Tocotrienols Provide Radioprotection to Multiple Organ Systems through Complementary Mechanisms of Antioxidant and Signaling Effects
Abstract
1. Introduction
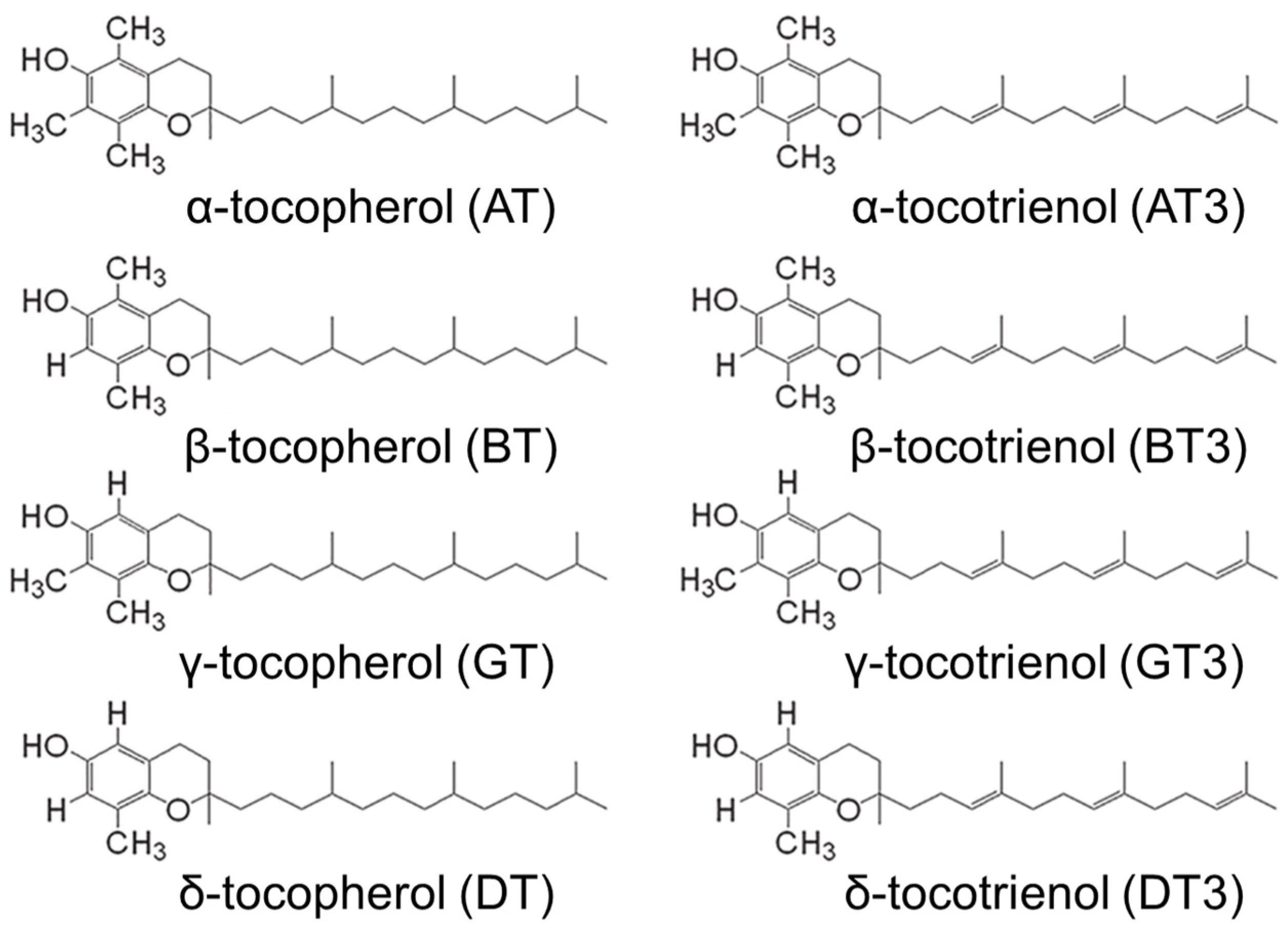
2. Pharmacological Action of Tocotrienols
2.1. Antioxidant Effects
2.1.1. Free Radical Scavenging
2.1.2. Induction of Antioxidant Enzymes
2.2. Modulation of Cellular Signaling Pathways
2.3. Mitochondrial-Protective Effects
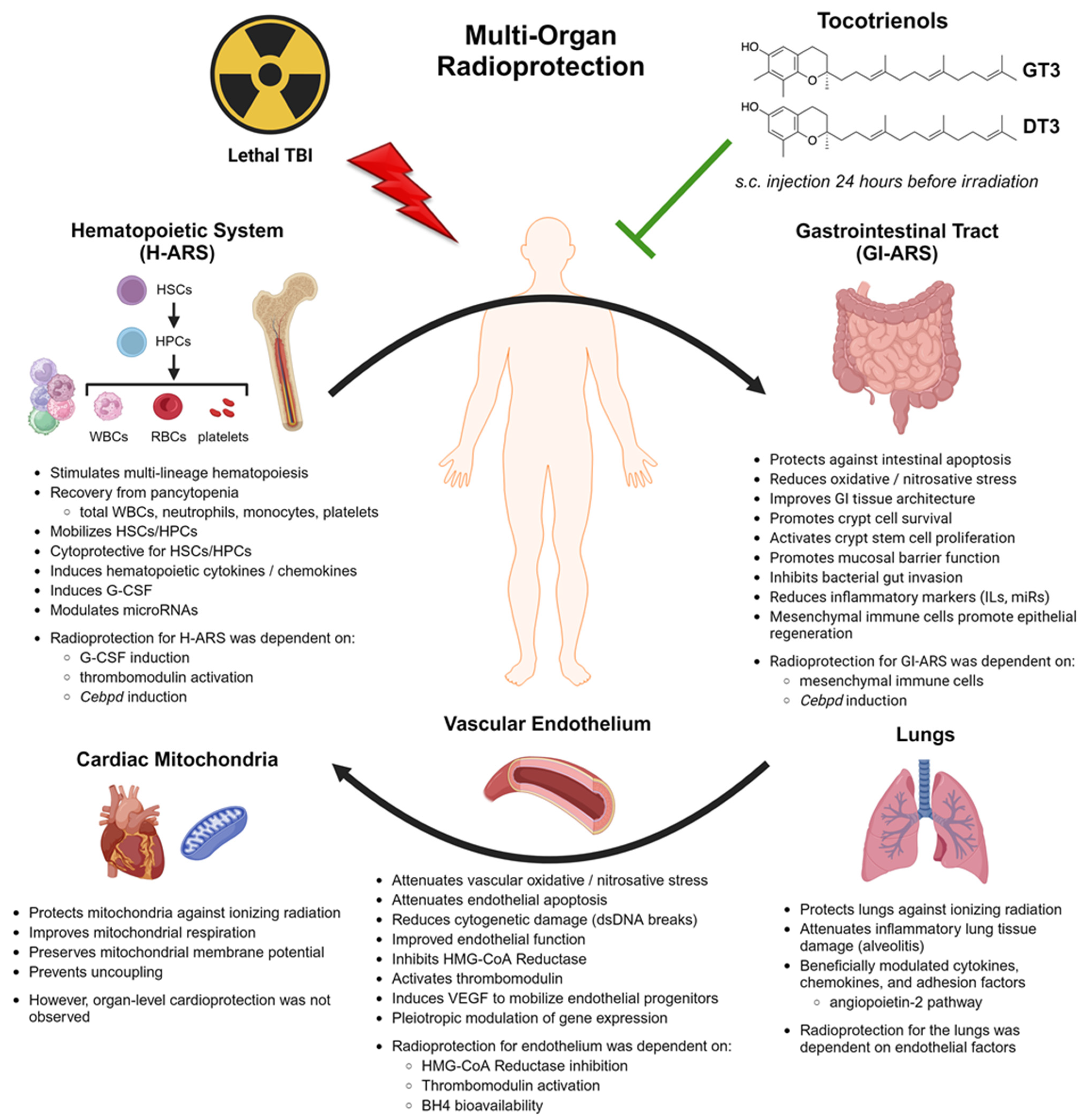
3. Tocotrienols as a Potential Radioprotective Countermeasure
Investigating the Multi-Organ Radioprotective Mechanisms of Action
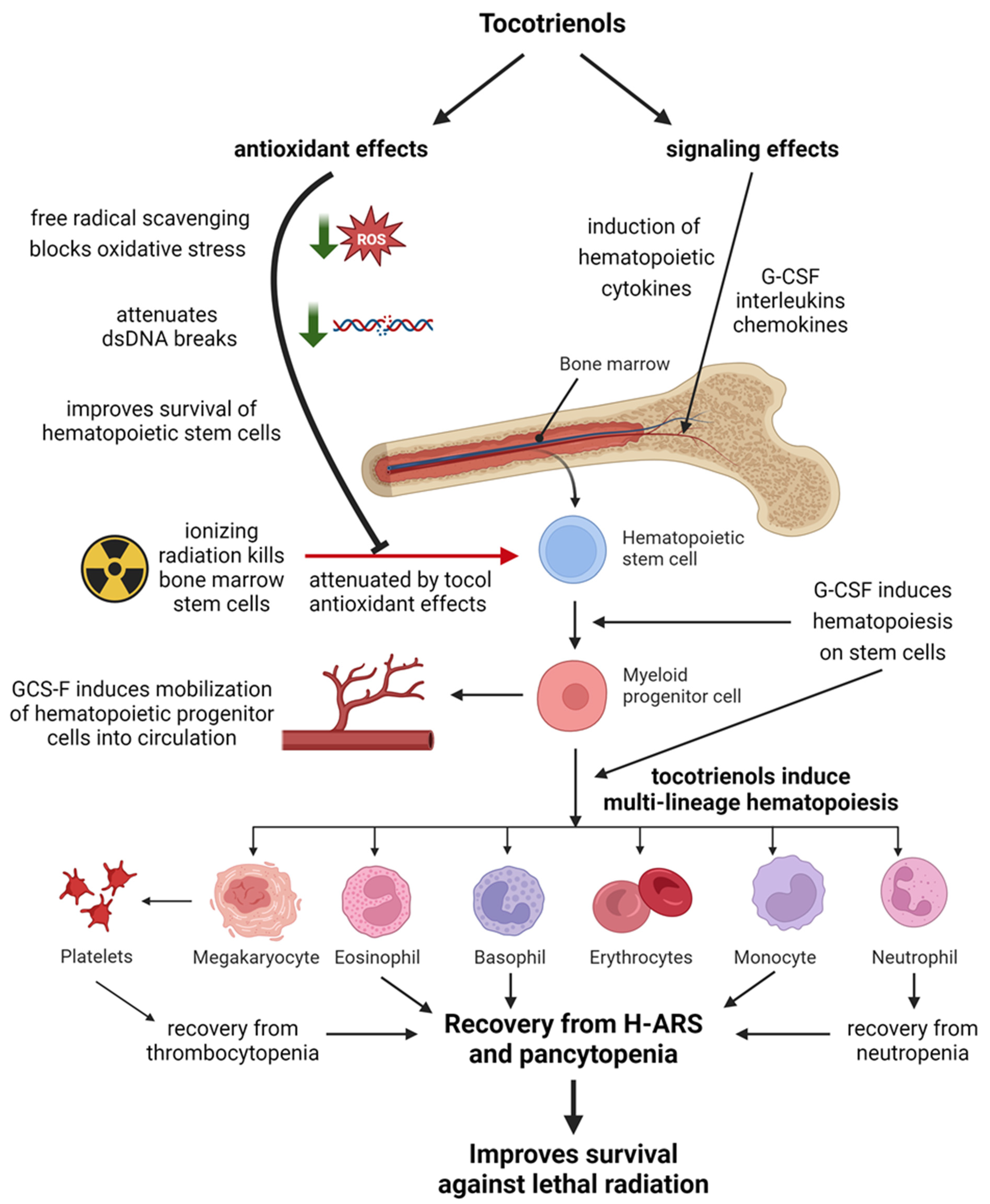
4. Radioprotection in the Hematopoietic System
4.1. Hematopoietic Factors
4.1.1. Granulocyte Colony-Stimulating Factor
4.1.2. Other Hematopoietic Cytokines
4.1.3. Hematopoietic Chemokines
4.2. Transcription Factor Cebpd
4.3. MicroRNAs
4.4. Direct Cytoprotective Effects
4.5. Summary of the Effects on the Hematopoietic System
5. Radioprotection in the Gastrointestinal Tract
5.1. Apoptosis Regulation
5.2. Mesenchymal Immune Cells
5.3. Inflammatory Markers
5.4. Transcription Factor Cebpd
5.5. Summary of the Effects on the Gastrointestinal Tract
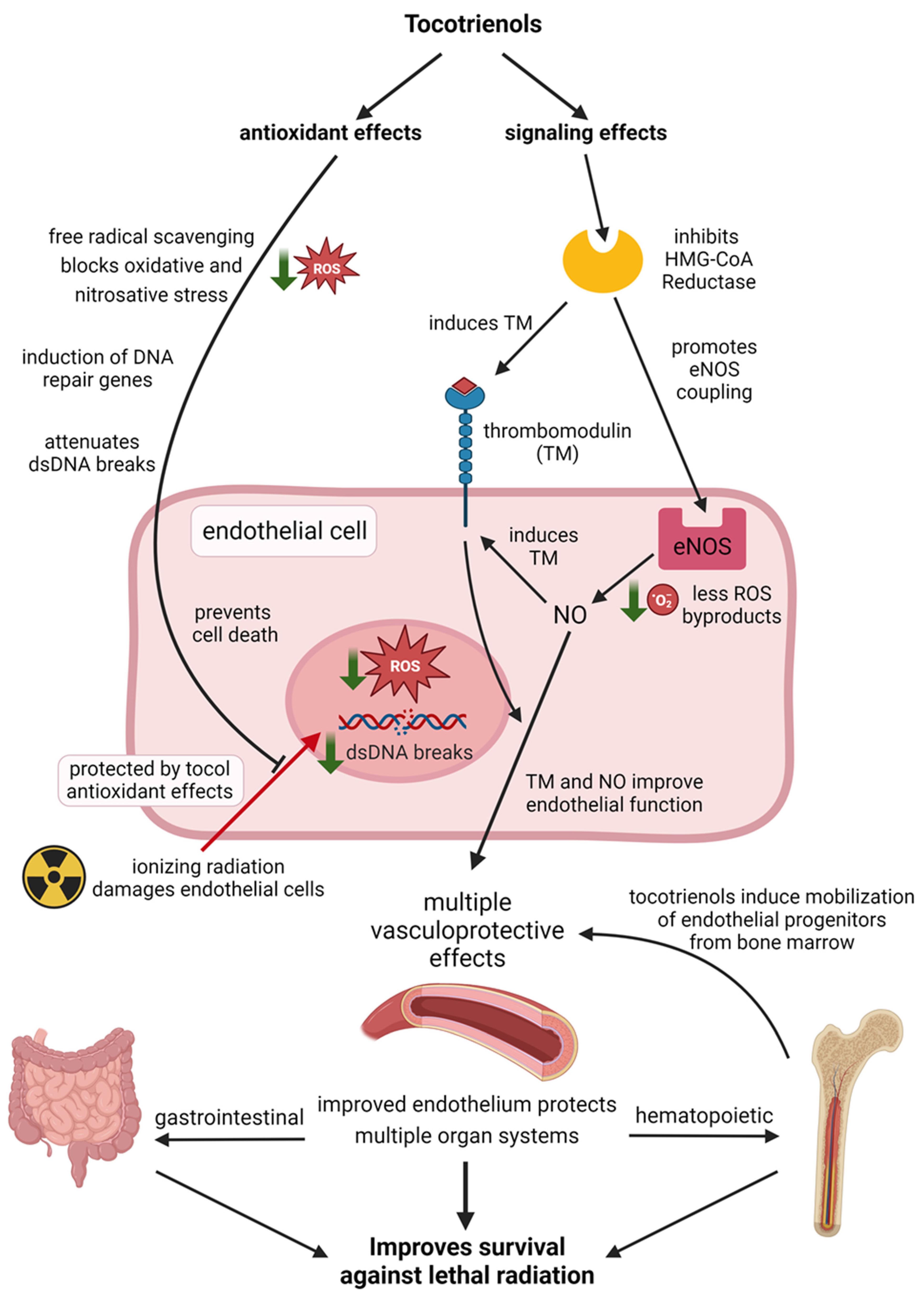
6. Radioprotection in the Vascular Endothelium
6.1. Oxidative and Nitrosative Stress
6.2. HMG-CoA Reductase and Thrombomodulin
6.3. VEGF and Endothelial Progenitors
6.4. Summary of the Effects on the Vascular Endothelium
7. Radioprotection in Other Organ Systems
7.1. Lung
7.2. Cardiac Mitochondria
7.3. Summary of the Effects on Other Organ Systems
8. Overview of Radioprotective Signaling Mechanisms for Tocotrienols
Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pellmar, T.C.; Rockwell, S.; Radiological/Nuclear Threat Countermeasures Working Group. Priority List of Research Areas for Radiological Nuclear Threat Countermeasures. Radiat. Res. 2005, 163, 115–123. [Google Scholar] [CrossRef]
- Valentin, J.; International Commission on Radiological Protection. Protecting People against Radiation Exposure in the Event of a Radiological Attack. A Report of The International Commission on Radiological Protection. Ann. ICRP 2005, 35, iii–iv. [Google Scholar] [CrossRef]
- Singh, V.K.; Romaine, P.L.P.; Seed, T.M. Medical Countermeasures for Radiation Exposure and Related Injuries: Characterization of Medicines, FDA-Approval Status and Inclusion into the Strategic National Stockpile. Health Phys. 2015, 108, 607–630. [Google Scholar] [CrossRef]
- Radiation and Nuclear Countermeasures Program. Strategic Plan and Research Agenda for Medical Countermeasures against Radiological and Nuclear Threats Progress Report: 2005–2011 and Future Directions: 2012–2016. Available online: https://www.niaid.nih.gov/sites/default/files/radnucprogressreport.pdf (accessed on 22 March 2022).
- Singh, V.K.; Ducey, E.J.; Brown, D.S.; Whitnall, M.H. A Review of Radiation Countermeasure Work Ongoing at the Armed Forces Radiobiology Research Institute. Int. J. Radiat. Biol. 2012, 88, 296–310. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador, R.; Villaescusa, J.I.; Soriano, J.M.; Estrela, J.M.; Montoro, A. Radioprotection and Radiomitigation: From the Bench to Clinical Practice. Biomedicines 2020, 8, 461. [Google Scholar] [CrossRef]
- Obrador, E.; Salvador-Palmer, R.; Villaescusa, J.I.; Gallego, E.; Pellicer, B.; Estrela, J.M.; Montoro, A. Nuclear and Radiological Emergencies: Biological Effects, Countermeasures and Biodosimetry. Antioxidants 2022, 11, 1098. [Google Scholar] [CrossRef]
- Singh, V.K.; Newman, V.L.; Romaine, P.L.; Wise, S.Y.; Seed, T.M. Radiation Countermeasure Agents: An Update (2011–2014). Expert Opin. Ther. Pat. 2014, 24, 1229–1255. [Google Scholar] [CrossRef]
- Nukala, U.; Thakkar, S.; Krager, K.J.; Breen, P.J.; Compadre, C.M.; Aykin-Burns, N. Antioxidant Tocols as Radiation Countermeasures (Challenges to Be Addressed to Use Tocols as Radiation Countermeasures in Humans). Antioxidants 2018, 7, 33. [Google Scholar] [CrossRef]
- Singh, V.K.; Seed, T.M. Development of Gamma-Tocotrienol as a Radiation Medical Countermeasure for the Acute Radiation Syndrome: Current Status and Future Perspectives. Expert Opin. Investig. Drugs 2023, 32, 25–35. [Google Scholar] [CrossRef]
- Ghosh, S.P.; Kulkarni, S.; Hieber, K.; Toles, R.; Romanyukha, L.; Kao, T.-C.; Hauer-Jensen, M.; Kumar, K.S. Gamma-Tocotrienol, a Tocol Antioxidant as a Potent Radioprotector. Int. J. Radiat. Biol. 2009, 85, 598–606. [Google Scholar] [CrossRef]
