Abstract
Quercetin is a monomeric polyphenol of plant origin that belongs to the flavonol-type flavonoid subclass. Extensive studies using cultured cells and experimental model animals have demonstrated the anti-atherosclerotic effects of dietary quercetin in relation to the prevention of cardiovascular disease (CVD). As quercetin is exclusively present in plant-based foods in the form of glycosides, this review focuses on the bioavailability and bioefficacy of quercetin glycosides in relation to vascular health effects. Some glucose-bound glycosides are absorbed from the small intestine after glucuronide/sulfate conjugation. Both conjugated metabolites and deconjugated quercetin aglycones formed by plasma β-glucuronidase activity act as food-derived anti-atherogenic factors by exerting antioxidant, anti-inflammatory, and plasma low-density lipoprotein cholesterol-lowering effects. However, most quercetin glycosides reach the large intestine, where they are subject to gut microbiota-dependent catabolism resulting in deglycosylated aglycone and chain-scission products. These catabolites also affect vascular health after transfer into the circulation. Furthermore, quercetin glycosides may improve gut microbiota profiles. A variety of human cohort studies and intervention studies support the idea that the intake of quercetin glycoside-rich plant foods such as onion helps to prevent CVD. Thus, quercetin glycoside-rich foods offer potential benefits in terms of cardiovascular health and possible clinical applications.
1. Introduction
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is categorized within the flavonol subgroup of flavonoids that is ubiquitously distributed throughout the plant kingdom. Flavonoids are known to participate in plant defense (for example, protection of plants from herbivores, microbial infections, and UV light) and act as attractants for pollinators and seed-dispersing animals, as well as allelopathic agents [1]. Quercetin is frequently found in vegetables, fruits, and cereals in the form of monomeric glycosides, in which various sugars are bound to its hydroxyl groups. Biosynthesis of quercetin starts with the condensation of three malonyl CoA and one p-coumaroyl CoA molecule, while quercetin aglycone is formed through successive reactions that produce naringenin–chalcone, naringenin, dihydrokaempferol, and dihydroquercetin [2]. Conversion from aglycone to glycoside is catalyzed by UDP-sugar-dependent glycosyltransferase [3]. Quercetin aglycone acts as a powerful antioxidant because of its ability to scavenge free radicals and chelate transition metal ions [4,5,6,7]. It has been predicted to act as a food-derived physiological antioxidant capable of attenuating oxidative stress, which underlies a variety of degenerative diseases [8,9], in-depth studies into the role of quercetin in the prevention of cardiovascular disease (CVD) strongly suggest that it exerts cardioprotective effects, as oxidative stress is tightly correlated with the onset and progression of CVD [10,11].
CVD is the leading global cause of mortality. In 2019, 17.9 million people died because of CVD, corresponding to 32% of total deaths worldwide [12]. This non-communicable disease includes coronary heart disease (CHD), cerebrovascular disease, and peripheral artery disease. In addition, ischemic heart disease is a type of CVD in which blood flow to heart muscle is reduced or restricted by atherosclerosis. The clinical application of fruits and vegetables has been developed as part of preventive strategies to combat this common chronic disease [13,14]. In 1993, the Zutphen Elderly Study showed for the first time that flavonoid intake from onion, black tea, and apples was inversely associated with mortality from CHD in elderly men [15]. The Seven Countries Study [16] also demonstrated an inverse association between average flavonoid intake and mortality from CHD. Thereafter, large-scale intervention studies, namely the Iowa Women’s Health study [17] and the Danish Cancer and Health cohort study [18] showed that habitual flavonoid intake was inversely associated with all-cause and cardiovascular mortality. Thus, a plentiful intake of dietary flavonoids and flavonoid-rich foods appears to be associated with the prevention of CVD. Recently, numerous in vitro and in vivo studies have proposed a wide variety of mechanisms for the prevention of CVD by flavonoids, such as the improvement of endothelial cell function, suppression of oxidized low-density lipoprotein (oxLDL) accumulation, and anti-inflammatory effects [19,20].
Dietary quercetin appears to act as a powerful preventative factor for CVD by exerting anti-atherosclerotic effects. In plant-based foods, quercetin is mainly present in its glycoside form rather than its aglycone form [21]. This review aims to discuss the significance of the anti-atherosclerotic effects of dietary quercetin glycosides in human health, as well as the prospects of quercetin glycosides as preventive and therapeutic drugs.
2. Dietary Intake and Composition of Quercetin Glycosides from Foods
Figure 1 shows the structures of quercetin glycosides, which are mainly present in vegetables. Onion and shallot are examples of vegetables rich in quercetin glycosides, the major components being quercetin 3,4′-O-β-diglucoside (Q3,4′diG) and quercetin 4′-O-β-glucoside (Q4′G, spiraeoside). These two glucosides, in which the glucose group is bound at the 4′-position, are unique in onion and shallot. In other vegetables, the glucose group is mainly bound at position three in the flavonol structure, forming quercetin 3-O-β-glucoside (Q3G, isoquercitrin). Quercetin 3-O-β-(6”-malonyl-glucoside) and quercetin 3-O-β-glucuronide (miquelianin), quercetin 3-O-β-sophoroside (biamaside), and quercetin 3-O-β-rutinoside (rutin) are present in lettuce, broccoli, and asparagus, respectively. In addition, onion skin contains considerable amounts of quercetin aglycone, together with Q4′G and Q3,4′diG, although this part of the onion is generally inedible.
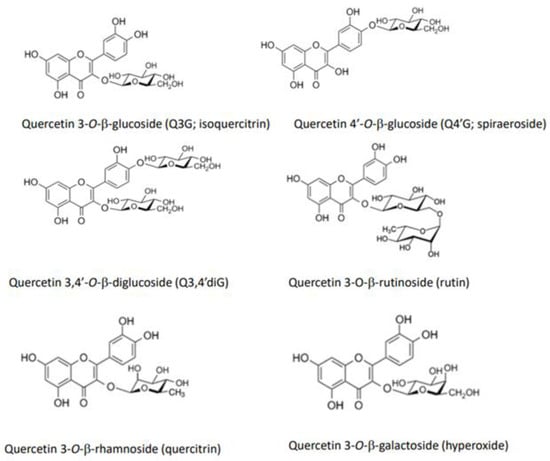
Figure 1.
Structures of major quercetin glycosides present in vegetables.
Two databases are frequently used to estimate the content of flavonoids in a wide range of foods [22,23]. Table 1 shows the quercetin glycoside content for several vegetables, obtained from the Phenol-Explorer database [22]. In other plant foods, quercetin-3-O-β-galactoside (hyperoside) is present in black chokeberry at 46.46 mg/100 g and lingonberry at 13.22 mg/100 g, while Q3G is present in black chokeberry and bottled black tea at 41.95 mg/100 g and 10.87 mg/100 g, respectively. In apple, hyperoside, quercetin-3-O-β-alabinoside, and quercetin-3-O-β-rhamnoside (quercitrin) are present at 2.36 mg/100 g, 1.40 mg/100 g, and 1.33 mg/100 g, respectively [22]. Furthermore, rutin is found in whole grain buckwheat (36.14 mg/100 g), red raspberry (11.0 mg/100 g), bottled black tea (19.68 mg/100 g), and caper spice (332.29 mg/100 g) [22].

Table 1.
Quercetin glycoside content of vegetables [22].
Globally, onion (Allium cepa L.) production has reached 44 million tonnes, representing 10% of worldwide vegetable production [24]. According to data provided by the 2002–2004 World Health Survey of fruit and vegetable intake [25], average quercetin intake was estimated at 4.1–5.9 mg/day for groups consuming < 5 servings/day and 9.0–18.0 mg/day for groups consuming > 5 servings/day of fruits and vegetables. Onions accounted for the largest proportion of quercetin intake in all geographic regions.
3. Intestinal Absorption and Bioavailability of Dietary Quercetin Glycosides
3.1. General Behavior in the Digestive Tract
Figure 2 shows the general scheme for the processing of quercetin glycosides in the digestive tract. Glucose-bound quercetin glycosides (quercetin glucosides) including Q4′G and Q3G—but not glycosides bound to other sugars, such as rutin, quercitrin, and hyperoside—can be hydrolyzed in the oral cavity by saliva excreted from oral epithelial cells, although hydrolytic activity can vary greatly between individuals [26]. However, most quercetin glycosides reach the small intestine through the esophagus and the stomach. Quercetin glucosides are partially deglycosylated and rapidly absorbed in the small intestine; in addition, conjugation reactions are carried out by phase II drug-metabolizing enzymes present in intestinal epithelial cells [27]. Then, the conjugated metabolites are transported to the liver via the portal route where they undergo secondary metabolism followed by enterohepatic circulation or blood circulation [28]. Conjugated metabolites in the blood circulation are finally excreted into the urine within a few days. In contrast, glycosides bound to sugars other than glucose and unabsorbed quercetin glucosides move to the large intestine, where they are subject to gut microbiota-dependent deglycosylation and degradation reactions, resulting in the production of quercetin aglycone and several types of hydroxyphenyl acid derivatives. Although they are mostly excreted into the feces, some are likely to be absorbed in the large intestine, entering the blood circulation with or without undergoing conjugation reactions, similar to quercetin glucosides in the small intestine [29]. Donovan et al. [30] generalized the pharmacokinetics of quercetin glycosides obtained from different human studies, converting the data to apply to 50 mg doses. Times to reach maximum plasma concentration (Tmax) were estimated at 1.5 h and 6 h for quercetin glucosides and rutin, respectively. The longer Tmax for rutin is consistent with the absorption of rutin in a more distal part of the intestine following hydrolysis into aglycone by the gut microbiota. In addition, the mean urinary excretion level was calculated to be higher for glucosides (2.5%) than rutin (0.7%).
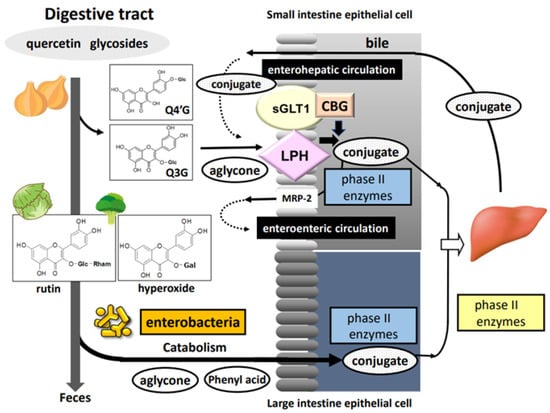
Figure 2.
Processing of dietary quercetin glycosides in the intestinal tract (CBG: cellular β-glucosidase; LPH: lactase phlorizin hydrolase; MRP-2: multidrug resistance-associated protein-2; sGLT1: sodium glucose cotranporter-1; Glc: glucose; Gal: galactose; Rham; rhamnose).
3.2. Absorption and Conjugation of Quercetin Glucosides in the Small Intestine
Quercetin glucosides are deglycosylated to form quercetin aglycone by cellular β-glucosidase (CBG), located in the cytosol of intestinal epithelial cells, and/or lactase phlorizin hydrolase (LPH), a plasma membrane-bound enzyme at the surface [31]. Enzymatic deglycosylation is a critical determinant of bioavailability. Quercetin aglycone produced by the action of LPH is easily absorbed by epithelial cells through passive diffusion because of its enhanced lipophilicity [32]. However, active transport of glucose-bound quercetin glycosides via sodium/glucose cotransporter-1 is required before deglycosylation by CBG in epithelial cells [33].
In the human small intestine, LPH is capable of deglycosylating both Q3G and Q4′G [34]. In contrast, CBG has very little effect on Q3G or Q3,4′diG [35]. Although a preference for deglycosylation of Q4′G rather than Q3G was identified in rat small intestine [36], a previous report [37] demonstrated that the bioavailabilities of Q3G and Q4′G did not differ in humans. Furthermore, a human study showed that Q3,4′diG and Q3G were similarly absorbed and transferred into the bloodstream [38]. Q3,4′diG appears to be absorbed into the body after simultaneous deglycosylation of the two glucose-bound groups at the 3- and 4′-positions by LPH or after Q4′G formation by LPH, followed by complete deglycosylation by CBG to release quercetin aglycone.
Neither hydrolyzing enzyme acts on glycosides bounds to sugars other than glucose, resulting in little absorption of such glycosides in the small intestine [39]. A study in rats suggested that rutin was absorbed more slowly than quercetin aglycone because of a requirement for deglycosylation by the cecal microbiota [40,41]. In addition, a human study with 15 subjects showed that the level of excretion of unmodified quercetin was 0.5% of intake after the consumption of strong black tea and 1.1% after the consumption of onions [42]. The lower level of excretion following tea intake may have resulted from the lower efficiency of intestinal absorption of rutin (present in tea), compared with the absorption of quercetin glucosides (present in onion). However, Erlund et al. [43] reported that the mean area under the curve (AUC) for plasma concentration–time and the maximum plasma concentration (Cmax) of the two compounds were similar, although the Tmax was significantly shorter after quercetin aglycone treatment than after rutin treatment. Jaganath et al. [44] demonstrated that rutin was mostly absorbed in the large intestine by comparing the metabolic profiles of healthy volunteers and ileostomy patients who had ingested rutin.
3.3. Metabolism of Quercetin Glucosides and Circulation of Their Metabolites
During intestinal absorption, quercetin glucosides are converted to their conjugated metabolites, glucuronides and sulfates, by UDP-glucuronosyl transferase and phenol sulfate transferase, respectively. The phenol groups of these conjugates can also be subject to O-methylation by the action of catechol-O-methyl transferase [45]. These conjugated metabolites are further metabolized to produce more complex compounds in the liver after transfer via the portal route. Conjugated metabolites derived from quercetin glucosides have also been suggested to be partly transferred to the blood circulation via the lymphatics [46]. The enterohepatic circulation of quercetin metabolites between the liver and the digestive tract via the gallbladder may affect their bioavailability as a result of reabsorption in the digestive tract. Epithelial multidrug resistance-associated protein 2 plays a role in the enteroenteric circulation of conjugated metabolites and modulation of quercetin glucoside bioavailability.
In a human study, 23 species of conjugated metabolites, including quercetin 3-O-β-glucuronide (Q3GA), quercetin 3′-O-β-glucuronide (Q3′GA), quercetin 4′-O-β-glucuronide (Q4′GA), quercetin 3′-O-sulfate (Q3′S), and isorhamnetin 3-O-β-glucuronide (IR3GA), were identified in the plasma 1 h to 4 h after the intake of red onion (Figure 3) [47]. In addition, comparing human plasma after the consumption of onion powder containing Q4′G and Q3,4′diG or apple skin containing quercetin-3-O-arabinoside, hyperoside, Q3G, and quercitrin revealed that similar conjugated metabolites (identified as quercetin sulfate, quercetin glucuronide, and quercetin diglucuronide) were produced, although their relative ratios differed [48,49]. Using physiologically based kinetic modeling to predict the plasma concentration of flavonoids, Boonpawa et al. [50] suggested that Q3′GA was the major circulating metabolite in 19 out of 20 individuals.
