Abstract
Plants and brown algae avoid photoinhibition (decline in photosystem II efficiency, Fv/Fm) caused by excess light energy and oxidative stress through several photoprotective mechanisms, such as antioxidant xanthophyll production and heat dissipation. The heat dissipation can be measured as non-photochemical quenching (NPQ) and is strongly driven by de-epoxidation of xanthophyll cycle pigments (XCP). Although NPQ is known to increase under high light acclimation and nutrient-deficient conditions, a few studies have investigated the combined effects of the conditions on both NPQ and associated xanthophyll-to-chlorophyll (Chl) a ratio. The present study investigated the photosynthetic parameters of the brown alga Sargassum fusiforme acclimated to three irradiance levels combined with three nutrient levels. Elevated irradiance decreased Fv/Fm but increased NPQ, XCP/Chl a ratio, and fucoxanthin/Chl a ratio, suggesting the photoprotective role of antioxidant fucoxanthin in brown algae. Reduced nutrient availability increased NPQ but had no effect on the other variables, including XCP/Chl a ratio and its de-epoxidation state. The results indicate that NPQ can be used as a sensitive stress marker for nutrient deficiency, but cannot be used to estimate XCP pool size and state.
1. Introduction
In photosynthetic organisms, high irradiance (i.e., high light intensity) under environmental stresses, such as low and high temperature, often causes photoinhibition, which is described as a decline in photosystem II efficiency (Fv/Fm), because excess light energy enhances the production of harmful reactive oxygen species in chloroplasts [1,2,3]. To cope with the oxidative stress, such organisms have evolved several photoprotective mechanisms, including the production of antioxidants and dissipation of the excess light energy as heat [2,3,4]. Both mechanisms are strongly associated with carotenoids (carotenes and xanthophylls) because several compounds, such as β-carotene (β-Car), zeaxanthin (Zx), and fucoxanthin (Fx) act as antioxidants [3,5,6], and the de-epoxidation of xanthophyll cycle pigments (XCP) from violaxanthin (Vx) to Zx through antheraxanthin (Ax) is one of major drivers of the heat dissipation in various organisms, including plants and brown algae [4,7,8].
As one of the pigments involved in the carotenoid biosynthetic pathway that leads to the formation of xanthophylls from carotene, β-Car can be converted to β-cryptoxanthin, Zx, Ax, Vx, and neoxanthin, while Fx in brown algae is hypothesized to be synthesized from neoxanthin [6]. Some of these carotenoids bind with protein and chlorophyll (Chl) a, constituting photosystem II reaction center [9] and light-harvesting complex II [10]. The carotenoid-to-Chl a ratio of various organisms is reported to increase in response to high light acclimation [11,12,13]. Moreover, our previous studies have shown the effects of elevated irradiance and nutrient availability on Chl a content, Chl c/ Chl a, Fx/Chl a, and XCP/Chl a ratios in brown algae [14,15]. However, the role of Fx as a photoprotective compound in brown algae remains unclear because the Fx/Chl a ratio does not change under elevated irradiance acclimation [14,15].
The heat dissipation can be quantified as non-photochemical quenching (NPQ) using pulse amplitude modulation (PAM) chlorophyll fluorometers [4,5,6]. Previous studies have shown that high light acclimation enhanced NPQ (or the similar indicator, qN) of several organisms, including brown algae [4,16,17,18]. Moreover, NPQ is reported to increase under nutrient-deficient conditions in plants [19,20] and algae [17,21,22,23], although the underlying physiological mechanisms remain uncertain [14]. Meanwhile, NPQ generally correlates with de-epoxidation states (DES) of XCP in plants [24] and brown algae [11], and it is also strongly affected by the total amount of XCP (i.e., XCP pool size) in brown algae [4,11]. Hence, increased NPQ of brown algae under reduced nutrient availability may be caused by changing XCP pool size and/or DES of XCP. However, the nutrient effects on NPQ and XCP of brown algae have rarely been quantified at the same time.
Large brown algae (kelps and fucoids) are the dominant taxa in temperate reef ecosystems [25,26,27]. The present study investigated the combined effects of irradiance and nutrient availability on photosynthetic parameters (Fv/Fm and NPQ) and pigments (Chl a content, Chl c/ Chl a ratio, β-Car/Chl a ratio, Fx/Chl a ratio, XCP/Chl a ratio, and DES) in the fucoid brown alga Sargassum fusiforme, which is one of the common and commercially important species in Asian countries. Holdfasts of the species were used in the present study instead of the shoots because they have important ecological traits, including the ability to grow without shoots, regeneration ability via germination of new shoots (i.e., vegetative reproduction) [28,29], and high tolerance to warm and nutrient-poor conditions [28].
