell. Longev. 2021, 2021, 6685612. [Google Scholar] [CrossRef]
- Kim, D.; Nam, H.J.; Lee, W.; Yim, H.Y.; Ahn, J.Y.; Park, S.W.; Shin, H.J.R.; Yu, R.; Won, K.J.; Bae, J.S.; et al. PKCα-LSD1-NF-κB-signaling cascade is crucial for epigenetic control of the inflammatory response. Mol. Cell. 2018, 69, 398–411.e6. [Google Scholar] [CrossRef]
- Haines, R.R.; Scharer, C.D.; Lobby, J.L.; Boss, J.M. LSD1 cooperates with noncanonical NF-κB signaling to regulate marginal zone B cell development. J. Immunol. 2019, 203, 1867–1881. [Google Scholar] [CrossRef]
- Liang, L.; Sun, M.; Qi, Z.; Li, W. Lysine demethylase 1A exacerbates LPS-induced inflammation of vascular smooth muscle cells through modulation of NF-κB activation. Trop. J. Pharm. Res. 2020, 19, 481–487. [Google Scholar] [CrossRef]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Manea, S.A.; Gafencu, A.V.; Raicu, M. Regulation of NADPH oxidase subunit p22(phox) by NF-kB in human aortic smooth muscle cells. Arch. Physiol. Biochem. 2007, 113, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Manea, A.; Tanase, L.I.; Raicu, M.; Simionescu, M. Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2010, 396, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Vasilatos, S.N.; Boric, L.; Shaw, P.G.; Davidson, N.E. Inhibitors of histone demethylation and histone deacetylation cooperate in regulating gene expression and inhibiting growth in human breast cancer cells. Breast Cancer Res. Treat. 2012, 131, 777–789. [Google Scholar] [CrossRef]
- Vasilatos, S.N.; Katz, T.A.; Oesterreich, S.; Wan, Y.; Davidson, N.E.; Huang, Y. Crosstalk between lysine-specific demethylase 1 (LSD1) and histone deacetylases mediates antineoplastic efficacy of HDAC inhibitors in human breast cancer cells. Carcinogenesis 2013, 34, 1196–1207. [Google Scholar] [CrossRef]
- Nalawansha, D.A.; Pflum, M.K.H. LSD1 substrate binding and gene expression are affected by HDAC1-mediated deacetylation. ACS Chem. Biol. 2017, 12, 254–264. [Google Scholar] [CrossRef]
- Manea, S.A.; Antonescu, M.L.; Fenyo, I.M.; Raicu, M.; Simionescu, M.; Manea, A. Epigenetic regulation of vascular NADPH oxidase expression and reactive oxygen species production by histone deacetylase-dependent mechanisms in experimental diabetes. Redox Biol. 2018, 16, 332–343. [Google Scholar] [CrossRef]
- Yang, Y.T.; Wang, X.; Zhang, Y.Y.; Yuan, W.J. The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis. Cell Death Dis. 2019, 10, 278. [Google Scholar] [CrossRef]
- Wojtala, M.; Rybaczek, D.; Wielgus, E.; Sobalska-Kwapis, M.; Strapagiel, D.; Balcerczyk, A. The role of lysine-specific demethylase 1 (LSD1) in shaping the endothelial inflammatory response. Cell. Physiol. Biochem. 2021, 55, 569–589. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, T.; Zhai, H.; Peng, W.; Zhou, Y.; Li, Q.; Yang, H. Inhibition of lysine-specific demethylase 1A suppresses neointimal hyperplasia by targeting bone morphogenetic protein 2 and mediating vascular smooth muscle cell phenotype. Cell Prolif. 2020, 53, e12711. [Google Scholar] [CrossRef]
- Yuan, B.; Liu, H.; Pan, X.; Dong, X.; Qu, L.F.; Sun, J.; Pan, L.L. LSD1 downregulates p21 expression in vascular smooth muscle cells and promotes neointima formation. Biochem. Pharmacol. 2022, 198, 114947. [Google Scholar] [CrossRef] [PubMed]
- Abdulla, A.; Zhang, Y.; Hsu, F.N.; Xiaoli, A.M.; Zhao, X.; Yang, E.S.T.; Ji, J.Y.; Yang, F. Regulation of lipogenic gene expression by lysine-specific histone demethylase-1 (LSD1). J. Biol. Chem. 2014, 289, 29937–29947. [Google Scholar] [CrossRef] [PubMed]
