Biogenic Selenium Nanoparticles Synthesized Using Alginate Oligosaccharides Attenuate Heat Stress-Induced Impairment of Breast Meat Quality via Regulating Oxidative Stress, Metabolome and Ferroptosis in Broilers
Abstract
1. Introduction
2. Materials and Methods
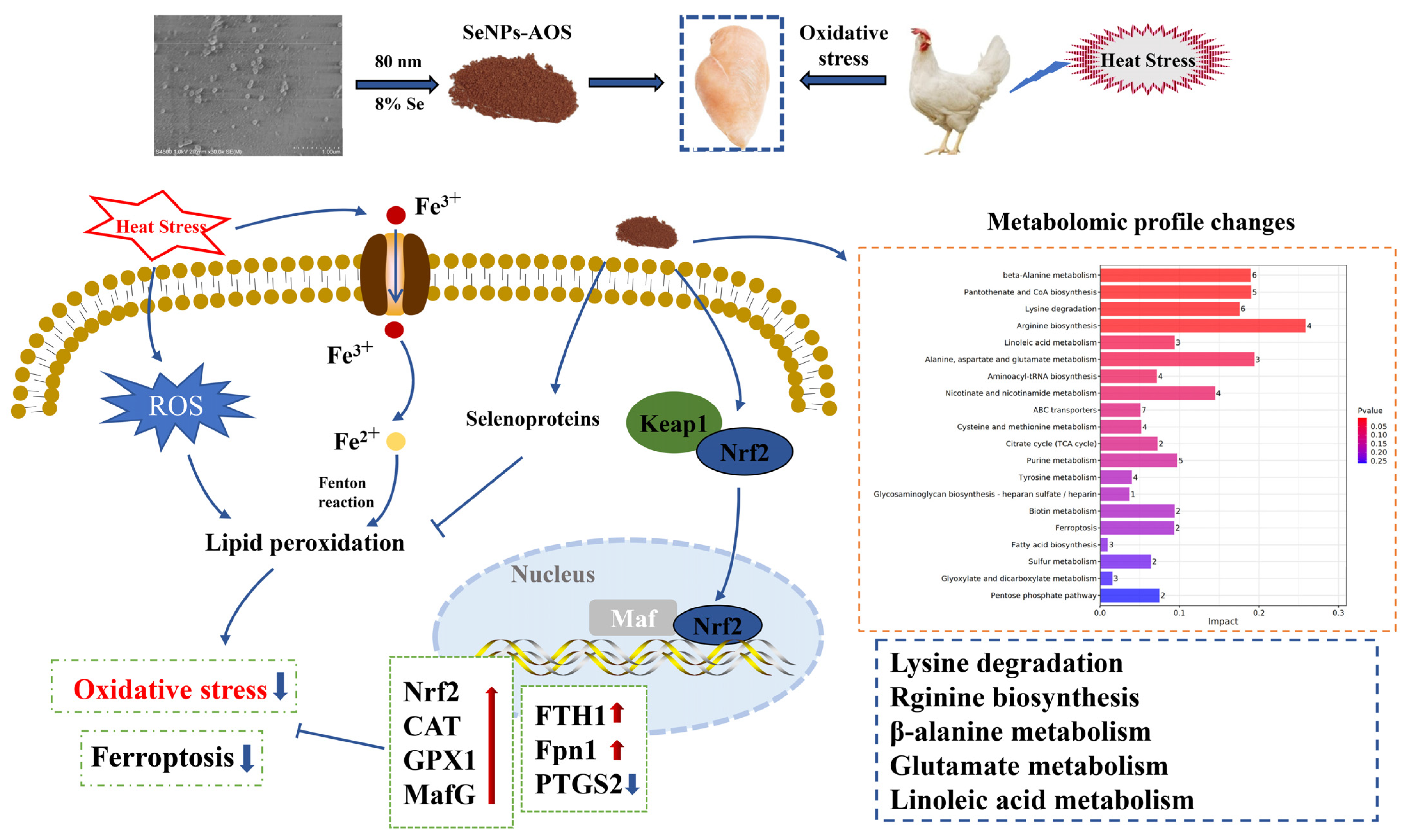
2.1. Biosynthesis of SeNPs-AOS
2.2. Birds, Experimental Design, and Management
2.3. Sample Collection
2.4. Measurement of Meat Quality
2.5. Non-Targeted Metabolomics Analysis
2.6. Antioxidant Capacity Analysis
2.7. Determination of Antioxidant, Selenoprotein, and Ferroptosis-Related Gene Expression
2.8. Statistical Analysis
3. Results
3.1. Meat Quality
3.2. Non-Targeted Metabolomics
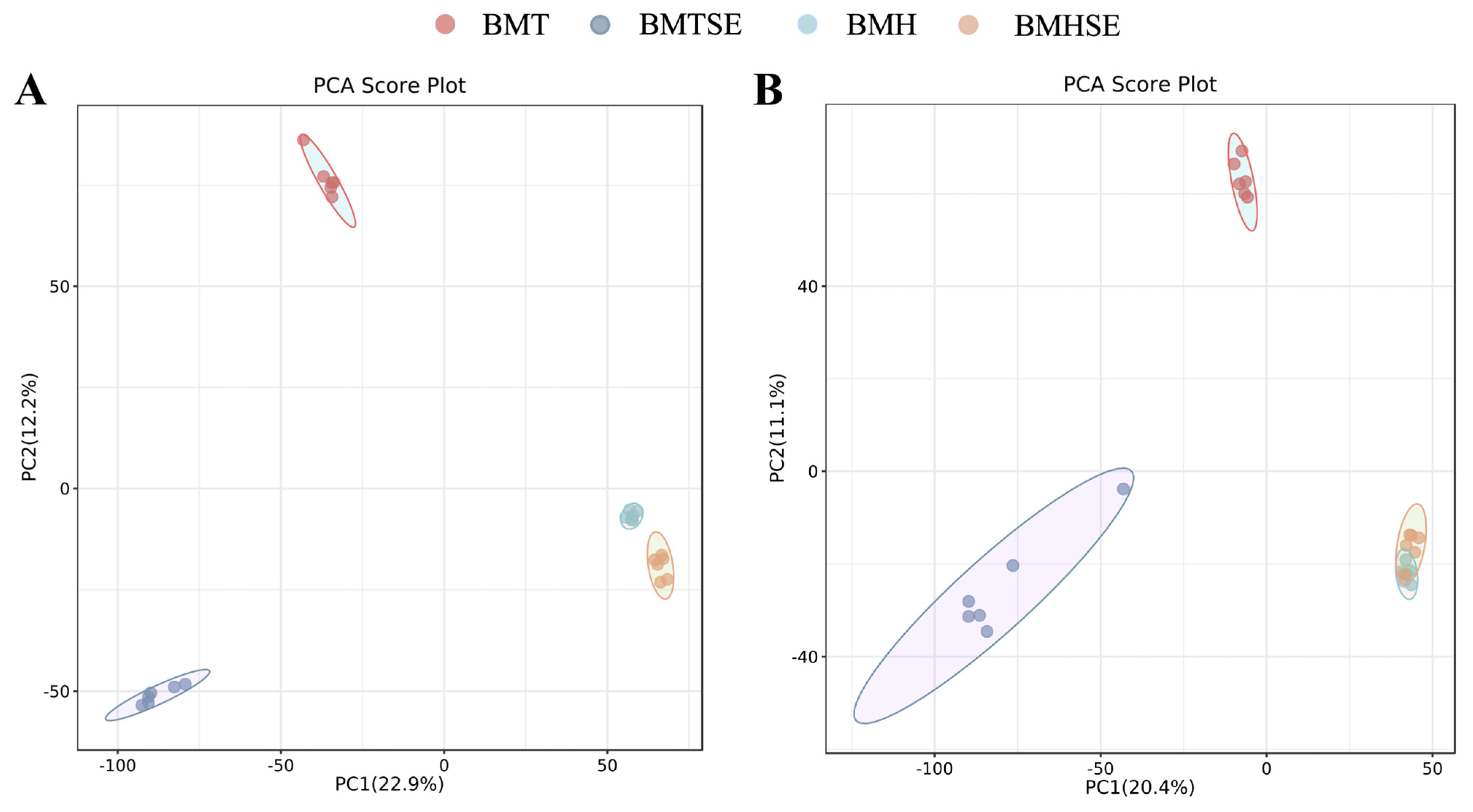
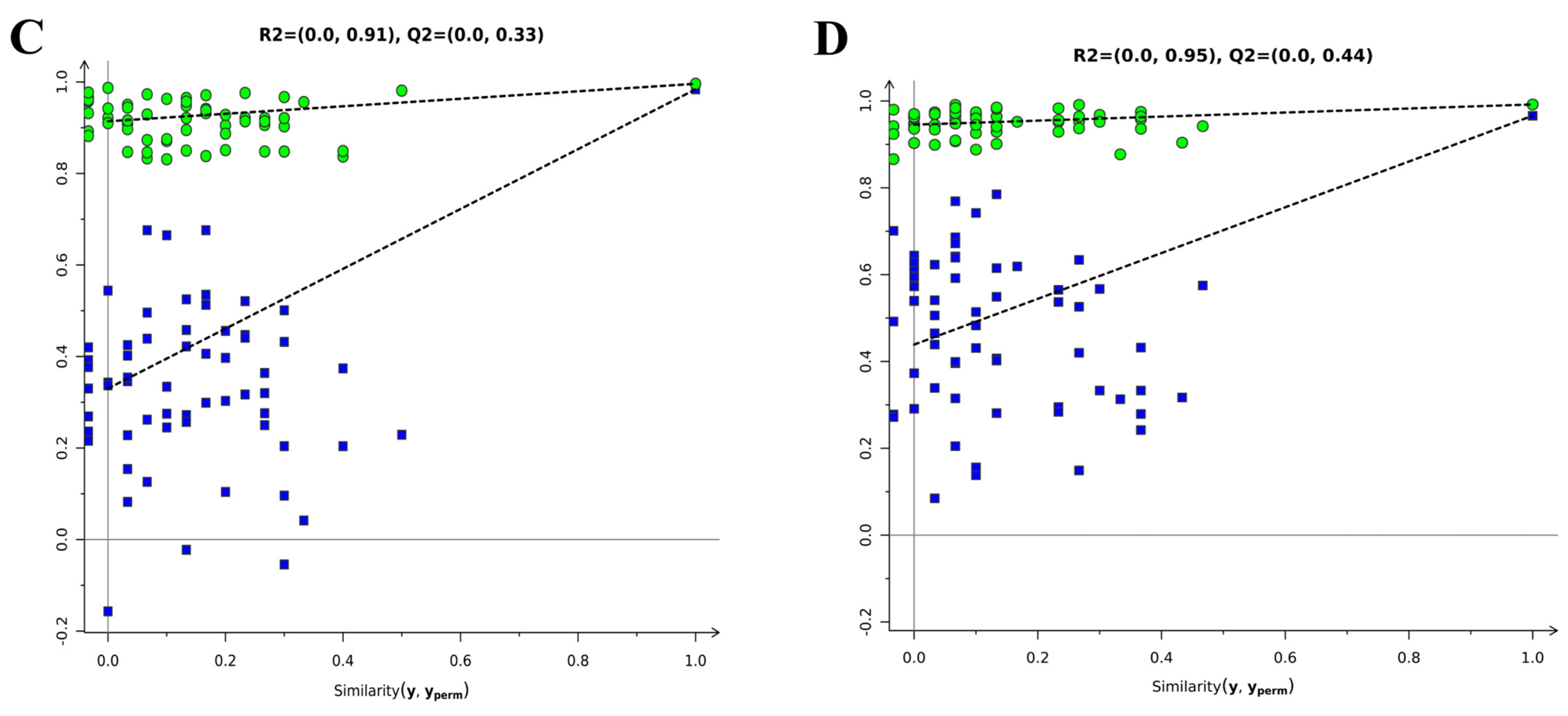
3.2.1. Multivariate Statistical Analysis
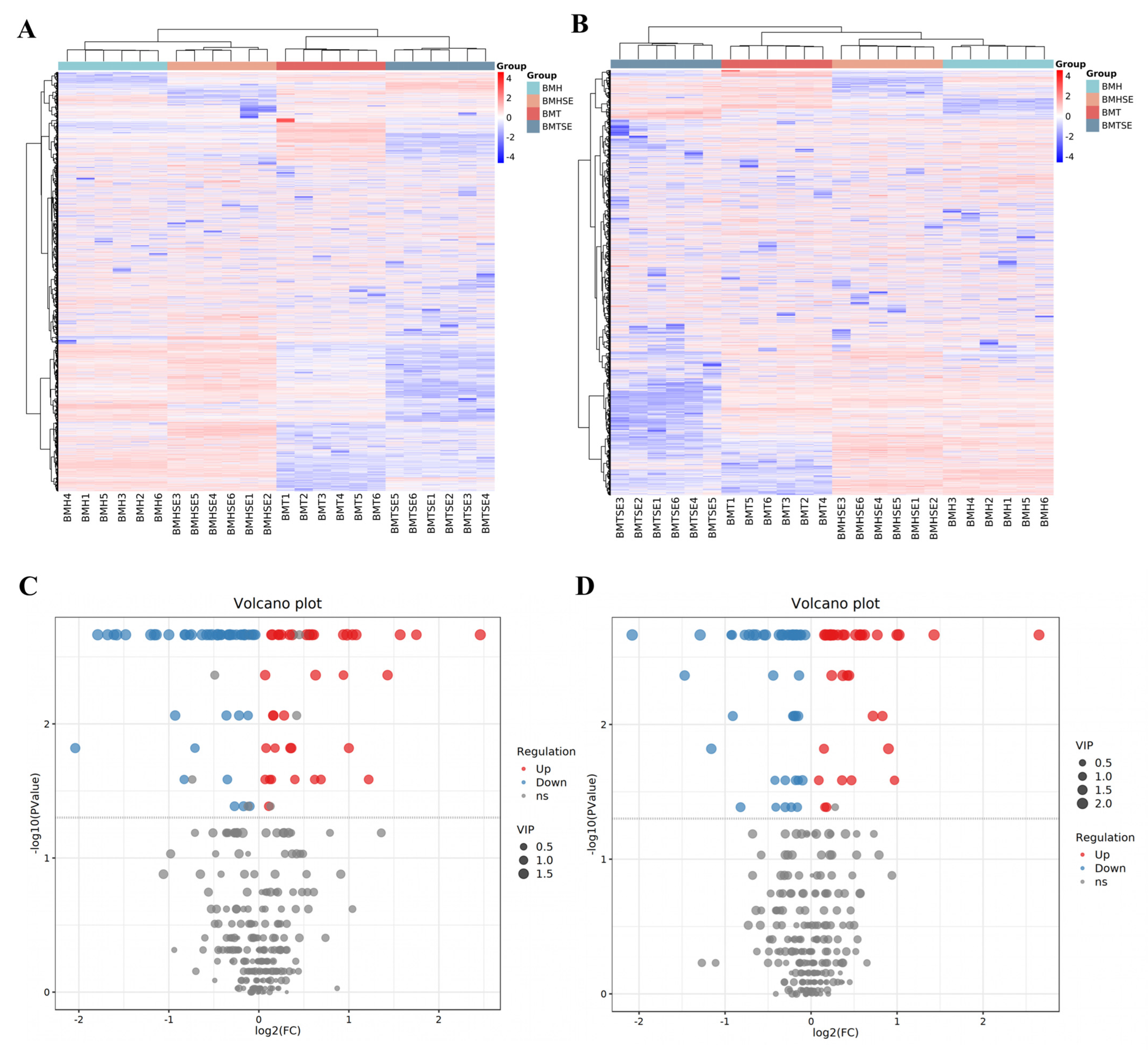
3.2.2. Differential Metabolites Identification
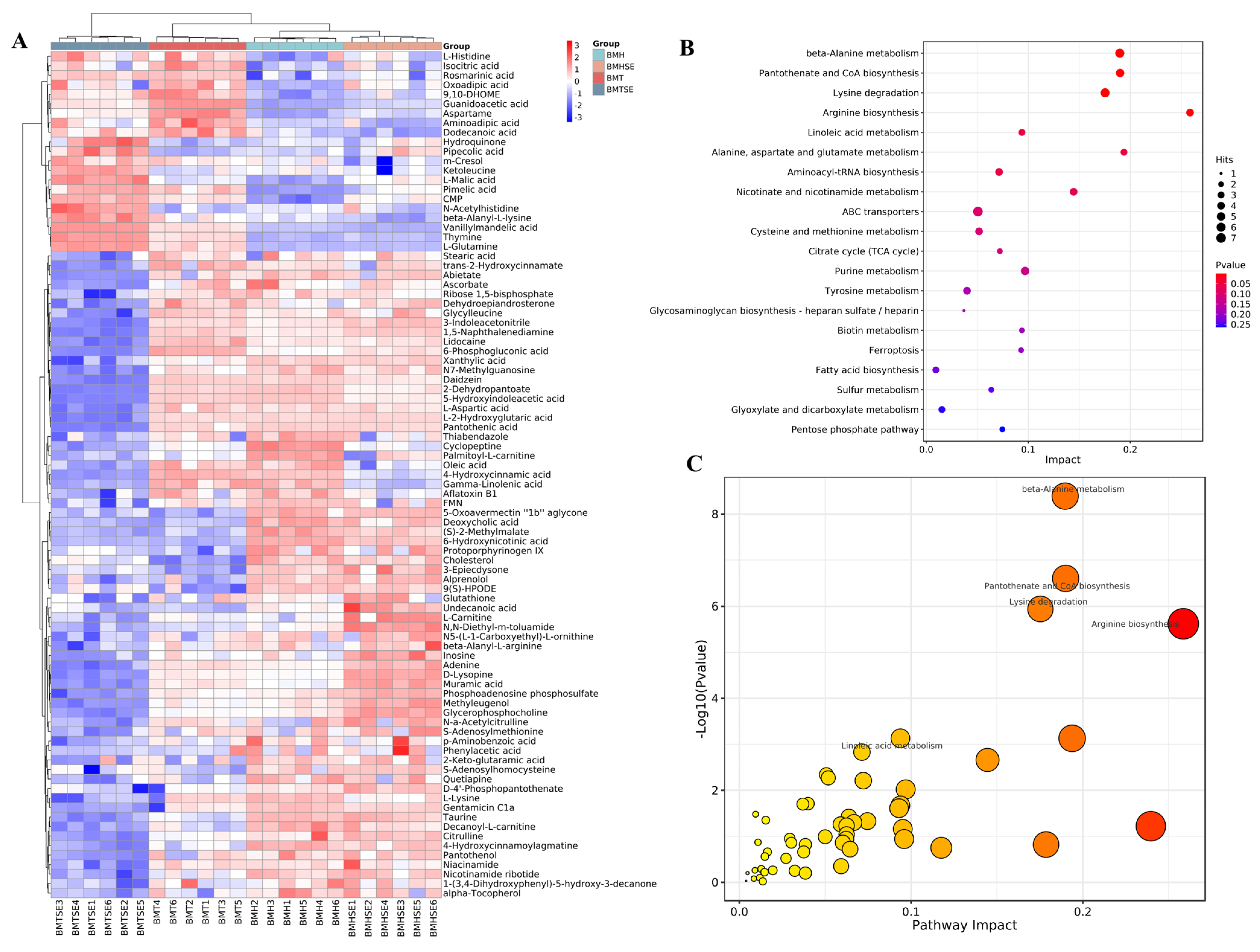
3.2.3. Metabolic Pathways of Differential Metabolites
3.3. Antioxidant Capacity
3.4. Determination of Antioxidant, Selenoprotein, and Ferroptosis-Related Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Rostagno, M.H. Effects of heat stress on the gut health of poultry. J. Anim. Sci. 2020, 98, skaa090. [Google Scholar] [CrossRef] [PubMed]
- Song, D.J.; King, A.J. Effects of heat stress on broiler meat quality. World’s Poult. Sci. J. 2015, 71, 701–709. [Google Scholar] [CrossRef]
- Ebrahim, R.; Liang, J.B.; Jahromi, M.F.; Shokryazdan, P.; Ebrahimi, M.; Chen, W.L.; Goh, Y.M. Effects of tannic acid on performance and fatty acid composition of breast muscle in broiler chickens under heat stress. Ital. J. Anim. Sci. 2015, 14, 3956. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef]
- Pardo, Z.; Lara, L.; Nieto, R.; Fernández-Fígares, I.; Seiquer, I. Muscle quality traits and oxidative status of Iberian pigs supplemented with zinc and betaine under heat stress. Meat Sci. 2023, 198, 109119. [Google Scholar] [CrossRef]
- Chen, Y.P.; Cheng, Y.F.; Du, M.F.; Zhou, Y.M. Protective effects of dietary synbiotic supplementation on meat quality and oxidative status in broilers under heat stress. Environ. Sci. Pollut. Res. 2021, 28, 30197–30206. [Google Scholar] [CrossRef]
- Song, Z.H.; Cheng, K.; Zheng, X.C.; Ahmad, H.; Zhang, L.L.; Wang, T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult. Sci. 2018, 97, 430–437. [Google Scholar] [CrossRef]
- Chen, Z.; Xing, T.; Li, J.; Zhang, L.; Jiang, Y.; Gao, F. Oxidative stress impairs the meat quality of broiler by damaging mitochondrial function, affecting calcium metabolism and leading to ferroptosis. Anim. Biosci. 2022, 35, 1616. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, X.; Zeng, Y.; Mo, X.; Hong, S.; He, H.; Li, J.; Fatima, S.; Liu, Q. Oxidative stress induces mitochondrial iron overload and ferroptotic cell death. Sci. Rep. 2023, 13, 15515. [Google Scholar] [CrossRef]
- Chen, G.H.; Song, C.C.; Pantopoulos, K.; Wei, X.L.; Zheng, H.; Luo, Z. Mitochondrial oxidative stress mediated Fe-induced ferroptosis via the NRF2-ARE pathway. Free Radic. Biol. Med. 2022, 180, 95–107. [Google Scholar] [CrossRef]
