Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Standards
2.2. Samples
2.3. Infusions Preparation
2.4. In Vitro Antioxidant Activity
2.4.1. DPPH• Radical-Scavenging Activity
2.4.2. Ferric Reducing Antioxidant Power (FRAP)
2.4.3. Oxygen Radical Absorbance Capacity (ORAC)
2.4.4. Determination of Total Phenolic Content
2.4.5. Determination of Total Flavonoids Content
2.5. Analysis of Phenolic Compounds by UHPLC-ESI-QTOF-MS
2.6. Analysis of Free Amino Acids by RP-HPLC-FLD
2.7. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Activity and Total Contents in Phenolics and Flavonoids
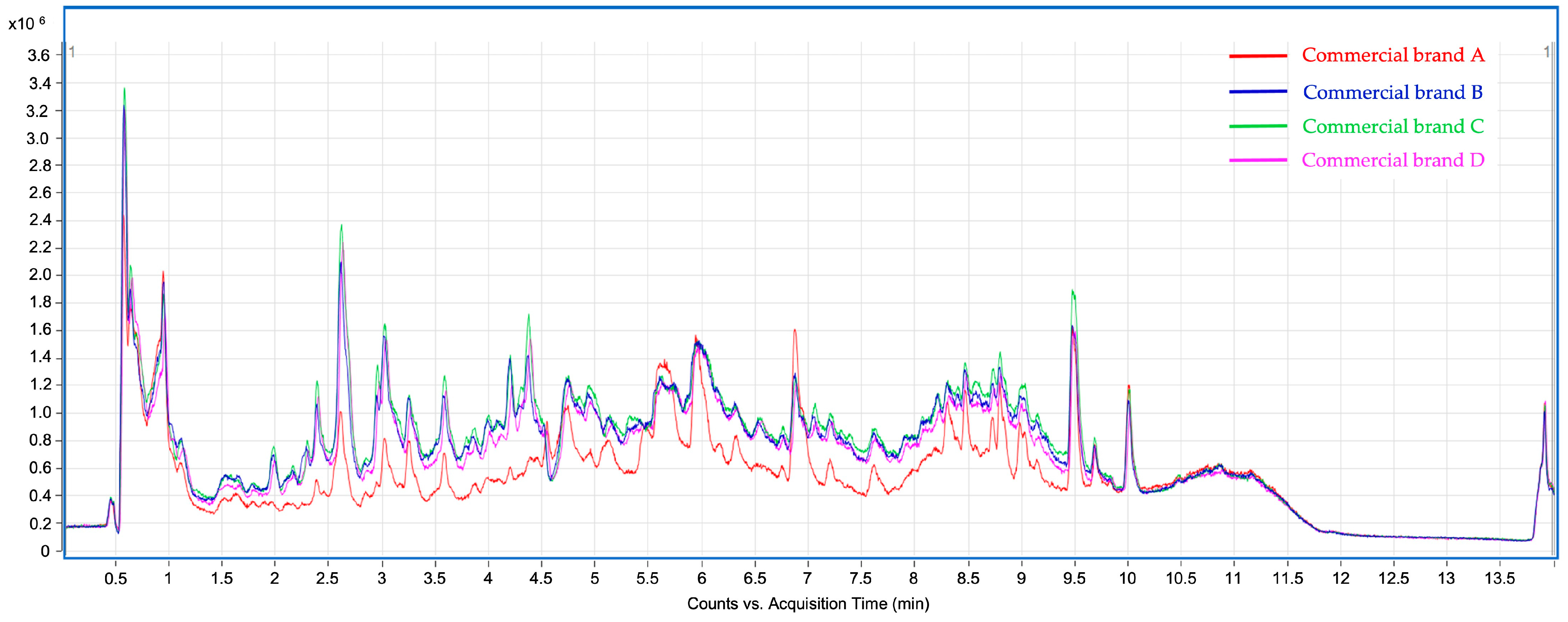
3.2. Chemical Characterization by UHPLC-ESI-QTOF-MS
3.3. Free Amino Acid Profile by RP-HPLC-FLD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahramsoltani, R.; Rostamiasrabadi, P.; Shahpiri, Z.; Marques, A.M.; Rahimi, R.; Farzaei, M.H. Aloysia citrodora Paláu (Lemon verbena): A review of phytochemistry and pharmacology. J. Ethnopharmacol. 2018, 222, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Quirantes-Piné, R.; Arráez-Román, D.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Characterization of phenolic and other polar compounds in a lemon verbena extract by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Carnat, A.; Carnat, A.P.; Fraisse, D.; Lamaison, J.L. The aromatic and polyphenolic composition of lemon verbena tea. Fitoterapia 1999, 70, 44–49. [Google Scholar] [CrossRef]
- Farzaneh, V.; Carvalho, I.S. A review of the health benefit potentials of herbal plant infusions and their mechanism of actions. Ind. Crops Prod. 2015, 65, 247–258. [Google Scholar] [CrossRef]
- Attia, Y.M.; El-Kersh, D.M.; Wagdy, H.A.; Elmazar, M.M. Verbascoside: Identification, quantification, and potential sensitization of colorectal cancer cells to 5-FU by targeting PI3K/AKT pathway. Sci. Rep. 2018, 8, 16939. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio)synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Bilia, A.R.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC–DAD–ESI–MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Funes, L.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography with diode array detection coupled to electrospray time-of-flight and ion-trap tandem mass spectrometry to identify phenolic compounds from a lemon verbena extract. J. Chromatogr. A 2009, 1216, 5391–5397. [Google Scholar] [CrossRef]
- Ren, L.; Xue, X.; Zhang, F.; Wang, Y.; Liu, Y.; Li, C.; Liang, X. Studies of iridoid glycosides using liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2007, 21, 3039–3050. [Google Scholar] [CrossRef]
- Garrett, A.R.; Weagel, E.G.; Martinez, A.D.; Heaton, M.; Robison, R.A.; O’Neill, K.L. A novel method for predicting antioxidant activity based on amino acid structure. Food Chem. 2014, 158, 490–496. [Google Scholar] [CrossRef]
