Ecotoxicity of Copper(I) Chloride in Grooved Carpet Shell (Ruditapes decussatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling and Acclimation Phase
2.3. Experimental Design
2.3.1. Preparation and Quantification of Copper in Water
2.3.2. Lethal Concentration (LC50)
2.3.3. Sub-Acute Trial and Sampling Procedures
2.4. Oxidative Stress Biomarkers in Clams
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Copper-Environmental Health Criteria 200; World Health Organization: Geneva, Switzerland, 1998; pp. 1–389. [Google Scholar]
- Mikesell, R.F. The Global Copper Industry: Problems and Prospects, 1st ed.; Routledge: London, UK, 1988; pp. 1–174. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Osdaghi, E.; Behlau, F.; Köhl, J.; Jones, J.B.; Aubertot, J.N. Thirteen decades of antimicrobial copper compounds applied in agriculture. A review. Agron. Sustain. Dev. 2018, 38, 28. [Google Scholar] [CrossRef]
- Garside, M. Global Copper Usage 2010–2020. Statista. Available online: https://www.statista.com/topics/1409/copper/#dossierKeyfigures (accessed on 23 July 2022).
- Sikamo, J.; Mwanza, A.; Mweemba, C. Copper mining in Zambia-history and future. J. South. Afr. Inst. Min. Metall. 2016, 116, 491–496. [Google Scholar] [CrossRef]
- Lagos, G.; Peters, D.; Videla, A.; Jara, J.J. The effect of mine aging on the evolution of environmental footprint indicators in the Chilean copper mining industry 2001–2015. J. Clean. Prod. 2018, 174, 389–400. [Google Scholar] [CrossRef]
- Pietrzyk, S.; Tora, B. Trends in global copper mining—A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 427, 12002. [Google Scholar] [CrossRef]
- Traulsen, H.R.; Taylor, J.C.; George, D.B. Copper smelting-an overview. JOM 1982, 34, 35–40. [Google Scholar] [CrossRef]
- Newhook, R.; Hirtle, H.; Byrne, K.; Meek, M.E. Releases from copper smelters and refineries and zinc plants in Canada: Human health exposure and risk characterization. Sci. Total Environ. 2003, 301, 23–41. [Google Scholar] [CrossRef]
- Nikolić, I.P.; Milošević, I.M.; Milijić, N.N.; Mihajlović, I.N. Cleaner production and technical effectiveness: Multi-criteria analysis of copper smelting facilities. J. Clean. Prod. 2019, 215, 423–432. [Google Scholar] [CrossRef]
- Davis, A.P.; Shokouhian, M.; Ni, S. Loading estimates of lead, copper, cadmium, and zinc in urban runoff from specific sources. Chemosphere 2001, 44, 997–1009. [Google Scholar] [CrossRef]
- Luo, W.; Lu, Y.; Tong, X.; Wang, B.; Wang, G.; Shi, Y.; Wang, T.; Naile, J.; Giesy, J.P. Distribution of copper, cadmium, and lead in soils from former industrialized urban areas of Beijing, China. Bull. Environ. Contam. Toxicol. 2009, 82, 378–383. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Custódio, F.B.; Knupp, E.A.; Palmieri, H.E.; Silva, J.B.B.; Glória, M.B.A. Cadmium, copper and lead levels in different cultivars of lettuce and soil from urban agriculture. Environ. Pollut. 2018, 242, 383–389. [Google Scholar] [CrossRef]
- Schiff, K.; Diehl, D.; Valkirs, A. Copper emissions from antifouling paint on recreational vessels. Mar. Pollut. Bull. 2004, 48, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Swain, G.W. Managing the use of copper-based antifouling paints. Environ. Manag. 2007, 39, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, A.S.; Oranu, E.A.; Tao, M.; Keller, A.A. Release and detection of nanosized copper from a commercial antifouling paint. Water Res. 2016, 102, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Elia, A.C.; Magara, G.; Pastorino, P.; Zaccaroni, A.; Caldaroni, B.; Andreini, R.; Righetti, M.; Silvi, M.; Dörr, A.J.M.; Prearo, M. Ecotoxicity in Hyriopsis bialatus of copper and zinc biocides used in metal-based antifouling paints. Environ. Sci. Pollut. Res. 2022, 29, 18245–18258. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: London, UK, 2014; pp. 1–1542. [Google Scholar]
