DHA and EPA Alleviate Epileptic Depression in PTZ-Treated Young Mice Model by Inhibiting Neuroinflammation through Regulating Microglial M2 Polarization and Improving Mitochondrial Metabolism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subject Animals and Experimental Design
2.2. The PTZ-Kindled Epilepsy Model Mice Construction
2.3. Behavioral Assays
2.3.1. Tail Suspension Test (TST)
2.3.2. Forced Swimming Test (FST)
2.3.3. Open Field Test (OFT)
2.4. Western Blot and ELISA Analysis
2.5. Nissl Staining
2.6. Fluoro-Jade B (FJB) Staining
2.7. Neuronal Nuclei (NeuN) Staining
2.8. Immunofluorescence
2.9. Statistical Analysis
3. Results
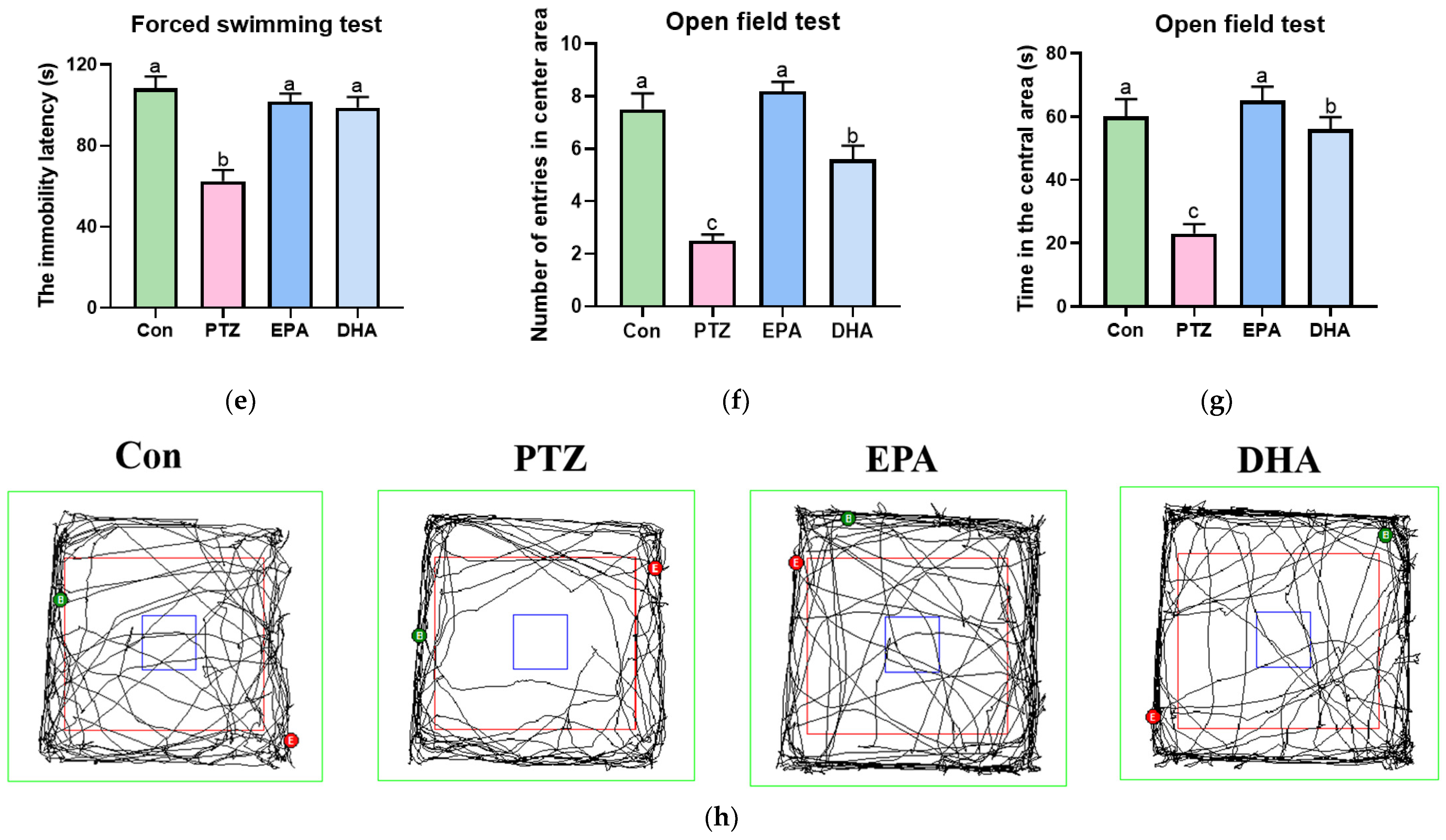
3.1. Effects of EPA and DHA on PTZ-Induced Epileptic Seizure and Depressive-like Behaviors in Young Model Mice
3.2. Effects of EPA and DHA on Hippocampal Neuron Injury in PTZ-Treated Young Mice
3.3. Effects of EPA and DHA on Hippocampal Myelin Damage in PTZ-Treated Young Mice
3.4. Effects of EPA and DHA on Hippocampal Microglia Polarization in PTZ-Treated Young Mice
3.5. Effects of EPA and DHA on NLRP3 Inflammasome Activation in PTZ-Treated Young Mice
3.6. Effects of EPA and DHA on NOX2-Mitochondrial Oxidative Stress in PTZ-Treated Young Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berg, A.T. Epilepsy, cognition, and behavior: The clinical picture. Epilepsia 2011, 52 (Suppl. S1), 7–12. [Google Scholar] [CrossRef] [PubMed]
- Austin, J.K.; Harezlak, J.; Dunn, D.W.; Huster, G.A.; Rose, D.F.; Ambrosius, W.T. Behavior problems in children before first recognized seizures. Pediatrics 2001, 107, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Hague, C.; Waber, D.; Rotenberg, A.; Vega, C. Prevalence of suicidality in children and adolescents with depressive disorders with and without epilepsy. Epilepsy Behav. 2023, 148, 109467. [Google Scholar] [CrossRef] [PubMed]
- Josephson, C.B.; Lowerison, M.; Vallerand, I.; Sajobi, T.T.; Patten, S.; Jette, N.; Wiebe, S. Association of Depression and Treated Depression with Epilepsy and Seizure Outcomes: A Multicohort Analysis. JAMA Neurol. 2017, 74, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Holmes, G.L. Drug Treatment of Epilepsy Neuropsychiatric Comorbidities in Children. Paediatr. Drugs 2021, 23, 55–73. [Google Scholar] [CrossRef]
- Hitiris, N.; Mohanraj, R.; Norrie, J.; Sills, G.J.; Brodie, M.J. Predictors of pharmacoresistant epilepsy. Epilepsy Res. 2007, 75, 192–196. [Google Scholar] [CrossRef]
- Cramer, J.A.; Blum, D.; Reed, M.; Fanning, K. The influence of comorbid depression on seizure severity. Epilepsia 2003, 44, 1578–1584. [Google Scholar] [CrossRef]
- Tao, W.; Hu, Y.; Chen, Z.; Dai, Y.; Hu, Y.; Qi, M. Magnolol attenuates depressive-like behaviors by polarizing microglia towards the M2 phenotype through the regulation of Nrf2/HO-1/NLRP3 signaling pathway. Phytomed. Int. J. Phytother. Phytopharm. 2021, 91, 153692. [Google Scholar] [CrossRef]