- Guidea, A.; Zagrean-Tuza, C.; Mot, A.C.; Sarbu, C. Comprehensive evaluation of radical scavenging, reducing power and chelating capacity of free proteinogenic amino acids using spectroscopic assays and multivariate exploratory techniques. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 233, 118158. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sharma, A.; Kaur, R.; Thukral, A.K.; Bhardwaj, R.; Ahmad, P. Differential distribution of amino acids in plants. Amino Acids 2017, 49, 821–869. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, J.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Andrade, N.; Moreira, A.; Cifuentes, A.; Martel, F.; Oliveira, M.B.P.P.; Ibáñez, E. Cherry stem infusions: Antioxidant potential and phenolic profile by UHPLC-ESI-QTOF-MS. Food Funct. 2020, 11, 3471–3482. [Google Scholar] [CrossRef]
- Machado, S.; Costa, A.S.G.; Pimentel, F.B.; Oliveira, M.B.P.P.; Alves, R.C. A study on the protein fraction of coffee silverskin: Protein/non-protein nitrogen and free and total amino acid profiles. Food Chem. 2020, 326, 126940. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Zamora, A.; Delgado-Andrade, C.; Rufián-Henares, J.A. Antioxidant capacity, total phenols and color profile during the storage of selected plants used for infusion. Food Chem. 2016, 199, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Sánchez-Marzo, N.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.; Herranz-López, M.; Micol, V.; Segura-Carretero, A. Relationships between chemical structure and antioxidant activity of isolated phytocompounds from lemon verbena. Antioxidants 2019, 8, 324. [Google Scholar] [CrossRef] [PubMed]
- Barreto Peixoto, J.A.; Álvarez-Rivera, G.; Alves, R.C.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P. Comprehensive phenolic and free amino acid analysis of rosemary infusions: Influence on the antioxidant potential. Antioxidants 2021, 10, 500. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal medicine: An introduction to its history, usage, regulation, current trends, and research needs. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011; Chapter 1. [Google Scholar]
- Barreira, J.C.M. Caracterização Biológica, Química e Nutricional de Castanea sativa Miller e Prunus dulcis (Miller) D.A. Webb. Ph.D. Thesis, Faculty of Pharmacy University of Porto, Porto, Portugal, 2011. [Google Scholar]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef]
- Pereira, E.; Pimenta, A.I.; Calhelha, R.C.; Antonio, A.L.; Barros, L.; Santos-Buelga, C.; Verde, S.C.; Ferreira, I.C.F.R. Infusions of gamma irradiated Aloysia citrodora L. and Mentha x piperita L.: Effects on phenolic composition, cytotoxicity, antibacterial and virucidal activities. Ind. Crops Prod. 2017, 97, 582–590. [Google Scholar] [CrossRef]
- Filho, J.G.S.; Duringer, J.M.; Uchoa, D.E.A.; Xavier, H.S.; Filho, J.M.B.; Filho, R.B. Distribution of iridoid glucosides in plants from the genus Lippia (Verbenaceae): An investigation of Lippia alba (Mill.) N.E. Brown. Nat. Prod. Commun. 2019, 2, 715–716. [Google Scholar] [CrossRef]
- Rimpler, H.; Sauerbier, H. Iridoid glucosides as taxonomic markers in the genera Lantana, Lippia, Aloysia and Phyla. Biochem. Syst. Ecol. 1986, 14, 307–310. [Google Scholar] [CrossRef]