- Hall, L.W., Jr.; Anderson, R.D. A deterministic ecological risk assessment for copper in European saltwater environments. Mar. Pollut. Bull. 1999, 38, 207–218. [Google Scholar] [CrossRef]
- Bighiu, M.A.; Gorokhova, E.; Carney Almroth, B.; Eriksson Wiklund, A.-K. Metal contamination in harbours impacts life-history traits and metallothionein levels in snails. PLoS ONE 2017, 12, e0180157. [Google Scholar] [CrossRef]
- Muller-Karanassos, C.; Arundel, W.; Lindeque, P.K.; Vance, T.; Turner, A.; Cole, M. Environmental concentrations of antifouling paint particles are toxic to sediment-dwelling invertebrates. Environ. Pollut. 2021, 268, 115754. [Google Scholar] [CrossRef]
- Freitas, R.; Pinto, L.R.; Sampaio, M.; Costa, A.; Silva, M.; Rodrigues, A.M.; Quintino, V.; Figueira, E. Effects of depuration on the element concentration in bivalves: Comparison between sympatric Ruditapes decussatus and Ruditapes philippinarum. Estuar. Coast. Shelf Sci. 2012, 110, 43–53. [Google Scholar] [CrossRef]
- Freitas, R.; Martins, R.; Campino, B.; Figueira, E.; Soares, A.M.V.M.; Montaudouin, X. Trematode communities in cockles (Cerastoderma edule) of the Ria de Aveiro (Portugal): Influence of inorganic contamination. Mar. Pollut. Bull. 2014, 82, 117–126. [Google Scholar] [CrossRef]
- Esposito, G.; Meloni, D.; Abete, M.C.; Colombero, G.; Mantia, M.; Pastorino, P.; Prearo, M.; Pais, A.; Antuofermo, E.; Squadrone, S. The bivalve Ruditapes decussatus: A biomonitor of trace elements pollution in Sardinian coastal lagoons (Italy). Environ. Pollut. 2018, 242, 1720–1728. [Google Scholar] [CrossRef]
- Sfriso, A.A.; Chiesa, S.; Sfriso, A.; Buosi, A.; Gobbo, L.; Gnolo, A.B.; Argese, E. Spatial distribution, bioaccumulation profiles and risk for consumption of edible bivalves: A comparison among razor clam, Manila clam and cockles in the Venice Lagoon. Sci. Total Environ. 2018, 643, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Burioli, E.A.V.; Squadrone, S.; Stella, C.; Foglini, C.; Abete, M.C.; Prearo, M. Trace element occurrence in the Pacific oyster Crassostrea gigas from coastal marine ecosystems in Italy. Chemosphere 2017, 187, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Katranitsas, A.; Castritsi-Catharios, J.; Persoone, G. The effects of a copper-based antifouling paint on mortality and enzymatic activity of a non-target marine organism. Mar. Pollut. Bull. 2003, 46, 1491–1494. [Google Scholar] [CrossRef]
- Miller, R.J.; Adeleye, A.S.; Page, H.M.; Kui, L.; Lenihan, H.S.; Keller, A.A. Nano and traditional copper and zinc antifouling coatings: Metal release and impact on marine sessile invertebrate communities. J. Nanoparticle Res. 2020, 22, 129. [Google Scholar] [CrossRef]
- Langston, W.J.; Bebianno, M.J.; Burt, G.R. Metal handling strategies in molluscs. In Metal Metabolism in the Aquatic Environment; Langston, W.J., Bebiano, M.J., Eds.; Chapman and Hall: London, UK, 1998; pp. 219–272. [Google Scholar]
- Mouneyrac, C.; Amiard, J.C.; Amiard-Triquet, C. Effect of natural factors (salinity and body weight) on cadmium, copper, zinc and metallothionein-like protein levels in resident populations of oysters (Crassostrea gigas) from a polluted estuary. Mar. Ecol. Prog. Ser. 1998, 162, 125–135. [Google Scholar] [CrossRef]