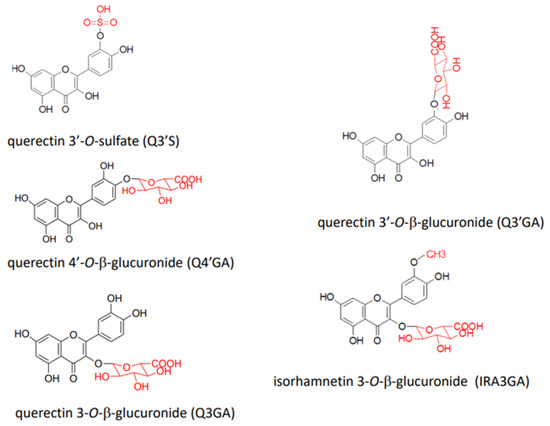
Figure 3.
Structures of representative quercetin conjugated metabolites detected in human plasma.
3.4. Catabolism of Quercetin Glycosides by the Microbiota in the Large Intestine
In the large intestine, quercetin glycosides are subject to enzymatic ring-fission reactions and deglycosylation by the gut microbiota (Figure 4). Although the products are mostly excreted into the feces, a portion can reach the circulation following absorption in the large intestine. The gut microbiota can convert rutin to phenyl acid derivatives, namely 3-hydroxy phenylacetic acid (3HPAA) (36%), 3-methoxy-4-hydroxyphenylacetic acid (8%), and 3,4-dihydroxyphenylacetic acid (3,4-DHPAA) (5%) [51]. Degradation of quercetin aglycone by Eubacterium ramulus, a strict anaerobe resident in the human intestinal tract, also produces 3,4-DHPAA [52]. Furthermore 3,4-DHPAA and 4-hydroxybenzoic acid, in addition to quercetin aglycone, were detected as catabolites following the anaerobic incubation of quercitrin with human intestinal bacteria [53]. In the case of rutin catabolism by human fecal bacteria, deglycosylation to produce quercetin aglycone and subsequent degradation to form hydroxyphenyl acid derivatives depend on interindividual gut microbiota composition [54]. In addition, the catabolic profile of hyperoside produced by human intestinal bacteria revealed six metabolites, including quercetin aglycone, 3,4-DHPAA, and 3,4-dihydroxyphenylbenzoic acid [55].
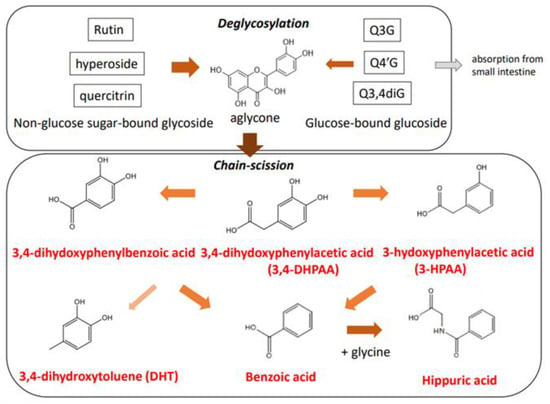
Figure 4.
Gut microbiota-dependent catabolic pathway of quercetin glycosides in the large intestine.
These degradation products are suggested to enhance intestinal health by modulating direct or indirect immune functions such as T-cell differentiation and gut microbiota turnover [56]. Hydroxyphenyl acid derivatives are also expected to exert their effects in the blood circulation after transfer from the large intestine. However, little is known about absorption mechanisms in the large intestine. Nonetheless, these hydroxyphenyl acid derivatives and their conjugated metabolites appear to be present in human plasma following the intake of quercetin glycosides, as demonstrated for hesperetin-7-rutinoside and naringenin 7-rutinoside after the ingestion of orange juice [57].
4. Modulation of Bioavailability
4.1. General Comments
The bioavailability of quercetin glycosides is highly influenced by the type and amount ingested. Interindividual differences in intestinal deglycosylation and absorption efficiencies and microbiota profiles are also endogenous determinants of quercetin glycoside bioavailability [58]. In a human study whereby large amounts of quercetin aglycone (500 mg/day or 1000 mg/day) were used as dietary supplements for 12 weeks, the overnight-fasted plasma concentrations of quercetin metabolites were significantly increased. Although the increases were highly variable between individuals, they were not related to gender, BMI, fitness level, or dietary intake [59]. Nevertheless, several strategies aimed at improving the bioavailability of quercetin glycosides have been applied to better realize the cardioprotective activities of these compounds in vivo [60].
4.2. Effects of the Food Matrix
The food matrix has been shown to increase the bioavailability of quercetin aglycone. For example, a randomized, single-blind, diet-controlled study demonstrated that for equivalent intakes of quercetin (130 mg), quercetin-enriched cereal bars were more effective at increasing the plasma levels of quercetin metabolites than quercetin powder-filled capsules [61]. In addition, comparison of AUC and Cmax following the ingestion of quercetin aglycone derived from onion skin extract powder (163 mg) or pure quercetin dihydrate (136 mg) showed that the values were 4.8-fold and 5.4-fold higher, respectively, for the onion skin extract [62]. Thus, the amorphous structure of quercetin aglycone from onion skin extract appears to improve solubility in the intestinal tract, in comparison with the crystal structure of quercetin dihydrate.
A cross-over trial was carried out to compare the bioavailability of quercetin administered in the form of fresh red onion (quercetin glycosides: 47 mg quercetin aglycone equivalent) with that of quercetin in the form of a dietary supplement (quercetin aglycone tablet: 544 mg quercetin aglycone equivalent) [63]. The results showed that the 24-h urinary excretion levels of total quercetin following the consumption of onion (1.69 ± 0.79 μmol) or quercetin supplement tablets (1.17 ± 0.44 μmol) were not significantly different. These findings indicated that quercetin aglycone was more efficiently available from onions than from quercetin supplements. The food matrix is likely to contribute to these differences in bioavailability. In addition, Wiczkowski et al. [64] compared the bioavailability of 10 mg quercetin aglycone equivalent in dry shallot skin (containing quercetin aglycone at 83.3% and quercetin glucosides at 16.7%) and shallot flesh (containing quercetin glucosides at 99.2% at intake). The results showed that the AUC value for intake via dry shallot skin was more than twice as high as the value for shallot flesh, indicating that the bioavailability of quercetin aglycone was dependent on dietary source in humans. It is therefore clear that the lower bioavailability of quercetin aglycone, compared with quercetin glucosides, is caused by the lower solubility of pure quercetin aglycone (supplied in the form of tablets or capsules) in the digestive tract. In addition, a human ingestion study confirmed that onion peel powder performed better than onion peel extract at increasing the plasma concentration of quercetin [65]. Thus, the food matrix acts as a key factor for improving the bioavailability of quercetin in either aglycone or glucoside form. Therefore, lower levels of quercetin ingested from foods may be as effective as higher levels of pure quercetin ingested in the form of supplements at inducing the vascular functions of this compound.
4.3. Effect of Coexisting Ingredients
Studies have shown that dietary fat increases the micellization efficiency of quercetin aglycone, improving its intestinal absorption. For example, a randomized cross-over trial in overweight adults showed that the inclusion of 15.4 g of fat alongside 1095 mg quercetin aglycone resulted in increases of 45% and 32% in the Cmax and AUC values, respectively [66]. Moreover, a study conducted in rats demonstrated that the addition of more than 4.6% of soybean oil to an onion powder-containing diet for one or two weeks enhanced the plasma concentration of quercetin [67]. Therefore, it is likely that fat and oil increase the absorption of quercetin glucosides in the intestinal tract by assisting micellization, regardless of supplementary or dietary source. However, the effect of fat on the bioavailability of quercetin glucosides in humans is unknown. In addition, combining cooked sweet potato with fried onion was found to suppress increases in plasma quercetin metabolite concentrations in humans. This suppressive effect was probably caused by excess carbohydrate digestion products from the sweet potato interfering with quercetin glucoside deglycosylation during intestinal epithelial uptake [68]. Combining onion and tofu also affected the plasma profiles of quercetin metabolites, with quercetin sulfate only detected after combined intake [69]. Therefore, specific food combinations may considerably affect the bioavailability and metabolic profiles of quercetin glucosides after dietary ingestion.
4.4. Effects of Cooking and Processing
Cooking lowered the quercetin content of tomatoes and onions, with greater reductions caused by microwaving and boiling than by frying [70]. Ioku et al. [71] demonstrated that microwaving and frying did not affect the content of quercetin glucosides in onion flesh, although boiling led to a loss of approximately 30% because of transfer to the boiling water. The effects of food processing (including mechanical processing, thermal and non-thermal treatments, oral delivery and nanoformulations, enzymatic hydrolysis, and fermentation) on the bioavailability of flavonoids have been extensively reviewed by Polia et al. [72]. In addition, to increase water solubility, quercetin glucoside can be enzymatically modified to produce isoquercitrin derivatives containing 1–6 glucose moieties at the 3-glucoside position of Q3G (EMIQ). In a human study with 100 mg quercetin equivalent intake, EMIQ produced a higher plasma concentration of quercetin conjugates 90 min after ingestion, compared with intact Q3G [38]. EMIQ appears to be effectively transported to intestinal epithelial cells through the “unstirred” water layer before being hydrolyzed by mucosal maltase–glucoamylase and LPH and efficiently incorporated into cells as aglycone.
5. Mechanisms of Onset of Atherosclerosis
Atherosclerosis is a potentially serious condition whereby arteries become clogged with fatty streaks termed plaques or atheroma. The resulting constriction and injury of vessel lumens cause a deficiency in the supply of oxygen from the blood to the heart, damaging the cardiac muscle and leading to CVD. Arterial blood vessels are composed of adventitia, media, and intima. The surface of the intima is covered by the vascular endothelium, which is formed of a monolayer of endothelial cells. Endothelium represents the first barrier encountered by molecules, cells, and pathogens circulating in the bloodstream and performs a wide variety of vascular functions including control of vascular permeability, vasoconstriction, vasodilation, and thrombus formation. The steps that lead to the development of atherosclerosis are described below [73,74] (see Figure 5).
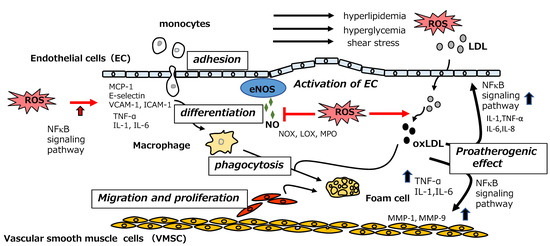
Figure 5.
Vascular events in the initial stage of atherosclerosis (eNOS: endothelial nitric oxide synthase; ICAM-1: intermolecular adhesion molecule-1; IL-1; interleukin-1, IL-6: interleukin-6; IL-8: interleukin-8; LDL; low-density lipoprotein; LOX; lipoxygenase; MCP-1: monocyte chemotactic protein-1; MMP-1: matrix metalloproteinase-1; MMP-9: matrix metalloproteinase-9; MPO: myeloperoxidase; NFκB: nuclear factor kappa B; NOX; NADPH oxygenase; oxLDL: oxidized low-density lipoprotein; ROS: reactive oxygen species; TNF-α: tumor necrosis factor-α; VCAM-1: vascular cell adhesion molecule-1).
5.1. Endothelial Dysfunction and Formation of oxLDL
Endothelial cells act as sensors for mechanical stress on the vascular wall (wall shear stress, WSS) caused by disturbances in blood flow. They also act as signal transducers through the production of biologically active substances, inducing the expression of monocyte chemotactic protein-1 (MCP-1) and the proliferation of vascular smooth muscle cells (VSMCs) in the vascular media. Nitric oxide (NO) produced by endothelial NO synthase (eNOS) acts as an endothelially-derived vasodilator, exerting an anti-atherosclerotic effect. However, NO is deactivated by the overproduction of reactive oxygen species (ROS) under conditions of oxidative stress such as hyperlipidemia, hypertension, and diabetes. Furthermore, oxidative stress leads to the production of pro-inflammatory cytokines including tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), as well as the upregulation of adhesion molecules such as vascular cell adhesion molecule-1 (VCAM-1) and intermolecular adhesion molecule-1 (ICAM-1), through activation of the nuclear factor kappa B (NFκB) signaling pathway.
WSS distorts the structure of the vascular endothelium, leading to the subendothelial infiltration of plasma LDL via caveolae-dependent transcytosis. In addition, the activation of endothelial cells by inflammatory cytokines results in the recruitment of monocytes to the endothelium. After migrating to the endothelial cell surface, monocytes are subjected to rolling by E-selectin molecules and firm attachment to the endothelium through interactions with VCAM-1 and ICAM-1. Then, monocytes infiltrate the intima via paracellular or transcellular processes. Once within the intima, monocytes differentiate into macrophages, which can be polarized to M1 (pro-inflammatory) or M2 (anti-inflammatory) phenotypes; atherosclerotic conditions favor M1 polarization. M1 macrophages release the inflammatory cytokines IL-1β, IL-6, and TNF-α, which promote monocyte recruitment and propagate the inflammatory response. Infiltrated LDL particles are converted to oxLDL by oxidative modification of the tyrosine residue of apolipoprotein B-100. Myeloperoxidase (MPO), 15-lipoxygenase, and NADPH oxygenase (NOX), which are activated by endothelial dysfunction, are suggested to be responsible for this oxidative modification.
5.2. Phagocytosis of oxLDL by Monocyte-Derived Macrophages and Foam Cell Formation
Monocyte-derived macrophages internalize oxLDL by phagocytosis through LDL scavenger receptors, CD36, scavenger receptor A1 (SRA-1), and oxLDL receptor (lectin-like oxidized LDL receptor-1, LOX-1). The accumulation of oxLDL within macrophages leads to foam cell formation. Following the lysosomal degradation of oxLDL, the released cholesterol is esterified by acyl-CoA cholesterol acyltransferase at the endoplasmic reticulum and distributed as droplets in the cytosol. Next, free cholesterol hydrolyzed from esterified cholesterol by the action of cholesteryl ester hydrolase is transferred into high-density lipoprotein (HDL) via ABC transporter A1 (ABCA1) and ABC transporter G1 (ABCG1). This event is an essential step for the recovery of excess cholesterol from peripheral tissue and an important process in the prevention of atherosclerosis. However, under inflammatory conditions, the excretion of excess cholesterol by ABC transporters does not occur, thus promoting foam cell formation.
