Abstract
Marine pollution, due to the regular discharge of contaminants by various anthropogenic sources, is a growing problem that imposes detrimental influences on natural species. Sharks, because of a diet based on smaller polluted animals, are exposed to the risk of water contamination and the subsequent bioaccumulation and biomagnification. Trace elements are very diffuse water pollutants and able to induce oxidative stress in a variety of marine organisms. However, to date, studies on sharks are rather scarce and often limited to mercury. In this context, the present study aimed to analyze the accumulation of trace elements and their putative correlation with the onset of an oxidative status in the muscle of the lesser spotted dogfish Scyliorhinus canicula, from the Central Mediterranean Sea. Ecotoxicological analysis detected the presence of Pb, As, Cd, Mn, Zn, Ni, Cu, and Fe; no significant differences were observed between sexes, while a negative correlation was found between Pb and animal length. Analysis of oxidative stress markers showed either positive or negative correlation with respect to the presence of trace elements. Lipid peroxidation (TBARS) positively correlated with Zn, Ni, and Fe; SOD enzyme activity negatively correlated with Cu and Ni; LDH was negatively correlated with Fe and positively correlated with Pb. Moreover, positive correlations between the leukocyte count and Mn and Zn, as well as with LDH activity, were also observed. The data suggested that, in sharks, trace elements accumulation may affect oxidant and antioxidant processes with important outcomes for their physiology and health.
1. Introduction
Aquatic environment contamination by trace elements, resulting from geological and biological cycles, as well as from anthropogenic activities, represents a severe and growing challenge for resident organisms [1,2,3,4]. Both non-essential trace elements (e.g., arsenic (As), mercury (Hg), lead (Pb), and cadmium (Cd)), without known physiological functions, and essential trace elements (e.g., zinc (Zn), copper (Cu), nickel (Ni), and iron (Fe)), necessary for basic physiological processes, are able to accumulate in fish tissues [1,5,6,7,8], with potential detrimental effects on the physiology of target organs. Trace elements can interact with diatomic molecular oxygen from metabolic pathways to produce reactive oxygen species (ROS), such as the superoxide anion radical (O2•−), the hydrogen peroxide (H2O2), the hydroxyl radical (•OH), and the superoxide radical (O2). When ROS production exceeds removal, antioxidant defenses can be deactivated; this results in oxidative stress and macromolecule damage (lipid peroxidation, protein degradation, and disruption of DNA), cellular dysfunction, and/or death [9]. A correlation between trace elements accumulation and oxidative stress has been reported in aquatic organisms [1,10,11,12,13,14,15,16]. However, several species show the ability to prevent oxidative damage caused by trace elements. This may occur by scavenging and/or neutralization (e.g., through metallothionein), by increasing the activity of antioxidant enzymes (i.e., superoxide dismutase (SOD), catalase (CAT), and glutathione S-transferase (GST)), and by excretion [1,13].
Sharks, due to the high position in the food chain, slow growth rates, and low elimination abilities [10,17], are able to bioaccumulate, or worse, to biomagnify several elements through the food chain [5,15,18,19,20]. So far, information regarding the influence of the exposure to trace elements on shark health is scarce and fragmentary, often obtained in a limited number of species or in a restricted region of the world [21,22]. Preliminary data revealed that in several species, such as the nurse shark and the spiny dogfish shark, trace elements, such as Pb, As, and Cd, affect reproduction, cardiac performance, homeostasis balance, and vascular dynamics [23,24,25]. Little is known on the putative impact of trace elements accumulation on the oxidative status of animals. In this regard, the few data available, mainly derived from studies performed on epipelagic and demersal species (e.g., blue sharks [1], mako sharks [12], the Atlantic sharpnose shark [10]), suggested a correlation between several trace elements and the activity of antioxidant enzymes (e.g., SOD, CAT, and glutathione peroxidase (GPx)), as well as the levels of oxidation products (e.g., protein carbonylation and lipid peroxidation). However, a significant variability, possibly depending on environmental exposure and/or behavioral differences between populations, sex, or maturity stage, has emerged.
The lesser spotted dogfish Scyliorhinus canicula (Linnaeus, 1758) is a small benthic shark, widely distributed in the Mediterranean Sea, in which it represents one of the most abundant species, and in the North-Eastern Atlantic Ocean. It can be found from the intertidal zone to the continental slopes, from 10 to 780 m, with highest abundances at depths of 200–500 m [26,27]. It is an opportunistic and a generalist scavenger mesopredator that feeds mostly on small invertebrates, such as crustaceans and cephalopods, and on small fishes [28]. S. canicula is a robust species that well tolerates stress conditions and parasitic load [5]. For this reason, it represents a model useful to assess the phenotypic resilience to both environmental and anthropogenic stressors. To date, the risk assessment has been mainly based on population reduction due to fishing overexploitation [29,30], with limited attention on the effects of environmental challenges due to the increasing pollution of the Mediterranean Sea. Recently, Reinero and coworkers [5] detected the presence of trace elements in vertebrae, skin, and liver of S. canicula from the Central Tyrrhenian Sea. However, very few information is available on the skeletal muscle that, for its high activity during continuous swimming, is susceptible of bioaccumulation, with potential crucial outcomes for whole animal fitness [31]. Several shark species, such as S. canicula in the Mediterranean, are edible. Thus, contaminated skeletal muscle may represent a major route for transfer of trace elements into humans [32], with possible aftermath for population health.
Based on these observations and by integrating mass spectrometer and biomolecular evaluations, this study aimed: (i) to determine the concentrations of trace elements (Pb, As, Mn, Zn, Ni, Cu, Cd, and Fe); (ii) to quantify main indicators of oxidative stress; and (iii) to assess the potential correlation between trace elements and oxidative stress indicators in the skeletal muscle of the lesser spotted dogfish S. canicula from the Central Tyrrhenian Sea.