2. Materials and Methods
2.1. Sample Preparation
Five individuals of S. fusiforme with relatively large holdfasts were collected in June 2021 at a depth of 0–1 m along the Yojiro coast (31°33′30″ N, 130°33′47″ E), Kagoshima Bay, southern Japan, and were transported to the laboratory in insulated cool boxes. Ten holdfast segments (5 mm in length) without shoots and other sessile organisms were cut from each specimen, yielding a total of 50 segments. Each of the 10 segments derived from the S. fusiforme individuals were placed in petri dishes containing 30 mL sterilized seawater (a total of five dishes). Subsequently, the petri dishes were incubated for 24 h at an optimal temperature of 20 °C [28] and irradiance of 130 μmol photons m−2 s−1 with a 12 h light (L): 12 h dark (D) photoperiod.
2.2. Culture Experiment
At the beginning of the experiment, the wet weight of each segment (initial value) was measured using an electronic balance (0.1 mg accuracy) after the removal of excess moisture by blotting on paper towels. The 50 segments were divided into 10 groups, with each group having five specimens with a similar size distribution. One of the 10 groups was used to evaluate initial photosynthetic parameters and pigment content. Each of the remaining nine groups were subjected to one of the nine different treatments, consisting of three irradiance levels (30, 130, and 300 μmol photons m−2 s−1) and three nutrient levels.
The three irradiance levels were set based on compensation (i.e., 5–37 μmol photons m−2 s−1) [30], optimal growth (i.e., 100–180 μmol photons m−2 s−1) [31], and saturation irradiance values of the species (i.e., 391 μmol photons m−2 s−1) [30]. Light with wavelength of 400–700 nm (i.e., photosynthetically active radiation) was provided by white fluorescent tubes. The nutrient levels were achieved by adjusting the dilution ratio of Provasoli’s enriched seawater (PESI) to 0%, 5%, and 25%. The 0% PESI was prepared using artificial seawater without nitrates or phosphates (Marine art SF-1, Tomita Pharmaceutical Co., Ltd., Naruto, Japan). The dissolved inorganic nitrogen concentrations in the 5% and 25% PESI are theoretically 40 and 200 μM, respectively [14,15].
The holdfast segments were cultured for 28 d at 20 °C under a 12 h L: 12 h D photoperiod. The culture media in each petri dish were changed every 7 d. Each treatment was replicated using five segments derived from five different individuals (one segment per petri dish, five petri dishes per treatment) because physiological responses may differ among individuals [32]. At the end of the culture experiment, the wet weights of the segments (final value) were measured and relative growth rates (RGR, % d−1) were calculated as 100 × ln(final value/initial value)/28 d. The Fv/Fm of each segment was measured using a JUNIOR-PAM chlorophyll fluorometer (Heinz Walz GmbH, Effeltrich, Germany) after more than 1 h of dark acclimation to exclude energy-dependent fluorescence quenching and state transitions [33,34]. The maximum value of NPQ was also measured during the exposure of segments to actinic light intensity of 1150 μmol photons m−2 s−1 for 5 min.
2.3. Pigment Analyses
Pigment contents, including Chl a, Chl c2, β-Car, Zx, Ax, Vx, and Fx were measured using high-performance liquid chromatography (HPLC) after transferring the samples into 6 mL bottles containing 4 mL dimethylformamide. The samples were diluted in distilled water to achieve 80% concentration and then analyzed by an HPLC system (Shimadzu Corp., Kyoto, Japan), according to the method described by Zapata et al. [35]. Afterward, the total content of XCP (the sum of Vx, Ax, and Zx) and DES (the ratio of 0.5Ax + Zx to total XCP) [10] were calculated. Only Chl a content and the ratios of other pigments (Chl c2, Fx, and XCP) to Chl a in S. fusiforme holdfasts are presented in this study because the responses to abiotic factors among the pigment contents were similar; however, the responses differed among the ratios of the pigments to Chl a content of the kelp Undaria pinnatifida [14].
