Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Unripe Grape Extracts
2.2. Winemaking Process
2.3. Chemicals
2.4. General Analysis
2.5. Color Intensity and Hue
2.6. CIELab Chromatic Characteristics
2.7. Copigmentation
2.8. Total Phenols
2.9. DPPH
2.10. FRAP
2.11. ABTS
2.12. Microbiological Analysis
2.13. Sensory Evaluation
2.14. Economic Evaluation
2.15. Statistical Analysis
3. Results and Discussion
3.1. Chemical Analyses
3.2. Microbiological Analysis
3.3. Sensory Evaluations
3.4. Economic Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bartowsky, E.J. Bacterial spoilage of wine and approaches to minimize it. Lett. Appl. Microbiol. 2009, 48, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Doneche, B.; Lonvaud, A. Handbook of Enology: The Microbiology of Wine and Vinifications; John Wiley & Sons Ltd: Chichester, UK, 2006; Volume 1. [Google Scholar]
- Guerrero, R.F.; Cantos-Villar, E. Demonstrating the Efficiency of Sulphur Dioxide Replacements in Wine: A Parameter Review. Trends Food Sci. Technol. 2015, 42, 27–43. [Google Scholar] [CrossRef]
- Comuzzo, P.; Rauhut, D.; Werner, M.; Lagazio, C.; Zironi, R. A Survey on Wines from Organic Viticulture from Different European Countries. Food Control. 2013, 34, 274–282. [Google Scholar] [CrossRef]
- Lisanti, T.; Blaiotta, G.; Nioi, C.; Moio, L. Alternative methods to SO2 for microbiological stabilization of wine. Compr. Rev. Food Sci. Food Saf. 2019, 18, 455–479. [Google Scholar] [CrossRef]
- Motta, S.; Guaita, M.; Cassino, C.; Bosso, A. Relationship between Polyphenolic Content, Antioxidant Properties and Oxygen Consumption Rate of Different Tannins in a Model Wine Solution. Food Chem. 2020, 313, 126045. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Ruiz-Moreno, M.J.; Garde-Cerdán, T.; Puertas, B.; Moreno-Rojas, J.M.; Gonzalo-Riago, A. Effect of Hydroxytyrosol on Quality of Sulfur Dioxide-Free Red Wine. Food Chem. 2016, 192, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Raposo, R.; Chinnici, F.; Ruiz-Moreno, M.J.; Puertas, B.; Cuevas, F.J.; Carbú, M.; Guerrero, R.F.; Ortíz-Somovilla, V.; Morenorojas, J.M.; Cantos-Villar, E. Sulfur Free Red Wines through the Use of Grapevine Shoots: Impact on the Wine Quality. Food Chem. 2018, 243, 453–460. [Google Scholar] [CrossRef]
- Esparza, I.; Martínez-Inda, B.; Cimminelli, M.J.; Jimeno-Mendoza, M.C.; Moler, J.A.; Jiménez-Moreno, N.; Ancín-Azpilicueta, C. Reducing SO2 Doses in Red Wines by Using Grape Stem Extracts as Antioxidants. Biomolecules 2020, 10, 1369. [Google Scholar] [CrossRef]
- Sonni, F.; Jesus, M.; Bastante, C.; Chinnici, F.; Natali, N.; Riponi, C. Replacement of Sulfur Dioxide by Lysozyme and Oenological Tannins during Fermentation: Influence on Volatile Composition of White Wines. J. Sci. Food Agric. 2009, 89, 688–696. [Google Scholar] [CrossRef]
- González-Rompinelli, E.M.; Rodríguez-Bencomo, J.J.; García-Ruiz, A.; Sánchez-Patán, F.; Martín-álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. A Winery-Scale Trial of the Use of Antimicrobial Plant Phenolic Extracts as Preservatives during Wine Ageing in Barrels. Food Control. 2013, 33, 440–447. [Google Scholar] [CrossRef]
- Salaha, M.I.; Kallithraka, S.; Marmaras, I.; Koussissi, E.; Tzourou, I. A natural alternative to sulphur dioxide for red wine production: Influence on colour, antioxidant activity and anthocyanin content. J. Food Compos. Anal. 2008, 21, 660–666. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of crop level on growth, yield and wine quality of a high yielding Carignane vineyard. Am. J. Enol. Vitic. 1984, 35, 247–252. [Google Scholar] [CrossRef]
