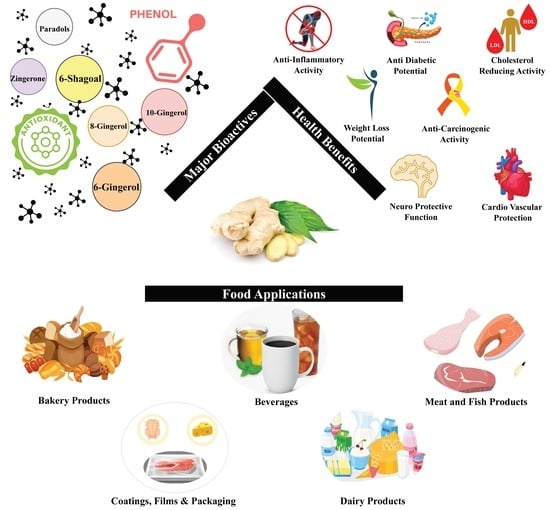
Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications
Abstract
1. Introduction
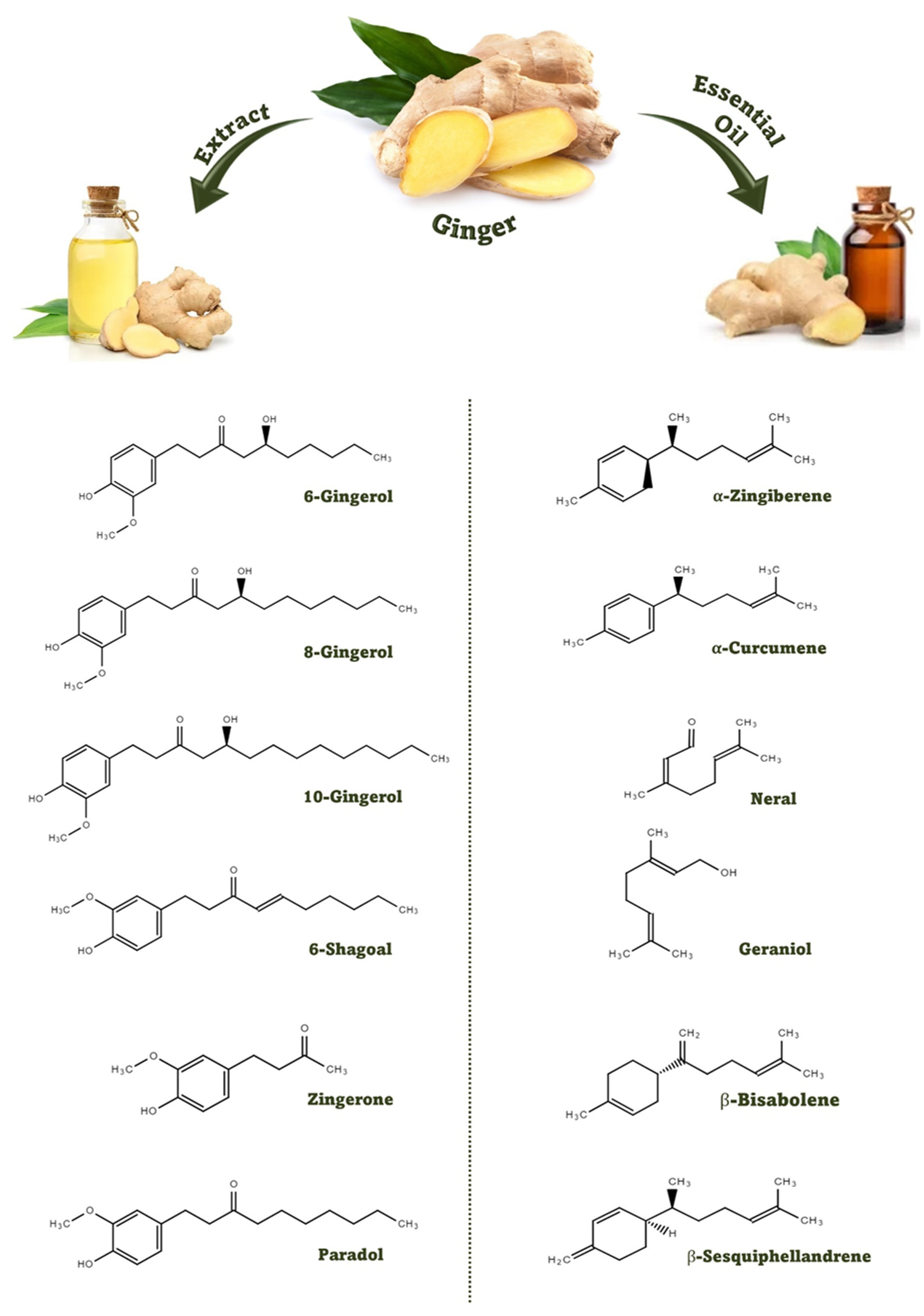
2. Bioactive Compounds in Ginger
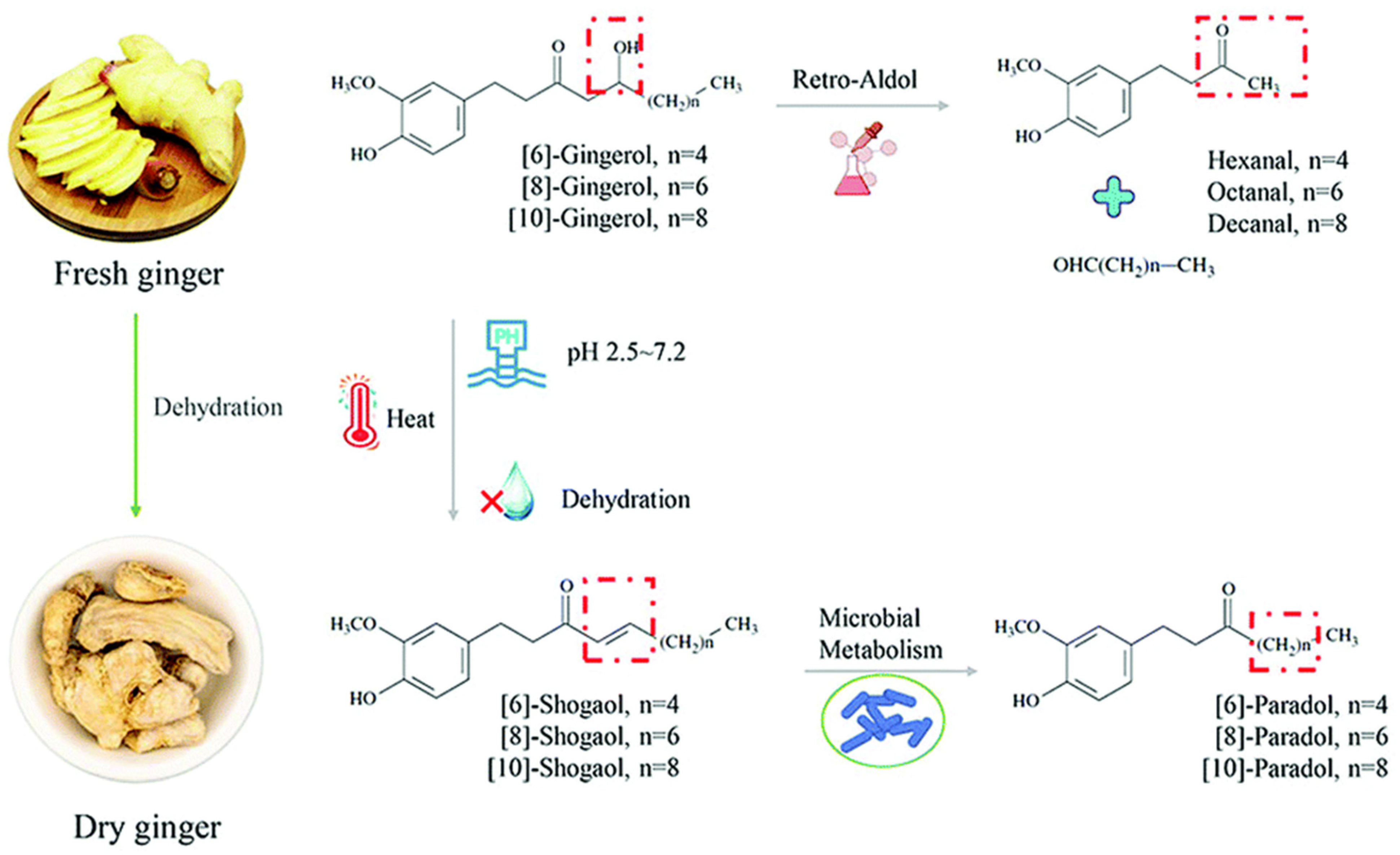
3. Effect of Processing and Extraction Process on Ginger Bioactive Compounds
4. Beneficial Aspects of Bioactive Compounds
4.1. Antioxidant Activity
4.2. Anti-Inflammatory Activity
4.3. Antimicrobial Activity
4.4. Anti-Carcinogenic Potential
4.5. Neuroprotective Activity
4.6. Cardiovascular Protection
4.7. Anti-Obese Activity
4.8. Anti-Diabetic Effect
5. Food Applications
5.1. Bakery Products
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Wheat Bread | ↑ Antioxidant Activity; Minerals; Total Phenolics; Textural Properties; Shelf Life; Sensorial Characteristics; ↓ Microbial Growth | [73,77] |
| Cookies | ↑ Antioxidants; Phenolic Contents; Shelf Life; ↓ Acrylamide Content; Microbial Growth; ≈ Sensorial Characteristics | [82,83,85,86] | |
| Cake | ↑ Antioxidant Activity; Total Phenolics, Shelf Life; Sensorial Properties; ↓ Bacterial and Fungal growth | [89] | |
| Ginger Juice | Wheat Flour Muffin | ↑ Total Phenolics; Antioxidants; Sensorial Characteristics; ≈ Textural Attributes | [90] |
| Ginger Extract | Baked Bars | ↑ Antioxidants; Phenolic Contents; Antioxidant Retention during Storage; Flavor and Color; ≈ Overall Acceptability | [91] |
5.2. Dairy Products
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Yogurt | ↑ Antioxidant Activity; Phenolic Contents; Total Solids; Viscosity; Textural Properties, Storage Stability; ↓ Syneresis; pH; Time; Energy; ≈ Sensorial Characteristics | [92,93] |
| PEDA | ↑ Functionality; Shelf Life; ↓ Microbial Growth; Cost | [96] | |
| Ginger Juice | Yogurt | ↑ Total Phenolic Contents; Antioxidant Total Solids; Viscosity; ↓ Coagulation Time; pH | [97,98] |
| Ice Cream | ↑ Antioxidant Activity; Phenolic Content; Sensorial Characteristics; Melting Resistance | [101,102,104] | |
| Ginger Extract | Yogurt | ↑ Antioxidants; Phenolic Contents; Sensorial Properties; ↓ Syneresis; pH | [105,107] |
| Labneh | ↑ Antimicrobial Activity; Antioxidants; Phenolic Contents; Sensorial Characteristics; ↓ Microbial Growth; pH | [109] | |
| Cheese | ↑ Antioxidant Activity; Total Phenols; Oxidative Stability; Storage Stability; Sensorial Characteristics; Coagulation ↓ Microbial Growth | [110,112] | |
| Ice Cream | ↑ Antioxidants; Total Phenolic Contents; Sensorial Properties; ↓ Microbial Count; ≈ Viscosity; Overrun | [113,114] | |
| Ginger Protease | Cheese | ↑ Proteolysis; Functionality; Coagulation; Sensorial Properties | [111] |
| Ginger Essential Oil | Soft Cheese | ↑ Antioxidants; Phenolic Contents; Sensorial Characteristics; ↓ Microbial Growth | [117] |
5.3. Beverages
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Cocoa Beverage | ↑ Total Phenolic Contents; Antioxidant Activity; Viscosity | [118] |
| Ginger Extract | Carrot and Kinnow RTS Drink | ↑ Antioxidant Activity; Preservation; ↓ Microbial Growth; ≈ Sensorial Properties | [120] |
| Zobo Drink | ↑ Functionality; Shelf Life; Sensorial Properties; ↓ Microbial Count | [126] | |
| Black Tea | ↑ Antioxidant Activity; Phenolic Contents; Sensorial Properties | [131] | |
| Ginger Iced Tea | ↑ Nutraceutical Compounds; Shelf Life; Organoleptic Attributes | [133] | |
| Robusta Coffee | ↑ Antioxidant Activity; Phenolic Contents; ≈ Sensorial Properties | [135] |
5.4. Meat and Fish Products
| Derivative | Product | Positive Effect | Reference |
|---|---|---|---|
| Ginger Powder | Rabbit Burgers | ↑ Antioxidant Activity; Fatty Acid Profile; Shelf Life; ↓ Lipid Peroxidation | [136] |
| Pork Burger | ↑ Antioxidant Activity; PUFA; Oxidative Stability; Sensorial Characteristics; ↓ Microbial Load | [137] | |
| Shredded Ginger | Canned Pork | ↑ Antioxidant Activity; Tenderness; Organoleptic Properties; ↓ Microbial Load | [138] |
| Ginger Paste | Chicken Meat | ↑ Antioxidants; Antimicrobial Activity; Oxidative Stability ↓ Microbial Load | [140,143] |
| Ginger Juice | Goat Meat | ↑ Antioxidant Activity; Tenderness; Oxidative Resistance; Storage Stability; Overall Acceptability; ↓ Gamey Odor | [141] |
| Beef | ↑ Antioxidant Activity; Tenderness; Sensorial Properties; ↓ Cooking Loss | [145] | |
| Ginger Extract | Camel Meat Patties | ↑ Antioxidants; Lipid Stability; Collagen Solubility; Sensorial Properties | [144] |
| Smoked Buffalo Meat | ↑ Antioxidant Activity; Tenderness; Sensorial Characteristics; ↓ Cooking Loss; Microbial Load | [146] | |
| Smoked Fish | ↑ Antioxidants; Shelf Life; Organoleptic Attributes; ↓ Mold Count; Oxidative Rancidity | [147] | |
| Tilapia Fish Burger | ↑ Antioxidant Activity; Shelf Life; ↓ Lipid Oxidation | [148] | |
| Yak Meat | ↑ Antioxidant Activity; Tenderness; Oxidative Resistance; Cooking Yield; ↓ Microbial Load | [150] | |
| Gingerol Rich Extract | Chicken Feed | ↑ Antioxidant Activity; Bioactive Compounds; Meat Quality | [155] |
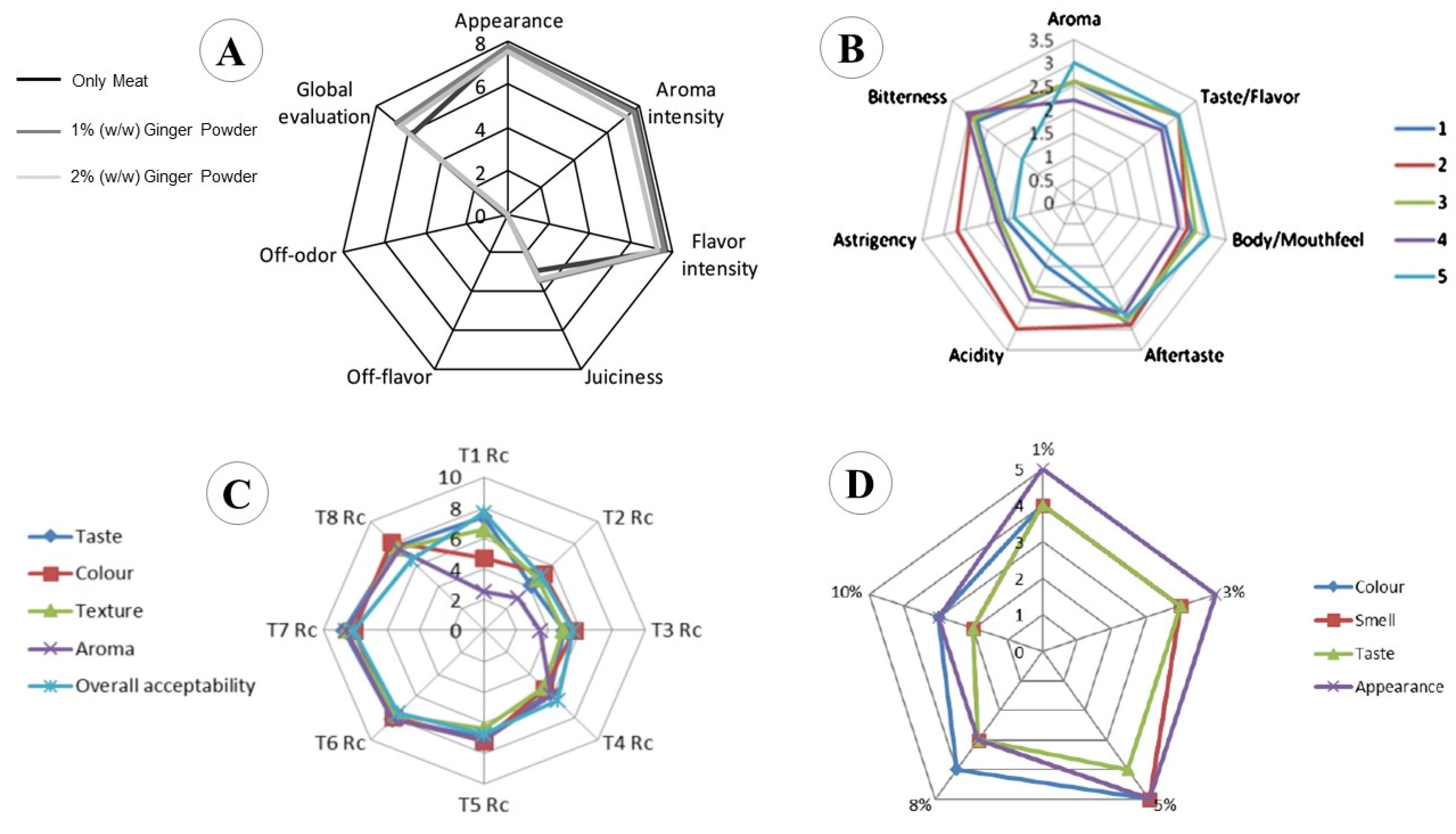
5.5. Film, Coating, and Packaging
| Derivative | Product | Positive Effects | Reference |
|---|---|---|---|
| Ginger Extract | Chitosan-Based Edible Coating (Walnut) | ↑ Antioxidant Activity; Shelf Life; Oxidative Stability; ↓ Fungal Spoilage | [159] |
| Garlic Extract cum Vacuum Packaging (Fish Fillet) | ↑ Antioxidant Activity; Shelf Life; Oxidative Stability; Sensorial Properties; ↓ Microbial Spoilage | [160] | |
| Ginger Essential Oil | Whey Protein Isolate Coating (Cheese) | ↑ Anti-bacterial Activity; Shelf Life; Water and Fat Barrier Properties | [161] |
| Edible Coating (Chicken Meat) | ↑ Shelf Life; Sensorial Characteristics ↓ Microbial Growth; ≈ Antioxidant Activity | [164] | |