2.4. Statistical Analyses
Differences in the initial wet weights of S. fusiforme segments among the ten treatment groups were analyzed using analysis of variance (ANOVA). The effects of irradiance and nutrient levels on all variables of the holdfasts were analyzed by two-way ANOVA. Some variables were logarithmically transformed because the data were not normally distributed (Shapiro-Wilk test, p < 0.05) and did not show homogeneous variances (Bartlett’s test, p < 0.05). All analyses were performed using R (https://www.r-project.org, accessed on 1 November 2022). Data are presented as means ± standard deviation.
3. Results
The average initial wet weight of S. fusiforme holdfasts was 12.0 ± 4.07 mg. No significant differences were observed in the initial wet weights among the ten groups (df = 9, MS =13.670, F = 0.790, p = 0.627). The initial Fv/Fm and NPQ values were 0.67 ± 0.06 and 3.25 ± 0.60, respectively. The initial values of Chl a content, Chl c/Chl a ratio, β-Car/Chl a ratio, Zx/Chl a ratio, Vx/Chl a ratio, Fx/Chl a ratio, XCP/Chl a ratio, and DES were 0.201 ± 0.029 mg/g, 0.081 ± 0.007, 0.068 ± 0.005, 0.002 ± 0.001, 0.051 ± 0.005, 0.246 ± 0.014, 0.053 ± 0.005, and 0.039 ± 0.016, respectively.
According to the two-way ANOVA results, irradiance had a significant effect on RGR, Fv/Fm, Chl a content; however, the effect on Chl c/Chl a ratio was not significant (Table 1 and Figure 1). RGR was significantly higher at medium and high irradiance levels than at low irradiance levels. In contrast, Fv/Fm was lower at medium and high irradiance levels than at low irradiance levels. Chl a content decreased in response to elevated irradiance. The effects of nutrient levels and interaction between irradiance and nutrient levels on the variables were not detected.

Table 1.
Results of two-way ANOVA on the effects of irradiance and nutrients on the variables of Sargassum fusiforme holdfasts. MS, F and p indicate mean square, F value, and significance probability, respectively. Asterisks indicate the significant effects (p < 0.05).
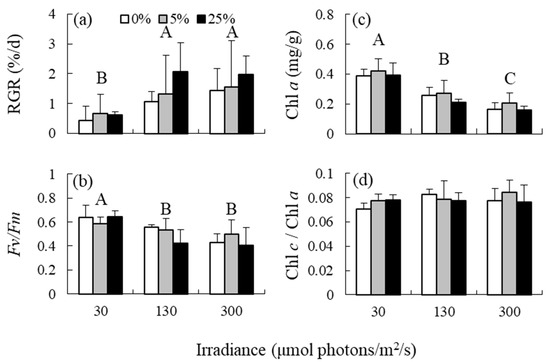
Figure 1.
(a) Relative growth rates (RGRs), (b) photosystem II efficiency (Fv/Fm), (c) chlorophyll (Chl) a content, and (d) Chl c2/Chl a ratios of the brown alga Sargassum fusiforme acclimated to three irradiance levels (30, 130, and 300 μmol photons m−2 s−1) and three nutrient levels (0, 5, and 25% Provasoli’s enriched seawater, PESI). Values are expressed as means ± standard deviation. Different large letters indicate statistical significances (p < 0.05) among the different treatments.
Irradiance had no effect on the β-Car/Chl a ratio, but had an effect on the Zx/Chl a, Vx/Chl a, and Fx/Chl a ratios (Table 1 and Figure 2). The Zx/Chl a ratio was significantly higher at medium and high irradiance levels than at low irradiance levels. The Vx/Chl a and Fx/Chl a ratios increased in response to elevated irradiance. The effects of nutrient levels and interaction between irradiance and nutrient levels on the variables included in the analysis were not detected. Additionally, high correlations were observed between the β-Car/Chl a and Zx/Chl a ratios, and between the Vx/Chl a and Fx/Chl a ratios, but not between the Zx/Chl a and Vx/Chl a ratios, and between the Zx/Chl a and Fx/Chl a ratios (Figure 3).
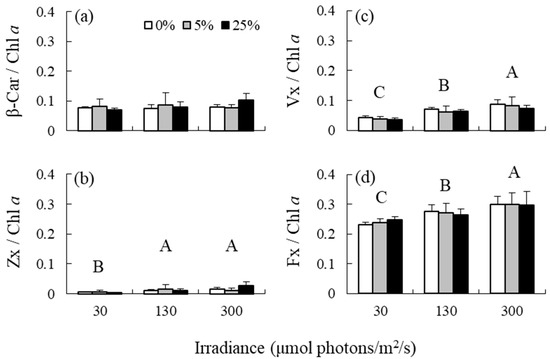
Figure 2.