- El-Basyouni, S.Z.; Chen, D.; Ibrahim, R.K.; Neish, A.C.; Towers, G.H.N. The biosynthesis of hydroxybenzoic acids in higher plants. Phytochemistry 1964, 3, 485–492. [Google Scholar] [CrossRef]
- Klick, S.; Herrmann, K. Glucosides and glucose esters of hydroxybenzoic acids in plants. Phytochemistry 1988, 27, 2177–2180. [Google Scholar] [CrossRef]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of phenolic acids: Metabolites versus parent compounds: A review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef]
- Rashmi, H.B.; Negi, P.S. Phenolic acids from vegetables: A review on processing stability and health benefits. Food Res. Int. 2020, 136, 109298. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Barontini, M.; Tofani, D.; Balducci, V.; Gambacorta, A. Synthesis and structure/antioxidant activity relationship of novel catecholic antioxidant structural analogues to hydroxytyrosol and its lipophilic esters. J. Agric. Food Chem. 2012, 60, 7408–7416. [Google Scholar] [CrossRef]
- Espíndola, K.M.M.; Ferreira, R.G.; Narvaez, L.E.M.; Silva Rosario, A.C.R.; da Silva, A.H.M.; Silva, A.G.B.; Vieira, A.P.O.; Monteiro, M.C. Chemical and pharmacological aspects of caffeic acid and its activity in hepatocarcinoma. Front. Oncol. 2019, 9, 541. [Google Scholar] [CrossRef]
- Razzaghi-Asl, N.; Garrido, J.; Khazraei, H.; Borges, F.; Firuzi, O. Antioxidant properties of hydroxycinnamic acids: A review of structure–activity relationships. Curr. Med. Chem. 2013, 20, 4436–4450. [Google Scholar] [CrossRef]
- Chang, X.; Liu, F.; Lin, Z.; Qiu, J.; Peng, C.; Lu, Y.; Guo, X. Phytochemical profiles and cellular antioxidant activities in chestnut (Castanea mollissima BL.) kernels of five different cultivars. Molecules 2020, 25, 178. [Google Scholar] [CrossRef]
- Lopes, C.L.; Pereira, E.; Soković, M.; Carvalho, A.M.; Barata, A.M.; Lopes, V.; Rocha, F.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Phenolic composition and bioactivity of Lavandula pedunculata (Mill.) cav. samples from different geographical origin. Molecules 2018, 23, 1037. [Google Scholar] [CrossRef] [PubMed]
- Vinha, A.F.; Ferreres, F.; Silva, B.M.; Valentão, P.; Gonçalves, A.; Pereira, J.A.; Oliveira, M.B.; Seabra, R.M.; Andrade, P.B. Phenolic profiles of Portuguese olive fruits (Olea europaea L.): Influences of cultivar and geographical origin. Food Chem. 2005, 89, 561–568. [Google Scholar] [CrossRef]
- Chaaban, H.; Ioannou, I.; Paris, C.; Charbonnel, C.; Ghoul, M. The photostability of flavanones, flavonols and flavones and evolution of their antioxidant activity. J. Photochem. Photobiol. A Chem. 2017, 336, 131–139. [Google Scholar] [CrossRef]
- Gião, M.S.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Effect of technological processing upon the antioxidant capacity of aromatic and medicinal plant infusions: From harvest to packaging. LWT Food Sci. Technol. 2013, 50, 320–325. [Google Scholar] [CrossRef]