2. Materials and Methods
2.1. Sampling Collection
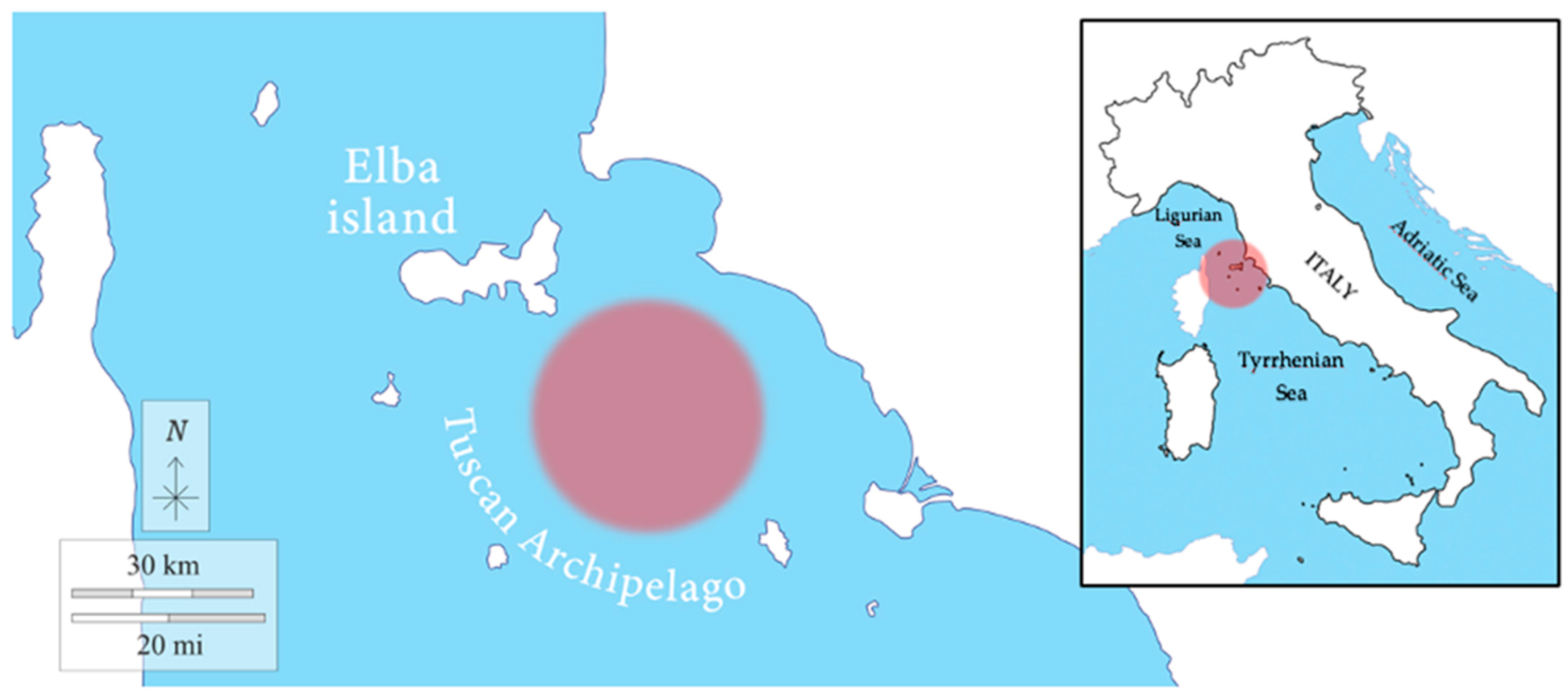
Sampling was carried out in January 2018 at 12 nautical miles off Rocchette Punta Ala (Tuscany, Italy), between the islands of the Tuscan Archipelago (Elba Island, Giglio Island, Pianosa Island, Montecristo Island, and Capraia Island) and the Gulf of Follonica (geographic coordinates: 42.565655° N, 10.601167° E) (Figure 1), in collaboration with the local fishing boat “Mare Blu”. The chondrichthyan specimens enrolled in the present work were obtained from commercial fisheries. The activity was conducted with the observation of the Regulation of the European Parliament and the Council for fishing in the General Fisheries Commission for the Mediterranean (GFCM) Agreement area and amending Council Regulation (EC) No. 1967/2006. All procedures were carried out with the approval by the “Ministero della transizione ecologica—MiTE” (n. authorization 0008263, 25/01/2022).
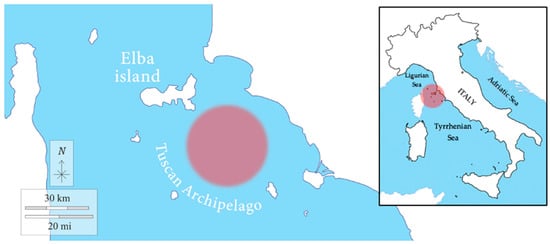
Figure 1.
Sampling area. The fishing area of the samples is indicated by the red circle in the figure.
In total, 24 specimens of S. canicula (n = 18 females and n = 6 males) were collected by commercial bottom trawling at a 150 m depth and transferred to a laboratory for subsequent processing. Total length and weight were measured for each sample, while sex was established by the presence of claspers in males and their absence in females. Maturity was confirmed by the presence of oocytes and eggs in females and calcified claspers and sperm in males [33]. For the leukocyte count, a blood sample was collected from the caudal vein of each specimen and immediately placed at +4 °C [5]. Specimens were then dissected, and samples of muscle, taken from the area near the dorsal fin, were removed from each sample and stored at −20 °C for ecotoxicological and oxidative stress analyses.
2.2. Hematological Analyses
Blood samples were analyzed using the DasitSysmex SX-1000i instrument; the leukocyte formula was obtained by fluorescence flow cytometry [34].
2.3. Trace Elements
Analysis of trace elements was performed by using a combination of acid attacks and an Elan DRCe (Perkin Elmer/SCIEX) Inductively Coupled Plasma Mass Spectrometer (ICP-MS), which is routinely used to determine almost all chemical elements in solid or soft tissues [5,20,35].
In total, 24 muscle samples were removed from 24 different sharks. Samples were first dehydrated under a hood for 24 h at a laminar flow without pulverization. They were then weighed in Teflon containers on a high-precision analytical scale after its calibration. Subsequently, samples were placed in 10 mL of an ultra-pure nitric acid (HNO3) solution, digested in a CEM MARS-5 microwave oven (50 min at 180 °C; power: 400 W; pressure: 300 PSI) and evaporated on a pre-heated plate at 200 °C. They were brought to a volume with ultrapure water in flasks of known volume (50 or 100 mL depending on the weight of the sample) and stored at −4 °C. The same procedure was applied to prepare a Tort (lobster hepatopancreas)-certified reference material (CRM), used as an unknown sample for quality control standards during the analytical sequence. After acid attack, samples were analyzed with the ICP-MS by introducing them into the spectrometer through a peristaltic pump and transformed into an aerosol by a nebulizer supplied with the instrument. In total, 46 trace elements were analyzed, but only Pb, As, Mn, Zn, Ni, Cd, Cu, and Fe were taken into consideration, because of the very low concentration of all the other elements.
2.4. Oxidative Stress Indicators
Evaluation of oxidative stress biomarkers was performed as described in detail by Filice et al. [36]. Muscle samples (N = 24) were homogenized in cold Tris/HCl buffer (100 mM; pH 7.2), containing a mixture of protease inhibitors. An aliquot of homogenates was used to determine TBARS levels; the remaining part was centrifuged at 5000× g for 5 min at 4 °C; the supernatant was tested for protein oxidation. Protein concentration was determined with the Bradford method by using a commercial kit (Bio-Rad Laboratories) and bovine serum albumin (BSA) as a standard.
2.4.1. Lipid Peroxidation
To assess lipid peroxidation (LPO), the concentration of 2-thiobarbituric acid-reacting substances (TBARS) was measured according to Tkachenko and Grudniewska [37]. Briefly, a reaction mixture containing the sample homogenate (0.2 mL, 10% w/v), 2-thiobarbituric acid (TBA; 0.8%, 0.2 mL), and trichloroacetic acid (TCA; 20%, 0.2 mL) was boiled in a water bath at 100 °C for 10 min and then centrifuged at 7000 rpm for 10 min. The supernatant was assessed at 540 nm to determine TBARS levels by measuring malondialdehyde (MDA) content, the major lipid oxidation product. TBARS values were expressed as MDA concentration (μM) per gram of tissue (MDA extinction coefficient: 156,000 M−1 cm−1).
2.4.2. Protein Oxidation
The levels of oxidatively modified protein (OMP) were evaluated by measuring carbonyl groups content with the traditional 2,4-dinitrophenylhydrazine (DNPH) method [38]. Aliquots of the supernatant were incubated at room temperature for 1 h with 10 mM DNPH in 2 M HCl and then precipitated with 2 volumes of TCA. The solution was centrifuged for 20 min at 7000 rpm; to remove DNPH excess, the pellet was washed thrice with ethanol-ethyl acetate (1:1; v/v); then, it was dissolved in 6M guanidine in 2N HCl. The concentration of carbonyl groups was measured spectrophotometrically at 370 nm (aldehydic derivates) and at 430 nm (ketonic derivates) using an extinction coefficient of 22,000 M−1 cm−1. Results were expressed as nmol per mg protein.