(a) β-carotene (β-Car)/Chl a ratios, (b) zeaxanthin (Zx)/Chl a ratios, (c) violaxanthin (Vx)/Chl a ratios, and (d) fucoxanthin (Fx) /Chl a ratios of the brown alga Sargassum fusiforme acclimated to three irradiance levels (30, 130, and 300 μmol photons m−2 s−1) and three nutrient levels (0, 5, and 25% Provasoli’s enriched seawater, PESI). Values are expressed as means ± standard deviation. Different large letters indicate statistically significant differences (p < 0.05) among different treatments.
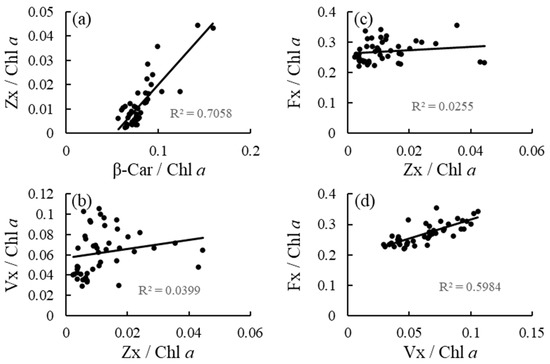
Figure 3.
(a) Correlations between β-Car/Chl a and Zx/Chl a ratios, (b) between Zx/Chl a and Vx/Chl a ratios, (c) between Zx/Chl a and Fx/Chl a ratios, and (d) between Vx/Chl a and Fx/Chl a ratios of the brown alga Sargassum fusiforme acclimated to three irradiance levels (30, 130, and 300 μmol photons m−2 s−1) and three nutrient levels (0, 5, and 25% Provasoli’s enriched seawater, PESI).
Irradiance had a significant effect on the XCP/Chl a ratio and NPQ (Table 1 and Figure 4); however, its effect on DES was not significant (Table 1). The XCP/Chl a ratio increased in response to elevated irradiance. NPQ was significantly higher at medium and high irradiance levels than at low irradiance levels. Nutrient levels had an effect on NPQ only, and NPQ was higher in 0% PESI treatments than in the 5% and 25% PESI treatments. No significant interaction was observed between irradiance and nutrient levels on the variables included in the analysis.
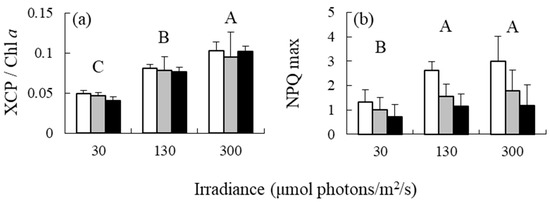
Figure 4.
(a) Xanthophyll cycle pigment (XCP)/Chl a ratios and (b) non-photochemical quenching (NPQ) values of the brown alga Sargassum fusiforme acclimated to three irradiance levels (30, 130, and 300 μmol photons m−2 s−1) and three nutrient levels (0, 5, and 25% Provasoli’s enriched seawater, PESI). Values are expressed as means ± standard deviation. Different large letters indicate statistically significant differences (p < 0.05) among different treatments.
4. Discussion
The indicator of photoinhibition, Fv/Fm of photosynthetic organisms is known to decrease in response to high irradiance under environmental stresses, such as high or low temperature [1,2,3]. For example, Balfagón et al. [36] showed that a negative effect of increased irradiance (50–600 μmol photons m−2 s−1) on Fv/Fm of the terrestrial plant Arabidopsis thaliana was synergized by elevated temperature from 23 °C to 42 °C. Similarly, Xu et al. [29] reported that Fv/Fm of the brown algae S. fusiforme reduced in response to increased irradiance (70–140 μmol photons m−2 s−1) at a high temperature of 24 °C but not at 16–20 °C. Machalek et al. [37] also reported that Fv/Fm of the kelp Saccharina latissima decreased in response to elevated irradiance (15–150 μmol photons m−2 s−1) at a low temperature of 5 °C but not at 17 °C. Meanwhile, nutrient-poor conditions during summer are one of the environmental stresses for brown algae, including S. fusiforme holdfasts [28,38]. In the present study, Fv/Fm of S. fusiforme holdfasts decreased by elevated irradiance (30–300 μmol photons m−2 s−1), while no significant interaction between irradiance and nutrient conditions was observed. This result suggests that the negative effect of high irradiance on Fv/Fm of this species was not strengthened by nutrient-poor conditions.
