Antioxidant Capacity of Free and Bound Phenolics from Olive Leaves: In Vitro and In Vivo Responses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Reagents
2.3. Extraction of Free and Bound Phenolic Fractions from Olive Leaves
2.4. Identification and Quantification of Free and Bound Antioxidants in Olive Leaves
2.5. Determination of TPC and TFC
2.6. Chemical Antioxidant Activity Evaluation
2.7. Cellular Antioxidant Activity Evaluation
2.7.1. Cell Culture and Cytotoxicity Assay
2.7.2. H2O2-Induced Oxidative Stress in HepG2 Cells
2.7.3. CAA Assay
2.8. In Vivo Antioxidant Activity Evaluation
2.8.1. Animals and Experimental Design
2.8.2. Histological Analysis
2.8.3. Biochemical Assays
2.8.4. RT-qPCR Analysis
2.9. Statistical Analysis
3. Results and Discussion
3.1. Identification and Quantification of Free and Bound Antioxidants in Olive Leaves
3.2. Chemical Antioxidant Activities of FP and BP Fractions in Olive Leaves
3.3. Cellular Antioxidant Activities of FP and BP Fractions in Olive Leaves
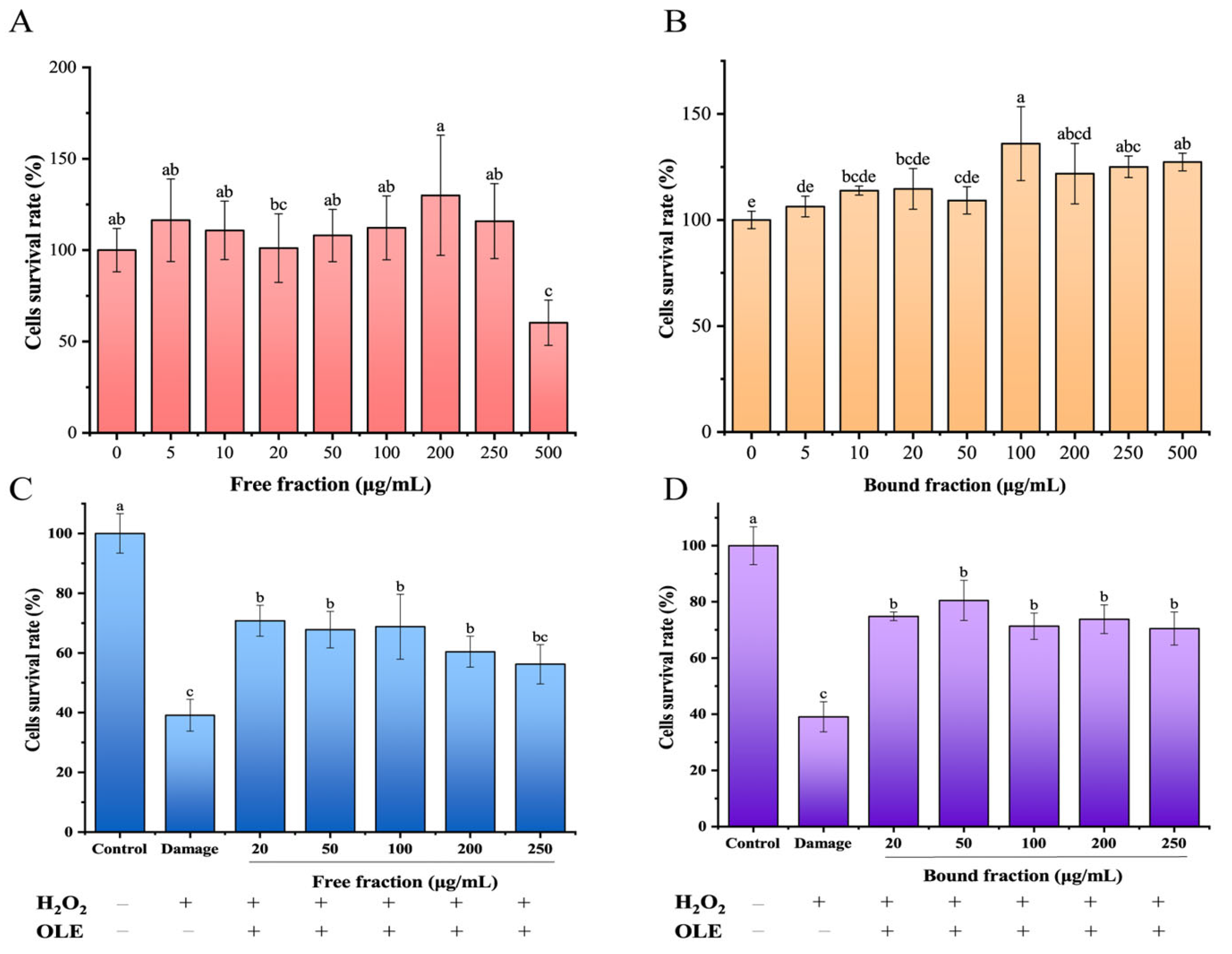
3.3.1. FP and BP Fractions in Olive Leaves Resist H2O2-Induced Oxidative Stress in HepG2 Cells
3.3.2. FP and BP Fractions in Olive Leaves Exhibited Cellular Antioxidant Activity
3.4. In Vivo Antioxidant Activities of FP and BP Fractions in Olive Leaves
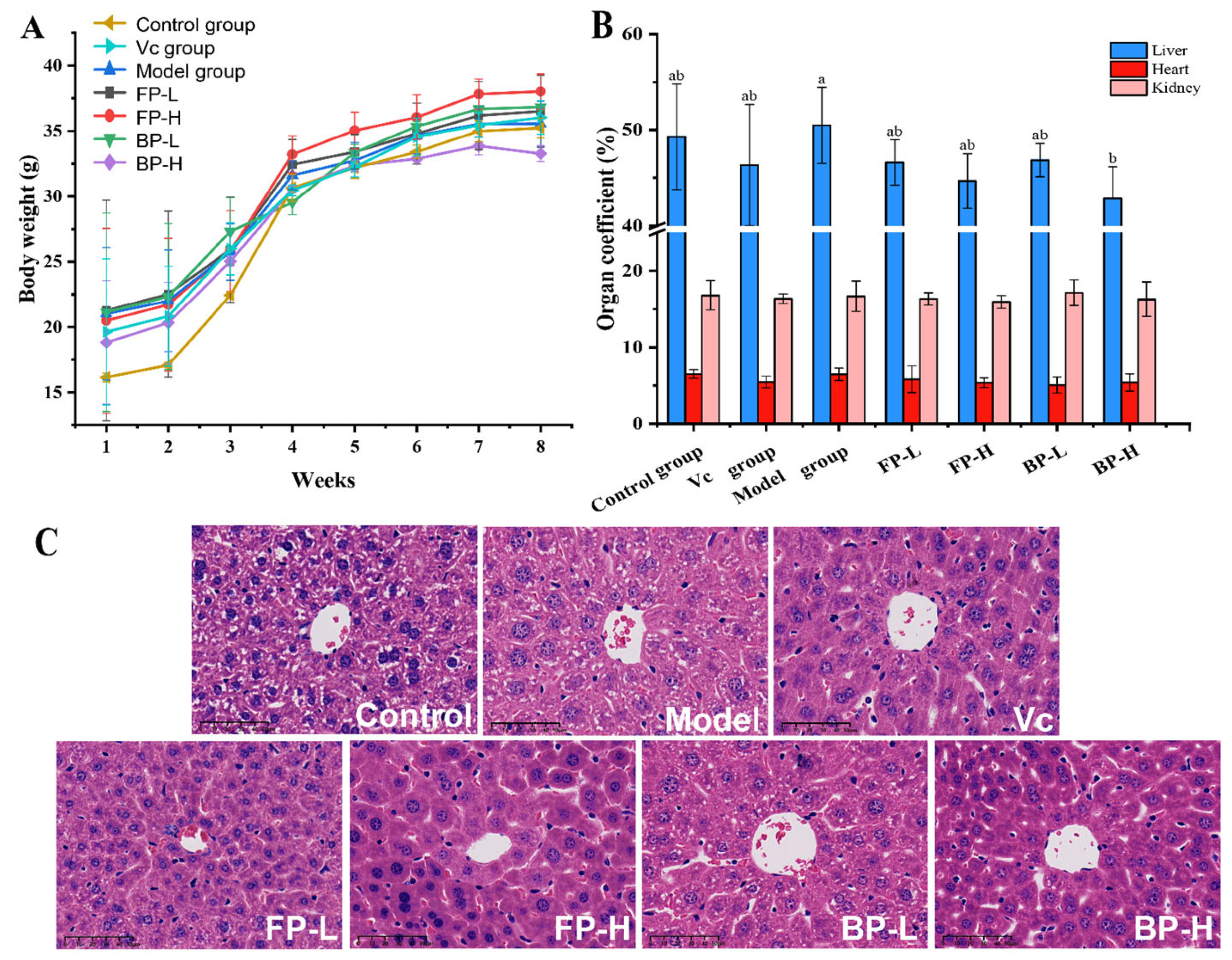
3.4.1. FP and BP Fractions in Olive Leaves Protect Liver Tissue in Aging Mice
3.4.2. FP and BP Fractions in Olive Leaves Relieve Oxidative Stress in Aging Mice
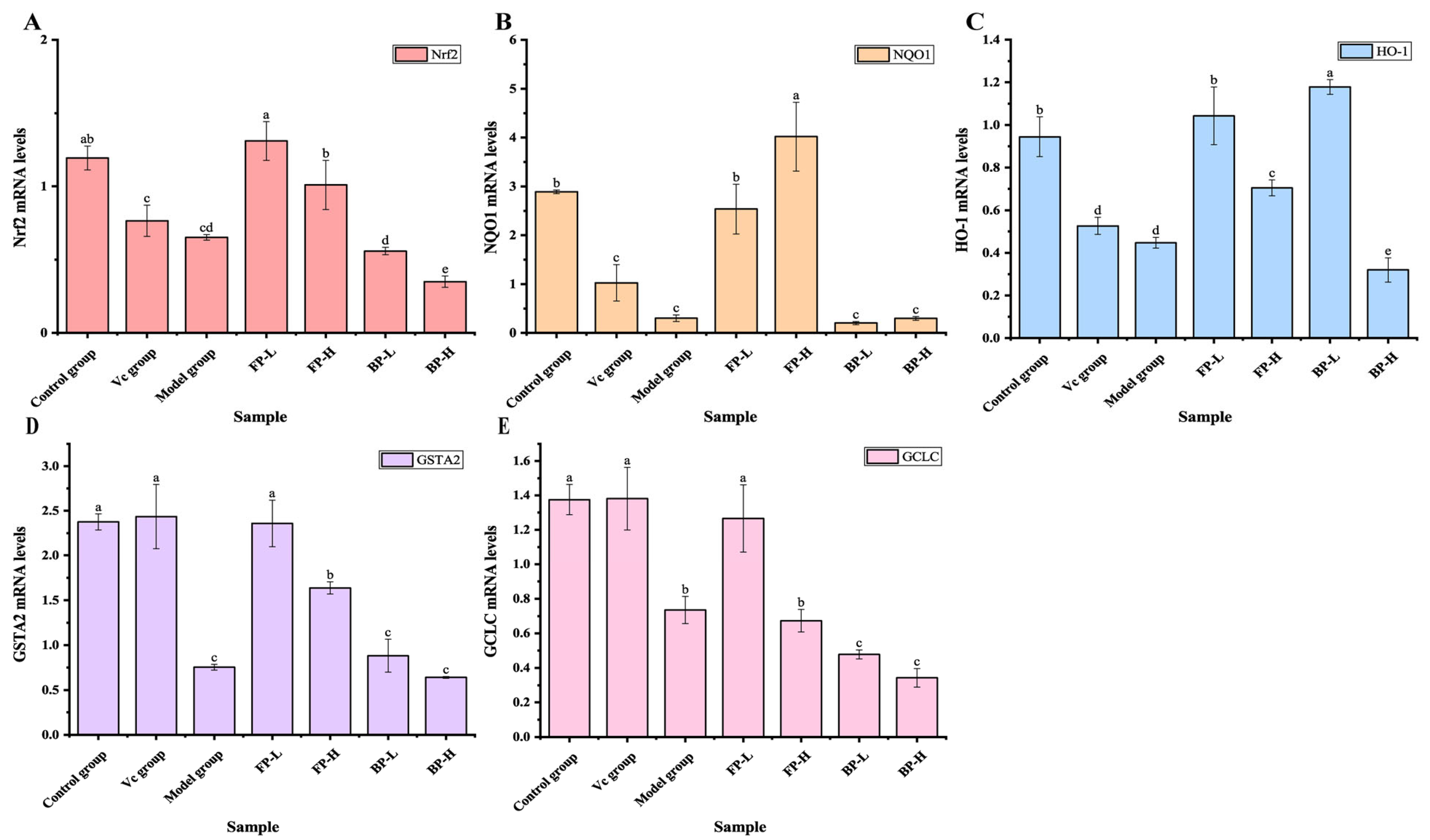
3.4.3. FP Fractions in Olive Leaves Activate Nrf2 Signaling in Aging Mice
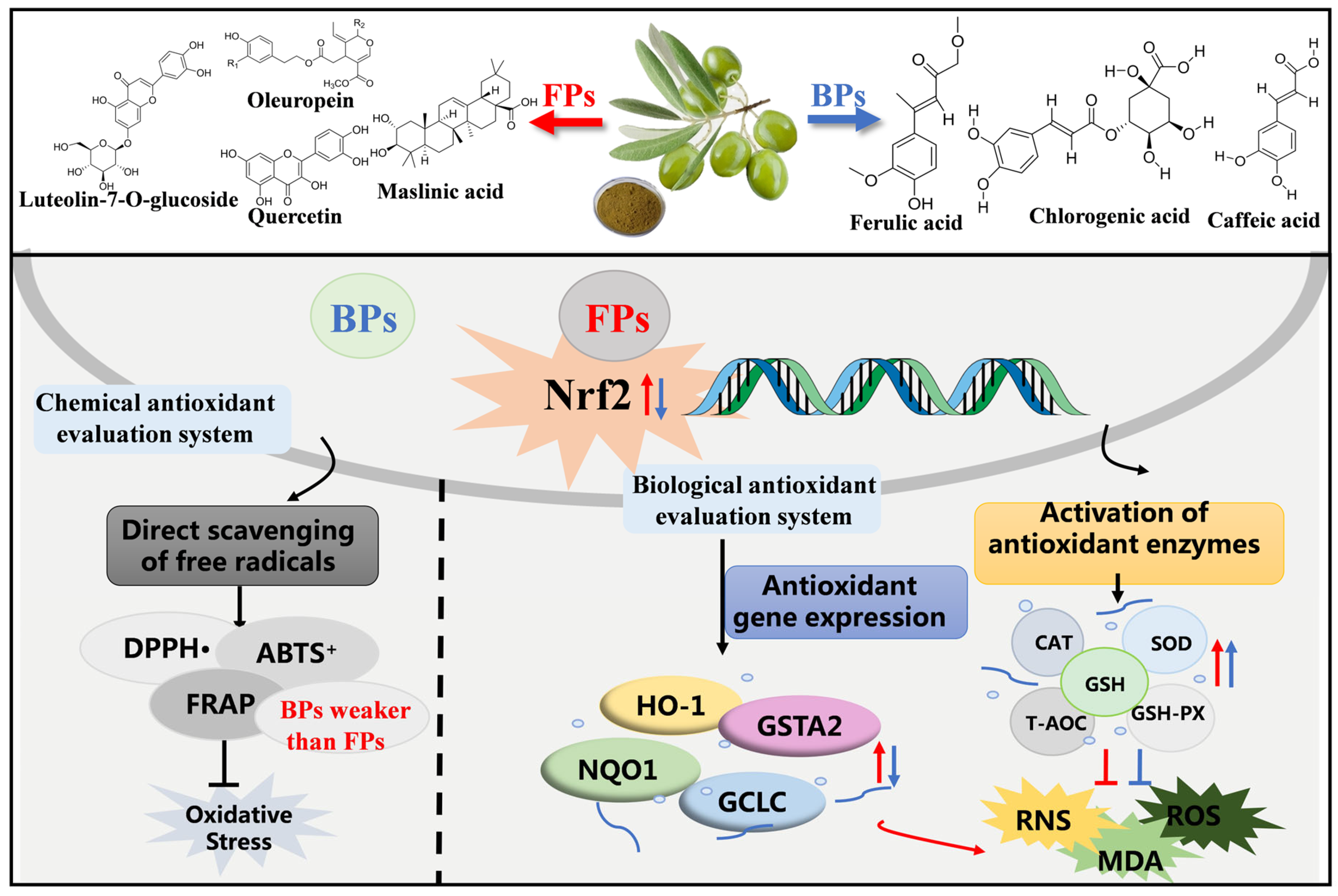
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rani, V.; Deep, G.; Singh, R.K.; Palle, K.; Yadav, U.C. Oxidative stress and metabolic disorders: Pathogenesis and therapeutic strategies. Life Sci. 2016, 148, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, R.; Ortega-Camarillo, C.; Ferreira-Hermosillo, A.; Diaz-Velazquez, M.F.; Meixueiro-Calderon, C.; Valencia-Ortega, J. Role of oxidative stress and inflammation in gestational diabetes mellitus. Antioxidants 2023, 12, 1812. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.R. Oxidative stress and the cardiovascular effects of air pollution. Free. Radic. Biol. Med. 2020, 151, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Mehdi, M.M. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Chen, X.; Deng, J.; Ouyang, D.; Wang, D.; Liang, Y.; Chen, Y.; Sun, Y. Effect of thermal processing on free and bound phenolic compounds and antioxidant activities of hawthorn. Food Chem. 2020, 332, 127429. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.; Peng, Z.; Guo, Z.; Wu, C.; Liu, J.; Wang, L.; Xiao, J.; Li, X. Deep eutectic solvent-based ultrasonic-assisted extraction of phenolic compounds from different potato genotypes: Comparison of free and bound phenolic profiles and antioxidant activity. Food Chem. 2022, 388, 133058. [Google Scholar] [CrossRef]
- Lucas-Gonzalez, R.; Viuda-Martos, M.; Perez-Alvarez, J.A.; Fernandez-Lopez, J. In vitro digestion models suitable for foods: Opportunities for new fields of application and challenges. Food Res. Int. 2018, 107, 423–436. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Meng, W.; Sun, H.; Mu, T.; Garcia-Vaquero, M. Extraction, purification, chemical characterization and antioxidant properties in vitro of polyphenols from the brown macroalga Ascophyllum nodosum. Algal Res. 2023, 70, 102989. [Google Scholar] [CrossRef]
- Gamboa-Gomez, C.I.; Simental-Mendia, L.E.; Gonzalez-Laredo, R.F.; Alcantar-Orozco, E.J.; Monserrat-Juarez, V.H.; Ramirez-Espana, J.C.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Rocha-Guzman, N.E. In vitro and in vivo assessment of anti-hyperglycemic and antioxidant effects of oak leaves (Quercus convallata and Quercus arizonica) infusions and fermented beverages. Food Res. Int. 2017, 102, 690–699. [Google Scholar] [CrossRef]
- Shang, H.M.; Zhou, H.Z.; Yang, J.Y.; Li, R.; Song, H.; Wu, H.X. In vitro and in vivo antioxidant activities of inulin. PLoS ONE 2018, 13, e192273. [Google Scholar] [CrossRef] [PubMed]
- Mota, J.C.; Almeida, P.P.; Freitas, M.Q.; Stockler-Pinto, M.B.; Guimaraes, J.T. Far from being a simple question: The complexity between in vitro and in vivo responses from nutrients and bioactive compounds with antioxidant potential. Food Chem. 2023, 402, 134351. [Google Scholar] [CrossRef] [PubMed]