- Madireddy, S.; Madireddy, S. Therapeutic Strategies to Ameliorate Neuronal Damage in Epilepsy by Regulating Oxidative Stress, Mitochondrial Dysfunction, and Neuroinflammation. Brain Sci. 2023, 13, 784. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, Y.; Morgan, S.; Mathur, R.; Feustel, P.; Mazurkiewicz, J.; Qian, J.; Chang, J.; Mathern, G.W.; Adamo, M.A.; et al. Noninflammatory Changes of Microglia Are Sufficient to Cause Epilepsy. Cell Rep. 2018, 22, 2080–2093. [Google Scholar] [CrossRef]
- Attia, G.M.; Elmansy, R.A.; Elsaed, W.M. Neuroprotective effect of nilotinib on pentylenetetrazol-induced epilepsy in adult rat hippocampus: Involvement of oxidative stress, autophagy, inflammation, and apoptosis. Folia Neuropathol. 2019, 57, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.; Li, K.; Chen, T.; Liu, S.; He, L. Geniposide protects depression through BTK/JAK2/STAT1 signaling pathway in lipopolysaccharide-induced depressive mice. Brain Res. Bull. 2021, 170, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Cao, P.; Zhang, H.; Meng, H.; Cheng, Y.; Xu, H.; Zang, S.; Li, Z.; Cui, J.; Li, Y. Celecoxib Exerts a Therapeutic Effect against Demyelination by Improving the Immune and Inflammatory Microenvironments. J. Inflamm. Res. 2020, 13, 1043–1055. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Dissing-Olesen, L.; Stevens, B. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol. 2016, 36, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Wang, J.; Yang, J.; Wang, X.P.; Huang, J.J.; Xue, T.F.; Sun, X.L. The Intra-nuclear SphK2-S1P Axis Facilitates M1-to-M2 Shift of Microglia via Suppressing HDAC1-Mediated KLF4 Deacetylation. Front. Immunol. 2019, 10, 1241. [Google Scholar] [CrossRef]
- Deng, X.L.; Feng, L.; Wang, Z.X.; Zhao, Y.E.; Zhan, Q.; Wu, X.M.; Xiao, B.; Shu, Y. The Runx1/Notch1 Signaling Pathway Participates in M1/M2 Microglia Polarization in a Mouse Model of Temporal Lobe Epilepsy and in BV-2 Cells. Neurochem. Res. 2020, 45, 2204–2216. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, M.; Li, Y.; Liu, S.; He, L. The role of CCR5 in the protective effect of Esculin on lipopolysaccharide-induced depressive symptom in mice. J. Affect. Disord. 2020, 277, 755–764. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zheng, Y.; Huang, L.; Ye, J.; Ye, Y.; Luo, H.; Chen, X.; Yao, W.; Chen, J.; Zhang, J.C. Nrf2 regulates the arginase 1(+) microglia phenotype through the initiation of TREM2 transcription, ameliorating depression-like behavior in mice. Transl. Psychiatry 2022, 12, 459. [Google Scholar] [CrossRef]
