The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. The Animals, Primary Hepatocyte Isolation, and Cell Culture
2.2. Chemicals
2.3. Fatty Acid Preparation
2.4. Detection of Intracellular Lipid Content and Cellular Triglycerides
2.5. Viability and Cell Death Assays
2.6. Measurement of Intracellular Reactive Oxygen Species (ROS) Generation
2.7. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
3.1. Esculetin Attenuates Palmitic-Acid-Induced Toxicity and Necrosis in Primary Rat Hepatocytes
3.2. Esculetin Attenuates Palmitic Acid-Induced Oxidative Stress in Primary Rat Hepatocytes
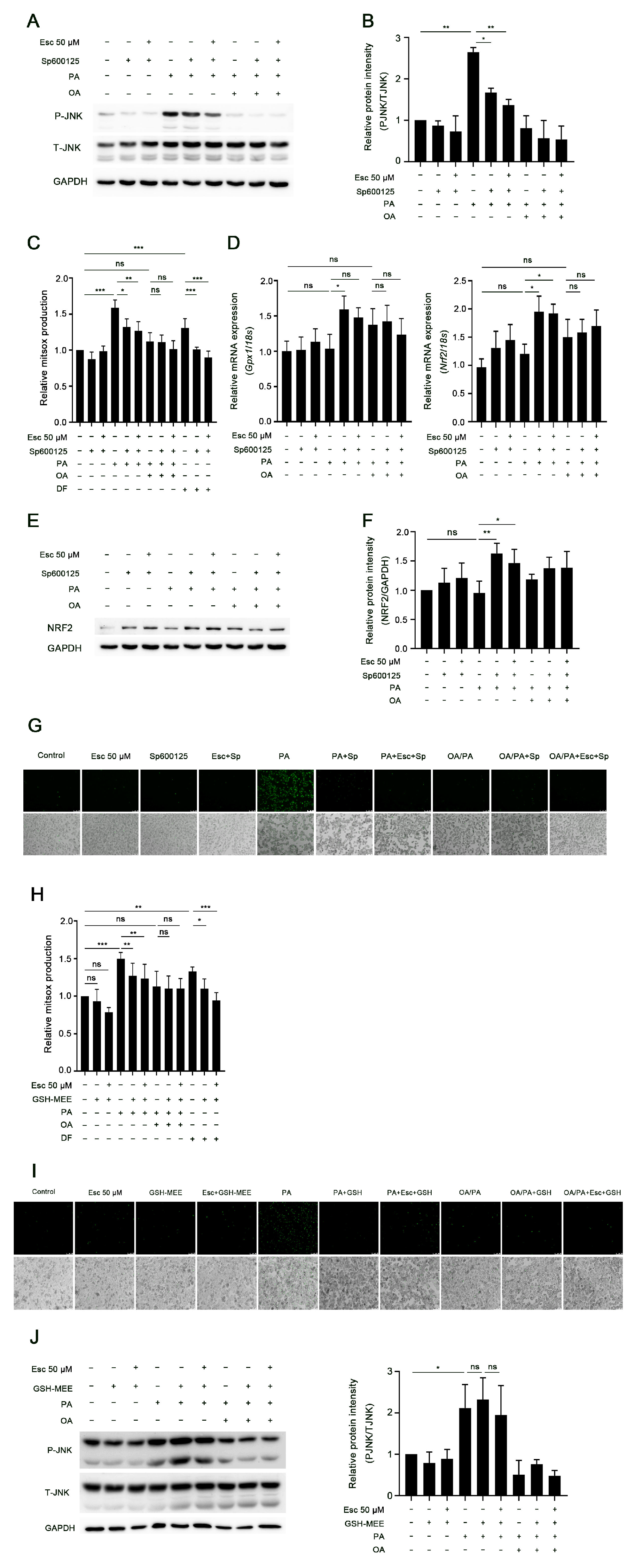
3.3. Esculetin Attenuates JNK Activation in Palmitic-Acid-Treated Hepatocytes
3.4. JNK Activation Induced by Palmitic Acid Leads to Increased Oxidative Stress and Lipotoxicity in Hepatocytes
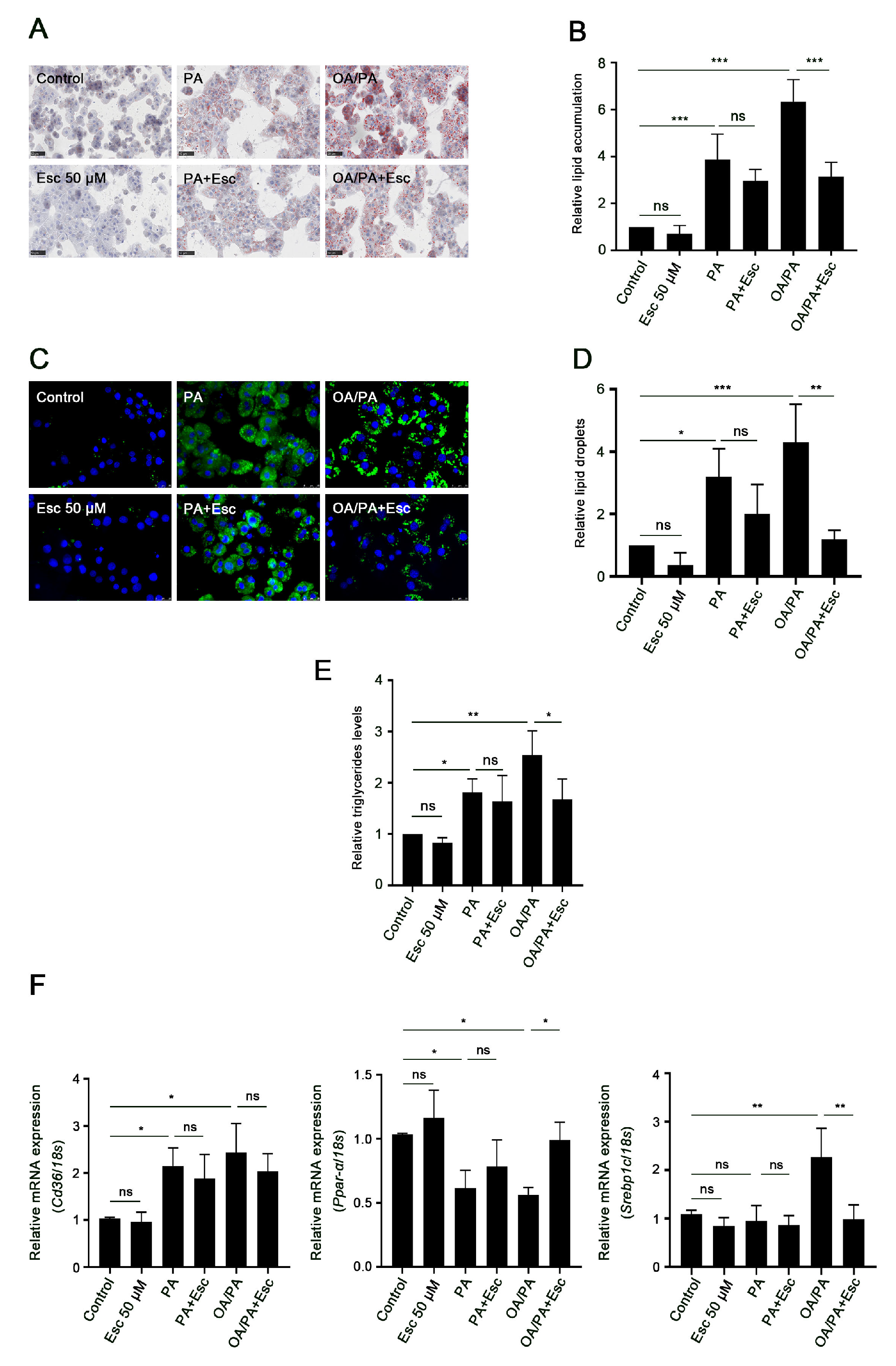
3.5. Esculetin Improves Free Fatty Acid-Induced Lipid Accumulation and Metabolism
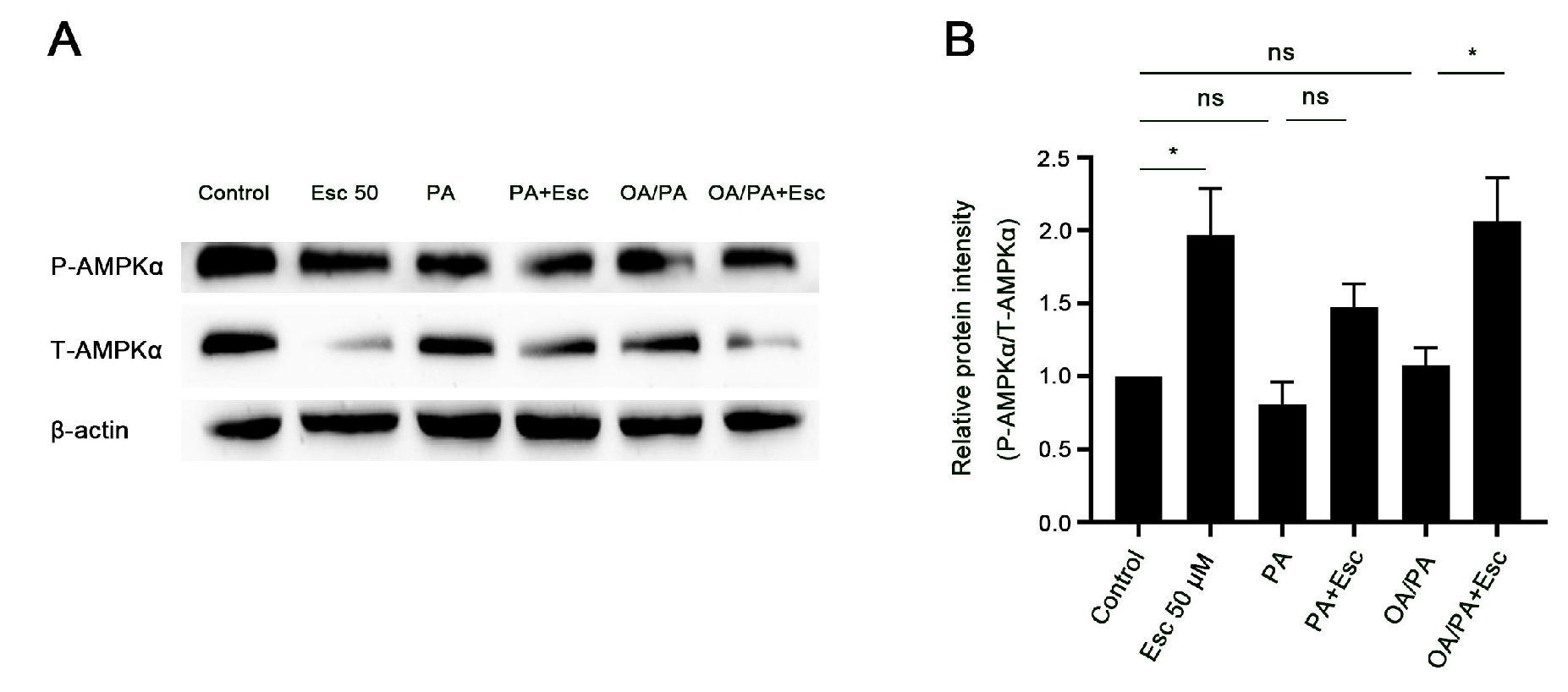
3.6. Esculetin Promotes AMPKα Phosphorylation in Free Fatty Acid-Treated Hepatocytes
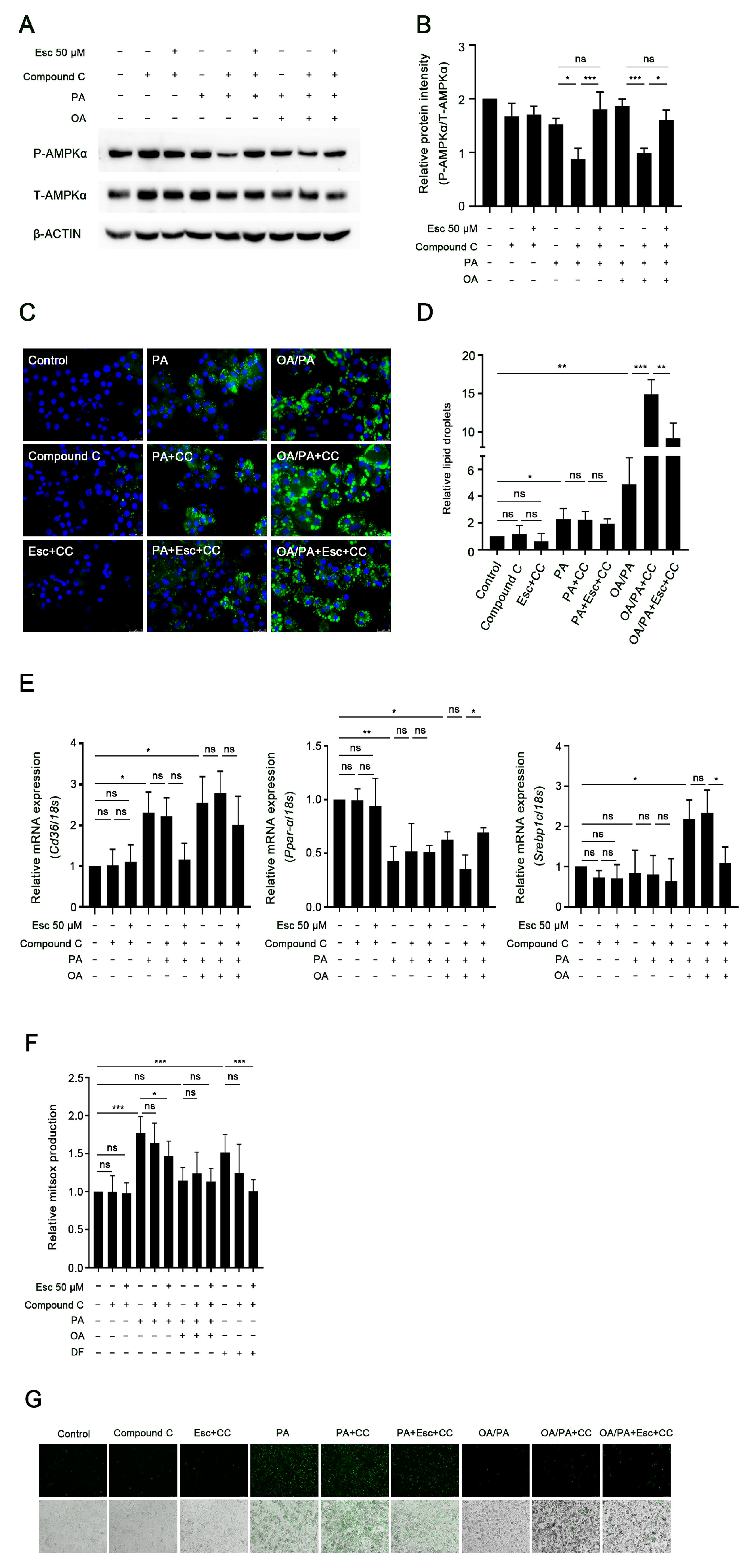
3.7. AMPKα Activation Mediates the Effects of Esculetin on Lipid Accumulation but Not on Oxidative Stress and Necrosis
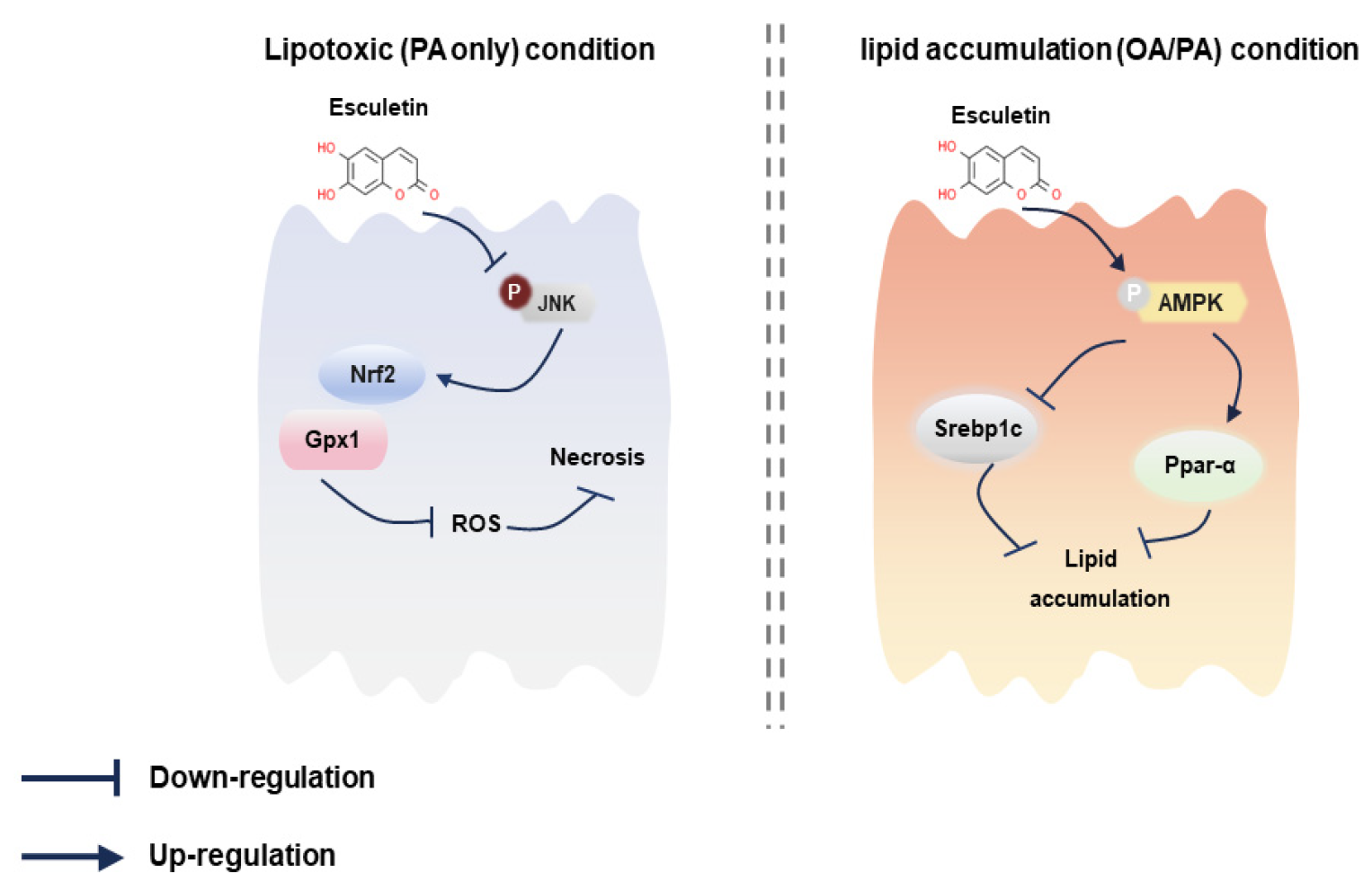