- Zhang, M.; Dunshea, F.R.; Warner, R.D.; DiGiacomo, K.; Osei-Amponsah, R.; Chauhan, S.S. Impacts of heat stress on meat quality and strategies for amelioration: A review. Int. J. Biometeorol. 2020, 64, 1613–1628. [Google Scholar] [CrossRef]
- Gu, Y.; Qiu, Y.; Wei, X.; Li, Z.; Hu, Z.; Gu, Y.; Zhao, Y.; Wang, Y.; Yue, T.; Yuan, Y. Characterization of selenium-containing polysaccharides isolated from selenium-enriched tea and its bioactivities. Food Chem. 2020, 316, 126371. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, A.; Basu, A.; Bhattacharya, S. Selenium nanoparticles are less toxic than inorganic and organic selenium to mice in vivo. Nucleus 2019, 62, 259–268. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, M.; Guan, Z.; Yang, J.; Liu, Z.; Xu, S. Disbalance of calcium regulation-related genes in broiler hearts induced by selenium deficiency. Avian Pathol. 2017, 46, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Bakhshalinejad, R.; Hassanabadi, A.; Swick, R.A. Dietary sources and levels of selenium supplements affect growth performance, carcass yield, meat quality and tissue selenium deposition in broilers. Anim. Nutr. 2019, 5, 256–263. [Google Scholar] [CrossRef]
- Xiao, D.; Li, T.; Huang, X.; Zhu, K.; Li, Z.; Dong, Y.; Wang, L.; Huang, J. Advances in the Study of Selenium-Enriched Probiotics: From the Inorganic Se into Se Nanoparticles. Mol. Nutr. Food Res. 2023, 2300432. [Google Scholar] [CrossRef]
- Saffari, S.; Keyvanshokooh, S.; Zakeri, M.; Johari, S.; Pasha-Zanoosi, H. Effects of different dietary selenium sources (sodium selenite, selenomethionine and nanoselenium) on growth performance, muscle composition, blood enzymes and antioxidant status of common carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 611–617. [Google Scholar] [CrossRef]
- Bień, D.; Michalczuk, M.; Łysek-Gładysińska, M.; Jóźwik, A.; Wieczorek, A.; Matuszewski, A.; Kinsner, M.; Konieczka, P. Nano-Sized Selenium Maintains Performance and Improves Health Status and Antioxidant Potential While Not Compromising Ultrastructure of Breast Muscle and Liver in Chickens. Antioxidants 2023, 12, 905. [Google Scholar] [CrossRef]
- Xu, X.; Pan, Y.; Liu, X.; Han, Z.; Chen, S. Constructing Selenium Nanoparticles with Enhanced Storage Stability and Antioxidant Activities via Conformational Transition of Curdlan. Foods 2023, 12, 563. [Google Scholar] [CrossRef]
- Ji, H.; Lou, X.; Jiao, J.; Li, Y.; Dai, K.; Jia, X. Preliminary Structural Characterization of Selenium Nanoparticle Composites Modified by Astragalus Polysaccharide and the Cytotoxicity Mechanism on Liver Cancer Cells. Molecules 2023, 28, 1561. [Google Scholar] [CrossRef]
- Tritean, N.; Dima, Ș.O.; Trică, B.; Stoica, R.; Ghiurea, M.; Moraru, I.; Cimpean, A.; Oancea, F.; Constantinescu-Aruxandei, D. Selenium-Fortified Kombucha–Pollen Beverage by In Situ Biosynthesized Selenium Nanoparticles with High Biocompatibility and Antioxidant Activity. Antioxidants 2023, 12, 1711. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.W.; Baowei, W.; Guoqing, H.; Qiaoli, W.; Bin, Y. Effects of yeast selenium supplementation on the growth performance, meat quality, immunity, and antioxidant capacity of goose. J. Anim. Physiol. Anim. Nutr. 2011, 95, 440–448. [Google Scholar]