- Ramms, B.; Pollow, D.P.; Zhu, H.; Nora, C.; Harrington, A.R.; Omar, I.; Gordts, P.L.S.M.; Wortham, M.; Sander, M. Systemic LSD1 inhibition prevents aberrant remodeling of metabolism in obesity. Diabetes 2022, 71, 2513–2529. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, X.; Liu, S.; Zeng, S.; Yu, L.; Yang, G.; Guo, J.; Xu, Y. BRG1 regulates NOX gene transcription in endothelial cells and contributes to cardiac ischemia-reperfusion injury. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3477–3486. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, J.; Yang, J.; Xu, L.; Hu, Q.; Xu, C.; Yang, S.; Jiang, H. Histone demethylase KDM3a, a novel regulator of vascular smooth muscle cells, controls vascular neointimal hyperplasia in diabetic rats. Atherosclerosis 2017, 257, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, S.; Yao, G.; Yu, D.; Chen, K.; Tong, Q.; Ye, L.; Wu, C.; Sun, Y.; Li, H.; et al. Identification of the histone lysine demethylase KDM4A/JMJD2A as a novel epigenetic target in M1 macrophage polarization induced by oxidized LDL. Oncotarget 2017, 8, 114442–114456. [Google Scholar] [CrossRef]
- Mokou, M.; Klein, J.; Makridakis, M.; Bitsika, V.; Bascands, J.L.; Saulnier-Blache, J.S.; Mullen, W.; Sacherer, M.; Zoidakis, J.; Pieske, B.; et al. Proteomics based identification of KDM5 histone demethylases associated with cardiovascular disease. EBioMedicine 2019, 41, 91–104. [Google Scholar] [CrossRef]
- Han, M.; Xu, W.; Cheng, P.; Jin, H.; Wang, X. Histone demethylase lysine demethylase 5B in development and cancer. Oncotarget 2017, 8, 8980–8991. [Google Scholar] [CrossRef]
- Rao, C.V.; Kawamori, T.; Hamid, R.; Reddy, B.S. Chemoprevention of colonic aberrant crypt foci by an inducible nitric oxide synthase-selective inhibitor. Carcinogenesis 1999, 20, 641–644. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, S.-A.; Vlad, M.-L.; Lazar, A.-G.; Muresian, H.; Simionescu, M.; Manea, A. Pharmacological Inhibition of Lysine-Specific Demethylase 1A Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice by a Mechanism Involving Decreased Oxidative Stress and Inflammation; Potential Implications in Human Atherosclerosis. Antioxidants 2022, 11, 2382. https://doi.org/10.3390/antiox11122382
Manea S-A, Vlad M-L, Lazar A-G, Muresian H, Simionescu M, Manea A. Pharmacological Inhibition of Lysine-Specific Demethylase 1A Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice by a Mechanism Involving Decreased Oxidative Stress and Inflammation; Potential Implications in Human Atherosclerosis. Antioxidants. 2022; 11(12):2382. https://doi.org/10.3390/antiox11122382
Chicago/Turabian StyleManea, Simona-Adriana, Mihaela-Loredana Vlad, Alexandra-Gela Lazar, Horia Muresian, Maya Simionescu, and Adrian Manea. 2022. "Pharmacological Inhibition of Lysine-Specific Demethylase 1A Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice by a Mechanism Involving Decreased Oxidative Stress and Inflammation; Potential Implications in Human Atherosclerosis" Antioxidants 11, no. 12: 2382. https://doi.org/10.3390/antiox11122382
APA StyleManea, S.-A., Vlad, M.-L., Lazar, A.-G., Muresian, H., Simionescu, M., & Manea, A. (2022). Pharmacological Inhibition of Lysine-Specific Demethylase 1A Reduces Atherosclerotic Lesion Formation in Apolipoprotein E-Deficient Mice by a Mechanism Involving Decreased Oxidative Stress and Inflammation; Potential Implications in Human Atherosclerosis. Antioxidants, 11(12), 2382. https://doi.org/10.3390/antiox11122382