- Berbée, M.; Fu, Q.; Boerma, M.; Wang, J.; Kumar, K.S.; Hauer-Jensen, M. γ-Tocotrienol Ameliorates Intestinal Radiation Injury and Reduces Vascular Oxidative Stress after Total-Body Irradiation by an HMG-CoA Reductase-Dependent Mechanism. Radiat. Res. 2009, 171, 596–605. [Google Scholar] [CrossRef]
- Berbée, M.; Fu, Q.; Garg, S.; Kulkarni, S.; Kumar, K.S.; Hauer-Jensen, M. Pentoxifylline Enhances the Radioprotective Properties of γ-Tocotrienol: Differential Effects on the Hematopoietic, Gastrointestinal and Vascular Systems. Radiat. Res. 2011, 175, 297–306. [Google Scholar] [CrossRef]
- Kulkarni, S.; Chakraborty, K.; Kumar, K.S.; Kao, T.-C.; Hauer-Jensen, M.; Ghosh, S.P. Synergistic Radioprotection by Gamma-Tocotrienol and Pentoxifylline: Role of cAMP Signaling. ISRN Radiol. 2013, 2013, 390379. [Google Scholar] [CrossRef]
- Pathak, R.; Shao, L.; Ghosh, S.P.; Zhou, D.; Boerma, M.; Weiler, H.; Hauer-Jensen, M. Thrombomodulin Contributes to Gamma Tocotrienol-Mediated Lethality Protection and Hematopoietic Cell Recovery in Irradiated Mice. PLoS ONE 2015, 10, e0122511. [Google Scholar] [CrossRef]
- Singh, V.K.; Kulkarni, S.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.P.; Hendrickson, H.; Gulani, J.; Ghosh, S.P.; Kumar, K.S.; et al. Radioprotective Efficacy of Gamma-Tocotrienol in Nonhuman Primates. Radiat. Res. 2016, 185, 285–298. [Google Scholar] [CrossRef]
- Garg, T.K.; Garg, S.; Miousse, I.R.; Wise, S.Y.; Carpenter, A.D.; Fatanmi, O.O.; van Rhee, F.; Singh, V.K.; Hauer-Jensen, M. Gamma-Tocotrienol Modulates Total-Body Irradiation-Induced Hematopoietic Injury in a Nonhuman Primate Model. Int. J. Mol. Sci. 2022, 23, 16170. [Google Scholar] [CrossRef]
- Satyamitra, M.M.; Kulkarni, S.; Ghosh, S.P.; Mullaney, C.P.; Condliffe, D.; Srinivasan, V. Hematopoietic Recovery and Amelioration of Radiation-Induced Lethality by the Vitamin E Isoform δ-Tocotrienol. Radiat. Res. 2011, 175, 736–745. [Google Scholar] [CrossRef]
- Satyamitra, M.; Ney, P.; Graves, J.; Mullaney, C.; Srinivasan, V. Mechanism of Radioprotection by δ-Tocotrienol: Pharmacokinetics, Pharmacodynamics and Modulation of Signalling Pathways. Br. J. Radiol. 2012, 85, e1093–e1103. [Google Scholar] [CrossRef]
- Suman, S.; Datta, K.; Chakraborty, K.; Kulkarni, S.S.; Doiron, K.; Fornace, A.J.; Sree Kumar, K.; Hauer-Jensen, M.; Ghosh, S.P. Gamma Tocotrienol, a Potent Radioprotector, Preferentially Upregulates Expression of Anti-Apoptotic Genes to Promote Intestinal Cell Survival. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 60, 488–496. [Google Scholar] [CrossRef]
- Banerjee, S.; Shah, S.K.; Melnyk, S.B.; Pathak, R.; Hauer-Jensen, M.; Pawar, S.A. Cebpd Is Essential for Gamma-Tocotrienol Mediated Protection against Radiation-Induced Hematopoietic and Intestinal Injury. Antioxidants 2018, 7, 55. [Google Scholar] [CrossRef]
- Garg, S.; Sadhukhan, R.; Banerjee, S.; Savenka, A.V.; Basnakian, A.G.; McHargue, V.; Wang, J.; Pawar, S.A.; Ghosh, S.P.; Ware, J.; et al. Gamma-Tocotrienol Protects the Intestine from Radiation Potentially by Accelerating Mesenchymal Immune Cell Recovery. Antioxidants 2019, 8, 57. [Google Scholar] [CrossRef]
- Garg, S.; Garg, T.K.; Wise, S.Y.; Fatanmi, O.O.; Miousse, I.R.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of Gamma-Tocotrienol on Intestinal Injury in a GI-Specific Acute Radiation Syndrome Model in Nonhuman Primate. Int. J. Mol. Sci. 2022, 23, 4643. [Google Scholar] [CrossRef]
- Garg, S.; Garg, T.K.; Miousse, I.R.; Wise, S.Y.; Fatanmi, O.O.; Savenka, A.V.; Basnakian, A.G.; Singh, V.K.; Hauer-Jensen, M. Effects of Gamma-Tocotrienol on Partial-Body Irradiation-Induced Intestinal Injury in a Nonhuman Primate Model. Antioxidants 2022, 11, 1895. [Google Scholar] [CrossRef]
- Li, X.H.; Ghosh, S.P.; Ha, C.T.; Fu, D.; Elliott, T.B.; Bolduc, D.L.; Villa, V.; Whitnall, M.H.; Landauer, M.R.; Xiao, M. Delta-Tocotrienol Protects Mice from Radiation-Induced Gastrointestinal Injury. Radiat. Res. 2013, 180, 649–657. [Google Scholar] [CrossRef]
- Li, X.H.; Ha, C.T.; Fu, D.; Landauer, M.R.; Ghosh, S.P.; Xiao, M. Delta-Tocotrienol Suppresses Radiation-Induced microRNA-30 and Protects Mice and Human CD34+ Cells from Radiation Injury. PLoS ONE 2015, 10, e0122258. [Google Scholar] [CrossRef]
- Berbee, M.; Fu, Q.; Boerma, M.; Pathak, R.; Zhou, D.; Kumar, K.S.; Hauer-Jensen, M. Reduction of Radiation-Induced Vascular Nitrosative Stress by the Vitamin E Analog γ-Tocotrienol: Evidence of a Role for Tetrahydrobiopterin. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 884–891. [Google Scholar] [CrossRef]