- Fan, C.; Yu, B.; Chen, D.W. Effects of different sources and levels of selenium on performance, thyroid function and antioxidant status in stressed broiler chickens. Int. J. Poult. Sci. 2009, 8, 583–587. [Google Scholar] [CrossRef][Green Version]
- Ibrahim, R.Y.M.; Saber, A.A.; Hammad, H.B.I. The possible role of the seaweed Ulva fasciata on ameliorating hyperthyroidism-associated heart inflammations in a rat model. Environ. Sci. Pollut. Res. 2021, 28, 6830–6842. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.J. Effects of Nanoselenium-Fucoidan Oligosaccharides on Growth Performance and Intestinal Mechanical Barrier in Heat-Stressed Broilers. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2023. [Google Scholar]
- Khajeh Bami, M.; Afsharmanesh, M.; Ebrahimnejad, H. Effect of dietary Bacillus coagulans and different forms of zinc on performance, intestinal microbiota, carcass and meat quality of broiler chickens. Probiotics Antimicrob. Proteins 2020, 12, 461–472. [Google Scholar] [CrossRef]
- Yue, Y.B.; Zou, L.; Tao, J.; Yin, L.; Xie, Z.R.; Xia, Y.; Zhang, Z.Y.; Wang, K.H.; Zhu, M. Transcriptomics and metabolomics together reveal the underlying mechanism of heroin hepatotoxicity. Toxicology 2023, 483, 153393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ji, O.; Zheng, L.; Lo, H.K. Effect of dietary β-1,3-glucan supplementation and heat stress on growth performance, nutrient digestibility, meat quality, organ weight, ileum microbiota, and immunity in broilers. Poult. Sci. 2020, 99, 4969–4977. [Google Scholar] [CrossRef]
- Tavaniello, S.; Slawinska, A.; Prioriello, D.; Petrecca, V.; Bertocchi, M.; Zampiga, M.; Salvatori, G.; Maiorano, G. Effect of galactooligosaccharides delivered in ovo on meat quality traits of broiler chickens exposed to heat stress. Poult. Sci. 2020, 99, 612–619. [Google Scholar] [CrossRef]
- Wen, C.; Chen, Y.; Leng, Z.; Ding, L.; Wang, T.; Zhou, Y. Dietary betaine improves meat quality and oxidative status of broilers under heat stress. J. Sci. Food Agric. 2019, 99, 620–623. [Google Scholar] [CrossRef]
- Suliman, G.M.; Hussein, E.O.; Al-Owaimer, A.N.; Alhotan, R.A.; AlGaradi, M.A.; Mahdi, J.M.; Ba-Awadh, H.A.; Qaid, M.M.; Swelum, A.A.A. Betaine and nano-emulsified vegetable oil supplementation for improving carcass and meat quality characteristics of broiler chickens under heat stress conditions. Front. Vet. Sci. 2023, 10, 1147020. [Google Scholar] [CrossRef]
- Choi, J.; Kong, B.; Bowker, B.C.; Zhuang, H.; Kim, W.K. Nutritional Strategies to Improve Meat Quality and Composition in the Challenging Conditions of Broiler Production: A Review. Animals 2023, 13, 1386. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Wang, X.; Zhao, F.; Wang, C.; Zhang, Q.; Chen, X.; Geng, Z.; Zhang, C. Resveratrol Attenuates Heat Stress-Induced Impairment of Meat Quality in Broilers by Regulating the Nrf2 Signaling Pathway. Animals 2022, 12, 1889. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H.; Zhang, J.; Zhou, B.; He, X.; Wang, T.; Wang, C. Dietary rutin improves breast meat quality in heat-stressed broilers and protects mitochondria from oxidative attack via the AMPK/PINK1–Parkin pathway. J. Sci. Food Agric. 