2.4.3. Superoxide Dismutase (SOD) Activity
To determine SOD activity, the pyrogallol method of Marklund and Marklund [39], modified by Tresnakova et al. 2023 [40], was used. In brief, the SOD-dependent inhibition of the auto-oxidation of pyrogallol at pH 8.20 was assayed spectrophotometrically at 420 nm and 25 °C. The reaction was prepared in 50 mM Tris-HCl, 1 mM EDTA, and 0.2 mM pyrogallol and monitored every 30 s for 5 min. One unit of SOD activity was defined as the amount of the enzyme that inhibits 50% of pyrogallol auto-oxidation. Results were expressed in U/mL protein.
2.5. Lactate Dehydrogenase Assay
Muscle samples were homogenized in a cold homogenization buffer (0.025 M EDTA, 0.01 M NaCl, 0.01 M Tris-HCl (pH 7.4), and 0.1% Triton) containing a protease inhibitors mixture and then centrifuged at 10,000× g for 10 min at 4 °C. LDH activity was determined as described by Imbrogno et al. [41], by monitoring NADH oxidation at 340 nm in a 3 mL reaction mixture containing potassium phosphate (0.1 M, pH 7.5), sodium pyruvate (0.66 mM), and NADH (0.2 mM), supplemented with 5 μL of the tissue supernatant. All assays were performed at 25 °C. LDH activity was expressed as UI/mL protein.
2.6. Data Analysis
The statistical significance of correlation analyses was assessed using linear correlation tests: not significant (N.S., p > 0.05), significant (*: 0.01 ≤ p ≤ 0.001), very significant (**: 0.001 ≤ p < 0.0001), extremely significant (***: p ≤ 0.0001). Statistical tests were carried out in Instat 3.0 for Mac.
3. Results
A total of 24 S. canicula (n = 18 females; n = 6 males) were sampled in January 2018 in the Central Tyrrhenian Sea. All specimens were found to be sexually mature. Biometric evaluations revealed an average total length (TL) (±sd) of 39.77 ± 2.95 cm (range: 33–47 cm) and a total weight (TW) (±sd) of 200.06 ± 51.56 g (range: 135–317.50 g) (Table S1). No significant differences were detected between the two sexes, although a tendency was observed with males being larger and heavier than females.
3.1. Trace Elements Concentrations
Trace element concentrations measured in muscle samples of S. canicula are summarized in Table 1. No differences were observed between males and females. Only Pb levels were found to be significantly lower in animals with higher TL (p < 0.001).

Table 1.
Trace elements concentrations (mg Kg−1) in muscle samples of the lesser spotted dogfish. Data are shown as the average value, the standard deviation (sd), and the range (minimum–maximum).
3.2. Oxidative Status
The average values for TBARS levels, protein carbonyl concentrations, and SOD and LDH activity, measured in muscle homogenates of S. canicula are reported in Table 2.

Table 2.
Oxidative stress indicators in the muscle tissue of the lesser spotted dogfish S. canicula. Data are shown as the average value, the standard deviation (sd), and the range (minimum–maximum).
Statistical correlation analysis revealed that protein carbonyl levels (OMP-ALD and OMP-KET) were not influenced by sex but were significantly lower in specimens with higher TL (p < 0.01 in both cases). TBARS levels and LDH activity were higher in females (p < 0.001 for TBARS and p < 0.01 for LDH), while SOD levels were higher in males (p < 0.01); no correlation was observed between these markers and TL (Table 3).

Table 3.
Differences in oxidative stress indicators in the muscle of the lesser spotted dogfish S. canicula between sexes and correlation with TL. N.S.—not significant; * p < 0.01; ** p < 0.001.
Significant correlations were found between oxidative stress indicators (Table 4): TBARS were negatively correlated with SOD (p < 0.01); OMPALD and OMPKET were positively correlated with each other (p < 0.0001) and with SOD (p < 0.01); LDH was not correlated with any of the other oxidative stress indicators.

Table 4.
Correlations between oxidative stress indicators in the muscle of the lesser spotted dogfish S. canicula. N.S.—not significant; * p < 0.01; *** p < 0.0001.
3.3. Leukocyte Count
Blood samples to perform the leukocyte count (103/µL) was collected from 16 sharks. The average value (±sd) of white blood cells (WBCs) was 186.35 ± 51.41 103/µL (range: 77.56–274.54 103/µL).
3.4. Correlations between Trace Elements, Oxidative Stress Indicators, and the Leukocyte Count
The correlations between trace elements and oxidative stress indicators are shown in Table 5. TBARS positively correlated with Zn, Ni, and Fe (p < 0.01 in all cases), while SOD activity was negatively correlated with Cu and Ni (p < 0.01 in both cases). LDH was negatively correlated with Fe (p < 0.01) and positively correlated with Pb (p < 0.01).

Table 5.
Correlations between oxidative stress indicators and trace elements accumulation in the muscle of the lesser spotted dogfish S.canicula. (N.S.—not significant; * p < 0.01).
The leukocyte count showed a significant and positive correlation (Table 6) with Mn and Zn (p < 0.01), as well as with LDH activity (p < 0.01).

Table 6.
Correlations between oxidative stress indicators and trace elements accumulation with the leukocytes count (N.S.—not significant; * p < 0.01).
4. Discussion
The present study highlighted the correlations between trace elements accumulation and markers of oxidative status in the muscle of the lesser spotted dogfish S. canicula, collected at a 150 m depth in the Central Tyrrhenian Sea.
As revealed by animal inspection, all specimens were sexually mature, and a prevalence of females with respect to males was observed in the whole sample. This different sex ratio is not unusual in sharks, being possibly related to the breeding behavior of the species. For example, unisexual aggregations of S. canicula have been observed in certain geographical areas and at different times of the year [17,29,42,43]. The biometric analysis of the samples used in this study showed no significant differences between males and females in terms of length and weight. However, a tendency was observed, with males longer and heavier than females. This is interesting since, despite females are usually larger than males, sometimes the opposite is observed due to a differential growth rate between the sexes [42]. In fact, after sexual maturity, females spend more energy into egg development and ovulation and this results in a slower growth, while males of the same size maintain a constant growth rate [42]. By spectrometer analysis, the presence of trace elements was evaluated in the skeletal muscle of all sharks here considered. The comparison of the data with those obtained on different anatomical districts of the same animals and already reported [5] revealed that the concentrations of trace elements were similar to those found in the vertebrae but higher than those detected in the skin and liver, suggesting that, in the lesser spotted dogfish, the vertebrae and the skeletal muscle may represent a major bioaccumulating district of the body. Exceptions are represented by Ni and Fe that, due to their high affinity with placoid scales [44,45,46], are mainly accumulated in the skin [5] and by Cu that, in association with Zn and Cd, is mainly accumulated in liver [47].
