Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline
Abstract
:1. Introduction
2. Materials and Methods
2.1. Molecules
2.2. Acanthamoeba spp. Strains
2.3. In Vitro Effect against the Trophozoite Stage
2.4. In Vitro Effect against the Cyst Stage
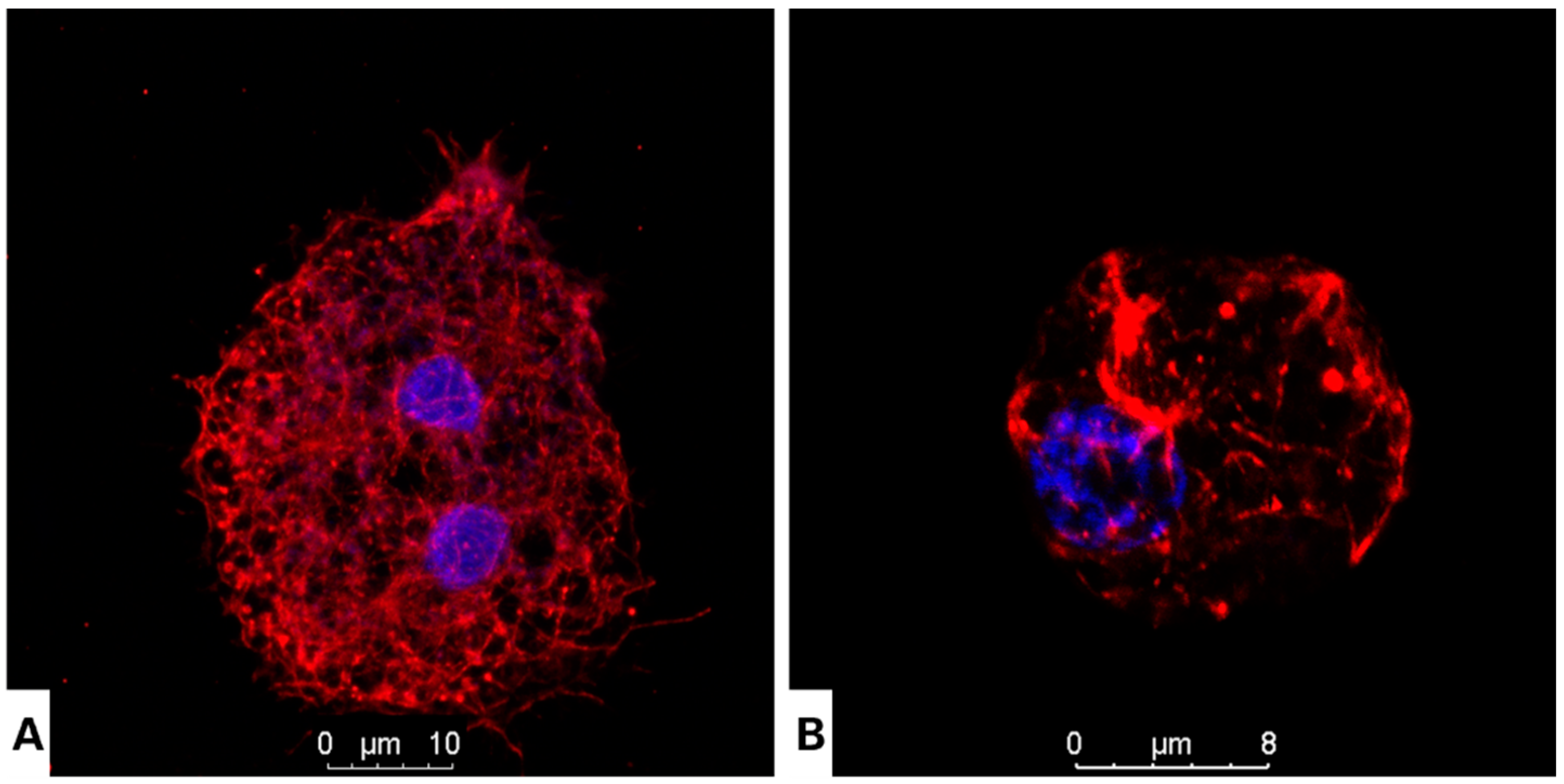
2.5. Evaluation of Actin Distribution
2.6. Immunofluorescence Staining of Intracellular Tubulin in Acanthamoeba culbertsoni
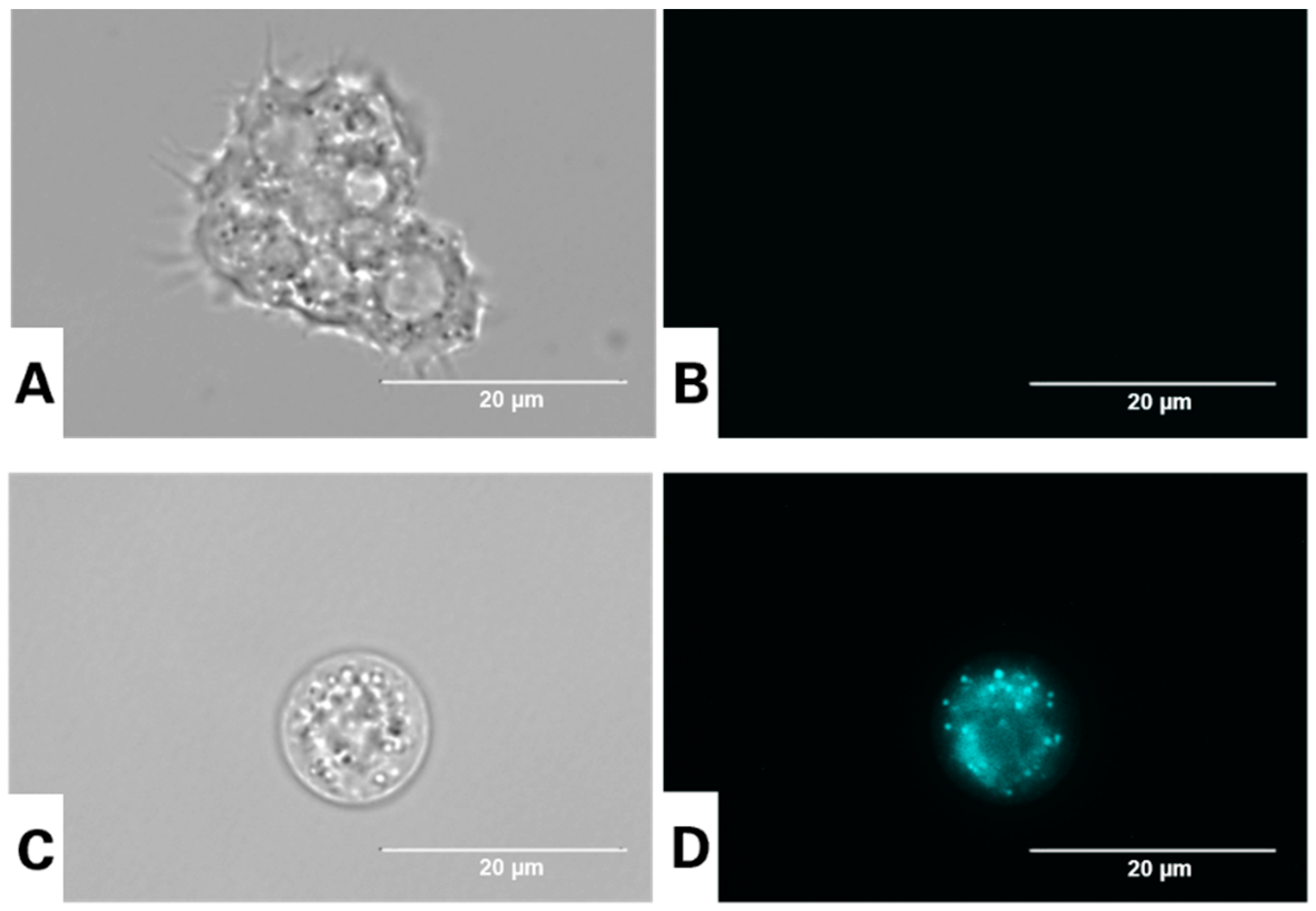
2.7. In Vitro Labelling of Autophagic Vacuoles in Acanthamoeba culbertsoni
2.8. Proteomic Analysis of the Effect of Nitroxoline in Acanthamoeba Castellanii L-10 Trophozoites
2.8.1. Comparative Label-Free Proteomics
2.8.2. Protein Digestion
2.8.3. nLC-MS 2 Analysis
2.8.4. Data Analysis
2.9. Evaluation of Nitroxoline Mechanism of Action
2.9.1. Analysis of Mitochondrial Function Disruption
2.9.2. Measurement of ATP Production
2.9.3. Chromatin Condensation Detection
2.9.4. Plasma Membrane Permeability
2.9.5. Evaluation of Intracellular ROS Production
2.10. Statistical Analysis
3. Results
3.1. In Vitro Activity of Nitroxoline against Trophozoites and Cysts of Acanthamoeba spp.
Evaluation of Nitroxoline Effect on Cellular Events
3.2. Nitroxoline Affects Both Cellular Morphology and Cytoskeleton Structure in Fixed Cells
3.3. Visualization of Autophagic Vacuoles in A. culbertsoni Using the Dye Monodansylcadaverine
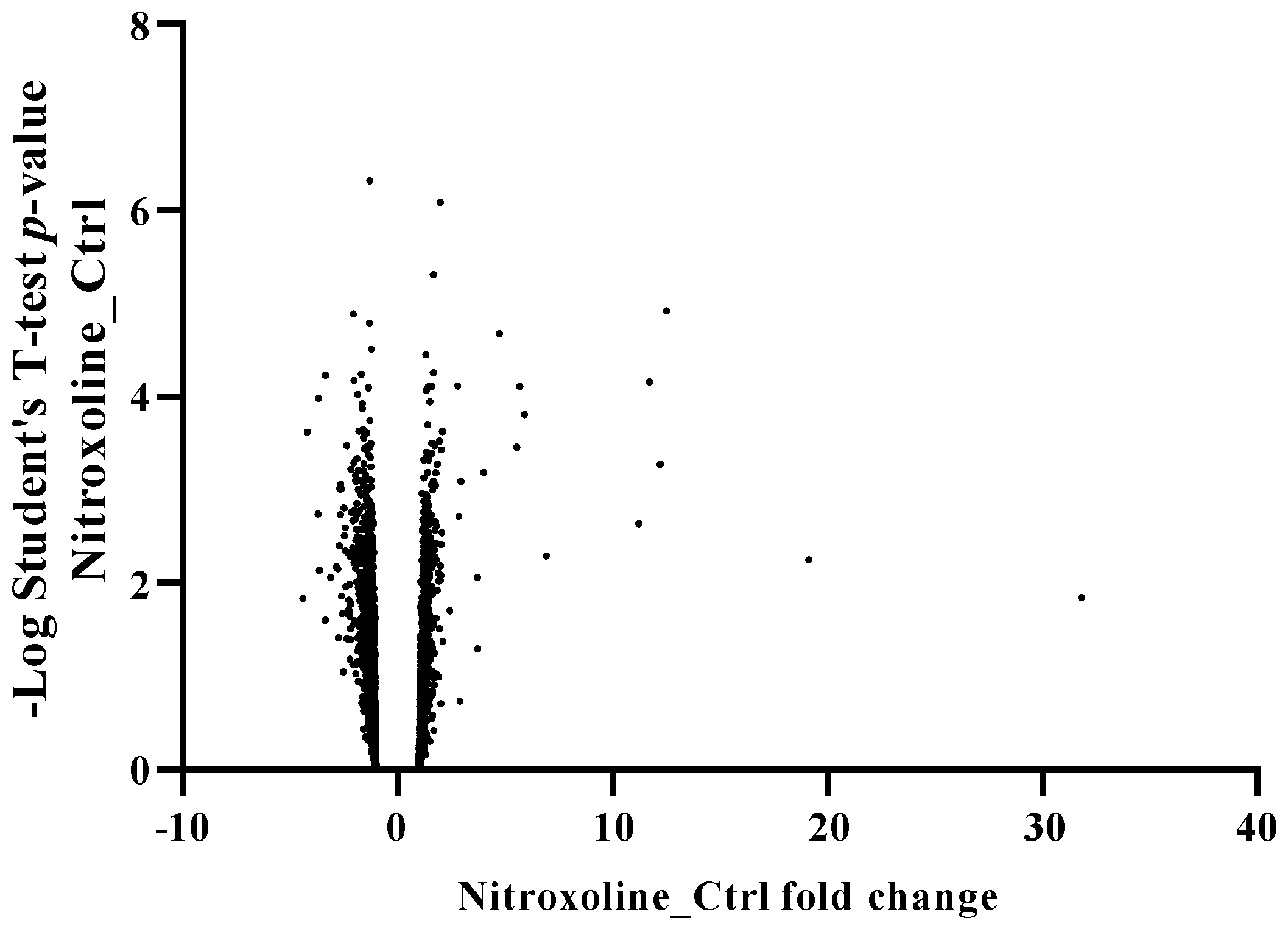
3.4. Nitroxoline Effect on the Proteomic Profile of Acanthamoeba Castellanii L-10
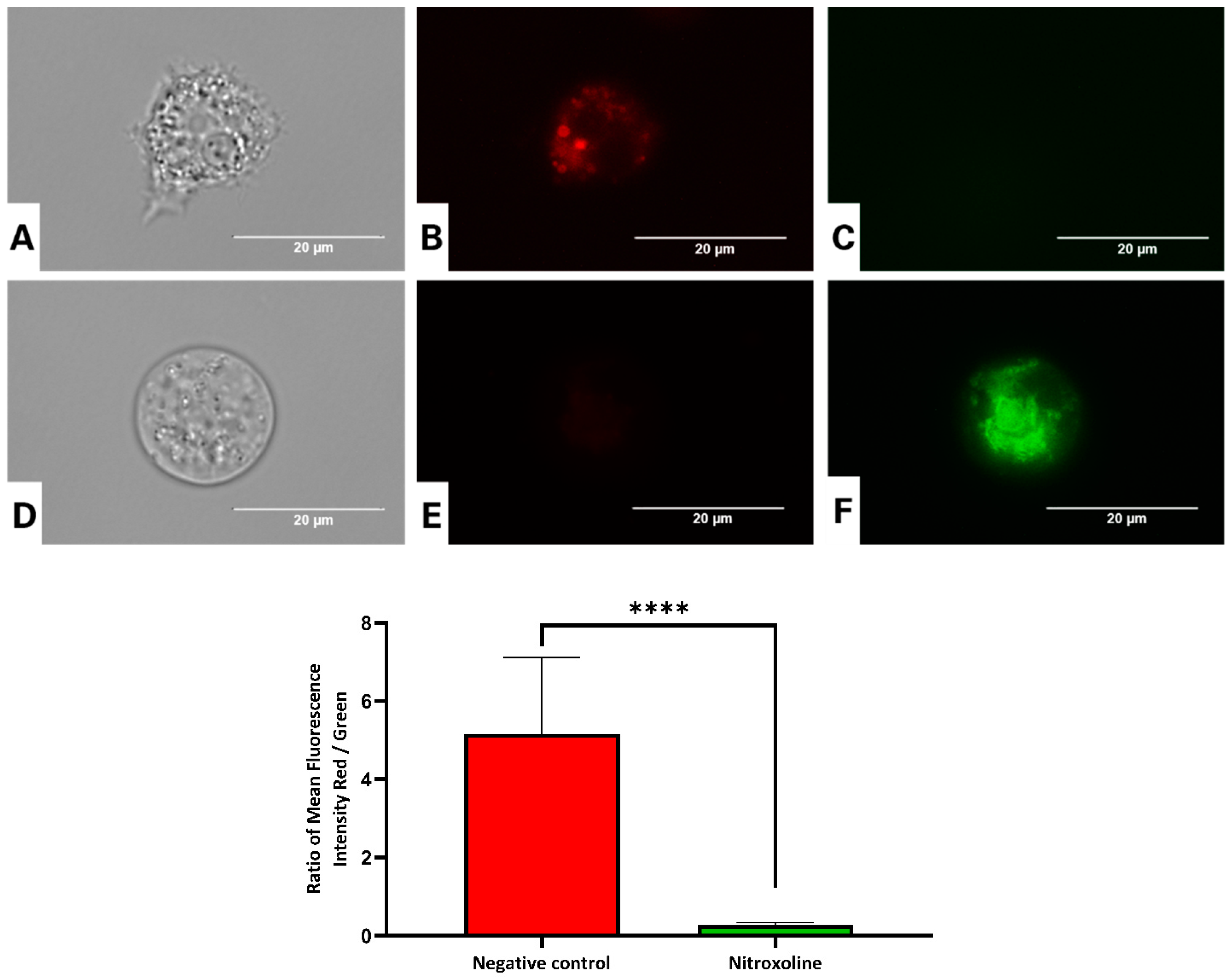
3.5. Nitroxoline Causse Mitochondrial Dysfunction in A. culbertsoni
3.5.1. Nitroxoline Prevents the Aggregation of the JC-1 Dye
3.5.2. Nitroxoline Induces a Decrease in the ATP Levels
3.6. In Vitro Determination of the Cell Death Mode Induced by the Nitroxoline
Analyzing Cell Death by Double Nuclear Staining with Hoechst 33342/PI
3.7. Nitroxoline Could Alter Plasma Membrane Permeability in Treated Cells
3.8. Nitroxoline Increases the Cytosolic Level of Reactive Oxygen Species (ROS) in A. culbertsoni
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheid, P.L. Free-Living Amoebae as Human Pathogens: (Genus) Acanthamoeba. In Encyclopedia of Parasitology; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1078–1080. [Google Scholar]
- Siddiqui, R.; Khan, N.A. Biology and Pathogenesis of Acanthamoeba. Parasit. Vectors 2012, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Morales, J.; Khan, N.A.; Walochnik, J. An Update on Acanthamoeba Keratitis: Diagnosis, Pathogenesis and Treatment. Parasite 2015, 22, 10. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A. Acanthamoeba Invasion of the Central Nervous System. Int. J. Parasitol. 