2023, 103, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Teyssier, J.R.; Preynat, A.; Cozannet, P.; Briens, M.; Mauromoustakos, A.; Greene, E.S.; Owens, C.M.; Dridi, S.; Rochell, S.J. Constant and cyclic chronic heat stress models differentially influence growth performance, carcass traits and meat quality of broilers. Poult. Sci. 2022, 101, 101963. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.S.; Xing, T.; Li, J.L.; Zhang, L.; Jiang, Y.; Gao, F. Transcriptome analysis reveals the mechanism of chronic heat stress on meat quality of broilers. J. Anim. Sci. Biotechnol. 2022, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, X.M.; Zhao, J.S.; Wang, Y.; Hao, X.J.; Liu, K.D.; Liu, H.W. Protective effects of chlorogenic acid on the meat quality of oxidatively stressed broilers revealed by integrated metabolomics and antioxidant analysis. Food Funct. 2022, 13, 2238–2252. [Google Scholar] [CrossRef]
- De Grande, A.; Ducatelle, R.; Leleu, S.; Rapp, C.; Torres, C.; Petracci, M.; De Smet, S.; Michiels, J.; Haesebrouck, F.; Van Immerseel, F. Effects of the dietary zinc source and vitamin E level on live weight and carcass yield and meat quality in male broilers reared under chronic cyclic heat stress conditions in the finisher phase. Front. Physiol. 2022, 13, 992689. [Google Scholar] [CrossRef]
- Mohamed, D.A.; Sazili, A.Q.; Teck Chwen, L.; Samsudin, A.A. Effect of microbiota-selenoprotein on meat selenium content and meat quality of broiler chickens. Animals 2020, 10, 981. [Google Scholar] [CrossRef]
- Lochi, G.M.; Shah, M.G.; Gandahi, J.A.; Gadahi, J.A.; Hadi, S.A.; Farooq, T.; Vistro, W.A.; Rahmani, M.M. Effect of selenium nanoparticles and chitosan on production performance and antioxidant integrity of heat-stressed broiler. Biol. Trace Elem. Res. 2023, 201, 1977–1986. [Google Scholar] [CrossRef]
- Wang, C.L.; Xing, G.Z.; Wang, L.S.; Li, S.F.; Zhang, L.Y.; Lu, L.; Luo, X.G.; Liao, X.D. Effects of selenium source and level on growth performance, antioxidative ability and meat quality of broilers. J. Integr. Agric. 2021, 20, 227–235. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, Y.F.; Wei, Y.; Chen, X.F.; Li, R.; Xie, J.H.; Wang, G.G.; Ming, J.J.; Yin, H.Q.; Xiang, J.Q.; et al. Dietary Se-Enriched Cardamine enshiensis Supplementation Alleviates Transport-Stress-Induced Body Weight Loss, Anti-Oxidative Capacity and Meat Quality Impairments of Broilers. Animals 2022, 12, 3193. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Niazi, A.S.; Arslan, M.; Azhar, M.; Asad, T.; Raziq, F.; Gondal, M.A.; Rauf, M.; Liaqat, S.; Naz, S.; et al. Effects of selenium supplementation on the growth performance, slaughter characteristics, and blood biochemistry of naked neck chicken. Poult. Sci. 2023, 102, 102420. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, X.; Xue, Y.; Lin, G.; Xiong, Y.L. Dietary linseed oil supplemented with organic selenium improved the fatty acid nutritional profile, muscular selenium deposition, water retention, and tenderness of fresh pork. Meat Sci. 2017, 131, 99–106. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult. Sci. 2011, 90, 680–686. [Google Scholar] [CrossRef]