| Fish Protein and Chitosan Composite Films | ↑ Antioxidant Activity; Shelf Life; Water Barrier Properties | [166] | |
| Cinnamon EO Chitosan Films (Pork) | ↑ Antioxidant Activity; Shelf Life; Film Thickness; ↓ Microbial Spoilage; Lipid Oxidation | [167] |
6. Conclusions and Future Prospectus
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Elzebroek, A.T.G.; Wind, K. Guide to Cultivated Plants; CABI: Wallingford, CT, USA, 2008; p. 540. [Google Scholar]
- Srinivasan, K. Ginger Rhizomes (Zingiber officinale): A Spice with Multiple Health Beneficial Potentials. Pharma Nutr. 2017, 5, 18–28. [Google Scholar] [CrossRef]
- Nair, K.P. Turmeric (Curcuma longa L.) and Ginger (Zingiber Oo Cinale Rosc.)—World’s Invaluable Medicinal Spices the Agronomy and Economy of Turmeric and Ginger; Springer: Cham, Switzerland, 2019; p. 568. [Google Scholar]
- Baliga, M.S.; Shivashankara, A.R.; Haniadka, R.; Palatty, P.L.; Arora, R.; Fayad, R. Ginger (Zingiber officinale Roscoe): An Ancient Remedy and Modern Drug in Gastrointestinal Disorders. In Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease, 1st ed.; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 187–199. [Google Scholar] [CrossRef]
- Mahboubi, M. Zingiber officinale Rosc. Essential Oil, a Review on Its Composition and Bioactivity. Clin. Phytosci. 2019, 5, 12. [Google Scholar] [CrossRef]
- Ma, R.H.; Ni, Z.J.; Zhu, Y.Y.; Thakur, K.; Zhang, F.; Zhang, Y.Y.; Hu, F.; Zhang, J.G.; Wei, Z.J. A Recent Update on the Multifaceted Health Benefits Associated with Ginger and Its Bioactive Components. Food Funct. 2021, 12, 519–542. [Google Scholar] [CrossRef]
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Ginger: A Potential Neutraceutical, An Updated Review. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 1227–1238. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H. Bin Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Kamaruddin, M.S.H.; Chong, G.H.; Mohd Daud, N.; Putra, N.R.; Md Salleh, L.; Suleiman, N. Bioactivities and Green Advanced Extraction Technologies of Ginger Oleoresin Extracts: A Review. Food Res. Int. 2023, 164, 112283. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Valencia, G.A.; Monteiro, A.R. Impact of Drying and Extractions Processes on the Recovery of Gingerols and Shogaols, the Main Bioactive Compounds of Ginger. Food Res. Int. 2022, 154I, 111043. [Google Scholar] [CrossRef]
- Garza-Cadena, C.; Ortega-Rivera, D.M.; Machorro-García, G.; Gonzalez-Zermeño, E.M.; Homma-Dueñas, D.; Plata-Gryl, M.; Castro-Muñoz, R. A Comprehensive Review on Ginger (Zingiber officinale) as a Potential Source of Nutraceuticals for Food Formulations: Towards the Polishing of Gingerol and Other Present Biomolecules. Food Chem. 2023, 413, 1–18. [Google Scholar] [CrossRef]
- Tavares, L.; Smaoui, S.; Pinilla, C.M.B.; Ben Hlima, H.; Lopes Barros, H. Ginger: A Systematic Review of Clinical Trials and Recent Advances in Encapsulation of Its Bioactive Compounds. Food Funct. 2022, 13, 1078–1091. [Google Scholar] [CrossRef]
- Beristain-Bauza, S.D.C.; Hernández-Carranza, P.; Cid-Pérez, T.S.; Ávila-Sosa, R.; Ruiz-López, I.I.; Ochoa-Velasco, C.E. Antimicrobial Activity of Ginger (Zingiber officinale) and Its Application in Food Products. Food Rev. Int. 2019, 35, 407–426. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research Progress on Chemical Constituents of Zingiber officinale Roscoe. Biomed. Res. Int. 2019, 2019, 29. [Google Scholar] [CrossRef]
- Jolad, S.D.; Lantz, R.C.; Guan, J.C.; Bates, R.B.; Timmermann, B.N. Commercially Processed Dry Ginger (Zingiber officinale): Composition and Effects on LPS-Stimulated PGE2 Production. Phytochemistry 2005, 66, 1614–1635. [Google Scholar] [CrossRef]
- Sang, S.; Snook, H.D.; Tareq, F.S.; Fasina, Y. Precision Research on Ginger: The Type of Ginger Matters. J. Agric. Food Chem. 2020, 68, 8517–8523. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Roshan, S.; Hammad, S.; Pandohee, J.; Hu, X.; Zengin, G. Ginger and Its Active Compounds in Cancer Therapy: From Folk Uses to Nano-Therapeutic Applications. Semin. Cancer Biol. 2021, 69, 140–149. [Google Scholar] [CrossRef]
- Afzal, M.; Al-Hadidi, D.; Menon, M.; Pesek, J.; Dhami, M.S.I. Ginger: An Ethnomedical, Chemical and Pharmacological Review. Drug Metabol. Drug Interact. 2001, 18, 159–190. [Google Scholar] [CrossRef]
- Vasala, P.A. Ginger. In Handbook of Herbs and Spices; Peter, K.V., Ed.; Woodhead Publishing: Boca Raton FL, USA, 2012; Volume 1, pp. 195–206. [Google Scholar]
- Kuo, P.L.; Hsu, Y.L.; Huang, M.S.; Tsai, M.J.; Ko, Y.C. Ginger Suppresses Phthalate Ester-Induced Airway Remodeling. J. Agric. Food Chem. 2011, 59, 3429–3438. [Google Scholar] [CrossRef]
- Weng, C.J.; Wu, C.F.; Huang, H.W.; Ho, C.T.; Yen, G.C. Anti-Invasion Effects of 6-Shogaol and 6-Gingerol, Two Active Components in Ginger, on Human Hepatocarcinoma Cells. Mol. Nutr. Food Res. 2010, 54, 1618–1627. [Google Scholar] [CrossRef]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, Cytoprotective and Antioxidant Effects of Isolated Phenolic Compounds from Fresh Ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Nemati, M. Therapeutic Potentials of Ginger for Treatment of Multiple Sclerosis: A Review with Emphasis on Its Immunomodulatory, Anti-Inflammatory and Anti-Oxidative Properties. J. Neuroimmunol. 2018, 324, 54–75. [Google Scholar] [CrossRef]
- Abdullahi, A.; Ahmad, K.; Ismail, I.S.; Asib, N.; Ahmed, O.H.; Abubakar, A.I.; Siddiqui, Y.; Ismail, M.R. Potential of Using Ginger Essential Oils-Based Nanotechnology to Control Tropical Plant Diseases. Plant Pathol. J. 2020, 36, 515–535. [Google Scholar] [CrossRef]
- Malik, A.; Shimpy; Kumar, M. Advancements in Ginger Drying Technologies. J. Stored Prod. Res. 2023, 100, 102058. [Google Scholar] [CrossRef]