5.3. Proatherogenic Effect of oxLDL on Endothelial Cells and VSMCs
oxLDL activates the NFκB signaling pathway in macrophages, resulting in the production of inflammatory cytokines, activation of endothelial cells, monocyte infiltration, and, finally, foam cell formation. In addition, the uptake of oxLDL by endothelial cells via LOX-1 accelerates endothelial dysfunction by inducing the production of pro-inflammatory cytokines. Furthermore, unrestricted uptake of oxLDL by VSMCs occurs via the receptors SRA-1, CD-36, and LOX-1, leading to foam cell formation. Once taken up by VMSCs, oxLDL induces the expression of the collagen-decomposing enzymes matrix metalloproteinase-1 and matrix-metalloproteinase-9, resulting in the degradation of extracellular collagen, which finally leads to destabilization of the foam cells.
5.4. Fibrosis of Blood Vessels and Thrombus Formation
Elevation of plasma LDL levels by hypercholesterolemia leads to continual repetition of the above processes. Atheroma (fibrous plaques) develops gradually, and the arteries become narrow and hard. Decreased vessel flexibility results in atherosclerosis and the induction of hypertension. Hypertrophied plaques can become vulnerable, and then thrombosis occurs in the vessel because of plaque disruption, resulting in the blockage of blood flow.
6. Proposed Mechanism for CVD Prevention by Quercetin Glycosides
Scavenging of oxidative stress-inducing free radicals by the phenolic hydroxyl groups of quercetin and its glycosides enables these compounds to act as powerful antioxidants. However, it is worth pointing out that the bioavailability of quercetin glycosides appears to be lower than that of antioxidant vitamins such as vitamin C and vitamin E. Indeed, an essential mechanism of the anti-atherosclerotic effects of quercetin and its glycosides appears to be indirect antioxidant activity. That is, these compounds modulate the activity and expression of oxidative/antioxidative enzymes responsible for the regulation of oxidative stress, attenuating inflammatory activity by suppressing the production of pro-inflammatory enzymes and cytokines [74,75].
6.1. Mechanism of Anti-Inflammatory Activity of Phenolic Compounds
A series of anti-inflammatory functions performed by phenolic compounds (summarized in a review by Rhaman et al. [76]) is outlined below.
- Phenolic compounds inhibit the NFκB signaling pathway, resulting in downregulated gene expression of inflammation-inducing enzymes such as cyclooxygenase-2 (COX-2), inducible NOS (iNOS), and 5-lipoxygenase (5-LOX) and inflammatory cytokines such as IL-1β, IL-6, and TNF-α.
- Phenolic compounds prevent the adhesion of monocytes to endothelial cells by suppressing the expression of E-selectin, MCP-1, VCAM-1, and ICAM-1.
- Phenolic compounds attenuate endothelial oxidative stress by inhibiting NOX-dependent ROS production. They also elevate levels of the endothelial anti-atherosclerotic factor NO by accelerating the phosphorylation of eNOS via an AMP-activated protein kinase (AMPK)-dependent mechanism. In addition, phenolic compounds elevate eNOS expression by activating the nuclear factor E2-related factor 2 (Nrf2) pathway.
- Phenolic compounds suppress the migration of VSMCs by inhibiting platelet-derived growth factor-related signaling molecules such as phosphoinositide-3-kinase (PI3K). They also suppress VSMC growth by inducing apoptosis via activation of p38 mitogen-activated protein kinase and P53 signaling pathways.
In vitro cultured cell experiments showed that quercetin aglycone inhibited the generation of ROS by COX-2 and 5-LOX, downregulated the production of COX-2 and NFκB, and suppressed the secretion of IL-6 and TNF-α by lipopolysaccharide (LPS)-stimulated macrophages [77]. Quercetin aglycone also inhibited the secretion of IL-1β, IL-6, IFN-γ, and TNF-α by LPS-stimulated blood cells [77]. Moreover, quercetin aglycone was reported to suppress the production of NO, IL-6, MCP-1, TNF-α, iNOS, and COX-2 by downregulating the NFκB signaling pathway [77] and to exert anti-inflammatory effects by inhibiting tyrosine kinase, Src, and Syk-mediated PI3K phosphorylation and expression of VCAM-1 and CD80 [78]. Activation of the Nrf-2 signaling pathway may contribute to the suppression of ICAM-1 and E-selectin [79].
6.2. Insights from In Vivo Animal Experiments
6.2.1. Modulation of Inflammatory Cytokines and Oxidative Stress
Carrageenan-induced inflammation in rats under oxidative stress was found to be reduced by pretreatment with 10 mg/kg body weight (BW) of quercetin aglycone, isoquercitrin, or rutin, which inhibited prostanoid synthesis and cytokine production [80]. In addition, oral administration of quercetin aglycone (five doses of 150 mg/kg BW) resulted in reduced clinical signs in an experimental rat model of arthritis, correlating with decreased levels of macrophage inflammatory mediators [81]. In rats with experimental autoimmune myocarditis, quercetin aglycone administered at a dose of 20 mg/kg BW for 21 days reduced clinical symptoms and serum levels of TNF-α and IL-17 while increasing IL-10 levels [82]. Furthermore, the intraperitoneal injection of quercetin aglycone (100–200 mg/kg BW) in a hypertriglyceridemia-related rat model of acute pancreatitis led to attenuated pancreatic histopathological damage and decreased mRNA and protein expression of NF-κB, IL-1β, IL-6, and TNFα [83].
Both quercetin aglycone and rutin partially but significantly attenuated lipid peroxidation in normal and diabetic model rats with ischemia-reperfusion injury, suggestive of cardioprotective effects [84]. Meanwhile, quercetin treatment (50 mg/kg BW) was found to enhance the activity of Cu/Zu-superoxide dismutase (SOD) and glutathione peroxidase and reduce the level of lipid peroxidation products in brain edema induced in an experimental rat model of subarachnoid hemorrhage [85]. Furthermore, administration of quercetin (50–150 mg/kg BW) for 30 days significantly ameliorated myocardial oxidative injury and immune function impairment in a rat model of myocardial ischemia [86]. In the same ischemia model, the cardioprotective effect of rutin was found to be weaker than that of quercetin aglycone at the same dose, despite the cardioprotective effects of both phenolic compounds in cardiac fibrosis [87].
6.2.2. Modulation of Plasma LDL Levels
Elevation of plasma LDL levels is one of the risk factors for atherosclerosis. In terms of atherosclerosis prevention, quercetin and its glycosides have generated interest because of their potential LDL-lowering effects during various processes, including intestinal cholesterol absorption, very-low-density lipoprotein assembly, LDL metabolism and endocytosis, and bile acid metabolism [88]. For example, quercetin aglycone has been shown to influence the activity of the sterol transporter protein Nieman-Pick C1-like 1 (NPC1L1). Quercetin aglycone decreased serum cholesterol levels when administered alongside a high-cholesterol diet, likely because of its specific inhibition of intestinal cholesterol absorption mediated by NPC1L1 [89].
Several in vivo experiments have demonstrated the effects of dietary quercetin glucosides administered to animals on high-cholesterol diets. For example, New Zealand white rabbits were maintained on a 2.0% cholesterol diet with 0.1% Q3G for a month [90]. Triacylglycerol and cholesterol levels in the plasma and aorta were lowered and lipid peroxidation biomarkers were decreased by the co-administration of Q3G, indicating that Q3G attenuated lipid peroxidation in the aorta, as well as suppressing hyperlipidemia. In mice fed a 1.0% cholesterol diet for 12 weeks, supplementation with Q3G (0.05%–0.1%) led to the attenuation of hyperinsulinemia, as determined by decreased plasma levels of hepatic LDL receptor and proprotein convertase subtilisin kexin 9 (PCSK9), a regulator of plasma LDL cholesterol clearance [91]. Additionally, in apo-E−/− mice fed a high-fat diet for 12 weeks, plaque formation and levels of PCSK9, TNF-α, and IL-6 were significantly reduced by co-administration of quercetin aglycone at 12.5 mg/kg BW; meanwhile, the expression of ABCA1 and anti-atherosclerotic liver X receptor-α (LXR-α) was significantly increased [92].
Liver cholesterol is converted to bile acid by the action of the drug-metabolizing enzymes CYP7A1, CYP27A1, and CYP7B. Bile acid is released into the duodenum via the bile duct and reabsorbed by intestinal epithelial cells via apical sodium-dependent bile acid transporter (ASBT) and ileal bile acid-binding protein. Bile acid is then subject to reuptake into the liver by sodium-dependent taurocholate co-transporting peptide in a recycling process known as enterohepatic circulation. Therefore, increased excretion of bile acid leads to accelerated bile acid synthesis. Quercetin has been suggested to accelerate bile acid synthesis by upregulating CYP7A1 expression, resulting in lower cholesterol efflux. For example, in a rat study, a quercetin-based diet increased expression of CYP7A1, LXR-α, and ABCG1 [93]. In a different study carried out in mice, dietary supplementation with black bean extract containing Q3G resulted in elevated CYP7A1 expression and increased excretion of bile acid and cholesterol via bile acid synthesis [94]. Quercetin may also decrease plasma LDL levels by downregulating the expression of ASBT, which is responsible for normal enterohepatic circulation [95]. These reports indicate that quercetin has the potential to exert anti-atherosclerotic effects by regulating plasma LDL concentration.
6.3. In Vivo Animal Studies on the Anti-Atherogenic Effects of Onion Intake
Onions have been reported to protect against experimentally induced atherosclerosis. For example, in rats fed on a high-fat diet for 8 weeks, co-administration of onion peel extract (0.2% in the diet) increased ABCA1 expression, lowered LDL levels, and increased HDL levels [96]. In another study, co-administration of 10% onion powder to rats on a high-cholesterol diet (2%) for 7 weeks resulted in increased antioxidant activity in mesenteric microvessels, with the upregulation of SOD and GPX activity and the suppression of NOX activity [97]. Thus, enriching the diet with onion as a functional ingredient is proposed to prevent the vascular dysfunction associated with the initial development of atherosclerosis. In the same experimental system, onion powder increased anti-inflammatory ω-3 oxylipins levels in the liver, thus apparently exerting anti-inflammatory effects through the regulation of pro-inflammatory mediators [98]. Including onion in the diet of rats has also been found to influence the levels of specific primary and secondary bile acids, indicating that onion acts as a critical modifier of fecal bile acid composition linked to hypercholesterolemia [99]. In addition, administration of onion peel extract at 200 mg/kg BW to mice maintained on a 1.25% cholesterol diet for 12 weeks resulted in decreased LDL cholesterol levels, lower atherogenic index values, and concomitant increases in LDL receptor and CYP7A1 mRNA levels; these findings indicated that onion intake lowered cholesterol in the plasma and liver by inducing the excretion of cholesterol into the feces [100]. In a similar experiment, intake of onion extract (2.42 mg/kg BW quercetin aglycone plus 4.60 mg/kg BW Q4′G) ameliorated hyperlipidemia in rats by upregulating liver LDL receptor and downregulating 3-hydroxy-3-methylglutaryl-CoA reductase, an essential enzyme for cholesterol synthesis [101]. Nevertheless, it should be noted that active components other than quercetin aglycone and its glycosides may be responsible (independently or synergistically) for the cardioprotective effects of such extracts.
7. Disadvantages of In Vitro Cultured Cell and In Vivo Animal Studies for Elucidating the Mechanism of Action of Quercetin Glycosides
Quercetin glycosides are suggested to exert antioxidant and anti-inflammatory effects by modulating the activities of inflammation-related enzymes and cytokines directly, at the target site, or indirectly, through the regulation of gene expression via specific cellular signaling pathways. However, our mechanistic understanding of the cardioprotective effects of quercetin glycosides remains poor, as individual reports often fail to provide a comprehensive overview of potential integrative mechanisms. In addition, unphysiologically-high concentrations of quercetin compounds (>μM) have frequently been used in cultured cell studies as well as animal studies, which may not be appropriate for disease prevention in humans. So far, it is unknown to what extent the mechanisms proposed in these studies are responsible for the prevention of CVD in humans. It should be emphasized that neither quercetin aglycone nor quercetin glycosides are easily absorbed into the body, and even after absorption, they are mostly present in the form of conjugated metabolites in the blood. Therefore, the functions of these conjugated metabolites should be clarified in cultured cell studies to fully explore their protective mechanisms.
7.1. Mechanisms of Action of Conjugated Metabolites
7.1.1. Modulation of Oxidative Enzymes and Inflammatory Enzymes
Quercetin glucuronides have been found to exert inhibitory effects on xanthin oxidase (XOD) and LOX at expected plasma concentrations, depending on the conjugation position [102]. Q3′S retained considerable LOX inhibitory activity, while Q3GA had no LOX inhibitory activity [103]. Nonetheless, Q3GA was found to act as an effective inhibitor of MPO, an inflammatory enzyme secreted by activated neutrophils and macrophages [104,105]. Another study showed that quercetin plasma metabolites, including Q3GA, inhibited COX-2 mRNA expression in human lymphocytes ex vivo, although, in vivo, a single high dose of quercetin glucosides from onions had no effect on COX-2 mRNA levels [106].
7.1.2. Protection of Endothelial Function
Quercetin plasma metabolites including Q3GA and IR3GA induced the activation of AMPK and eNOS in human aortic endothelial cells (HAECs), indicating that these metabolites may protect endothelial function in part via the AMPK pathway [107]. Q3GA also ameliorated endothelial insulin resistance by suppressing excess ROS-induced inflammation via inhibition of IL-6 and TNF-α production [108]. However, Q3GA did not suppress TNF-α-induced ICAM-1 expression in IL-1α-stimulated HAECs, despite elevated permeability [109]. Furthermore, although Q3′S, Q3GA, and IR3GA suppressed the recruitment of monocytes to endothelial cells by inhibiting the cell-surface expression of VCAM-1 [110], neither Q3GA nor Q3′S affected the expression of cell adhesion molecules in LPS-stimulated human neutrophils [111].
Caveolin-1 plays a key role in endothelial dysfunction, acting as a platform for proatherogenic signal transduction in the caveolar membrane of endothelial cells [112]. Physiological concentrations (1 μM) of quercetin aglycone and Q3GA were equally effective at inhibiting oxLDL-induced expression of caveolin-1; both compounds also downregulated adhesion molecule expression in human umbilical vein endothelial cells (HUVECs) [113]. However, Q3GA did not inhibit the hydrogen peroxide-induced phosphorylation of caveolin-1, which increases the permeability of endothelial cells [114].
7.1.3. VSMCs as Cellular Targets
Neither Q3GA, Q3′-S, nor IR3GA affected the expression of VCAM-1, ICAM-1, or MCP-1 in TNF-α-stimulated human umbilical artery smooth muscle cells (HUASMCs) [115], suggesting that the effects of conjugated metabolites in blood vessels are not mediated via VSMCs. Nevertheless, Q3GA was found to suppress angiotensin II-induced c-Jun-N-terminal kinase (JNK) activation, thereby inhibiting DNA binding by activator protein-1 (AP-1) and preventing rat aortic smooth muscle cell hypertrophy [116]. Q3GA also suppressed platelet-derived growth factor-induced cell migration and proliferation by inhibiting the JNK and AP-1 signaling pathways [117].