- Yang, M.X.; Lu, Z.J.; Li, F.L.; Shi, F.; Zhan, F.B.; Zhang, Y.L.; Zhao, L.J.; Li, Y.; Li, J.; Lin, L.; et al. Alginate oligosaccharide improves fat metabolism and antioxidant capacity in the liver of grass carp (Ctenopharyngodon idellus). Aquaculture 2021, 540, 736664. [Google Scholar] [CrossRef]
- Ramanathan, R.; Kiyimba, F.; Suman, S.P.; Mafi, G.G. The potential of metabolomics in meat science: Current applications, future trends, and challenges. J. Proteom. 2023, 104926. [Google Scholar] [CrossRef]
- Shi, K.; Zhao, Q.; Shao, M.H.; Duan, Y.; Li, D.F.; Lu, Y.Q.; Tang, Y.F.; Feng, C.G. Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat. Metabolites 2022, 12, 367. [Google Scholar] [CrossRef]
- Fang, M.X.; Hu, W.; Liu, B. Effects of nano-selenium on cecum microbial community and metabolomics in chickens challenged with Ochratoxin A. Front. Vet. Sci. 2023, 10, 1228360. [Google Scholar] [CrossRef]
- Bejaoui, B.; Sdiri, C.; Souf, I.B.; Slimen, I.B.; Larbi, M.B.; Koumba, S.; Martin, P.; M’Hamdi, N. Physicochemical Properties, Antioxidant Markers, and Meat Quality as Affected by Heat Stress: A Review. Molecules 2023, 28, 3332. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Majdeddin, M.; Degroote, J.; Liefferinge, E.V.; Noten, N.V.; Kerschaver, C.V.; Vandaele, M.; Dorigam, J.C.D.P.; Michiels, J. Effect of supplemental methyl sulfonyl methane on performance, carcass and meat quality and oxidative status in chronic cyclic heat-stressed finishing broilers. Poult. Sci. 2023, 102, 102321. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Liu, Y.; Ye, Y.W.; Tao, Z.G.; Cheng, Z.J.; Wang, T.; Zhou, Y.M. Effects of gingerols-rich extract of ginger on growth performance, serum metabolites, meat quality and antioxidant activity of heat-stressed broilers. J. Therm. Biol. 2020, 89, 102544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Su, S.F.; Zhao, J.; He, X.L.; Fu, S.Y.; Wang, B.; Wang, Y.F.; Wang, D.Q.; Yun, N.N.; Chen, X.; et al. Effects of dietary oat supplementation on carcass traits, muscle metabolites, amino acid profiles, and its association with meat quality of Small-tail Han sheep. Food Chem. 2023, 411, 135456. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yu, M.; Liu, Z.; Deng, D.; Cui, Y.; Tian, Z.; Wang, G. Effect of amino acids and their derivatives on meat quality of finishing pigs. J. Food Sci. Technol. 2020, 57, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat Sci. 2020, 162, 108025. [Google Scholar] [CrossRef]
- Yin, C.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens. Animals 2021, 11, 630. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Wang, Y.; Zhao, G.; Wen, J.; Cui, H. Metabolomics-Based Analysis of the Major Taste Contributors of Meat by Comparing Differences in Muscle Tissue between Chickens and Common Livestock Species. Foods 2022, 11, 3586. [Google Scholar] [CrossRef]
- Tian, X.Z.; Li, J.X.; Luo, Q.Y.; Wang, X.; Xiao, M.M.; Zhou, D.; Lu, Q.; Chen, X. Effect of supplementation with selenium-yeast on muscle antioxidant activity, meat quality, fatty acids and amino acids in goats. Front. Vet. Sci. 2022, 8, 813672. [Google Scholar] [CrossRef]