- Elia, A.C.; Magara, G.; Righetti, M.; Dörr, A.J.M.; Scanzio, T.; Pacini, N.; Abete, M.C.; Prearo, M. Oxidative stress and related biomarkers in cupric and cuprous chloride-treated rainbow trout. Environ. Sci. Pollut. Res. 2017, 24, 10205–10219. [Google Scholar] [CrossRef]
- Alzieu, C.; Thibaud, Y.; Heral, M.; Boutier, B. Evaluation des Risques dus à l’emploi des Peintures Anti-Salissures Dansales Zones Conchylicoles. Rev. Trav. L’institut Pech. Marit. 1980, 44, 305–348. [Google Scholar]
- Regoli, F.; Principato, G. Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: Implications for the use of biochemical biomarkers. Aquat. Toxicol. 1995, 31, 143–164. [Google Scholar] [CrossRef]
- Canesi, L.; Viarengo, A.; Leonzio, C.; Filippelli, M.; Gallo, G. Heavy metals and glutathione metabolism in mussel tissues. Aquat. Toxicol. 1999, 46, 67–76. [Google Scholar] [CrossRef]
- Goswami, P.; Hariharan, G.; Godhantaraman, N.; Munuswamy, N. An integrated use of multiple biomarkers to investigate the individual and combined effect of copper and cadmium on the marine green mussel (Perna viridis). J. Environ. Sci. Health Part A-Toxic/Hazard. Subst. Environ. Eng. 2014, 49, 1564–1577. [Google Scholar] [CrossRef]
- De Vico, G.; Carella, F. Elementi di Patologia Comparata dei Molluschi; Paolo Loffredo Iniziative Editoriali Srl: Napoli, Italy, 2016; pp. 1–320. [Google Scholar]
- Tebble, N. British bivalve seashells. A Handbook for Identification; The British Museum: London, UK, 1966; pp. 1–211. [Google Scholar]
- Breber, P. On-growing of the carpet shell clam (Tapes decussatus (L.)): Two years’ experience in Venice Lagoon. Aquaculture 1985, 44, 51–56. [Google Scholar] [CrossRef]
- Viale, I. Il Comparto dell’Acquacoltura in Sardegna alla Luce dei Risultati dell’Indagine Conoscitiva Condotta dall’Agenzia Laore. Acquacoltura in Sardegna: Le Opportunità del Biologico e dei Disciplinari di Produzione. Siamaggiore, 2 Ottobre 2009. Available online: https://www.sardegnaagricoltura.it/documenti/14_43_20091201175044.pdf (accessed on 13 June 2022).
- Parisi, G.; Centoducati, G.; Gasco, L.; Gatta, P.P.; Moretti, V.M.; Piccolo, G.; Roncarati, A.; Terova, G.; Pais, A. Molluscs and echinoderms aquaculture: Biological aspects, current status, technical progress and future perspectives for the most promising species in Italy. Ital. J. Anim. Sci. 2012, 11, e72. [Google Scholar] [CrossRef]
- Degetto, S.; Schintu, M.; Contu, A.; Sbrignadello, G. Santa Gilla lagoon (Italy): A mercury sediment pollution case study. Contamination assessment and restoration of the site. Sci. Total Environ. 1997, 204, 49–56. [Google Scholar] [CrossRef]
- Cottiglia, M. The Santa Gilla Lagoon; Reports of the seminar of the University of Cagliari, Italy LXV; 1995; pp. 15–19. [Google Scholar]
- Frontalini, F.; Buosi, C.; Da Pelo, S.; Coccioni, R.; Cherchi, A.; Bucci, C. Benthic foraminifera as bio-indicators of trace element pollution in the heavily contaminated Santa Gilla lagoon (Cagliari, Italy). Mar. Pollut. Bull. 2009, 58, 858–877. [Google Scholar] [CrossRef]
- Atzori, G.; Aru, V.; Cesare Marincola, F.; Chiarantini, L.; Medas, D.; Sarais, G.; Cabiddu, S. Sediments distribution of trace metals in a coastal lagoon (Southern Sardinia, Mediterranean Sea): Assessment of contamination and ecological risk. Chem. Ecol. 2018, 34, 727–746. [Google Scholar] [CrossRef]
- Agenzia Regionale per la Protezione dell’Ambiente e della Sardegna (ARPAS). Relazione Finale, Tavolo Tecnico Fluorsid, Piano di Monitoraggio Straordinario Matrici Ambientali. 2017, pp. 1–68. Available online: https://www.sardegnaambiente.it/documenti/21_393_20170825110926.pdf (accessed on 24 January 2022).