- Shin, H.J.; Jeong, E.A.; Lee, J.Y.; An, H.S.; Jang, H.M.; Ahn, Y.J.; Lee, J.; Kim, K.E.; Roh, G.S. Lipocalin-2 Deficiency Reduces Oxidative Stress and Neuroinflammation and Results in Attenuation of Kainic Acid-Induced Hippocampal Cell Death. Antioxidants 2021, 10, 100. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Maes, M.; Galecki, P.; Chang, Y.S.; Berk, M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 676–692. [Google Scholar]
- Yang, Y.; Wang, X.; Xiao, A.; Han, J.; Wang, Z.; Wen, M. Ketogenic diet prevents chronic sleep deprivation-induced Alzheimer’s disease by inhibiting iron dyshomeostasis and promoting repair via Sirt1/Nrf2 pathway. Front. Aging Neurosci. 2022, 14, 998292. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, X.; Chen, L.; Wang, S.; Han, J.; Wang, Z.; Wen, M. A Compared Study of Eicosapentaenoic Acid and Docosahexaenoic Acid in Improving Seizure-Induced Cognitive Deficiency in a Pentylenetetrazol-Kindling Young Mice Model. Mar. Drugs 2023, 21, 464. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, F.N.; Costa, A.P.; Ghisleni, G.; Diaz, A.P.; Rodrigues, A.L.S.; Peluffo, H.; Kaster, M.P. NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain Behav. Immun. 2017, 64, 367–383. [Google Scholar] [CrossRef] [PubMed]
- Saadi, A.; Sandouka, S.; Grad, E.; Singh, P.K.; Shekh-Ahmad, T. Spatial, temporal, and cell-type-specific expression of NADPH Oxidase isoforms following seizure models in rats. Free Radic. Biol. Med. 2022, 190, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Huang, J.; Liu, A.; Wu, J.; Fan, H.; Shen, M.; Lai, X.; Ma, H.; Sun, W.; Yang, J.; et al. Atorvastatin improves motor function, anxiety and depression by NOX2-mediated autophagy and oxidative stress in MPTP-lesioned mice. Aging 2020, 13, 831–845. [Google Scholar] [CrossRef]
- Yingze, Y.; Zhihong, J.; Tong, J.; Yina, L.; Zhi, Z.; Xu, Z.; Xiaoxing, X.; Lijuan, G. NOX2-mediated reactive oxygen species are double-edged swords in focal cerebral ischemia in mice. J. Neuroinflamm. 2022, 19, 184. [Google Scholar] [CrossRef]
- Kim, J.E.; Cho, K.O. Functional Nutrients for Epilepsy. Nutrients 2019, 11, 1309. [Google Scholar] [CrossRef]
- Ortega, M.A.; Fraile-Martínez, Ó.; García-Montero, C.; Alvarez-Mon, M.A.; Lahera, G.; Monserrat, J.; Llavero-Valero, M.; Gutiérrez-Rojas, L.; Molina, R.; Rodríguez-Jimenez, R.; et al. Biological Role of Nutrients, Food and Dietary Patterns in the Prevention and Clinical Management of Major Depressive Disorder. Nutrients 2022, 14, 3099. [Google Scholar] [CrossRef]
- Reda, D.M.; Abd-El-Fatah, N.K.; Omar Tel, S.; Darwish, O.A. Fish Oil Intake and Seizure Control in Children with Medically Resistant Epilepsy. N. Am. J. Med. Sci. 2015, 7, 317–321. [Google Scholar] [CrossRef] [PubMed]
