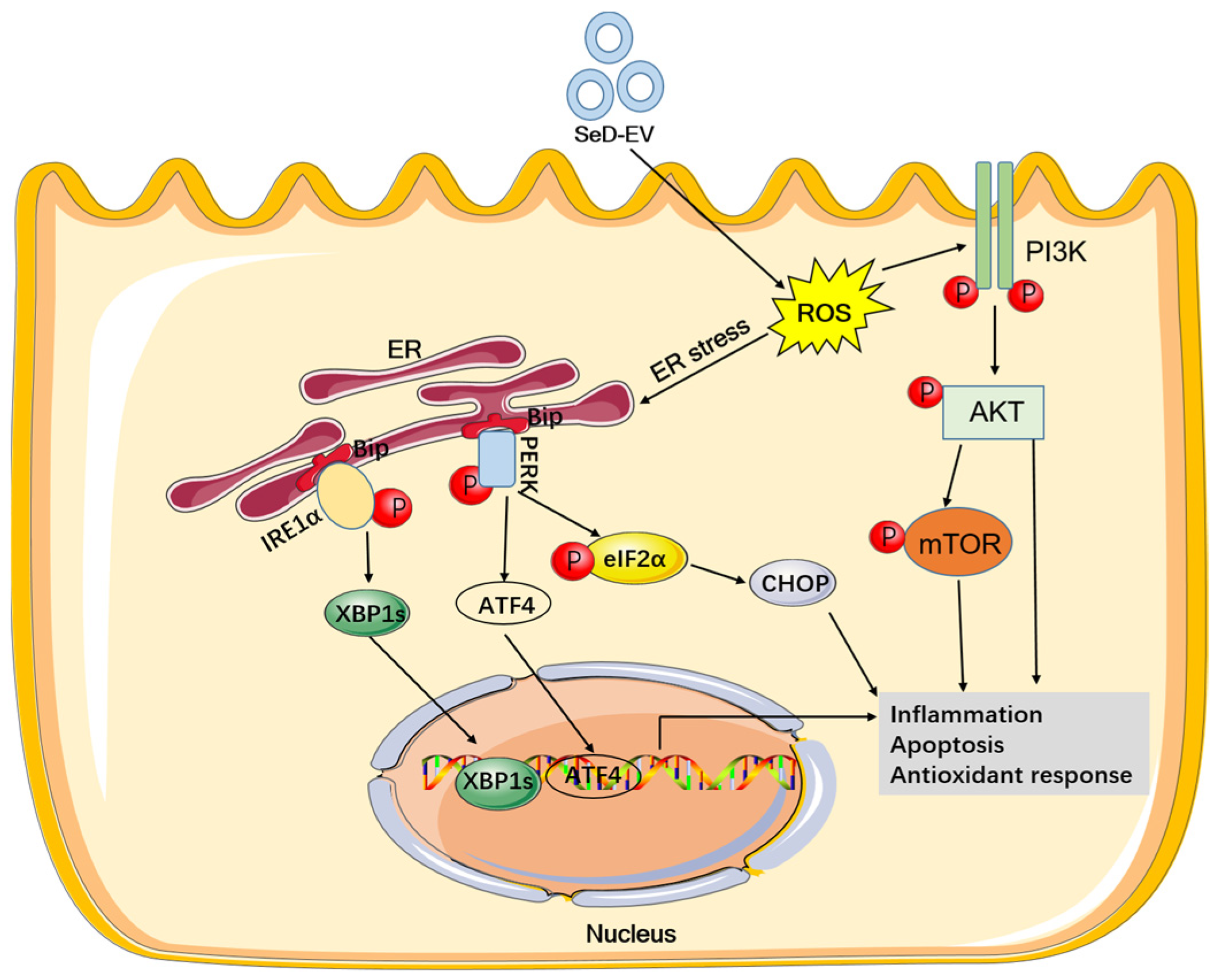
Extracellular Vesicles Derived from Selenium-Deficient MAC-T Cells Aggravated Inflammation and Apoptosis by Triggering the Endoplasmic Reticulum (ER) Stress/PI3K-AKT-mTOR Pathway in Bovine Mammary Epithelial Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Detection of Intracellular Se Levels
2.3. ROS Analysis
2.4. Evaluation of Antioxidant Biochemical Indexes
2.5. ELISA
2.6. TUNEL Assay
2.7. Isolation of Extracellular Vesicles
2.8. TEM
2.9. Particle Size Analysis
2.10. RNA Sequencing and Analysis
2.11. RT-qPCR
2.12. Western Blot
2.13. Extracellular Vesicles Tracing
2.14. Statistical Analysis
3. Results
3.1. Se Deficiency Promoted MAC-T Cell Damage through Oxidative Stress
3.2. Identification of Extracellular Vesicles
3.3. Overview of RNA Sequencing
3.4. Differential Expression of mRNA
3.5. Differential Expression of lncRNA
3.6. Prediction of lncRNA Target Genes
3.7. GO and KEGG Analysis of lncRNA Target Genes
3.8. SeD-EV Induced Apoptosis and Inflammation of Normal MAC-T Cells
3.9. SeD-EV Promoted ER Stress by Oxidative Stress
3.10. SeD-EV Inhibited PI3K-AkT-mTOR Signaling Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.Z.; Liu, L.; Zhang, Z.; Khan, A.; Wang, D.; Mi, S.; Usman, T.; Liu, G.; Guo, G.; Li, X.; et al. Folic acid supplementation regulates milk production variables, metabolic associated genes and pathways in perinatal Holsteins. J. Anim. Physiol. Anim. Nutr. 2020, 104, 483–492. [Google Scholar] [CrossRef]
- Contreras, G.A.; Strieder-Barboza, C.; Raphael, W. Adipose tissue lipolysis and remodeling during the transition period of dairy cows. J. Anim. Sci. Biotechnol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Bernabucci, U.; Ronchi, B.; Lacetera, N.; Nardone, A. Influence of body condition score on relationships between metabolic status and oxidative stress in periparturient dairy cows. J. Dairy Sci. 2005, 88, 2017–2026. [Google Scholar] [CrossRef] [PubMed]
- Ayemele, A.G.; Tilahun, M.; Lingling, S.; Elsaadawy, S.A.; Guo, Z.; Zhao, G.; Xu, J.; Bu, D. Oxidative Stress in Dairy Cows: Insights into the Mechanistic Mode of Actions and Mitigating Strategies. Antioxidants 2021, 10, 1918. [Google Scholar] [CrossRef]
- Sordillo, L.M. Nutritional strategies to optimize dairy cattle immunity. J. Dairy Sci. 2016, 99, 4967–4982. [Google Scholar] [CrossRef]
- Khan, M.Z.; Ma, Y.; Xiao, J.; Chen, T.; Ma, J.; Liu, S.; Wang, Y.; Khan, A.; Alugongo, G.M.; Cao, Z. Role of Selenium and Vitamins E and B9 in the Alleviation of Bovine Mastitis during the Periparturient Period. Antioxidants 2022, 11, 657. [Google Scholar] [CrossRef]
- Surai, P.F.; Kochish, I.I.; Fisinin, V.I.; Juniper, D.T. Revisiting Oxidative Stress and the Use of Organic Selenium in Dairy Cow Nutrition. Animals 2019, 9, 462. [Google Scholar] [CrossRef]