Balfagón et al. [36] also showed that the increased irradiance combined with the elevated temperature caused not only decreased Fv/Fm but also leaf senescence and reduced survival rate of A. thaliana. Similarly, Endo et al. [39] reported that elevated temperature (23–26 °C) at high irradiance (180 μmol photons m−2 s−1) resulted in reduced Fv/Fm and blade erosion of the kelp Eisenia bicyclis. Thus, photoinhibition is often accompanied by physiological damages in plant and algal bodies. However, the elevated irradiance (30–300 μmol photons m−2 s−1) reduced Fv/Fm but enhanced growth of S. fusiforme holdfasts in the present study. Therefore, the decline of Fv/Fm under high light acclimation seem not necessarily to accompany growth suppression in the absence of environmental stresses.
Chl a content of plants and algae is known to decrease under high light acclimation [14,15,40,41] and this response contributes to limiting absorption of excess light energy [19]. In addition, in the present study, Chl a content of S. fusiforme holdfasts decreased in response to increased acclimation irradiance from 30 to 300 μmol photons m−2 s−1. Moreover, microalgae are known to decrease their ratio of accessory Chl to Chl a under high light acclimation [41]. Previous studies have shown that Chl c/Chl a ratio of brown algae was lower in the thalli grown at a sun-exposed shallower depth than the same species collected at greater depths or shaded site [42]. However, in the present study, no significant difference was found in Chl c2/Chl a ratio of S. fusiforme holdfasts acclimated in three different irradiance conditions. Charan et al. [15] also reported that the Chl c2/Chl a ratio of S. fusiforme shoots was not affected by irradiance at 23 °C, although the value decreased in response to increased irradiance (30–150 μmol photons m−2 s−1) combined with heat stress (26 °C). Moreover, Endo et al. [14] reported that increased irradiance (30–180 μmol photons m−2 s−1) caused a decrease in the Chl c1/Chl a ratio but an increase in the Chl c2/Chl a ratio of the kelp U. pinnatifida. Hence, the variation in the Chl c/Chl a ratio of brown algae along depth and sun-exposure gradients found in previous studies [42] cannot be explained by the response to changing irradiance. It might be affected by changes in light quality associated with depth or sun-exposure, although this hypothesis needs to be tested.
In the carotenoid biosynthetic pathway, β-Car can be converted to β-cryptoxanthin, Zx, Ax, Vx, and neoxanthin, while Fx in brown algae is hypothesized to be synthesized from neoxanthin [6]. The hypothesis is partially supported by the results of the present study, which showed a stronger correlation between the Vx/Chl a and Fx/Chl a ratio than between the Zx/Chl a and Vx/Chl a or Fx/Chl a ratios in S. fusiforme holdfasts acclimated to nine types of treatments. In addition, a strong correlation was observed between the β-Car/Chl a and Zx/Chl a ratios, nevertheless elevated irradiance did not affect β-Car/Chl a ratio but affected Zx/Chl a ratio, implying that β-Car might be rapidly converted to Zx in this alga. Xie et al. [12] reported that elevated irradiance increased β-Car content of the red alga Neopyropia yezoensis, while such an increase in β-Car/Chl a ratio was not observed in the brown alga S. fusiforme in the present study. In contrast, irradiance elevation did not affect the Fx/Chl a ratio of U. pinnatifida and S. fusiforme shoots but increased the ratio of S. fusiforme holdfasts in the present study, suggesting that Fx is one of photoprotective compounds in this alga. Hence, the chemical form of carotenoids accumulated as a result of high light acclimation may differ among species and parts within the species.