- Berbée, M.; Fu, Q.; Boerma, M.; Sree Kumar, K.; Loose, D.S.; Hauer-Jensen, M. Mechanisms Underlying the Radioprotective Properties of γ-Tocotrienol: Comparative Gene Expression Profiling in Tocol-Treated Endothelial Cells. Genes Nutr. 2012, 7, 75–81. [Google Scholar] [CrossRef]
- Pathak, R.; Bachri, A.; Ghosh, S.P.; Koturbash, I.; Boerma, M.; Binz, R.K.; Sawyer, J.R.; Hauer-Jensen, M. The Vitamin E Analog Gamma-Tocotrienol (GT3) Suppresses Radiation-Induced Cytogenetic Damage. Pharm. Res. 2016, 33, 2117–2125. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sundaram, C.; Prasad, S.; Kannappan, R. Tocotrienols, the Vitamin E of the 21st Century: It’s Potential Against Cancer and Other Chronic Diseases. Biochem. Pharmacol. 2010, 80, 1613–1631. [Google Scholar] [CrossRef]
- Peh, H.Y.; Tan, W.S.D.; Liao, W.; Wong, W.S.F. Vitamin E Therapy beyond Cancer: Tocopherol versus Tocotrienol. Pharmacol. Ther. 2016, 162, 152–169. [Google Scholar] [CrossRef]
- Singh, V.K.; Beattie, L.A.; Seed, T.M. Vitamin E: Tocopherols and Tocotrienols as Potential Radiation Countermeasures. J. Radiat. Res. 2013, 54, 973–988. [Google Scholar] [CrossRef]
- Ahsan, H.; Ahad, A.; Iqbal, J.; Siddiqui, W.A. Pharmacological Potential of Tocotrienols: A Review. Nutr. Metab. 2014, 11, 52. [Google Scholar] [CrossRef]
- Kanchi, M.M.; Shanmugam, M.K.; Rane, G.; Sethi, G.; Kumar, A.P. Tocotrienols: The Unsaturated Sidekick Shifting New Paradigms in Vitamin E Therapeutics. Drug Discov. Today 2017, 22, 1765–1781. [Google Scholar] [CrossRef]
- Aykin-Burns, N.; Pathak, R.; Boerma, M.; Kim, T.; Hauer-Jensen, M. Utilization of Vitamin E Analogs to Protect Normal Tissues While Enhancing Antitumor Effects. Semin. Radiat. Oncol. 2019, 29, 55–61. [Google Scholar] [CrossRef]
- Ranasinghe, R.; Mathai, M.; Zulli, A. Revisiting the Therapeutic Potential of Tocotrienol. Biofactors 2022, 48, 813–856. [Google Scholar] [CrossRef]
- Kulkarni, S.; Singh, P.K.; Ghosh, S.P.; Posarac, A.; Singh, V.K. Granulocyte Colony-Stimulating Factor Antibody Abrogates Radioprotective Efficacy of Gamma-Tocotrienol, a Promising Radiation Countermeasure. Cytokine 2013, 62, 278–285. [Google Scholar] [CrossRef]
- Singh, V.K.; Wise, S.Y.; Scott, J.R.; Romaine, P.L.P.; Newman, V.L.; Fatanmi, O.O. Radioprotective Efficacy of Delta-Tocotrienol, a Vitamin E Isoform, Is Mediated through Granulocyte Colony-Stimulating Factor. Life Sci. 2014, 98, 113–122. [Google Scholar] [CrossRef]
- Ray, S.; Kulkarni, S.S.; Chakraborty, K.; Pessu, R.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Mobilization of Progenitor Cells into Peripheral Blood by Gamma-Tocotrienol: A Promising Radiation Countermeasure. Int. Immunopharmacol. 2013, 15, 557–564. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free Radical Recycling and Intramembrane Mobility in the Antioxidant Properties of Alpha-Tocopherol and Alpha-Tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Yamaoka, M.; Carrillo, M.J.H. Effect of Tocopherols and Tocotrienols on the Physicochemical Property of the Liposomal Membrane in Relation to Their Antioxidative Activity. Chem. Phys. Lipids 1990, 55, 295–300. [Google Scholar] [CrossRef]
- Suzuki, Y.J.; Tsuchiya, M.; Wassall, S.R.; Choo, Y.M.; Govil, G.; Kagan, V.E.; Packer, L. Structural and Dynamic Membrane Properties of Alpha-Tocopherol and Alpha-Tocotrienol: Implication to the Molecular Mechanism of Their Antioxidant Potency. Biochemistry 1993, 32, 10692–10699. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, Y.; Niki, E.; Noguchi, N. Comparative Study on the Action of Tocopherols and Tocotrienols as Antioxidant: Chemical and Physical Effects. Chem. Phys. Lipids 2003, 123, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Shafran, R.L.; Jackson, W.E.; Seed, T.M.; Kumar, K.S. Induction of Cytokines by Radioprotective Tocopherol Analogs. Exp. Mol. Pathol. 2006, 81, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.S.; Cary, L.H.; Gambles, K.; Hauer-Jensen, M.; Kumar, K.S.; Ghosh, S.P. Gamma-Tocotrienol, a Radiation Prophylaxis Agent, Induces High Levels of Granulocyte Colony-Stimulating Factor. Int. Immunopharmacol. 2012, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Pall, M.L.; Levine, S. Nrf2, a Master Regulator of Detoxification and also Antioxidant, Anti-Inflammatory and Other Cytoprotective Mechanisms, is Raised by Health Promoting Factors. Sheng Li Xue Bao 2015, 67, 1–18. [Google Scholar] [PubMed]
- Atia, A.; Alrawaiq, N.S.; Abdullah, A. Tocotrienols Activate Nrf2 Nuclear Translocation and Increase the Antioxidant- Related Hepatoprotective Mechanism in Mice Liver. Curr. Pharm. Biotechnol. 2021, 22, 1085–1098. [Google Scholar] [CrossRef]