- Okumoto, S.; Funck, D.; Trovato, M.; Forlani, G. Editorial: Amino acids of the glutamate family: Functions beyond primary metabolism. Front. Plant Sci. 2016, 7, 318. [Google Scholar] [CrossRef]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Tzin, V.; Galili, G. New insights into the shikimate and aromatic amino acids biosynthesis pathways in plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, J.; Zhao, C.; Zhou, H.; Li, Y.; Zhang, J.; Li, L.; Hu, C.; Li, W.; Peng, X.; et al. A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci. Rep. 2015, 5, 16346. [Google Scholar] [CrossRef]
- Maeda, H.A. Evolutionary diversification of primary metabolism and its contribution to plant chemical diversity. Front. Plant Sci. 2019, 10, 881. [Google Scholar] [CrossRef]

| Commercial Brand | Antioxidant Activity | Bioactive Compounds | |||
|---|---|---|---|---|---|
| DPPH• Inhibition (μg TE/mL) | FRAP (μg FSE/mL) | ORAC (μg TE/mL) | Total Phenolic Content (μg GAE/mL) | Total Flavonoids Content (μg CE/mL) | |
| A | 23.07 ± 1.89 b | 1030.50 ± 123.74 c | 300.10 ± 4.97 c | 59.72 ± 4.44 c | 55.42 ± 0.00 b |
| B | 67.40 ± 1.48 a | 3805.00 ± 35.36 b | 1022.59 ± 20.90 a | 227.22 ± 5.56 b | 210.28 ± 18.03 a |
| C | 66.34 ± 1.11 a | 4755.00 ± 176.78 a | 853.21 ± 19.54 ab | 269.44 ± 8.89 a | 254.72 ± 21.40 a |
| D | 67.18 ± 0.92 a | 3563.33 ± 381.88 b | 707.03 ± 66.80 b | 227.78 ± 7.22 b | 215.14 ± 19.34 a |
| DPPH• Inhibition | FRAP | ORAC | Total Phenolic Content | Total Flavonoids Content | |
|---|---|---|---|---|---|
| DPPH• Inhibition | 1 | 0.949 * | 0.923 * | 0.970 * | 0.966 * |
| FRAP | 0.949 * | 1 | 0.879 * | 0.990 * | 0.983 * |
| ORAC | 0.923 * | 0.879 * | 1 | 0.895 * | 0.883 * |
| Total Phenolic Content | 0.970 * | 0.990 * | 0.895 * | 1 | 0.998 * |
| Total Flavonoids Content | 0.966 * | 0.983 * | 0.883 * | 0.998 * | 1 |
| Peak | Retention Time (min) | [M–H]− Experimental | [M–H]− Theoretical | Error (ppm) | MS2 Productions | Molecular Formula | Family | Tentative Identification | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2.111 | 315.0722 | 315.0722 | −0.13 | 108 (68), 109 (42), 152 (100), 153 (53) | C13H16O9 | Hydroxybenzoic acid | Dihydroxybenzoic acid glucoside | |
| 2 | 2.298 | 373.1144 | 373.1140 | −1.01 | 123 (81), 149 (75), 167 (53), 193 (100) | C16H22O10 | Iridoid | Gardoside (I) | [2,8,21] |
| 3 | 2.358 | 581.1730 | 581.1723 | −1.16 | 149 (10), 167 (9), 211 (13), 373 (100) | C23H34O17 | Iridoid | Gardoside-O-methylglucuronide | |
| 4 | 2.391 | 391.1256 | 391.1246 | −2.59 | 101 (21), 119 (32), 123 (57), 149 (30), 167 (100), 185 (24), 211 (15), 229 (28) | C16H24O11 | Iridoid | Shanzhiside | [2,21] |
| 5 | 2.605 | 387.0938 | 387.0933 | −1.32 | 101 (45), 119 (64), 137 (79), 163 (64), 181 (100), 343 (29) | C16H20O11 | Iridoid | Ixoside | [2] |
| 6 | 2.638 | 373.1148 | 373.1140 | −2.08 | 123 (100), 149 (87), 167 (24), 193 (7) 211 (18) | C16H22O10 | Iridoid | Gardoside (II) | [2,8,21] |
| 7 | 2.885 | 299.0779 | 299.0772 | −2.20 | 137 (100) | C13H16O8 | Hydroxybenzoic acid | Hydroxybenzoic acid-O-glucoside (I) | |
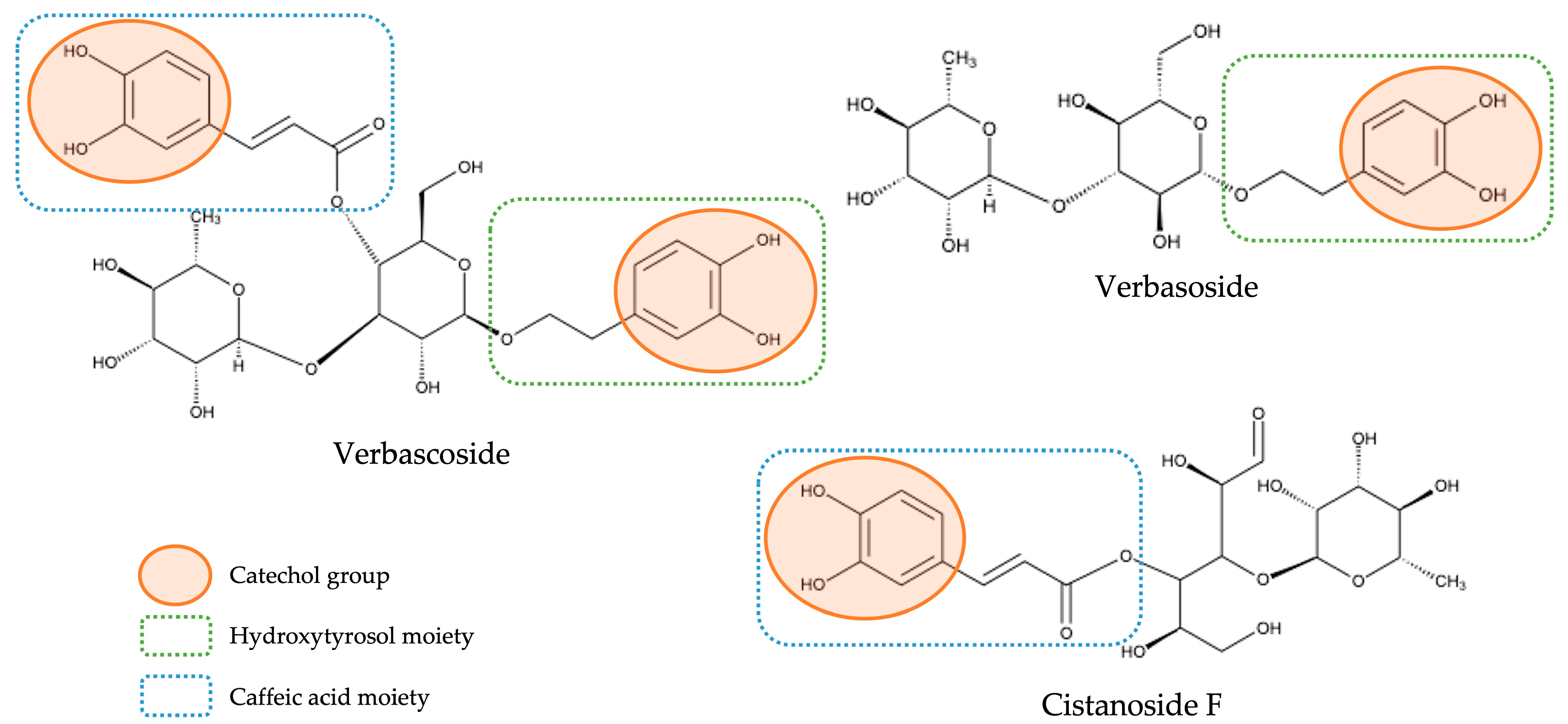
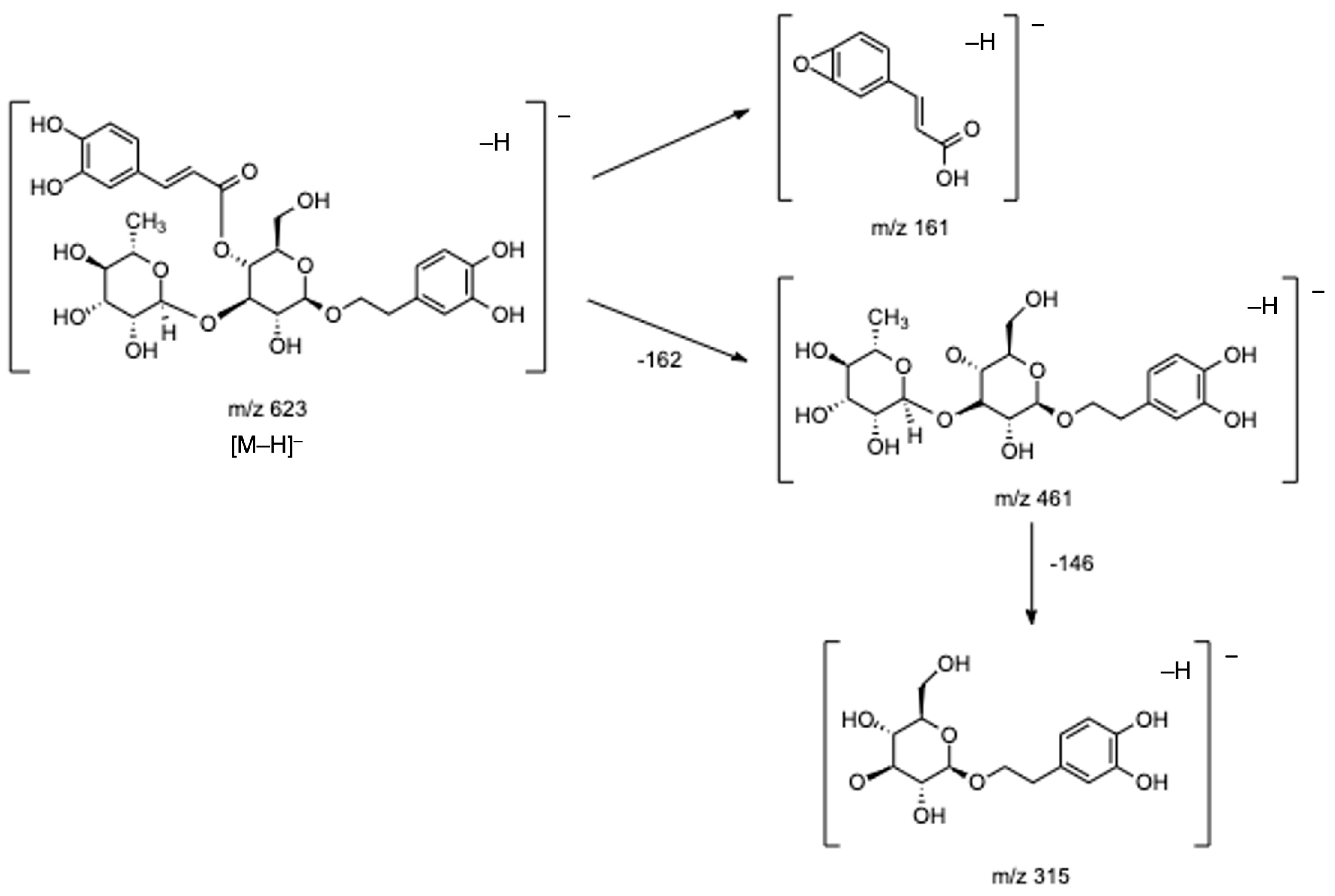
| 8 | 3.028 | 461.1680 | 461.1665 | −3.35 | 113 (45), 135 (28), 161 (12), 297 (5), 315 (10), 461 (100) | C20H30O12 | Phenylethanoid | Verbasoside | [2,8,22] |