Ocampo-Alvarez et al. [11] observed that the blades of the kelp Macrocystis pyrifera acclimated to shallow depths exhibited higher XCP/Chl a ratios and NPQ than those grown at greater depths. Moreover, previous studies have reported that high light acclimation resulted in increases in XCP/Chl a ratios [14] and NPQ [18] of brown algae. In addition, in the present study, the elevated irradiance increased both XCP/Chl a ratios and NPQ of S. fusiforme holdfasts. Hence, increased NPQ under high light acclimation can be explained by increased XCP pool size of the brown algae. Meanwhile, NPQ decreased in response to nutrient enrichment in S. fusiforme, which is consistent with the observation made in plants [19,20] and algae [17,21,22,23]. Although the XCP/Chl a ratio can decrease under nutrient enriched conditions [14,15], XCP/Chl a ratios and DES observed in our study did not change. The results suggest that the decrease in NPQ in response to nutrient enrichment was not due to the decreases in XCP pool size and de-epoxidation rate of Vx to Zx. Therefore, NPQ can be used as a sensitive stress marker for nutrient deficiency but cannot be used to estimate XCP pool size and state. Such a reduction in NPQ without any changes in XCP might be associated with state transitions between photosystems I and II [43,44], although this possibility needs to be examined.
5. Conclusions
The results of the present study showed that elevated irradiance caused photoinhibition and Chl a degradation but enhanced growth and photoprotection, which were reflected by the increases in Fx/Chl a ratios, XCP/Chl a ratios, and NPQ in S. fusiforme holdfasts, while nutrient availability had negligible effects on the variables other than NPQ. These traits may enhance the survival and growth of S. fusiforme holdfasts, without forming a canopy of its own shoots under high irradiance and nutrient-poor conditions that occur during summer [28]. However, little is known regarding the combined effects of elevated summer temperatures under climate change and increased irradiance on XCP/Chl a ratios and NPQ in brown algae, including S. fusiforme. Furthermore, the mechanisms of associated with decreases in NPQ under nutrient enrichment conditions still remain unknown. Therefore, further studies should be conducted to investigate the effects of abiotic factors on NPQ and associated variables to enhance our understanding of photoprotection in photosynthetic organisms, including brown macroalgae.
Author Contributions
Conceptualization, H.E. and Y.O.; methodology, H.E. and Y.O.; software, H.E.; validation, H.E. and Y.O.; formal analysis, H.E. and Y.O.; investigation, H.M. and Y.O.; resources, H.E. and H.M.; data curation, H.E. and Y.O.; writing—original draft preparation, H.E. and H.M.; writing—review and editing, H.E. and Y.O.; visualization, H.E.; supervision, H.E. and Y.O.; project administration, H.E. and Y.O.; funding acquisition, H.E. and Y.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI Grant Number 20K06187.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data and any statistical analysis are available from the corresponding author upon request.
Acknowledgments
We thank R. Terada and T. Kobari of Kagoshima University for lending us some incubators.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta Bioenerg. 2007, 1767, 414–421. [Google Scholar] [CrossRef]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant. Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef]
- Pintó-Marijuan, M.; Munné-Bosch, S. Photo-oxidative stress markers as a measure of abiotic stress-induced leaf senescence: Advantages and limitations. J. Exp. Bot. 2014, 65, 3845–3857. [Google Scholar] [CrossRef]
- Goss, R.; Lepetit, B. Biodiversity of NPQ. J. Plant Physiol. 2015, 172, 13–32. [Google Scholar] [CrossRef]
- Havaux, M.; Dall’Osto, L.; Bassi, R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol. 2007, 145, 1506–1520. [Google Scholar] [CrossRef]
- Mikami, K.; Hosokawa, M. Biosynthetic pathway and health benefits of fucoxanthin, an algae-specific xanthophyll in brown seaweeds. Int. J. Mol. Sci. 2013, 14, 13763–13781. [Google Scholar] [CrossRef]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef]