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Davis, T.M.E. Diabetes and metabolic dysfunction-associated fatty liver disease. Metabolism 2021, 123, 154868. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Sanchez, N.; Cruz-Ramon, V.C.; Ramirez-Perez, O.L.; Hwang, J.P.; Barranco-Fragoso, B.; Cordova-Gallardo, J. New Aspects of Lipotoxicity in Nonalcoholic Steatohepatitis. Int. J. Mol. Sci. 2018, 19, 2034. [Google Scholar] [CrossRef]
- Geng, Y.; Faber, K.N.; de Meijer, V.E.; Blokzijl, H.; Moshage, H. How does hepatic lipid accumulation lead to lipotoxicity in non-alcoholic fatty liver disease? Hepatol. Int. 2021, 15, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Arroyave-Ospina, J.C.; Wu, Z.; Geng, Y.; Moshage, H. Role of Oxidative Stress in the Pathogenesis of Non-Alcoholic Fatty Liver Disease: Implications for Prevention and Therapy. Antioxidants 2021, 10, 174. [Google Scholar] [CrossRef]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free. Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Egnatchik, R.A.; Leamy, A.K.; Noguchi, Y.; Shiota, M.; Young, J.D. Palmitate-induced activation of mitochondrial metabolism promotes oxidative stress and apoptosis in H4IIEC3 rat hepatocytes. Metabolism 2014, 63, 283–295. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Arif, Z.; Kabir, A.; Mehmood, I.; Munir, D.; Razzaq, A.; Ali, A.; Goksen, G.; Coşier, V.; Ahmad, N.; et al. Oxidative stress and metabolic diseases: Relevance and therapeutic strategies. Front. Nutr. 2022, 9, 994309. [Google Scholar] [CrossRef]
- Mannaa, F.A.; Abdel-Wahhab, K.G. Physiological potential of cytokines and liver damages. Hepatoma. Res. 2016, 2, 131–143. [Google Scholar] [CrossRef]