- Ottaviano, F.G.; Tang, S.S.; Handy, D.E.; Loscalzo, J. Regulation of the extracellular antioxidant selenoprotein plasma glutathione peroxidase (GPx-3) in mammalian cells. Mol. Cell. Biochem. 2009, 327, 111–126. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Jankowiak, D.; Tomza-Marciniak, A.; Pilarczyk, R.; Sablik, P.; Drozd, R.; Tylkowska, A.; Skólmowska, M. Selenium concentration and glutathione peroxidase (GSH-Px) activity in serum of cows at different stages of lactation. Biol. Trace Elem. Res. 2012, 147, 91–96. [Google Scholar] [CrossRef]
- Brigelius-Flohé, R.; Banning, A.; Schnurr, K. Selenium-dependent enzymes in endothelial cell function. Antioxid. Redox Signal. 2003, 5, 205–215. [Google Scholar] [CrossRef]
- Miranda, S.G.; Wang, Y.J.; Purdie, N.G.; Osborne, V.R.; Coomber, B.L.; Cant, J.P. Selenomethionine stimulates expression of glutathione peroxidase 1 and 3 and growth of bovine mammary epithelial cells in primary culture. J. Dairy Sci. 2009, 92, 2670–2683. [Google Scholar] [CrossRef]
- Malbe, M.; Klaassen, E.; Kaartinen, L.; Attila, M.; Atroshi, F. Effects of oral selenium supplementation on mastitis markers and pathogens in Estonian cows. Vet. Ther. Res. Appl. Vet. Med. 2003, 4, 145–154. [Google Scholar]
- Wang, D.; Jia, D.; He, R.; Lian, S.; Wang, J.; Wu, R. Association Between Serum Selenium Level and Subclinical Mastitis in Dairy Cattle. Biol. Trace Elem. Res. 2021, 199, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Di Conza, G.; Ho, P.C. ER Stress Responses: An Emerging Modulator for Innate Immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef]
- Choi, J.A.; Song, C.H. Insights Into the Role of Endoplasmic Reticulum Stress in Infectious Diseases. Front. Immunol. 2019, 10, 3147. [Google Scholar] [CrossRef]
- Li, K.; Wu, J.; Xu, S.; Li, X.; Zhang, Y.; Gao, X.J. Rosmarinic acid alleviates intestinal inflammatory damage and inhibits endoplasmic reticulum stress and smooth muscle contraction abnormalities in intestinal tissues by regulating gut microbiota. Microbiol. Spectr. 2023, 11, e0191423. [Google Scholar] [CrossRef]
- Bettigole, S.E.; Glimcher, L.H. Endoplasmic reticulum stress in immunity. Annu. Rev. Immunol. 2015, 33, 107–138. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2018, 9, 3083. [Google Scholar] [CrossRef] [PubMed]
- So, J.S. Roles of Endoplasmic Reticulum Stress in Immune Responses. Mol. Cells 2018, 41, 705–716. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- Cintio, M.; Polacchini, G.; Scarsella, E.; Montanari, T.; Stefanon, B.; Colitti, M. MicroRNA Milk Exosomes: From Cellular Regulator to Genomic Marker. Animals 2020, 10, 1126. [Google Scholar] [CrossRef] [PubMed]
- Zempleni, J.; Sukreet, S.; Zhou, F.; Wu, D.; Mutai, E. Milk-Derived Exosomes and Metabolic Regulation. Annu. Rev. Anim. Biosci. 2019, 7, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Xia, B.; Shan, S.; Zheng, A.; Zhang, S.; Chen, J.; Liang, X.J. High-quality milk exosomes as oral drug delivery system. Biomaterials 2021, 277, 121126. [Google Scholar] [CrossRef] [PubMed]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Gupta, R.C. Bovine milk-derived exosomes for drug delivery. Cancer Lett. 2016, 371, 48–61. [Google Scholar] [CrossRef]