- Fia, G.; Gori, C.; Bucalossi, G.; Borghini, F.; Zanoni, B. A Naturally Occurring Antioxidant Complex from Unripe Grapes: The Case of Sangiovese (v. Vitis Vinifera). Antioxidants 2018, 7, 27. [Google Scholar] [CrossRef]
- Fia, G.; Bucalossi, G.; Gori, C.; Borghini, F.; Zanoni, B. Recovery of Bioactive Compounds from Unripe Red Grapes (Cv.Sangiovese) through a Green Extraction. Foods 2020, 9, 566. [Google Scholar] [CrossRef] [PubMed]
- Fia, G.; Bucalossi, G.; Zanoni, B. Characterisation of Extracts Obtained from Unripe Grapes and Evaluation of Their Potential Protective Effects against Oxidation of Wine Colour in Comparison with Different Oenological Products. Foods 2021, 10, 1499. [Google Scholar] [CrossRef]
- Bucalossi, G.; Fia, G.; Dinnella, C.; De Toffoli, A.; Canuti, V.; Zanoni, B.; Servili, M.; Pagliarini, E.; Gallina Toschi, T.; Monteleone, E. Functional and Sensory Properties of Phenolic Compounds from Unripe Grapes in Vegetable Food Prototypes. Food Chem. 2020, 315, 126291. [Google Scholar] [CrossRef]
- Proserpio, C.; Fia, G.; Bucalossi, G.; Zanoni, B.; Spinelli, S.; Dinnella, C.; Monteleone, E.; Pagliarini, E. Winemaking Byproducts as Source of Antioxidant Components: Consumers’ Acceptance and Expectations of Phenol-enriched Plant-based Food. Antioxidants 2020, 9, 661. [Google Scholar] [CrossRef]
- De Dupas Matos, A.; Marangon, M.; Magli, M.; Cianciabella, M.; Predieri, S.; Curioni, A.; Vincenzi, S. Sensory Characterization of Cucumbers Pickled with Verjuice as Novel Acidifying Agent. Food Chem. 2019, 286, 78–86. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of Dipping Pre-Treatment with Unripe Grape Juice on Dried “Golden Delicious” Apple Slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and ripening in grape berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar] [CrossRef]
- Hemmelskamp, J. Environmental policy instruments and their effects on innovation. Eur. Plan. Stud. 1997, 5, 177–194. [Google Scholar] [CrossRef]
- Fabbrizzi, S.; Alampi Sottini, V.; Cipollaro, M.; Menghini, S. Sustainability and Natural Wines: An Exploratory Analysis on Consumers. Sustainability 2021, 13, 7645. [Google Scholar] [CrossRef]
- Costanigro, M.; Appleby, C.; Menke, S.D. The wine headache: Consumer perceptions of sulfites and willingness to pay for non-sulfited wines. Food Qual. Prefer. 2014, 31, 81–89. [Google Scholar] [CrossRef]
- D’Amico, M.; Di Vita, G.; Monaco, L. Exploring environmental consciousness and consumer preferences for organic wines without sulphites. J. Clean. Prod. 2016, 12, 64–71. [Google Scholar] [CrossRef]
- Pícha, K.; Navrátil, J. The factors of Lifestyle of Health and Sustainability influencing pro-environmental buying behaviour. J. Clean. Prod. 2019, 234, 233–241. [Google Scholar] [CrossRef]
- Fia, G.; Gori, C. Process for the extraction of antioxidants from plant matrices. Italian Patent Number 102016000022015, 2 March 2016. [Google Scholar]
- OIV. International Organisation of the Vine and Wine. Compendium of International Analysis of Methods. Available online: http://www.oiv.int/oiv/info/frmethodesanalyses (accessed on 31 October 2019).
- Glories, Y. La couleur des vins rouges 2. Mesure, origine et interprétation. OENO ONE 1984, 18, 253–271. [Google Scholar] [CrossRef]
- OIV. International Organisation of the Vine and Wine. Compendium of International Analysis of Methods—OIV Chromati Characteristics. Available online: http://www.oiv.int/public/medias/2478/oiv-ma-as2-11.pdf (accessed on 31 October 2019).