- Tang, S.P.; Mao, X.L.; Chen, Y.H.; Yan, L.L.; Ye, L.P.; Li, S.W. Reactive Oxygen Species Induce Fatty Liver and Ischemia-Reperfusion Injury by Promoting Inflammation and Cell Death. Front. Immunol. 2022, 13, 870239. [Google Scholar] [CrossRef] [PubMed]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Lu, Z.; Miao, Y.; Muhammad, I.; Tian, E.; Hu, W.; Wang, J.; Wang, B.; Li, R.; Li, J. Colistin-induced autophagy and apoptosis involves the JNK-Bcl2-Bax signaling pathway and JNK-p53-ROS positive feedback loop in PC-12 cells. Chem. Biol. Interact. 2017, 277, 62–73. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metabolism 2018, 83, 256–270. [Google Scholar] [CrossRef]
- Li, B.; Zhou, P.; Xu, K.; Chen, T.; Jiao, J.; Wei, H.; Yang, X.; Xu, W.; Wan, W.; Xiao, J. Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int. J. Biol. Sci. 2020, 16, 74–84. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell. Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef]
- Li, L.; Fu, J.; Sun, J.; Liu, D.; Chen, C.; Wang, H.; Hou, Y.; Xu, Y.; Pi, J. Is Nrf2-ARE a potential target in NAFLD mitigation? Curr. Opin. Toxicol. 2019, 13, 35–44. [Google Scholar] [CrossRef]
- Feng, X.; Yu, W.; Li, X.; Zhou, F.; Zhang, W.; Shen, Q.; Li, J.; Zhang, C.; Shen, P. Apigenin, a modulator of PPARγ, attenuates HFD-induced NAFLD by regulating hepatocyte lipid metabolism and oxidative stress via Nrf2 activation. Biochem. Pharmacol. 