2007, 37, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kalra, S.K.; Sharma, P.; Shyam, K.; Tejan, N.; Ghoshal, U. Acanthamoeba and Its Pathogenic Role in Granulomatous Amebic Encephalitis. Exp. Parasitol. 2020, 208, 107788. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.C.; Fiester, S.E.; Madeline, L.A.; Fulcher, J.W.; Ward, M.E.; Schammel, C.M.-G.; Hakimi, R.K. Acanthamoeba spp. and Balamuthia mandrillaris Leading to Fatal Granulomatous Amebic Encephalitis. Forensic Sci. Med. Pathol. 2020, 16, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Fanselow, N.; Sirajuddin, N.; Yin, X.-T.; Huang, A.J.W.; Stuart, P.M. Acanthamoeba Keratitis, Pathology, Diagnosis and Treatment. Pathogens 2021, 10, 323. [Google Scholar] [CrossRef]
- Maycock, N.J.R.; Jayaswal, R. Update on Acanthamoeba Keratitis: Diagnosis, Treatment, and Outcomes. Cornea 2016, 35, 713–720. [Google Scholar] [CrossRef]
- Diehl, M.L.N.; Paes, J.; Rott, M.B. Genotype Distribution of Acanthamoeba in Keratitis: A Systematic Review. Parasitol. Res. 2021, 120, 3051–3063. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Galindo, R.L.; Kraus, E.S.; Ghanem, K.G. Disseminated Acanthamebiasis in a Renal Transplant Recipient with Osteomyelitis and Cutaneous Lesions: Case Report and Literature Review. Clin. Infect. Dis. 2002, 35, e43–e49. [Google Scholar] [CrossRef]
- Torno, M.S.; Babapour, R.; Gurevitch, A.; Witt, M.D. Cutaneous Acanthamoebiasis in AIDS. J. Am. Acad. Dermatol. 2000, 42, 351–354. [Google Scholar] [CrossRef]
- Walia, R.; Montoya, J.G.; Visvesvera, G.S.; Booton, G.C.; Doyle, R.L. A Case of Successful Treatment of Cutaneous Acanthamoeba Infection in a Lung Transplant Recipient. Transpl. Infect. Dis. 2007, 9, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Saberi, R.; Fakhar, M.; Makhlough, A.; Sedighi, O.; Tabaripour, R.; Asfaram, S.; Latifi, A.; Espahbodi, F.; Sharifpour, A. First Evidence for Colonizing of Acanthamoeba T4 Genotype in Urinary Tracts of Patients with Recurrent Urinary Tract Infections. Acta Parasitol. 2021, 66, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.J.; Gomes, K.X.; Cortines, J.R.; Peralta, J.M.; Peralta, R.H.S. Acanthamoeba spp. as a Universal Host for Pathogenic Microorganisms: One Bridge from Environment to Host Virulence. Microbiol. Res. 2016, 193, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Mungroo, M.R.; Siddiqui, R.; Khan, N.A. War of the Microbial World: Acanthamoeba spp. Interactions with Microorganisms. Folia Microbiol. 2021, 66, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Scheid, P.; Zöller, L.; Pressmar, S.; Richard, G.; Michel, R. An Extraordinary Endocytobiont in Acanthamoeba sp. Isolated from a Patient with Keratitis. Parasitol. Res. 2008, 102, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Scheid, P. Relevance of Free-Living Amoebae as Hosts for Phylogenetically Diverse Microorganisms. Parasitol. Res. 2014, 113, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Rayamajhee, B.; Subedi, D.; Peguda, H.K.; Willcox, M.D.; Henriquez, F.L.; Carnt, N. A Systematic Review of Intracellular Microorganisms within Acanthamoeba to Understand Potential Impact for Infection. Pathogens 2021, 10, 225. [Google Scholar] [CrossRef]