- Determinazione N. 748/Det/13 del 16.01.2014. Zone Classificate ai Fini della Produzione di Molluschi Bivalvi Vivi ai Sensi del Regolamento (CE) n. 854/2004 del Parlamento Europeo e del Consiglio del 29 Aprile 2004 e della Delibera della Giunta Regionale n. 26/9 del 3.6.2009. Riclassificazione delle Zone Classificate Presenti nella Laguna di Santa Gilla Denominate “Santa Gilla Mitili Zona Nord”, “Santa Gilla Mitili Zona Sud”, “Santa Gilla Veneroidi”. Available online: https://www.regione.sardegna.it/documenti/1_19_20140121173803.pdf (accessed on 24 July 2022).
- Søndergaard, J.; Asmund, G.; Larsen, M.M. Trace elements determination in seawater by ICP-MS with on-line pre-concentration on a Chelex-100 column using a ”standard”instrument setup. MethodsX 2015, 2, 323–330. [Google Scholar] [CrossRef]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test. In OECD Guidelines for the Testing of Chemicals; Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- Elia, A.C.; Burioli, E.; Magara, G.; Pastorino, P.; Caldaroni, B.; Menconi, V.; Dörr, A.J.M.; Colombero, G.; Abete, M.C.; Prearo, M. Oxidative Stress Ecology on Pacific Oyster Crassostrea Gigas from Lagoon and Offshore Italian Sites. Sci. Total Environ. 2020, 739, 139886. [Google Scholar] [CrossRef]
- Akerboom, T.P.M.; Sies, H. Assay of glutathione disulfide and glutathione mixed disulfide in biological samples. In Methods in Enzymology; Academic Press: London, UK, 1981; Volume 77, pp. 373–382. [Google Scholar]
- McCord, J.M.; Fridovich, I. Superoxide dismutase an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 1969, 244, 6049–6055. [Google Scholar] [CrossRef]
- Greenwald, R.A. Handbook of Methods for Oxygen Radicals Research. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 314–316. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosenbrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 266–275. [Google Scholar] [CrossRef]
- Latorre, M.; Troncoso, R.; Uauy, R. Biological aspects of copper. In Clinical and Translational Perspectives on Wilson Disease; Academic Press: Cambridge, MA, USA, 2019; pp. 25–31. [Google Scholar]
- Moffett, J.W.; Zika, R.G. Oxidation kinetics of Cu (I) in seawater: Implications for its existence in the marine environment. Mar. Chem. 1983, 13, 239–251. [Google Scholar] [CrossRef]
- Mota, A.M.; Correia dos Santos, M.M. Trace metal speciation of labile chemical species in natural waters: Electrochemical methods. In Metal Speciation and Bioavailability in Aquatic Systems; Tessier, A., Turner, D.R., Eds.; Wiley: New York, NY, USA, 1995; pp. 205–257. [Google Scholar]