- Zugcic, T.; Abdelkebir, R.; Alcantara, C.; Carmen Collado, M.; Vicente Garcia-Perez, J.; Melendez-Martinez, A.J.; Jambrak, A.R.; Lorenzo, J.M.; Barba, F.J. From extraction of valuable compounds to health promoting benefits of olive leaves through bioaccessibility, bioavailability and impact on gut microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, J.; Xin, X.; Zhu, S.; Niu, E.; Wu, Q.; Li, T.; Liu, D. Changes in phytochemical profiles and biological activity of olive leaves treated by two drying methods. Front. Nutr. 2022, 9, 854680. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-products of fruit and vegetables: Antioxidant properties of extractable and non-extractable phenolic compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Shahidi, F.; Hossain, A. Importance of insoluble-bound phenolics to the antioxidant potential is dictated by source material. Antioxidants 2023, 12, 203. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutierrez-Uribe, J.A.; Serna-Saldivar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Chandrasekara, A.; Shahidi, F. Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. J. Agric. Food. Chem. 2010, 58, 6706–6714. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.D.; Xue, Q.W.; Zhao, T.R.; Khan, A.; Wang, Y.F.; Liu, Y.P.; Cao, J.X.; Cheng, G.G. The effect of ultra-high pretreatment on free, esterified and insoluble-bound phenolics from mango leaves and their antioxidant and cytoprotective activities. Food Chem. 2022, 368, 130864. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Importance of insoluble-bound phenolics to antioxidant properties of wheat. J. Agric. Food. Chem. 2006, 54, 1256–1264. [Google Scholar] [CrossRef]
- You, B.; Yang, S.; Yu, J.; Xian, W.; Deng, Y.; Huang, W.; Li, W.; Yang, R. Effect of thermal and dry salt-curing processing on free and bound phenolics and antioxidant activity in prunus mume fruits together with the phenolic bioaccessibility. LWT 2021, 145, 111355. [Google Scholar] [CrossRef]
- Zhang, C.; Xin, X.; Zhang, J.; Zhu, S.; Niu, E.; Zhou, Z.; Liu, D. Comparative evaluation of the phytochemical profiles and antioxidant potentials of olive leaves from 32 cultivars grown in china. Molecules 2022, 27, 1292. [Google Scholar] [CrossRef]
- Martinović, A.; Cavoski, I. The exploitation of cornelian cherry (Cornus mas L.) Cultivars and genotypes from montenegro as a source of natural bioactive compounds. Food Chem. 2020, 318, 126549. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The frap assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Li, F.; Liao, X.; Jiang, L.; Zhao, J.; Wu, S.; Ming, J. Orientin attenuated d-galn/lps-induced liver injury through the inhibition of oxidative stress via Nrf2/Keap1 pathway. J. Agric. Food. Chem. 2022, 70, 7953–7967. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Peng, L.; Xiong, H.; Liu, W.; Zhang, H.; Peng, X.; Zhu, X.; Guo, F.; Sun, Y. The profiles of durian (Durio zibethinus murr.) Shell phenolics and their antioxidant effects on H2O2-treated HepG2 cells as well as the metabolites and organ distribution in rats. Food Res. Int. 2023, 163, 112122. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular antioxidant activity (CAA) assay for assessing antioxidants, foods, and dietary supplements. J. Agric. Food. Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef]
- Shahidi, F.; Dissanayaka, C.S. Phenolic-protein interactions: Insight from in-silico analyses—A review. Food Prod. Process. Nutr. 2023, 5, 2. [Google Scholar] [CrossRef]
- Contreras, M.; Lama-Munoz, A.; Espinola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef]
- Dias, M.C.; Figueiredo, C.; Pinto, D.C.G.A.; Freitas, H.; Santos, C.; Silva, A.M.S. Heat shock and UV-B episodes modulate olive leaves lipophilic and phenolic metabolite profiles. Ind. Crop. Prod. 2019, 133, 269–275. [Google Scholar] [CrossRef]
- Li, M.; Xu, T.; Zheng, W.; Gao, B.; Zhu, H.; Xu, R.; Deng, H.; Wang, B.; Wu, Y.; Sun, X.; et al. Triacylglycerols compositions, soluble and bound phenolics of red sorghums, and their radical scavenging and anti-inflammatory activities. Food Chem. 2021, 340, 128123. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, Y.; Wang, L.; Tan, Y.; Shi, Y.; Sedjoah, R.A.; Shao, Y.; Li, L.; Wang, M.; Wan, J.; et al. Ultrasound-assisted extraction of bound phenolic compounds from the residue of apocynum venetum tea and their antioxidant activities. Food Biosci. 2022, 47, 101646. [Google Scholar] [CrossRef]
- Liu, Z. Why natural antioxidants are readily recognized by biological systems? 3D architecture plays a role! Food Chem. 2022, 380, 132143. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Lee, J.W.; Jang, H.; Kim, J.G.; Lee, M.K.; Hong, J.T.; Lee, M.S.; Hwang, B.Y. Quinic acid esters from erycibe obtusifolia with antioxidant and tyrosinase inhibitory activities. Nat. Prod. Res. 2019, 35, 3026–3032. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Shahidi, F. Identification and quantification of soluble and insoluble-bound phenolics in lentil hulls using HPLC-ESI-MS/MS and their antioxidant potential. Food Chem. 2020, 315, 126202. [Google Scholar] [CrossRef] [PubMed]