- Taravaud, A.; Fechtali-Moute, Z.; Loiseau, P.M.; Pomel, S. Drugs Used for the Treatment of Cerebral and Disseminated Infections Caused by Free-Living Amoebae. Clin. Transl. Sci. 2021, 14, 791–805. [Google Scholar] [CrossRef]
- Anwar, A.; Khan, N.A.; Siddiqui, R. Combating Acanthamoeba spp. Cysts: What Are the Options? Parasit. Vectors 2018, 11, 26. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Zhao, Y.; Ju, X.; Wang, L.; Jin, L.; Fine, R.D.; Li, M. Biological Characteristics and Pathogenicity of Acanthamoeba. Front. Microbiol. 2023, 14, 1147077. [Google Scholar] [CrossRef]
- Wolf, M.A.; Thielman, N.M.; Kraft, B.D. Treatment of Acanthamoeba Encephalitis. Am. J. Med. 2022, 135, e20–e21. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Anwar, A.; Ong, S.; Anwar, A.; Khan, N.A. Applications of Medicinal Chemistry for Drug Discovery against Acanthamoeba Infections. Med. Res. Rev. 2022, 42, 462–512. [Google Scholar] [CrossRef] [PubMed]
- Schor, S.; Einav, S. Combating Intracellular Pathogens with Repurposed Host-Targeted Drugs. ACS Infect. Dis. 2018, 4, 88–92. [Google Scholar] [CrossRef]
- Supuran, C.T. Antiprotozoal Drugs: Challenges and Opportunities. Expert. Opin. Ther. Pat. 2023, 33, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Naber, K.G.; Niggemann, H.; Stein, G.; Stein, G. Review of the Literature and Individual Patients’ Data Meta-Analysis on Efficacy and Tolerance of Nitroxoline in the Treatment of Uncomplicated Urinary Tract Infections. BMC Infect. Dis. 2014, 14, 628. [Google Scholar] [CrossRef]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.; Schmiemann, G.; et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients. Part II: Therapy and Prevention. Urol. Int. 2018, 100, 271–278. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Kresken, M.; Wohlfarth, E.; Bahrs, C.; Grabein, B.; Strohmaier, W.L.; Naber, K.G. Therapie Der Zystitis Mit Nitroxolin—NitroxWin. Die Urol. 2023, 62, 1186–1192. [Google Scholar] [CrossRef]
- Lin, W.; Sun, J.; Sadahira, T.; Xu, N.; Wada, K.; Liu, C.; Araki, M.; Xu, A.; Watanabe, M.; Nasu, Y.; et al. Discovery and Validation of Nitroxoline as a Novel STAT3 Inhibitor in Drug-Resistant Urothelial Bladder Cancer. Int. J. Biol. Sci. 2021, 17, 3255–3267. [Google Scholar] [CrossRef]
- Wykowski, R.; Fuentefria, A.M.; de Andrade, S.F. Antimicrobial Activity of Clioquinol and Nitroxoline: A Scoping Review. Arch. Microbiol. 2022, 204, 535. [Google Scholar] [CrossRef]