- Boulton, R. The Copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Di Stefano, R.; Cravero, M.C.; Gentilini, N. Metodi per Lo Studio Dei Polifenoli Dei Vini. Enotecnico 1989, 25, 83–89. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C.L.W.T. Use of free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cavazza, A.; Grando, M.S.; Zini, C. Rilevazione della flora microbica di mosti e vini. Vignevini 1992, 9, 17–20. [Google Scholar]
- De Man, J.C.; Rogosa, D.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Granchi, L.; Bosco, M.; Messini, A.; Vincenzini, M. Rapid detection and quantification of yeast species during spontaneous wine fermentation by PCR-RFLP analysis of the rDNA ITS region. J. Appl. Microbiol. 1999, 87, 949–956. [Google Scholar] [CrossRef]
- ISO 4120:2021; Sensory Analysis—Methodology—Triangle Test. ISO: Geneva, Switzerland, 2021.
- ISO 8589. 2007; Sensory Analysis—General Guidance for the Design of Testrooms. ISO: Geneva, Switzerland, 2007.
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Effect of substituents on the ionisation of flavylium salts and anthocyanins and their reactions with sulphur dioxide. Chem. Ind. 1966, 2, 1965–1966. [Google Scholar]
- Sánchez-Gómez, R.; Alamo-Sanza, M.; Martínez-Gil, A.M.; Nevares, I. Red wine aging by different micro-oxygenation systems and oak wood-effects on anthocyanins, copigmentation and color evolution. Processes 2020, 8, 1250. [Google Scholar] [CrossRef]
- Marchante, L.; Loarce, L.; Izquierdo-Cañas, P.M.; Alañón, M.E.; García-Romero, E.; Pérez-Coello, M.S.; Díaz-Maroto, M.C. Natural extracts from grapes seed and stem by-products in combination with colloidal silver as alternative preservatives to SO2 for white wines: Effects on chemical composition and sensorial properties. Food Res. Int. 2019, 125, 108594. [Google Scholar] [CrossRef]
- Marchante, L.; Márquez, K.; Contreras, D.; Izquierdo-Cañas, P.M.; García-Romero, E.; Díaz-Maroto, M.C. Impact of oenological antioxidant substances on the formation of 1-hydroxyethyl radical and phenolic composition in SO2 free red wines. J. Sci. Food Agric. 2020, 100, 3401–3407. [Google Scholar] [CrossRef]
- Fan, L.; Wang, Y.; Xie, P.; Zhang, L.; Li, Y.; Zhou, J. Copigmentation effects of phenolics on color enhancement and stability of blackberry wine residue anthocyanins: Chromaticity, kinetics and structural simulation. Food Chem. 2019, 275, 299–308. [Google Scholar] [CrossRef] [PubMed]