7.1.4. Vasodilation and Vasoconstriction
Q3′S but not Q3GA was shown to inhibit endothelin-induced contraction of porcine coronary artery segments [118]. In addition, a HUASMC and HUVEC co-culture model study confirmed the beneficial effects of conjugated metabolites of quercetin on vascular function [119].
7.2. Deconjugation of Quercetin Glucuronides
As described above, quercetin is mainly present in the blood circulation in the form of hydrophilic conjugated metabolites. In general, conjugation reactions constitute a detoxification process for xenobiotics, as well as an inactivation process for dietary flavonoids. However, the anti-inflammatory effects of conjugated quercetin glucuronides have been suggested to result from deconjugation reactions that occur during inflammation and produce the parent aglycone [120,121]. Shimoi et al. [122] first reported that a flavone-type flavonoid, luteolin, was deconjugated by stimulated human neutrophils, while β-glucuronidase activity was increased in the plasma of LPS-injected rats and serum of patients undergoing hemodialysis. These findings indicated that the deconjugation of flavonoid metabolites was carried out in the plasma by β-glucuronidase, derived from stimulated neutrophils under inflammatory conditions. Kawai et al. [123] demonstrated the uptake of Q3GA by activated macrophages in human atherosclerotic lesions and showed that the anti-inflammatory effects of Q3GA were mediated by the release of its aglycone. In addition, they found that the extracellular efflux of β-glucuronidase from macrophages was triggered by increased levels of cellular calcium ions caused by mitochondrial dysfunction, while acidification around the cellular surface was also necessary for efficient enzyme activity [124]. The effects of activated macrophages on deconjugated quercetin aglycone levels represent a plausible explanation for the anti-atherosclerotic effects of dietary quercetin glycosides, which are characterized by low bioavailability and high susceptibility to metabolic conversion (Figure 6).
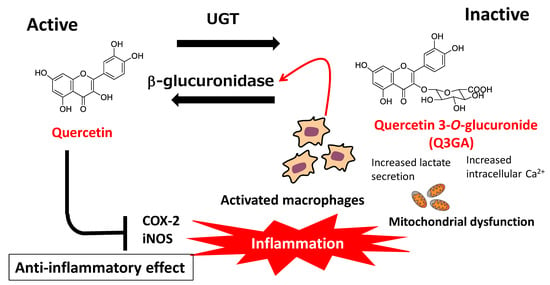
Figure 6.
Physiological significance of deconjugation of quercetin conjugated metabolites: conjugation–deconjugation cycle (COX-2: cyclooxyganse-2; iNOS: inducible nitric oxide synthase; UGT: UDP glucuronosyl transferase).
Further experiments showed that when Q3GA was perfused through isolated rat mesenteric vascular beds, Q3GA was slowly deconjugated to its aglycone in the perfusate and tissue by β-glucuronidase activity [125]. In addition, the administration of Q3GA or IR3GA (10 mg/kg BW) to spontaneously hypertensive rats (SHRs) resulted in the progressive lowering of mean blood pressure; this effect was abolished in rats treated with a specific β-glucuronidase inhibitor [126]. Q3GA was also found to effectively inhibit NOX activity in VSMCs from both normotensive rats and SHRs, while this effect was abrogated by the addition of a β-glucuronidase inhibitor [127]. These reports indicate the importance of deconjugation reactions for the vascular function of quercetin glucuronides. A human clinical study carried out using 15 healthy volunteers by Peretz et al. [128] also demonstrated the importance of quercetin metabolite deconjugation. This double-blind, randomized, placebo-controlled trial showed that increased branchial arterial diameter following the intake of 200 mg or 400 mg quercetin aglycone for 5 h was positively correlated with the products of Q3GA levels and plasma β-glucuronidase activity. It is therefore likely that dietary quercetin exerts acute vasodilator effects in vivo because of the deconjugation of the metabolite Q3GA. Interestingly, the comparison of Q3GA, Q3′GA, Q7GA, and Q4′GA showed that Q3GA was the most responsive to activated macrophage-induced deconjugation [129].
8. Contribution of the Microbiota to the Anti-Atherosclerotic Effect of Quercetin Glycosides
Dietary flavonoids are only partially absorbed by epithelial cells in the small intestine and mostly pass through the intestinal tract before being excreted in feces. Until recently, the functions of unabsorbed flavonoids have been limited to the inhibition of digestive enzymes such as lipase and amylase, resulting in the regulation of lipid metabolism and blood glucose levels, respectively. However, considerable attention is now being paid to novel functions relating to the gut microbiota such as the physiological role of catabolites produced by intestinal microorganisms and the prevention of dysbiosis in the microbiota [130].
8.1. Function of Catabolites Produced by the Action of the Microbiota
A catabolite of quercetin in the large intestine, 3-(3-hydroxyphenyl) propionic acid, has been suggested to exert anti-inflammatory effects by inhibiting the TNFα-induced adhesion of monocytes to HAECs and suppressing the upregulation of E-selectin, without modulating the expression of ICAM-1/VCAM-1 [131]. In addition, both 3,4-dihydroxyphenyl acetic acid (3,4-DHPAA) and 3,4-dihydroxyphenyl propionic acid (3,4-DHPPA), typical chain-scission products of quercetin, suppressed the production of TNFα, IL-1β, and IL-6 in LPS-stimulated peripheral blood mononuclear cells [132]. Analysis of an acetaminophen-induced mouse model of liver injury suggested that 3,4-DHPAA protected against liver injury by activation of the Nrf-2 signaling pathway, as the resulting upregulation of phase II drug-detoxifying enzymes and antioxidant enzymes was observed to promote the nuclear translocation of Nrf-2 [133]. Another study showed that 3,4-DHPAA upregulated the expression of phase-II drug-metabolizing enzymes, thereby suppressing oxidative stress in hepatocytes [134]. Thus, this catabolite may play a key role within the digestive tract and blood circulation. Furthermore, 3,4-dihydroxytoluene (DHT), suggested to be a catabolite of rutin, inhibited the production of inflammatory cytokines by reducing NFκB signaling in LPS-activated macrophages [135]. DHT was also shown to inhibit cholesterol synthesis in primary rat hepatocytes [136], while treatment with DHT or 3,4-DHPAA decreased blood pressure in normotensive rats and SHRs [137].
8.2. Modulation of Gut Microbiota Profiles
Quercetin and its glycosides have been shown to exert a variety of effects on the gut microbiota. The human gut microbiota is mainly composed of the Gram-positive phyla Firmicutes and Actinobacteria and Gram-negative phyla Bacteroidetes and Proteobacteria. A study comparing the gut biota in coronary artery disease patients and healthy volunteers showed that the order Lactobacillales (belonging to the phylum Firmicutes) was increased and the phylum Bacteroidetes was decreased in the coronary artery disease patients [138]. This dysbiosis may be a risk factor for atherosclerotic events. Nonetheless, unabsorbed flavonoids located in the large intestine have been suggested to act as prebiotics by increasing the abundance of Lactobacillus and Bifidobacterium, resulting in the improvement of the barrier function of the large intestine [139]. In addition, quercetin aglycone (but not rutin) strongly inhibited the growth of the human intestinal microorganisms Ruminococcus gauvreauii, Bacteroides galacturonicus, and Lactobacillus spp. [140]. Furthermore, the administration of quercetin aglycone to LDL receptor-null mice maintained on a high-fat diet reduced the abundance of Verrocomicrobia and increased the abundance of Actinobacteria, Cyanobacteria, and Firmicutes while reducing the extent of atherosclerotic lesions [141].
Short-chain fatty acids (SCFAs) including acetic acid, propionic acid, and butyric acid, are produced primarily from the microbial fermentation of dietary fiber. These compounds are known to regulate microbial activity in the gut and participate in host health through tissue-specific mechanisms relating to gut barrier function, glucose homeostasis, and immunomodulation [142]. In a multi-reactor gastrointestinal model, rutin increased total SCFA levels following digestion for 2 h [143]. In addition, high-pressure processed onion was able to increase the abundance of beneficial colon bacteria Bifidobacterium spp. and Lactobacillus spp. and enhance the production of total SCFAs in a colon fermentation simulator [144].
Further studies indicated the protective effects exerted by quercetin on the probiotic Lactobacillus strain, leading to improved functionality in the host [145]. Moreover, quercetin aglycone, Q3G, and A4′G were suggested to enhance the production of anti-inflammatory substances by Bifidobacterium adolescentis [146,147]. Quercetin aglycone also affects the mucosal barrier covering the intestinal epithelial surface, which is vital for maintaining intestinal health [148]; in vitro studies using a small intestinal cell co-culture system showed that quercetin aglycone modulated the expression and secretion of mucin, leading to improved barrier function [149].
Choline (a component of phosphatidylcholine found in eggs and other common foodstuffs) and L-carnitine (found in red meat) are converted to trimethylamine (TMA) by the action of the gut microbiota and transported to the liver via the portal vein following absorption in the large intestine [150]. TMA is oxidized to trimethylamine-N-oxide (TMAO) by the liver enzyme flavin-monooxygenase-3 and then delivered into the bloodstream [151]. Interestingly, TMAO appeared to promote atherosclerosis by accelerating the induction of platelet aggregation and inflammation in blood vessels. Hu et al. [152] reported that TMAO-induced vascular dysfunction and hepatic injury were significantly attenuated by the administration of tartary buckwheat flavonoids (consisting of rutin and quercetin) to TMA-fed mice for 8 weeks at 400 or 800 mg/kg BW. In addition, quercetin 3-ramnosylgalactoside was suggested to reduce the production of TMAO from TMA by acting as a potential inhibitor of TMA-lyase [153]. The possible functions of quercetin glycosides in the large intestine are summarized in Figure 7.
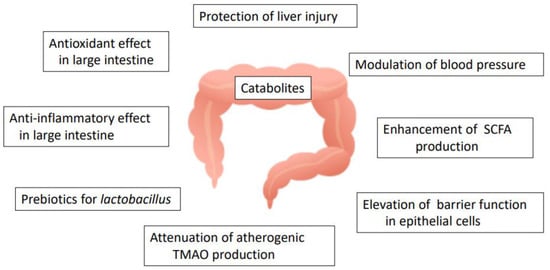
Figure 7.
Possible role of quercetin glycosides in relation to gut microbiota activity (SCFA: short chain fatty acids; TMAO: trimethylamine oxide).
9. Quercetin Glucosides as Senolytics
Senescent cells are defined as cells in a permanent state of growth arrest following the gradual loss of proliferative ability. Senescent cells are characterized by the spontaneous secretion of physiologically-active substances including inflammatory cytokines, growth factors, extracellular matrix metalloproteinases, and exosomes. This senescent-associated secretory phenotype (SASP) induces chronic inflammation in the surrounding tissue and contributes to the onset or exacerbation of CVD and other age-related diseases [154]. As atherosclerosis progresses with aging, senescent endothelial cells accumulate in atherosclerotic lesions and induce the infiltration of inflammatory cells by SASP [155]. The application of reagents capable of senolysis (i.e., the depletion of senescent cells), known as senolytics, is expected to aid in the development of novel treatments for CVD and other age-related diseases [156]. A powerful therapeutic strategy for the application of senolysis is the induction of senescent cell-specific apoptosis through the downregulation of Bcl-2 and Bcl-xL, members of the anti-apoptotic Bcl-2 family of proteins [157]. Quercetin aglycone was found to specifically deplete senescent cells in a study carried out in HUVECs and to act as a senolytic agent when combined with an anticancer drug (dasatinib) in a rodent study [158]. A pilot study for a human clinical trial has also been conducted, whereby a combination of dasatinib (100 mg/day) and quercetin (1000 mg/day) was administered for 3 days to nine patients with diabetic kidney disease (67.7 ± 3.1 years old) [159]. The results showed significantly decreased numbers of senescent cells in adipose tissue after 14 days and lower levels of SASP-related substances in plasma. This study suggested that high doses of quercetin may be required for the induction of senolytic effects in humans because of the low bioavailability of quercetin supplements. However, lower dosages may be sufficient when quercetin glucosides with higher bioavailability are supplied by cooked or processed foods.
10. Human Studies on the CVD-Preventive Effects of Quercetin Glycosides
10.1. Observational Studies
Multiple studies have investigated the role of quercetin glycosides in preventing CVD. For example, in their 1993 study, Hertog et al. [15] assessed flavonoid intake in a cohort of elderly men and followed them up for 5 years. The study results showed that flavonols (quercetin, kaempferol, and myricetin) and flavones (apigenin and luteolin), ingested mainly from tea, onions, and apples, were inversely associated with mortality from CHD. Flavonoids supplied in regularly consumed foods were therefore proposed to reduce the risk of death from CHD in this cohort. Analysis of sixteen cohorts in the Seven Countries Study (followed up for 25 years) also demonstrated that average flavonoid intake was inversely associated with CHD-related mortality [16]. Meanwhile, a French study showed that high consumption of flavonoid-rich foods may prevent CVD in women, but not in men [160]. Similarly, a 5-year follow-up study of Australian women aged > 75 years indicated that high levels of total flavonoid intake were associated with lower risks of all-cause and CVD mortality [161]. In 2014, a large-scale cohort study of 13,818 women in their 50 s, followed up for 15 years, discovered that greater intake of flavonoids from foods including oranges, berries, onions, and apples was associated with increased odds of healthy aging (defined as no chronic disease, no cognitive impairment, no impairment of physical function, and no limitation of mental health) [162]. The results of a large-scale cohort study on the prevention of CVD were also published recently by an Australian group. This study, which used the Danish Diet Cancer and Health Cohort (56,048 participants aged 52–60 years followed up for 23 years [18]) indicated that all-cause mortality and CVD mortality were inversely associated with dairy flavonoid intake (up to approximately 500 mg). In addition, a recent comprehensive meta-analysis of 39 prospective cohort studies on dietary flavonoids and CVD clarified that increased dietary intake of total flavonoids was linearly associated with lower risk of CVD, while quercetin intake was linearly associated with lower CHD risk [163].
Concerning flavonoid-rich foods, Galeone et al. [164] reported a case-control study of non-fatal acute myocardial infarction (AMI) in which the risk of AMI was decreased by the consumption of one or more portion of onion per week. In addition, an Iranian cohort study with a 6-year follow-up period showed that a higher habitual intake of allium vegetables (i.e., garlic and onion) was associated with reduced risk of CVD outcomes [165]. These observational studies strongly suggest the beneficial effects of onion and other quercetin glucoside-rich foods in terms of CVD prevention. Nevertheless, there may be inaccuracies in the reported levels of quercetin consumption by individuals. Metabolomic analyses using urine and blood samples may facilitate the accurate assessment of the amounts of flavonoids to which individuals have been exposed.