- Laurie, M.T.; White, C.V.; Retallack, H.; Wu, W.; Moser, M.S.; Sakanari, J.A.; Ang, K.; Wilson, C.; Arkin, M.R.; DeRisi, J.L. Functional Assessment of 2,177 U.S. and International Drugs Identifies the Quinoline Nitroxoline as a Potent Amoebicidal Agent against the Pathogen Balamuthia mandrillaris. mBio 2018, 9, e02051-18. [Google Scholar] [CrossRef]
- Hoffmann, A.M.; Wolke, M.; Rybniker, J.; Plum, G.; Fuchs, F. In Vitro Activity of Repurposed Nitroxoline Against Clinically Isolated Mycobacteria Including Multidrug-Resistant Mycobacterium tuberculosis. Front. Pharmacol. 2022, 13, 906097. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Hamprecht, A. Susceptibility of Carbapenemase-Producing Enterobacterales (CPE) to Nitroxoline. J. Antimicrob. Chemother. 2019, 74, 2934–2937. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Becerra-Aparicio, F.; Xanthopoulou, K.; Seifert, H.; Higgins, P.G. In Vitro Activity of Nitroxoline against Carbapenem-Resistant Acinetobacter Baumannii Isolated from the Urinary Tract. J. Antimicrob. Chemother. 2022, 77, 1912–1915. [Google Scholar] [CrossRef]
- Abouelhassan, Y.; Yang, Q.; Yousaf, H.; Nguyen, M.T.; Rolfe, M.; Schultz, G.S.; Huigens, R.W. Nitroxoline: A Broad-Spectrum Biofilm-Eradicating Agent against Pathogenic Bacteria. Int. J. Antimicrob. Agents 2017, 49, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, F.; Hof, H.; Hofmann, S.; Kurzai, O.; Meis, J.F.; Hamprecht, A. Antifungal Activity of Nitroxoline against Candida auris Isolates. Clin. Microbiol. Infect. 2021, 27, 1697.e7–1697.e10. [Google Scholar] [CrossRef] [PubMed]
- Knez, D.; Brus, B.; Coquelle, N.; Sosič, I.; Šink, R.; Brazzolotto, X.; Mravljak, J.; Colletier, J.-P.; Gobec, S. Structure-Based Development of Nitroxoline Derivatives as Potential Multifunctional Anti-Alzheimer Agents. Bioorganic Med. Chem. 2015, 23, 4442–4452. [Google Scholar] [CrossRef]
- Ren, L.; Jiang, M.; Xue, D.; Wang, H.; Lu, Z.; Ding, L.; Xie, H.; Wang, R.; Luo, W.; Xu, L.; et al. Nitroxoline Suppresses Metastasis in Bladder Cancer via EGR1/CircNDRG1/MiR-520h/Smad7/EMT Signaling Pathway. Int. J. Biol. Sci. 2022, 18, 5207–5220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, S.; Yang, D.; Pan, K.; Li, L.; Yuan, S. Preclinical Pharmacodynamic Evaluation of Antibiotic Nitroxoline for Anticancer Drug Repurposing. Oncol. Lett. 2016, 11, 3265–3272. [Google Scholar] [CrossRef]
- Lazovic, J.; Guo, L.; Nakashima, J.; Mirsadraei, L.; Yong, W.; Kim, H.J.; Ellingson, B.; Wu, H.; Pope, W.B. Nitroxoline Induces Apoptosis and Slows Glioma Growth in Vivo. Neuro Oncol. 2015, 17, 53–62. [Google Scholar] [CrossRef]
- Bojkova, D.; Zöller, N.; Tietgen, M.; Steinhorst, K.; Bechtel, M.; Rothenburger, T.; Kandler, J.D.; Schneider, J.; Corman, V.M.; Ciesek, S.; et al. Repurposing of the Antibiotic Nitroxoline for the Treatment of Mpox. J. Med. Virol. 2023, 95, e28652. [Google Scholar] [CrossRef]
