Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish
Abstract
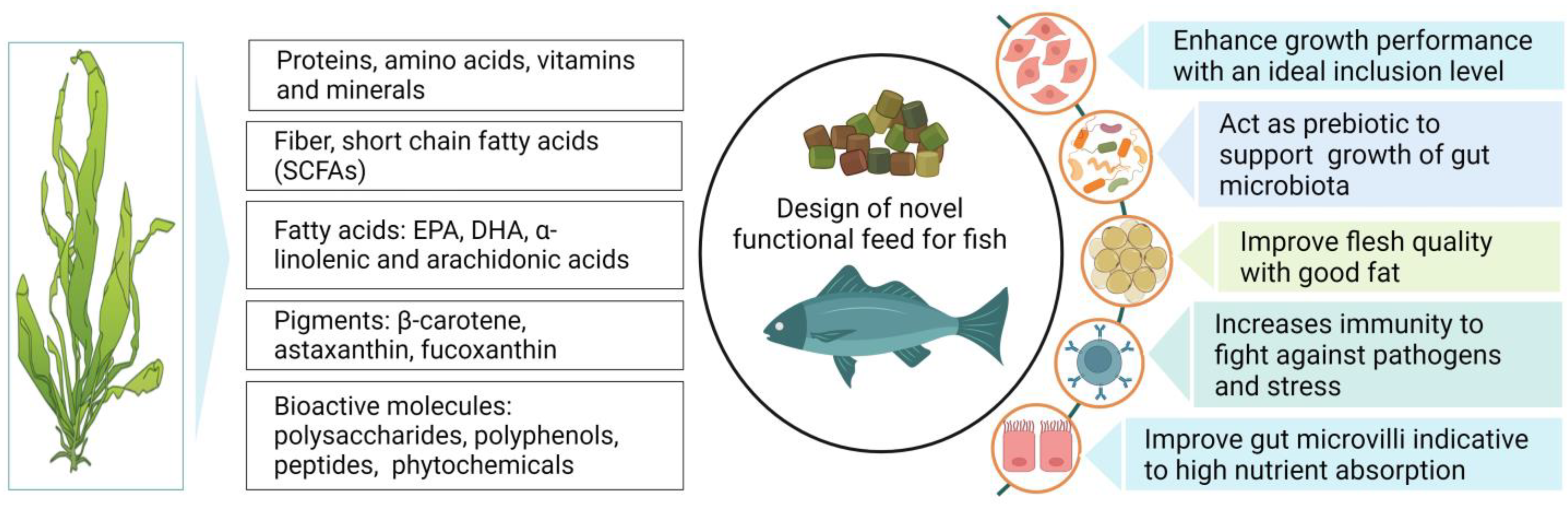
:1. Introduction
2. Seaweed Resources and Seaweed’s Nutritional Composition
2.1. Brown Seaweed
2.2. Red Seaweed
2.3. Green Seaweed
3. Seaweed-Based Functional Metabolites
3.1. Polysaccharides
3.2. Phenolic Compounds
3.3. Pigments
4. Most Commonly Utilized Seaweeds in Aquafeed
5. The Role of Seaweed in Aquaculture Production
5.1. Growth Performance and Feed Utilization
5.1.1. Growth Performance
5.1.2. Feed Conversion Ratio (FCR)
5.1.3. Feed Palatability
5.1.4. Feed Digestibility
| Seaweed and Derivatives | Fish Species | Applied Levels | Effective Level | Trial Period (Day) | Response | Reference |
|---|---|---|---|---|---|---|
| Sargassum portieranum (Phaeophyceae) | Oreochromis niloticus | 5 and 10% | 10% | 84 | SW inclusion resulted in significant growth enhancement | [117] |
| Grateloupia acuminata and G. doryphora (Rhodophyta) | O. niloticus | 0.1, 0.25, 0.5, and 1.0% | 0.5 and 1.0% | 60 | Growth and digestibility increased compared to control | [118] |
| Polyphenols from Eisenia arborea (Phaeophyceae) | Haliotis fulgens | 13.9 and 33.3 mg/g | - | 12 | Polyphenol reduction in feed promoted feed attractiveness and consumption | [119] |
| Fucoidan from Fucus vesiculosus (Phaeophyceae) | Danio rerio | 100 μg/mL | - | 5 | Fucoidan reduced NO and ROS accumulation in D. rerio larvae, which indicated therapeutic role of fucodian against inflammatory disorder | [120] |
| Fucoidan from Saccharina japonica (Phaeophyceae) | Clarias gariepinus | 0.04 and 0.06% | - | 21 | Dietary fucodian significantly enhanced the phagocytic activity, serum lysozyme, and bactericidal activity | [121] |
| Fucodian from Cladosiphon okamuranus (Phaeophyceae) | Pagrus major | 0.4% | - | 56 | Fucoidan supplementation showed nonsignificant improvement in feed utilization. Catalase activity is significantly influenced by fucodian | [122] |
| Fucodian from Undaria pinnatifida (Phaeophyceae) | Marsupenaeus japonicus | 0.01, 0.05, and 0.10% | 0.05% | 56 | 0.05% fucodian supplementation remarkably increased the growth and immune performances | [123] |
| Fucodian from Undaria pinnatifida (Phaeophyceae) | Lates calcarifer | 0.5 and 1.0% | 1.0% | 52 | 1% fucoidan inclusion diet exhibited enhanced growth | [124] |
| Gracilaria persica (Rhodophyta) | Acipenser persicus | 0.25, 0.5, and 1.0% | 0.5 and 1.0% | 56 | No significant improvement in growth due to SW provision | [125] |
| Mixture of Ulva lactuca (Chlorophyta), Jania rubens, and Pterocladia capillacea (Rhodophyta) | O. niloticus | 0.5, 1, 1.5, and 2.0% | 2.0% | 70 | Growth promoted at 2% dietary SW | [94] |
| Gracilaria sp. (Rhodophyta), Ulva sp. (Chlorophyta), or Fucus sp. (Phaeophyceae) | D. labrax | 2.5 and 7.5% | 7.5% | 49 | Immunity and antioxidant status improved at 7.5% SW inclusion compared to control | [81] |
| Laminaria sp. (Phaeophyceae) | S. salar | 3, 6, and 10% | 10% | 30 | Growth and immune status developed at 10% SW inclusion | [82] |
| Gracilaria pygmaea (Rhodophyta) | O. mykiss | 3, 6, 9, and 12% | 9% | 56 | Growth improved at 9% SW, while it was reduced at 12% SW level | [84] |
| Fucodian from Cladosiphon okamuranus (Phaeophyceae) | P. major | 0.05, 0.1, 0.2, 0.4, and 0.8% | 0.4% | 60 | Growth promoted at 0.4% dietary SW. Enhanced immune response and disease resistance at 0.3–0.4% SW | [83] |
| Ulva lactuca (Chlorophyta) Jania rubens and Pterocladia capillacea (Rhodophyta) | Pangasianodon hypophthalmus | 1, 2, and 3% | 2% | 60 | SW at a level of 2% improved the growth and resistance against Aeromonous. hydrophila infection. | [126] |
| Pelvetia canaliculata (Phaeophyceae) | Sparus aurata | 1, 5, and 10% | - | 56 | SW inclusion produced no changes in proximate composition and the fatty acid profile of fish when compared to control | [127] |
| Gracilariopsis lemaneiformis (Rhodophyta) | Pagrosomus major | 3, 6, 9, 12, and 15% | 3% | 56 | Growth improved at 3% SW. Liver glycogen and hepatic AST were significantly higher in supplemented group | [128] |
| Sargassum wightii (Phaeophyceae) | L. rohita | 2% | - | 45 | Growth promoted by dietary SW without compromising its immune-modulating effects | [96] |
| Ulva prolifera (formerly Enteromorpha prolifera) (Chlorophyta) | O. mossambicus × O. niloticus | 1, 2, 3, 4, and 5% | 5% | 49 | Growth was enhanced by dietary U. prolifera. SOD, LYZ, acid phosphatase and alkaline phosphatase activities were enhanced | [129] |
| Gracilaria arcuata (Rhodophyta) | O. niloticus | 20, 40, and 60% | 20% | 84 | Growth and feed utilization improved at 20% SW | [130] |
| Gracilariopsis persica, Hypnea flagelliformis (Rhodophyta), and Sargassum boveanum (Phaeophyceae) | O. mykiss | 5 and 10% | - | 83 | Serum LYZ, SOD, and CAT activity increased by SW provision | [22] |
| Gracilaria pulvinata (Rhodophyta) | Lates calcarifer | 3, 6, and 9% | 3% | 40 | No growth retardation up to 3% SW. Serum LYZ activitywas significantly enhanced at 3% supplementation, while ACH50 was lowered at 9% SW | [131] |
| Ulva rigida (Chlorophyta) and Undaria pinnatifida (Phaeophyceae) | Solea senegalensis | 10% | - | 150 | Growth retardation observed in growing stage for Undaria-based diet | [101] |
| Mixture of Gracilaria sp. (Rhodophyta), Ulva sp. (Chlorophyta), and Fucus sp. (Phaeophyceae) | D. labrax | 7.5% | - | 63 | Did not mitigate negative effects of environmental oscillations on growth and immunity by dietary SW | [132] |
| Ulva sp. (Chlorophyta) | Argyrosomus japonicus | 5, 10, and 20% | 5% | 63 | Growth and feed utility increased at 5% SW | [133] |
| Ulva lactuca (Chlorophyta) | S. aurata | 2.6 and 7.8%, 14.6 and 29.1% | - | 140 | No growth retardation observed by dietary SW | [134] |
| Gracilaria pygmaea (Rhodophyta) | O. mykiss | 3, 6, 9, and 12% | 6% | 49 | Growth was enhanced at 6% SW | [135] |
| Gracilaria sp. (Rhodophyta) and Alaria sp. (Phaeophyceae) | A. regius | 5% | - | 69 | No growth retardation by SW addition. Lipid peroxidation lowered | [136] |
| Gracilariopsis lemaneiformis (Rhodophyta) and Sargassum horneri (Phaeophyceae) | Lutjanus stellatus | 5, 10, 15, and 20% | 15% | 60 | Growth retardation at 20% SW | [137] |
| Taonia atomaria (Phaeophyceae) | O. niloticus | 5, 10, and 15% | 5% | 84 | Significant growth improvement by SW inclusion | [138] |
| Palmaria palmata (Rhodophyta) | S. salar | 5, 10, and 15% | - | 98 | ALT activity significantly decreased with no effects on LYZ or ACH50 activity | [139] |
| Ulva lactuca (Chlorophyta) | Lutjanus stellatus | 5, 10, 15, and 20% | 5% | 60 | Growth promoted at 5% SW | [140] |
| Sargassum angustifolium (Phaeophyceae) | O. mykiss | 0.005, 0.01, 0.02, and 0.04% | - | 56 | Immune status and lower mortality against Yersinia rukeri by dietary SW | [141] |
| Gracilaria sp. (Rhodophyta) | D. labrax | 0.5 and 4.5% | - | 42 | ACH50 activity was enhanced, while no effect was observed on LYZ and PO activity by SW inclusion | [142] |
| Sargassum dentifolium (Phaeophyceae) | O. mossambicus × O. niloticus | 1, 2, and 3% | 3% | 84 | Significantly increased GOT and triglycerides level, while no impact was noticed for total plasma protein, albumin, and globulin | [143] |
| Saccharina latissimi (Phaeophyceae) | O. mykiss | 1, 2, and 4% | 1 and 2% | 84 | Significantly downregulated the expression of stress marker (gpx1b2) | [144] |
| Ulva prolifera, Ulva australis (formerly U. pertusa) (Chlorophyta), or G. lemaneiformis (Rhodophyta) | Siganus canaliculatus | 12% | - | 70 | LYZ, dismutase, and acid phosphatase were significantly enhanced. Enhanced resistance against Vibrio parahaemolyticus | [145] |
| Ulva sp. (Chlorophyta) | O. niloticus | 5 and 10% | 10% | 68 | Significantly enhanced ACH50 activity, while no effects were observed in the cases of LYZ and PO activity | [146] |
| Padina gymnospora (Phaeophyceae) | Cyprinus carpio | 0.01, 0.1, or 1% | - | 21 | Remarkably improved serum LYZ, MPO, and antibody responses | [147] |
| Enteromorpha intestinalis (Chlorophyta) | O. niloticus | 10, 20, 30, and 40% | 20% | 42 | Significantly improved growth performance at 20% inclusion level | [148] |
| Sargassum fusiformis (formerly Hizikia fusiformis) (Phaeophyceae) | Paralichthys olivaceus | 0, 0.5, and 1% | - | 84 | Significantly upgraded the immune status of fish by raising the level of hepatic IL-2 and IL-6 | [149] |
| S. fusiforme and Ecklonia cava (Phaeophyceae) | Paralichthys olivaceus | 6% | - | 42 | Hb level and RBC count were significantly elevated. Exhibited higher resistance against Edwardsiella tarda challenge | [150] |
| Ulva lactuca (Chlorophyta) and Pterocladia capillacea (Rhodophyta) | D. labrax | 5, 10, and 15% | - | 56 | P. capillacea exhibited high-stress resistance capacity compared to U. lactuca | [14] |
| Eucheuma denticulatum (Rhodophyta) and Sargassum fulvellum (Phaeophyceae) | P. olivaceus | 3 and 6% | 6% | 56 | Significantly lowered the level of blood cholesterol and triglycerides. Serum LYZ activity was significantly enhanced | [151] |
| Sargassum whitti (Phaeophyceae) | M. cephalus | 0.5, 1.0, and 1.5.0% | - | WBC, LYZ, and RBC significantly elevated in seaweed-supplemented groups. Mortality rate decreased after exposure to Pseudomonas fluorescence | [152] | |