To the best of our knowledge, this is the first description of trace elements accumulation in the muscle of S. canicula from individuals collected in the wild in the Mediterranean. In fact, most of the ecotoxicological analyses carried out in S. canicula in target organs and tissues, including the muscle, are from studies performed during artificial experiments in a controlled environment [45,48,49]. This prevents a conclusive comparison of our data with already available observations. At the same time, our results may be considered a reference point for future investigations with the ICP-MS technique on small benthic or demersal mesopredator sharks coming from other Mediterranean areas and naturally exposed to aquatic contaminants. With the exception of Cu, our results showed higher concentrations of trace elements in the muscle of S. canicula than in the same tissue of Galeus melastomus (Rafinesque, 1810) from the Northern Tyrrhenian Sea [50], G. melastomus and Centrophorus granulosus (Bloch and Schneider, 1801) coming from the Eastern Mediterranean Sea [51], and Mustelus mustelus (Linnaeus, 1758) coming from the southeastern waters of the Mediterranean Sea [52]. With respect to the data reported for other Mediterranean sharks, the relatively higher levels of trace elements we detected in the skeletal muscle of S. canicula could be in part related to its sedentary lifestyle, with the consequent chronic exposure to bioaccumulation when inhabiting degraded environments [5]. This possibility is supported by data in benthic or demersal sharks showing a high vulnerability to pollution than pelagic and migratory animals [30]. Of note, the high concentrations of trace elements we discovered are consistent with the data from ARPAT [53] that reported high levels of pollution in waters around the Tuscan Archipelago. As already shown for other target tissues of the same species, such as vertebrae, skin, and liver [5], for the skeletal muscle, no significant correlation was observed between contamination levels and animal sexes. Only Pb was negatively correlated with the size of the sharks, being lower in animals with higher TL. This trend is well-known in fishes [54,55]. In S. canicula, this may be sustained by a growth-related change of the diet [17] and the higher detoxifying activity of the rectal gland observed in relation to animal growth (and the consequent increase in TL) that may contribute to excrete some toxic trace elements, as in the case of Pb [45,46]. Of note, in the skeletal muscle, the levels of Mn and Zn significantly and negatively correlated with the leukocyte count. A decrease in leukocytes has been demonstrated in the vertebrae of S. canicula in relation to an increased concentration of As [5]. In different marine and terrestrial animals (e.g., glaucous gull Larus hyperboreus [56]; African common toad Amietophrynus regularis [57]; green frog Pelophylax synkl. hispanicus [35]), the increase of the parasitic load, together with the accumulation of trace elements, has been linked to a general condition of immunosuppression. Based on our results, it can be hypothesized that in S. canicula, the presence of Mn and Zn could compromise the activity of the immune system.
The exposure of marine organisms to pollutants results in molecular alterations and in structural and functional changes that can be detrimental for animal fitness [40,58,59]. The evaluation of these events takes advantage of the analysis of well-established biological markers. Among others, the activity of antioxidant enzymes and the levels of oxidation products (TBARS and protein carbonyls) are very useful to detect alterations of the oxidative status of the whole organism and of specific tissue targets [12]. Antioxidant enzymes prevent oxidative tissue damage by minimizing either the production of ROS or their interaction with other molecules [60]. In the present study, we observed in the skeletal muscle of S. canicula an activity of SOD which is in line with values reported in the same tissue for other pelagic shark species, such as the blue sharks Prionace glauca [11] and the shortfin mako Isurus oxyrinchus [12]. This is the first study reporting reference values for SOD activity in S. canicula. Only one work analyzed antioxidant efficiency in the lesser spotted dogfish, with reference values relative only to CAT and GST [61]. Different from the blue shark, in S. canicula SOD activity is sex-correlated, with higher values in males than females. Moreover, SOD is inversely correlated to lipid peroxidation (TBARS), whose levels in males are lower than in females. It cannot be excluded that the differences in the levels of oxidative stress indicators in target tissues are related to the reproductive status and to sex, since during gestation females have higher metabolic rates, which may increase ROS production by affecting the balance between pro-oxidants and antioxidants factors [60,62]. The levels of TBARS here reported are in line with that previously described for S. canicula [63,64]. Of note, in the skeletal muscle of cartilaginous fishes, including S. canicula, TBARS levels are significantly higher than those reported in teleost [63]. This has been attributed to the presence in elasmobranchs of squalene, a low-density polyunsaturated hydrocarbon necessary to ensure neutral buoyancy [65]. Notably, in S. canicula, high lipid peroxidation levels are paralleled by lower antioxidant activity [64]; this may support the negative correlation between TBARS and SOD observed in the present study.
When the oxidative status was correlated with the presence of Ni, Zn, and Fe, we found a positive correlation with TBARS levels. In aquatic animals, exposure to these metals, particularly Fe and Ni, induces oxidative stress by inhibiting the activity of antioxidant enzymes [66,67] and by promoting ROS generation. This, in turn, may increase lipid peroxidation [68,69]. For example, in Oryzias latipes embryo, exposure to nano-iron inhibits antioxidant enzymes activity in a dose-dependent manner with consequent induction of lipid peroxidation [66]. Iron overload increases lipid peroxidation also in liver and heart tissues of Clarius gariepinus [70], as well as in gills and liver of Labeo rohita [69]. Similarly, the capacity of Ni to induce lipid peroxidation has been demonstrated in various fish species, such as Salvelinus namaycush [71], Coregonus clupeaformis [72], Cirrhinus mrigala [67], Carassius auratus [73], and Prochilodus lineatus [68]. Of note, in all these species, the Ni-dependent increase of TBARS levels was associated with a significant reduction in antioxidant enzyme activity, including SOD, a trend similar to that observed in the present study. This suggests that in shark skeletal muscle Ni is able to inhibit the antioxidant capacity of SOD and to induce lipid peroxidation, although it is present at lower concentration than in other tissues [5]. No evidence showing a direct correlation between Ni and Fe and oxidative markers is available in sharks. However, in the muscle of mako sharks from Baja California Sur, the activity of antioxidant enzymes, such as SOD and CAT, is significantly correlated with As, and other correlations were found between GPx and Pb, As, and Cd [12]. Similarly, in the liver of blue sharks from Baja California Sur, a significant correlation was found between CAT, TBARS, As, and Cd and between SOD, Zn, and Pb in the kidneys [1].
Together with lipid peroxidation, carbonyl derivatives have become one of the most frequently used markers of oxidative stress [74]. An increase of protein carbonyl (PC) levels has been observed in some marine species exposed to pollutants [75,76]; however, only few studies assessed the potential of this biomarker in shark species [11,12]. Higher PCs amounts were found in muscle samples from males of P. glauca, when compared to females [11], while a different tissue expression was observed in juvenile individuals of I. oxyrinchus, with the highest PCs levels detected in the kidneys, followed by liver and muscle [12]. In our study, the levels of oxidatively modified proteins, estimated by OMP-ALD and OMP-KET, resulted independent of sex but inversely correlated to TL. The nature of this correlation remains unclear. However, as proposed for trace elements, the possibility that the antioxidant capacity to remove oxidative products over time increases with animal size cannot be excluded. The positive correlation that we observed between OMP and SOD activity may support this hypothesis. Moreover, no correlation with OMP levels and trace elements has been found in this study. Although different studies analyzed this biomarker in response to pollutants, a correlation between PC and trace elements was reported only for P. glauca [1], in which PC levels are negatively correlated with Fe and positively correlated with As and Cu. In this context, the use of PCs as biomarkers in studies aimed to assess the effects of pollution in elasmobranchs requires to be further confirmed.
Lactate dehydrogenase (LDH), the terminal enzyme in vertebrate’s anaerobic glycolysis, has recently emerged as an important biomarker useful to determine, in fish, tissue and cellular damage caused by pollutants [77]. Trace metals exposure on LDH activity in fish is controversial. An increased LDH activity in response to different trace metals (Cu, Cd, Pb, and Hg) has been reported in Cyprinus carpio [78], P. lineatus [79], Synechogobius hasta [80], and Catla catla [81]. Conversely, a reduction of enzyme activity has been observed in Sparus aurata and Clarias gariepinus exposed to Cu and Pb, respectively [82,83,84]. In our study, LDH was correlated positively with Pb and negatively with Fe. In aquatic species, exposure to Pb is proposed to contribute to a reduced oxygen uptake rate [85,86] and a decreased activity of glycolytic enzymes [87,88]. Particularly in tissue with high metabolic rates, as brain and muscle, this may induce the activation of anaerobic pathways. As reported above, the skeletal muscle of S. canicula accumulates Pb more than other tissues [5]. The positive correlation we observed between this metal and LDH activity may suggest that in the skeletal muscle, anaerobic pathways are induced for energy release. This mechanism was already observed in fish in response to mercury exposure [89,90,91]. Different from Pb, information on the effect of Fe accumulation on LDH activity in fish is currently very scarce. Only one study carried out in trout showed an increase in LDH activity in the serum of tilapia Oreochromis niloticus exposed to iron oxide nanoparticles [92]. Whether in S. canicula Fe accumulation may negatively affect muscle energy metabolism deserves further investigation.