- Spottiswoode, N.; Pet, D.; Kim, A.; Gruenberg, K.; Shah, M.; Ramachandran, A.; Laurie, M.T.; Zia, M.; Fouassier, C.; Boutros, C.L.; et al. Successful Treatment of Balamuthia mandrillaris Granulomatous Amebic Encephalitis with Nitroxoline. Emerg. Infect. Dis. 2023, 29, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Chao-Pellicer, J.; Arberas-Jiménez, I.; Fuchs, F.; Sifaoui, I.; Piñero, J.E.; Lorenzo-Morales, J.; Scheid, P. Repurposing of Nitroxoline as an Alternative Primary Amoebic Meningoencephalitis Treatment. Antibiotics 2023, 12, 1280. [Google Scholar] [CrossRef] [PubMed]
- González-Robles, A.; Salazar-Villatoro, L.; Omaña-Molina, M.; Reyes-Batlle, M.; Martín-Navarro, C.M.; Lorenzo-Morales, J. Morphological Features and In Vitro Cytopathic Effect of Acanthamoeba griffini Trophozoites Isolated from a Clinical Case. J. Parasitol. Res. 2014, 2014, 256310. [Google Scholar] [CrossRef] [PubMed]
- Omaña-Molina, M.; González-Robles, A.; Iliana Salazar-Villatoro, L.; Lorenzo-Morales, J.; Cristóbal-Ramos, A.R.; Hernández-Ramírez, V.I.; Talamás-Rohana, P.; Méndez Cruz, A.R.; Martínez-Palomo, A. Reevaluating the Role of Acanthamoeba Proteases in Tissue Invasion: Observation of Cytopathogenic Mechanisms on MDCK Cell Monolayers and Hamster Corneal Cells. Biomed. Res. Int. 2013, 2013, 461329. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Expósito, R.L.; Nicolás-Hernández, D.S.; Sifaoui, I.; Cuadrado, C.; Salazar-Villatoro, L.; Reyes-Batlle, M.; Hernández-Daranas, A.; Omaña-Molina, M.; Fernández, J.J.; Díaz-Marrero, A.R.; et al. Gongolarones as Antiamoeboid Chemical Scaffold. Biomed. Pharmacother. 2023, 158, 114185. [Google Scholar] [CrossRef]
- Sifaoui, I.; Díaz-Rodríguez, P.; Rodríguez-Expósito, R.L.; Reyes-Batlle, M.; López-Arencibia, A.; Salazar Villatoro, L.; Castelan-Ramírez, I.; Omaña-Molina, M.; Oliva, A.; Piñero, J.E.; et al. Pitavastatin Loaded Nanoparticles: A Suitable Ophthalmic Treatment for Acanthamoeba Keratitis Inducing Cell Death and Autophagy in Acanthamoeba polyphaga. Eur. J. Pharm. Biopharm. 2022, 180, 11–22. [Google Scholar] [CrossRef]
- Arbon, D.; Ženíšková, K.; Šubrtová, K.; Mach, J.; Štursa, J.; Machado, M.; Zahedifard, F.; Leštinová, T.; Hierro-Yap, C.; Neuzil, J.; et al. Repurposing of MitoTam: Novel Anti-Cancer Drug Candidate Exhibits Potent Activity against Major Protozoan and Fungal Pathogens. Antimicrob. Agents Chemother. 2022, 66, e0072722. [Google Scholar] [CrossRef]
- Hughes, C.S.; Moggridge, S.; Müller, T.; Sorensen, P.H.; Morin, G.B.; Krijgsveld, J. Single-Pot, Solid-Phase-Enhanced Sample Preparation for Proteomics Experiments. Nat. Protoc. 2019, 14, 68–85. [Google Scholar] [CrossRef]