| Gracilariopsis lemaneiformis (Rhodophyta) | Siganus canaliculatus | 33% | - | 56 | LYZ and ACH50 activity was remarkably enhanced in the group provided seaweed | [153] |
| Ecklonia cava (Phaeophyceae) | P. olivaceus | 2, 4, and 6% | - | 42 | Serum LYZ, MPO, and NBT activities were significantly increased | [154] |
| Macrocystis pyrifera (Phaeophyceae) and Chondrus crispus (Rhodophyta) | Epinephelus coicoides | 0.001, 0.002, and 0.003% | - | 5 | Significantly enhanced RBC, SOD, and phagocytic activity. Exhibited resistance against V. alginolyticus | [155] |
| Sargassum fusiforme (formerly Hizikia fusiformis) (Phaeophyceae) | P. olivaceus | 2, 4, and 6% | - | 56 | Phagocyte activity was elevated with the increase of S. fusiforme in diet. Improved resistance to Streptococcus iniae | [156] |
| Ulva lactuca (Chlorophyta) Pterocladia capillacea (Rhodophyta) | S. aurata | 5, 10, and 15% | 5 and 10% | 56 | Enhanced stress response ability | [157] |
5.2. Immune Status, Antioxidant Response, and Gut Health in Fish
5.2.1. Immunity and Disease Resistance
5.2.2. Antioxidant Response
5.3. Intestinal Morphology
5.4. Gut Microbiota Composition
6. Potential Limitations and Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garlock, T.; Asche, F.; Anderson, J.; Ceballos-concha, A.; Love, D.C.; Osmundsen, T.C.; Beatriz, R.; Pincinato, M. Aquaculture: The missing contributor in the food security agenda. Glob. Food Secur. 2022, 32, 100620. [Google Scholar] [CrossRef]
- Barasa, J.E.; Mukhongo, P.N.; Ngetich, C.C. Perspectives on salmon aquaculture: Current status, challenges and genetic improvement for future growth. In Salmon Aquaculture; IntechOpen: London, UK, 2022; pp. 1–28. [Google Scholar]
- Afewerki, S.; Asche, F.; Misund, B.; Thorvaldsen, T.; Tveteras, R. Innovation in the Norwegian aquaculture industry. Rev. Aquac. 2023, 15, 759–771. [Google Scholar] [CrossRef]
- Wan, A.H.L.; Davies, S.J.; Soler-vila, A.; Fitzgerald, R.; Johnson, M.P. Macroalgae as a sustainable aquafeed ingredient. Rev. Aquac. 2019, 11, 458–492. [Google Scholar] [CrossRef]
- Akbary, P.; Aminikhoei, Z. Effect of water-soluble polysaccharide extract from the green alga Ulva rigida on growth performance, antioxidant enzyme activity, and immune stimulation of grey mullet Mugil cephalus. J. Appl. Phycol. 2018, 30, 1345–1353. [Google Scholar] [CrossRef]
- Thepot, V.; Campbell, A.H.; Rimmer, M.A.; Paul, N.A. Meta-analysis of the use of seaweeds and their extracts as immunostimulants for fish: A systematic review. Rev. Aquac. 2021, 13, 907–933. [Google Scholar] [CrossRef]
- Dawczynski, C.; Schubert, R.; Jahreis, G. Amino acids, fatty acids, and dietary fibre in edible seaweed products. Food Chem. 2007, 103, 891–899. [Google Scholar] [CrossRef]
- Saleh, H.H. Review on using of macroalgae (seaweeds) in fish nutrition. J. Zool. Res. 2020, 2, 6–11. [Google Scholar] [CrossRef]
- Øverland, M.; Mydland, L.T.; Skrede, A. Marine macroalgae as sources of protein and bioactive compounds in feed for monogastric animals. J. Sci. Food Agric. 2019, 99, 13–24. [Google Scholar] [CrossRef]
- Martínez-Antequera, F.P.; Martos-Sitcha, J.A.; Reyna, J.M.; Moyano, F.J. Evaluation of the Inclusion of the Green Seaweed Ulva ohnoi as an Ingredient in Feeds for Gilthead Sea Bream (Sparus aurata) and European Sea Bass (Dicentrarchus labrax). Animals 2021, 11, 1684. [Google Scholar] [CrossRef]
- Satoh, K.-I.; Nakagawa, H.; Kasahara, S. Effect of Ulva meal supplementation on disease resistance of red sea bream. Bull. Jpn. Soc. Sci. Fish. 1987, 53, 1115–1120. [Google Scholar] [CrossRef]
- Soler-vila, A.; Coughlan, S.; Guiry, M.D. The red alga Porphyra dioica as a fish-feed ingredient for rainbow trout (Oncorhynchus mykiss): Effects on growth, feed efficiency, and carcass composition. J. Appl. Phycol. 2009, 21, 617–624. [Google Scholar] [CrossRef]
- An, B.N.T.; Anh, N.T.N. Co-culture of Nile tilapia (Oreochromis niloticus) and red seaweed (Gracilaria tenuistipitata) under different feeding rates: Effects on water quality, fish growth and feed efficiency. J. Appl. Phycol. 2020, 32, 2031–2040. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-Sayed, A.; Sakr, E.M. Pterocladia (Rhodophyta) and Ulva (Chlorophyta) as feed supplements for European seabass, Dicentrarchus labrax L., fry. J. Appl. Phycol. 2013, 25, 1369–1376. [Google Scholar] [CrossRef]
- Azaza, B.M.S.; Mensi, F.; Ksouri, J.; Dhraief, M.N.; Brini, B.; Abdelmouleh, A.; Kraı, M.M. Growth of Nile tilapia (Oreochromis niloticus L.) fed with diets containing graded levels of green algae Ulva meal (Ulva rigida) reared in geothermal waters of southern Tunisia. J. Appl. Ichthyol. 2008, 24, 202–207. [Google Scholar] [CrossRef]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as valuable sources of essential fatty acids for human nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, C.C.C.R.; Caramujo, M.J. The Various Roles of Fatty Acids. Molecules 2018, 23, 2583. [Google Scholar] [CrossRef] [PubMed]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a functional ingredient for a healthy diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef]
- Gora, A.H.; Sahu, N.P.; Sahoo, S.; Rehman, S.; Dar, S.A.; Ahmad, I.; Agarwal, D. Effect of dietary Sargassum wightii and its fucoidan-rich extract on growth, immunity, disease resistance and antimicrobial peptide gene expression in Labeo rohita. Int. Aquat. Res. 2018, 10, 115–131. [Google Scholar] [CrossRef]
- Safavi, S.V.; Kenari, A.A.; Tabarsa, M.; Esmaeili, M. Effect of sulfated polysaccharides extracted from marine macroalgae (Ulva intestinalis and Gracilariopsis persica) on growth performance, fatty acid profile, and immune response of rainbow trout (Oncorhynchus mykiss). J. Appl. Phycol. 2019, 31, 4021–4035. [Google Scholar] [CrossRef]
- Thanigaivel, S.; Chandrasekaran, N.; Mukherjee, A.; Thomas, J. Protective efficacy of microencapsulated seaweed extracts for preventing Aeromonas infections in Oreochromis mossambicus. Comp. Biochem. Physiol. 2019, 218, 36–45. [Google Scholar] [CrossRef]
- Vazirzadeh, A.; Marhamati, A.; Rabiee, R.; Faggio, C. Immunomodulation, antioxidant enhancement and immune genes up-regulation in rainbow trout (Oncorhynchus mykiss) fed on seaweeds included diets. Fish Shellfish Immunol. 2020, 106, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Thepot, V.; Campbell, A.H.; Rimmer, M.A.; Jelocnik, M.; Johnston, C. Dietary inclusion of the red seaweed Asparagopsis taxiformis boosts production, stimulates immune response and modulates gut microbiota in Atlantic salmon, Salmo salar. Aquaculture 2022, 546, 737286. [Google Scholar] [CrossRef]
- Siddik, M.A.B.; Vatsos, I.N.; Rahman, M.A.; Pham, H.D. Selenium-Enriched Spirulina (SeE-SP) Enhance Antioxidant Response, Immunity, and Disease Resistance in Juvenile Asian Seabass, Lates calcarifer. Antioxidants 2022, 11, 1572. [Google Scholar] [CrossRef]
- Murty, U.S.; Banerjee, A.K. Seaweeds: The wealth of oceans. In Handbook of Marine Macroalgae: Biotechnology and Applied Phycology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 36–44. [Google Scholar]
- Din, N.A.S.; Mohd Alayudin, S.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown algae as functional food source of fucoxanthin: A review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Misurcova, L. Isolation and chemical properties of molecules derived from seaweeds chemical composition of seaweeds. In Handbook of Marine Macroalgae; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 173–192. [Google Scholar]
- Salehi, B.; Sharifi-rad, J.; Seca, A.M.L.; Pinto, D.C.G.A. Current trends on seaweeds: Looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules 2019, 24, 4182. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y.; Zhang, Y.; Yang, Y.; Wang, P.; Imre, B.; Wong, A.C.Y.; Hsieh, Y.S.Y.; Wang, D. Brown Algae Carbohydrates: Structures, Pharmaceutical Properties, and Research Challenges. Mar. Drugs 2021, 19, 620. [Google Scholar] [CrossRef]
- D’Armas, H.D.; Jaramillo, C.; Armas, M.D.; Echavarría, A.; Valverde, P. Proximate composition of several macroalgae from the coast of Salinas Bay, Ecuador. Rev. Biol. Trop. 2019, 67, 61–68. [Google Scholar]
- Schmid, M.; Kraft, L.G.K.; Van Der Loos, L.; Kraft, G.T.; Virtue, P.; Nichols, P.D.; Hurd, C.L. Southern Australian seaweeds: A promising resource for omega-3 fatty acids. Food Chem. 2018, 265, 70–77. [Google Scholar] [CrossRef]
- Airanthi, M.K.W.; Sasaki, N.; Iwasaki, S.; Baba, N.; Abe, M.; Hosokawa, M.; Miyashita, K. Effect of brown seaweed lipids on fatty acid composition and lipid hydroperoxide levels of mouse liver. J. Agric. Food Chem. 2011, 59, 4156–4163. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Özogul, Y.; Regenstein, J.M. Marine bioactive compounds and their health benefits: A review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 446–465. [Google Scholar] [CrossRef]