- He, F.; Ru, X.; Wen, T. NRF2, a Transcription Factor for Stress Response and Beyond. Int. J. Mol. Sci. 2020, 21, 4777. [Google Scholar] [CrossRef]
- Wang, Y.; Park, N.-Y.; Jang, Y.; Ma, A.; Jiang, Q. Vitamin E γ-Tocotrienol Inhibits Cytokine-Stimulated NF-κB Activation by Induction of Anti-Inflammatory A20 via Stress Adaptive Response Due to Modulation of Sphingolipids. J. Immunol. Baltim. Md 1950 2015, 195, 126–133. [Google Scholar] [CrossRef]
- Singh, V.; Gupta, D.; Arora, R. NF-kB as a Key Player in Regulation of Cellular Radiation Responses and Identification of Radiation Countermeasures. Discoveries 2015, 3, e35. [Google Scholar] [CrossRef]
- Agarwal, M.K.; Agarwal, M.L.; Athar, M.; Gupta, S. Tocotrienol-Rich Fraction of Palm Oil Activates P53, Modulates Bax/Bcl2 Ratio and Induces Apoptosis Independent of Cell Cycle Association. Cell Cycle 2004, 3, 205–211. [Google Scholar] [CrossRef]
- Ghosh, S.P.; Pathak, R.; Kumar, P.; Biswas, S.; Bhattacharyya, S.; Kumar, V.P.; Hauer-Jensen, M.; Biswas, R. Gamma-Tocotrienol Modulates Radiation-Induced MicroRNA Expression in Mouse Spleen. Radiat. Res. 2016, 185, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lu, X.; Feng, J.B.; Tian, M.; Liu, Q.J. Identification and Validation of Candidate Radiation-Responsive Genes for Human Biodosimetr. Biomed. Environ. Sci. BES 2017, 30, 834–840. [Google Scholar] [PubMed]
- Pearce, B.C.; Parker, R.A.; Deason, M.E.; Qureshi, A.A.; Wright, J.J. Hypocholesterolemic Activity of Synthetic and Natural Tocotrienols. J. Med. Chem. 1992, 35, 3595–3606. [Google Scholar] [CrossRef]
- Parker, R.A.; Pearce, B.C.; Clark, R.W.; Gordon, D.A.; Wright, J.J. Tocotrienols Regulate Cholesterol Production in Mammalian Cells by Post-Transcriptional Suppression of 3-Hydroxy-3-Methylglutaryl-Coenzyme A Reductase. J. Biol. Chem. 1993, 268, 11230–11238. [Google Scholar] [PubMed]
- Ramanathan, N.; Tan, E.; Loh, L.J.; Soh, B.S.; Yap, W.N. Tocotrienol Is a Cardioprotective Agent against Ageing-Associated Cardiovascular Disease and Its Associated Morbidities. Nutr. Metab. 2018, 15, 6. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic Effects of Statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89. [Google Scholar] [CrossRef]
- Laufs, U.; La Fata, V.; Plutzky, J.; Liao, J.K. Upregulation of Endothelial Nitric Oxide Synthase by HMG CoA Reductase Inhibitors. Circulation 1998, 97, 1129–1135. [Google Scholar] [CrossRef]
- Nowak, G.; Bakajsova, D.; Hayes, C.; Hauer-Jensen, M.; Compadre, C.M. γ-Tocotrienol Protects against Mitochondrial Dysfunction and Renal Cell Death. J. Pharmacol. Exp. Ther. 2012, 340, 330–338. [Google Scholar] [CrossRef]
- Nowak, G.; Megyesi, J. γ-Tocotrienol Protects against Mitochondrial Dysfunction, Energy Deficits, Morphological Damage, and Decreases in Renal Functions after Renal Ischemia. Int. J. Mol. Sci. 2021, 22, 12674. [Google Scholar] [CrossRef]
- Sridharan, V.; Tripathi, P.; Aykin-Burns, N.; Krager, K.J.; Sharma, S.K.; Moros, E.G.; Melnyk, S.B.; Pavliv, O.; Hauer-Jensen, M.; Boerma, M. A Tocotrienol-Enriched Formulation Protects against Radiation-Induced Changes in Cardiac Mitochondria without Modifying Late Cardiac Function or Structure. Radiat. Res. 2015, 183, 357–366. [Google Scholar] [CrossRef]
- Krager, K.J.; Pineda, E.N.; Kharade, S.V.; Kordsmeier, M.; Howard, L.; Breen, P.J.; Compadre, C.M.; Hauer-Jensen, M.; Aykin-Burns, N. Tocotrienol-Rich Fraction from Rice Bran Demonstrates Potent Radiation Protection Activity. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 148791. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Shafie, N.H.; Kaniappan, P.; Bahari, H. An Interactive Review on the Role of Tocotrienols in the Neurodegenerative Disorders. Front. Nutr. 2021, 8, 754086. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, A.; Esatbeyoglu, T.; Piegholdt, S.; Dose, J.; Ikuta, N.; Okamoto, H.; Ishida, Y.; Terao, K.; Matsugo, S.; Rimbach, G. Dietary Tocotrienol/γ-Cyclodextrin Complex Increases Mitochondrial Membrane Potential and ATP Concentrations in the Brains of Aged Mice. Oxid. Med. Cell. Longev. 2015, 2015, 789710. [Google Scholar] [CrossRef] [PubMed]
- Li, X.H.; Fu, D.; Latif, N.H.; Mullaney, C.P.; Ney, P.H.; Mog, S.R.; Whitnall, M.H.; Srinivasan, V.; Xiao, M. δ-Tocotrienol Protects Mouse and Human Hematopoietic Progenitors from γ-Irradiation through Extracellular Signal-Regulated Kinase/Mammalian Target of Rapamycin Signaling. Haematologica 2010, 95, 1996–2004. [Google Scholar] [CrossRef]