- Rappsilber, J.; Mann, M.; Ishihama, Y. Protocol for Micro-Purification, Enrichment, Pre-Fractionation and Storage of Peptides for Proteomics Using StageTips. Nat. Protoc. 2007, 2, 1896–1906. [Google Scholar] [CrossRef]
- Hebert, A.S.; Richards, A.L.; Bailey, D.J.; Ulbrich, A.; Coughlin, E.E.; Westphall, M.S.; Coon, J.J. The One Hour Yeast Proteome. Mol. Cell. Proteom. 2014, 13, 339–347. [Google Scholar] [CrossRef]
- Cox, J.; Mann, M. MaxQuant Enables High Peptide Identification Rates, Individualized p.p.b.-Range Mass Accuracies and Proteome-Wide Protein Quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-Wide Label-Free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Tyanova, S.; Temu, T.; Sinitcyn, P.; Carlson, A.; Hein, M.Y.; Geiger, T.; Mann, M.; Cox, J. The Perseus Computational Platform for Comprehensive Analysis of (Prote)Omics Data. Nat. Methods 2016, 13, 731–740. [Google Scholar] [CrossRef]
- Baig, A.M.; Lalani, S.; Khan, N.A. Apoptosis in Acanthamoeba castellanii Belonging to the T4 Genotype. J. Basic Microbiol. 2017, 57, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowski, S.; Sajid, M.; Reece, S.E. Evolution of Apoptosis-like Programmed Cell Death in Unicellular Protozoan Parasites. Parasit. Vectors 2011, 4, 44. [Google Scholar] [CrossRef]
- Niemann, A.; Takatsuki, A.; Elsässer, H.-P. The Lysosomotropic Agent Monodansylcadaverine Also Acts as a Solvent Polarity Probe. J. Histochem. Cytochem. 2000, 48, 251–258. [Google Scholar] [CrossRef]
- Biederbick, A.; Kern, H.F.; Elsässer, H.P. Monodansylcadaverine (MDC) Is a Specific in Vivo Marker for Autophagic Vacuoles. Eur. J. Cell Biol. 1995, 66, 3–14. [Google Scholar]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Wijma, R.A.; Huttner, A.; Koch, B.C.P.; Mouton, J.W.; Muller, A.E. Review of the Pharmacokinetic Properties of Nitrofurantoin and Nitroxoline. J. Antimicrob. Chemother. 2018, 73, 2916–2926. [Google Scholar] [CrossRef]
- Bergogne-Berezin, E.; Berthelot, G.; Muller-Serieys, C. [Present Status of Nitroxoline]. Pathol. Biol. 1987, 35, 873–878. [Google Scholar]
- Mrhar, A.; Kopitar, Z.; Kozjek, F.; Presl, V.; Karba, R. Clinical Pharmacokinetics of Nitroxoline. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 476–481. [Google Scholar]
- Sharma, G.; Kalra, S.K.; Tejan, N.; Ghoshal, U. Nanoparticles Based Therapeutic Efficacy against Acanthamoeba: Updates and Future Prospect. Exp. Parasitol. 2020, 218, 108008. [Google Scholar] [CrossRef]