- Holdt, S.L.; Kraan, S. Bioactive compounds in seaweed: Functional food applications and legislation. J. Appl. Phycol. 2011, 23, 543–597. [Google Scholar] [CrossRef]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.; Quek, S. Red seaweed: A promising alternative protein source for global food sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Cian, R.E.; Drago, S.R.; de Medina, F.S.; Martínez-Augustin, O. Proteins and carbohydrates from red seaweeds: Evidence for beneficial effects on gut function and microbiota. Mar. Drugs 2015, 13, 5358–5383. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.M.; Luong-van, J.T. Seasonal variation in the chemical composition of tropical Australian marine macroalgae. J. Appl. Phycol. 2006, 18, 381–387. [Google Scholar] [CrossRef]
- Pereira, L. A review of the nutrient composition of selected edible seaweeds. In Seaweed; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2011; pp. 15–47. ISBN 9781614708780. [Google Scholar]
- Sanchez-Machado, D.I.; Lopez-Cervantes, J.; López-Hernández, J.; Paseiro-Losada, P. Fatty acids, total lipid, protein and ash contents of processed edible seaweeds. Food Chem. 2004, 85, 439–444. [Google Scholar] [CrossRef]
- Gaspar, R.; Fonseca, R.; Pereira, L. Illustrated Guide to the Macroalgae of Buarcos Bay, Figueira da Foz, Portugal; MARE UC, DCV, FCT: Coimbra, Portugal, 2020; 128p. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.K. Nutritional evaluation of some subtropical red and green seaweeds: Part I—Proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000, 71, 475–482. [Google Scholar] [CrossRef]
- Benjama, O.; Masniyom, P. Nutritional composition and physicochemical properties of two green seaweeds (Ulva pertusa and U. intestinalis) from the Pattani bay in southern Thailand. Songklanakarin J. Sci. Technol. 2011, 33, 575–583. [Google Scholar]
- Rajapakse, N.; Kim, S.K. Nutritional and digestive health benefits of seaweed. In Advances in Food and Nutrition; Academic Press: Cambridge, MA, USA, 2011; pp. 18–27. [Google Scholar]
- Corino, C.; Modina, S.C.; Di Giancamillo, A.; Chiapparini, S. Seaweeds in pig nutrition. Animals 2019, 9, 1126. [Google Scholar] [CrossRef]
- Garcı, M.N.; Pereira, A.C.; Leets, I.; Quiroga, M.F. High iron content and bioavailability in humans from four species of marine algae. J. Nutr. 2018, 137, 2691–2695. [Google Scholar] [CrossRef]
- Kumar, C.S.; Ganesan, P.; Pv, S.; Bhaskar, N. Seaweeds as a source of nutritionally beneficial compounds—A review. J. Food Sci. Technol. 2008, 45, 1–13. [Google Scholar]
- Charoensiddhi, S.; Conlon, M.A.; Vuaran, M.S.; Franco, C.M.M.; Zhang, W. Polysaccharide and phlorotannin-enriched extracts of the brown seaweed Ecklonia radiata influence human gut microbiota and fermentation in vitro. J. Appl. Phycol. 2017, 29, 2407–2416. [Google Scholar] [CrossRef]
- Sari-chmayssem, N.; Taha, S.; Mawlawi, H.; Guégan, J.; Jefti, J.; Benvegnu, T. Extracted ulvans from green algae Ulva linza of lebanese origin and amphiphilic derivatives: Evaluation of their physico-chemical and rheological properties. J. Appl. Phycol. 2018, 31, 1931–1946. [Google Scholar] [CrossRef]
- Murata, M.; Nakazoe, J. Production and use of marine algae in Japan. Jpn. Agric. Res. Q. 2001, 35, 281–290. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Fonseca, P.; Carneiro, M.; Moreira, W. Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour. Technol. 2006, 97, 2402–2406. [Google Scholar] [CrossRef] [PubMed]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Devill, C.; Damas, J.; Forget, P.; Dandrifosse, G.; Peulen, O. Laminarin in the dietary fibre concept. J. Sci. Food Agric. 2004, 84, 1030–1038. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef]
- Gunathilake, T.; Akanbi, T.O.; Suleria, H.A.R.; Nalder, T.D.; Francis, D.S.; Barrow, C.J. Seaweed phenolics as natural antioxidants, aquafeed additives, veterinary treatments and cross-linkers for microencapsulation. Mar. Drugs 2022, 20, 445. [Google Scholar] [CrossRef]
- López, A.; Rico, M.; Rivero, A.; de Tangil, M.S. The effects of solvents on the phenolic contents and antioxidant activity of Stypocaulon scoparium algae extracts. Food Chem. 2011, 125, 1104–1109. [Google Scholar] [CrossRef]
- Yumiko, Y.; Ya-Pei, H.; Takeshi, S. Distribution of flavinoids and related compounds from seaweeds in Japan. J. Tokyo Univ. Fish. 2003, 89, 1–6. [Google Scholar]
- Onofrejová, L.; Vašíčková, J.; Klejdus, B.; Stratil, P.; Mišurcová, L.; Kráčmar, S.; Kopecký, J.; Vacek, J. Bioactive phenols in algae: The application of pressurized-liquid and solid-phase extraction techniques. J. Pharm. Biomed. Anal. 2010, 51, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Catarino, M.D.; Silva, A.M.S.; Cardoso, S.M. Fucaceae: A source of bioactive phlorotannins. Int. J. Mol. Sci. 2017, 18, 1327. [Google Scholar] [CrossRef] [PubMed]
- El-beltagi, H.S.; Mohamed, A.A.; Mohamed, H.I.; Ramadan, K.M.A. Phytochemical and potential properties of seaweeds and their recent applications: A review. Mar. Drugs 2022, 20, 342. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A. Carotenoids of Norwegian Brown Seaweeds and of Seaweed Meals; Report No. 3t; Norwegian Institute of Seaweed Research: Trondheim, Norway, 1966. [Google Scholar]
- Balasubramaniam, V.; Chelyn, L.J.; Vimala, S.; Fairulnizal, M.N.M.; Brownlee, I.A.; Amin, I. Carotenoid composition and antioxidant potential of Eucheuma denticulatum, Sargassum polycystum and Caulerpa lentillifera. Heliyon 2020, 6, e04654. [Google Scholar] [CrossRef]
- Osório, C.; Machado, S.; Peixoto, J.; Bessada, S.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Pigments content (Chlorophylls, Fucoxanthin and Phycobiliproteins) of different commercial dried algae. Separations 2020, 7, 33. [Google Scholar] [CrossRef]
- Cofrades, S.; López-Lopez, I.; Bravo, L.; Ruiz-Capillas, C.; Bastida, S. Nutritional and antioxidant properties of different brown and red spanish edible seaweeds. Food Sci. Technol. Int. 2010, 16, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.V.; In, L.G.; Martins, M.; Pereira, L.; Mouga, T. Primary composition and pigments of 11 red seaweed species from the center of Portugal. J. Mar. Sci. Eng. 2022, 10, 1168. [Google Scholar] [CrossRef]
- Chakraborty, K.; Joseph, D. Antioxidant activities and phenolic contents of three red seaweeds (Division: Rhodophyta) harvested from the gulf of mannar of Peninsular India. J. Food Sci. Technol. 2015, 52, 1924–1935. [Google Scholar] [CrossRef]
- Gunathilaka, T.L.; Samarakoon, K.W.; Ranasinghe, P.; Peiris, L.D.C. In-Vitro Antioxidant, Hypoglycemic Activity, and Identification of Bioactive Compounds in Phenol-Rich Extract from the Marine Red Algae Gracilaria edulis (Gmelin) Silva. Molecules 2019, 24, 3708. [Google Scholar] [CrossRef]
- Sobuj, M.K.A.; Islam, M.A.; Islam, M.S.; Islam, M.M.; Mahmud, Y.; Rafiquzzaman, S.M. Effect of Solvents on Bioactive Compounds and Antioxidant Activity of Padina tetrastromatica and Gracilaria tenuistipitata Seaweeds Collected from Bangladesh. Sci. Rep. 2021, 11, 19082. [Google Scholar] [CrossRef]
- Zakaria, N.A.; Ibrahim, D.; Sulaiman, S.F.; Supardy, N.A.; Biotechnology, I.; Sains, U.; Pinang, P.; Pinang, P. Assessment of antioxidant activity, total phenolic content and in-vitro toxicity of Malaysian red seaweed, Acanthophora spicifera. J. Chem. Pharm. Res. 2011, 3, 182–191. [Google Scholar]
- El-Baky, H.A.; El Baz, F.K.; Baroty, G.S.E. Evaluation of marine alga Ulva lactuca L. as a source of natural preservative ingredient. Am.-Eurasian J. Agric. Environ. Sci. 2008, 3, 434–444. [Google Scholar]
- Roh, M.; Uddin, S.; Chun, B. Extraction of fucoxanthin and polyphenol from Undaria pinnatifida using supercritical carbon dioxide with co-solvent. Biotechnol. Bioprocess Eng. 2008, 13, 724–729. [Google Scholar] [CrossRef]
- Srikong, W.; Bovornreungroj, N.; Mittraparparthorn, P. Antibacterial and antioxidant activities of differential solvent extractions from the green seaweed Ulva intestinalis. ScienceAsia 2017, 43, 88–95. [Google Scholar] [CrossRef]
- Rattaya, S.; Benjakul, S. Extraction, antioxidative, and antimicrobial activities of brown seaweed extracts, Turbinaria ornata and Sargassum polycystum, grown in Thailand. Int. Aquat. Res. 2015, 7, 1–16. [Google Scholar] [CrossRef]
- Saravana, P.; Cho, Y.; Park, Y.; Woo, H.; Chun, B. Structural, antioxidant, and emulsifying activities of fucoidan from Saccharina japonica using pressurized liquid extraction. Carbohydr. Polym. 2016, 153, 518–525. [Google Scholar] [CrossRef]
- Subbiah, V.; Ebrahimi, F.; Agar, O.T.; Dunshea, F.R.; Barrow, C.J.; Suleria, H.A.R. Comparative Study on the Effect of Phenolics and Their Antioxidant Potential of Freeze-Dried Australian Beach-Cast Seaweed Species upon Different Extraction Methodologies. Pharmaceuticals 2023, 16, 773. [Google Scholar] [CrossRef]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant mediated α-glucosidase and α-amylase inhibition. J. Food Sci. 2010, 75, 97–102. [Google Scholar] [CrossRef]
- Rajauria, G.; Jaiswal, A.K.; Abu-gannam, N.; Gupta, S. Antimicrobial, antioxidant and free radical-scavenging capacity of brown seaweed Himanthalia elongata from western coast of Ireland. J. Food Biochem. 2013, 37, 322–335. [Google Scholar] [CrossRef]
- FAO. Top 10 Species Groups in Global Aquaculture 2019. World Aquaculture Performance Indicators (WAPI) Factsheet. 2021. 4p. Available online: www.fao.org/3/cb5186en/cb5186en.pdf (accessed on 14 October 2023).