- García-Marino, M.; Escudero-Gilete, M.L.; Heredia, F.J.; Escribano-Bailón, M.T.; Rivas-Gonzalo, J.C. Color-copigmentation study by tristimulus colorimetry (CIELAB) in red wines obtained from Tempranillo and Graciano varieties. Food Res. Int. 2013, 51, 123–131. [Google Scholar] [CrossRef]
- Van Wyk, S.; Silva, F.V.M. Non thermal Preservation of Wine. In Preservatives and Preservation Approaches in Beverages; Mihai Grumezescu, A., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 203–235. [Google Scholar]
- Izquierdo-Cañas, P.M.; García-Romero, E.; Huertas-Nebreda, B.; Gómez-Alonso, S. Colloidal silver complex as an alternative to sulphur dioxide in winemaking. Food Control. 2012, 23, 73–81. [Google Scholar] [CrossRef]
- Santos, M.C.; Nunes, C.; Cappelle, J.; Gonçalves, F.J.; Rodrigues, A.; Saraiva, J.A.; Coimbra, M.A. Effect of high pressure treatments on the physicochemical properties of a sulphur dioxide-free red wine. Food Chemistry 2013, 141, 2558–2566. [Google Scholar] [CrossRef] [PubMed]
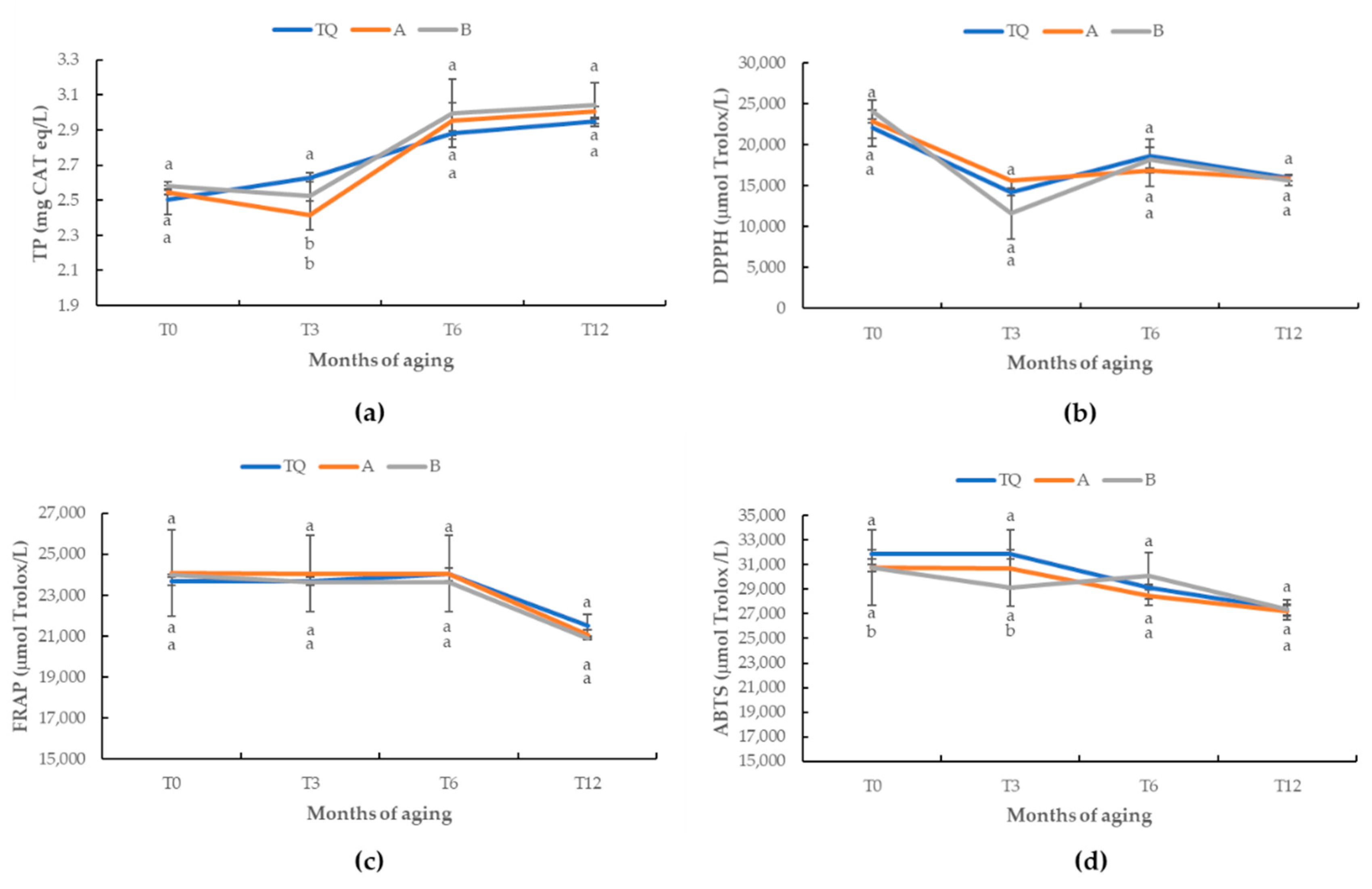

| TQ | A | B | F-Value | |
|---|---|---|---|---|
| Abs420 | 3.43 ± 0.01 c | 3.68 ± 0.01 b | 3.73 ± 0.02 a | 749.65 *** |
| Abs520 | 4.88 ± 0.02 b | 5.42 ± 0.01 a | 5.43 ± 0.01 a | 2067.66 *** |
| Abs620 | 1.03 ± 0.01 c | 1.12 ± 0.01 b | 1.14 ± 0.00 a | 810.00 *** |
| ABS420% | 36.70 ± 0.08 a | 36.03 ± 0.07 c | 36.22 ± 0.09 b | 98.73 *** |
| Abs520% | 52.26 ± 0.08 c | 52.99 ± 0.10 a | 52.66 ± 0.08 b | 93.22*** |
| Abs620% | 11.02 ± 0.04 b | 10.98 ± 0.03 b | 11.11 ± 0.01 a | 27.07 *** |
| CI | 9.34 ± 0.03 c | 10.23 ± 0.02 b | 10.31 ± 0.03 a | 1846.91 *** |
| Hue | 0.702 ± 0.003 a | 0.680 ± 0.003 c | 0.682 ± 0.003 b | 98.90 *** |
| L* | 73.50 ± 0.38 a | 70.99 ± 0.11 b | 71.23 ± 0.21 b | 164.18 *** |
| a* | 27.92 ± 0.28 b | 30.92 ± 0.15 a | 30.90 ± 0.17 a | 388.31 *** |
| b* | 2.09 ± 0.09 a | 2.23 ± 0.15 a | 2.05 ± 0.11 a | 3.27 (*) |
| C* | 28.01 ± 0.28 b | 31.02 ± 0.14 a | 30.97 ± 0.16 a | 404.00 *** |
| H* | 13.31 ± 0.63 a | 13.89 ± 1.08 a | 13.77 ± 0.23 a | 1.07 |
| AC | 0.79 ± 0.03 a | 0.79 ± 0.06 a | 0.83 ± 0.02 a | 2.11 |
| COP | 18.43 ± 0.78 a | 18.03 ± 1.19 a | 18.73 ± 0.33 a | 1.04 |