- Singh, P.K.; Krishnan, S. Vitamin E Analogs as Radiation Response Modifiers. Evid.-Based Complement. Altern. Med. ECAM 2015, 2015, 741301. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Hauer-Jensen, M. γ-Tocotrienol as a Promising Countermeasure for Acute Radiation Syndrome: Current Status. Int. J. Mol. Sci. 2016, 17, 663. [Google Scholar] [CrossRef]
- Compadre, C.M.; Singh, A.; Thakkar, S.; Zheng, G.; Breen, P.J.; Ghosh, S.; Kiaei, M.; Boerma, M.; Varughese, K.I.; Hauer-Jensen, M. Molecular Dynamics Guided Design of Tocoflexol: A New Radioprotectant Tocotrienol with Enhanced Bioavailability. Drug Dev. Res. 2014, 75, 10–22. [Google Scholar] [CrossRef]
- Liu, X.; Gujarathi, S.; Zhang, X.; Shao, L.; Boerma, M.; Compadre, C.M.; Crooks, P.A.; Hauer-Jensen, M.; Zhou, D.; Zheng, G. Synthesis of (2R,8′S,3′E)-δ-Tocodienol, a Tocoflexol Family Member Designed to Have a Superior Pharmacokinetic Profile Compared to δ-Tocotrienol. Tetrahedron 2016, 72, 4001–4006. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Gao, Z.; Fu, Q.; Song, L.; Zhang, P.; Zhang, X.; Hendrickson, H.; Crooks, P.A.; Zhou, D.; Zheng, G. Deuteration of the Farnesyl Terminal Methyl Groups of δ-Tocotrienol and Its Effects on the Metabolic Stability and Ability of Inducing G-CSF Production. Bioorg. Med. Chem. 2020, 28, 115498. [Google Scholar] [CrossRef]
- Kumar, V.P.; Stone, S.; Biswas, S.; Sharma, N.; Ghosh, S.P. Gamma Tocotrienol Protects Mice from Targeted Thoracic Radiation Injury. Front. Pharmacol. 2020, 11, 587970. [Google Scholar] [CrossRef]
- Singh, V.K.; Olabisi, A.O. Nonhuman Primates as Models for the Discovery and Development of Radiation Countermeasures. Expert Opin. Drug Discov. 2017, 12, 695–709. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Fatanmi, O.O.; Verma, A.; Newman, V.L.; Wise, S.Y.; Romaine, P.L.P.; Berg, A.N. Progenitor Cell Mobilization by Gamma-Tocotrienol: A Promising Radiation Countermeasure. Health Phys. 2016, 111, 85–92. [Google Scholar] [CrossRef]
- Kulkarni, S.; Ghosh, S.P.; Satyamitra, M.; Mog, S.; Hieber, K.; Romanyukha, L.; Gambles, K.; Toles, R.; Kao, T.-C.; Hauer-Jensen, M.; et al. Gamma-Tocotrienol Protects Hematopoietic Stem and Progenitor Cells in Mice after Total-Body Irradiation. Radiat. Res. 2010, 173, 738–747. [Google Scholar] [CrossRef] [PubMed]
- Theyab, A.; Algahtani, M.; Alsharif, K.F.; Hawsawi, Y.M.; Alghamdi, A.; Alghamdi, A.; Akinwale, J. New Insight into the Mechanism of Granulocyte Colony-Stimulating Factor (G-CSF) That Induces the Mobilization of Neutrophils. Hematology 2021, 26, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Romaine, P.L.P.; Newman, V.L. Biologics as Countermeasures for Acute Radiation Syndrome: Where Are We Now? Expert Opin. Biol. Ther. 2015, 15, 465–471. [Google Scholar] [CrossRef]
- Singh, V.K.; Fatanmi, O.O.; Wise, S.Y.; Newman, V.L.; Romaine, P.L.P.; Seed, T.M. The Potentiation of the Radioprotective Efficacy of Two Medical Countermeasures, Gamma-Tocotrienol and Amifostine, by a Combination Prophylactic Modality. Radiat. Prot. Dosim. 2016, 172, 302–310. [Google Scholar] [CrossRef]
- Bonavita, O.; Mollica Poeta, V.; Massara, M.; Mantovani, A.; Bonecchi, R. Regulation of Hematopoiesis by the Chemokine System. Cytokine 2018, 109, 76–80. [Google Scholar] [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A Guide to Chemokines and Their Receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Sokol, C.L.; Luster, A.D. The Chemokine System in Innate Immunity. Cold Spring Harb. Perspect. Biol. 2015, 7, a016303. [Google Scholar] [CrossRef]
- David, B.A.; Kubes, P. Exploring the Complex Role of Chemokines and Chemoattractants In Vivo on Leukocyte Dynamics. Immunol. Rev. 2019, 289, 9–30. [Google Scholar] [CrossRef]
- Pawar, S.A.; Shao, L.; Chang, J.; Wang, W.; Pathak, R.; Zhu, X.; Wang, J.; Hendrickson, H.; Boerma, M.; Sterneck, E.; et al. C/EBPδ Deficiency Sensitizes Mice to Ionizing Radiation-Induced Hematopoietic and Intestinal Injury. PLoS ONE 2014, 9, e94967. [Google Scholar] [CrossRef] [PubMed]
- Fendler, W.; Malachowska, B.; Meghani, K.; Konstantinopoulos, P.A.; Guha, C.; Singh, V.K.; Chowdhury, D. Evolutionarily Conserved Serum microRNAs Predict Radiation-Induced Fatality in Nonhuman Primates. Sci. Transl. Med. 2017, 9, eaal2408. [Google Scholar] [CrossRef] [PubMed]
- Cheema, A.K.; Byrum, S.D.; Sharma, N.K.; Altadill, T.; Kumar, V.P.; Biswas, S.; Balgley, B.M.; Hauer-Jensen, M.; Tackett, A.J.; Ghosh, S.P. Proteomic Changes in Mouse Spleen after Radiation-Induced Injury and Its Modulation by Gamma-Tocotrienol. Radiat. Res. 2018, 190, 449–463. [Google Scholar] [CrossRef] [PubMed]