- Benavente-Garcia, O.; Castillo, J.; Lorente, J. Antioxidant activity of phenolics extracted from Olea Europaea L. Leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ros scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ros associated degenerative disorders. Biochim. Biophys. Acta-Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Flores-Felix, J.D.; Costa, A.R.; Falcao, A.; Alves, G.; Silva, L.R. Hepatoprotective effects of sweet cherry extracts (cv. Saco). Foods 2021, 10, 2623. [Google Scholar] [CrossRef]
- Gao, S.H.; Zhao, T.R.; Liu, Y.P.; Wang, Y.F.; Cheng, G.G.; Cao, J.X. Phenolic constituents, antioxidant activity and neuroprotective effects of ethanol extracts of fruits, leaves and flower buds from Vaccinium Dunalianum wight. Food Chem. 2022, 374, 131752. [Google Scholar] [CrossRef]
- Chu, Q.; Jia, R.; Chen, C.; Wang, Y.; Yu, X.; Zhang, Y.; Wu, T.; Zheng, X. Apios americana medik leaf extracts attenuate H2O2-induced hepatotoxicity. Food Biosci. 2021, 41, 100996. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Liu, Q.; Lin, Y.; Zhang, Z.; Li, S. Study on extraction and antioxidant activity of flavonoids from Hemerocallis Fulva (daylily) leaves. Molecules 2022, 27, 2916. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jin, R.; Chen, J.; Cao, J.; Xiao, J.; Li, X.; Sun, C. Tangeretin maintains antioxidant activity by reducing Cul3 mediated Nrf2 ubiquitination. Food Chem. 2021, 365, 130470. [Google Scholar] [CrossRef] [PubMed]
- Sha, A.; Liu, Y.; Qiu, X.; Xiong, B. Polysaccharide from paris polyphylla improves learning and memory ability in d-galactose-induced aging model mice based on antioxidation, p19/p53/p21, and wnt/β-catenin signaling pathways. Int. J. Biol. Macromol. 2023, 251, 126311. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Prakash, A.; Dogra, S. Protective effect of curcumin (Curcuma Longa) against D-galactose-induced senescence in mice. J. Asian Nat. Prod. Res. 2011, 13, 42–55. [Google Scholar] [CrossRef]
- Sun, W.; Zhu, J.; Qin, G.; Huang, Y.; Cheng, S.; Chen, Z.; Zhang, Y.; Shu, Y.; Zeng, X.; Guo, R. Lonicera japonica polysaccharides alleviate D-galactose-induced oxidative stress and restore gut microbiota in icr mice. Int. J. Biol. Macromol. 2023, 245, 125517. [Google Scholar] [CrossRef]