- Ruban, A.V.; Wilson, S. The mechanism of non-photochemical quenching in plants: Localization and driving forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Umena, Y.; Kawakami, K.; Shen, J.R.; Kamiya, N. Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 2011, 473, 55–60. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, H.; Wang, K.; Kuang, T.; Zhang, J.; Gui, L.; An, X.; Chang, W. Crystal structure of spinach major light-harvesting complex at 2.72 Å resolution. Nature 2004, 428, 287–292. [Google Scholar] [CrossRef]
- Ocampo-Alvarez, H.; García-Mendoza, E. Antagonist effect between violaxanthin and de-epoxidated pigments in nonphotochemical quenching induction in the qE deficient brown alga Macrocystis pyrifera. Biochim. Biophys. Acta Bioenerg. 2013, 1827, 427–437. [Google Scholar] [CrossRef]
- Xie, X.; Lu, X.; Wang, L.; He, L.; Wang, G. High light intensity increases the concentrations of β-carotene and zeaxanthin in marine red macroalgae. Algal. Res. 2020, 47, 101852. [Google Scholar] [CrossRef]
- Izuhara, T.; Kaihatsu, I.; Jimbo, H.; Takaichi, S.; Nishiyama, Y. Elevated levels of specific carotenoids during acclimation to strong light protect the repair of photosystem II in Synechocystis sp. PCC 6803. Front. Plant Sci. 2020, 11, 1030. [Google Scholar] [CrossRef]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 1683–1693. [Google Scholar] [CrossRef]
- Charan, H.; Inomata, E.; Endo, H.; Sato, Y.; Okumura, Y.; Aoki, M.N. Decreased irradiance and nutrient enrichment mitigate the negative effect of ocean warming on growth and biochemical compositions of a canopy-forming marine macroalga. J. Mar. Sci. Eng. 2022, 10, 479. [Google Scholar] [CrossRef]
- Król, M.; Ivanov, A.G.; Jansson, S.; Kloppstech, K.; Huner, N.P. Greening under high light or cold temperature affects the level of xanthophyll-cycle pigments, early light-inducible proteins, and light-harvesting polypeptides in wild-type barley and the Chlorina f2 mutant. Plant Physiol. 1999, 120, 193–204. [Google Scholar] [CrossRef]
- Harrison, J.W.; Smith, R.E. Effects of nutrients and irradiance on PSII variable fluorescence of lake phytoplankton assemblages. Aquat. Sci. 2013, 75, 399–411. [Google Scholar] [CrossRef]
- Zhong, Z.H.; Wang, Y.; Qin, S.; Zhuang, L.C.; Li, J.J.; Song, W.L.; Liu, Z.Y. Short-term photoacclimation and photoregulation strategies of Sargassum horneri in response to temperature and light. Photosynthetica 2021, 59, 268–276. [Google Scholar] [CrossRef]
- Morales, F.; Abadía, J.; Abadía, A. Thermal energy dissipation in plants under unfavorable soil conditions. In Non-Photochemical Quenching and Energy Dissipation in Plants, Algae and Cyanobacteria; Demmig-Adams, B., Garab, G., Adams, W., III, Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 605–630. [Google Scholar]
- Su, B.; Wang, L.; Shangguan, Z. Morphological and physiological responses and plasticity in Robinia pseudoacacia to the coupling of water, nitrogen and phosphorus. J. Plant. Nutr. Soil Sci. 2021, 184, 271–281. [Google Scholar] [CrossRef]
- Petrou, K.; Kranz, S.A.; Doblin, M.A.; Ralph, P.J. Photophysiological responses of Fragilariopsis cylindrus (Bacillariophyceae) to nitrogen depletion at two temperatures. J. Phycol. 2012, 48, 127–136. [Google Scholar] [CrossRef]
- Rocha, G.S.; Parrish, C.C.; Lombardi, A.T.; da Graça Gama Melão, M. Biochemical and physiological responses of Selenastrum gracile (Chlorophyceae) acclimated to different phosphorus concentrations. J. Appl. Phycol. 2018, 30, 2167–2177. [Google Scholar] [CrossRef]
- Celis-Plá, P.S.; Brown, M.T.; Santillán-Sarmiento, A.; Korbee, N.; Sáez, C.A.; Figueroa, F.L. Ecophysiological and metabolic responses to interactive exposure to nutrients and copper excess in the brown macroalga Cystoseira tamariscifolia. Mar. Pollut. Bull. 2018, 128, 214–222. [Google Scholar] [CrossRef]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature: Uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]