Our study revealed a positive correlation between muscle LDH activity and serum leukocytes. LDH not only functions as a marker of energy metabolism but, together with other oxidative and metabolic enzymes, it also represents an indicator of stress [93] that may reflect animal health. In addition, blood cells count is indicative of the health status of an animal under challenging conditions, including contaminants exposure [94]. It is known that trace elements bioaccumulation is associated with immunosuppression in different shark species, including S. canicula [5], thus making animals more sensitive to parasite infestation. Our data pave the way to further investigations aimed to assess the value of the correlation between muscle LDH activity and serum leukocytes as a marker of health in fish under metals bioaccumulation.
5. Conclusions
In this work, we found that the skeletal muscle of a Mediterranean population of S. canicula contained eight trace elements (Pb, As, Cd, Mn, Zn, Ni, Cu, and Fe), whose amounts, except for Ni, Cu, and Fe, were higher than those present in skin and liver. The correlation between the levels of these contaminants, the leukocytes count, and biological markers of oxidative stress suggested a possible role of Pb, Zn, Ni, and Fe as predictive markers of oxidative stress in the lesser spotted shark. In addition, Mn and Zn, together with LDH, could be considered indicators of animal health.
Marine pollution, mostly resulting from urban and industrial activity, is going to be a serious growing problem. Although, at the moment, the risk assessment of S. canicula mainly relies on fishing overexploitation, the results of the present study suggest that the impact of environmental pollution must be taken into serious consideration. In this regard, analysis of the correlation between the levels of trace elements and the stress-related response could represent a useful tool for monitoring the health status of this species in the wild.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antiox12020524/s1, Table S1: Biological and biometric data of the 24 specimens of S. canicula enrolled for the present study. All individuals were sexually mature.
Author Contributions
Conceptualization, S.I., E.S., P.M. and M.C.C.; methodology, S.I., E.S., D.B. and P.M.; formal analysis, E.S., G.G., F.L.L., M.F. and F.R.R.; investigation, M.F., F.R.R., G.G., E.S., S.I., F.L.L. and D.B.; resources, C.F., D.B. and M.C.C.; data curation, M.F. and F.R.R.; writing—original draft preparation, M.F., F.R.R., E.S. and S.I.; writing—review and editing, M.C.C., C.F., S.I., D.B. and P.M.; supervision, S.I., E.S., M.C.C., C.F. and D.B.; funding acquisition, M.C.C., S.I., C.F., P.M., D.B. and E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The chondrichthyan specimens enrolled in the present work were obtained from commercial fisheries. The activity was conducted with the observation of the Regulation of the European Parliament and the Council for fishing in the General Fisheries Commission for the Mediterranean (GFCM) Agreement area and amending Council Regulation (EC) No. 1967/2006. All procedures were carried out with the approval by the “Ministero della transizione ecologica—MiTE” (n. authorization 0008263, 25 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request due to restrictions, e.g., privacy or ethical.
Acknowledgments
The authors are grateful to the professional fishermen who provided S. canicula specimens and to Serena Leo and Samira Gallo for their help during collection and analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Barrera-García, A.; O’Hara, T.; Galván-Magaña, F.; Méndez-Rodríguez, L.C.; Castellini, J.M.; Zenteno-Savín, T. Trace elements and oxidative stress indicators in the liver and kidney of the blue shark (Prionace glauca). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Impellitteri, F.; Curpan, A.S.; Plavan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public Health 2022, 19, 16830. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, D.; Sinha, R.; Faggio, C. Advances in biological methods for the sequestration of heavy metals from water bodies: A review. Environ. Toxicol. Pharm. 2022, 94, 103927. [Google Scholar] [CrossRef] [PubMed]
- Shahjahan, M.; Taslima, K.; Rahman, M.S.; Al-Emran, M.; Alam, S.I.; Faggio, C. Effects of heavy metals on fish physiology—A review. Chemosphere 2022, 300, 134519. [Google Scholar] [CrossRef] [PubMed]
- Reinero, F.R.; Milazzo, C.; Minervino, M.; Marchio, C.; Filice, M.; Bevacqua, L.; Giglio, G.; Leonetti, F.L.; Micarelli, P.; Tripepi, S.; et al. Parasitic Load, Hematological Parameters, and Trace Elements Accumulation in the Lesser Spotted Dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Biology 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Shiry, N.; Derakhshesh, N.; Gholamhosseini, A.; Pouladi, M.; Faggio, C. Heavy Metal Concentrations in Cynoglossus arel (Bloch & Schneider, 1801) and Sediment in the Chabahar Bay, Iran. Int. J. Environ. Res. 2021, 15, 773–784. [Google Scholar] [CrossRef]
- Terrazas-Lopez, R.; Arreola-Mendoza, L.; Galvan-Magana, F.; Anguiano-Zamora, M.; Sujitha, S.B.; Jonathan, M.P. Cadmium concentration in liver and muscle of silky shark (Carcharhinus falciformis) in the tip of Baja California south, Mexico. Mar. Pollut. Bull. 2016, 107, 389–392. [Google Scholar] [CrossRef] [PubMed]
- Nędzarek, A.; Rybczyk, A.; Bonisławska, M.; Tański, A.; Tórz, A. Bioaccumulation of metals in tissues of Rutilus rutilus and Perca fluviatilis from lakes with poor ecological status—Human health risk assessment. Eur. Zool. J. 2021, 88, 1084–1095. [Google Scholar] [CrossRef]
- Ercal, N.; Gurer-Orhan, H.; Aykin-Burns, N. Toxic metals and oxidative stress part I: Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001, 1, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Somerville, R.; Fisher, M.; Persson, L.; Ehnert-Russo, S.; Gelsleichter, J.; Bielmyer-Fraser, G. Analysis of Trace Element Concentrations and Antioxidant Enzyme Activity in Muscle Tissue of the Atlantic Sharpnose Shark, Rhizoprionodon terraenovae. Arch. Environ. Contam. Toxicol. 2020, 79, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Barrera-García, A.; O’Hara, T.; Galván-Magaña, F.; Méndez-Rodríguez, L.C.; Castellini, J.M.; Zenteno-Savín, T. Oxidative stress indicators and trace elements in the blue shark (Prionace glauca) off the east coast of the Mexican Pacific Ocean. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2012, 156, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Alavez, M.; Labrada-Martagón, V.; Méndez-Rodriguez, L.C.; Galván-Magaña, F.; Zenteno-Savín, T. Oxidative stress indicators and trace element concentrations in tissues of mako shark (Isurus oxyrinchus). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Lushchak, V.I. Environmentally induced oxidative stress in aquatic animals. Aquat. Toxicol. 2011, 101, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.M.; Nunes, M.; Marchand, P.; Le Bizec, B.; Mendes, S.; Correia, J.P.; Lemos, M.F.; Novais, S.C. Blue sharks (Prionace glauca) as bioindicators of pollution and health in the Atlantic Ocean: Contamination levels and biochemical stress responses. Sci. Total Environ. 2016, 563, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Wosnick, N.; Chaves, A.P.; Leite, R.D.; Nunes, J.L.S.; Saint’Pierre, T.D.; Willmer, I.Q.; Hauser-Davis, R.A. Nurse sharks, space rockets and cargo ships: Metals and oxidative stress in a benthic, resident and large-sized mesopredator, Ginglymostoma cirratum. Environ. Pollut. 2021, 288, 117784. [Google Scholar] [CrossRef] [PubMed]