- DeGiorgio, C.M.; Taha, A.Y. Omega-3 fatty acids (ῳ-3 fatty acids) in epilepsy: Animal models and human clinical trials. Expert Rev. Neurother. 2016, 16, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramanieapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of omega-3 PUFAs in depression: A meta-analysis. Transl. Psychiatry 2019, 9, 190. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, F.A.S.; Ghebremeskel, K.; Abdel-Rahman, M.E.; Ahmed, A.A.M.; Mohmed, I.M.; Osman, G.; Elseed, M.; Hamed, A.; Rabinowicz, A.L.; Salih, M.A.M.; et al. The differential effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on seizure frequency in patients with drug-resistant epilepsy—A randomized, double-blind, placebo-controlled trial. Epilepsy Behav. 2018, 87, 32–38. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, A.; Yang, Y.; Zhao, Y.; Wang, C.C.; Wang, Y.; Han, J.; Wang, Z.; Wen, M. DHA and EPA Prevent Seizure and Depression-Like Behavior by Inhibiting Ferroptosis and Neuroinflammation via Different Mode-of-Actions in a Pentylenetetrazole-Induced Kindling Model in Mice. Mol. Nutr. Food Res. 2022, 66, e2200275. [Google Scholar] [CrossRef]
- Nieoczym, D.; Socała, K.; Zelek-Molik, A.; Pieróg, M.; Przejczowska-Pomierny, K.; Szafarz, M.; Wyska, E.; Nalepa, I.; Wlaź, P. Anticonvulsant effect of pterostilbene and its influence on the anxiety- and depression-like behavior in the pentetrazol-kindled mice: Behavioral, biochemical, and molecular studies. Psychopharmacology 2021, 238, 3167–3181. [Google Scholar] [CrossRef]
- Mensch, S.; Baraban, M.; Almeida, R.; Czopka, T.; Ausborn, J.; El Manira, A.; Lyons, D.A. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 2015, 18, 628–630. [Google Scholar] [CrossRef]
- Jain, S.; Bharal, N.; Khurana, S.; Mediratta, P.K.; Sharma, K.K. Anticonvulsant and antioxidant actions of trimetazidine in pentylenetetrazole-induced kindling model in mice. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2011, 383, 385–392. [Google Scholar] [CrossRef]
- Tzeng, T.T.; Tsay, H.J.; Chang, L.; Hsu, C.L.; Lai, T.H.; Huang, F.L.; Shiao, Y.J. Caspase 3 involves in neuroplasticity, microglial activation and neurogenesis in the mice hippocampus after intracerebral injection of kainic acid. J. Biomed. Sci. 2013, 20, 90. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, C.; Zhang, R.; Jin, L.; Yin, X.; Zheng, X.; Siebert, H.C.; Li, Y.; Wang, Z.; Loers, G.; et al. Amelioration of clinical course and demyelination in the cuprizone mouse model in relation to ketogenic diet. Food Funct. 2020, 11, 5647–5663. [Google Scholar] [CrossRef]
- Mondal, A.; Bose, D.; Saha, P.; Sarkar, S.; Seth, R.; Kimono, D.; Albadrani, M.; Nagarkatti, M.; Nagarkatti, P.; Chatterjee, S. Lipocalin 2 induces neuroinflammation and blood-brain barrier dysfunction through liver-brain axis in murine model of nonalcoholic steatohepatitis. J. Neuroinflamm. 2020, 17, 201. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, J.F.; Tseng, M.; Young, L.T. Downregulation in components of the mitochondrial electron transport chain in the postmortem frontal cortex of subjects with bipolar disorder. J. Psychiatry Neurosci. JPN 2006, 31, 189–196. [Google Scholar] [PubMed]