- Muller, F.L.L. Interactions of copper, lead and cadmium with the dissolved, colloidal and particulate components of estuarine and coastal waters. Mar. Chem. 1996, 52, 245–268. [Google Scholar] [CrossRef]
- Kozelka, P.B.; Bruland, K.W. Chemical speciation of dissolved Cu, Zn, Cd, Pb in Narragansett Bay, Rhode Island. Mar. Chem. 1998, 60, 267–282. [Google Scholar] [CrossRef]
- Cobelo-Garcìa, A.; Prego, R. Chemical speciation of dissolved copper, lead and zinc in a ria coastal system: The role of resuspended sediments. Anal. Chim. Acta 2004, 524, 109–114. [Google Scholar] [CrossRef]
- Sánchez-Marín, P. A review of chemical speciation techniques used for predicting dissolved copper bioavailability in seawater. Environ. Chem. 2020, 17, 469–478. [Google Scholar] [CrossRef]
- Thompson, C.M.; Ellwood, M.J.; Sander, S.G. Dissolved copper speciation in the Tasman Sea, SW Pacific ocean. Mar. Chem. 2014, 164, 84–94. [Google Scholar] [CrossRef]
- Livingstone, D.R.; Lips, F.; Garcia, M.P.; Pipe, R.K. Antioxidant enzymes in the digestive gland of the common mussel Mytilus edulis. Mar. Biol. 1992, 112, 265–276. [Google Scholar] [CrossRef]
- Magara, G.; Sangsawang, A.; Pastorino, P.; Oddon, S.B.; Caldaroni, B.; Menconi, V.; Kovitvadhi, U.; Gasco, L.; Meloni, D.; Dörr, A.J.M.; et al. First Insights into Oxidative Stress and Theoretical Environmental Risk of Bronopol and Detarox® AP, Two Biocides Claimed to Be Ecofriendly for a Sustainable Aquaculture. Sci. Total Environ. 2021, 778, 146375. [Google Scholar] [CrossRef]
- Piras, P.L.; Chessa, G.; Cossu, M.; Fiori, G.; Piras, P.; Ledda, G. Lead and other heavy metals (cadmium and mercury) accumulation in bivalve mollusks (Mytilus galloprovincialis, Ruditapes spp. and Crassostrea gigas) sampled in Sardinia in 2008-2012. Ital. J. Food Saf. 2013, 2, e49. [Google Scholar] [CrossRef]
- Paçal, E.; Gümüş, B.A.; Günal, A.C.; Erkmen, B.; Arslan, P.; Yıldırım, Z.; Erkoç, F. Oxidative stress response as biomarker of exposure of a freshwater invertebrate model organism (Unio mancus Lamarck, 1819) to antifouling copper pyrithione. Pestic. Phytomed. 2022, 37, 63–76. [Google Scholar] [CrossRef]
- Marigómez, I.; Soto, M.; Cajaraville, M.P.; Angulo, E.; Giamberini, L. Cellular and subcellular distribution of metals in molluscs. Microsc. Res. Tech. 2002, 56, 358–392. [Google Scholar] [CrossRef] [PubMed]
- Paris-Palacios, S.; Biagianti-Risbourg, S.; Vernet, G. Biochemical and (ultra)structural hepatic perturbations of Brachydanio rerio (Teleostei, Cyprinidae) exposed to two sublethal concentrations of copper sulfate. Aquat. Toxicol. 2000, 50, 109–124. [Google Scholar] [CrossRef]