- Rashidi, M.; Bijari, S.; Khazaei, A.H.; Shojaei-Ghahrizjani, F.; Rezakhani, L. The role of milk-derived exosomes in the treatment of diseases. Front. Genet. 2022, 13, 1009338. [Google Scholar] [CrossRef]
- Tong, C.; Chen, Q.; Zhao, L.; Ma, J.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of long intergenic noncoding RNAs in bovine mammary glands. BMC Genom. 2017, 18, 468. [Google Scholar] [CrossRef]
- Ma, M.; Pei, Y.; Wang, X.; Feng, J.; Zhang, Y.; Gao, M.Q. LncRNA XIST mediates bovine mammary epithelial cell inflammatory response via NF-κB/NLRP3 inflammasome pathway. Cell Prolif. 2019, 52, e12525. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Li, X.; Wang, Q.; Qing, S.; Zhang, Y.; Gao, M.Q. A novel long non-coding RNA regulates the immune response in MAC-T cells and contributes to bovine mastitis. FEBS J. 2019, 286, 1780–1795. [Google Scholar] [CrossRef]
- Yang, W.; Li, X.; Qi, S.; Li, X.; Zhou, K.; Qing, S.; Zhang, Y.; Gao, M.Q. lncRNA H19 is involved in TGF-β1-induced epithelial to mesenchymal transition in bovine epithelial cells through PI3K/AKT Signaling Pathway. Peer J. 2017, 5, e3950. [Google Scholar] [CrossRef]
- Wang, X.; Wang, H.; Zhang, R.; Li, D.; Gao, M.Q. LRRC75A antisense lncRNA1 knockout attenuates inflammatory responses of bovine mammary epithelial cells. Int. J. Biol. Sci. 2020, 16, 251–263. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, J.; Huang, Z.; Jing, H.; Yin, B.; Guo, S.; Deng, G.; Guo, M. Exosomal lnc-AFTR as a novel translation regulator of FAS ameliorates Staphylococcus aureus-induced mastitis. BioFactors 2022, 48, 148–163. [Google Scholar] [CrossRef]
- Chen, Y.; Jing, H.; Chen, M.; Liang, W.; Yang, J.; Deng, G.; Guo, M. Transcriptional Profiling of Exosomes Derived from Staphylococcus aureus-Infected Bovine Mammary Epithelial Cell Line MAC-T by RNA-Seq Analysis. Oxidative Med. Cell. Longev. 2021, 2021, 8460355. [Google Scholar] [CrossRef]
- Ma, S.; Tong, C.; Ibeagha-Awemu, E.M.; Zhao, X. Identification and characterization of differentially expressed exosomal microRNAs in bovine milk infected with Staphylococcus aureus. BMC Genom. 2019, 20, 934. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, T.A.; Sacco, R.E.; Nonnecke, B.J.; Lippolis, J.D. Bovine milk proteome: Quantitative changes in normal milk exosomes, milk fat globule membranes and whey proteomes resulting from Staphylococcus aureus mastitis. J. Proteom. 2013, 82, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, Y.; He, W.; Wang, Y.; Cao, Z.; Yang, H.; Wang, W.; Li, S. Novel organic selenium source hydroxy-selenomethionine counteracts the blood-milk barrier disruption and inflammatory response of mice under heat stress. Front. Immunol. 