- Sumagin, R.; Brazil, J.C.; Nava, P.; Nishio, H.; Alam, A.; Luissint, A.C.; Weber, D.A.; Neish, A.S.; Nusrat, A.; Parkos, C.A. Neutrophil Interactions with Epithelial-Expressed ICAM-1 Enhances Intestinal Mucosal Wound Healing. Mucosal Immunol. 2016, 9, 1151–1162. [Google Scholar] [CrossRef] [PubMed]
- Dahan, S.; Roda, G.; Pinn, D.; Roth-Walter, F.; Kamalu, O.; Martin, A.P.; Mayer, L. Epithelial: Lamina Propria Lymphocyte Interactions Promote Epithelial Cell Differentiation. Gastroenterology 2008, 134, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Pollard, H.B. Ionizing Radiation-Induced Altered microRNA Expression as Biomarkers for Assessing Acute Radiation Injury. Expert Rev. Mol. Diagn. 2017, 17, 871–874. [Google Scholar] [CrossRef]
- Venkatesulu, B.P.; Mahadevan, L.S.; Aliru, M.L.; Yang, X.; Bodd, M.H.; Singh, P.K.; Yusuf, S.W.; Abe, J.; Krishnan, S. Radiation-Induced Endothelial Vascular Injury. JACC Basic Transl. Sci. 2018, 3, 563–572. [Google Scholar] [CrossRef]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of Radiation-Induced Endothelium Damage: Emerging Models and Technologies. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial Apoptosis as the Primary Lesion Initiating Intestinal Radiation Damage in Mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef]
- Satyamitra, M.M.; DiCarlo, A.L.; Taliaferro, L. Understanding the Pathophysiology and Challenges of Development of Medical Countermeasures for Radiation-Induced Vascular/Endothelial Cell Injuries: Report of a NIAID Workshop, August 20, 2015. Radiat. Res. 2016, 186, 99–111. [Google Scholar] [CrossRef]
- Pathak, R.; Kumar, V.P.; Hauer-Jensen, M.; Ghosh, S.P. Enhanced Survival in Mice Exposed to Ionizing Radiation by Combination of Gamma-Tocotrienol and Simvastatin. Mil. Med. 2019, 184 (Suppl. 1), 644–651. [Google Scholar] [CrossRef] [PubMed]
- Fu, Q.; Wang, J.; Boerma, M.; Berbée, M.; Qiu, X.; Fink, L.M.; Hauer-Jensen, M. Involvement of Heat Shock Factor 1 in Statin-Induced Transcriptional Upregulation of Endothelial Thrombomodulin. Circ. Res. 2008, 103, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Wang, J.; Zheng, H.; Ling, W.; Joseph, J.; Li, D.; Mehta, J.L.; Ponnappan, U.; Lin, P.; Fink, L.M.; et al. Statins Increase Thrombomodulin Expression and Function in Human Endothelial Cells by a Nitric Oxide-Dependent Mechanism and Counteract Tumor Necrosis Factor Alpha-Induced Thrombomodulin Downregulation. Blood Coagul. Fibrinolysis Int. J. Haemost. Thromb. 2003, 14, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, H.; Ou, X.; Fink, L.M.; Hauer-Jensen, M. Deficiency of Microvascular Thrombomodulin and Up-Regulation of Protease-Activated Receptor-1 in Irradiated Rat Intestine: Possible Link between Endothelial Dysfunction and Chronic Radiation Fibrosis. Am. J. Pathol. 2002, 160, 2063–2072. [Google Scholar] [CrossRef]
- Ito, T.; Maruyama, I. Thrombomodulin: Protectorate God of the Vasculature in Thrombosis and Inflammation. J. Thromb. Haemost. JTH 2011, 9 (Suppl. 1), 168–173. [Google Scholar] [CrossRef]
- Richter, K.K.; Fink, L.M.; Hughes, B.M.; Sung, C.C.; Hauer-Jensen, M. Is the Loss of Endothelial Thrombomodulin Involved in the Mechanism of Chronicity in Late Radiation Enteropathy? Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 1997, 44, 65–71. [Google Scholar] [CrossRef]
- Hicklin, D.J.; Ellis, L.M. Role of the Vascular Endothelial Growth Factor Pathway in Tumor Growth and Angiogenesis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2005, 23, 1011–1027. [Google Scholar] [CrossRef]
- Kam, W.W.-Y.; Banati, R.B. Effects of Ionizing Radiation on Mitochondria. Free Radic. Biol. Med. 2013, 65, 607–619. [Google Scholar] [CrossRef]
- Livingston, K.; Schlaak, R.A.; Puckett, L.L.; Bergom, C. The Role of Mitochondrial Dysfunction in Radiation-Induced Heart Disease: From Bench to Bedside. Front. Cardiovasc. Med. 2020, 7, 20. [Google Scholar] [CrossRef]
- Tomasetti, M.; Neuzil, J. Vitamin E Analogues and Immune Response in Cancer Treatment. Vitam. Horm. 2007, 76, 463–491. [Google Scholar] [CrossRef]
- Neuzil, J.; Tomasetti, M.; Zhao, Y.; Dong, L.-F.; Birringer, M.; Wang, X.-F.; Low, P.; Wu, K.; Salvatore, B.A.; Ralph, S.J. Vitamin E Analogs, a Novel Group of “Mitocans,” as Anticancer Agents: The Importance of Being Redox-Silent. Mol. Pharmacol. 2007, 71, 1185–1199. [Google Scholar] [CrossRef] [PubMed]