- Freedman, J.H.; Ciriolo, M.R.; Peisach, J. The role of glutathione in copper metabolism and toxicity. J. Biol. Chem. 1989, 264, 5598–5605. [Google Scholar] [CrossRef]
- Bigot, A.; Minguez, L.; Giambérini, L.; Rodius, F. Early defense responses in the freshwater bivalve Corbicula fluminea exposed to copper and cadmium: Transcriptional and histochemical studies. Environ. Toxicol. 2011, 26, 623–632. [Google Scholar] [CrossRef]
- Gomes, T.; Pinheiro, J.P.; Cancio, I.; Pereira, C.G.; Cardoso, C.; Bebianno, M.J. Effects of copper nanoparticles exposure in the mussel Mytilus galloprovincialis. Environ. Sci. Technol. 2011, 45, 9356–9362. [Google Scholar] [CrossRef]
- Gupta, Y.R.; Sellegounder, D.; Kannan, M.; Deepa, S.; Senthilkumaran, B.; Basavaraju, Y. Effect of copper nanoparticles exposure in the physiology of the common carp (Cyprinus carpio): Biochemical, histological and proteomic approaches. Aquac. Fish. 2016, 1, 15–23. [Google Scholar] [CrossRef]
- Aliaga, M.E.; López-Alarcón, C.; Bridi, R.; Speisky, H. Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione. J. Inorg. Biochem. 2016, 54, 78–88. [Google Scholar] [CrossRef]
- Al Kaddissi, S.; Legeay, A.; Elia, A.C.; Gonzalez, P.; Floriani, M.; Cavalie, I.; Massabuau, J.C.; Gilbin, R.; Simon, O. Mitochondrial gene expression, antioxidant responses, and histopathology after cadmium exposure. Environ. Toxicol. 2014, 29, 893907. [Google Scholar] [CrossRef]
- Cozzari, M.; Elia, A.C.; Pacini, N.; Smith, B.D.; Boyle, D.; Rainbow, P.S.; Khan, F.R. Bioaccumulation and oxidative stress responses measured in the estuarine ragworm (Nereis diversicolor) exposed to dissolved, nano and bulksized silver. Environ. Pollut. 2015, 198, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Imlay, J.A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc. Natl. Acad. Sci. USA 2009, 106, 8344–8349. [Google Scholar] [CrossRef] [PubMed]
- Maria, V.; Bebianno, M. Antioxidant and lipid peroxidation responses in Mytilus galloprovincialis exposed to mixtures of benzo (a) pyrene and copper. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2011, 154, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Company, R.; Serafim, A.; Bebianno, M.J.; Cosson, R.; Shillito, B.; Fiala-Médioni, A. Effect of cadmium, copper and mercury on antioxidant enzyme activities and lipid peroxidation in the gills of the hydrothermal vent mussel Bathymodiolus azoricus. Mar. Environ. Res. 2004, 58, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Fridovich, I. Superoxide dismutases. J. Biol. Chem. 1989, 264, 7761–7764. [Google Scholar] [CrossRef]