- Cruvinel, J.M.; Groff Urayama, P.M.; Oura, C.Y.; de Lima Krenchinski, F.K.; dos Santos, T.S.; de Souza, B.A.; Kadri, S.M.; Correa, C.R.; Sartori, J.R.; Pezzato, A.C. Pequi Oil (Caryocar brasiliense Camb.) Attenuates the Adverse Effects of Cyclical Heat Stress and Modulates the Oxidative Stress-Related Genes in Broiler Chickens. Animals 2023, 13, 1896. [Google Scholar] [CrossRef]
- Hu, H.; Bai, X.; Xu, K.; Zhang, C.; Chen, L. Effect of phloretin on growth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 2021, 100, 101217. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.P.; Liu, Y.L.; Zhang, J.X.; Ding, K.N.; Lu, M.H.; He, Y.M. Heat stress in broilers of liver injury effects of heat stress on oxidative stress and autophagy in liver of broilers. Poult. Sci. 2022, 101, 102085. [Google Scholar] [CrossRef] [PubMed]
- Alian, H.A.; Samy, H.M.; Ibrahim, M.T.; Mahmoud, M.M. Nanoselenium effect on growth performance, carcass traits, antioxidant activity, and immune status of broilers. Environ. Sci. Pollut. Res. 2020, 27, 38607–38616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Li, X.; Wei, Y. Selenium and Selenoproteins in Health. Biomolecules 2023, 13, 799. [Google Scholar] [CrossRef]
- Jing, J.Z.; Zeng, H.J.; Shao, Q.J.; Tang, J.Y.; Wang, L.Q.; Jia, G.; Liu, G.M.; Chen, X.L.; Tian, G.; Cai, J.Y.; et al. Selenomethionine alleviates environmental heat stress induced hepatic lipid accumulation and glycogen infiltration of broilers via maintaining mitochondrial and endoplasmic reticulum homeostasis. Redox Biol. 2023, 67, 102912. [Google Scholar] [CrossRef]
- Cao, L.; Tang, J.; Li, Q.; Xu, J.; Jia, G.; Liu, G.; Chen, X.; Shang, H.; Cai, J.; Zhao, H. Expression of selenoprotein genes is affected by heat stress in IPEC-J2 cells. Biol. Trace Elem. Res. 2016, 172, 354–360. [Google Scholar] [CrossRef]
- Abo El-Magd, N.F.; Barbosa, P.O.; Nick, J.; Covalero, V.; Grignetti, G.; Bermano, G. Selenium, as selenite, prevents adipogenesis by modulating selenoproteins gene expression and oxidative stress–related genes. Nutrition 2022, 93, 111424. [Google Scholar] [CrossRef]
- Hu, H.; Dai, S.; Li, J.; Wen, A.; Bai, X. Glutamine improves heat stress–induced oxidative damage in the broiler thigh muscle by activating the nuclear factor erythroid 2–related 2/Kelch-like ECH-associated protein 1 signaling pathway. Poult. Sci. 2020, 99, 1454–1461. [Google Scholar] [CrossRef]
- Song, D.Z.; Cheng, Y.Z.; Li, X.X.; Wang, F.Q.; Lu, Z.Q.; Xiao, X.; Wang, Y.Z. Biogenic Nanoselenium Particles Effectively Attenuate Oxidative Stress-Induced Intestinal Epithelial Barrier Injury by Activating the Nrf2 Antioxidant Pathway. ACS Appl. Mater. Interfaces 2017, 9, 14724–14740. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, S.; Song, Y.; Yuan, J.; Hu, S.; Chen, M.; Li, L. Alginate oligosaccharide alleviated cisplatin-induced kidney oxidative stress via lactobacillus genus-FAHFAs-Nrf2 Axis in mice. Front. Immunol. 2022, 13, 857242. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Y.; Liu, M.; Lu, J.; Guan, S. Toward improved human health: Nrf2 plays a critical role in regulating ferroptosis. Food Funct. 2021, 12, 9583–9606. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Huang, C.; Liu, J.; Meng, C.; Gu, Q.; Du, X.; Yan, M.; Yu, Y.; Liu, F.; Xia, C. Nrf2 and its dependent autophagy activation cooperatively counteract ferroptosis to alleviate acute liver injury. Pharmacol. Res. 2023, 187, 106563. [Google Scholar] [CrossRef] [PubMed]