- Zagórska, J.; Czernicka-Boś, L.; Kukula-Koch, W.; Szalak, R.; Koch, W. Impact of Thermal Processing on the Composition of Secondary Metabolites of Ginger Rhizome—A Review. Foods 2022, 11, 3484. [Google Scholar] [CrossRef]
- Ghafoor, K.; Al Juhaimi, F.; Özcan, M.M.; Uslu, N.; Babiker, E.E.; Mohamed Ahmed, I.A. Total Phenolics, Total Carotenoids, Individual Phenolics and Antioxidant Activity of Ginger (Zingiber officinale) Rhizome as Affected by Drying Methods. LWT 2020, 126, 109354. [Google Scholar] [CrossRef]
- Schwertner, H.A.; Rios, D.C. High-Performance Liquid Chromatographic Analysis of 6-Gingerol, 8-Gingerol, 10-Gingerol, and 6-Shogaol in Ginger-Containing Dietary Supplements, Spices, Teas, and Beverages. J. Chromatogr. B 2007, 856, 41–47. [Google Scholar] [CrossRef]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.T. Occurrence, Biological Activity and Metabolism of 6-Shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef]
- Ho, S.C.; Su, M.S. Optimized Heat Treatment Enhances the Anti-Inflammatory Capacity of Ginger. Int. J. Food Prop. 2016, 19, 1884–1898. [Google Scholar] [CrossRef]
- Ozola, B.; Augspole, I.; Duma, M.; Kreicbergs, V. Bioactive Compounds in Fresh and Dried Ginger Root (Zingiber officinale). Food Balt. 2019, 8, 265–268. [Google Scholar]
- Tanweer, S.; Mehmood, T.; Zainab, S.; Ahmad, Z.; Shehzad, A. Comparison and HPLC Quantification of Antioxidant Profiling of Ginger Rhizome, Leaves and Flower Extracts. Clin. Phytosci. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of Emerging Technologies for the Extraction of Bioactive Compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Bairaktari, M.; Kostopoulou, I.; Pontillo, A.R.N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–599. [Google Scholar] [CrossRef]
- Jang, T.W.; Choi, J.S.; Park, J.H. Protective and Inhibitory Effects of Acteoside from Abeliophyllum Distichum Nakai against Oxidative DNA Damage. Mol. Med. Rep. 2020, 22, 2076–2084. [Google Scholar] [CrossRef]
- Sousa, C.M.d.M.; Silva, H.R.; Vieira, G.M., Jr.; Ayres, M.C.C.; da Costa, C.L.S.; Delton, S.A.; Cavalcante, L.C.D.; Barros, E.D.S.; Araújo, P.B.d.M.; Brandão, M.S.; et al. Total Phenolic and Antioxidant Activity of Five Medicinal Plants. Quim. Nova 2007, 30, 351–355. [Google Scholar] [CrossRef]
- Yehya, A.H.S.; Asif, M.; Tan, Y.J.; Sasidharan, S.; Abdul Majid, A.M.S.; Oon, C.E. Broad Spectrum Targeting of Tumor Vasculature by Medicinal Plants: An Updated Review. J. Herb. Med. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M.U.R. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-Methoxyphenyl)-2-Butanone). Sci. World J. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Sikdar, S.; Paul, A.; Ghosh, S.; Khuda-Bukhsh, A.R. [6]-Gingerol Isolated from Ginger Attenuates Sodium Arsenite Induced Oxidative Stress and Plays a Corrective Role in Improving Insulin Signaling in Mice. Toxicol. Lett. 2012, 210, 34–43. [Google Scholar] [CrossRef]
- Ali, A.M.A.; El-Nour, M.E.A.M.; Yagi, S.M. Total Phenolic and Flavonoid Contents and Antioxidant Activity of Ginger (Zingiber officinale Rosc.) Rhizome, Callus and Callus Treated with Some Elicitors. J. Genet. Eng. Biotechnol. 2018, 16, 677–682. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative Antioxidant and Anti-Inflammatory Effects of [6]-Gingerol, [8]-Gingerol, [10]-Gingerol and [6]-Shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Morvaridzadeh, M.; Fazelian, S.; Agah, S.; Khazdouz, M.; Rahimlou, M.; Agh, F.; Potter, E.; Heshmati, S.; Heshmati, J. Effect of Ginger (Zingiber officinale) on Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cytokine 2020, 135, 155224. [Google Scholar] [CrossRef]
- Ho, S.C.; Chang, K.S.; Lin, C.C. Anti-Neuroinflammatory Capacity of Fresh Ginger Is Attributed Mainly to 10-Gingerol. Food Chem. 2013, 141, 3183–3191. [Google Scholar] [CrossRef]
- Zhang, M.; Viennois, E.; Prasad, M.; Zhang, Y.; Wang, L.; Zhang, Z.; Han, M.K.; Xiao, B.; Xu, C.; Srinivasan, S.; et al. Edible Ginger-Derived Nanoparticles: A Novel Therapeutic Approach for the Prevention and Treatment of Inflammatory Bowel Disease and Colitis-Associated Cancer. Biomaterials 2016, 101, 321–340. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlina, D. Oral Delivery of Nanoparticles Loaded with Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C. Medicinal Plants and Antimicrobial Activity. J. Ethnopharmacol. 2005, 100, 80–84. [Google Scholar] [CrossRef]
- Moon, Y.S.; Lee, H.S.; Lee, S.E. Inhibitory Effects of Three Monoterpenes from Ginger Essential Oil on Growth and Aflatoxin Production of Aspergillus Flavus and Their Gene Regulation in Aflatoxin Biosynthesis. Appl. Biol. Chem. 2018, 61, 243–250. [Google Scholar] [CrossRef]
- Chakotiya, A.S.; Tanwar, A.; Narula, A.; Sharma, R.K. Zingiber officinale: Its Antibacterial Activity on Pseudomonas Aeruginosa and Mode of Action Evaluated by Flow Cytometry. Microb. Pathog. 2017, 107, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.G.; Hu, F.; Wei, Z.J. Antibacterial Activity and Mechanism of Ginger Essential Oil against Escherichia coli and Staphylococcus aureus. Molecules 2020, 25, 3955. [Google Scholar] [CrossRef]
- Sasidharan, I.; Menon, A.N. Comparative Chemical Composition and Antimicrobial Activity Fresh & Dry Ginger Oils (Zingiber officinale Roscoe). Int. J. Curr. Pharm. Res. 2010, 2, 40–43. [Google Scholar]
- Wang, H.M.; Chen, C.Y.; Chen, H.A.; Huang, W.C.; Lin, W.R.; Chen, T.C.; Lin, C.Y.; Chien, H.J.; Lu, P.L.; Lin, C.M.; et al. Zingiber officinale (Ginger) Compounds Have Tetracycline-Resistance Modifying Effects against Clinical Extensively Drug-Resistant Acinetobacter Baumannii. Phytother. Res. 2010, 24, 1825–1830. [Google Scholar] [CrossRef]