- Valente, L.M.P.; Gouveia, A.; Rema, P.; Matos, J. Evaluation of three seaweeds Gracilaria bursapastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European seabass (Dicentrarchus labrax) juveniles. Aquaculture 2006, 252, 85–91. [Google Scholar] [CrossRef]
- Tolentino-Pablico, G.; Bailly, N.; Froese, R.; Elloran, C. Seaweeds preferred by herbivorous fishes. J. Appl. Phycol. 2008, 20, 933–938. [Google Scholar] [CrossRef]
- Norambuena, F.; Hermon, K.; Skrzypczyk, V.; Emery, J.A.; Sharon, Y.; Beard, A.; Turchini, G.M. Algae in fish feed: Performances and fatty acid metabolism in juvenile Atlantic salmon. PLoS ONE 2015, 10, e0124042. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, M.J.; Svendsen, J.C.; Malte, H.; Pereira, L.F.; Carvalho, P.; Pereira, R.; Gonçalves, J.F.M.; Ozório, R.O.A. Diets supplemented with seaweed affect metabolic rate, innate immune, and antioxidant responses, but not individual growth rate in European seabass (Dicentrarchus labrax). J. Appl. Phycol. 2016, 28, 2061–2071. [Google Scholar] [CrossRef]
- Kamunde, C.; Sappal, R.; Melegy, T.M. Brown seaweed (AquaArom) supplementation increases food intake and improves growth, antioxidant status and resistance to temperature stress in Atlantic salmon, Salmo salar. PLoS ONE 2019, 14, e0219792. [Google Scholar] [CrossRef]
- Sony, N.M.; Ishikawa, M.; Hossain, S.; Koshio, S.; Yokoyama, S. The effect of dietary fucoidan on growth, immune functions, blood characteristics and oxidative stress resistance of juvenile red sea bream, Pagrus major. Fish Physiol. Biochem. 2019, 45, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Sotoudeh, E.; Mardani, F. Antioxidant- related parameters, digestive enzyme activity and intestinal morphology in rainbow trout (Oncorhynchus mykiss) fry fed graded levels of red seaweed, Gracilaria pygmaea. Aquac. Nutr. 2018, 24, 777–785. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-ghannam, N. Bioactive potential and possible health effects of edible brown seaweeds. Trends Food Sci. Technol. 2011, 22, 315–326. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of chronic diseases. Mar. Drugs 2011, 9, 1056–1100. [Google Scholar] [CrossRef]
- Yone, Y.; Furuichi, M.; Urano, K. Effects of wakame Undaria pinnatifida and Ascophyllum nodosum on absorption of dietary nutrients, and blood sugar and plasma free amino-N levels of red sea bream. Bull. Jpn. Soc. Sci. Fish. 1985, 10, 1817–1819. [Google Scholar] [CrossRef]
- Hashim, R.; Azam, N.; Saat, M. The utilization of seaweed meals as binding agents in pelleted feeds for snakehead (Chalzna striatzcs) fry and their effects on growth. Aquaculture 1992, 108, 299–308. [Google Scholar] [CrossRef]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Ray, A.K. Applications of plant ingredients for tropical and subtropical freshwater finfish: Possibilities and challenges. Rev. Aquac. 2018, 11, 793–815. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Fumanal, M.; Sáez, M.I.; Martínez, T.F.; Moriñigo, M.A.; Fernández-díaz, C. Evaluation of Ulva ohnoi as functional dietary ingredient in juvenile senegalese sole (Solea senegalensi): Effects on the structure and functionality of the intestinal mucosa. Algal Res. 2019, 42, 101608. [Google Scholar] [CrossRef]
- Vigors, S.; O’Doherty, J.V.; Rattigan, R.; McDonnell, M.J.; Rajauria, G.; Sweeney, T. Effect of a Laminarin Rich Macroalgal Extract on the Caecal and Colonic Microbiota in the Post-Weaned Pig. Mar. Drugs 2020, 18, 157. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Mendes, S.I.; Varela, J.L.; Ruiz-Jarabo, I.; Rico, R.; Figueroa, F.L.; Abdala, R.; Moriñigo, M.Á.; Mancera, J.M.; Alarcón, F.J. Growth, tissue metabolites and digestive functionality in Sparus aurata juveniles fed different levels of macroalgae, Gracilaria cornea and Ulva rigida. Aquac. Res. 2015, 47, 3224–3238. [Google Scholar] [CrossRef]
- Ashour, M.; Mabrouk, M.M.; Ayoub, H.F.; El-feky, M.M.M.M.; Zaki, S.Z. Effect of dietary seaweed extract supplementation on growth, feed utilization, hematological indices, and non-specific immunity of Nile tilapia, Oreochromis niloticus challenged with Aeromonas hydrophila. J. Appl. Phycol. 2020, 32, 3467–3479. [Google Scholar] [CrossRef]
- Shi, Q.; Rong, H.; Hao, M.; Zhu, D.; Aweya, J.J.; Li, S. Effects of dietary Sargassum horneri on growth performance, serum biochemical parameters, hepatic antioxidant status, and immune responses of juvenile black sea bream Acanthopagrus schlegelii. J. Appl. Phycol. 2019, 31, 2103–2113. [Google Scholar] [CrossRef]
- Sajina, K.A.; Sahu, N.P.; Varghese, T.; Jain, K.K. Fucoidan-rich Sargassum wightii extract supplemented with α -amylase improve growth and immune responses of Labeo rohita (Hamilton, 1822) fingerlings. J. Appl. Phycol. 2019, 31, 2469–2480. [Google Scholar] [CrossRef]
- Sattanathan, G.; Palanisamy, T.; Padmapriya, S.; Anand, V.; Park, S.; Ho, I.; Balasubramanian, B. Influences of dietary inclusion of algae Chaetomorpha aerea enhanced growth performance, immunity, haematological response and disease resistance of Labeo rohita challenged with Aeromonas hydrophila. Aquac. Rep. 2020, 17, 100353. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Eissa, E.H.; Tawfik, W.A.; Elnabi, H.E.A.; Saadony, S.; Bazina, W.K.; Ahmed, R.A. Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol. Biochem. 2022, 48, 585–601. [Google Scholar] [CrossRef]
- de Oliveira, M.N.; Freitas, A.L.P.; Carvalho, A.F.U.; Sampaio, T.M.T.; Farias, D.F.; Teixeira, D.I.A.; Gouveia, S.T.; Pereira, J.G.; de Castro Catanho de Sena, M.M. Nutritive and non-nutritive attributes of washed-up seaweeds from the coast of Ceará, Brazil. Food Chem. 2009, 115, 254–259. [Google Scholar] [CrossRef]
- Sharawy, Z.; Ashour, M.; Abbas, E.M.; Ashry, O.A. Effects of dietary marine microalgae, Tetraselmis suecica, on production, gene expression, protein markers and bacterial count of Pacific white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 51, 2216–2228. [Google Scholar] [CrossRef]
- Moutinho, S.; Linares, F.; Rodríguez, J.L.; Sousa, V.; Valente, L.M.P. Inclusion of 10% seaweed meal in diets for juvenile and on-growing life stages of senegalese sole (Solea senegalensis). J. Appl. Phycol. 2018, 30, 3589–3601. [Google Scholar] [CrossRef]
- Queiroz, A.; Pereira, R.; Domingues, A. Effect of seaweed supplementation on growth performance, immune and oxidative stress responses in gilthead seabream (Sparus aurata). In Proceedings of the International Meeting on Marine Research, Lisbon, Portugal, 10–11 July 2014; pp. 2–4. [Google Scholar]
- Yu, Y.; Chen, W.; Liu, Y.; Niu, J.; Chen, M.; Tian, L. Effect of different dietary levels of Gracilaria lemaneiformis dry power on growth performance, haematological parameters and intestinal structure of juvenile pacific white shrimp (Litopenaeus vannamei). Aquaculture 2016, 450, 356–362. [Google Scholar] [CrossRef]
- Dworjanyn, S.A.; Pirozzi, I.; Liu, W. The effect of the addition of algae feeding stimulants to artificial diets for the sea urchin Tripneustes gratilla. Aquaculture 2007, 273, 624–633. [Google Scholar] [CrossRef]
- Tantikitti, C. Feed Palatability and the alternative protein sources in shrimp feed. Songklanakarin J. Sci. Technol. 2014, 36, 51–55. [Google Scholar]
- Al-souti, A.; Gallardo, W.; Claereboudt, M.; Mahgoub, O. Attractability and palatability of formulated diets incorporated with chicken feather and algal meals for juvenile gilthead seabream, Sparus aurata. Aquac. Rep. 2019, 14, 100199. [Google Scholar] [CrossRef]
- Bowker, J. Attractant Properties of Chemical Constituents of the Green Macroalga Ulva and Their Response Effects on the Commercially Important Sea Urchin Tripneustes gratilla. Bachelor’s Thesis, Department of Biological Sciences, University of Cape Town, Cape Town, South Africa, 2013; pp. 1–29. [Google Scholar]
- Van Alstyne, K.L.; Wolfe, G.V.; Freidenburg, T.L.; Neill, A.; Hicken, C. Activated defense systems in marine macroalgae: Evidence for an ecological role for DMSP cleavage. Mar. Ecol. Prog. Ser. 2001, 213, 53–65. [Google Scholar] [CrossRef]
- Rajauria, G. Seaweeds: A sustainable feed source for livestock and aquaculture. In Seaweed Sustainability; Tiwari, B.K., Troy, D.J., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 389–420. [Google Scholar] [CrossRef]
- Cruz, E.; Ricque, D.; Tapia, M.; Guajardo, C.; Obaldo, L.; Velasco, M.; Carrasco, A. Water Stability, Texture of Shrimp Feeds Formulated with Natural, Synthetic Binders; Global Seafood Alliance: Portsmouth, NH, USA, 2020. [Google Scholar]
- Moura, P.C.; Fernandes, J.M.; Diniz, M.S.; Fetter, V.; Vassilenko, V. Differentiation of the organoleptic volatile organic compound profile of three edible seaweeds. Metabolites 2023, 13, 713. [Google Scholar] [CrossRef]
- Marinho, G.; Nunes, C.; Sousa-Pinto, I.; Pereira, R.; Rema, P.; Valente, L.M.P. The IMTA-cultivated chlorophyta Ulva spp. as a sustainable ingredient in Nile tilapia (Oreochromis niloticus) diets. J. Appl. Phycol. 2013, 25, 1359–1367. [Google Scholar] [CrossRef]
- Pereira, R.; Valente, L.M.P.; Sousa-pinto, I.; Rema, P. Apparent nutrient digestibility of seaweeds by rainbow trout (Oncorhynchus mykiss) and Nile tilapia (Oreochromis niloticus). Algal Res. 2012, 1, 77–82. [Google Scholar] [CrossRef]
- Halver, J.E. Nutrient flow and retention. In Fish Nutrition, 3rd ed.; Academic Press: Cambridge, MA, USA, 2002; pp. 755–770. [Google Scholar]
- Montgomery, W.L.; Gerking, S.D. Marine macroalgae as foods for fishes: An evaluation of potential food quality. Environ. Biol. Fish. 1980, 5, 143–153. [Google Scholar] [CrossRef]
- Hidalgo, M.C.; Urea, E.; Sanz, A. Comparative study of digestive enzymes in fish with different nutritional habits. Proteolytic and amylase activities. Aquaculture 1999, 170, 267–283. [Google Scholar] [CrossRef]
- Mwendwa, R.; Wawire, M.; Kahenya, P. Effect of dietary supplementation with seaweed on growth and nutritional quality of Nile tilapia. J. Agric. Sci. Technol. 2023, 22, 100–116. [Google Scholar]