2017, 136, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.; Theise, N.D.; Loneker, A.E.; Janmey, P.A.; Wells, R.G. Lipid droplets disrupt mechanosensing in human hepatocytes. Am. J. Physiol. Gastrointest. Liver. Physiol. 2020, 319, G11–G22. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Carvalho, P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell. Biol. 2019, 20, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, Q.; Geng, Y.; Ma, C.; Shao, Q. Dynamic Regulation of Lipid Droplet Biogenesis in Plant Cells and Proteins Involved in the Process. Int. J. Mol. Sci. 2023, 24, 7476. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Sala, P.; Peña-Quintana, L. Biochemical Markers for the Diagnosis of Mitochondrial Fatty Acid Oxidation Diseases. J. Clin. Med. 2021, 10, 4855. [Google Scholar] [CrossRef] [PubMed]
- Zadoorian, A.; Du, X.; Yang, H. Lipid droplet biogenesis and functions in health and disease. Nat. Rev. Endocrinol. 2023, 19, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Eynaudi, A.; Díaz-Castro, F.; Bórquez, J.C.; Bravo-Sagua, R.; Parra, V.; Troncoso, R. Differential Effects of Oleic and Palmitic Acids on Lipid Droplet-Mitochondria Interaction in the Hepatic Cell Line HepG2. Front. Nutr. 2021, 8, 775382. [Google Scholar] [CrossRef]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2021, 13, 376–388. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell. Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Durazzo, A.; Lucarini, M. Advances in Research on Food Bioactive Molecules and Health. Molecules 2021, 26, 7678. [Google Scholar] [CrossRef]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef]