- Varshosaz, J.; Fard, M.M.; Mirian, M.; Hassanzadeh, F. Targeted Nanoparticles for Co-Delivery of 5-FU and Nitroxoline, a Cathepsin B Inhibitor, in HepG2 Cells of Hepatocellular Carcinoma. Anti-Cancer Agents Med. Chem. 2020, 20, 346–358. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Matsui, Y.; Bhat, S.; Nacev, B.A.; Xu, J.; Bhang, H.C.; Dhara, S.; Han, K.C.; Chong, C.R.; Pomper, M.G.; et al. Effect of Nitroxoline on Angiogenesis and Growth of Human Bladder Cancer. JNCI J. Natl. Cancer Inst. 2010, 102, 1855–1873. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-L.; Hsu, L.-C.; Leu, W.-J.; Chen, C.-S.; Guh, J.-H. Repurposing of Nitroxoline as a Potential Anticancer Agent against Human Prostate Cancer—A Crucial Role on AMPK/MTOR Signaling Pathway and the Interplay with Chk2 Activation. Oncotarget 2015, 6, 39806–39820. [Google Scholar] [CrossRef] [PubMed]
- Thomson, S.; Rice, C.A.; Zhang, T.; Edrada-Ebel, R.; Henriquez, F.L.; Roberts, C.W. Characterisation of Sterol Biosynthesis and Validation of 14α-Demethylase as a Drug Target in Acanthamoeba. Sci. Rep. 2017, 7, 8247. [Google Scholar] [CrossRef] [PubMed]
- Sobke, A.; Makarewicz, O.; Baier, M.; Bär, C.; Pfister, W.; Gatermann, S.G.; Pletz, M.W.; Forstner, C. Empirical Treatment of Lower Urinary Tract Infections in the Face of Spreading Multidrug Resistance: In Vitro Study on the Effectiveness of Nitroxoline. Int. J. Antimicrob. Agents 2018, 51, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Veschi, S.; Ronci, M.; Lanuti, P.; De Lellis, L.; Florio, R.; Bologna, G.; Scotti, L.; Carletti, E.; Brugnoli, F.; Di Bella, M.C.; et al. Integrative Proteomic and Functional Analyses Provide Novel Insights into the Action of the Repurposed Drug Candidate Nitroxoline in AsPC-1 Cells. Sci. Rep. 2020, 10, 2574. [Google Scholar] [CrossRef] [PubMed]
- Sobke, A.; Klinger, M.; Hermann, B.; Sachse, S.; Nietzsche, S.; Makarewicz, O.; Keller, P.M.; Pfister, W.; Straube, E. The Urinary Antibiotic 5-Nitro-8-Hydroxyquinoline (Nitroxoline) Reduces the Formation and Induces the Dispersal of Pseudomonas Aeruginosa Biofilms by Chelation of Iron and Zinc. Antimicrob. Agents Chemother. 2012, 56, 6021–6025. [Google Scholar] [CrossRef]
- Conte, L.; Zara, V. The Rieske Iron-Sulfur Protein: Import and Assembly into the Cytochrome Complex of Yeast Mitochondria. Bioinorg. Chem. Appl. 2011, 2011, 363941. [Google Scholar] [CrossRef]
- Kresken, M.; Körber-Irrgang, B. In Vitro Activity of Nitroxoline against Escherichia coli Urine Isolates from Outpatient Departments in Germany. Antimicrob. Agents Chemother. 2014, 58, 7019–7020. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Kang, J.-M.; Joo, S.-Y.; Song, S.-M.; Lê, H.G.; Thái, T.L.; Lee, J.; Goo, Y.-K.; Chung, D.-I.; Sohn, W.-M.; et al. Molecular and Biochemical Properties of a Cysteine Protease of Acanthamoeba castellanii. Korean J. Parasitol. 2018, 56, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Song, S.-M.; Moon, E.-K.; Lee, Y.-R.; Jha, B.K.; Danne, D.-B.S.; Cha, H.-J.; Yu, H.S.; Kong, H.-H.; Chung, D.-I.; et al. Cysteine Protease Inhibitor (AcStefin) Is Required for Complete Cyst Formation of Acanthamoeba. Eukaryot. Cell 2013, 12, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirova, T.S.; Selivanova, O.M.; Galzitskaya, O.V. α-Crystallins Are Small Heat Shock Proteins: Functional and Structural Properties. Biochemistry 2017, 82, 106–121. [Google Scholar] [CrossRef]