- Duckworth, C.G.; Picariello, C.R.; Thomason, R.K.; Patel, K.S.; Bielmyer-Fraser, G.K. Responses of the sea anemone, Exaiptasia pallida, to ocean acidification conditions and zinc or nickel exposure. Aquat. Toxicol. 2017, 182, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Reinero, F.R.; Becerril-GarcÍA, E.E.; Elorriagaverplancken, F.R.; Melo-Barrera, F.N.; Toraldo-Serra, M.L.; Giglio, G.; Micarelli, P.; Tripepi, S.; GalvÁN-MagaÑA, F.; Sperone, E. Stable isotopes provide evidence of a trophic shift in the lesser spotted dogfish Scyliorhinus canicula from the Central Tyrrhenian Sea. Mediterr. Mar. Sci. 2023, 24, 1–6. [Google Scholar] [CrossRef]
- Olmedo, P.; Hernandez, A.F.; Pla, A.; Femia, P.; Navas-Acien, A.; Gil, F. Determination of essential elements (copper, manganese, selenium and zinc) in fish and shellfish samples. Risk and nutritional assessment and mercury-selenium balance. Food Chem. Toxicol. 2013, 62, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Matulik, A.G.; Kerstetter, D.W.; Hammerschlag, N.; Divoll, T.; Hammerschmidt, C.R.; Evers, D.C. Bioaccumulation and biomagnification of mercury and methylmercury in four sympatric coastal sharks in a protected subtropical lagoon. Mar. Pollut. Bull. 2017, 116, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Bevacqua, L.; Reinero, F.R.; Becerril-García, E.E.; Elorriaga-Verplancken, F.R.; Juaristi-Videgaray, D.; Micarelli, P.; Galván-Magaña, F.; Curiel-Godoy, P.; Giglio, G.; Tripepi, S.; et al. Trace elements and isotopes analyses on historical samples of white sharks from the Mediterranean Sea. Eur. Zool. J. 2021, 88, 132–141. [Google Scholar] [CrossRef]
- Boldrocchi, G.; Monticelli, D.; Omar, Y.M.; Bettinetti, R. Trace elements and POPs in two commercial shark species from Djibouti: Implications for human exposure. Sci. Total Environ. 2019, 669, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.A. Integrating Biomechanics, Ecomorphology, and Ecotoxicology to Characterize the Roles of Coastal Sharks from Separate Ecological Niches. Doctoral Dissertation, Texas A&M University, College Station, TX, USA, 2019. [Google Scholar]
- Evans, D.H.; Weingarten, K. The effect of cadmium and other metals on vascular smooth muscle of the dogfish shark, Squalus acanthias. Toxicology 1990, 61, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wang, X.T.; Wu, L.; Mateescu, M.A. Toxic effects of cadmium and copper on the isolated heart of dogfish shark, Squalus acanthias. J. Toxicol. Environ. Health. Part A 1999, 57, 507–519. [Google Scholar] [CrossRef]
- Wosnick, N.; Niella, Y.; Hammerschlag, N.; Chaves, A.P.; Hauser-Davis, R.A.; da Rocha, R.C.C.; Jorge, M.B.; de Oliveira, R.W.S.; Nunes, J.L.S. Negative metal bioaccumulation impacts on systemic shark health and homeostatic balance. Mar. Pollut. Bull. 2021, 168, 112398. [Google Scholar] [CrossRef] [PubMed]
- Mancusi, C.; Massi, D.; Baino, R.; Cariani, A.; Crobe, V.; Ebert, D.A.; Ferrari, A.; Gordon, C.A.; Hoff, G.R.; Iglesias, S.P.; et al. An identification key for Chondrichthyes egg cases of the Mediterranean and Black Sea. Eur. Zool. J. 2021, 88, 436–448. [Google Scholar] [CrossRef]
- Leonetti, F.L.; Giglio, G.; Leone, A.; Coppola, F.; Romano, C.; Bottaro, M.; Reinero, F.R.; Milazzo, C.; Micarelli, P.; Tripepi, S.; et al. An updated checklist of chondrichthyans of Calabria (Central Mediterranean, southern Italy), with emphasis on rare species. Mediterr. Mar. Sci. 2020, 21, 794–807. [Google Scholar] [CrossRef]
- Kousteni, V.; Karachle, P.K.; Megalofonou, P. Diet of the small-spotted catshark Scyliorhinus canicula in the Aegean Sea (eastern Mediterranean). Mar. Biol. Res. 2017, 13, 161–173. [Google Scholar] [CrossRef]
- Finotto, L.; Gristina, M.; Garofalo, G.; Riginella, E.; Mazzoldi, C. Contrasting life history and reproductive traits in two populations of Scyliorhinus canicula. Mar. Biol. 2015, 162, 1175–1186. [Google Scholar] [CrossRef]
- Consales, G.; Marsili, L. Assessment of the conservation status of Chondrichthyans: Underestimation of the pollution threat. Eur. Zool. J. 2021, 88, 165–180. [Google Scholar] [CrossRef]
- Marques, A.; Alves, L.; Moutinho, A.; Lemos, M.; Novais, S. Scyliorhinus canicula (Linnaeus, 1758) metal accumulation: A public health concern for Atlantic fish consumers? Mar. Pollut. Bull. 2021, 169, 112477. [Google Scholar] [CrossRef] [PubMed]
- Rajeshkumar, S.; Li, X. Bioaccumulation of heavy metals in fish species from the Meiliang Bay, Taihu Lake, China. Toxicol. Rep. 2018, 5, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Compagno, L.J.V.; Dando, M.; Fowler, S.L. A Field Guide to the Sharks of the World; Collins: London, UK, 2005. [Google Scholar]
- Arnold, J.E. Hematology of the sandbar shark, Carcharhinus plumbeus: Standardization of complete blood count techniques for elasmobranchs. Vet. Clin. Pathol. 2005, 34, 115–123. [Google Scholar] [CrossRef] [PubMed]
- De Donato, C.; Barca, D.; Milazzo, C.; Santoro, R.; Giglio, G.; Tripepi, S.; Sperone, E. Is trace element concentration correlated to parasite abundance? A case study in a population of the green frog Pelophylax synkl. hispanicus from the Neto River (Calabria, southern Italy). Parasitol. Res. 2017, 116, 1745–1753. [Google Scholar] [CrossRef] [PubMed]
- Filice, M.; Leo, S.; Mazza, R.; Amelio, D.; Garofalo, F.; Imbrogno, S.; Cerra, M.C.; Gattuso, A. The heart of the adult goldfish Carassius auratus as a target of Bisphenol A: A multifaceted analysis. Environ. Pollut. 2021, 269, 116177. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, H.; Grudniewska, J. Evaluation of oxidative stress markers in the heart and liver of rainbow trout (Oncorhynchus mykiss walbaum) exposed to the formalin. Fish Physiol. Biochem. 2016, 42, 1819–1832. [Google Scholar] [CrossRef] [PubMed]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Tresnakova, N.; Famulari, S.; Zicarelli, G.; Impellitteri, F.; Pagano, M.; Presti, G.; Filice, M.; Caferro, A.; Gulotta, E.; Salvatore, G.; et al. Multi-characteristic toxicity of enantioselective chiral fungicide tebuconazole to a model organism Mediterranean mussel Mytilus galloprovincialis Lamarck, 1819 (Bivalve: Mytilidae). Sci. Total Environ. 2023, 862, 160874. [Google Scholar] [CrossRef] [PubMed]