10.2. Intervention Studies
Some small-scale, double-blind, randomized, controlled studies have been carried out to assess the effects of flavonoid intake on CVD prevention. These intervention studies frequently involve flow-mediated dilatation (FMD), a non-invasive test of endothelial function that indicates the severity and extent of coronary atherosclerosis.
In a systematic review of seven trials involving healthy volunteers and patients without hypertension, Serban et al. [166] showed that supplementation with >500 mg/day of quercetin aglycone appeared to be required for the reduction of both systolic blood pressure and diastolic blood pressure. This high dosage may reflect the poor bioavailability of quercetin aglycone administered as a supplement. Another systematic review, which examined 18 randomized controlled trials concerning the effects of flavonoid-rich food products on endothelial function, showed that catechol-type flavonoids, including quercetin, improved FMD, albeit with a non-linear dose-response relationship [167]. In addition, several small-scale intervention studies have explored inflammation-related biomarkers as endpoints for the effectiveness of quercetin supplementation. For example, a randomized, placebo-controlled, crossover trial in 12 healthy men showed that oral administration of 200 mg quercetin resulted in significant increases in biomarkers of NO production (S-nitrosothiols, plasma nitrite, and urinary nitrate) and reductions in plasma and urinary endothelin concentrations [168]. Meanwhile, the administration of 150 mg quercetin/day to 93 overweight/obese subjects (aged 25–65 years) resulted in reduced systolic blood pressure after 6 weeks [169]. In this trial, quercetin intake significantly lowered the concentration of oxLDL in plasma without affecting TNF-α or C-reactive protein (CRP). Another trial, conducted in healthy male smokers, showed that supplementation with 100 mg quercetin (in capsules) for 10 weeks significantly reduced serum total cholesterol and LDL cholesterol concentrations and increased HDL cholesterol concentration; decreases in systolic and diastolic blood pressures were also observed, despite the lack of changes in IL-6 and VCAM-1 levels [170]. An intervention study that included 37 healthy hypertensive men and women aged 40–80 years receiving daily Q3G supplements (160 mg in capsules for 4 weeks) showed reduced levels of several inflammatory biomarkers, including soluble endothelial selectin, 1L-1β, and Z-score [171], although no changes in FMD or insulin resistance were seen [172]. Thus, quercetin glucosides may mediate cardioprotective effects by improving endothelial function and reducing inflammation. Nonetheless, one study indicated that acute administration of Q3G (up to 400 mg/day) to 15 healthy volunteers did not lead to any improvement in endothelial function, blood pressure, or NO production [173]. In contrast, in 25 participants with at least one CVD risk factor, increased FMD was observed 1.5 h after the administration of EMIQ (2 mg quercetin equivalent/kg BW), which is more water-soluble than Q3G [174]. Finally, a meta-analysis of 4–6 eligible randomized control studies evaluating the impact of quercetin supplementation on inflammatory biomarkers indicated that there were no relevant overall effects on peripheral CRP, IL6, or TNF-α [175]. However, subgroup analysis revealed significant reductions in circulating CRP and IL-6 in patients with diagnosed diseases and high-dose intervention. Thus, the authors concluded that the consumption of quercetin represented a promising therapeutic strategy for chronic disease management.
Regarding quercetin glucoside-rich foods, nine published works on onion intake are summarized in Table 2. In one study, continual onion consumption for 7 days did not result in any effects on platelet aggregation or other homeostatic variables, although mean plasma quercetin concentration was raised [176]. In another study, the treatment of stable type 2-diabetic patients with daily quercetin supplements (76–110 mg) provided by onion and tea for 2 weeks lowered the oxidative damage caused by hydrogen peroxide to freshly prepared lymphocyte DNA [177]. Ingestion of fried onions and fresh cherry tomatoes by non-obese normocholesterolemic female volunteers increased the resistance of lymphocyte DNA to strand breakage and decreased the levels of urinary 8-hydroxyguanosine, an oxidative stress biomarker, 4 h after ingestion of the meal [178]. In a different trial, intake of onion extracts for 30 days significantly improved FMD postprandially, while fasting FMD was not affected [179]. Moreover, in an 8-week trial, lower levels of total cholesterol and LDL cholesterol were found in patients allocated to a high-onion intervention group, compared with a low-onion control group [180]. A hypertensive subgroup of participants in a randomized 6-week control trial who received onion skin extracts showed decreases in systolic blood pressure [181]. Meanwhile, for healthy overweight and obese participants receiving daily soft capsules of onion peel extract, FMD was improved, and numbers of endothelial progenitor cells were significantly increased after 12 weeks [182]. In addition, healthy hypercholesterolemic volunteers who ingested wine containing extracts of onion exhibited substantially suppressed total cholesterol and LDL cholesterol levels after 10 weeks [183]. Nevertheless, another study showed that the daily intake of onion skin extracts did not significantly affect parameters of systemic or adipose tissue inflammation [184].

Table 2.
Intervention trials examining the anti-atherosclerotic effects of onion intake *.
Overall, both observational and intervention studies strongly imply the beneficial cardioprotective effects of a plentiful intake of quercetin glycoside-rich foods, although definitive conclusions cannot be derived at present. Intervention trials suffer from interindividual differences in the inherent and food style-dependent bioavailability of quercetin glycosides. Large-scale, long-term intervention studies are required to precisely assess the cardioprotective effects of quercetin glycoside-rich foods. Moreover, quercetin glycoside intakes calculated from common flavonoid-rich food databases [22,23] do not show the true levels of quercetin exposure in humans. This is a problematic issue for epidemiological studies attempting to explore the relationship between the intake of quercetin glycoside-rich foods and their CVD-preventive effects. Thus, novel biomarkers that reflect real individual exposure to quercetin should be developed to facilitate the accurate assessment of the cardioprotective effects of these foods.
11. Conclusions and Future Perspectives
Humans consume a wide variety of polyphenols from plant foods every day. The physiological functions of polyphenols are currently of great interest in relation to their protective effects against chronic diseases [185]. Notably, 2022 saw the publication of a pioneering large-scale intervention study suggesting that the habitual intake of cacao polyphenols reduced CVD-related mortality [186]. Quercetin, a member of the monomeric flavonoid subgroup of polyphenols, accounts for a major part of the dietary flavonoids originating from vegetables. The bioactivity and bioavailability of quercetin and its related flavonoids have been extensively investigated over the last 20 years to elucidate their cardioprotective role. It should be emphasized that glycosides, rather than aglycone, are the dietary source of quercetin. Glucose-bound glucosides, which are representative of the glycosides present in onion, should be distinguished from glycosides bound to non-glucose sugars in terms of absorption from the small intestine. Nevertheless, both types of glycosides are mostly catabolized and decomposed by gut microbiota to yield aglycone and ring-scission products that can exert dietary fiber-like effects on the digestive tract and gut microbiome. In the blood, quercetin glycosides are converted to conjugated metabolites, like invading xenobiotics. Under inflammatory conditions, conjugated metabolites may be returned to their aglycone form because of the activation of deconjugation enzymes. Both conjugates and deconjugated aglycone can suppress atherosclerosis via multiple functions, such as antioxidant, anti-inflammatory, and plasma cholesterol-lowering effects, directed against a broad range of targets including endothelial cells, oxidized LDL, VSMCs, and hepatic cells. However, a comprehensive mechanism that integrates each of the functions proposed to underlie the protective effects of dietary quercetin is yet to be established. Elucidation of these cardioprotective effects will require new techniques that enable the visualization of time-dependent changes occurring at cellular and intracellular target sites following quercetin glycoside ingestion/intestinal absorption.
In general, the most abundant dietary sources of quercetin are Q3,4′diG and Q4′G, supplied from onion. Onions are considered a functional food of vegetable origin as they contain two types of bioactive compounds, sulfur-containing compounds and quercetin glycosides [187]. Here, we described the results of short-term intervention trials indicating the cardioprotective effects of onion intake (Table 2). However, the relationship between the bioefficacy and bioavailability of quercetin glycosides from onions is not yet known. A large-scale intervention trial is necessary to clarify this issue. Extensive metabolomic analysis of plasma is required, as the bioavailability of plant bioactives, including flavonoids, differs between individuals [188]. Such a study would help to confirm the role of onion quercetin glycosides as a beneficial food source capable of preventing CVD.
The therapeutic effects and safe uses of plant polyphenols have also been explored in the context of clinical treatments for CVD [189]. Interestingly, pilot studies on the senolytic properties of quercetin (i.e., the selective removal of senescent cells from the human body) have recently been initiated, using high-dose supplementation of pure quercetin aglycone in capsules or tablets. However, we have confirmed that the bioavailability of quercetin glucosides from onion or processed onion is much higher than that of pure quercetin aglycone in capsules or tablets because of the effect of the food matrix, as well as other unknown factors. Thus, onion itself could be used clinically for the prevention or treatment of CVD [190]. The high bioavailability of onion quercetin glucosides warrants their potential application as a source of dietary quercetin for CVD treatment.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank Sarah Ivins, from Edanz (https://jp.edanz.com/ac, accessed on 6 December 2022) for editing a draft of this manuscript.
Conflicts of Interest
The author declares no conflict of interest.
References
- Mathesius, U. Flavonoid functions in plants and their interactions with other organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, M.L.F.; Rius, S.P.; Casati, P. Flavonoids: Biosynthesis, biological functions, and biotechnological applications. Front. Plant Sci. 2012, 3, 222. [Google Scholar] [CrossRef]
- Biswas, A.; Thattai, M. Promiscuity and specificity of eukaryotic glycosyltransferases. Biochem. Soc. Transact. 2020, 48, 891–900. [Google Scholar] [CrossRef]
- Terao, J.; Piskula, M.K.; Yao, Q. Protective effect of epicatechin, epicatechin gallate and quercetin on lipid peroxidation in phospholipid bilayers. Arch. Biochem. Biophys. 1994, 308, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 4, 375–383. [Google Scholar] [CrossRef]
- Bors, W.; Michel, C.; Stettmaier, K. Antioxidant effects of flavonoids. Biofactors 1997, 6, 399–402. [Google Scholar] [CrossRef]
- Ferrali, M.; Signorini, C.; Caciotti, B.; Sugherini, L.; Ciccoli, L.; Giachetti, D.; Comporti, M. Protection against oxidative damage of erythrocyte membrane by the flavonoid quercetin and its relation to iron chelating activity. FEBS Lett. 1997, 416, 123–129. [Google Scholar] [CrossRef]
- Oboh, G.; Ademosun, A.O.; Ogunsuyi, O.B. Quercetin and its role in chronic diseases. Adv. Exp. Med. Biol. 2016, 929, 377–387. [Google Scholar] [CrossRef]
- Deepika; Maurya, P.K. Health benefits of quercetin in age-related diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef]
- Ferrenczyova, K.; Kalocayova, B.; Bartekova, M. Potential implications of quercetin and its derivatives in cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- WHO Fact Sheets Cardiovascular Diseases 11 June 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvd) (accessed on 14 September 2022).
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencout, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers. 2019, 5, 56. [Google Scholar] [CrossRef]
- Nanri, A.; Mizoue, T.; Shimazu, T.; Ishihara, J.; Takachi, R.; Noda, M.; Iso, H.; Sasazuki, S.; Sawada, N.; Tugane, S. Dietary patterns and all-cause, cancer, and cardiovascular disease mortality in Japanese men and women: The Japan public health center-based prospective study. PLoS ONE 2017, 12, e0174848. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Hertog, M.G.; Kromhout, D.; Aravanis, C.; Blackburn, H.; Buzina, R.; Fidanza, F.; Giampaoli, S.; Jansen, A.; Menotti, A.; Nedeljkovic, S. Flavonoid intake and long-term risk of coronary heart disease and cancer in the seven countries study. Arch. Intern. Med. 1995, 155, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Mink, P.J.; Scrafford, C.G.; Barraj, L.M.; Harnack, L.; Hong, C.-P.; Nettleton, J.A.; Jacobs, D.R., Jr. Flavonoid intake and cardiovascular disease mortality: A prospective study in postmenopausal women. Am. J. Clin. Nutr. 2007, 85, 895–909. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Dalgaard, F.; Kyro, C.; Murray, K.; Bondonno, C.P.; Lewis, J.R.; Croft, K.D.; Gislason, G.; Scalbert, A.; Cassidy, A.; et al. Flavonoid intake is associated with lower mortality in the Daish Diet Cancer and Health Cohort. Nat. Commun. 2019, 10, 3651. [Google Scholar] [CrossRef]
- Kozlowska, A.; Szostak-Wegierek, D. Targeting cardiovascular diseases by flavonols: An update. Nutrients 2022, 14, 1439. [Google Scholar] [CrossRef]
- Grosso, G.J.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernandez, T.Y.; Quiles, J.L.; Battino, M.; et al. The effect of dietary polyphenols on vascular health and hypertension: Current evidence and mechanisms of action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Crozier, A. Dietary flavonoids and phenolic compounds. In Plant Phenolics and Human Health; Fraga, C.G., Ed.; Wiley &Sons Inc: Hoboken, NJ, USA, 2010; pp. 1–51. [Google Scholar]
- Rothwell, J.A.; Pérez-Jiménez, J.; Neveu, V.; Medina-Ramon, A.; M’Hiri, N.; Garcia Lobato, P.; Manach, C.; Knox, K.; Eisner, R.; Wishart, D.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- USDA: USDA Special Interest Databases on Flavonoids. 2015. Available online: https://data.nal.usda.gov/dataset/usda-special-interest-databases-flavonoids (accessed on 14 September 2022).