- El-Benhawy, S.A.; Abdelrhman, I.G.; Sadek, N.A.; Fahmy, E.I.; AboGabal, A.A.; Elmasry, H.; Saleh, S.A.; Sakr, O.A.; Elwany, M.N.; Rabie, M.A.F. Studying ferroptosis and iron metabolism pre-and post-radiotherapy treatment in breast cancer patients. J. Egypt. Natl. Cancer Inst. 2023, 35, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yang, K.T.; Lee, W.S.; Ting, P.C.; Luo, Y.P.; Lin, D.J.; Wang, Y.S.; Chang, J.C. Xanthohumol Protects the Rat Myocardium against Ischemia/Reperfusion Injury-Induced Ferroptosis. Oxid. Med. Cell. Longev. 2022, 2022, 9523491. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Ye, J.; Su, Y.; Zhang, X.; Pang, X.; Liao, J.; Wang, R.; Zhao, C.; Zhang, H.; Hu, L.; et al. Co-exposure to environmentally relevant concentrations of cadmium and polystyrene nanoplastics induced oxidative stress, ferroptosis and excessive mitophagy in mice kidney. Environ. Pollut. 2023, 333, 121947. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, N.; Ji, Y.; Sheng, X.; Huo, J.; Zhu, Y.; Huang, M.; He, W.; Ma, J. Brusatol induces ferroptosis in oesophageal squamous cell carcinoma by repressing GSH synthesis and increasing the labile iron pool via inhibition of the NRF2 pathway. Biomed. Pharmacother. 2023, 167, 115567. [Google Scholar] [CrossRef]
- Schimanski, L.M.; Drakesmith, H.; Merryweather-Clarke, A.T.; Viprakasit, V.; Edwards, J.P.; Sweetland, E.; Bastin, J.M.; Cowley, D.; Chinthammitr, Y.; Robson, K.J.; et al. In vitro functional analysis of human ferroportin (FPN) and hemochromatosis-associated FPN mutations. Blood 2005, 105, 4096–4102. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Yuan, T.; Xie, L.; Zhang, L. EPC-exosomal miR-26a-5p improves airway remodeling in COPD by inhibiting ferroptosis of bronchial epithelial cells via PTGS2/PGE2 signaling pathway. Sci. Rep. 2023, 13, 6126. [Google Scholar] [CrossRef]
- Yi, T.T.; Zhang, L.M.; Huang, X.N. Glycyrrhizic acid protects against temporal lobe epilepsy in young rats by regulating neuronal ferroptosis through the miR-194-5p/PTGS2 axis. Kaohsiung J. Med. Sci. 2023, 39, 154–165. [Google Scholar] [CrossRef]
- Zhao, L.; Feng, Y.; Xu, Z.J. Selenium mitigated aflatoxin B1-induced cardiotoxicity with potential regulation of 4 selenoproteins and ferroptosis signaling in chicks. Food Chem. Toxicol. 2021, 154, 112320. [Google Scholar] [CrossRef]
- Jung, Y.J.; Choi, H.; Oh, E. Selenium mitigates ferroptosis-mediated dopaminergic cell death by regulating the Nrf2/GPX4 pathway. Neurosci. Lett. 2023, 810, 137314. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Luan, Y.; Wang, H.; Zhang, P.; Liu, S.; Wang, P.; Cao, Y.; Sun, H.; Wu, L. Selenium inhibits ferroptosis and ameliorates autistic-like behaviors of BTBR mice by regulating the Nrf2/GPx4 pathway. Brain Res. Bull. 2022, 183, 38–48. [Google Scholar] [CrossRef] [PubMed]

| Items | TN | HS | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CON | SeNPs-AOS | CON | SeNPs-AOS | Temp. | SeNPs-AOS | Temp. × SeNPs-AOS | ||