- Castillo, C.; Salazar, V.; Ariznavarreta, C.; Vara, E.; Tresguerres, J.A. Effect of isoflavone administration on age-related hepatocyte changes in old ovariectomized femal wistar rats. Phytomedicine 2006, 13, 468–476. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Lakshmi, S.P.; Reddy, A.T.; Kodidhela, L.D.; Varadacharyulu, N.C. Epigallocatechin gallate diminishes cigarette smoke-induced oxidative stress, lipid peroxidation, and inflammation in human bronchial epithelial cells. Life Sci. 2020, 259, 118260. [Google Scholar] [CrossRef]
- Kang, Y.P.; Mockabee-Macias, A.; Jiang, C.; Falzone, A.; Prieto-Farigua, N.; Stone, E.; Harris, I.S.; Denicola, G.M. Non-canonical glutamate-cysteine ligase activity protects against ferroptosis. Cell Metab. 2021, 33, 174–189. [Google Scholar] [CrossRef]
- Wang, C.; Cui, C.; Li, N.; Sun, X.; Wen, L.; Gao, E.; Wang, F. Antioxidant activity and protective effect of wheat germ peptides in an in vitro celiac disease model via Keap1/Nrf2 signaling pathway. Food Res. Int. 2022, 161, 111864. [Google Scholar] [CrossRef] [PubMed]



| No | Rt (min) | Measured m/z | MS/MS Fragments | Molecular Formula | CAS | Compounds | Class | FP | BP |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3.912 | 153.0550 [M − H]− | 123.04, 153.05 | C8H10O3 | 10597-60-1 | Hydroxytyrosol | Simple phenols | X | X |
| 2 | 5.296 | 353.0880 [M − H]− | 191.06, 209.66 | C16H18O9 | 327-97-9 | Chlorogenic acid | Phenolic acids | X | X |
| 3 | 5.790 | 163.0390 [M + H]+ | 163.04, 145.03, 135.04 | C9H8O4 | 331-39-5 | Caffeic acid | Phenolic acids | X | |
| 4 | 6.605 | 147.0440 [M + H]+ | 147.04, 119.05 | C9H8O3 | 7400-08-0 | 4-Coumaric acid | Phenolic acids | X | |
| 5 | 7.110 | 223.0610 [M − H]− | 164.05, 208.04, 223.06 | C11H12O5 | 530-59-6 | Sinapinic acid | Phenolic acids | X | |
| 6 | 7.155 | 177.0646 [M + H]+ | 177.05, 145.03 | C10H10O4 | 537-98-4 | Ferulic acid | Phenolic acids | X | |
| 7 | 5.698 | 403.1250 [M − H]− | 59.01, 71.01, 89.02 | C17H24O11 | 58822-47-2 | Secoxyloganin | Iridoids | X | |
| 8 | 7.525 | 539.1776 [M-H]− | 539.18, 377.12, 275.09 | C25H32O13 | 32619-42-4 | Oleuropein | Iridoids | X | |
| 9 | 5.916 | 609.1469 [M − H]− | 447.09, 609.15, 285.04 | C27H30O16 | 52187-80-1 | Luteolin-3′,7-di-O-glucoside | Flavonoids | X | |
| 10 | 6.523 | 609.1470 [M − H]− | 609.15, 300.03, 301.04 | C27H30O16 | 153-18-4 | Rutin | Flavonoids | X | X |
| 11 | 6.528 | 611.1608 [M + H]+ | 303.05, 466.11, 85.03 | C27H30O16 | 18016-58-5 | Quercetin 3-O-glucoside-7-O-rhamnoside | Flavonoids | X | |