- Sarfati, D.; Gurney, J. Preventing Cancer: The Only Way Forward. Lancet 2022, 400, 540–541. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, N.F.; El-Bahrawy, H.A.; Abo Mansour, H.E. Ginger Extract Adjuvant to Doxorubicin in Mammary Carcinoma: Study of Some Molecular Mechanisms. Eur. J. Nutr. 2018, 57, 981–989. [Google Scholar] [CrossRef]
- Rajagopal, C.; Lankadasari, M.B.; Aranjani, J.M.; Harikumar, K.B. Targeting Oncogenic Transcription Factors by Polyphenols: A Novel Approach for Cancer Therapy. Pharmacol. Res. 2018, 130, 273–291. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Srzednicki, G.; Konczak, I. Antioxidant, Enzyme Inhibitory and Antiproliferative Activity of Polyphenolic-Rich Fraction of Commercial Dry Ginger Powder. Int. J. Food Sci. Technol. 2015, 50, 2229–2235. [Google Scholar] [CrossRef]
- Liu, C.M.; Kao, C.L.; Tseng, Y.T.; Lo, Y.C.; Chen, C.Y. Ginger Phytochemicals Inhibit Cell Growth and Modulate Drug Resistance Factors in Docetaxel Resistant Prostate Cancer Cell. Molecules 2017, 22, 1477. [Google Scholar] [CrossRef]
- Brahmbhatt, M.; Gundala, S.R.; Asif, G.; Shamsi, S.A.; Aneja, R. Ginger Phytochemicals Exhibit Synergy to Inhibit Prostate Cancer Cell Proliferation. Nutr. Cancer 2013, 65, 263–272. [Google Scholar] [CrossRef]
- Mansingh, D.P.; Sunanda, O.J.; Sali, V.K.; Vasanthi, H.R. [6]-Gingerol–Induced Cell Cycle Arrest, Reactive Oxygen Species Generation, and Disruption of Mitochondrial Membrane Potential Are Associated with Apoptosis in Human Gastric Cancer (AGS) Cells. J. Biochem. Mol. Toxicol. 2018, 32, e22206. [Google Scholar] [CrossRef]
- Peng, S.; Yao, J.; Liu, Y.; Duan, D.; Zhang, X.; Fang, J. Activation of Nrf2 Target Enzymes Conferring Protection against Oxidative Stress in PC12 Cells by Ginger Principal Constituent 6-Shogaol. Food Funct. 2015, 6, 2813–2823. [Google Scholar] [CrossRef]
- Lim, S.; Moon, M.; Oh, H.; Kim, H.G.; Kim, S.Y.; Oh, M.S. Ginger Improves Cognitive Function via NGF-Induced ERK/CREB Activation in the Hippocampus of the Mouse. J. Nutr. Biochem. 2014, 25, 1058–1065. [Google Scholar] [CrossRef]
- Huh, E.; Lim, S.; Kim, H.G.; Ha, S.K.; Park, H.Y.; Huh, Y.; Oh, M.S. Ginger Fermented with: Schizosaccharomyces Pombe Alleviates Memory Impairment via Protecting Hippocampal Neuronal Cells in Amyloid Beta1-42 Plaque Injected Mice. Food Funct. R. Soc. Chem. 2018, 9, 171–178. [Google Scholar] [CrossRef]
- Iranshahy, M.; Javadi, B. Diet Therapy for the Treatment of Alzheimer’s Disease in View of Traditional Persian Medicine: A Review. Iran. J. Basic. Med. Sci. 2019, 22, 1102–1117. [Google Scholar]
- Du, H.; Li, L.; Bennett, D.; Guo, Y.; Key, T.J.; Bian, Z.; Sherliker, P.; Gao, H.; Chen, Y.; Yang, L.; et al. Fresh Fruit Consumption and Major Cardiovascular Disease in China. N. Engl. J. Med. 2016, 374, 1332–1343. [Google Scholar] [CrossRef]
- Khosravani, M.; Azarbayjani, M.A.; Abolmaesoomi, M.; Yusof, A.; Zainal Abidin, N.; Rahimi, E.; Feizolahi, F.; Akbari, M.; Seyedjalali, S.; Dehghan, F.; et al. Ginger Extract and Aerobic Training Reduces Lipid Profile in High-Fat Fed Diet Rats. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 1617–1622. [Google Scholar]
- Oh, S.; Lee, M.S.; Jung, S.; Kim, S.; Park, H.; Park, S.; Kim, S.Y.; Kim, C.T.; Jo, Y.H.; Kim, I.H.; et al. Ginger Extract Increases Muscle Mitochondrial Biogenesis and Serum HDL-Cholesterol Level in High-Fat Diet-Fed Rats. J. Funct. Foods 2017, 29, 193–200. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X.; Shen, Z.; Dong, J.; Huang, J. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1ɑ Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Dong, L.; Hu, X.; Feng, F.; Chen, F. 6-Gingerol, a Functional Polyphenol of Ginger, Promotes Browning through an AMPK-Dependent Pathway in 3T3-L1 Adipocytes. J. Agric. Food Chem. 2019, 67, 14056–14065. [Google Scholar] [CrossRef]
- Suk, S.; Kwon, G.T.; Lee, E.; Jang, W.J.; Yang, H.; Kim, J.H.; Thimmegowda, N.R.; Chung, M.Y.; Kwon, J.Y.; Yang, S.; et al. Gingerenone A, a Polyphenol Present in Ginger, Suppresses Obesity and Adipose Tissue Inflammation in High-Fat Diet-Fed Mice. Mol. Nutr. Food Res. 2017, 61, 1–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhao, Y.; Wang, P.; Ahmedna, M.; Sang, S. Bioactive Ginger Constituents Alleviate Protein Glycation by Trapping Methylglyoxal. Chem. Res. Toxicol. 2015, 28, 1842–1849. [Google Scholar] [CrossRef]
- Samad, M.B.; Mohsin, M.N.A.B.; Razu, B.A.; Hossain, M.T.; Mahzabeen, S.; Unnoor, N.; Muna, I.A.; Akhter, F.; Kabir, A.U.; Hannan, J.M.A. [6]-Gingerol, from Zingiber officinale, Potentiates GLP-1 Mediated Glucose-Stimulated Insulin Secretion Pathway in Pancreatic β-Cells and Increases RAB8/RAB10-Regulated Membrane Presentation of GLUT4 Transporters in Skeletal Muscle to Improve Hyperglycemia in Leprdb/Db Type 2 Diabetic Mice. BMC Complement. Altern. Med. 2017, 17, 395. [Google Scholar] [CrossRef]
- Amjad, A.; Sohaib, M.; Nawaz, H.; Javed, M.S.; Shah, M.; Shah, F.U.H.; Tariq, M.R.; Sajid, M.W.; Khan, A.A.; Bilal, M.; et al. Assessment of Rheological and Quality Characteristics of Bread Made by the Addition of Ginger Powder in Wheat Flour. Food Sci. Technol. Camp. 2022, 42, 47820. [Google Scholar] [CrossRef]
- Balestra, F.; Cocci, E.; Pinnavaia, G.G.; Romani, S. Evaluation of Antioxidant, Rheological and Sensorial Properties of Wheat Flour Dough and Bread Containing Ginger Powder. LWT Food Sci. Technol. 2011, 44, 700–705. [Google Scholar] [CrossRef]
- Özcan, M.M. The Effect of Ginger (Zingiber officinale) Powders at Different Concentrations on Bioactive Compounds, Antioxidant Activity, Phenolic Constituents, Nutrients and Sensory Characteristics of Wheat Bread. Int. J. Gastron. Food Sci. 2022, 28, 100532. [Google Scholar] [CrossRef]