| TA | 1.85 ± 0.15 a | 1.93 ± 0.01 a | 1.97 ± 0.01 a | 2.46 |
| AL | 43.06 ± 3.65 a | 44.19 ± 0.74 a | 44.18 ± 0.48 a | 0.54 |
| Ep | 1.66 ± 0.14 a | 1.651 ± 0.004 a | 1.65 ± 0.01 a | 0.02 |
| PP | 38.50 ± 3.32 a | 37.77 ± 0.45 a | 37.08 ± 0.20 a | 0.80 |
| FC | 6.84 ± 0.11 b | 6.93 ± 0.05 ab | 7.00 ± 0.03 a | 6.77 ** |
| TPI280 | 54.50 ± 0.21 a | 54.22 ± 0.11 b | 53.99 ± 0.13 b | 14.29 *** |
| TP | 2.50 ± 0.13 a | 2.55 ± 0.06 a | 2.58 ± 0.07 a | 0.95 |
| DPPH | 22,052 ± 2211 a | 22,891 ± 1221 a | 24,076 ± 1400 a | 0.68 |
| FRAP | 23,677 ± 178 a | 24,091 ± 2102 a | 24,009 ± 2291 a | 0.06 |
| ABTS | 31,842 ± 386 a | 30,770 ± 3048 a | 30734 ± 270 a | 0.20 |
| TQ | A | B | F-Value | |
|---|---|---|---|---|
| Abs420 | 4.00 ± 0.09 b | 4.32 ± 0.12 a | 4.30 ± 0.11 a | 11.60 ** |
| Abs520 | 4.77 ± 0.08 b | 4.99 ± 0.02 a | 4.95 ± 0.05 a | 16.81 *** |
| Abs620 | 1.20 ± 0.01 b | 1.37 ± 0.05 a | 1.34 ± 0.03 a | 25.37 *** |
| ABS420% | 40.09 ± 0.71 a | 40.42 ± 0.41 a | 40.58 ± 0.53 a | 0.75 |
| Abs520% | 47.83 ± 0.81 a | 46.71 ± 0.70 a | 46.75 ± 0.70 a | 3.10 (*) |
| Abs620% | 12.07 ± 0.12 b | 12.85 ± 0.30 a | 12.66 ± 0.16 a | 18.69 *** |
| CI | 9.99 ± 0.11 b | 10.69 ± 0.21 a | 10.60 ± 0.16 a | 28.84 *** |
| Hue | 0.83 ± 0.03 a | 0.86 ± 0.02 a | 0.86 ± 0.02 a | 1.75 |
| L* | 74.58 ± 0.02 a | 73.75 ± 0.41 b | 74.05 ± 0.47 ab | 6.19 * |
| a* | 21.55 ± 0.37 b | 22.03 ± 0.05 a | 22.22 ± 0.04 a | 44.35 *** |
| b* | 9.98 ± 0.47 a | 9.80 ± 1.35 a | 9.77 ± 0.61 a | 0.09 |
| C* | 23.66 ± 0.15 b | 24.40 ± 0.52 a | 24.44 ± 0.29 a | 20.96 *** |
| H* | 2.11 ± 0.15 a | 2.04 ± 0.33 a | 2.03 ± 0.14 a | 0.21 |
| AC | 0.63 ± 0.01 a | 0.33 ± 0.03 c | 0.47 ± 0.07 b | 66.86 *** |
| COP | 13.57 ± 0.11 a | 10.01 ± 0.24 c | 11.62 ± 0.32 b | 251.60 *** |
| TA | 1.54 ± 0.10 a | 1.41 ± 0.09 a | 1.42 ± 0.12 a | 2.16 |
| AL | 35.88 ± 0.48 a | 32.46 ± 0.33 b | 32.72 ± 1.63 b | 19.97 *** |
| Ep | 2.265 ± 0.002 c | 2.57 ± 0.01 a | 2.528 ± 0.004 b | 3383.12 *** |
| PP | 48.88 ± 0.07 c | 56.68 ± 0.35 a | 54.70 ± 0.41 b | 954.49 *** |
| FC | 7.44 ± 0.04 b | 7.93 ± 0.03 a | 7.91 ± 0.03 a | 309.03 *** |
| TPI280 | 59.11 ± 0.46 b | 60.58 ± 0.66 a | 60.37 ± 0.45 a | 14.33 *** |
| TP | 2.88 ± 0.09 a | 2.89 ± 0.13 a | 2.99 ± 0.15 a | 1.17 |
| DPPH | 15,931 ± 312 a | 15,812 ± 606 a | 15,812 ± 606 a | 1.54 |