- Griffith, G.; Trueman, L.; Crowther, T.; Thomas, B.; Smith, B. Onions—A global benefit to health. Phytother. Res. 2002, 16, 603–615. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.M.; Barraj, L.M.; Spungen, J.H.; Herman, D.R.; Randolph, K. Global assessment of select phytonutrient intakes by level of fruit and vegetable consumption. Br. J. Nutr. 2014, 112, 1004–1018. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef]
- Crespy, V.; Morand, C.; Manach, C.; Besson, C.; Demigne, C.; Remesy, C. Part of quercetin absorbed in the small intestine is conjugated and further secreted in the intestinal lumen. Am. J. Physiol. 1999, 277, G120–G126. [Google Scholar] [CrossRef] [PubMed]
- Murota, K.; Terao, J. Antioxidative flavonoid quercetin: Implication of its intestinal absorption and metabolism. Arch. Biochem. Biophys. 2003, 417, 12–17. [Google Scholar] [CrossRef]
- Kawabata, K.; Mukai, R.; Ishisaka, A. Quercetin and related polyphenols: New insights and implications for their bioactivity and bioavailability. Food Funct. 2015, 6, 1399–1417. [Google Scholar] [CrossRef]
- Donovan, C.; Manach, R.M.; Faulks, P.A.; Kroon, P.A. Plant Secondary Metabolites: Occurrence, Structure and Role in the Human Diet; Crozier, A., Clifford, M.N., Ashihara, H., Eds.; Blackwell Publishing: Oxford, UK, 2006; pp. 303–351. [Google Scholar]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotech. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Day, A.J.; Morgan, M.R.A. Experimental determination of octanol-water partition coefficients of quercetin and related flavonoids. J. Agric. Food Chem. 2005, 53, 4355–4360. [Google Scholar] [CrossRef]
- Wolffram, S.; Block, M.; Ader, P. Quercetin-3-glucoside is transported by the glucose carrier SGLT1 across the brush border membrane of rat small intestine. J. Nutr. 2002, 132, 630–635. [Google Scholar] [CrossRef]
- Day, A.J.; Canada, F.J.; Diaz, J.C.; Kroon, P.A.; Mclauchlan, R.; Faulds, C.B.; Plumb, G.W.; Morgan, M.R.A.; Williamson, G. Dietary flavonoid and isoflavone glycosides are hydrolysed by the lactase site of lactase phlorizin hydrolase. FEBS Lett. 2000, 46, 166–170. [Google Scholar] [CrossRef]
- Day, A.J.; Dupont, M.S.; Ridley, S.; Rhodes, M.; Rhodes, M.J.C.; Morgan, M.R.A.; Williamson, G. Deglycosylation of flavonoid and isoflavonoid glycosides by human small intestine and liver β-glucosidase activity. FEBS Lett. 1998, 436, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Ioku, K.; Pongpiriyadacha, Y.; Konishi, Y.; Takei, Y.; Nakatani, N.; Terao, J. β-Glucosidase activity in the rat small intestine toward quercetin monoglucosides. Biosci. Biotechnol. Biochem. 1998, 62, 1428–1431. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Vree, T.B.; Katan, M.B. Bioavailability of quercetin-3-glucoside and quercetin-4′-glucoside do not differ in humans. J. Nutr. 2000, 130, 1200–1203. [Google Scholar] [CrossRef]
- Murota, K.; Matsuda, N.; Kashino, Y.; Fujikura, Y.; Nakamura, T.; Kato, Y.; Shimizu, R.; Okuyama, S.; Tanaka, H.; Koda, T.; et al. α-Oligoglucoylation of a sugar moiety enhances the bioavailability of quercetin glucosides in humans. Arch. Biochem. Biophys. 2010, 501, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Arts, I.C.W.; Sesink, A.L.A.; Faassen-Peters, M.; Hollman, P.C.H. The type of sugar moiety is a determinant of the small intestinal uptake and subsequent biliary excretion of dietary quercetin glycosides. Br. J. Nutr. 2004, 91, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Morand, C.; Demigne, C.; Texier, O.; Regerat, F.; Remesy, C. Bioavailability of rutin and quercetin in rats. FEBS Lett. 1997, 409, 12–16. [Google Scholar] [CrossRef]
- Carbonaro, M.; Grant, G. Absorption of quercetin and rutin in rat small intestine. Ann. Nutr. Metab. 2005, 49, 178–182. [Google Scholar] [CrossRef]
- De Vries, J.H.M.; Hollman, P.C.H.; Meyboom, S.; Buysman, M.N.C.P.; Zock, P.L.; van Staveren, W.A.; Katan, M.B. Plasma concentrations and urinary excretion of the antioxidant flavonols quercetin and kaempferol as biomarkers for dietary intake. Am. J. Clin. Nutr. 1998, 68, 60–65. [Google Scholar] [CrossRef]
- Erlund, I.; Kosonen, T.; Alfthan, G.; Maenpaa, J.; Perttunen, K.; Kenraali, J.; Parantainen, J.; Aro, A. Pharmacokinetics of quercetin from quercetin aglycone and rutin in healthy volunteers. Eur. J. Clin. Pharmacol. 2000, 56, 545–553. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Edwards, C.A.; Crozier, A. The relative contribution of the small and large intestine to the absorption and metabolism of rutin in man. Free Radic. Res. 2006, 40, 1035–1046. [Google Scholar] [CrossRef]
- Boonpawa, R.; Spenkelink, A.; Rietjens, I.M.C.M.; Punt, A. A physiologically based kinetic (PBK) model describing plasma concentrations of quercetin and its metabolites in rats. Biochem. Pharmacol. 2014, 89, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Kinjo, C.; Nakamura, Y.; Kato, Y.; Nishikawa, M.; Hamada, M.; Nakajima, N.; Ikushiro, S.; Murota, K. Lymphatic metabolites of quercetin after intestinal administration of quercetin-3-glucoside and its aglycone in rats. Arch. Biochem. Biophys. 2018, 645, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Mullen, W.; Boitier, A.; Stewart, A.J.; Crozier, A. Flavonoid metabolites in human plasma and urine after the consumption of red onions: Analysis by liquid chromatography with photodiode array and full scan tandem mass spectrometric detection. J. Chromatogr. A 2004, 1058, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Mitchell, A.E. Pharmacokinetics of quercetin absorption from apples and onions in healthy humans. J. Agric. Food Chem. 2012, 60, 3874–3881. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ebeler, S.E.; Zweigenbaum, J.A.Z.; Mitchell, A.E. UHPLC-(ESI)QTOF MS/MS profiling of quercetin metabolites in human plasma postconsumption of applesauce enriched with apple peel and onion. J. Agric. Food Chem. 2012, 60, 8510–8520. [Google Scholar] [CrossRef] [PubMed]
- Boonpawa, R.; Moradi, N.; Spenkelink, A.; Rietjens, I.M.C.M.; Punt, A. Use of physiologically based kinetic (PBK) modeling to study interindividual human variation and species differences in plasma concentrations of quercetin and its metabolites. Biochem. Pharmacol. 2015, 98, 690–702. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.H.; Buijsman, M.N.C.P.; van Amelsvoort, M.M.; Katan, M.B. Chlorogenic acid, quercetin-3-rutinoside and black tea phenols are extensively metabolized in humans. J. Nutr. 2003, 133, 1806–1814. [Google Scholar] [CrossRef]
- Braune, A.; Gutschow, M.; Engst, W.; Blaut, M. Degradation of quercetin and luteolin by Eubacterium ramulus. Appl. Environ. Microbiol. 2001, 67, 5558–5567. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, S.Y.; Park, S.Y.; Han, M.J. Metabolism of quercitrin by human intestinal bacteria and its relation to some biological activities. Biol. Pharm. Bull. 1999, 22, 749–751. [Google Scholar] [CrossRef]
- Jaganath, I.B.; Mullen, W.; Lean, M.E.J.; Edwards, C.A.; Crozier, A. In vitro catabolism of rutin by human fecal bacteria and the antioxidant capacity of its catabolites. Free Radic. Biol. Med. 2009, 47, 1180–1189. [Google Scholar] [CrossRef]
- Yang, J.; Qian, D.; Guo, J.; Jiang, S.; Shang, E.; Duan, J.; Xu, J. Identification of the major metabolites of hyperoxide produced by the human intestinal bacteria using the ultra performance liquid chromatography/quadrupole-time-of-flight mass spectrometry. J. Ethnopharmacol. 2013, 147, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Liu, X.; Bolling, B. Flavonoids and gut health. Curr. Opin. Biotechnol. 2020, 61, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Caro, G.; Ludwig, I.A.; Polyviou, T.; Malkova, D.; Garcia, A.; Moreno-Rojas, J.M.; Clozier, A. Identification of plasma and urinary metabolites and catabolites derived from orange juice (poly)phenols: Analysis by high-performance liquid chromatography-high resolution mass spectrometry. J Agric. Food Chem. 2016, 64, 5724–5735. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 7, 429–452. [Google Scholar] [CrossRef]
- Jin, F.; Nieman, D.C.; Shanely, R.A.; Knab, A.M.; Austin, M.D.; Sha, W. The variable plasma quercetin response to 12-week quercetin supplementation in humans. Eur. J. Clin. Nutr. 2010, 64, 692–697. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bruno, R.S. Endogenous and exogenous mediators of quercetin bioavailability. J. Nutr. Biochem. 2015, 26, 201–210. [Google Scholar] [CrossRef]
- Egert, S.; Wolffram, S.; Schulze, B.; Langguth, P.; Hubbermann, E.M.; Schwarz, K.; Adolphi, B.; Bosy-Westphal, A.; Rimbach, G.; Muller, M.J. Enriched cereal bars are more effective in increasing plasma quercetin compared with quercetin from powder-filled hard capsules. Br. J. Nutr. 2012, 107, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Burak, C.; Brull, V.; Langguth, P.; Zimmerman, B.F.; Stoffel-Wagner, B.; Sausen, U.; Stehle, P.; Wolffram, S.; Egert, S. Higher plasma quercetin levels following oral administration of an onion skin extract compared with pure quercetin dihydrate in humans. Eur. J. Nutr. 2017, 56, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Williamson, G. Comparison of the urinary excretion of quercetin glycosides from red onion and aglycone from dietary supplements in healthy subjects: A randomized single-blinded cross-over study. Food Funct. 2015, 6, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Kashino, Y.; Murota, K.; Matsuda, N.; Tomotake, M.; Hamano, T.; Mukai, R.; Terao, J. Effect of processed onions on the plasma concentration of quercetin in rats and humans. J. Food Sci. 2015, 80, H2597–H2602. [Google Scholar] [CrossRef]
- Guo, Y.; Mah, E.; Davis, C.G.; Jalili, T.; Ferruzzi, M.G.; Chun, O.K.; Bruno, R.S. Dietary fat increases quercetin bioavailability in overweight adults. Mol. Nutr. Food Res. 2013, 57, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Ippoushi, K.; Ito, H.; Horie, H.; Terao, J. Enhancing effect of lipids and emulsifiers on the accumulation of quercetin metabolites in blood plasma after the short-term ingestion of onion by rats. Biosci. Biotechnol. Biochem. 2003, 67, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Nuka, E.; Takahashi, M.; Okitsu, M.; Nayama, C.; Nishijima, H.; Sogawa, R.; Kawabata, K.; Terao, J.; Mukai, R. Lowering effect of combined sweet potato and onion intake on plasma quercetin concentration and underlying mechanism involving intestinal b-glucosidase activity. Biosci. Biotech. Biochem. 2022, 86, 1695–1698. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Murota, K.; Kumamoto, S.; Misumi, K.; Bando, N.; Ikushiro, S.; Takahashi, N.; Sekido, K.; Kato, Y.; Terao, J. Plasma metabolites of dietary flavonoids after combination meal consumption with onion and tofu in humans. Mol. Nutr. Food Res. 2014, 58, 310–317. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Ioku, K.; Aoyama, Y.; Tokuno, A.; Terao, J.; Nakatani, N.; Takei, Y. Various cooking methods and the flavonoid content in onion. J. Nutr. Sci. Vitaminol. 2001, 47, 78–83. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martinez-Blazquez, A.; Horcajada, M.-N.; Tomas-Barberan, F.A.; Garcia-Villalba, R. Technological and biotechnological process to enhance the bioavailability of dietary (poly)phenols in humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-Garcia, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martin, C. Pathophysiology of atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Li, R.-L.; Wang, L.-Y.; Liu, S.; Duan, H.-X.; Zhang, Q.; Zhang, T.; Peng, W.; Huang, Y.; Wu, C. Natural Flavonoids derived from fruits are potential agents against atherosclerosis. Front. Nutr. 2022, 9, 862277. [Google Scholar] [CrossRef]
- Terao, J. Factors affecting bioavailability of quercetin-related flavonoids and the consequences of their vascular function. Biochem. Pharmacol. 2017, 139, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahaman, S.; Islam, R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, S.; et al. Role of phenolic compounds in human disease: Current knowledge and future prospects. Molecules 2022, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016, 2016, 9340637. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Li, C.; Zhang, W.-J.; Frei, B. Quercetin inhibits LPS-induced adhesion molecule expression and oxidant production in human aortic endothelial cells by p38-mediated Nrf2 activation and antioxidant enzyme induction. Redox Biol. 2016, 9, 104–113. [Google Scholar] [CrossRef]
- Morikawa, K.; Nonaka, M.; Narahara, M.; Torii, I.; Kawaguchi, K.; Yoshikawa, T.; Kumazawa, Y.; Morikawa, S. Inhibitory effect of quercetin on carrageenan-induced inflammation in rats. Life Sci. 2003, 74, 709–721. [Google Scholar] [CrossRef]
- Mamani-Matsuda, M.; Kauss, T.; Al-Kharrat, A.; Rambert, J.; Fawaz, F.; Thiolat, D.; Moynet, D.; Coves, S.; Malvy, D.; Mossalayi, M.D. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem. Pharmacol. 2006, 72, 1304–1310. [Google Scholar] [CrossRef]
- Milenkovic, M.; Arsenovic-Ranin, N.; Stojic-Vukanic, Z.; Bufan, B.; Vucicevic, D.; Jancic, I. Quercetin ameliorates experimental autoimmune myocarditis in rats. J. Pharm. Pharmaceut. Sci. 2010, 13, 311–319. [Google Scholar] [CrossRef]