- Imbrogno, S.; Aiello, D.; Filice, M.; Leo, S.; Mazza, R.; Cerra, M.C.; Napoli, A. MS-based proteomic analysis of cardiac response to hypoxia in the goldfish (Carassius auratus). Sci. Rep. 2019, 9, 18953. [Google Scholar] [CrossRef] [PubMed]
- Ivory, P.; Jeal, F.; Nolan, C. Age Determination, Growth and Reproduction in the Lesser-spotted Dogfish, Scyliorhinus canicula (L.). J. Northwest. Atl. Fish. Sci. 2004, 37, 89–106. [Google Scholar] [CrossRef]
- Kousteni, V.; Megalofonou, P. Reproductive strategy of Scyliorhinus canicula (L., 1758): A holistic approach based on macroscopic measurements and microscopic observations of the reproductive organs. Mar. Freshw. Res. 2019, 71, 596–616. [Google Scholar] [CrossRef]
- Mathews, T.; Fisher, N.; Jeffree, R. Assimilation and retention of metals in teleost and elasmobranch fishes following dietary exposure. Mar. Ecol Progr. Ser. 2008, 360, 1–12. [Google Scholar] [CrossRef]
- De Boeck, G.; Eyckmans, M.; Lardon, I.; Bobbaers, R.; Sinha, A.K.; Blust, R. Metal accumulation and metallothionein induction in the spotted dogfish Scyliorhinus canicula. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 155, 503–508. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Oberhansli, F.; Teyssie, J.L. Phylogenetic consistencies among chondrichthyan and teleost fishes in their bioaccumulation of multiple trace elements from seawater. Sci. Total Environ. 2010, 408, 3200–3210. [Google Scholar] [CrossRef]
- Marcovecchio, J.E.; Moreno, V.J.; Pérez, A. Metal accumulation in tissues of sharks from the Bahía Blanca estuary, Argentina. Mar. Environ. Res. 1991, 31, 263–274. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Warnau, M.; Oberhansli, F.; Teyssie, J.L. Bioaccumulation of heavy metals and radionuclides from seawater by encased embryos of the spotted dogfish Scyliorhinus canicula. Mar. Pollut. Bull. 2006, 52, 1278–1286. [Google Scholar] [CrossRef]
- Jeffree, R.A.; Warnau, M.; Teyssie, J.L.; Markich, S.J. Comparison of the bioaccumulation from seawater and depuration of heavy metals and radionuclides in the spotted dogfish Scyliorhinus canicula (Chondrichthys) and the turbot Psetta maxima (Actinopterygii: Teleostei). Sci. Total Environ. 2006, 368, 839–852. [Google Scholar] [CrossRef] [PubMed]
- Gaion, A.; Scuderi, A.; Sartori, D.; Pellegrini, D.; Ligas, A. Trace metals in tissues of Galeus melastomus Rafinesque, 1810 from the northern Tyrrhenian Sea (NW Mediterranean). Acta Adriat. 2016, 57, 165–172. [Google Scholar]
- Hornung, H.; Krom, M.D.; Cohen, Y.; Bernhard, M. Trace metal content in deep-water sharks from the eastern Mediterranean Sea. Mar. Biol. 1993, 115, 331–338. [Google Scholar] [CrossRef]
- Storelli, M.M.; Cuttone, G.; Marcotrigiano, G.O. Distribution of trace elements in the tissues of smooth hound Mustelus mustelus (Linnaeus, 1758) from the southern–eastern waters of Mediterranean Sea (Italy). Environ. Monit. Assess. 2011, 174, 271–281. [Google Scholar] [CrossRef] [PubMed]
- ARPAT. Relazione Sullo Stato Dell’ambiente in Toscana: Acque Marine ed Erosione Costiera; ARPAT: Florence, Italy, 2014; pp. 65–71. [Google Scholar]
- Nussey, G.; van Vuren, J.; Preez, H. Bioaccumulation of Chromium, Manganese, Nickel and Lead in the Tissues of the Moggel, Labeo umbratus (Cyprinidae), From Witbank Dam, Mpumalanga. Water SA 2000, 26, 269–284. [Google Scholar]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Sagerup, K.; Helgason, L.B.; Polder, A.; Strom, H.; Josefsen, T.D.; Skare, J.U.; Gabrielsen, G.W. Persistent organic pollutants and mercury in dead and dying glaucous gulls (Larus hyperboreus) at Bjornoya (Svalbard). Sci. Total Environ. 2009, 407, 6009–6016. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshani, S.; Madhushani, W.A.; Jayawardena, U.A.; Wickramasinghe, D.D.; Udagama, P.V. Heavy metal mediated immunomodulation of the Indian green frog, Euphlyctis hexadactylus (Anura:Ranidae) in urban wetlands. Ecotoxicol. Environ. Saf. 2015, 116, 40–49. [Google Scholar] [CrossRef]
- Saha, S.; Dhara, K.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Sub-lethal acute effects of environmental concentrations of inorganic mercury on hematological and biochemical parameters in walking catfish, Clarias batrachus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109511. [Google Scholar] [CrossRef]
- Barathinivas, A.; Ramya, S.; Neethirajan, K.; Jayakumararaj, R.; Pothiraj, C.; Balaji, P.; Faggio, C. Ecotoxicological Effects of Pesticides on Hematological Parameters and Oxidative Enzymes in Freshwater Catfish, Mystus keletius. Sustainability 2022, 14, 9529. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J. Free Radicals in Biology and Medicine; Oxford University Press: Oxford, OH, USA, 2015. [Google Scholar]
- Gorbi, S.; Pellegrini, D.; Tedesco, S.; Regoli, F. Antioxidant efficiency and detoxification enzymes in spotted dogfish Scyliorhinus canicula. Mar. Environ. Res. 2004, 58, 293–297. [Google Scholar] [CrossRef]
- Garratt, M.; Vasilaki, A.; Stockley, P.; McArdle, F.; Jackson, M.; Hurst, J.L. Is oxidative stress a physiological cost of reproduction? An experimental test in house mice. Proc. R. Soc. B Biol. Sci. 2011, 278, 1098–1106. [Google Scholar] [CrossRef]
- Solé, M.; Baena, M.; Arnau, S.; Carrasson, M.; Maynou, F.; Cartes, J.E. Muscular cholinesterase activities and lipid peroxidation levels as biomarkers in several Mediterranean marine fish species and their relationship with ecological variables. Environ. Int. 2010, 36, 202–211. [Google Scholar] [CrossRef]
- Solé, M.; Lobera, G.; Aljinovic, B.; Ríos, J.; García de la Parra, L.M.; Maynou, F.; Cartes, J.E. Cholinesterases activities and lipid peroxidation levels in muscle from shelf and slope dwelling fish from the NW Mediterranean: Its potential use in pollution monitoring. Sci. Total Environ. 2008, 402, 306–317. [Google Scholar] [CrossRef]
- Wilhelm Filho, D.; Giulivi, C.; Boveris, A. Antioxidant defences in marine fish—I. Teleosts. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 1993, 106, 409–413. [Google Scholar]
- Li, H.; Zhou, Q.; Wu, Y.; Fu, J.; Wang, T.; Jiang, G. Effects of waterborne nano-iron on medaka (Oryzias latipes): Antioxidant enzymatic activity, lipid peroxidation and histopathology. Ecotoxicol. Environ. Saf. 2009, 72, 684–692. [Google Scholar] [CrossRef]