- Yang, G.; Pan, W.; Zhang, R.; Pan, Y.; Guo, Q.; Song, W.; Zheng, W.; Nie, X. Genome-Wide Identification and Characterization of Caffeoyl-Coenzyme A O-Methyltransferase Genes Related to the Fusarium Head Blight Response in Wheat. BMC Genom. 2021, 22, 504. [Google Scholar] [CrossRef]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial Biogenesis and Mitochondrial DNA Maintenance of Mammalian Cells under Oxidative Stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef]






| Acanthamoeba Strains | Nitroxoline IC50 (µM) | Chlorhexidine IC50 (µM) | Voriconazole IC50 (µM) | |||
|---|---|---|---|---|---|---|
| Trophozoites | Cysts | Trophozoites | Cysts | Trophozoites | Cysts | |
| A. castellanii Neff | 0.87 ± 0.19 aA | 0.28 ± 0.12 aA | 3.02 ± 0.89 aB | 5.97 ± 1.76 cC | 0.99 ± 0.04 aA | 3.45 ± 0.17 bB |
| A. polyphaga | 0.95 ± 0.01 aA | 0.81 ± 0.03 aA | 5.59 ± 0.04 bB | 9.41 ± 0.16 dC | 1.07 ± 0.02 aA | 6.98 ± 0.05 cB |
| A. griffini | 0.69 ± 0.01 aA | 0.84 ± 0.01 aA | 5.60 ± 0.07 bB | 7.38 ± 1.94 cB | 0.32 ± 0.01 aA | 0.92 ± 0.06 aA |
| A. quina | 3.24 ± 0.56 bB | 0.31 ± 0.05 aA | 5.31 ± 0.48 bCB | 4.04 ± 0.48 b | 0.54 ± 0.01 aA | 4.69 ± 0.09 bB |
| A. L-10 | 2.85 ± 0.58 bB | 0.11 ± 0.03 aA | 9.11 ± 0.29 dC | 1.30 ± 0.36 aA | 1.77 ± 0.15 abA | 0.51 ± 0.10 aA |
| A. culbertsoni | 1.17 ± 0.09 aA | 0.98 ± 0.23 aA | 8.11 ± 0.17 cC | 2.92 ± 0.28 bB | 1.93 ± 0.09 bB | 1.24 ± 0.15 aA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Expósito, R.L.; Sifaoui, I.; Reyes-Batlle, M.; Fuchs, F.; Scheid, P.L.; Piñero, J.E.; Sutak, R.; Lorenzo-Morales, J. Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants 2023, 12, 2081. https://doi.org/10.3390/antiox12122081
Rodríguez-Expósito RL, Sifaoui I, Reyes-Batlle M, Fuchs F, Scheid PL, Piñero JE, Sutak R, Lorenzo-Morales J. Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants. 2023; 12(12):2081. https://doi.org/10.3390/antiox12122081
Chicago/Turabian StyleRodríguez-Expósito, Rubén L., Ines Sifaoui, María Reyes-Batlle, Frieder Fuchs, Patrick L. Scheid, José E. Piñero, Robert Sutak, and Jacob Lorenzo-Morales. 2023. "Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline" Antioxidants 12, no. 12: 2081. https://doi.org/10.3390/antiox12122081
APA StyleRodríguez-Expósito, R. L., Sifaoui, I., Reyes-Batlle, M., Fuchs, F., Scheid, P. L., Piñero, J. E., Sutak, R., & Lorenzo-Morales, J. (2023). Induction of Programmed Cell Death in Acanthamoeba culbertsoni by the Repurposed Compound Nitroxoline. Antioxidants, 12(12), 2081. https://doi.org/10.3390/antiox12122081