- Hu, C.; Wu, Z.; Huang, Z.; Hao, X.; Wang, S.; Deng, J.; Yin, Y.; Tan, C. Nox2 impairs VEGF-A-induced angiogenesis in placenta via mitochondrial ROS-STAT3 pathway. Redox Biol. 2021, 45, 102051. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Nihonmatsu-Kikuchi, N.; Yu, X.; Ishimoto, K.; Hisanaga, S.I.; Tatebayashi, Y. A novel, rapid, quantitative cell-counting method reveals oligodendroglial reduction in the frontopolar cortex in major depressive disorder. Mol. Psychiatry 2011, 16, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
- Miyata, S.; Taniguchi, M.; Koyama, Y.; Shimizu, S.; Tanaka, T.; Yasuno, F.; Yamamoto, A.; Iida, H.; Kudo, T.; Katayama, T.; et al. Association between chronic stress-induced structural abnormalities in Ranvier nodes and reduced oligodendrocyte activity in major depression. Sci. Rep. 2016, 6, 23084. [Google Scholar] [CrossRef]
- Makinodan, M.; Rosen, K.M.; Ito, S.; Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 2012, 337, 1357–1360. [Google Scholar] [CrossRef]
- Deng, S.L.; Chen, J.G.; Wang, F. Microglia: A Central Player in Depression. Curr. Med. Sci. 2020, 40, 391–400. [Google Scholar] [CrossRef]
- Peet, M.; Horrobin, D.F. A dose-ranging study of the effects of ethyl-eicosapentaenoate in patients with ongoing depression despite apparently adequate treatment with standard drugs. Arch. Gen. Psychiatry 2002, 59, 913–919. [Google Scholar] [CrossRef]
- Nemets, B.; Stahl, Z.; Belmaker, R.H. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am. J. Psychiatry 2002, 159, 477–479. [Google Scholar] [CrossRef]
- Martins, J.G. EPA but not DHA appears to be responsible for the efficacy of omega-3 long chain polyunsaturated fatty acid supplementation in depression: Evidence from a meta-analysis of randomized controlled trials. J. Am. Coll. Nutr. 2009, 28, 525–542. [Google Scholar] [CrossRef]
- Guan, Y.F.; Huang, G.B.; Xu, M.D.; Gao, F.; Lin, S.; Huang, J.; Wang, J.; Li, Y.Q.; Wu, C.H.; Yao, S.; et al. Anti-depression effects of ketogenic diet are mediated via the restoration of microglial activation and neuronal excitability in the lateral habenula. Brain Behav. Immun. 2020, 88, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, S.; Lu, Y.; Li, X.; Liao, Y.; Peng, Z.; Li, Y.; Hou, L.; Yuan, Z.; Cheng, J. Stress-induced NLRP3 inflammasome activation negatively regulates fear memory in mice. J. Neuroinflamm. 2020, 17, 205. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Zhang, Y.; Liang, P.; He, Y.; Peng, B.; Liu, W.; Han, S.; Yin, J.; He, X. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- von Herrmann, K.M.; Anderson, F.L.; Martinez, E.M.; Young, A.L.; Havrda, M.C. Slc6a3-dependent expression of a CAPS-associated Nlrp3 allele results in progressive behavioral abnormalities and neuroinflammation in aging mice. J. Neuroinflamm. 