- Zheng, J.Y.; Wu, J.H.; Chen, J.; Liu, J.; Lu, Y.Y.; Huang, C.L.; Hu, G.Y.; Wang, X.P.; Zeng, Y. Therapeutic effects of quercetin on early inflammation in hypertriglyceridemia-related acute pancreatitis and its mechanism. Pancreatology 2016, 16, 200–210. [Google Scholar] [CrossRef]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R.C. Cardioprotective actions of two bioflavonoids, quercetin and rutin, in experimental myocardial infarction in both normal and streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.; Feng, D.; Qin, H.; Wen, H.; Yin, Z.; Gao, G.; Li, C. Protective effect of quercetin against oxidative stress and brain edema in an experimental rat model of subarachnoid hemorrhage. Int. J. Med. Sci. 2014, 11, 282–290. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, L.; Lu, S. Evaluation of antioxidant and immunity activities of quercetin in isoproterenol-treated rats. Molecules 2012, 17, 4281–4291. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jiang, Y.; Jing, W.; Sun, B.; Miao, C.; Ren, L. Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can. J. Physiol. Pharmacol. 2013, 91, 951–959. [Google Scholar] [CrossRef]
- Sun, P.; Zhao, L.; Zhang, N.; Zhou, J.; Zhang, L.; Wu, W.; Ji, B.; Zhou, F. Bioactivity of dietary polyphenols: The role in LDL-C lowering. Foods 2021, 10, 2666. [Google Scholar] [CrossRef]
- Nekohashi, M.; Ogawa, M.; Ogihara, T.; Nakazawa, K.; Kato, H.; Misaka, T.; Abe, K.; Kobayashi, S. Luteolin and quercetin affect the cholesterol absorption mediated by epithelial cholesterol transporter Niemann-Pick C1-Like 1 in Caco-2 cells and rats. PLoS ONE 2014, 9, e97901. [Google Scholar] [CrossRef]
- Kamada, C.; da Silva, E.L.; Ohnishi-Kameyama, M.; Moon, J.-H.; Terao, J. Attenuation of lipid peroxidation and hyperlipidemia by quercetin glucoside in the Aorta of high cholesterol-fed rabbit. Free Radic. Res. 2005, 39, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Mbikay, M.; Mayne, J.; Sirois, F.; Fedoryak, O.; Raymond, A.; Noad, J.; Chretien, M. Mice fed a high-cholesterol diet supplemented with quercetin-3-glucoside show attenuated hyperlipidemia and hyperinsulinemia associated with differential regulation of PCSK9 and LDLR in their liver and pancreas. Mol. Nutr. Food Res. 2018, 62, e1700729. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cao, H.; Shen, D.; Chen, C.; Xing, S.; Dou, F.; Jia, Q. Effect of quercetin on atherosclerosis based on expressions of ABCA1, LXR-α, and PCSK9 in ApoE-/- mice. Chin. J. Integr. Med. 2020, 26, 114–121. [Google Scholar] [CrossRef]
- Zhang, M.; Xie, Z.; Gao, W.; Pu, L.; Wei, J.; Guo, C. Quercetin regulates hepatic cholesterol metabolism by promoting cholesterol-to-bile acid conversion and cholesterol efflux in rats. Nutr. Res. 2016, 36, 271–279. [Google Scholar] [CrossRef]
- Chavez-Santoscoy, R.A.; Gutierrez-Uribe, J.A.; Granados, O.; Torre-Villalvazo, I.; Serna-Saldivar, S.O.; Torres, N.; Palacios-Gonzalez, B.; Tovar, A.R. Flavonoids and saponins extracted from black bean (Phaseolus vulgaris L.) seed coats modulate lipid metabolism and biliary cholesterol secretion in C57BL/6 mice. Br. J. Nutr. 2014, 112, 886–899. [Google Scholar] [CrossRef]
- Chambers, K.E.; Day, P.E.; Aboufarrag, H.T.; Kroon, P.A. Polyphenol effects of cholesterol metabolism via bile acid biosynthesis, CYP7A1: A review. Nutrients 2019, 11, 2588. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-M.; Moon, J.; Do, H.J.; Chung, J.H.; Lee, K.-H.; Cha, Y.-J.; Shin, M.-J. Onion peel extract increases hepatic low-density lipoprotein receptor and ATP-binding cassette transporter A1 messenger RNA expression in Sprague-Dawley rats fed a high fat diet. Nutr. Res. 2012, 32, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pena, D.; Angulo, J.; Vallejo, S.; Colina-Coca, C.; de Ancos, B.; Sanchez-Ferrer, C.F.; Peiro, C.; Sanchez-Moreno, C. High-cholesterol diet enriched with onion affects endothelium-dependent relaxation and NADPH oxidase activity in mesenteric microvessels from Wistar rats. Nutr. Metab. 2014, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pena, D.; Checa, A.; de Ancos, B.; Wheelock, C.E. New insights into the effects of onion consumption on lipid mediators using a diet-induced model of hypercholesterolemia. Redox Biol. 2017, 11, 205–212. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Gimenez, L.; de Ancos, B.; Sanchez-Moreno, C. Role of dietary onion in modifying the faecal bile acid content in rats fed a high-cholesterol diet. Food Func. 2017, 8, 2184–2192. [Google Scholar] [CrossRef]
- Kang, H.-J.; Balasubramanian, P.; Pichiah, T.; Abinaya, R.V.; Sohn, H.-S.; Cha, Y.-S. Hypocholesterolemic effect of quercetin-rich onion peel extract in C57BL/6J mice fed with high cholesterol diet. Food Sci. Biotechnol. 2016, 25, 855–860. [Google Scholar] [CrossRef]
- Li, W.; Yang, C.; Mei, X.; Huang, R.; Zhang, S.; Tang, Y.; Dong, Q.; Zhou, C. Effect of the polyphenol-rich extract from Allium cepa on hyperlipidemic spraque-dawley rats. J. Food Biochem. 2020, 45, e13565. [Google Scholar] [CrossRef]
- Day, A.J.; Bao, Y.; Morgan, M.R.; Williamson, G. Conjugation position of quercetin glucuronides and effect on biological activity. Free Radic. Biol. Med. 2000, 29, 1234–1243. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Stewart, S.; Mckinley, A.J.; Needs, P.W.; Kroon, P.A.; Hodgson, J.M.; Croft, K.D. Metabolic transformation has a profound effect on anti-inflammatory activity of flavonoids such as quercetin: Lack of association between antioxidant and lipoxygenase inhibitory activity. Biochem. Pharmacol. 2008, 75, 1045–1053. [Google Scholar] [CrossRef]
- Shiba, Y.; Kinoshita, T.; Chuman, H.; Taketani, Y.; Takeda, E.; Kato, Y.; Naito, M.; Kawabata, K.; Ishisaka, A.; Terao, J.; et al. Flavonoids as substrates and inhibitors of myeloperoxidase: Molecular actions of aglycone and metabolites. Chem Res. Toxicol. 2008, 21, 1600–1609. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Mckinley, A.J.; Needs, P.W.; Kroon, P.A.; Hodgson, J.M.; Croft, K.D. Quercetin and its in vivo metabolites inhibit neutrophil-mediated low-density lipoprotein oxidation. J. Agric. Food Chem. 2008, 56, 3609–3615. [Google Scholar] [CrossRef] [PubMed]
- De Pascual-Teresa, S.; Johnston, K.L.; Dupont, M.S.; O’Leary, K.A.; Needs, P.W.; Morgan, L.M.; Clifford, M.N.; Bao, Y.; Williamson, G. Quercetin metabolites downregulate cyclooxygenase-2 transcription in human lymphocytes ex vivo but not in vivo. J. Nutr. 2004, 134, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Croft, K.D.; Hodgson, J.M.; Kyle, R.; Lee, I.-L.E.; Wang, Y.; Stocker, R.; Ward, N.C. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem. Pharmacol. 2012, 84, 1036–1044. [Google Scholar] [CrossRef]
- Guo, X.-D.; Zhang, D.-Y.; Gao, X.-J.; Parry, J.; Liu, K.; Liu, B.-L.; Wang, M. Quercetin and quercetin-3-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, M.; Kajiya, K.; Terao, J.; Kaji, K.; Kumazawa, S.; Nakayama, T.; Shimoi, K. Effect of quercetin conjugates on vascular permeability and expression of adhesion molecules. Biofactors 2004, 22, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Tribolo, S.; Lodi, F.; Connor, C.; Suri, S.; Wilson, V.G.; Taylor, M.A.; Needs, P.W.; Kroon, P.A.; Hughes, D.A. Comparative effects of quercetin and its predominant human metabolites on adhesion molecule expression in activated human vascular endothelial cells. Atherosclerosis 2008, 197, 50–56. [Google Scholar] [CrossRef]
- Suri, S.; Taylor, M.A.; Verity, A.; Tribolo, S.; Needs, P.W.; Kroon, P.A.; Hughes, D.A.; Wilson, V.G. A comparative study of the effects of quercetin and its glucuronide and sulfate metabolites on human neutrophil function in vitro. Biochem. Pharmacol. 2008, 76, 645–653. [Google Scholar] [CrossRef]
- Filippini, A.; Sica, G.; D’Alessio, A. The caveolar membrane system in endothelium: From cell signaling to vascular pathology. J. Cell Biochem. 2018, 119, 5060–5071. [Google Scholar] [CrossRef]
- Kamada, C.; Mukai, R.; Kondo, A.; Sato, S.; Terao, J. Effect of quercetin and its metabolite on caveolin-1 expression induced by oxidized LDL and lysophosphatidylcholine in endothelial cells. J. Clin. Biochem. Nutr. 2016, 58, 193–201. [Google Scholar] [CrossRef]
- Kondo-Kawai, A.; Sakai, T.; Terao, J.; Mukai, R. Suppressive effects of quercetin on hydrogen peroxide-induced caveolin-1 phosphorylation in endothelial cells. J. Clin. Biochem. Nutr. 2021, 69, 28–36. [Google Scholar] [CrossRef]
- Winterbone, M.S.; Tribolo, S.; Needs, P.W.; Kroon, P.A.; Hughes, D.A. Physiologically relevant metabolites of quercetin have no effect on adhesion molecule or chemokine expression in human vascular smooth muscle cells. Atherosclerosis 2009, 202, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, M.; Tsuchiya, K.; Suzaki, Y.; Kirima, K.; Kyaw, M.; Moon, J.-H.; Terao, J.; Tamaki, T. Quercetin glucuronide prevents VSMC hypertrophy by angiotensin II via the inhibition of JNK and AP-1 signaling pathway. Biochem. Biophys. Res. Commun. 2002, 293, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Ishizawa, K.; Izawa-Ishizawa, Y.; Ohnishi, S.; Motobayashi, Y.; Kawazoe, K.; Hamano, S.; Tsuchiya, K.; Tomita, S.; Minakuchi, K.; Tamaki, T. Quercetin glucuronide inhibits cell migration and proliferation by platelet-derived growth factor in vascular smooth muscle cells. J. Pharmacol. Sci. 2009, 109, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Rayment, S.; Hughes, D.A.; Kroon, P.A.; Needs, P.W.; Taylor, M.A.; Tribolo, S.; Wilson, V.G. Quercetin and its major metabolites selectively modulate cyclic GMP-dependent relaxations and associated tolerance in pig isolated coronary artery. Br. J. Pharmacol. 2010, 159, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Lodi, F.; Winterbone, M.S.; Tribolo, S.; Needs, P.W.; Hughes, D.A.; Kroon, P.A. Human quercetin conjugated metabolites attenuate TNF-α induced changes in vasomodulatory molecules in an HUASMCs/HUVECs co-culture model. Planta Med. 2012, 78, 1571–1573. [Google Scholar] [CrossRef]
- Terao, J.; Murota, K.; Kawai, Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Func. 2011, 2, 11–17. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J.; Santos-Buelga, C. The Flavonoid paradox: Conjugation and deconjugation as key steps for the biological activity of flavonoids. J. Sci. Food Agric. 2012, 92, 1822–1825. [Google Scholar] [CrossRef]
- Shimoi, K.; Saka, N.; Nozawa, R.; Sato, M.; Amano, I.; Nakayama, T.; Kinae, N. Deglucuronidation of a flavonoid, luteolin monoglucuronide, during inflammation. Drug Metab. Dispos. 2001, 29, 1521–1524. [Google Scholar]
- Kawai, Y.; Nishikawa, T.; Shiba, Y.; Saito, S.; Murota, K.; Shibata, N.; Kobayashi, M.; Kanayama, M.; Uchida, K.; Terao, J. Macrophage as a target of quercetin glucuronides in human atherosclerotic arteries. J. Biol. Chem. 2008, 283, 9424–9434. [Google Scholar] [CrossRef]
- Ishisaka, A.; Kawabata, K.; Miki, S.; Shiba, Y.; Minekawa, S.; Nishikawa, T.; Mukai, R.; Terao, J.; Kawai, Y. Mitochondrial dysfunction leads to deconjugation of quercetin glucuronides in inflammatory macrophages. PLoS ONE 2013, 8, e80843. [Google Scholar] [CrossRef]
- Menendez, C.; Duenas, M.; Galindo, P.; Gonzalez-Manzano, S.; Jimenez, R.; Moreno, L.; Zarzuelo, M.J.; Rodoriguez-Gomez, I.; Duarte, J.; Santos-Buelga, C.; et al. Vascular deconjugation of quercetin glucuronide: The flavonoid paradox revealed? Mol. Nutr. Food Res. 2011, 55, 1780–1790. [Google Scholar] [CrossRef] [PubMed]
- Galindo, P.; Rodoriguez-Gomez, I.; Gonzalez-Manzano, S.; Duenas, M.; Jimenez, R.; Menendez, C.; Vargas, F.; Tamargo, J.; Santos-Buelga, C.; Perez-Vizcaino, F.; et al. Glucuronidated quercetin lowers blood pressure in spontaneously hypertensive rats via deconjugation. PLoS ONE 2012, 7, e32673. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R.; Lopez-Sepulveda, R.; Romero, M.; Toral, M.; Cogolludo, A.; Perez-Vizcaino, F.; Duarte, J. Quercetin and its metabolites inhibit the membrane NADPH activity in vascular smooth muscle cells from normotenstive and spontaneously hypertensive rats. Food Funct. 2015, 6, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Gonzalez-Manzano, S.; Jimenez, R.; Perez-Abud, R.; Haro, J.M.; Osuna, A.; Santos-Buelga, C.; Duarte, J. The flavonoid quercetin induces acute vasodilator effects in healthy volunteers: Correlation with beta-glucuronidase activity. Pharm. Res. 2014, 89, 11–18. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kada, Y.; Kimata, M.; Sakaki, T.; Ikushiro, S. Comparison of metabolism and biological properties among positional isomers of quercetin glucuronide in LPS- and RANKL-challenged RAW264.7 cells. Biosci. Biotechnol. Biochem. 2022, 86, 1670–1679. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Minihane, A.-M. The role of metabolism (and the microbiome) in defining the clinical efficacy of dietary flavonoids. Am. J. Clin. Nutr. 2017, 105, 10–22. [Google Scholar] [CrossRef]
- Feng, J.; Ge, C.; Li, W.; Li, R. 3-(3-hydroxyphenyl)propionic acid, a microbial metabolite of quercetin, inhibits monocyte binding to endothelial cells via modulating E-selectin expression. Fitoterapia 2022, 156, 105071. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andres-Lacueva, C.; Urpi-Sarda, M.; Vazquez-Agell, M.; Lamuela-Raventos, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Xue, H.; Xie, W.; Jiang, Z.; Wang, M.; Wang, J.; Zhao, H.; Zhang, X. 3,4-Dihydroxyphenylacetic acid, a microbiota-derived metabolite of quercetin, attenuates acetoaminophen (APAP)-induced liver injury through activation of Nrf-2. Xenobiotica 2016, 46, 931–939. [Google Scholar] [CrossRef]
- Tang, Y.; Nakashima, S.; Saiki, S.; Myoi, Y.; Abe, N.; Kuwazuru, S.; Zhu, B.; Ashida, H.; Murata, Y.; Nakamura, Y. 3,4-Dihydroxyphenylacetic acid is a predominant biologically-active catabolite of quercetin glycosides. Food Res. Int. 2016, 89, 716–723. [Google Scholar] [CrossRef]