- Savitskaya, M.A.; Onischenko, G.E. α-Tocopheryl Succinate Affects Malignant Cell Viability, Proliferation, and Differentiation. Biochem. Biokhimiia 2016, 81, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Satoh, H.; Fukumoto, K.; Kumadaki, I.; Ichikawa, T.; Yamada, K.; Hagiwara, K.; Yano, T. Induction of Cytotoxicity in Human Lung Adenocarcinoma Cells by 6-O-Carboxypropyl-Alpha-Tocotrienol, a Redox-Silent Derivative of Alpha-Tocotrienol. Int. J. Cancer 2005, 115, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Ishii, K.; Hido, M.; Sakamura, M.; Virgona, N.; Yano, T. α-Tocotrienol and Redox-Silent Analogs of Vitamin E Enhances Bortezomib Sensitivity in Solid Cancer Cells through Modulation of NFE2L1. Int. J. Mol. Sci. 2023, 24, 9382. [Google Scholar] [CrossRef]
- Ishii, K.; Fusegi, M.; Mori, T.; Teshima, K.; Ninomiya, N.; Kohno, K.; Sato, A.; Ishida, T.; Miyakoshi, Y.; Yano, T. A Redox-Silent Analogue of Tocotrienol May Break the Homeostasis of Proteasomes in Human Malignant Mesothelioma Cells by Inhibiting STAT3 and NRF1. Int. J. Mol. Sci. 2022, 23, 2655. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Harada, K.; Yano, Y.; Kumadaki, I.; Hagiwara, K.; Takebayashi, J.; Kido, W.; Virgona, N.; Yano, T. A Redox-Silent Analogue of Tocotrienol Inhibits Hypoxic Adaptation of Lung Cancer Cells. Biochem. Biophys. Res. Commun. 2008, 365, 875–881. [Google Scholar] [CrossRef]
- Yano, T.; Sato, A.; Sekine, M.; Virgona, N.; Ota, M. Redox-Inactive Analogue of Tocotrienol as a Potential Anti-Cancer Agent. Anticancer Agents Med. Chem. 2013, 13, 496–501. [Google Scholar]
- Singh, V.K.; Brown, D.S.; Kao, T.-C. Tocopherol Succinate: A Promising Radiation Countermeasure. Int. Immunopharmacol. 2009, 9, 1423–1430. [Google Scholar] [CrossRef]
- Singh, V.K.; Brown, D.S.; Kao, T.-C. Alpha-Tocopherol Succinate Protects Mice from Gamma-Radiation by Induction of Granulocyte-Colony Stimulating Factor. Int. J. Radiat. Biol. 2010, 86, 12–21. [Google Scholar] [CrossRef]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Brown, D.S.; Singh, V.K. Radioprotective Efficacy of Tocopherol Succinate Is Mediated through Granulocyte-Colony Stimulating Factor. Cytokine 2011, 56, 411–421. [Google Scholar] [CrossRef]
- Singh, P.K.; Wise, S.Y.; Ducey, E.J.; Fatanmi, O.O.; Elliott, T.B.; Singh, V.K. α-Tocopherol Succinate Protects Mice against Radiation-Induced Gastrointestinal Injury. Radiat. Res. 2012, 177, 133–145. [Google Scholar] [CrossRef] [PubMed]
| Hematopoietic System | ||
|---|---|---|
| Major Radioprotective Effects of Tocotrienols for H-ARS | Model | References |
Improves 30-day survival against H-ARS from lethal doses of total body irradiation
| Mice | GT3: [11] DT3: [18,65] |
| Stimulates multilineage hematopoiesis | Mice Primate | GT3: [11,16,17] DT3: [18,19] |
Protects against pancytopenia
| Mice Primate | GT3: [11,12,13,14,15,16,17] DT3: [18,19] |
| Particularly effective for neutropenia and thrombocytopenia | Mice Primate | GT3: [11,12,15,16,17] DT3: [18,19] |
Induces hematopoietic cytokines/hormones/chemokines
| Mice | GT3: [13,14,15,37,44,45] DT3: [38,70] |
| G-CSF induction is required for radioprotective efficacy | Mice | GT3: [37] DT3: [38] |
Promotes survival for HSCs and HPCs in the bone marrow
| Mice Primate hCD34+ cells | GT3: [13,14,29,37,74] DT3: [19,65] |
| Promotes spleen cell survival | Mice | GT3: [12,13] |
| Promotes recovery and proliferation of HSCs/HPCs | Mice | GT3: [37,74] DT3: [65] |
Mobilizes progenitor cells into the peripheral circulation
| Mice without radiation | GT3: [39] |
Reduces inflammatory and apoptotic markers
| Mice hCD34+ cells | DT3: [26] |
| Modulates gene expression with microRNAs and modulates protein expression in mouse spleen | Mice | GT3: [52,84] |
| Gastrointestinal Tract | ||
|---|---|---|
| Major Radioprotective Effects of Tocotrienols for GI-ARS | Model | References |
Prevents apoptosis of intestinal tissue cells
| Mice Primate | GT3: [20,23] DT3: [25] |
| Reduces GI-tract oxidative and nitrosative stress | Mice | GT3: [21] |
| Improves gastrointestinal tissue architecture | Mice Primate | GT3: [13,22,23] DT3: [25] |
| Promotes crypt cell survival | Mice | GT3: [12,13,20,21,22] DT3: [25] |
| Activates crypt stem cell proliferation | Primate | GT3: [23,24] |
| Promotes mucosal barrier function | Mice | GT3: [12,22] DT3: [25] |
| Inhibits bacterial gut invasion | Mice | GT3: [12] DT3: [25] |
| Preserves plasma citrulline levels | Mice | GT3: [12] |
Accelerated mesenchymal immune cell recovery to protect the intestine