| 9 | 3.158 | 299.0773 | 299.0772 | −0.19 | 93 (59), 137 (100) | C13H16O8 | Hydroxybenzoic acid | Hydroxybenzoic acid-O-glucoside (II) | |
| 10 | 3.255 | 487.1464 | 487.1457 | −1.40 | 179 (100) | C21H28O13 | Free hydroxycinnamic acid | Cistanoside F | [2,8,21] |
| 11 | 3.318 | 341.0884 | 341.0878 | −1.69 | 135 (42), 161 (47), 179 (100), 221 (30), 251 (13), 281 (34) | C15H18O9 | Free hydroxycinnamic acid | Caffeic acid-O-hexoside (I) | |
| 12 | 3.495 | 341.0887 | 341.0878 | −2.57 | 135 (47), 161 (31), 179 (100), 221 (50), 251 (22), 281 (30) | C15H18O9 | Free hydroxycinnamic acid | Caffeic acid-O-hexoside (II) | |
| 13 | 3.588 | 389.1102 | 389.1089 | −3.24 | 101 (50), 121 (100), 165 (53), 183 (24), 209 (26), 345 (29) | C16H22O11 | Iridoid | Theveside | [2,8,21] |
| 14 | 3.858 | 179.0345 | 179.0350 | 2.70 | 135 (100) | C9H8O4 | Free hydroxycinnamic acid | Caffeic acid | |
| 15 | 4.671 | 637.1064 | 637.1046 | −2.76 | 193 (10), 285 (61), 351 (87), 637 (100) | C27H26O18 | Flavone | Luteolin-7-diglucuronide | [2,7,8,21,22] |
| 16 | 4.758 | 639.1941 | 639.1931 | −1.62 | 161 (22), 179 (15), 459 (8), 487 (9), 529 (5), 621 (46), 639 (100) | C29H36O16 | Hydroxycinnamic acid conjugated to phenylethanoid | Hydroxy(iso)verbascoside (I) | [7,8,21] |
| 17 | 4.775 | 163.0399 | 163.0401 | 1.03 | 119 (100) | C9H8O3 | Free hydroxycinnamic acid | p-coumaric acid | [22] |
| 18 | 5.078 | 621.1106 | 621.1097 | −1.40 | 113 (11), 193 (18), 269 (12), 351 (100) | C27H26O17 | Flavone | Apigenin-7-diglucuronide | [7,8,22] |
| 19 | 5.132 | 639.1934 | 639.1931 | −0.53 | 161 (11), 179 (10), 251 (9), 323 (13), 459 (10), 487 (8), 529 (6), 621 (47), 639 (100) | C29H36O16 | Hydroxycinnamic acid conjugated to phenylethanoid | Hydroxy(iso)verbascoside (II) | [7,8,21] |
| 20 | 5.462 | 651.1226 | 651.1203 | −3.54 | 193 (9), 351 (100) | C28H28O18 | Flavone | Chrysoeriol-7-diglucuronide | [2,8,21,22] |
| 21 | 5.615 | 623.2001 | 623.1981 | −3.13 | 161 (33), 315 (1), 461 (8), 623 (100) | C29H36O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Verbascoside/Isoverbascoside/Forsythoside A (I) | [2,7,8,21,22] |
| 22 | 5.838 | 491.0839 | 491.0831 | −1.59 | 300 (35), 315 (100) | C22H20O13 | Flavonol | Isorhamnetin-7-O-glucuronide | [22] |
| 23 | 5.965 | 623.2000 | 623.1981 | −2.97 | 161 (20), 315 (1), 461 (8), 623 (100) | C29H36O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Verbascoside/Isoverbascoside/Forsythoside A (II) | [2,7,8,21,22] |
| 24 | 6.318 | 637.2146 | 637.2138 | −1.26 | 175 (30), 193 (8), 315 (3), 461 (27), 637 (100) | C30H38O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Eukovoside (I) | [2,7,8,21,22] |
| 25 | 6.462 | 475.0886 | 475.0882 | −0.79 | 113 (39), 175 (7), 284 (37), 299 (100) | C22H20O12 | Flavone | Trihydroxymethoxyflavone glucuronide | |
| 26 | 6.615 | 635.1258 | 635.1254 | −0.66 | 113 (8), 175 (9), 193 (13), 285 (7), 351 (100), 461 (5) | C28H28O17 | Flavone | Acacetin-7-diglucuronide | [2,8,21] |