- Coleman, M.A.; Wernberg, T. Forgotten underwater forests: The key role of fucoids on Australian temperate reefs. Ecol. Evol. 2017, 7, 8406–8418. [Google Scholar] [CrossRef]
- Wernberg, T.; Filbee-Dexter, K. Missing the marine forest for the trees. Mar. Ecol. Prog. Ser. 2019, 612, 209–215. [Google Scholar] [CrossRef]
- Smale, D.A. Impacts of ocean warming on kelp forest ecosystems. New Phytol. 2020, 225, 1447–1454. [Google Scholar] [CrossRef]
- Endo, H.; Sugie, T.; Yonemori, Y.; Nishikido, Y.; Moriyama, H.; Ito, R.; Okunishi, S. Vegetative reproduction is more advantageous than sexual reproduction in a canopy-forming clonal macroalga under ocean warming accompanied by oligotrophication and intensive herbivory. Plants 2021, 10, 1522. [Google Scholar] [CrossRef]
- Xu, L.; Luo, L.; Zuo, X.; Cao, C.; Lin, L.; Zheng, H.; Ma, Z.; Chen, B.; Wu, M. Effects of temperature and irradiance on the regeneration of juveniles from the holdfasts of Sargassum fusiforme, a commercial seaweed. Aquaculture 2022, 557, 738317. [Google Scholar] [CrossRef]
- Kokubu, S.; Nishihara, G.N.; Watanabe, Y.; Tsuchiya, Y.; Amamo, Y.; Terada, R. The effect of irradiance and temperature on the photosynthesis of a native alga Sargassum fusiforme (Fucales) from Kagoshima, Japan. Phycologia 2015, 54, 235–247. [Google Scholar] [CrossRef]
- Baba, M. Effects of temperature and irradiance on germling growth in eight Sargassaceous species. Rep. Mar. Ecol. Res. Inst. 2007, 10, 9–20. [Google Scholar]
- Jormalainen, V.; Ramsay, T. Resistance of the brown alga Fucus vesiculosus to herbivory. Oikos 2009, 118, 713–722. [Google Scholar] [CrossRef]
- Hanelt, D. Capability of dynamic photoinhibition in Arctic macroalgae is related to their depth distribution. Mar. Biol. 1998, 131, 361–369. [Google Scholar] [CrossRef]
- Bischof, K.; Hanelt, D.; Wiencke, C. Acclimation of maximal quantum yield of photosynthesis in the brown alga Alaria esculenta under high light and UV radiation. Plant Biol. 1999, 1, 435–444. [Google Scholar] [CrossRef]
- Zapata, M.; Rodríguez, F.; Garrido, J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: A new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Mar. Ecol. Prog. Ser. 2000, 195, 29–45. [Google Scholar] [CrossRef]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef]
- Machalek, K.M.; Davison, I.R.; Falkowski, P.G. Thermal acclimation and photoacclimation of photosynthesis in the brown alga Laminaria saccharina. Plant Cell Environ. 1996, 19, 1005–1016. [Google Scholar] [CrossRef]
- Endo, H.; Gao, X.A. New classification tool and a systematic review of macroalgal studies disentangle the complex interactive effects of warming and nutrient enrichment on primary production. Front. Mar. Sci. 2022, 9, 774801. [Google Scholar] [CrossRef]
- Endo, H.; Inomata, E.; Gao, X.; Kinoshita, J.; Sato, Y.; Agatsuma, Y. Heat stress promotes nitrogen accumulation in meristems via apical blade erosion in a brown macroalga with intercalary growth. Front. Mar. Sci. 2020, 7, 575721. [Google Scholar] [CrossRef]
- Dietz, K.J. Efficient high light acclimation involves rapid processes at multiple mechanistic levels. J. Exp. Bot. 2015, 66, 2401–2414. [Google Scholar] [CrossRef]
- MacIntyre, H.L.; Kana, T.M.; Anning, T.; Geider, R.J. Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. J. Phycol. 2002, 38, 17–38. [Google Scholar] [CrossRef]
- Perez-Bermudez, P.; Garcia-Carrascosa, M.; Cornejo, M.J.; Segura, J. Water-depth effects in photosynthetic pigment content of the benthic algae Dictyota dichotoma and Udotea petiolata. Aquat. Bot. 1981, 11, 373–377. [Google Scholar] [CrossRef]
- Shelly, K.; Higgins, T.; Beardall, J.; Wood, B.; McNaughton, D.; Heraud, P. Characterising nutrient-induced fluorescence transients (NIFTs) in nitrogen-stressed Chlorella emersonii (Chlorophyta). Phycologia 2007, 46, 503–512. [Google Scholar] [CrossRef]
- Haan, J.D.; Huisman, J.; Dekker, F.; Ten Brinke, J.L.; Ford, A.K.; van Ooijen, J.; van Duyl, F.C.; Vermeij, M.J.A.; Visser, P.M. Fast detection of nutrient limitation in macroalgae and seagrass with nutrient-induced fluorescence. PloS ONE 2013, 8, e68834. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).