- Wang, Z.; Efferth, T.; Hua, X.; Zhang, X.A. Medicinal plants and their secondary metabolites in alleviating knee osteoarthritis: A systematic review. Phytomedicine 2022, 105, 154347. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef]
- Garg, S.S.; Gupta, J.; Sahu, D.; Liu, C.J. Pharmacological and Therapeutic Applications of Esculetin. Int. J. Mol. Sci. 2022, 23, 12643. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef] [PubMed]
- Kadakol, A.; Goru, S.K.; Malek, V.; Gaikwad, A.B. Esculetin ameliorates vascular perturbation by intervening in the occupancy of H2BK120Ub at At1, At2, Tgfβ1 and Mcp1 promoter gene in thoracic aorta of IR and T2D rats. Biomed. Pharmacother. 2017, 95, 1461–1468. [Google Scholar] [CrossRef]
- Kadakol, A.; Pandey, A.; Goru, S.K.; Malek, V.; Gaikwad, A.B. Insulin sensitizing and cardioprotective effects of Esculetin and Telmisartan combination by attenuating Ang II mediated vascular reactivity and cardiac fibrosis. Eur. J. Pharmacol. 2015, 765, 591–597. [Google Scholar] [CrossRef]
- Choi, R.Y.; Ham, J.R.; Lee, M.K. Esculetin prevents non-alcoholic fatty liver in diabetic mice fed high-fat diet. Chem. Biol. Interact. 2016, 260, 13–21. [Google Scholar] [CrossRef]