| Breast muscle rate | 0.25 | 0.25 | 0.24 | 0.25 | 0.009 | 0.516 | 0.986 | 0.870 |
| pH (0 min) | 6.77 a | 6.69 ab | 6.55 b | 6.63 ab | 0.055 | 0.022 | 0.952 | 0.145 |
| pH (24 h) | 6.49 a | 6.32 b | 6.38 ab | 6.34 ab | 0.051 | 0.400 | 0.063 | 0.214 |
| Shear force | 5.94 b | 5.91 b | 8.24 a | 5.88 b | 0.360 | 0.180 | 0.001 | 0.016 |
| Drip loss | 0.05 | 0.05 | 0.06 | 0.05 | 0.006 | 0.005 | 0.004 | 0.004 |
| Cooking loss | 0.23 b | 0.29 a | 0.31 a | 0.29 a | 0.010 | 0.002 | 0.048 | 0.001 |
| Freezing loss | 0.07 b | 0.06 bc | 0.09 a | 0.06 c | 0.003 | 0.045 | <0.001 | 0.001 |
| Lightness L* | 47.65 a | 46.45 ab | 46.11 ab | 43.70 b | 1.071 | 0.059 | 0.106 | 0.579 |
| Redness a* | 3.30 ab | 3.47 a | 2.44 bc | 2.12 c | 0.293 | 0.001 | 0.792 | 0.413 |
| Yellowness b* | 12.23 ab | 13.88 a | 12.70 ab | 11.59 a | 0.685 | 0.197 | 0.698 | 0.056 |
| Items | TN | HS | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|---|
| CON | SeNPs-AOS | CON | SeNPs-AOS | Temp. | SeNPs-AOS | Temp. × SeNPs-AOS | ||
| T-AOC, mmol/mg prot | 0.29 a | 0.25 ab | 0.24 c | 0.23 bc | 19.747 | 0.002 | 0.804 | 0.037 |
| T-SOD, U/mg prot | 239.96 a | 231.04 a | 172.42 b | 186.94 b | 13.039 | 0.001 | 0.832 | 0.380 |
| CAT, U/mg prot | 5.20 a | 5.05 a | 2.10 b | 4.02 a | 0.404 | <0.001 | 0.041 | 0.018 |
| MDA, nmol/mg prot | 0.39 b | 0.40 b | 0.66 a | 0.48 b | 0.037 | 0.001 | 0.030 | 0.015 |
| GSH-Px, U/mg prot | 25.96 a | 29.54 a | 11.23 b | 26.02 a | 1.804 | <0.001 | <0.001 | 0.006 |
| GST, U/mg prot | 29.26 ab | 35.55 a | 20.74 b | 30.43 ab | 3.428 | 0.06 | 0.030 | 0.625 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.-Y.; An, Y.-C.; Zhang, S.-Y.; Huang, M.-Y.; Ye, X.-Q.; Zhao, Z.-H.; Liu, W.-C. Biogenic Selenium Nanoparticles Synthesized Using Alginate Oligosaccharides Attenuate Heat Stress-Induced Impairment of Breast Meat Quality via Regulating Oxidative Stress, Metabolome and Ferroptosis in Broilers. Antioxidants 2023, 12, 2032. https://doi.org/10.3390/antiox12122032
Yang Y-Y, An Y-C, Zhang S-Y, Huang M-Y, Ye X-Q, Zhao Z-H, Liu W-C. Biogenic Selenium Nanoparticles Synthesized Using Alginate Oligosaccharides Attenuate Heat Stress-Induced Impairment of Breast Meat Quality via Regulating Oxidative Stress, Metabolome and Ferroptosis in Broilers. Antioxidants. 2023; 12(12):2032. https://doi.org/10.3390/antiox12122032
Chicago/Turabian StyleYang, Yu-Ying, Yu-Chen An, Shu-Yue Zhang, Meng-Yi Huang, Xue-Qing Ye, Zhi-Hui Zhao, and Wen-Chao Liu. 2023. "Biogenic Selenium Nanoparticles Synthesized Using Alginate Oligosaccharides Attenuate Heat Stress-Induced Impairment of Breast Meat Quality via Regulating Oxidative Stress, Metabolome and Ferroptosis in Broilers" Antioxidants 12, no. 12: 2032. https://doi.org/10.3390/antiox12122032
APA StyleYang, Y.-Y., An, Y.-C., Zhang, S.-Y., Huang, M.-Y., Ye, X.-Q., Zhao, Z.-H., & Liu, W.-C. (2023). Biogenic Selenium Nanoparticles Synthesized Using Alginate Oligosaccharides Attenuate Heat Stress-Induced Impairment of Breast Meat Quality via Regulating Oxidative Stress, Metabolome and Ferroptosis in Broilers. Antioxidants, 12(12), 2032. https://doi.org/10.3390/antiox12122032