| 12 | 6.753 | 465.1026 [M + H]+ | 303.05, 85.03 | C21H20O12 | 482-35-9 | Quercetin-3β-D-glucoside | Flavonoids | X | |
| 13 | 6.758 | 449.1077 [M + H]+ | 449.11, 287.05 | C21H20O11 | 5373/11/5 | Luteolin-7-O-glucoside | Flavonoids | X | X |
| 14 | 6.941 | 577.1570 [M − H]− | 269.05, 577.16 | C27H30O14 | 17306-46-6 | Apigenin-7-O-neohesperidoside | Flavonoids | X | X |
| 15 | 7.143 | 609.1815 [M + H]+ | 301.07, 609.18 | C28H32O15 | 38665-01-9 | Neodiosmin | Flavonoids | X | |
| 16 | 7.214 | 477.1043 [M − H]− | 431.10, 268.04, 269.05 | C21H20O10 | 578-74-5 | Apigenin-7-O-glucoside | Flavonoids | X | X |
| 17 | 7.236 | 447.0937 [M − H]− | 285.04, 447.09 | C21H20O11 | 16290-07-6 | Kaempferol-7-O-glucoside | Flavonoids | X | X |
| 18 | 7.390 | 447.0939 [M− H]− | 210.04, 285.04 | C21H20O11 | 6920-38-3 | Luteolin-4′-O-glucoside | Flavonoids | X | |
| 19 | 8.919 | 285.0407 [M − H]− | 285.04, 299.46 | C15H10O6 | 491-70-3 | Luteolin | Flavonoids | X | X |
| 20 | 8.922 | 287.0548 [M + H]+ | 287.05, 269.08 | C15H10O6 | 520-18-3 | Kaempferol | Flavonoids | X | |
| 21 | 9.012 | 303.0496 [M − H]− | 303.05, 285.15 | C15H10O7 | 117-39-5 | Quercetin | Flavonoids | X | |
| 22 | 9.882 | 301.0703 [M + H]+ | 301.07, 286.05 | C16H12O6 | 520-34-3 | Diosmetin | Flavonoids | X | |
| 23 | 10.001 | 315.0514 [M − H]− | 315.05, 300.03 | C16H12O7 | 1486-70-0 | 3-O-Methylquercetin | Flavonoids | X | |
| 24 | 11.579 | 487.3434 [M − H]− | 487.34, 469.33 | C30H48O5 | 464-92-6 | Asiatic acid | Triterpenoid acids | X | |
| 25 | 13.414 | 471.3483 [M − H]− | 471.35, 428.24 | C30H48O4 | 4373-41-5 | Maslinic acid | Triterpenoid acids | X | |
| 26 | 13.434 | 455.3517 [M + H]+ | 205.16, 189.16, 203.18 | C30H48O4 | 4547-24-4 | Corosolic acid | Triterpenoid acids | X | X |
| 27 | 12.759 | 457.3673 [M + H]+ | 457.37, 203.18, 191.18 | C30H48O3 | 508-02-1 | Oleanolic acid | Triterpenoid acids | X | |
| 28 | 16.492 | 439.3567 [M + H]+ | 411.36, 439.36, 203.18 | C30H48O3 | 77-52-1 | Ursolic acid | Triterpenoid acids | X | X |
| No. | Compounds | Content (mg/g DW) | No. | Compounds | Content (mg/g DW) | ||
|---|---|---|---|---|---|---|---|
| FP Fraction | BP Fraction | FP Fraction | BP Fraction | ||||
| 1 | Hydroxytyrosol | 0.11 ± 0.01 b | 0.19 ± 0.09 a | 15 | Apigenin-7-O-glucoside | 0.47 ± 0.03 a | 0.05 ± 0.01 b |
| 2 | Chlorogenic acid | 0.13 ± 0.02 a | 0.07 ± 0.01 b | 16 | Neodiosmin | 0.54 ± 0.03 | ND |