| 27 | 6.768 | 637.2145 | 637.2138 | −1.10 | 161 (10), 175 (28), 285 (6), 315 (3), 351 (9), 461 (18), 637 (100) | C30H38O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Eukovoside (II) | [2,7,8,21,22] |
| 28 | 7.212 | 651.2306 | 651.2294 | −1.77 | 175 (73), 193 (9), 475 (5), 591 (18), 651 (100) | C31H40O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Martynoside (I) | [2,7,8,21,22] |
| 29 | 7.592 | 651.2304 | 651.2294 | −1.46 | 175 (23), 193 (8), 265 (6), 475 (5), 505 (3), 591 (18), 651 (100) | C31H40O15 | Hydroxycinnamic acid conjugated to phenylethanoid | Martynoside (II) | [2,8,21,22] |
| 30 | 7.772 | 315.0516 | 315.0510 | −1.81 | 300 (100), 301 (33) | C16H12O7 | Flavone | Eupafolin (6-Methoxyluteolin) | [1] |
| 31 | 8.312 | 299.0563 | 299.0561 | −0.62 | 284 (100) | C16H12O6 | Flavone | Trihydroxymethoxyflavone: Hispidulin/Diosmetin/Chrysoeriol | [1] |
| 32 | 8.415 | 329.0678 | 329.0667 | −3.41 | 271 (12), 299 (61), 314 (100) | C17H14O7 | Flavone | Trihydroxydimethoxyflavone: Jaceosidin/Cirsilol | [1,2] |
| 33 | 8.712 | 313.0715 | 313.0718 | 0.84 | 283 (100), 284 (59), 298 (63) | C17H14O6 | Flavone | Dihydroxydimethoxyflavone: Cirsimaritin/Pectolinarigenin | [1] |
| 34 | 8.802 | 343.0834 | 343.0823 | −3.12 | 313 (74), 328 (100) | C18H16O7 | Flavone | Eupatorin (3′,5-Dihydroxy-4′,6,7-trimethoxyflavone) | [1] |
| Peak | Compound | Peak Areas | |||
|---|---|---|---|---|---|
| A | B | C | D | ||
| 1 | Dihydroxybenzoic acid glucoside | 33.7 ± 2.6 c | 69.8 ± 8.3 b | 115.7 ± 6.9 a | 71.5 ± 5.5 b |
| 2 | Gardoside (I) | 59.5 ± 2.3 c | 228.3 ± 11.9 b | 320.4 ± 41.7 a | 247.6 ± 20.9 ab |
| 3 | Gardoside-O-methylglucuronide | 26.3 ± 4.2 b | 149.9 ± 6.1 a | 153.5 ± 15.6 a | 149.4 ± 13.4 a |
| 4 | Shanzhiside | 180.1 ± 1.5 c | 561.6 ± 31.9 b | 639.2 ± 57.7 ab | 700.3 ± 32.8 a |
| 5 | Ixoside | 56.4 ± 3.5 c | 233.8 ± 10.5 b | 399.6 ± 16.9 a | 285.4 ± 39.3 b |
| 6 | Gardoside (II) | 563.9 ± 38.9 c | 1385.9 ± 11.4 b | 1539.4 ± 87.7 ab | 1587.0 ± 33.1 a |
| 7 | Hydroxybenzoic acid-O-glucoside (I) | 15.4 ± 1.1 b | 53.0 ± 2.8 a | 56.4 ± 3.0 a | 60.9 ± 3.1 a |
| 8 | Verbasoside | 472.2 ± 41.6 b | 1448.9 ± 41.3 a | 1602.3 ± 115.4 a | 1532.9 ± 101.2 a |
| 9 | Hydroxybenzoic acid-O-glucoside (II) | 44.9 ± 2.5 c | 125.0 ± 17.3 b | 188.9 ± 12.9 ab | 256.8 ± 22.9 a |
| 10 | Cistanoside F | 644.7 ± 74.4 b | 1067.7 ± 22.3 a | 1076.3 ± 69.2 a | 950.2 ± 62.8 a |
| 11 | Caffeic acid-O-hexoside (I) | 22.6 ± 2.7 b | 81.0 ± 11.3 a | 104.0 ± 6.9 a | 85.2 ± 9.3 a |
| 12 | Caffeic acid-O-hexoside (II) | 34.3 ± 2.1 b | 129.1 ± 11.1 a | 156.6 ± 4.7 a | 133.8 ± 17.4 a |
| 13 | Theveside | 707.2 ± 91.7 b | 951.5 ± 48.3 ab | 1177.1 ± 154.9 a | 1287.2 ± 94.0 a |
| 14 | Caffeic acid | 183.9 ± 28.6 b | 469.8 ± 71.0 a | 445.7 ± 3.5 a | 310.7 ± 24.3 ab |
| 15 | Luteolin-7-diglucuronide | 3857.9 ± 41.7 b | 4365.1 ± 172.0 a | 4466.2 ± 135.5 a | 4312.5 ± 75.2 a |
| 16 | Hydroxy(iso)verbascoside (I) | 476.1 ± 18.2 b | 971.0 ± 67.5 a | 894.5 ± 88.9 a | 813.0 ± 43.9 a |
| 17 | p-coumaric acid | 390.3 ± 10.1 c | 662.5 ± 67.1 b | nd | 735.6 ± 37.9 a |
| 18 | Apigenin-7-diglucuronide | 879.9 ± 88.1 b | 984.2 ± 48.2 ab | 1071.2 ± 42.4 a | 981.9 ± 15.6 ab |