- Radwan, M.; El-sharkawy, M.A.; Negm, M.A.; Mohammadein, A. Dual effect of dietary seaweed of extract nanoparticles (GNS) with bionanocomposite cellulose acetate membranes (CA/Bio-AgNps) on growth performance and health status of Nile tilapia (Oreochromis niloticus): Specification on feed utilization, immune system, and antiparasitic action. Front. Mar. Sci. 2022, 9, 1008397. [Google Scholar] [CrossRef]
- Villa-Arce, M.Á.; Muñoz-Ochoa, M.; Hernández-Carmona, G.; Mendoza-Cruz, M.; Godínez-Pérez, C.A.; Vélez-Arellano, N. Formulated algae-based feed with low polyphenol content and its effect on the feeding preference of juvenile blue abalone Haliotis fulgens. J. Appl. Phycol. 2023, 35, 2485–2493. [Google Scholar] [CrossRef]
- Jeong, J.; Hwang, S.J.; Han, M.H.; Lee, D.; Yoo, J.S.; Choi, I. Fucoidan inhibits lipopolysaccharide-induced inflammatory responses in RAW 264.7 macrophages and zebrafish larvae. Mol. Cell. Toxicol. 2017, 13, 405–417. [Google Scholar] [CrossRef]
- El-boshy, M.; El-ashram, A.; Risha, E.; Abdelhamid, F.; Zahran, E.; Gab-alla, A. Dietary fucoidan enhance the non-specific immune response and disease resistance in African catfish, Clarias gariepinus immunosuppressed by cadmium chloride. Vet. Immunol. Immunopathol. 2014, 162, 168–173. [Google Scholar] [CrossRef]
- Sony, N.M.; Hossain, S.; Ishikawa, M.; Koshio, S.; Yokoyama, S. Efficacy of mozuku fucoidan in alternative protein-based diet to improve growth, health performance, and stress resistance of juvenile red seabream, Pagrus major. Fish Physiol. Biochem. 2020, 46, 2437–2455. [Google Scholar] [CrossRef]
- Traifalgar, R.F.; Kira, H.; Tung, H.T.; Raafat, F.; Michael; Laining, A.; Yokoyama, S.; Ishikawa, M.; Koshio, S. Influence of dietary fucoidan supplementation on growth and immunological response of juvenile Marsupenaeus japonicus. J. World Aquac. Soc. 2010, 41, 235–244. [Google Scholar] [CrossRef]
- Tuller, J.; De Santis, C.; Jerry, D.R. Dietary influence of fucoidan supplementation on growth of Lates calcarifer (Bloch). Aquac. Res. 2014, 45, 749–754. [Google Scholar] [CrossRef]
- Adel, M.; Hossein, A.; Dawood, M.A.O.; Karimi, B. Dietary Gracilaria persica mediated the growth performance, fillet colouration, and immune response of Persian sturgeon (Acipenser persicus). Aquaculture 2021, 530, 735950. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, A.F.; Ayoub, H.F.; Abd El-Gawad, E.A.; Abdelghany, M.F.; AbdelTawwab, M. Potential effects of dietary seaweeds mixture on the growth performance, antioxidant status, immunity response, and resistance of striped catfish (Pangasianodon hypophthalmus) against Aeromonas hydrophila infection. Fish. Shellfish Immunol. 2021, 119, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Antunes, M.; Neves, M.; Pires, D.; Passos, R.; do Carmo, B.; Tchobanov, C.F.; Forte, S.; Vaz, M.; Baptista, T.; Tecelão, C. Proximate Composition and Fatty Acid Profile of Gilthead Seabream (Sparus aurata) Fed with Pelvetia canaliculate Supplemented Diets: An Insight towards the Valorization of Seaweed Biomass. Foods 2023, 12, 1810. [Google Scholar] [CrossRef] [PubMed]
- Xuan, X.; Li, W.; Zhu, W.; Wang, S. Effects of different levels of macroalga Gracilaria lemaneiformis on growth performance and feed utilization on the red sea bream, Pagrosomus major. J. Appl. Phycol. 2019, 31, 3213–3222. [Google Scholar] [CrossRef]
- Zhongbao, L.I.; Huan, Y.; Jingbo, S. Growth performance, digestive enzyme activities and serum nonspecific immunity of the red tilapia (Oreochromis mossambicus × Oreochromis niloticus) fed diets supplemented with ultrafi Ne powder of Enteromopha prolifera. J. Oceanol. Limnol. 2018, 36, 1843–1850. [Google Scholar]
- Younis, E.M.; Al-quffail, A.S.; Al-asgah, N.A.; Al-hafedh, Y.S. Effect of dietary fish meal replacement by red algae, Gracilaria arcuata, on growth performance and body composition of Nile tilapia Oreochromis niloticus. Saudi J. Biol. Sci. 2017. [Google Scholar] [CrossRef]
- Morshedi, V.; Bahabadi, M.N.; Sotoudeh, E.; Azodi, M.; Hafezieh, M. Nutritional evaluation of Gracilaria pulvinata as partial substitute with fish meal in practical diets of barramundi (Lates calcarifer). J. Appl. Phycol. 2018, 30, 619–628. [Google Scholar] [CrossRef]
- Lobo, G.; Pereira, L.F.; Gonçalves, J.F.M.; Peixoto, M.J.; Ozo´rio, R.O.A. Effect of dietary seaweed supplementation on growth performance, antioxidant and immune responses in European seabass (Dicentrarchus labrax) subjected to rearing temperature and salinity oscillations. Int. Aquat. Res. 2018, 10, 321–331. [Google Scholar] [CrossRef]
- Madibana, M.; Mlambo, V.; Lewis, B.; Fouché, C. Effect of graded levels of dietary seaweed (Ulva sp.) on growth, hematological and serum biochemical parameters in dusky kob, Argyrosomus japonicus, Sciaenidae. Egypt. J. Aquat. Res. 2017, 43, 249–254. [Google Scholar] [CrossRef]
- Shpigel, M.; Guttman, L.; Shauli, L.; Odintsov, V.; Harpaz, S. Ulva lactuca from an integrated multi-trophic aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus aurata) diet. Aquaculture 2017, 481, 112–118. [Google Scholar] [CrossRef]
- Sotoudeh, E.; Jafari, M. Effects of dietary supplementation with red seaweed, Gracilaria pygmae on growth, carcass composition and hematology of juvenile rainbow trout, Oncorhynchus mykiss. Aquac. Int. 2017, 25, 1857–1867. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Salas-leitón, E.; Brito, F.; Pereira, L.F.; Svendsen, J.C.; Baptista, T.; Pereira, R.; Abreu, H.; Reis, P.A.; Fernando, J.; et al. Effects of dietary Gracilaria sp. and Alaria sp. supplementation on growth performance, metabolic rates and health in meagre (Argyrosomus regius) subjected to pathogen infection. J. Appl. Phycol. 2017, 29, 433–447. [Google Scholar] [CrossRef]
- Zhu, D.; Wen, X.; Li, S.; Xuan, X.; Li, Y. Evaluation of the red alga Gracilaria lemaneiformis and brown alga Sargassum horneri as ingredients in diets for white spotted snapper Lutjanus stellatus akazaki juveniles. J. Appl. Phycol. 2017, 29, 3211–3219. [Google Scholar] [CrossRef]
- Hussein, E.E.M. Effect of seaweed supplemented diets on Nile tilapia, Oreochromis niloticus performance. Int. J. Fish. Aquat. Stud. 2017, 5, 205–210. [Google Scholar]
- Wan, A.H.L.; Soler-vila, A.; Keeffe, D.O.; Casburn, P.; Fitzgerald, R.; Johnson, M.P. The inclusion of Palmaria palmata macroalgae in Atlantic salmon (Salmo salar) diets: Effects on growth, haematology, immunity and liver function. J. Appl. Phycol. 2016, 28, 3091–3100. [Google Scholar] [CrossRef]
- Zhu, D.; Wen, X.; Xuan, X. The green alga Ulva lactuca as a potential ingredient in diets for juvenile white spotted snapper Lutjanus stellatus akazaki. J. Appl. Phycol. 2016, 28, 703–711. [Google Scholar] [CrossRef]
- Zeraatpisheh, F.; Firouzbakhsh, F.; Khalili, K.J. Effects of the macroalga Sargassum angustifolium hot water extract on hematological parameters and immune responses in rainbow trout (Oncohrynchus mykiss) infected with Yersinia rukeri. J. Appl. Phycol. 2018, 30, 2029–2037. [Google Scholar] [CrossRef]
- Peixoto, M.J.; Magnoni, L.; Gonçalves, J.F.M.; Twijnstra, R.H.; Kijjoa, A.; Pereira, R.; Palstra, A.P.; Ozório, R.O.A. Effects of dietary supplementation of Gracilaria sp. extracts on fillet quality, oxidative stress, and immune responses in European seabass (Dicentrarchus labrax). J. Appl. Phycol. 2019, 31, 761–770. [Google Scholar] [CrossRef]
- Abdelrhman, A.M.; Ashour, M.; Al-zahaby, M.A.; Sharawy, Z.Z.; Nazmi, H.; Zaki, M.A.A.; Ahmed, N.H.; Ahmed, S.R.; El-haroun, E.; Van Doan, H.; et al. Effect of polysaccharides derived from brown macroalgae Sargassum dentifolium on growth performance, serum biochemical, digestive histology and enzyme activity of hybrid red tilapia. Aquac. Rep. 2022, 25, 101212. [Google Scholar] [CrossRef]
- Ferreira, M.; Larsen, B.K.; Granby, K.; Cunha, S.C.; Fernandes, J.O.; Nunes, M.L.; Marques, A.; Dias, J.; Castro, L.F.C.; Valente, L.M.P. Diets supplemented with Saccharina latissima influence the expression of genes related to lipid metabolism and oxidative stress modulating rainbow trout (Oncorhynchus mykiss) fillet composition. Food Chem. Toxicol. 2020, 140, 111332. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Li, X.; You, C.; Wang, S.; Li, Y. Supplementation of macroalgae together with non-starch polysaccharide-degrading enzymes in diets enhanced growth performance, innate immune indexes, and disease resistance against Vibrio parahaemolyticus in rabbitfish Siganus canaliculatus. J. Appl. Phycol. 2019, 31, 2071–2083. [Google Scholar] [CrossRef]
- Valente, L.M.P.; Araújo, M.; Batista, S.; Peixoto, M.J.; Sousa-pinto, I.; Brotas, V.; Cunha, L.M.; Rema, P. Carotenoid deposition, flesh quality and immunological response of Nile tilapia fed increasing levels of IMTA-cultivated Ulva spp. J. Appl. Phycol. 2016, 28, 691–701. [Google Scholar] [CrossRef]
- Rajendran, P.; Subramani, P.A.; Michael, D. Polysaccharides from marine macroalga, Padina gymnospora improve the nonspecific and specific immune responses of Cyprinus carpio and protect it from different pathogens. Fish Shellfish Immunol. 2016, 58, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Siddik, M.A.B.; Rahman, M.M.; Anh, N.T.N.; Nevejan, N.; Bossier, P. Seaweed, Enteromorpha intestinalis, as a diet for Nile tilapia Oreochromis niloticus fry. J. Appl. Aquac. 2015, 27, 113–123. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, K.; Han, H.; Nam, T.; Lee, B. Dietary Hizikia fusiformis glycoprotein-induced IGF-I and IGFBP-3 associated to somatic growth, polyunsaturated fatty acid metabolism, and immunity in juvenile olive flounder Paralichthys olivaceus. Comp. Biochem. Physiol. Part A 2014, 167, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, S.; Khosravi, S.; Rahimnejad, S.; Lee, K. Evaluation of Sargassum fusiforme and Ecklonia cava as dietary additives for olive flounder (Paralichthys olivaceus). Turk. J. Fish. Aquat. Sci. 2014, 14, 321–330. [Google Scholar] [CrossRef]