| 3 | Caffeic acid | ND | 0.16 ± 0.01 | 17 | Kaempferol-7-O-glucoside | 2.07 ± 0.15 a | 0.14 ± 0.03 b |
| 4 | 4-Coumaric acid | ND | 0.21 ± 0.03 | 18 | Luteolin-4′-O-glucoside | 0.61 ± 0.04 | ND |
| 5 | Sinapinic acid | ND | 0.39 ± 0.03 | 19 | Luteolin | 0.38 ± 0.03 a | 0.03 ± 0.01 b |
| 6 | Ferulic acid | ND | 0.20 ± 0.08 | 20 | Kaempferol | 0.03 ± 0.01 | ND |
| 7 | Secoxyloganin | 2.11 ± 0.18 | ND | 21 | Quercetin | 0.12 ± 0.01 | ND |
| 8 | Oleuropein | 17.52 ± 2.61 | ND | 22 | Diosmetin | 0.03 ± 0.00 | ND |
| 9 | Luteolin-3′,7-di-O-glucoside | 0.44 ± 0.03 | ND | 23 | 3-O-Methylquercetin | 0.02 ± 0.00 | ND |
| 10 | Rutin | 1.31 ± 0.10 a | 0.12 ± 0.01 b | 24 | Asiatic acid | 0.26 ± 0.01 a | ND |
| 11 | Quercetin 3-O-glucoside-7-O-rhamnoside | 0.52 ± 0.03 | ND | 25 | Maslinic acid | 1.31 ± 0.02 a | 0.01 ± 0.00 b |
| 12 | Quercetin-3β-D-glucoside | 0.78 ± 0.06 | ND | 26 | Corosolic acid | 0.39 ± 0.08 | ND |
| 13 | Luteolin-7-O-glucoside | 2.80 ± 0.27 a | 0.12 ± 0.04 b | 27 | Oleanolic acid | 1.61 ± 0.07 a | 0.06 ± 0.00 b |
| 14 | Apigenin-7-O-neohesperidoside | 0.71 ± 0.04 a | 0.31 ± 0.02 b | 28 | Ursolic acid | ND | 0.02 ± 0.01 |
| TPC (mg GAE/g DW) | TFC (mg RE/g DW) | DPPH (μmol TE/gDW) | ABTS (μmol TE/gDW) | FRAP (μmol TE/gDW) | |
|---|---|---|---|---|---|
| FP fraction | 26.39 ± 1.26 a | 145.30 ± 9.11 a | 26.09 ± 0.03 a | 249.49 ± 0.29 a | 13.79 ± 0.03 a |
| BP fraction | 0.65 ± 0.05 b | 1.73 ± 0.29 b | 10.44 ± 0.01 b | 6.66 ± 0.01 b | 0.37 ± 0.00 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Wu, W.; Zhang, J.; Wu, Q.; Zhu, S.; Niu, E.; Wang, S.; Jiang, C.; Liu, D.; Zhang, C. Antioxidant Capacity of Free and Bound Phenolics from Olive Leaves: In Vitro and In Vivo Responses. Antioxidants 2023, 12, 2033. https://doi.org/10.3390/antiox12122033
Li T, Wu W, Zhang J, Wu Q, Zhu S, Niu E, Wang S, Jiang C, Liu D, Zhang C. Antioxidant Capacity of Free and Bound Phenolics from Olive Leaves: In Vitro and In Vivo Responses. Antioxidants. 2023; 12(12):2033. https://doi.org/10.3390/antiox12122033
Chicago/Turabian StyleLi, Ting, Wenjun Wu, Jianming Zhang, Qinghang Wu, Shenlong Zhu, Erli Niu, Shengfeng Wang, Chengying Jiang, Daqun Liu, and Chengcheng Zhang. 2023. "Antioxidant Capacity of Free and Bound Phenolics from Olive Leaves: In Vitro and In Vivo Responses" Antioxidants 12, no. 12: 2033. https://doi.org/10.3390/antiox12122033