| 19 | Hydroxy(iso)verbascoside (II) | 190.5 ± 10.3 c | 383.3 ± 26.8 a | 334.1 ± 18.4 ab | 270.8 ± 2.0 b |
| 20 | Chrysoeriol-7-diglucuronide | 1750.7 ± 34.4 b | 1992.2 ± 48.3 a | 1959.4 ± 51.7 a | 1917.0 ± 44.8 a |
| 21 | Verbascoside/Isoverbascoside/Forsythoside A (I) | 5188.3 ± 438.2 b | 7347.7 ± 34.3 a | 7730.9 ± 116.8 a | 7594.3 ± 252.4 a |
| 22 | Isorhamnetin-7-O-glucuronide | 171.6 ± 25.0 a | 149.4 ± 8.3 a | 158.2 ± 4.5 a | 172.5 ± 11.9 a |
| 23 | Verbascoside/Isoverbascoside/Forsythoside A (II) | 4957.7 ± 548.4 b | 8938.7 ± 21.4 a | 9092.4 ± 332.6 a | 8460.5 ± 161.6 a |
| 24 | Eukovoside (I) | 430.5 ± 27.3 b | 984.9 ± 43.4 a | 959.4 ± 90.6 a | 984.2 ± 103.1 a |
| 25 | Trihydroxymethoxyflavone glucuronide | 102.0 ± 9.5 a | 103.4 ± 5.5 a | 105.7 ± 7.2 a | 113.9 ± 6.2 a |
| 26 | Acacetin-7-diglucuronide | 244.2 ± 34.0 a | 272.9 ± 25.3 a | 267.1 ± 28.5 a | 252.9 ± 9.9 a |
| 27 | Eukovoside (II) | 134.7 ± 3.2 b | 227.3 ± 12.1 a | 230.2 ± 18.7 a | 186.5 ± 24.2 ab |
| 28 | Martynoside (I) | 448.3 ± 44.5 b | 827.5 ± 53.1 a | 952.9 ± 34.6 a | 812.7 ± 107.1 a |
| 29 | Martynoside (II) | 90.1 ± 14.4 b | 176.6 ± 12.1 a | 179.2 ± 0.2 a | 117.4 ± 8.8 b |
| 30 | Eupafolin (6-Methoxyluteolin) | 40.2 ± 5.0 b | 99.2 ± 0.5 a | 68.7 ± 5.2 ab | 44.5 ± 7.9 b |
| 31 | Trihydroxymethoxyflavone: Hispidulin/Diosmetin/Chrysoeriol | 68.8 ± 0.5 c | 157.2 ± 0.8 a | 119.4 ± 11.6 b | 92.4 ± 6.4 bc |
| 32 | Trihydroxydimethoxyflavone: Jaceosidin/Cirsilol | 106.9 ± 2.8 b | 241.0 ± 10.2 a | 227.8 ± 2.8 a | 133.7 ± 17.6 b |
| 33 | Dihydroxydimethoxyflavone: Cirsimaritin/Pectolinarigenin | 45.3 ± 2.5 c | 99.2 ± 13.4 ab | 113.6 ± 8.8 a | 64.7 ± 9.6 bc |
| 34 | Eupatorin (3′.5-Dihydroxy-4′.6.7-trimethoxyflavone) | 50.9 ± 3.3 c | 128.4 ± 11.1 a | 129.7 ± 2.7 a | 90.1 ± 9.9 b |
| Total | 22,669.9 | 36,067.0 | 37,035.8 | 35,809.9 | |
 | |||||
| % Phenolic acids | 58.6 | 62.4 | 60.8 | 61.0 | |
| % Hydroxybenzoic acids | 0.4 | 0.7 | 1.0 | 1.1 | |
| % Hydroxycinnamic acids | 58.2 | 61.7 | 59.8 | 59.9 | |
| % Free Hydroxycinnamic acids | 5.6 | 6.7 | 4.8 | 6.2 | |
| % Hydroxycinnamic acids conjugated to phenylethanoids | 52.6 | 55.1 | 55.0 | 53.7 | |
| % Flavonoids | 32.3 | 23.8 | 23.5 | 22.8 | |
| % Flavonols | 0.8 | 0.4 | 0.4 | 0.5 | |
| % Flavones | 31.5 | 23.4 | 23.0 | 22.4 | |
| % Phenylethanoids | 2.1 | 4.0 | 4.3 | 4.3 | |
| % Iridoids | 7.0 | 9.7 | 11.4 | 11.9 | |
| Free Amino Acid | Commercial Brand | |||
|---|---|---|---|---|
| A | B | C | D | |
| Aspartic Acid | 2.98 ± 0.04 a | 2.70 ± 0.12 b | 2.73 ± 0.05 b | 2.72 ± 0.06 b |
| Glutamic Acid | 2.61 ± 0.17 a | 2.59 ± 0.08 a | 2.52 ± 0.07 a | 2.37 ± 0.01 a |
| Asparagine | 3.10 ± 0.48 a | 2.38 ± 0.03 a | 2.55 ± 0.51 a | 2.01 ± 0.22 a |
| Serine | 1.80 ± 0.10 a | 1.70 ± 0.04 a | 1.75 ± 0.13 a | 1.65 ± 0.02 a |
| Glutamine | 1.77 ± 0.14 a | 2.01 ± 0.17 a | 1.32 ± 0.07 b | 1.17 ± 0.11 b |
| Histidine | nd | nd | nd | nd |
| Glycine | 0.33 ± 0.03 a | 0.28 ± 0.03 a | 0.29 ± 0.02 a | 0.27 ± 0.01 a |
| Threonine | 1.35 ± 0.03 a | 1.33 ± 0.01 a | 1.35 ± 0.05 a | 1.30 ± 0.02 a |
| Arginine | 1.34 ± 0.13 a | 1.26 ± 0.06 a | 1.43 ± 0.21 a | 1.32 ± 0.04 a |