- Su, K.-Y.; Yu, C.Y.; Chen, Y.-P.; Hua, K.-F.; Chen, Y.-L.S. 3,4-Dihydroxytoluene, a metabolite of rutin, inhibits inflammatory responses in lipopolysaccharide-activated macrophages by reducing the activation of NF-kB signaling. BMC Complement. Altern. Med. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Glasser, G.; Graefe, E.U.; Struck, G.; Veit, M.; Gebhardt, R. Comparison of antioxidative capacities and inhibitory effect on cholesterol biosynthesis of quercetin and potential metabolites. Phytomedicine 2002, 9, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pourova, J.; Najmanova, I.; Voprsalova, M.; Migkos, T.; Pilarova, V.; Applova, L.; Novakova, L.; Mladenk, P. Two flavonoid metabolites, 3,4-dihydroxyphenylacetic acid and 4-methylcatechol, relax arteries ex vivo and decrease blood pressure in vivo. Vascul. Pharmacol. 2018, 111, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Emoto, T.; Sasaki, N.; Hirata, K. Gut microbiota and coronary artery disease. Int. Heart J. 2016, 57, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Koudoufio, M.; Desjardins, Y.; Feldman, F.; Spahis, S.; Delvin, E.; Levy, E. Insight into polyphenol and gut microbiota crosstalk: Are their metabolites the key to understand protective effects against metabolic disorders? Antioxidants 2020, 9, 982. [Google Scholar] [CrossRef]
- Duda-Chodak, A. The inhibitory effect of polyphenols on human gut microbiota. J. Physiol. Pharmacol. 2012, 63, 497–503. [Google Scholar]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. App. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Ekbatan, S.S.; Sleno, L.; Sabally, K.; Khairallah, J.; Azadi, B.; Rodes, L.; Prakash, S.; Donnelly, D.J.; Kubow, S. Biotransformation of polyphenols in a dynamic multistage gastrointestinal model. Food Chem. 2016, 204, 453–462. [Google Scholar] [CrossRef]
- Fernandez-Jalao, I.; Balderas, C.; Calvo, M.V.; Fontecha, J.; Sanchez-Moreno, C.; De Ancos, B. Impact of high-pressure processed onion on colonic metabolism using a dynamic gastrointestinal digestion simulator. Metabolites 2021, 11, 262. [Google Scholar] [CrossRef]
- Dos Santos, A.S.; de Albuquerque, T.M.R.; de Brito Alves, J.L.; de Souza, E.L. Effects of quercetin and resveratrol on in vitro properties related to the functionality of potentially probiotic lactobacillus strains. Front. Microbiol. 2019, 10, 2229. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols enhanced production of anti-inflammatory substances by Bifidobacterium adolescentis: Prebiotic actions of galangin, quercetin, and fisetin. Biofactors 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Kato, Y.; Sakano, T.; Baba, N.; Hagiwara, K.; Tamura, A.; Baba, S.; Natsume, M.; Ohigashi, H. Effects of phytochemicals on in vitro anti-inflammatory activity of Bifidobacterium adolescentis. Biosci. Biotechnol. Biochem. 2015, 79, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Volstatova, T.; Marchica, A.; Hroncova, Z.; Bernardi, R.; Doskocil, I.; Havlik, J. Effects of chlorogenic acid, epicatechin gallate, and quercetin on mucin expression and secretion in the Caco-2/HT29-MTX cell model. Food Sci. Nutr. 2019, 7, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Klipfell, E.; Bennett, B.J.; Koeth, R.; Levison, B.S.; DuGar, B.; Feldstein, A.E.; Britt, E.B.; Fu, X.; Chung, Y.-M.; et al. flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011, 472, 57–63. [Google Scholar] [CrossRef]
- Tang, W.H.W.; Wang, Z.; Levison, B.S.; Koeth, R.A.; Britt, E.B.; Fu, X.; Wu, Y.; Hazen, S.L. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N. Eng. J. Med. 2013, 368, 1575–1584. [Google Scholar] [CrossRef]
- Hu, Y.; Zhao, Y.; Yuan, L.; Yang, X. Protective effects of tartary buckwheat flavonoids on high TMAO diet-induced vascular dysfunction and liver injury in mice. Food Funct. 2015, 6, 3359–3372. [Google Scholar] [CrossRef]
- Hua, F.; Zhou, P.; Bao, G.-H.; Ling, T.-J. Flavonoids in Lu’an Guapian tea as potential inhibitors of TMA-lyase in acute myocardial infarction. J. Food Biochem. 2022, 46, e14110. [Google Scholar] [CrossRef]
- Bloom, S.I.; Islam, M.T.; Lesniewski, L.A.; Donato, A.J. Mechanisms and consequences of endothelial cell senescence. Nat. Rev. Cardiol. 2023, 20, 38–51. [Google Scholar] [CrossRef]
- Shimizu, I.; Minamino, T. Cellular senescence in arterial diseases. J. Lipid Atheroscler. 2020, 9, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Gasek, N.; Kuchel, G.A.; Kirkland, J.L.; Xu, M. Strategies for targeting senescent cells in human disease. Nat. Aging 2021, 1, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, J.L.; Tchkonia, T. Selolytic drugs: From discovery to translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Zhu, Y.; Tchkonia, T.; Pirskhalava, T.; Gower, A.C.; Ding, H.; Giogadeze, N.; Palmer, A.K.; Ikeno, Y.; Hubbard, G.B.; Lenburg, M.; et al. The Achilles’ heel of senescent cells: From transcriptome to senolytic drugs. Aging Cell 2015, 14, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Hickson, L.J.; Prata, L.G.P.L.; Bobart, S.A.; Evans, T.K.; Giorgadze, N.; Hashmi, S.K.; Herrmann, S.M.; Jensen, M.D.; Jia, Q.; Jordan, K.L.; et al. Senolytics decrease senescent cells in humans: Preliminary report from a clinical trial of Dasatinib plus Quercetin in individuals with diabetic kidney disease. EBioMedicine 2019, 47, 446–456. [Google Scholar] [CrossRef]
- Mennen, L.I.; Sapinho, D.; de Bree, A.; Arnault, N.; Bertrais, S.; Galan, P.; Hercberg, S. Consumption of foods rich in flavonoids is related to a decreased cardiovascular risk in apparently healthy French women. J. Nutr. 2004, 134, 923–926. [Google Scholar] [CrossRef]
- Ivey, K.L.; Hodgson, J.M.; Croft, K.D.; Lewis, J.R.; Prince, R.L. Flavonoid intake and all-cause mortality. Am. J. Clin. Nutr. 2015, 101, 1012–1020. [Google Scholar] [CrossRef]
- Samieri, C.; Sun, Q.; Townsend, M.K.; Rimm, E.B.; Grodstein, F. Dietary flavonoid intake at midlife healthy aging in women. Am. J. Clin. Nutr. 2014, 100, 1489–1497. [Google Scholar] [CrossRef]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary flavonoids and cardiovascular disease: A comprehensive dose-response meta-analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef]
- Galeone, C.; Tavani, A.; Pelucchi, C.; Negri, E.; La Vecchia, C. Allium vegetable intake and risk of acute myocardial infarction in Italy. Eur. J. Nutr. 2009, 48, 120–123. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Momenan, A.A.; Azizi, F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: A longitudinal follow-up study. J. Hypertens. 2017, 35, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.-C.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of quercetin on blood pressure: A systematic review and meta-analysis of randomized controlled trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomized controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Loke, W.M.; Hodgson, J.M.; Proudfoot, J.M.; McKinley, A.J.; Puddey, I.B.; Croft, K.D. Pure dietary flavonoids quercetin and (−)-epicatechin augment nitric oxide products and reduce endothelin-1 acutely in healthy men. Am. J. Clin. Nutr. 2008, 88, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Egert, S.; Bosy-Westphal, A.; Seiberl, J.; Kurbitz, C.; Settler, U.; Plachta-Danielzik, S.; Wagner, A.E.; Frank, J.; Schrezenmeir, J.; Rimbach, G.; et al. Quercetin reduces systolic blood pressure and plasma oxidized low-density lipoprotein concentrations in overweight subjects with a high-cardiovascular disease risk phenotype: A double-blinded, placebo-controlled cross-over study. Br. J. Nutr. 2009, 102, 1065–1074. [Google Scholar] [CrossRef]
- Lee, K.-H.; Park, E.; Lee, H.-J.; Kim, M.-O.; Cha, Y.-J.; Kim, J.-M.; Lee, H.; Shin, M.-J. Effects of daily quercetin-rich supplementation on cardiometabolic risks in male smokers. Nutr. Res. Pract. 2011, 5, 28–33. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Schalkwijk, C.; Kromhout, D.; Hollman, P.C.H. Supplementation of the pure flavonoids epicatechin and quercetin affects some biomarkers of endothelial dysfunction and inflammation in (pre)hypertensive adults: A randomized double-blind, placebo-controlled, crossover trial. J. Nutr. 2015, 145, 1459–1463. [Google Scholar] [CrossRef]
- Dower, J.I.; Geleijnse, J.M.; Gijsbers, L.; Zock, P.L.; Kromhout, D.; Hollman, P.C.H. Effect of the pure flavonoids epicatechin and quercetin on vascular function and cardiometabolic health: A randomized, double-blind placebo-controlled, crossover trial. Am. J. Clin. Nutr. 2015, 101, 914–921. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Rich, L.; Mas, E.; Shinde, S.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. Acute effects of quercetin-3-O-glucoside on endothelial function and blood pressure: A randomized dose-response study. Am. J. Clin. Nutr. 2016, 104, 97–103. [Google Scholar] [CrossRef]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Woodman, R.J.; Hodgson, J.M.; Croft, K.D. Enzymatically modified isoquercitrin improves endothelial function in volunteers at risk of cardiovascular disease. Br. J. Nutr. 2020, 123, 182–189. [Google Scholar] [CrossRef]
- Ou, Q.; Zheng, Z.; Zhao, Y.; Lin, W. Impact of quercetin on systemic levels of inflammation: A meta-analysis of randomized controlled human trials. Int. J. Food Sci. Nutr. 2020, 71, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Janssen, K.; Mensink, R.P.; Cox, F.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavonoids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and dietary supplement study. Am. J. Clin. Nutr. 1998, 67, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Lean, M.E.J.; Noroozi, M.; Kelly, I.; Burns, J.; Talwar, D.; Sattar, N.; Crozier, A. Dietary flavonols protect diabetic human lymphocytes against oxidative damage to DNA. Diabetes 1999, 48, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Boyle, S.P.; Dobson, V.L.; Duthie, S.J.; Kyle, J.A.; Collins, A.R. Absorption and DNA protective effect of flavonoid glycosides from an onion meal. Eur. J. Nutr. 2000, 39, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Tsuge, N.; Sawada, H.; Higashi, Y. Chronic intake of onion extract containing quercetin improved postprandial endothelial dysfunction in healthy men. J. Am. Coll. Nutr. 2013, 32, 160–164. [Google Scholar] [CrossRef]
- Ebrahimi-Mamaghani, M.; Saghafi-Asl, M.; Pirouzpanah, S.; Asghari-Jafarabadi, M. Effects of raw red onion consumption on metabolic features in overweight or obese women with polycystic ovary syndrome: A randomized controlled clinical trial. J. Obstet. Gynaecol. Res. 2014, 40, 1067–1076. [Google Scholar] [CrossRef]
- Brull, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Muller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Naaf, S.; et al. Effects of a quercetin-rich onion skin extract on 24h ambulatory blood pressure and endothelial function in overweight-to-obese patients with (pre-)hypertension: A randomized double blinded placebo-controlled cross over trial. Br. J. Nutr. 2015, 114, 1263–1277. [Google Scholar] [CrossRef]
- Choi, E.-Y.; Lee, H.; Woom, J.S.; Jang, H.H.; Hwang, S.J.; Kim, H.S.; Kim, W.-S.; Kim, Y.-S.; Choue, R.; Cha, Y.-J.; et al. Effect of onion peel extract on endothelial function and endothelial progenitor cells in overweight and obese individuals. Nutrition 2015, 31, 1131–1135. [Google Scholar] [CrossRef]
- Chiu, H.-F.; Shen, Y.-C.; Huang, T.-Y.; Venkatakrishnan, K.; Wang, C.-K. Cardioprotective efficacy of red wine extract of onion in healthy hypercholesterolemic subjects. Phytother. Res. 2016, 30, 380–385. [Google Scholar] [CrossRef]
- Brull, V.; Burak, C.; Stoffel-Wagner, B.; Wolffram, S.; Nickenig, G.; Muller, C.; Langguth, P.; Alteheld, B.; Fimmers, R.; Stehle, P.; et al. No effects of quercetin from onion skin extract on serum leptin and adiponectin concentrations in overweight-to-obese patients with (pre-)hypertension: A randomized double-blinded, placebo-controlled crossover trial. Eur. J. Nur. 2017, 56, 2265–2275. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodoriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [PubMed]
- Sesso, H.D.; Manson, J.E.; Aragaki, A.K.; Rist, P.M.; Johnson, L.G.; Friedenberg, G.; Copeland, T.; Clar, A.; Mora, S.; Moorthy, M.V.; et al. Effect of cocoa flavanol supplementation for the prevention of cardiovascular disease events: The Cocoa Supplement and Multivitamin Outcomes Study (COSMOS) randomized clinical trial. Am. J. Clin. Nutr. 2022, 115, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.X.; Lin, F.-J.; Li, H.; Li, H.-B.; Wu, D.-T.; Geng, F.; Ma, W.; Wang, Y.; Miao, B.-H.; Gan, R.-Y. Recent advances in bioactive compounds, health functions, and safety concerns of onion (Allium cepa L.). Front. Nutr. 2021, 8, 669805. [Google Scholar] [CrossRef] [PubMed]
- Landerberg, R.; Manach, C.; Kerckhof, F.-M.; Minihane, A.-M.; Noureldin, R.; Saleh, M.; De Roos, B.; Tomas-Barberan, F.; Morand, C.; Van de Wiele, T. Future prospects for dissecting inter-individual variability in the absorption, distribtution and elimination of plant bioactives of relevance for cardiometabolic endpoints. Eur. J. Nutr. 2019, 58, S21–S36. [Google Scholar] [CrossRef]
- Alotaibi, B.S.; Ijaz, M.; Buabeid, M.; Kharaba, Z.J.; Yaseen, H.S.; Murtaza, G. Therapeutic effects of plant-derived polyphenolic compounds in cardiovascular diseases: A review. Drug Des. Devel. Ther. 2021, 15, 4713–4732. [Google Scholar] [CrossRef]
- Kumar, M.; Barbhai, M.D.; Hasan, M.; Punia, S.; Dhumal, S.; Radha; Rais, N.; Chandran, D.; Pandiselvam, R.; Kothakota, A.; et al. Onion (Allium cepa L.) peels: A review on bioactive compounds and biomedical activities. Biomed. Pharmacother. 2022, 146, 112498. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).