2022, 13, 1054128. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zeng, H.; Shao, Q.; Tang, J.; Wang, L.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; Cai, J.; et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. 2023, 67, 102912. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Y.; Chen, B.; Zhao, B.; Gao, X.J. Selenium Deficiency Promotes Oxidative Stress-Induced Mastitis via Activating the NF-κB and MAPK Pathways in Dairy Cow. Biol. Trace Elem. Res. 2022, 200, 2716–2726. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, W.; Shi, X.; Wang, Y.; Zhang, Y.; Liu, X.; Xu, S.; Zhang, J. Selenium deficiency caused hepatitis in chickens via the miR-138-5p/SelM/ROS/Ca(2+) overload pathway induced by hepatocyte necroptosis. Food Funct. 2023, 14, 9226–9242. [Google Scholar] [CrossRef]
- Kang, D.; Lee, J.; Jung, J.; Carlson, B.A.; Chang, M.J.; Chang, C.B.; Kang, S.B.; Lee, B.C.; Gladyshev, V.N.; Hatfield, D.L.; et al. Selenophosphate synthetase 1 deficiency exacerbates osteoarthritis by dysregulating redox homeostasis. Nat. Commun. 2022, 13, 779. [Google Scholar] [CrossRef]
- Xu, R.; Cao, J.W.; Xu, T.C.; Liu, T.J.; Zhu, M.R.; Guo, M.Y. Selenium deficiency induced inflammation and apoptosis via NF-κB and MAPKs pathways in muscle of common carp (Cyprinus carpio L.). Fish Shellfish. Immunol. 2023, 138, 108847. [Google Scholar] [CrossRef]
- Lei, L.; Jing, M.; Yingce, Z.; Pei, Z.; Yun, L. Selenium deficiency causes oxidative stress and activates inflammation, apoptosis, and necroptosis in the intestine of weaned calves. Met. Integr. Biometal Sci. 2023, 15, mfad028. [Google Scholar] [CrossRef]
- Samuel, M.; Sanwlani, R.; Pathan, M.; Anand, S.; Johnston, E.L.; Ang, C.S.; Kaparakis-Liaskos, M.; Mathivanan, S. Isolation and Characterization of Cow-, Buffalo-, Sheep- and Goat-Milk-Derived Extracellular Vesicles. Cells 2023, 12, 2491. [Google Scholar] [CrossRef]
- Aguilera, C.; Velásquez, A.E.; Gutierrez-Reinoso, M.A.; Wong, Y.S.; Melo-Baez, B.; Cabezas, J.; Caamaño, D.; Navarrete, F.; Rojas, D.; Riadi, G.; et al. Extracellular Vesicles Secreted by Pre-Hatching Bovine Embryos Produced In Vitro and In Vivo Alter the Expression of IFNtau-Stimulated Genes in Bovine Endometrial Cells. Int. J. Mol. Sci. 2023, 24, 7438. [Google Scholar] [CrossRef] [PubMed]
- Cappe, B.; Vandenabeele, P.; Riquet, F.B. A Guide to the expanding field of Extracellular Vesicles, and their release in Regulated Cell Death Programs. FEBS J. 2023. [Google Scholar] [CrossRef] [PubMed]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Sunde, R.A. Gene Set Enrichment Analysis of Selenium-Deficient and High-Selenium Rat Liver Transcript Expression and Comparison With Turkey Liver Expression. J. Nutr. 2021, 151, 772–784. [Google Scholar] [CrossRef]