| Alanine | 1.25 ± 0.08 a | 1.27 ± 0.03 a | 1.32 ± 0.03 a | 1.22 ± 0.03 a |
| Tyrosine | nd | nd | 0.06 ± 0.00 | nd |
| Valine | 0.93 ± 0.09 ab | 0.95 ± 0.05 ab | 1.10 ± 0.13 a | 0.79 ± 0.05 b |
| Methionine | nd | nd | nd | nd |
| Tryptophan | nd | nd | nd | nd |
| Phenylalanine | 0.50 ± 0.04 b | 0.71 ± 0.04 a | 0.71 ± 0.07 a | 0.69 ± 0.03 a |
| Isoleucine | 0.77 ± 0.05 a | 0.80 ± 0.03 a | 0.83 ± 0.02 a | 0.79 ± 0.03 a |
| Leucine | nd | 0.62 ± 0.05 a | 0.72 ± 0.06 a | 0.64 ± 0.03 a |
| Lysine | nd | nd | nd | nd |
| Hydroxyproline | nd | nd | 0.13 ± 0.02 | nd |
| Proline | 3.06 ± 0.35 a | 2.97 ± 0.54 a | 2.64 ± 0.62 a | 2.05 ± 0.04 a |
| Free Amino Acid | Spectrophotometric Assay | ||||
|---|---|---|---|---|---|
| DPPH• Scavenging | FRAP | ORAC | Total Phenolic Content | Total Flavonoid Content | |
| Aspartic Acid | −0.996 ** | −0.928 | −0.938 | −0.959 * | −0.954 * |
| Glutamic Acid | −0.524 | −0.400 | −0.129 | −0.485 | −0.498 |
| Asparagine | −0.873 | −0.684 | −0.654 | −0.771 | −0.770 |
| Serine | −0.764 | −0.519 | −0.543 | −0.623 | −0.621 |
| Glutamine | −0.328 | −0.369 | 0.067 | −0.402 | −0.423 |
| Histidine | - | - | - | - | - |
| Glycine | −0.959 * | −0.822 | −0.789 | −0.887 | −0.886 |
| Threonine | −0.467 | −0.167 | −0.210 | −0.291 | −0.291 |
| Arginine | −0.055 | 0.246 | −0.186 | 0.167 | 0.186 |
| Alanine | 0.263 | 0.562 | 0.423 | 0.452 | 0.453 |
| Tyrosine | - | - | - | - | - |
| Valine | 0.056 | 0.369 | 0.268 | 0.249 | 0.249 |
| Methionine | - | - | - | - | - |
| Tryptophan | - | - | - | - | - |
| Phenylalanine | 0.992 ** | 0.969 * | 0.938 | 0.986 * | 0.982 * |
| Isoleucine | 0.679 | 0.888 | 0.683 | 0.826 | 0.829 |
| Leucine | 0.989 * | 0.976 * | 0.890 | 0.996 ** | 0.995 ** |
| Lysine | - | - | - | - | - |
| Hydroxyproline | - | - | - | - | - |
| Proline | −0.557 | −0.436 | −0.168 | −0.520 | −0.533 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peixoto, J.A.B.; Álvarez-Rivera, G.; Costa, A.S.G.; Machado, S.; Cifuentes, A.; Ibáñez, E.; Oliveira, M.B.P.P.; Alves, R.C. Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions. Antioxidants 2023, 12, 251. https://doi.org/10.3390/antiox12020251
Peixoto JAB, Álvarez-Rivera G, Costa ASG, Machado S, Cifuentes A, Ibáñez E, Oliveira MBPP, Alves RC. Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions. Antioxidants. 2023; 12(2):251. https://doi.org/10.3390/antiox12020251
Chicago/Turabian StylePeixoto, Juliana A. Barreto, Gerardo Álvarez-Rivera, Anabela S. G. Costa, Susana Machado, Alejandro Cifuentes, Elena Ibáñez, M. Beatriz P. P. Oliveira, and Rita C. Alves. 2023. "Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions" Antioxidants 12, no. 2: 251. https://doi.org/10.3390/antiox12020251
APA StylePeixoto, J. A. B., Álvarez-Rivera, G., Costa, A. S. G., Machado, S., Cifuentes, A., Ibáñez, E., Oliveira, M. B. P. P., & Alves, R. C. (2023). Contribution of Phenolics and Free Amino Acids on the Antioxidant Profile of Commercial Lemon Verbena Infusions. Antioxidants, 12(2), 251. https://doi.org/10.3390/antiox12020251