- Ragaza, J.A.R.; Amauag, R.E.M.; Oshio, S.K. Comparative effects of dietary supplementation levels of Eucheuma denticulatum and Sargassum fulvellum in diet of juvenile japanese flounder Paralichthys olivaceus. Aquac. Sci. 2013, 61, 27–37. [Google Scholar]
- Kanimozhi, S.; Krishnaveni, M.; Deivasigmani, B.; Rajasekar, T.; Priyadarshni, P. Immunomo-stimulation effects of Sargassum whitti on Mugil cephalus against Pseudomonas fluorescence. Int. J. Curr. Microbiol. App. Sci. 2013, 2, 93–103. [Google Scholar]
- Xu, S.; Zhang, L.; Wu, Q.; Liu, X. Evaluation of dried seaweed Gracilaria lemaneiformis as an ingredient in diets for teleost fish Siganus canaliculatus. Aquac. Int. 2011, 19, 1007–1018. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K. Effects of dietary kelp (Ecklonia cava) on growth and innate immunity in juvenile olive flounder Paralichthys olivaceus (Temminck et Schlege). Aquac. Res. 2008, 39, 1687–1690. [Google Scholar] [CrossRef]
- Cheng, A.; Tu, C.; Chen, Y.; Nan, F.; Chen, J. The immunostimulatory effects of sodium alginate and iota-carrageenan on orange-spotted grouper Epinephelus coicoides and its resistance against Vibrio alginolyticus. Fish Shellfish Immunol. 2007, 22, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Pham, M.A.; Lee, K.; Lee, B.; Lim, S.; Kim, S.; Lee, Y.; Heo, M.; Lee, K. Effects of dietary Hizikia fusiformis on growth and immune responses in juvenile olive flounder (Paralichthys olivaceus). Asian-Aust. J. Anim. Sci. 2006, 19, 1769–1775. [Google Scholar] [CrossRef]
- Wassef, E.A.; El-sayed, A. Evaluation of Pterocladia (Rhodophyta) and Ulva (Chlorophyta) meals as additives to gilthead sea bream Sparus aurata diets. Egypt. J. Aquat. Res. 2005, 31, 321–332. [Google Scholar]
- Stentiford, G.D.; Sritunyalucksana, K.; Flegel, T.W.; Bryony, A.; Williams, P.; Withyachumnarnkul, B.; Itsathitphaisarn, O.; Bass, D. New paradigms to help solve the global aquaculture disease crisis. PLoS Pathog. 2017, 13, e1006160. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Baquero, F.; Martı´nez, J.-L.; Cantón, R. Antibiotics and antibiotic resistance in water environments. Curr. Opin. Biotechnol. 2008, 19, 260–265. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2020, 12, 640–663. [Google Scholar] [CrossRef]
- Done, H.Y.; Venkatesan, A.K.; Halden, R.U. Does the recent growth of aquaculture create antibiotic resistance threats different from those associated with land animal production in agriculture? AAPS J. 2015, 17, 513–524. [Google Scholar] [CrossRef]
- Mendonça, A.; VT, R.; Monserrat, J.; Romano, L.; Tesser, M. The inclusion of algae Gracilaria domingensis in the diet of mullet juveniles (Mugil liza) improves the immune response. J. Appl. Aquac. 2019, 31, 210–223. [Google Scholar] [CrossRef]
- Abo-Raya, M.H.; Alshehri, K.M.; Abdelhameed, R.F.A.; Elbialy, Z.I.; Elhady, S.S.; Mohamed, R.A. Assessment of growth- related parameters and immune-biochemical profile of Nile tilapia (Oreochromis niloticus) fed dietary Ulva fasciata extract. Aquac. Res. 2021, 52, 3233–3246. [Google Scholar] [CrossRef]
- Zhao, W.; Liu, H.F.Z.; Zhang, J.C.C.; Niu, B.G.J. Responses in growth performance, enzymatic activity, immune function and liver health after dietary supplementation of Porphyridium sp. in juvenile golden pompano (Trachinotus ovatus). Aquac. Nutr. 2021, 27, 679–690. [Google Scholar] [CrossRef]
- Rodrigues, M.V.; Zanuzzo, F.S.; De Oliveira, C.A.F.; Sima, P.; Vetvicka, V. Development of fish immunity and the role of β-glucan in immune responses. Molecules 2020, 25, 5378. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Hu, W.; Wang, L.; Qiao, H.; Wu, H.; Xu, Z. Effects of dietary supplementation with Sargassum horneri meal on growth performance, body composition, and immune response of juvenile turbot. J. Appl. Phycol. 2019, 31, 771–778. [Google Scholar] [CrossRef]
- Maldonado-Miranda, J.J.; Castillo-Pérez, L.J.; Ponce-Hernández, A.; Carranza-Álvarez, C. Summary of economic losses due to bacterial pathogens in aquaculture industry. In Bacterial Fish Diseases; Academic Press: Cambridge, MA, USA, 2022; Volume 1, pp. 399–417. [Google Scholar]
- Yeganeh, S.; Adel, M. Effects of dietary algae (Sargassum ilicifolium) as immunomodulator and growth promoter of juvenile great sturgeon (Huso huso Linnaeus, 1758). J. Appl. Phycol. 2019, 31, 2093–2102. [Google Scholar] [CrossRef]
- Fumanal, M.; Di Zeo, D.E.; Anguís, V.; Fernández-diaz, C.; Alarcón, J.; Piñera, R.; Albaladejo-riad, N.; Esteban, M.A.; Miguel, A.; Balebona, M.C. Inclusion of dietary Ulva ohnoi 5% modulates Solea senegalensis immune response during Photobacterium damselae subsp. piscicida infection. Fish Shellfish Immunol. 2020, 100, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Wen, C.M.; Nan, F.H.; Yeh, H.Y.; Lee, M.C. Immunomodulatory effects of Sarcodia suiae water extracts on Nile tilapia Oreochromis niloticus and its resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2020, 103, 159–168. [Google Scholar] [CrossRef]
- Leonard, S.G.; Sweeney, T.; Bahar, B.; Lynch, B.P.; Doherty, J.V.O. Effects of dietary seaweed extract supplementation in sows and post-weaned pigs on performance, intestinal morphology, intestinal microflora and immune status. Br. J. Nutr. 2011, 106, 688–699. [Google Scholar] [CrossRef]
- Narasimhan, M.K.; Pavithra, S.K.; Krishnan, V. In vitro analysis of antioxidant, antimicrobial and antiproliferative activity of Enteromorpha antenna, Enteromorpha linza and Gracilaria corticata extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 151–159. [Google Scholar] [CrossRef]
- Devi, G.K.; Manivannan, K.; Thirumaran, G.; Rajathi, A.A.; Anantharaman, P. In vitro antioxidant activities of selected seaweeds from southeast coast of India. Asian Pac. J. Trop. Med. 2011, 4, 205–211. [Google Scholar] [CrossRef]
- Martínez-páramo, S.; Diogo, P.; Dinis, M.T.; Soares, F.; Sarasquete, C.; Cabrita, E. Effect of two sulfur-containing amino acids, taurine and hypotaurine in European sea bass (Dicentrarchus labrax) sperm cryopreservation. Cryobiology 2013, 66, 333–338. [Google Scholar] [CrossRef]
- Kannan, G.; Saker, K.E.; Terrill, T.H.; Kouakou, B.; Galipalli, S.; Gelaye, S. Effect of seaweed extract supplementation in goats exposed to simulated preslaughter stress. Small Rumin. Res. 2007, 73, 221–227. [Google Scholar] [CrossRef]
- Makkar, H.P.; Tran, G.; Heuzé, V.; Giger-Reverdin, S.; Lessire, M.; Lebas, F.; Ankers, P. Seaweeds for livestock diets: A review. Anim. Feed Sci. Technol. 2015, 212, 1–17. [Google Scholar] [CrossRef]
- Montalban-Arques, A.; De Schryver, P.; Bossier, P.; Gorkiewicz, G.; Mulero, V.; Gatlin, D.M.; Galindo-Villegas, J. Selective manipulation of the gut microbiota improves immune status in vertebrates. Front. Immunol. 2015, 6, 512. [Google Scholar] [CrossRef]
- Gisbert, E.; Ortiz-Delgado, J.B.; Sarasquete, C. Nutritional cellular biomarkers in early life stages of fish. Histol. Histopathol. 2008, 23, 1525–1539. [Google Scholar]
- Zeynali, M.; Bahabadi, M.; Morshedi, V.; Qasemi, A.; Torfi Mozanzadeh, M. Effects of partial replacement of macroalgae (Sargassum ilicifolium) with fish meal on intestinal tissue structure in Asian seabass (Lates calcarifer). Vet. Res. Biol. Prod. 2021, 135, 110–119. [Google Scholar] [CrossRef]
- Rombout, J.H.W.M.; Abelli, L.; Picchietti, S.; Scapigliati, G.; Kiron, V. Teleost intestinal immunology. Fish Shellfish Immunol. 2011, 31, 616–626. [Google Scholar] [CrossRef]
- Abdel-mawla, M.S.; Magouz, F.I.; Khalafalla, M.M.; Amer, A.A.; Soliman, A.A.; Zaineldin, A.I.; Gewaily, M.S.; Dawood, M.A.O. Growth performance, intestinal morphology, blood biomarkers, and immune response of Thinlip grey mullet (Liza ramada) fed dietary laminarin supplement. J. Appl. Phycol. 2023, 35, 1801–1811. [Google Scholar] [CrossRef]
- Passos, R.; Patrícia, A.; Ferreira, I.; Pires, P.; Pires, D.; Gomes, E.; Santos, P.; Simões, M.; Afonso, C. Effect on health status and pathogen resistance of gilthead seabream (Sparus aurata) fed with diets supplemented with Gracilaria gracilis. Aquaculture 2021, 531, 735888. [Google Scholar] [CrossRef]
- Batista, S.; Pereira, R.; Oliveira, B.; Baião, L.F.; Jessen, F.; Tulli, F.; Messina, M.; Silva, J.L.; Abreu, H.; Valente, L.M.P. Exploring the potential of seaweed Gracilaria gracilis and microalga Nannochloropsis oceanica, single or blended, as natural dietary ingredients for European seabass Dicentrarchus labrax. J. Appl. Phycol. 2020, 32, 2041–2059. [Google Scholar] [CrossRef]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef]
- Niu, J.; Chen, X.; Lu, X.; Jiang, S.; Lin, H.; Liu, Y.; Huang, Z.; Wang, J.; Wang, Y.; Tian, L. Effects of different levels of dietary wakame (Undaria pinnatifida) on growth, immunity and intestinal structure of juvenile Penaeus monodon. Aquaculture 2015, 435, 78–85. [Google Scholar] [CrossRef]
- Silva, D.M.; Valente, L.M.P.; Pereira, R.; Pires, M.A.; Seixas, F.; Rema, P. Evaluation of IMTA-produced seaweeds (Gracilaria, Porphyra, and Ulva) as dietary ingredients in Nile tilapia, Oreochromis niloticus L., juveniles. Effects on growth performance and gut histology. J. Appl. Phycol. 2015, 27, 1671–1680. [Google Scholar] [CrossRef]
- Abdel-warith, A.A.; Younis, E.M.I.; Al-asgah, N.A. Potential use of green macroalgae Ulva lactuca as a feed supplement in diets on growth performance, feed utilization and body composition of the African catfish, Clarias gariepinus. Saudi J. Biol. Sci. 2016, 23, 404–409. [Google Scholar] [CrossRef]
- Mota, C.S.C.; Pinto, O.; Sá, T.; Ferreira, M.; Delerue-matos, C.; Cabrita, A.R.J.; Almeida, A.; Abreu, H.; Silva, J.; Fonseca, A.J.M.; et al. A commercial blend of macroalgae and microalgae promotes digestibility, growth performance, and muscle nutritional value of European seabass (Dicentrarchus labrax L.) juveniles. Front. Nutr. 2023, 10, 1165343. [Google Scholar] [CrossRef]