- Almasodi, A.G.S. Production and Evaluation of Some Bakery Products Containing Ginger Powder. J. Food Nutr. Res. 2018, 6, 205–215. [Google Scholar] [CrossRef][Green Version]
- Kaur, K.; Kaur, A. Effect of Supplementation of Garlic and Ginger Powder on Functional, Rheological and Bread Making Characteristics of Wheat Flour. J. Res. Punjab Agric. Univ. 2013, 50, 119–123. [Google Scholar]
- Muhammad, A.; Rahman, Z.; Ayub, M.; Durrani, Y.; Ali, S.; Tabassum, A.; Shakoor, A.; Khan, M.; Khan, A. Inhibitory Effect of Ginger and Turmeric on Rhizopus stolonifer Growth on Bread. J. Food Process Technol. 2014, 5, 1000325. [Google Scholar] [CrossRef]
- Cervenka, L.; Rezkova, S.; Kralovsky, J. Moisture Adsorption Characteristics of Gingerbread, a Traditional Bakery Product in Pardubice, Czech Republic. J. Food Eng. 2008, 84, 601–607. [Google Scholar] [CrossRef]
- Ghendov-Mosanu, A.; Cristea, E.; Patras, A.; Sturza, R.; Niculaua, M. Rose Hips, a Valuable Source of Antioxidants to Improve Gingerbread Characteristics. Molecules 2020, 25, 5659. [Google Scholar] [CrossRef]
- Lee, C.-S.; Lim, H.-S.; Cha, G.-H. Quality Characteristics of Cookies with Ginger Powder. Korean J. Food Cook. Sci. 2015, 31, 703–717. [Google Scholar] [CrossRef]
- Kaushal, M.; Vaidya, D.; Gupta, A.; Kaushik, R.; Verma, A.K. Bioactive Compounds and Acceptance of Cookies Supplemented with Ginger Flour. J. Pharmacogn. Phytochem. 2019, 8, 185–188. [Google Scholar]
- Yang, H.; Li, L.; Yin, Y.; Li, B.; Zhang, X.; Jiao, W.; Liang, Y. Effect of Ground Ginger on Dough and Biscuit Characteristics and Acrylamide Content. Food Sci. Biotechnol. 2019, 28, 1359–1366. [Google Scholar] [CrossRef]
- Kumari, S.; Gupta, A. Utilization of Ashwagandha, Ginger and Shatavari Root Powder for the Preparation of Value Added “Cookies”. Int. J. Home Sci. 2016, 2, 27–28. [Google Scholar]
- Marak, N.R.; Malemnganbi, C.C.; Marak, C.R.; Mishra, L.K. Functional and Antioxidant Properties of Cookies Incorporated with Foxtail Millet and Ginger Powder. J. Food Sci. Technol. 2019, 56, 5087–5096. [Google Scholar] [CrossRef]
- Abdel-Samie, M.A.S.; Wan, J.; Huang, W.; Chung, O.K.; Xu, B. Effects of Cumin and Ginger as Antioxidants on Dough Mixing Properties and Cookie Quality. Cereal Chem. 2010, 87, 454–460. [Google Scholar] [CrossRef]
- Al-Timim, S.S. Effect of Ginger on Organoleptic Characteristics and Prolonging the Duration of Conservation of Laboratory Biscuit. Iraq J. Mark. Res. Consum. Prot. 2011, 3, 37–51. [Google Scholar]
- Adebayo-Oyetoro, A.O.; Ogundipe, O.O.; Azoro, C.G.; Adeyeye, S.A.O. Production and Evaluation of Ginger Spiced Cookies from Wheat-Plantain Composite Flour. Pac. J. Sci. Technol. 2016, 17, 281–287. [Google Scholar]
- Almasodi, A.G.S. Utilization of ginger as a Natural Antioxidant and Antimicrobial in Bakery Products. Egypt. Arab. J. Appl. Sci. Technol. 2021, 1, 1–9. [Google Scholar] [CrossRef]
- Han, E.-J. Quality Characteristics of Muffins Containing Ginger Juice. Korean J. Culin. Res. 2012, 18, 256–266. [Google Scholar]
- Tanweer, S.; Shehzad, A.; Butt, M.S.; Shahid, M. Phytochemical Profiling of Conventional and Supercritical Ginger Extract Based Baked Bars. J. Food Process Technol. 2016, 7, 1000596. [Google Scholar] [CrossRef]
- Felfoul, I.; Borchani, M.; Samet-Bali, O.; Attia, H.; Ayadi, M.A. Effect of Ginger (Zingiber officinalis) Addition on Fermented Bovine Milk; Rheological Properties, Sensory Attributes and Antioxidanr Potential. J. New Sci. Agric. Biotechnol. 2017, 44, 2400–2409. [Google Scholar]
- Hanou, S.; Boukhemis, M.; Benatallah, L.; Djeghri, B.; Zidoune, M.-N. Research Article Effect of Ginger Powder Addition on Fermentation Kinetics, Rheological Properties and Bacterial Viability of Dromedary Yogurt. Adv. J. Food Sci. Technol. 2016, 10, 667–673. [Google Scholar] [CrossRef]
- Regu, M.; Yilma, Z.; Seifu, E. Effect of Garlic (Allium sativum) and Ginger (Zingiber officinale) Powder on Chemical Composition and Sensory Property of Ayib—Ethiopian Cottage Cheese. Int. Food Res. J. 2016, 23, 1226–1232. [Google Scholar]
- Yüksel, H.; Çalışkan Koç, G.; Dirim, S.N. Physical Characterization of Spray-Dried Milk Powders and Their Agglomerates with the Addition of Carob, Cinnamon, and Ginger Powders. Pamukkale Univ. J. Eng. Sci. 2019, 25, 824–833. [Google Scholar] [CrossRef]
- Gavhane, M.S.; Kamble, N.S.; Desale, R.J.; Ghule, B.K.; Mule, P.R. Studies on Preparation of PEDA with Ginger Powder. Int. J. Food Agric. Vet. Sci. 2014, 4, 64–68. [Google Scholar]
- Yang, G.H.; Guan, J.J.; Wang, J.S.; Yin, H.C.; Qiao, F.D.; Jia, F. Physicochemical and Sensory Characterization of Ginger-Juice Yogurt during Fermentation. Food Sci. Biotechnol. 2012, 21, 1541–1548. [Google Scholar] [CrossRef]
- Allam, M.G.; Gomaa, M.A.E.; Ayad, E.H.E.; Darwish, S.M. Application of Some Insensitive Probiotic Lactic Acid Bacteria and Ginger as Functional Dairy Products. Microb. Biosyst. 2018, 3, 60–73. [Google Scholar]
- Jadhav, S.; Patil, V.N.; Naik, P.; Kadam, R.M. Studies on Chemical Quality of Ginger (Zingiber officinale L.) Milk Shake. Asian J. Dairy Food Res. 2018, 37, 13–17. [Google Scholar] [CrossRef]
- Gaur, G.K.; Rani, R.; Dharaiya, C.N.; Solanki, K. Development of Herbal Milk Using Tulsi Juice, Ginger Juice and Turmeric Powder. Int. J. Chem. Stud. 2019, 7, 1150–1157. [Google Scholar]
- Jadhav, M.S.; Nimbalkar, C.A.; Kad, V.P. Effect of Different Levels of Ginger Juice on Physico-Chemical and Sensory Characteristics of Herbal Ice Cream. Res. J. Chem. Environ. Sci. 2017, 5, 45–50. [Google Scholar]
- David, J. Studies on Sensory Evaluation of Herbal Ice-Cream with Addition of Ginger (Zingiber officinale) Juice. J. Pharma Res. 2016, 5, 18–20. [Google Scholar]