- Tsuji, P.A.; Carlson, B.A.; Anderson, C.B.; Seifried, H.E.; Hatfield, D.L.; Howard, M.T. Dietary Selenium Levels Affect Selenoprotein Expression and Support the Interferon-γ and IL-6 Immune Response Pathways in Mice. Nutrients 2015, 7, 6529–6549. [Google Scholar] [CrossRef]
- Jehan, C.; Cartier, D.; Bucharles, C.; Anouar, Y.; Lihrmann, I. Emerging roles of ER-resident selenoproteins in brain physiology and physiopathology. Redox Biol. 2022, 55, 102412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Roh, Y.J.; Han, S.J.; Park, I.; Lee, H.M.; Ok, Y.S.; Lee, B.C.; Lee, S.R. Role of Selenoproteins in Redox Regulation of Signaling and the Antioxidant System: A Review. Antioxidants 2020, 9, 383. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, K.; Shi, J.; Shi, X.; Qi, X.; Lin, H. The decrease of selenoprotein K induced by selenium deficiency in diet improves apoptosis and cell progression block in chicken liver via the PTEN/PI3K/AKT pathway. Free Radic. Biol. Med. 2022, 189, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.J.; Liu, M.; Niu, Q.J.; Huang, Y.X.; Zhao, L.; Lei, X.G.; Sun, L.H. Both selenium deficiency and excess impair male reproductive system via inducing oxidative stress-activated PI3K/AKT-mediated apoptosis and cell proliferation signaling in testis of mice. Free Radic. Biol. Med. 2023, 197, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lian, S.; He, X.; Yu, D.; Liang, J.; Sun, D.; Wu, R. Selenium deficiency induces splenic growth retardation by deactivating the IGF-1R/PI3K/Akt/mTOR pathway. Met. Integr. Biometal Sci. 2018, 10, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Yang, J.; Yao, H.; Fan, R.; Cao, C.; Liu, C.; Zhang, S.; Lei, X.; Xu, S. The proteomic profiling of multiple tissue damage in chickens for a selenium deficiency biomarker discovery. Food Funct. 2020, 11, 1312–1321. [Google Scholar] [CrossRef] [PubMed]










| Gene | Primer Sequence (5′–3′) | NCBI Reference Sequence | Product Size |
|---|---|---|---|
| TNF-α | Sense: ACGGGCTTTACCTCATCTACTCA Anti-sense: GGCTCTTGATGGCAGACAGG | NM_173966.3 | 141 bp |
| IL-6 | Sense: ATGCTTCCAATCTGGGTTCA Anti-sense: GAGGATAATCTTTGCGTTCTTT | NM_173923.2 | 268 bp |
| IL-1β | Sense: GGCAACCGTACCTGAACCCA Anti-sense: CCACGATGACCGACACCACC | NM_174093.1 | 206 bp |
| GAPDH | Sense: TGCTGGTGCTGAGTATGTGGTG Anti-sense: CAGTCTTCTGGGTGGCAGTGAT | NM_001034034.2 | 296 bp |
| Sample | Total Reads | Total Reads (bp) | GC% | Error Rate (%) | Q20 (%) | Q30 (%) |
|---|---|---|---|---|---|---|
| SeC_1 | 61,456,724 | 9,135,825,342 | 49.01 | 0.12 | 98.82 | 96.45 |
| SeC_2 | 60,867,218 | 9,057,475,381 | 48.93 | 0.11 | 98.63 | 96.28 |
| SeD_1 | 59,355,422 | 8,903,313,300 | 49.35 | 0.12 | 98.57 | 95.68 |
| SeD_2 | 60,132,482 | 8,998,594,475 | 49.99 | 0.13 | 98.43 | 95.59 |
| Sample | Raw Reads | Raw Bases | Clean Reads | GC% | Q20 (%) | Q30 (%) | Clean Bases | Clean Bases% |
|---|---|---|---|---|---|---|---|---|