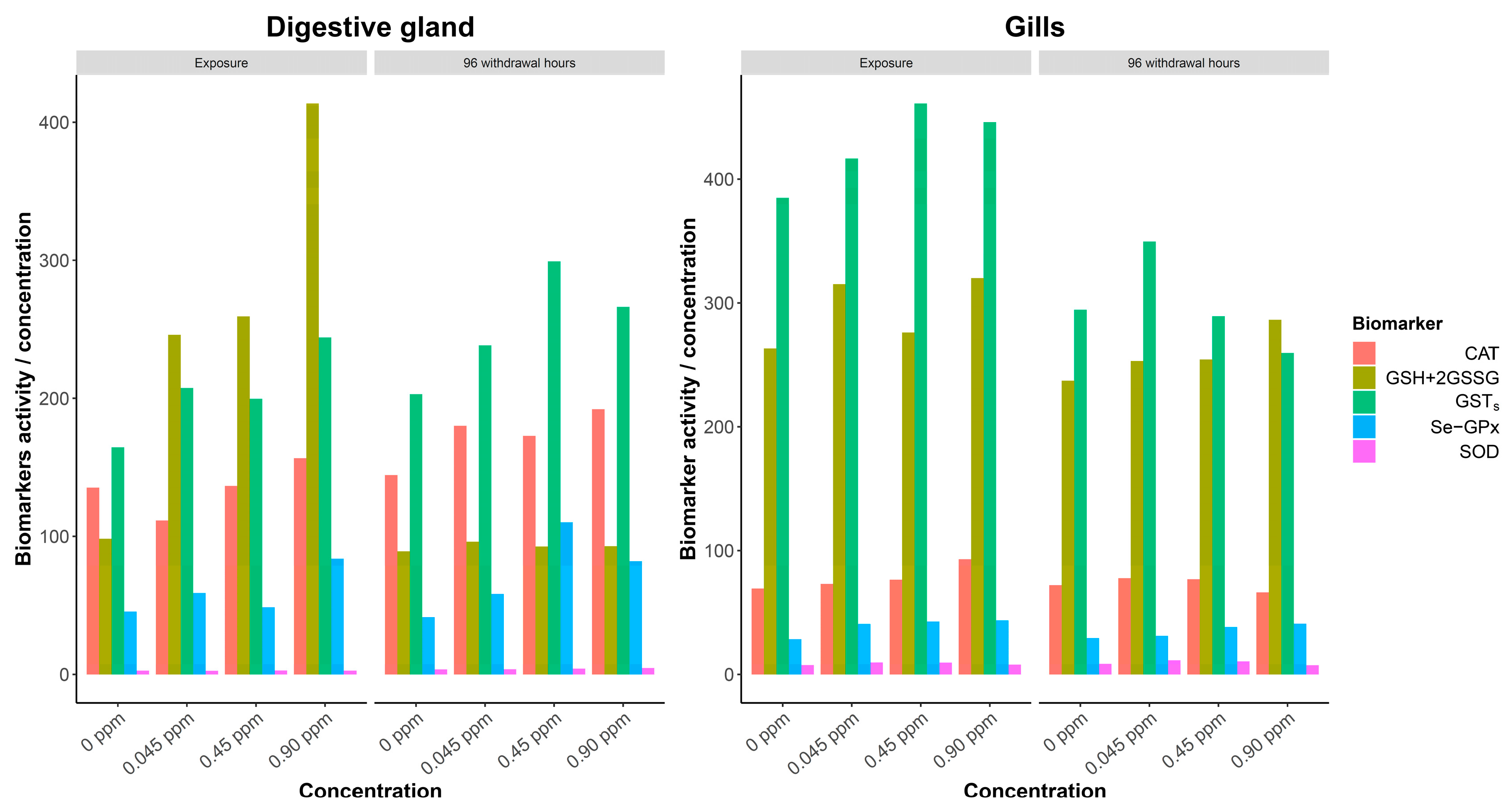
| Organ | Phase | Copper Treatment (CuCl mg/L) | SEM | Phase·mg/L | ||||
|---|---|---|---|---|---|---|---|---|
| Biomarker | Exposure | 96 Withdrawal Hours | Control | 0.045 | 0.45 | 0.90 | ||
| Digestive Gland | ||||||||
| CAT | 94.76 B | 133.47 A | 109.43 ab | 104.46 b | 118.70 ab | 123.88 a | 3.09 | NS |
| GSH+2GSSG | 120.37 A | 76.41 B | 75.10 | 101.96 | 105.43 | 11.05 | 5.88 | NS |
| GSTs | 139.95 B | 178.74 A | 134.52 b | 150.96 ab | 173.28 a | 178.61 a | 5.07 | NS |
| Se-GPx | 36.03 B | 50.13 A | 30.58 B | 36.59 B | 51.50 A | 53.64 A | 2.01 | *** |
| SOD | 2.18 B | 2.93 A | 2.33 | 2.49 | 2.66 | 2.74 | 0.07 | NS |
| Gills | ||||||||
| CAT | 55.56 | 51.82 | 55.45 | 54.99 | 54.18 | 50.12 | 1.51 | NS |
| GSH+2GSSG | 214.39 | 213.10 | 202.75 | 207.85 | 219.25 | 225.12 | 3.85 | NS |
| GSTs | 318.72 A | 238.48 B | 289.58 a | 309.54 a | 281.23 ab | 234.03 b | 8.35 | NS |
| Se-GPx | 20.21 b | 25.36 a | 21.58 | 21.25 | 23.71 | 24.61 | 0.88 | NS |
| SOD | 5.27 B | 6.56 A | 6.14 A | 6.84 A | 6.09 A | 4.58 B | 0.22 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, G.; Pastorino, P.; Prearo, M.; Magara, G.; Cesarani, A.; Freitas, R.; Caldaroni, B.; Meloni, D.; Pais, A.; Dondo, A.; et al. Ecotoxicity of Copper(I) Chloride in Grooved Carpet Shell (Ruditapes decussatus). Antioxidants 2022, 11, 2148. https://doi.org/10.3390/antiox11112148
Esposito G, Pastorino P, Prearo M, Magara G, Cesarani A, Freitas R, Caldaroni B, Meloni D, Pais A, Dondo A, et al. Ecotoxicity of Copper(I) Chloride in Grooved Carpet Shell (Ruditapes decussatus). Antioxidants. 2022; 11(11):2148. https://doi.org/10.3390/antiox11112148
Chicago/Turabian StyleEsposito, Giuseppe, Paolo Pastorino, Marino Prearo, Gabriele Magara, Alberto Cesarani, Rosa Freitas, Barbara Caldaroni, Domenico Meloni, Antonio Pais, Alessandro Dondo, and et al. 2022. "Ecotoxicity of Copper(I) Chloride in Grooved Carpet Shell (Ruditapes decussatus)" Antioxidants 11, no. 11: 2148. https://doi.org/10.3390/antiox11112148
APA StyleEsposito, G., Pastorino, P., Prearo, M., Magara, G., Cesarani, A., Freitas, R., Caldaroni, B., Meloni, D., Pais, A., Dondo, A., Antuofermo, E., & Elia, A. C. (2022). Ecotoxicity of Copper(I) Chloride in Grooved Carpet Shell (Ruditapes decussatus). Antioxidants, 11(11), 2148. https://doi.org/10.3390/antiox11112148