- Agrawal, A.K.; Karkhele, P.D.; Sandey, K.K.; Sahu, C.; Sinha, G. Effect of Incorporation of Ginger Juice in Various Rates on the Freezing and Thermal Properties of Ice Cream. Asian J. Dairy Food Res. 2015, 34, 92. [Google Scholar] [CrossRef]
- Mule, S.M.; Kadam, S.S.; Dandekar, V.; Ramod, S.; Desai, B.; Narkhede, S. Manufacturing Technology and Production Cost of Ginger (Zingiber officinale L.) and Aloe Vera (Aloe barbadensis) Juice Enriched Probiotic (L. acidophilus) Ice Cream. Int. J. Chem. Stud. 2020, 8, 185–188. [Google Scholar] [CrossRef]
- Molaie, S.; Latifi, Z.; Sabetgadam, M.; Khosrojerdi, M.; Razghandi, E. The Effect of Using Ginger Extract (Zingiber officinale) on the Antioxidant and Physicochemical Properties of Low-Fat Yogurt. J. Food Sci. Technol. 2022, 18, 265–273. [Google Scholar] [CrossRef]
- Srivastava, P.; Prasad, S.G.M.; Ali, M.N.; Prasad, M. Analysis of Antioxidant Activity of Herbal Yoghurt Prepared from Different Milk. Pharma Innov. J. 2015, 4, 18–20. [Google Scholar] [CrossRef]
- Simon, H.P.; Chandra, R.; Shukla, S.; Singh, S.S. Sensory Evaluation of Probiotic Herbal Yoghurt with Ginger and Garlic Extract. Pharma Innov. J. 2018, 7, 605–607. [Google Scholar] [CrossRef]
- Haji Ghafarloo, M.; Jouki, M.; Tabari, M. Production and Characterization of Synbiotic Doogh, a Yogurt-Based Iranian Drink by Gum Arabic, Ginger Extract and B. Bifidum. J. Food Sci. Technol. 2020, 57, 1158–1166. [Google Scholar] [CrossRef]
- Abd El-Khalek, A.B.; El-Sayed, H.S.; Ibrahim, G.A.; Sharaf, O.; Ibrahim, G.A.; El-Shafei, K.; El-Din, H.M.F.; Sharaf, O.M.; Tawfek, N.F.; Effat, B.A.; et al. Phenolic Compounds, Microbial Content and Sensory Evaluation of Synbiotic Labneh Conaining Ginger and Probiotic. Int. J. ChemTech Res. 2016, 9, 238–247. [Google Scholar]
- Hailu, Y.; Seifu, E.; Yilma, Z. Physicochemical Properties and Consumer Acceptability of Soft Unripened Cheese Made from Camel Milk Using Crude Extract of Ginger (Zingiber officinale) as Coagulant. Afr. J. Food Sci. 2014, 8, 87–91. [Google Scholar] [CrossRef]
- Hashim, M.M.; Dong, M.; Iqbal, M.F.; Li, W.; Chen, X. Ginger Protease Used as Coagulant Enhances the Proteolysis and Sensory Quality of Peshawari Cheese Compared to Calf Rennet. Dairy Sci. Technol. 2011, 91, 431–440. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.; Mohamed, S.H.S.; Seleet, F.L. Production and Evaluation of Soft Cheese Fortified with Ginger Extract as a Functional Dairy Food. Pol. J. Food Nutr. Sci. 2012, 62, 77–83. [Google Scholar] [CrossRef]
- Chamchan, R.; Sinchaipanit, P.; Disnil, S.; Jittinandana, S.; Nitithamyong, A.; On-nom, N. Formulation of Reduced Sugar Herbal Ice Cream Using Lemongrass or Ginger Extract. Brit. Food J. 2017, 119, 2172–2182. [Google Scholar] [CrossRef]
- Vedashree, M.; Asha, M.R.; Roopavati, C.; Naidu, M.M. Characterization of Volatile Components from Ginger Plant at Maturity and Its Value Addition to Ice Cream. J. Food Sci. Technol. 2020, 57, 3371–3380. [Google Scholar] [CrossRef]
- Gabbi, D.K.; Bajwa, U.; Goraya, R.K. Physicochemical, Melting and Sensory Properties of Ice Cream Incorporating Processed Ginger (Zingiber officinale). Int. J. Dairy Technol. 2018, 71, 190–197. [Google Scholar] [CrossRef]
- Mahalleh, A.A.; Mahalleh, A.A. Evaluation the Effect of Ginger (Zingiber officinale) Essential Oil on the Quality Characteristics Yogurt during Storage Time. Sustain. Agric. Sci. Res. 2021, 1, 71–87. [Google Scholar]
- Ahmed, L.I.; Ibrahim, N.; Abdel-Salam, A.B.; Fahim, K.M. Potential Application of Ginger, Clove and Thyme Essential Oils to Improve Soft Cheese Microbial Safety and Sensory Characteristics. Food Biosci. 2021, 42, 101177. [Google Scholar] [CrossRef]
- Faiqoh, K.E.N.; Muhammad, D.R.A.; Praseptiangga, D. Ginger-Flavoured Ready-to-Drink Cocoa Beverage Formulated with High and Low-Fat Content Powder: Consumer Preference, Properties and Stability. Food Res. 2021, 5, 7–17. [Google Scholar] [CrossRef]
- Wadikar, D.D.; Madhura, C.V.; Premavalli, K.S. Development of a Ginger-Based Appetizer in the Form of Ready-to-Reconstitute Mix and Ready-to-Drink Beverage. Int. J. Food Sci. Nutr. 2011, 62, 404–409. [Google Scholar] [CrossRef]
- Ullah, N.; Qazi, I.M.; Masroor, S.; Ali, I.; Khan, A.; Khan, M.; Gillani, A. Preservation of Ready to Serve Blended Carrot and Kinnow (Mandarin) Drink by Ginger Extract. J. Food Process Technol. 2015, 6, 1–6. [Google Scholar] [CrossRef]
- Gaikwad, K.K.; Singh, S.; Shakya, B.R. Studies on the Development and Shelf Life of Low Calorie Herbal Aonla-Ginger RTS Beverage by Using Artificial Sweeteners. J. Food Process Technol. 2013, 4, 1000200. [Google Scholar] [CrossRef]
- Liu, F.; Song, S.; Zhang, X.; Tan, C.; Karangwa, E. Effect of Sterilization Methods on Ginger Flavor Beverage Assessed by Partial Least Squares Regression of Descriptive Sensory Analysis and Gas Chromatography-Mass Spectrometry. Eur. Food Res. Technol. 2014, 238, 247–257. [Google Scholar] [CrossRef]
- Abdulkareem, S.A.; Uthman, H.; Jimoh, A. Development and Characterization of a Carbonated Ginger Drink. Leonardo J. Sci. 2011, 10, 45–54. [Google Scholar]
- Imran, A.; Quispe, C.; Zeeshan, A.; Imran, M.; Nadeem, M.; Gilani, S.A.; Gondal, T.A.; Tufail, T.; Aslam, F.; Rodrigues, C.F.; et al. Development and Antioxidant Characterization of Ginger-Mint Drink Prepared through Different Extraction Techniques. J. Food Meas. Charact. 2021, 15, 2576–2590. [Google Scholar] [CrossRef]
- Adelekan, A.O.; Arisa, N.U.; Alamu, A.E.; Adebayo, Y.O.; Popoola, G.J.T. Production and Acceptability of Fruits Enhanced Zobo Drink. Food Sci. Technol. Lett. 2014, 5, 46–51. [Google Scholar]