2020, 17, 213. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Casas-Barquero, N.; Williams, M.R.; Romero-Guillena, S.L.; Cañadas-Lozano, D.; Bullón, P.; Sánchez-Alcazar, J.A.; Navarro-Pando, J.M.; Cordero, M.D. Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol. Res. 2017, 121, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Du, Y.; Xie, Y.; Yu, X.; Chen, H.; Qiu, Y. Lipocalin-2 Regulates Hippocampal Microglial Activation in Poststroke Depression. Front. Aging Neurosci. 2021, 13, 798335. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.; Lee, S.; Kim, J.H.; Kim, J.H.; Seo, J.W.; Lee, W.H.; Mori, K.; Nakao, K.; Suk, K. Secreted protein lipocalin-2 promotes microglial M1 polarization. FASEB J. 2013, 27, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.L.; Shin, M.W.; Kim, S.W. Lipocalin 2 activates the NLRP3 inflammasome via LPS-induced NF-κB signaling and plays a role as a pro-inflammatory regulator in murine macrophages. Mol. Med. Rep. 2022, 26, 358. [Google Scholar] [CrossRef]
- Müller, N.; Scheld, M.; Voelz, C.; Gasterich, N.; Zhao, W.; Behrens, V.; Weiskirchen, R.; Baazm, M.; Clarner, T.; Beyer, C.; et al. Lipocalin-2 Deficiency Diminishes Canonical NLRP3 Inflammasome Formation and IL-1β Production in the Subacute Phase of Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 8689. [Google Scholar] [CrossRef]
- Giménez-Palomo, A.; Dodd, S.; Anmella, G.; Carvalho, A.F.; Scaini, G.; Quevedo, J.; Pacchiarotti, I.; Vieta, E.; Berk, M. The Role of Mitochondria in Mood Disorders: From Physiology to Pathophysiology and to Treatment. Front. Psychiatry 2021, 12, 546801. [Google Scholar] [CrossRef]
- Brown, N.C.; Andreazza, A.C.; Young, L.T. An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 2014, 218, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Valvassori, S.S.; Bavaresco, D.V.; Feier, G.; Cechinel-Recco, K.; Steckert, A.V.; Varela, R.B.; Borges, C.; Carvalho-Silva, M.; Gomes, L.M.; Streck, E.L.; et al. Increased oxidative stress in the mitochondria isolated from lymphocytes of bipolar disorder patients during depressive episodes. Psychiatry Res. 2018, 264, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Zvěřová, M.; Hroudová, J.; Fišar, Z.; Hansíková, H.; Kališová, L.; Kitzlerová, E.; Lambertová, A.; Raboch, J. Disturbances of mitochondrial parameters to distinguish patients with depressive episode of bipolar disorder and major depressive disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Villalpando-Rodriguez, G.E.; Gibson, S.B. Reactive Oxygen Species (ROS) Regulates Different Types of Cell Death by Acting as a Rheostat. Oxidative Med. Cell. Longev. 2021, 2021, 9912436. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, G.; Kumar, P. Neuroprotective role of apocynin against pentylenetetrazole kindling epilepsy and associated comorbidities in mice by suppression of ROS/RNS. Behav. Brain Res. 2022, 419, 113699. [Google Scholar] [CrossRef]