- Ma, J.; Deng, Y.; Yang, T.; Li, M.; Shang, J. Esculetin Alleviates Nonalcoholic Fatty Liver Disease on High-Cholesterol-Diet-Induced Larval Zebrafish and FFA-Induced BRL-3A Hepatocyte. Int. J. Mol. Sci. 2023, 24, 1593. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Kim, Y.; Staatz, C.E.; Baek, I.H. Oral bioavailability and pharmacokinetics of esculetin following intravenous and oral administration in rats. Xenobiotica 2021, 51, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Ritschel, W.A.; Brady, M.E.; Tan, H.S. First-pass effect of coumarin in man. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 99–103. [Google Scholar] [PubMed]
- Ritschel, W.A.; Brady, M.E.; Tan, H.S.; Hoffmann, K.A.; Yiu, I.M.; Grummich, K.W. Pharmacokinetics of coumarin and its 7-hydroxy-metabolites upon intravenous and peroral administration of coumarin in man. Eur. J. Clin. Pharmacol. 1977, 12, 457–461. [Google Scholar] [CrossRef]
- Pitaro, M.; Croce, N.; Gallo, V.; Arienzo, A.; Salvatore, G.; Antonini, G. Coumarin-Induced Hepatotoxicity: A Narrative Review. Molecules 2022, 27, 9063. [Google Scholar] [CrossRef]
- Li, Y.; Song, W.; Ou, X.; Luo, G.; Xie, Y.; Sun, R.; Wang, Y.; Qi, X.; Hu, M.; Liu, Z.; et al. Breast Cancer Resistance Protein and Multidrug Resistance Protein 2 Determine the Disposition of Esculetin-7-O-Glucuronide and 4-Methylesculetin-7-O-Glucuronide. Drug Metab. Dispos. 2019, 47, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Damba, T.; Wu, Z.; Serna-Salas, S.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Bioactive coumarin-derivative esculetin decreases hepatic stellate cell activation via induction of cellular senescence via the PI3K-Akt-GSK3β pathway. Food Biosci. 2022, 50, 102164. [Google Scholar] [CrossRef]