- Adesokan, I.A.; Abiola, O.P.; Adigun, M.O.; Anifowose, O.A. Analysis of Quality Attributes of Hibiscus sabdariffa (Zobo) Drinks Blended with Aqueous Extract of Ginger and Garlic. Afr. J. Food Sci. 2013, 7, 174–177. [Google Scholar] [CrossRef]
- Cardoso, M.H.; da Costa, J.F.; Marto, R.H.; Neves, M.F.T. Soybean, Mango and Ginger Beverage: Nutritional Information, Sensory Evaluation and Consumption Intent. Rev. Hosp. Univ. Pedro Ernesto 2015, 14, 18–26. [Google Scholar] [CrossRef]
- Septiana, A.T.; Triyanto, H.W. The Effect of Addition of Ginger Extract and Kencur Extract on the Physicochemical Properties of Instant Temulawak and the Sensory Properties of the Beverage. Sudirman J. Nutr. Food 2019, 3, 157–166. [Google Scholar] [CrossRef]
- Awe, F.B.; Fagbemi, T.N.; Ifesan, B.O.T.; Badejo, A.A. Antioxidant Properties of Cold and Hot Water Extracts of Cocoa, Hibiscus Flower Extract, and Ginger Beverage Blends. Food Res. Int. 2013, 52, 490–495. [Google Scholar] [CrossRef]
- Sugimoto, K.; Takeuchi, H.; Nakagawa, K.; Matsuoka, Y. Hyperthermic Effect of Ginger (Zingiber officinale) Extract-Containing Beverage on Peripheral Skin Surface Temperature in Women. Evid.-Based Complement. Altern. Med. 2018, 2018, 3207623. [Google Scholar] [CrossRef]
- Makanjuola, S.A.; Enujiugha, V.N. Enhancing Sensory Perception of Plant Based Nutraceutical Drinks by Combining Plants from Different Sources: A Preliminary Study of Tea and Ginger Blend. Prev. Nutr. Food Sci. 2017, 22, 372–375. [Google Scholar] [CrossRef][Green Version]
- Makanjuola, S.A.; Enujiugha, V.N.; Omoba, O.S.; Sanni, D.M. Optimization and Prediction of Antioxidant Properties of a Tea-Ginger Extract. Food Sci. Nutr. 2015, 3, 443–452. [Google Scholar] [CrossRef]
- Shoaib, M.; Khuram, H.; Aslam, W.; Muhmmad, S.; Shehzad, A.; Shakeel, A.; Sakandar, H.A.; Raza, H.; Omar, M.; Hafiz, A.; et al. Physico-Chemical and Sensory Profile of Conventional and Supercritical Ginger Extract Nutrified Ginger-Iced Tea. Am. J. Food Sci. Nutr. Res. 2015, 2, 154–158. [Google Scholar]
- Švarc-Gajić, J.; Cvetanović, A.; Segura-Carretero, A.; Mašković, P.; Jakšić, A. Functional Coffee Substitute Prepared from Ginger by Subcritical Water. J. Supercrit. Fluids 2017, 128, 32–38. [Google Scholar] [CrossRef]
- Kuswardhani, N.; Mukti, N.P.; Sari, P. Antioxidant and Sensory Properties of Ready to Drink Coffee-Ginger Made from Decaffeinated and Non-Decaffeinated Robusta Coffee Beans. IOP Conf. Ser. Earth Environ. Sci. 2021, 653, 012050. [Google Scholar] [CrossRef]
- Mancini, S.; Preziuso, G.; Dal Bosco, A.; Roscini, V.; Parisi, G.; Paci, G. Modifications of Fatty Acids Profile, Lipid Peroxidation and Antioxidant Capacity in Raw and Cooked Rabbit Burgers Added with Ginger. Meat Sci. 2017, 133, 151–158. [Google Scholar] [CrossRef]
- Mancini, S.; Paci, G.; Fratini, F.; Torracca, B.; Nuvoloni, R.; Dal Bosco, A.; Roscini, V.; Preziuso, G. Improving Pork Burgers Quality Using Zingiber officinale Roscoe Powder (Ginger). Meat Sci. 2017, 129, 161–168. [Google Scholar] [CrossRef]
- Draszanowska, A.; Karpińska-Tymoszczyk, M.; Olszewska, M.A. The Effect of Ginger Rhizome and Refrigerated Storage Time on the Quality of Pasteurized Canned Meat. Food Sci. Technol. Int. 2020, 26, 300–310. [Google Scholar] [CrossRef]
- Parkash, J.; Sharma, D.; Yadav, S. Application of Natural Tenderizers (Papain and Ginger) in Buffalo Calf Meat. Pharma Innov. 2021, 10, 203–205. [Google Scholar] [CrossRef]
- Singh, P.; Sahoo, J.; Chatli, M.K.; Biswas, A.K. Shelf Life Evaluation of Raw Chicken Meat Emuslison Incorporated with Clove Powder, Ginger and Garlic Paste as Natural Preservatives at Refrigerated Storage (4+1 OC). Int. Food Res. J. 2014, 21, 1363–1373. [Google Scholar]
- Putra, A.A.; Wattanachant, S.; Wattanachant, C. Sensory- Related Attributes of Raw and Cooked Meat of Culled Saanen Goat Marinated in Ginger and Pineapple Juices. Trop. Anim. Sci. J. 2019, 42, 59–67. [Google Scholar] [CrossRef]
- Gao, H.Y.; Zhou, G.H.; Zeng, J.; Ma, H.J.; Pan, R.S.; Yu, X.L. Tenderization of Goose Meat by Papain, Pineapple Juice and Ginger Juice Treatment. Adv. Mater. Res. 2011, 236–238, 2349–2352. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Kadum, H.; Fathallah, S.; Meor Hussin, A.S. Metabolomics Profiling and Antibacterial Activity of Fermented Ginger Paste Extends the Shelf Life of Chicken Meat. LWT Food Sci. Technol. 2020, 132, 109897. [Google Scholar] [CrossRef]
- Abdel-Naeem, H.H.S.; Mohamed, H.M.H. Improving the Physico-Chemical and Sensory Characteristics of Camel Meat Burger Patties Using Ginger Extract and Papain. Meat Sci. 2016, 118, 52–60. [Google Scholar] [CrossRef]
- Sukada, I.K.; Suberata, I.W.; Rasna, N.M.A. Immersion Effect with Extracts of Papaya Leaf, Pineapple, Ginger on Quality of Organoleptic and Bali Beef Nutrition. Int. J. Life Sci. 2019, 3, 12–24. [Google Scholar] [CrossRef]
- Anandh, M.A.; Lakshmanan, V. Storage Stability of Smoked Buffalo Rumen Meat Product Treated with Ginger Extract. J. Food Sci. Technol. 2014, 51, 1191–1196. [Google Scholar] [CrossRef]
- Iheagwara, M.C. Effect of Ginger Extract on Stability and Sensorial Quality of Smoked Mackerel (Scomber scombrus) Fish. J. Nutr. Food Sci. 2013, 3, 199. [Google Scholar] [CrossRef]
- Mattje, L.G.B.; Tormen, L.; Bombardelli, M.C.M.; Corazza, M.L.; Bainy, E.M. Ginger Essential Oil and Supercritical Extract as Natural Antioxidants in Tilapia Fish Burger. J. Food Process Preserv. 2019, 43, e13942. [Google Scholar] [CrossRef]
- Maiti, A.K.; Ahlawat, S.S.; Sharma, D.P.; Khanna, N. Application of Natural Tenderizers in Meat—A Review. Agric. Rev. 2008, 29, 226–230. [Google Scholar]