- Schmitz, G.; Ecker, J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008, 47, 147–155. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Bazan, N.G.; Birkle, D.L.; Tang, W.; Reddy, T.S. The accumulation of free arachidonic acid, diacylglycerols, prostaglandins, and lipoxygenase reaction products in the brain during experimental epilepsy. Adv. Neurol. 1986, 44, 879–902. [Google Scholar]
- Eraković, V.; Zupan, G.; Varljen, J.; Simonić, A. Pentylenetetrazol-induced seizures and kindling: Changes in free fatty acids, superoxide dismutase, and glutathione peroxidase activity. Neurochem. Int. 2003, 42, 173–178. [Google Scholar] [CrossRef]
- Peterson, L.D.; Jeffery, N.M.; Thies, F.; Sanderson, P.; Newsholme, E.A.; Calder, P.C. Eicosapentaenoic and docosahexaenoic acids alter rat spleen leukocyte fatty acid composition and prostaglandin E2 production but have different effects on lymphocyte functions and cell-mediated immunity. Lipids 1998, 33, 171–180. [Google Scholar] [CrossRef]
- Serhan, C.N.; Hong, S.; Gronert, K.; Colgan, S.P.; Devchand, P.R.; Mirick, G.; Moussignac, R.L. Resolvins: A family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J. Exp. Med. 2002, 196, 1025–1037. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Chen, L.; Zhang, N.; Zhao, Y.; Che, H.; Wang, Y.; Zhang, T.; Wen, M. DHA and EPA Alleviate Epileptic Depression in PTZ-Treated Young Mice Model by Inhibiting Neuroinflammation through Regulating Microglial M2 Polarization and Improving Mitochondrial Metabolism. Antioxidants 2023, 12, 2079. https://doi.org/10.3390/antiox12122079
Yang Y, Chen L, Zhang N, Zhao Y, Che H, Wang Y, Zhang T, Wen M. DHA and EPA Alleviate Epileptic Depression in PTZ-Treated Young Mice Model by Inhibiting Neuroinflammation through Regulating Microglial M2 Polarization and Improving Mitochondrial Metabolism. Antioxidants. 2023; 12(12):2079. https://doi.org/10.3390/antiox12122079
Chicago/Turabian StyleYang, Yueqi, Lu Chen, Ning Zhang, Yingcai Zhao, Hongxia Che, Yuming Wang, Tiantian Zhang, and Min Wen. 2023. "DHA and EPA Alleviate Epileptic Depression in PTZ-Treated Young Mice Model by Inhibiting Neuroinflammation through Regulating Microglial M2 Polarization and Improving Mitochondrial Metabolism" Antioxidants 12, no. 12: 2079. https://doi.org/10.3390/antiox12122079
APA StyleYang, Y., Chen, L., Zhang, N., Zhao, Y., Che, H., Wang, Y., Zhang, T., & Wen, M. (2023). DHA and EPA Alleviate Epileptic Depression in PTZ-Treated Young Mice Model by Inhibiting Neuroinflammation through Regulating Microglial M2 Polarization and Improving Mitochondrial Metabolism. Antioxidants, 12(12), 2079. https://doi.org/10.3390/antiox12122079