| Mice | GT3: [22] |
Reduces GI-tract inflammatory markers
| Mice | GT3: [21] DT3: [25,26] |
| Vascular Endothelium | ||
|---|---|---|
| Major Radioprotective Effects for Tocotrienols | Model | References |
Protects against vascular oxidative and nitrosative stress
| Mice | GT3: [12,13,27] |
Inhibits HMG-CoA reductase
| Mice | GT3: [12] |
Induces thrombomodulin
| Mice | GT3: [15,92] |
| Attenuates endothelial apoptosis | Mice | GT3: [27] |
Reduces cytogenetic damage
| HUVEC cells | GT3: [29] |
Improved endothelial function
| Mice | GT3: [12] |
Induction of VEGF
| Mice | GT3: [39] |
Modulates gene expression pathways
| HUVEC cells | GT3: [28] |
| Other Organ Systems | ||
|---|---|---|
| Major Radioprotective Effects for Tocotrienols | Model | References |
Protected the lung in a thoracic-targeted radiation injury model (lung-PBI)
| Mice | GT3: [71] |
Protected cardiac mitochondria against radiation damage in cell models and locally irradiated mouse heart
| H9c2 cells Mice | DT3 and tocotrienol extracts [61,62] |
| Signaling Mechanisms | Experimental Findings for Mechanistic Tocotrienol Radioprotection Research | Model | References |
|---|---|---|---|
| G-CSF induction | Both GT3 and DT3 strongly induce G-CSF within 24 h
| Mice | GT3: [37,45] DT3: [38] |
| Cytokines and chemokines induction | Both GT3 and DT3 induce circulating levels of interleukins (IL-1β, IL-6), other cytokines, and chemokines
| Mice | GT3: [13,14,15,37,39,44,45] DT3: [38] |
| Anti-inflammatory action in tissues | GI Tract: DT3 reduced inflammatory markers in the GI tract (IL-1β and IL-6), NF-κB, and miR-30 | Mice | DT3: [25,26] |
| Lungs: GT3 reduced inflammatory markers and protected the lungs against inflammatory tissue damage | Mice | GT3: [71] | |
| Progenitor mobilization | GT3 mobilizes hematopoietic, endothelial, and stromal progenitors
| Mice | GT3: [39] |
| HMG-CoA reductase inhibition | Tocotrienols potently inhibit HMG-CoA reductase to provide vasculoprotective effects against radiation
| Mice | GT3: [12] |
| Thrombomodulin activation | GT3 activates endothelial thrombomodulin, which contributes to radioprotective efficacy
| HUVEC cells Mice | GT3: [15,92] |
| Increasing tetrahydrobiopterin bioavailability | GT3 improves bioavailability of BH4, which is a cofactor for the vasculoprotective eNOS
| Mice | GT3: [27] |
| DNA repair gene induction | GT3 induces DNA repair genes: | HUVEC cells | GT3: [28,29] |
| Cebpd induction | GT3 induces the transcription factor Cebpd
| Mice | GT3: [21] |
| microRNAs | DT3 inhibits miR-30 levels in multiple cell types and tissues, which reduces apoptosis in hematopoietic cells
| Mice hCD34+ cells Primate | GT3: [52,83] DT3: [26] |
| Mitochondrial Function | Tocotrienols prevented radiation-induced mitochondrial respiratory dysfunction and uncoupling in cell models | H9c2 andHaCaT cells | DT3 and tocotrienol extract: [62] |
Tocotrienols prevented harmful mitochondrial changes in locally irradiated mouse heart
| Mice | tocotrienol extract: [61] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shrum, S.A.; Nukala, U.; Shrimali, S.; Pineda, E.N.; Krager, K.J.; Thakkar, S.; Jones, D.E.; Pathak, R.; Breen, P.J.; Aykin-Burns, N.; et al. Tocotrienols Provide Radioprotection to Multiple Organ Systems through Complementary Mechanisms of Antioxidant and Signaling Effects. Antioxidants 2023, 12, 1987. https://doi.org/10.3390/antiox12111987
Shrum SA, Nukala U, Shrimali S, Pineda EN, Krager KJ, Thakkar S, Jones DE, Pathak R, Breen PJ, Aykin-Burns N, et al. Tocotrienols Provide Radioprotection to Multiple Organ Systems through Complementary Mechanisms of Antioxidant and Signaling Effects. Antioxidants. 2023; 12(11):1987. https://doi.org/10.3390/antiox12111987
Chicago/Turabian StyleShrum, Stephen A., Ujwani Nukala, Shivangi Shrimali, Edith Nathalie Pineda, Kimberly J. Krager, Shraddha Thakkar, Darin E. Jones, Rupak Pathak, Philip J. Breen, Nukhet Aykin-Burns, and et al. 2023. "Tocotrienols Provide Radioprotection to Multiple Organ Systems through Complementary Mechanisms of Antioxidant and Signaling Effects" Antioxidants 12, no. 11: 1987. https://doi.org/10.3390/antiox12111987
APA StyleShrum, S. A., Nukala, U., Shrimali, S., Pineda, E. N., Krager, K. J., Thakkar, S., Jones, D. E., Pathak, R., Breen, P. J., Aykin-Burns, N., & Compadre, C. M. (2023). Tocotrienols Provide Radioprotection to Multiple Organ Systems through Complementary Mechanisms of Antioxidant and Signaling Effects. Antioxidants, 12(11), 1987. https://doi.org/10.3390/antiox12111987