- Cui, H.; Wang, Z.; Liu, J.; Wang, Y.; Wang, Z.; Fu, J.; Wan, Z.; Li, R.; Li, Q.; Helen, J. Effects of a highly purified fucoidan from Undaria pinnatifida on growth performance and intestine health status of Gibel carp Carassius auratus gibelio. Aquac. Nutr. 2020, 26, 47–59. [Google Scholar] [CrossRef]
- Nordvi, M.F.; Løvmo, S.D.; Bringslid, H.I.; Whatmore, P.; Sundh, H.; Reitan, K.I.; Aachmann, F.L.; Olsen, R.E. Fucoidan from Undaria pinnatifida mitigates intestinal inflammation in Atlantic salmon (Salmo salar). Aquaculture 2023, 575, 739777. [Google Scholar] [CrossRef]
- Mahgoub, H.A.; El-adl, M.A.M.; Ghanem, H.M.; Martyniuk, C.J. The effect of fucoidan or potassium permanganate on growth performance, intestinal pathology, and antioxidant status in Nile tilapia (Oreochromis niloticus). Fish Physiol. Biochem. 2020, 46, 2109–2131. [Google Scholar] [CrossRef]
- Van Vo, B.; Siddik, M.A.; Fotedar, R.; Chaklader, R.; Hanif, A.; Foysal, J.; Nguyen, H.Q. Progressive replacement of fishmeal by raw and enzyme-treated alga, Spirulina platensis influences growth, intestinal micromorphology and stress response in juvenile barramundi, Lates calcarifer. Aquaculture 2020, 529, 735741. [Google Scholar] [CrossRef]
- Pires, D.; Passos, R.; Carmo, B.; Tchobanov, C.F.; Forte, S.; Vaz, M.; Antunes, M.; Neves, M.; Tecel, C.; Baptista, T. Pelvetia canaliculata as an aquafeed supplement for gilthead seabream Sparus aurata: A biorefinery approach for seaweed biomass valorisation. Sustainability 2022, 14, 11469. [Google Scholar] [CrossRef]
- Guerreiro, I.; Magalhães, R.; Coutinho, F.; Couto, A.; Sousa, S.; Delerue-matos, C. Evaluation of the seaweeds Chondrus crispus and Ulva lactuca as functional ingredients in gilthead seabream (Sparus aurata). J. Appl. Phycol. 2019, 31, 2115–2124. [Google Scholar] [CrossRef]
- Cerezo, I.M.; Fumanal, M.; Tapia-paniagua, S.T.; Bautista, R.; Anguís, V.; Fernández-díaz, C.; Alarcón, F.J.; Moriñigo, M.A.; Balebona, M.C.; Xavier, R. Solea senegalensis bacterial intestinal microbiota is affected by low dietary inclusion of Ulva ohnoi diet composition and preparation. Front. Microbiol. 2022, 12, 801744. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, M.; Abdelha, Y.; Abreu, H.; Silva, J.; Valente, L.M.P.; Kiron, V. Gracilaria gracilis and Nannochloropsis oceanica, singly or in combination, in diets alter the intestinal microbiota of European seabass (Dicentrarchus labrax). Front. Mar. Sci. 2022, 9, 1001942. [Google Scholar] [CrossRef]
- Tapia-paniagua, S.T.; Fumanal, M.; Anguís, V.; Fernández-díaz, C.; Alarcón, F.J.; Moriñigo, M.A. Modulation of intestinal microbiota in Solea senegalensis fed low dietary level of Ulva ohnoi. Front. Microbiol. 2019, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.T.; Simões, M.; Costa, C.; Passos, R.; Baptista, T. Modulatory effect of Gracilaria gracilis on European seabass gut microbiota community and its functionality. Sci. Rep. 2022, 12, 14836. [Google Scholar] [CrossRef] [PubMed]
- Abdala-, R.T.; García-, D.J.; Rosa, M.; Rico, M.; Gómez-Pinchetti, J.L.; Juan, P.; Mancera, M.; Figueroa, F.L.; Javier, F.; Eduardo, A.; et al. Effects of a short pulse administration of Ulva rigida on innate immune response and intestinal microbiota in Sparus aurata juveniles. Aquac. Res. 2021, 52, 3038–3051. [Google Scholar] [CrossRef]
- Yazdanpanah, M.; Sotoudeh, E.; Mansouri Taee, H.; Habibi, H. Dietary administration of Sargassum angustifolium and Gracilaria pulvinata extracts affect antioxidant enzyme activities and Lactobacillus bacterial population in intestine of rainbow trout (Oncorhynchus mykiss) fry. Iran. J. Fish. Sci. 2021, 20, 926–944. [Google Scholar] [CrossRef]
- Silva-brito, F.; Alexandrino, D.A.M.; Jia, Z.; Mo, Y.; Kijjoa, A.; Abreu, H.; Carvalho, M.F.; Oz, R. Fish performance, intestinal bacterial community, digestive function and skin and fillet attributes during cold storage of gilthead seabream (Sparus aurata) fed diets supplemented with Gracilaria by-products. Aquaculture 2021, 541, 736808. [Google Scholar] [CrossRef]
- Keating, C.; Hinchcliffe, J.; Davies, R.; Whelan, S.; Wan, A.H.L.; Fitzgerald, R.D. Temporal changes in the gut microbiota in farmed Atlantic cod (Gadus morhua) outweigh the response to diet supplementation with macroalgae. Anim. Microbiome 2021, 3, 7. [Google Scholar] [CrossRef]
- Thepot, V.; Slinger, J.; Paul, N.A. Influence of seaweed supplements on the intestinal bacteria in the rabbit fish Siganus fuscescens: Evidence for a core microbiome. Res. Sq. 2020, 1–21. [Google Scholar] [CrossRef]
- Gupta, S.; Jep, L.; Abdelhafiz, Y.A.; Siriyappagouder, P.; Sørensen, M.; Fernandes, J.M.; Kiron, V. Macroalga-derived alginate oiligosaccharide alters intestinal bacteria of Atlantic salmon. Front. Microbiol. 2019, 10, 2037. [Google Scholar] [CrossRef] [PubMed]
- Xinxu, Z.; Huijuan, W.U.; Zhongzhen, L.I. Effects of dietary supplementation of Ulva pertusa and non- starch polysaccharide enzymes on gut microbiota of Siganus canaliculatus. J. Oceanol. Limnol. 2018, 36, 438–449. [Google Scholar]
- Rico, R.M.; Tejedor-Junco, M.T.; Tapia-Paniagua, S.T.; Alarcón, F.J.; Mancera, J.M.; López-Figueroa, F.; Balebona, M.C.; Abdala-Díaz, R.T.; Moriñigo, M.A. Influence of the dietary inclusion of Gracilaria cornea and Ulva rigida on the biodiversity of the intestinal microbiota of Sparus aurata juveniles. Aquac. Int. 2016, 24, 965–984. [Google Scholar] [CrossRef]
- Diwan, A.D.; Harke, S.N.; Archana, G. Aquaculture industry prospective from gut microbiome of fish and shellfish: An overview. Anim. Physiol. Anim. Nutr. 2021, 106, 441–469. [Google Scholar] [CrossRef] [PubMed]
- Llewellyn, M.S.; Boutin, S.; Hoseinifar, S.H.; Derome, N.; Biron, D.G.; Romero, J.; De, U. Teleost microbiomes: The state of the art in their characterization, manipulation and importance in aquaculture and fisheries. Front. Microbiol. 2014, 5, 207. [Google Scholar] [CrossRef] [PubMed]
- Egerton, S.; Culloty, S.; Whooley, J.; Stanton, C.; Ross, R.P.; Ross, R.P. The gut microbiota of marine fish. Front. Microbiol. 2018, 9, 873. [Google Scholar] [CrossRef] [PubMed]
- Eichmiller, J.J.; Hamilton, M.J.; Staley, C.; Sadowsky, M.J.; Sorensen, P.W. Environment shapes the fecal microbiome of invasive carp species. Microbiome 2016, 4, 44. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.T.; Gallardo-Escárate, C. Microbiome dynamic modulation through functional diets based on pre- and probiotics (Mannan-oligosaccharides and Saccharomyces cerevisiae) in juvenile rainbow trout (Oncorhynchus mykiss). J. Appl. Microbiol. 2017, 122, 1333–1347. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Koshio, S. Application of fermentation strategy in aquafeed for sustainable aquaculture. Rev. Aquac. 2020, 12, 987–1002. [Google Scholar] [CrossRef]
- Wilczynski, W.; Radlinska, M.; Wysujack, K.; Czub, M.; Brzeziński, T.; Kowalczyk, G.; Bełdowski, J.; Nogueira, P.; Maszczyk, P. Metagenomic analysis of the gastrointestinal microbiota of Gadus morhua callarias L. originating from a chemical munition dump site. Toxics 2022, 10, 206. [Google Scholar] [CrossRef]
- Chiheb, I.; Hassane, R.; José, M.; Seglar, D.; Francisco, J. Screening of antibacterial activity in marine green and brown macroalgae from the coast of Morocco. Afr. J. Biotechnol. 2010, 8, 1258–1262. [Google Scholar]
- Silva, M.; Vieira, L.; Almeida, A.P.; Kijjoa, A. The marine macroalgae of the genus Ulva: Chemistry, biological activities and potential applications. Oceanography 2013, 1, 101. [Google Scholar] [CrossRef]
- Kong, Y.; Teather, R.; Forster, R. Composition, spatial distribution, and diversity of the bacterial communities in the rumen of cows fed different forages. FEMS Microbiol. Ecol. 2010, 74, 612–622. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lahti, L.; Saloja, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Hehre, E.J.; Meeuwig, J.J. A global analysis of the relationship between farmed seaweed production and herbivorous fish catch. PLoS ONE 2016, 11, e0148250. [Google Scholar] [CrossRef]
- Xu, J.; Liao, W.; Liu, Y.; Guo, Y.; Jiang, S.; Zhao, C. An overview on the nutritional and bioactive components of green seaweeds. Food Prod. Process. Nutr. 2023, 5, 18. [Google Scholar] [CrossRef]
- López-vivas, J.M.; Riosmena-rodríguez, R.; Alfredo, A.; De Llave, J.; Pacheco-ruíz, I.; Yarish, C. Growth and reproductive responses of the conchocelis phase of Pyropia hollenbergii (Bangiales, Rhodophyta) to light and temperature. J. Appl. Phycol. 2015, 27, 1561–1570. [Google Scholar] [CrossRef]
- Loureiro, R.R.; Hurtado, A.Q.; Critchley, A.T. Impacts of AMPEP on epiphytes and diseases in kappaphycus and eucheuma cultivation. In Tropical Seaweed Farming Trends, Problems and Opportunities: Developments in Applied Phycology; Springer: Cham, Switzerland, 2017; pp. 111–119. ISBN 9783319634982. [Google Scholar]
- Mahrose, K.M.; Michalak, I. Seaweeds for animal feed, current status, challenges, and opportunities. In Sustainable Global Resources of Seaweeds; Ranga Rao, A., Ravishankar, G.A., Eds.; Springer: Cham, Switzerland, 2022; Volume 1. [Google Scholar] [CrossRef]
- Filippini, M.; Baldisserotto, A.; Menotta, S.; Fedrizzi, G.; Rubini, S.; Gigliotti, D.; Valpiani, G.; Buzzi, R.; Manfredini, S.; Vertuani, S. Heavy metals and potential risks in edible seaweed on the market in Italy. Chemosphere 2021, 263, 127983. [Google Scholar] [CrossRef]






| Seaweed Species | Tested Solvent | Bioactive Compounds | Concentration | Reference |
|---|---|---|---|---|
| Red seaweed (Rhodophyta) | ||||
| Porphyra umbilicalis | Methanol | TPC | 5.53 g GAE/100 g DW | [63] |
| P. umbilicalis | 90% acetone | Carotenoid Chl a | 1.88 µg/g WW | [64] |
| Jania rubens | 38.41 µg/mL WW | |||
| Hypnea musciformis | Ethyl acetate | TPC | 205.5 mg GAE/g DW | [65] |
| Gracilaria edulis | Aqueous fraction | TPC | 1704.69 µg GAE/g DW | [66] |