| FRAP | 21,514 ± 534 a | 21,050 ± 243 a | 20,902 ± 145 a | 0.04 |
| ABTS | 27,343 ± 457 a | 27,255 ± 431 a | 27,340 ± 843 a | 1.00 |
| Aging (Months) | Sample | Yeasts (UFC/mL) | LAB (UFC/mL) | AAB (UFC/mL) | ||
|---|---|---|---|---|---|---|
| TQ | <20 | (2.06 ± 0.65) × 105 | a | <10 | ||
| T0 | A | <20 | (2.16 ± 0.55) × 104 | b | <10 | |
| B | <20 | (8.06 ± 2.58) × 104 | c | <10 | ||
| TQ | (3.00 ± 0.56) × 102 | a | <10 | <10 | ||
| T3 | A | (1.00 ± 0.50) × 10 | b | <10 | <10 | |
| B | (5.33 ± 0.58) × 10 § | c | <10 | <10 | ||
| TQ | (1.30 ± 0.28) × 102 | a | <10 | <10 | ||
| T6 | A | (2.45 ± 0.77) × 102 | a | <10 | <10 | |
| B | (7.75 ± 4.86) × 102 † | a | <10 | <10 | ||
| TQ | (6.39 ± 9.70) × 103 | a | <10 | <10 | ||
| T12 | A | (6.35 ± 5.66) × 103 | a | <10 | <10 | |
| B | (6.24 ± 4.23) × 103 ‡ | a | (1.56 ± 0.40) × 104 | <10 |
| Aging (Months) | Sample | Correct Responses | p-Value |
|---|---|---|---|
| T0 | TQL–AL | 11 | 0.41 n.s. |
| TQL–BL | 8 | 0.83 n.s. | |
| T3 | TQL–AL | 12 | 0.20 n.s |
| TQL–BL | 8 | 0.75 n.s. | |
| T6 | TQL–AL | 14 | 0.09 n.s. |
| TQL–BL | 8 | 0.83 n.s. | |
| T12 | TQL–AL | 9 | 0.98 n.s. |
| TQL–BL | 5 | 0.71 n.s. |
| Yield | ||
|---|---|---|
| 20 g | 30 g | |
| EUR/L | ||
| Sample A | 0.47 | 0.31 |
| Sample B | 0.94 | 0.63 |
| EUR/bottle (0.75 L) | ||
| Sample A | 0.36 | 0.24 |
| Sample B | 0.71 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fia, G.; Menghini, S.; Mari, E.; Proserpio, C.; Pagliarini, E.; Granchi, L. Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine. Antioxidants 2023, 12, 365. https://doi.org/10.3390/antiox12020365
Fia G, Menghini S, Mari E, Proserpio C, Pagliarini E, Granchi L. Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine. Antioxidants. 2023; 12(2):365. https://doi.org/10.3390/antiox12020365
Chicago/Turabian StyleFia, Giovanna, Silvio Menghini, Eleonora Mari, Cristina Proserpio, Ella Pagliarini, and Lisa Granchi. 2023. "Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine" Antioxidants 12, no. 2: 365. https://doi.org/10.3390/antiox12020365
APA StyleFia, G., Menghini, S., Mari, E., Proserpio, C., Pagliarini, E., & Granchi, L. (2023). Replacement of SO2 with an Unripe Grape Extract and Chitosan during Oak Aging: Case Study of a Sangiovese Wine. Antioxidants, 12(2), 365. https://doi.org/10.3390/antiox12020365