| SeC_1 | 61,456,724 | 9,135,825,342 | 60,102,541 | 50.02 | 99.17 | 97.50 | 8,403,132,152 | 91.98 |
| SeC_2 | 60,867,218 | 9,057,475,381 | 59,987,628 | 49.93 | 99.12 | 97.45 | 8,271,286,526 | 91.32 |
| SeD_1 | 59,355,422 | 8,903,313,300 | 58,032,752 | 49.86 | 99.15 | 97.33 | 8,095,777,614 | 90.93 |
| SeD_2 | 60,132,482 | 8,998,594,475 | 59,824,232 | 49.91 | 99.20 | 97.36 | 8,344,396,659 | 92.73 |
| Sample | SeC_1 | SeC_2 | SeD_1 | SeD_2 |
|---|---|---|---|---|
| The effective reads | 110,240,527 (100%) | 109,856,243 (100%) | 99,225,814 (100%) | 102,453,768 (100%) |
| Total mapped | 87,839,652 (79.68%) | 86,654,605 (78.88%) | 77,776,870 (78.38%) | 800,368,834 (78.12%) |
| Multiple mapped | 5,975,037 (5.42%) | 5,888,295 (5.36%) | 5,336,626 (5.38%) | 5,399,313 (5.27%) |
| Uniquely mapped | 81,853,591 (74.25%) | 81,469,390 (74.16%) | 72,440,244 (73.01%) | 75,037,139 (73.24%) |
| Read1 mapped | 44,482,053 (40.35%) | 44,184,181 (40.22%) | 38,935,504 (39.24%) | 40,295,067 (39.33%) |
| Read2 mapped | 44,360,788 (40.24%) | 44,118,267 (40.16%) | 38,841,366 (39.14%) | 40,233,595 (39.27%) |
| Reads map to ‘+’ | 44,404,883 (40.28%) | 44,162,210 (40.20%) | 38,941,441 (39.25%) | 40,315,557 (39.35%) |
| Reads map to ‘−’ | 44,228,499 (40.12%) | 44,041,368 (40.09%) | 38,835,429 (39.14%) | 40,161,877 (39.20%) |
| Reads mapped in proper pairs | 82,526,059 (74.86%) | 82,018,671 (74.66%) | 73,512,266 (74.09%) | 76,369,038 (74.54%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, X.; Yang, J.; Feng, W.; Deng, G.; Xu, S.; Guo, M. Extracellular Vesicles Derived from Selenium-Deficient MAC-T Cells Aggravated Inflammation and Apoptosis by Triggering the Endoplasmic Reticulum (ER) Stress/PI3K-AKT-mTOR Pathway in Bovine Mammary Epithelial Cells. Antioxidants 2023, 12, 2077. https://doi.org/10.3390/antiox12122077
Chen Y, Zhang X, Yang J, Feng W, Deng G, Xu S, Guo M. Extracellular Vesicles Derived from Selenium-Deficient MAC-T Cells Aggravated Inflammation and Apoptosis by Triggering the Endoplasmic Reticulum (ER) Stress/PI3K-AKT-mTOR Pathway in Bovine Mammary Epithelial Cells. Antioxidants. 2023; 12(12):2077. https://doi.org/10.3390/antiox12122077
Chicago/Turabian StyleChen, Yu, Xiangqian Zhang, Jing Yang, Wen Feng, Ganzhen Deng, Shiwen Xu, and Mengyao Guo. 2023. "Extracellular Vesicles Derived from Selenium-Deficient MAC-T Cells Aggravated Inflammation and Apoptosis by Triggering the Endoplasmic Reticulum (ER) Stress/PI3K-AKT-mTOR Pathway in Bovine Mammary Epithelial Cells" Antioxidants 12, no. 12: 2077. https://doi.org/10.3390/antiox12122077
APA StyleChen, Y., Zhang, X., Yang, J., Feng, W., Deng, G., Xu, S., & Guo, M. (2023). Extracellular Vesicles Derived from Selenium-Deficient MAC-T Cells Aggravated Inflammation and Apoptosis by Triggering the Endoplasmic Reticulum (ER) Stress/PI3K-AKT-mTOR Pathway in Bovine Mammary Epithelial Cells. Antioxidants, 12(12), 2077. https://doi.org/10.3390/antiox12122077