- Parthiban, P.; Muniyan, M. Effect of heavy metal nickel on the lipid peroxidation and antioxidant parameters in the liver tissue of Cirrhinus mrigala (HAM.). Int. Dev. Res. 2011, 1, 1–4. [Google Scholar] [CrossRef]
- Palermo, F.F.; Risso, W.E.; Simonato, J.D.; Martinez, C.B.R. Bioaccumulation of nickel and its biochemical and genotoxic effects on juveniles of the neotropical fish Prochilodus lineatus. Ecotoxicol. Environ. Saf. 2015, 116, 19–28. [Google Scholar] [CrossRef]
- Singh, M.; Barman, A.S.; Devi, A.L.; Devi, A.G.; Pandey, P.K. Iron mediated hematological, oxidative and histological alterations in freshwater fish Labeo rohita. Ecotoxicol. Environ. Saf. 2019, 170, 87–97. [Google Scholar] [CrossRef]
- Baker, R.T.M.; Martin, P.; Davies, S.J. Ingestion of sub-lethal levels of iron sulphate by African catfish affects growth and tissue lipid peroxidation. Aquat. Toxicol. 1997, 40, 51–61. [Google Scholar] [CrossRef]
- Ptashynski, M.; Pedlar, R.; Evans, R.; Wautier, K.; Baron, C.; Klaverkamp, J. Accumulation, distribution and toxicology of dietary nickel in lake whitefish (Coregonus clupeaformis) and lake trout (Salvelinus namaycush). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 145–162. [Google Scholar] [CrossRef]
- Ptashynski, M.; Pedlar, R.; Evans, R.; Baron, C.; Klaverkamp, J. Toxicology of dietary nickel in lake whitefish (Coregonus clupeaformis). Aquat. Toxicol. 2002, 58, 229–247. [Google Scholar] [CrossRef]
- Zheng, G.-H.; Liu, C.-M.; Sun, J.-M.; Feng, Z.-J.; Cheng, C. Nickel-induced oxidative stress and apoptosis in Carassius auratus liver by JNK pathway. Aquat. Toxicol. 2014, 147, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Dalle-Donne, I.; Rossi, R.; Giustarini, D.; Milzani, A.; Colombo, R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta 2003, 329, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Almroth, B.C.; Sturve, J.; Stephensen, E.; Holth, T.F.; Förlin, L. Protein carbonyls and antioxidant defenses in corkwing wrasse (Symphodus melops) from a heavy metal polluted and a PAH polluted site. Mar. Environ. Res. 2008, 66, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Patetsini, E.; Dimitriadis, V.K.; Kaloyianni, M. Biomarkers in marine mussels, Mytilus galloprovincialis, exposed to environmentally relevant levels of the pesticides, chlorpyrifos and penoxsulam. Aquat. Toxicol. 2013, 126, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Bharose, R.; Singh, D. Effect of tannery waste water on lactate dehydrogenase (LDH) enzyme activity of fresh water fish, Channa punctatus. J. Entomol. Zool. Stud. 2017, 5, 643–647. [Google Scholar]
- Toth, L.; Juhasz, M.; Varga, T.; Csikkel-Szolnoki, A.; Nemcsok, J. Some effect of CuSO4 on carp. J. Environ. Sci. Health Part B 1996, 31, 627–635. [Google Scholar] [CrossRef]
- dos Santos Carvalho, C.; Fernandes, M.N. Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 437–442. [Google Scholar]
- Liu, X.; Luo, Z.; Xiong, B.; Liu, X.; Zhao, Y.; Hu, G.; Lv, G. Effect of waterborne copper exposure on growth, hepatic enzymatic activities and histology in Synechogobius hasta. Ecotoxicol. Environ. Saf. 2010, 73, 1286–1291. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.J.; Lokanatha, O.; Reddy, T.K.; Reddy, D. Effect of heavy metals on physiological parameters of the indian major carp catla catla. J. Ecotoxicol. Environ. Monit. 2011, 21, 453. [Google Scholar]
- Antognelli, C.; Romani, R.; Baldracchini, F.; De Santis, A.; Andreani, G.; Talesa, V. Different activity of glyoxalase system enzymes in specimens of Sparus auratus exposed to sublethal copper concentrations. Chem.-Biol. Interact. 2003, 142, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.; Mekkawy, I.; Verreth, J.; Kirschbaum, F. Effects of lead nitrate on the activity of metabolic enzymes during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol. Biochem. 2007, 33, 1–13. [Google Scholar] [CrossRef]
- Elarabany, N.; Bahnasawy, M. Comparative and Interactive Biochemical Effects of Sub-Lethal Concentrations of Cadmium and Lead on Some Tissues of the African Catfish (Clarias gariepinus). Toxicol. Res. 2019, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.; Van Aardt, W.; Ahern, M. The effect of lead on the metabolic and energetic status of the Yabby, Cherax destructor, during environmental hypoxia. Aquat. Toxicol. 2005, 75, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Ahern, M.D.; Morris, S. Respiratory, acid–base and metabolic responses of the freshwater crayfish Cherax destructor to lead contamination. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1999, 124, 105–111. [Google Scholar] [CrossRef]
- Tariang, K.-u.; Ramanujam, S.N.; Das, B. Effect of arsenic (As) and lead (Pb) on glycogen content and on the activities of selected enzymes involved in carbohydrate metabolism in freshwater catfish, Heteropneustes fossilis. Int. Aquat. Res. 2019, 11, 253–266. [Google Scholar] [CrossRef]
- Raibole, M.; Shaffi, S.; Raibole, M. Influence of selenium on lead caused variations of phosphofructokinase in different brain regions of fresh water teleosts. Int. J. Sci. Res. 2013, 2, 61–64. [Google Scholar]
- Vieira, L.R.; Gravato, C.; Soares, A.; Morgado, F.; Guilhermino, L. Acute effects of copper and mercury on the estuarine fish Pomatoschistus microps: Linking biomarkers to behaviour. Chemosphere 2009, 76, 1416–1427. [Google Scholar] [CrossRef] [PubMed]
- Barboza, L.G.A.; Vieira, L.R.; Branco, V.; Figueiredo, N.; Carvalho, F.; Carvalho, C.; Guilhermino, L. Microplastics cause neurotoxicity, oxidative damage and energy-related changes and interact with the bioaccumulation of mercury in the European seabass, Dicentrarchus labrax (Linnaeus, 1758). Aquat. Toxicol. 2018, 195, 49–57. [Google Scholar] [CrossRef]
- Radhakrishnaiah, K.; Suresh, A.; Sivaramakrishna, B. Effect of sublethal concentration of mercury and zinc on the energetics of a freshwater fish Cyprinus carpio (Linnaeus). Acta Biol. Hung. 1993, 44, 375–385. [Google Scholar]
- Ates, M.; Demir, V.; Arslan, Z.; Kaya, H.; Yılmaz, S.; Camas, M. Chronic exposure of tilapia (Oreochromis niloticus) to iron oxide nanoparticles: Effects of particle morphology on accumulation, elimination, hematology and immune responses. Aquat. Toxicol. 2016, 177, 22–32. [Google Scholar] [CrossRef]
- Abhijith, B.D.; Ramesh, M.; Poopal, R.K. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J. Basic Appl. Zool. 2016, 77, 31–40. [Google Scholar] [CrossRef]
- Corrêa, S.A.d.S.; Abessa, D.M.d.S.; Santos, L.G.d.; da Silva, E.B.; Seriani, R. Differential blood counting in fish as a non-destructive biomarker of water contamination exposure. Toxicol. Environ. Chem. 2017, 99, 482–491. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).