| TFC | 786.95 µg QE/g DW | |||
| Alkaloids | 522.34 µg PEG/g DW | |||
| Gracilaria tenuistipitata | Methanol | TPC | 68.20 mg GAE/g DW | [67] |
| TFC | 36.17 mg QE/g DW | |||
| Acanthophora spicifera | Ethyl acetate | TPC | 40.583 µg/mg DW | [68] |
| Green seaweed (Chlorophyta) | ||||
| Ulva lactuca | Water | TPC | 4.60 mg/g DW | [69] |
| TChl | 21.27 mg/g DW | |||
| Carotenoids | 12.73 mg/g DW | |||
| Ulva lactuca | Methanol | Fucoxanthin | 7.53 mg/g DW | [70] |
| Ulva intestinalis | Dichloromethane | TPC | 197.6 mg GAE/g extract | [71] |
| Caulerpa lentillifera | Methanol | TPC | 42.85 mg PGE/g DW | [72] |
| Brown seaweed (Phaeophyceae) | ||||
| Undaria pinnatifida | Methanol | TPC | 4.46 g GAE/100 g DW | [63] |
| Saccharina japonica | Distilled water | Carotenoids | 2.391 mg/g DW | [73] |
| Halopteris scoparia | Methanol | TPC | 328.7 mg GAE/100 g DW | [55] |
| Ethanol | 123.2 mg GAE/100 g DW | |||
| Water | 328.7 mg GAE/100 g DW | |||
| Sargassum sp. | Acetone | TPC | 14.6 mg GAE/g DW | [74] |
| TFC | 0.67 mg QE/g DW | |||
| Ecklonia radiata | Methanol | TPC | 12.19 mg GAE/g DW | [74] |
| TFC | 11.15 mg QE/g DW | |||
| Himanthalia elongata | Methanol | Polyphenol | 23.47 g GAE/100 g DW | [63] |
| Ascophyllum nodosum | Water | Fucoxanthin | 172–660 mg/kg DW | [75] |
| Himanthalia elongate | 60% methanol | TPC | 286.0 mg GAE/g DW | [76] |
| TFC | 109.8 mg QE/g DW | |||
| Seaweed Group | Genus | Species |
|---|---|---|
| Red seaweed (Rhodophyta) | Chondrus | C. crispus |
| Kappaphycopsis (formerly Eucheuma) | E. cottonii | |
| E. denticulatum | ||
| Palmaria | Palmaria palmata | |
| Gracilaria | G. edulis (formerly G. lichenoides) | |
| G. heteroclada | ||
| G. lichenoides | ||
| G. cornea | ||
| G. crassa | ||
| G. gracilis | ||
| G. persica | ||
| G. vermiculophylla | ||
| G. pulvinata | ||
| Porphyra | P. purpurea | |
| Pyropia yezoensis (Porphyra yezoensis) | ||
| Gracilariopsis | G. persica | |
| G. lemaneiformis | ||
| Brown seaweed (Phaeophyceae) | Sargassum | S. fusiforme |
| S. portieranum | ||
| S. aquifolium | ||
| S. horneri | ||
| S. boveanum | ||
| S. angustifolium | ||
| S. dentifolium | ||
| S. fulvellum | ||
| Saccharina (formerly Laminaria) | S. japonica | |
| S. latissimi | ||
| Ascophyllum | A. nodosum | |
| Saccharina | S. japonica | |
| S. latissimi | ||
| Undaria | U. pinnatifida | |
| Cladosiphon | C. okamuranus | |
| Padina | P. gymnospora | |
| P. pavonica | ||
| Macrocystis | M. pyrifera | |
| Green seaweed (Chlorophyta) | Ulva | U. lactuca |
| U. rigida | ||
| U. fascita | ||
| U. reticulata | ||
| U. autralis (formerly U. pertusa) | ||
| U. prolifera | ||
| U. intestinalis | ||
| U. ohnoi | ||
| Capsosiphon | C. fulvescens | |
| Codium | C. fragile | |
| Monostroma | M. nitidum | |
| Caulerpa | C. lentillifera |
| Seaweed and Derivatives | Fish Species | Applied Levels | Effective Level | Response | Reference |
|---|---|---|---|---|---|
| Ulva sp. (Chlorophyta), Gracilaria gracilis (Rhodophyta) | D. labrax | 2 and 4% | - | SW-blend-supplemented diet enhanced anterior intestinal absorption area by up to 45% | [189] |
| Fucoidan from Undaria pinnatifida (Phaeophyceae) | Carassius auratus gibelio | 0.1, 1.0 and 3.0% | 3.0% | Increased intestinal digestive enzyme activity, thereby enhancing intestinal microbial communities at a level of 3% dietary supplementation | [190] |
| Fucoidan from Undaria pinnatifida (Phaeophyceae) | Salmo salar | 1 and 3% | - | Fucoidan positively improved intestinal integrity and immune response | [191] |
| Fucoidan from Saccharina japonica (Phaeophyceae) | O. niloticus | 0.1, 0.2, 0.4, and 0.8% | - | Fucoidan in fish diets improved intestinal health and antioxidant status | [192] |
| Sargassum dentifolium (Phaeophyceae) | O. mossambicus × O. niloticus | 1, 2, and 3% | - | No abnormal or histological changes were detected due to the dietary SW supplementation | [143] |
| Sargassum ilicifolium (Phaeophyceae) | L. calcarfer | 3, 6, and 9% | 6% | No significant difference observed between enterocyte length, villi width, and muscle thickness in intestinal tissue between different treatments and the control group | [180] |
| Spirulina platensis | L. calcarifer | 10, 20, and 40% | 20% | Decreased intestinal fold and microvilli height were observed in fish fed 40% of Spirulina sp. in the diet | [193] |
| Pelvetia canaliculata (Phaeophyceae) | S. aurata | 1 and 10% | 10% | 10% SW supplementation led to greater thickness of the muscle layers and longer villi length | [194] |
| Gracilaria gracilis (Rhodophyta) | D. labrax | 0.35, 2.5, and 5% | 2.5% | 2.5% SW inclusion boosted the intestinal acid goblet cells | [183] |
| G. gracilis (Rhodophyta) and the microalga Nannochloropsis oceanica (Eustigmatophyceae) | D. labrax | 8% | - | All fish had well-preserved gut morphology; however, significant enhancement of goblet cells was observed in Nannochloropsis-based diet compared to Gracilaria-based feed | [184] |
| Ulva ohnoi (Chlorophyta) | S. senegalensis | 5% | - | SW significantly reduced damage to intestinal mucosa and enhanced the mucosal absorptive surface area | [91] |
| Laminaria sp. | S. salar | 3, 6, and 10% | - | Higher gut and intestinal weights and lengths were observed due to dietary SW provision. Lager surface area exhibited | [82] |
| Ulva lactuca (Chlorophyta), Chondrus crispus (Rhodophyta) | S. aurata | 2.5 and 5% | - | Dietary SW had no significant on distal intestine histomorphology | [195] |
| Gracilaria pygmaea (Rhodophyta) | O. mykiss | 3, 6, 9, and 12% | 9 and 12% | Normal histomorphology of anterior intestine and pyloric caeca was detected. Villi decreased due to 90 and 120 g/kg provision of SW | [84] |
| Ulva rigida (Chlorophyta), Undaria pinnatifida (Phaeophyceae) | S. senegalensis | 10% | - | Dietary Undaria significantly lowered the width of intestine villi | [101] |
| Taonia atomaria (Phaeophyceae) | O. niloticus | 5, 10, and 15% | - | No histopathological alterations were observed due to dietary SW provision | [138] |
| Asparagopsis taxiformis (Rhodophyta) | S. salar | 1.8, 2.6, and 3% | - | Increased bacteria diversity found in the hindgut | [23] |
| Gracilaria cornea (Rhodophyta), Ulva rigida (Chlorophyta) | S. aurata | 5, 15, and 25% | - | SW inclusion did not reveal any negative effects on gut structure | [93] |
| Gracilaria vermiculophylla, Porphyra dioica (Rhodophyta), and Ulva spp. (Chlorophyta) | O. niloticus | 10% | - | Exhibited a significant reduction in villi length in Gracilaria- and Porphyra-based diets, while no significant reduction was observed in case of Ulva spp. | [187] |
| Ulva ohnoi (Chlorophyta) | S. senegalensis | 5% | - | SW supplementation significantly enhanced the abundance of Vibrio while decreasing Stenotrophomonas abundance | [196] |
| Gracilaria gracilis (Rhodophyta) | D. labrax | 8% | - | G. gracilis supplementation promoted the growth of Sulfitobacter and Methylobacterium | [197] |
| Ulva ohnoi (Chlorophyta) | S. senegalensis | 5% | - | Pseudomonas and Mycopasmataceae were abundant in anterior and posterior GI tract, respectively | [198] |
| Gracilaria gracilis (Rhodophyta) | D. labrax | 2.5 and 5% | - | Gut microbiome diversity was not altered by SW supplementation. Abundance of Proteobacteria was reduced | [199] |
| Ulva rigida (Chlorophyta) | S. aurata | 25% | - | SW supplementation significantly modified intestinal microbiota | [200] |
| Sargassum angustifolium (Phaeophyceae), Gracilaria pulvinata (Rhodophyta) | O. mykiss | 0.025 and 0.05% | - | Supplementation of SW extracts did not affect total bacterial level; however, the abundance of Lactobacillus increased | [201] |
| Gracilaria sp. (Rhodophyta) | S. aurata | 2.5 and 5% | 5% | Abundance of Firmicutes phyla and Clostridium genera were enhanced with 5% SW | [202] |
| Ulva rigida (Chlorophyta), Ascophyllum nodosum (Rhodophyta) | Gadus morhua | 10% | - | U. rigida did not significantly influence the microbial composition of hindgut, while A. nodosum altered the scenario | [203] |
| Mixture of red, brown, and green SW | Siganus fuscescens | - | - | Increased abundance of Firmicutes and Proteobacteria while decreasing Bacteroides | [204] |
| Laminaria sp. (Alginates) | S. salar | 0.5 and 2.5% | 0.5% | Facilitated the abundance of several Proteobacteria such as Photobacterium phosphoreum, Aquabacterium parvum, and Achromobacter insolitus | [205] |
| Ulva australis (formerly Ulva pertusa) (Chlorophyta) | S. canaliculatus | 10% | - | SW in the diets enhanced the diversity of Firmicutes, Bacteroidetes, and Proteobacteria | [206] |
| Gracilaria cornea (Rhodophyta), Ulva rigida (Chlorophyta) | S. aurata | 5, 15, and 25% | 15% | Biodiversity of microbial community was significantly reduced with highest inclusion of U. rigida. Various Lactobacillus sp. were significantly stimulated, while Vibrio sp. was reduced | [207] |
| Strengths | Weaknesses |
|---|---|
|
|
| Threats | Opportunities |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddik, M.A.B.; Francis, P.; Rohani, M.F.; Azam, M.S.; Mock, T.S.; Francis, D.S. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants 2023, 12, 2066. https://doi.org/10.3390/antiox12122066
Siddik MAB, Francis P, Rohani MF, Azam MS, Mock TS, Francis DS. Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants. 2023; 12(12):2066. https://doi.org/10.3390/antiox12122066
Chicago/Turabian StyleSiddik, Muhammad A. B., Prue Francis, Md Fazle Rohani, Mohammed Shariful Azam, Thomas S. Mock, and David S. Francis. 2023. "Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish" Antioxidants 12, no. 12: 2066. https://doi.org/10.3390/antiox12122066
APA StyleSiddik, M. A. B., Francis, P., Rohani, M. F., Azam, M. S., Mock, T. S., & Francis, D. S. (2023). Seaweed and Seaweed-Based Functional Metabolites as Potential Modulators of Growth, Immune and Antioxidant Responses, and Gut Microbiota in Fish. Antioxidants, 12(12), 2066. https://doi.org/10.3390/antiox12122066