- Ruitong, D.; Zhi, Y.; Yuan, L.I.; Xingmin, L.I.; Lizhen, M.A. Tenderizing and Preserving Yak Meat by Ginger Extract (Zingiber officinale Rose). J. Muscle Foods 2010, 21, 757–768. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; Alagawany, M.; Shaheen, H.; Samak, D.; Othman, S.I.; Allam, A.A.; Taha, A.E.; Khafaga, A.F.; Arif, M.; Osman, A.; et al. Ginger and Its Derivatives as Promising Alternatives to Antibiotics in Poultry Feed. Animals 2020, 10, 452. [Google Scholar] [CrossRef]
- Khan, R.U.; Naz, S.; Nikousefat, Z.; Tufarelli, V.; Javdani, M.; Qureshi, M.S.; Laudadio, V. Potential Applications of Ginger (Zingiber officinale) in Poultry Diets. Worlds Poult. Sci. J. 2012, 68, 245–252. [Google Scholar] [CrossRef]
- Mancini, S.; Secci, G.; Preziuso, G.; Parisi, G.; Paci, G. Ginger (Zingiber officinale Roscoe) Powder as Dietary Supplementation in Rabbit: Life Performances, Carcass Characteristics and Meat Quality. Ital. J. Anim. Sci. 2018, 17, 867–872. [Google Scholar] [CrossRef]
- Herwati, M.; Marjuki, A. The Effect of Feeding Red Ginger (Zingiber officinale Rosc) as Phytobiotic on Broiler Slaughter Weight and Meat Quality. Int. J. Poult. Sci. 2011, 10, 983–986. [Google Scholar]
- Wen, C.; Liu, Y.; Ye, Y.; Tao, Z.; Cheng, Z.; Wang, T.; Zhou, Y. Effects of Gingerols-Rich Extract of Ginger on Growth Performance, Serum Metabolites, Meat Quality and Antioxidant Activity of Heat-Stressed Broilers. J. Therm. Biol. 2020, 89, 102544. [Google Scholar] [CrossRef]
- Elazab, M.A.; Khalifah, A.M.; Elokil, A.A.; Elkomy, A.E.; Rabie, M.M.; Mansour, A.T.; Morshedy, S.A. Effect of Dietary Rosemary and Ginger Essential Oils on the Growth Performance, Feed Utilization, Meat Nutritive Value, Blood Biochemicals, and Redox Status of Growing NZW Rabbits. Animals 2022, 12, 375. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential Oils as Natural Additives to Prevent Oxidation Reactions in Meat and Meat Products: A Review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Alibekov, R.S.; Gabrilyants, E.A.; Utebaeva, A.A.; Nurseitova, Z.T.; Konarbayeva, Z.K.; Khamitova, B.M. Cottage Cheese Fortified by Natural Additives. Food Res. 2021, 5, 152–159. [Google Scholar] [CrossRef]
- Shaukat, M.N.; Palmeri, R.; Restuccia, C.; Parafati, L.; Fallico, B. Glycerol Ginger Extract Addition to Edible Coating Formulation for Preventing Oxidation and Fungal Spoilage of Stored Walnuts. Food Biosci. 2023, 52, 102420. [Google Scholar] [CrossRef]
- Ahmed, E.S.; Shehata, M.G.; Abd-Rabou, H.S.; El-Menshawy, H. Extend Shelf-Life of Vacuum-Packaged Herring Fish Fillets Using Garlic and Ginger Extracts. J. Pure Appl. Microbiol. 2019, 13, 1571–1581. [Google Scholar] [CrossRef]
- Kavas, N.; Kavas, G.; Saygili, D. Use of Ginger Essential Oil-Fortified Edible Coatings in Kashar Cheese and Its Effects on Escherichia coli O157:H7 and Staphylococcus aureus. CyTA J. Food 2016, 14, 317–323. [Google Scholar] [CrossRef]
- Tsironi, M.; Kosma, I.S.; Badeka, A.V. The Effect of Whey Protein Films with Ginger and Rosemary Essential Oils on Microbiological Quality and Physicochemical Properties of Minced Lamb Meat. Sustainability 2022, 14, 3434. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Wang, H.; Liu, W.; Cheong, K.; Teng, B. Effect of Sodium Alginate-Agar Coating Containing Ginger Essential Oil on the Shelf Life and Quality of Beef. Food Control 2021, 130, 108216. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and Antioxidant Efficiency of Nanoemulsion-Based Edible Coating Containing Ginger (Zingiber officinale) Essential Oil and Its Effect on Safety and Quality Attributes of Chicken Breast Fillets. Food Control. 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Barkhordari, P.; Bazargani-Gilani, B. Effect of Apple Peel Extract and Zein Coating Enriched with Ginger Essential Oil on the Shelf Life of Chicken Thigh Meat. J. Food Meas. Charact. 2021, 15, 2727–2742. [Google Scholar] [CrossRef]
- Cai, L.; Wang, Y. Physicochemical and Antioxidant Properties Based on Fish Sarcoplasmic Protein/Chitosan Composite Films Containing Ginger Essential Oil Nanoemulsion. Food Bioprocess Technol. 2021, 14, 151–163. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Zhang, P.; Ye, L.; Wu, L.; He, S. Physical Characterization and Pork Packaging Application of Chitosan Films Incorporated with Combined Essential Oils of Cinnamon and Ginger. Food Bioprocess Technol. 2017, 10, 503–511. [Google Scholar] [CrossRef]
- Atarés, L.; Bonilla, J.; Chiralt, A. Characterization of Sodium Caseinate-Based Edible Films Incorporated with Cinnamon or Ginger Essential Oils. J. Food Eng. 2010, 100, 678–687. [Google Scholar] [CrossRef]
- Atarés, L.; De Jesús, C.; Talens, P.; Chiralt, A. Characterization of SPI-Based Edible Films Incorporated with Cinnamon or Ginger Essential Oils. J. Food Eng. 2010, 99, 384–391. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, A.; Wang, W.; Ye, R.; Liu, Y.; Xiao, J.; Wang, K. Characterisation of Microemulsion Nanofilms Based on Tilapia Fish Skin Gelatine and ZnO Nanoparticles Incorporated with Ginger Essential Oil: Meat Packaging Application. Int. J. Food Sci. Technol. 2017, 52, 1670–1679. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaukat, M.N.; Nazir, A.; Fallico, B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants 2023, 12, 2015. https://doi.org/10.3390/antiox12112015
Shaukat MN, Nazir A, Fallico B. Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants. 2023; 12(11):2015. https://doi.org/10.3390/antiox12112015
Chicago/Turabian StyleShaukat, Muhammad Nouman, Akmal Nazir, and Biagio Fallico. 2023. "Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications" Antioxidants 12, no. 11: 2015. https://doi.org/10.3390/antiox12112015
APA StyleShaukat, M. N., Nazir, A., & Fallico, B. (2023). Ginger Bioactives: A Comprehensive Review of Health Benefits and Potential Food Applications. Antioxidants, 12(11), 2015. https://doi.org/10.3390/antiox12112015