- Moshage, H.; Casini, A.; Lieber, C.S. Acetaldehyde selectively stimulates collagen production in cultured rat liver fat-storing cells but not in hepatocytes. Hepatology 1990, 12, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg-Vrenken, T.E.; Conde de la Rosa, L.; Buist-Homan, M.; Faber, K.N.; Moshage, H. Metformin protects rat hepatocytes against bile acid-induced apoptosis. PLoS ONE 2013, 8, e71773. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arroyave-Ospina, J.C.; Buist-Homan, M.; Schmidt, M.; Moshage, H. Protective effects of caffeine against palmitate-induced lipid toxicity in primary rat hepatocytes is associated with modulation of adenosine receptor A1 signaling. Biomed. Pharmacother. 2023, 165, 114884. [Google Scholar] [CrossRef]
- Geng, Y.; Wu, Z.; Buist-Homan, M.; Blokzijl, H.; Moshage, H. Hesperetin protects against palmitate-induced cellular toxicity via induction of GRP78 in hepatocytes. Toxicol. Appl. Pharmacol. 2020, 404, 115183. [Google Scholar] [CrossRef]
- Pruccoli, L.; Morroni, F.; Sita, G.; Hrelia, P.; Tarozzi, A. Esculetin as a Bifunctional Antioxidant Prevents and Counteracts the Oxidative Stress and Neuronal Death Induced by Amyloid Protein in SH-SY5Y Cells. Antioxidants 2020, 9, 551. [Google Scholar] [CrossRef]
- Karam, B.S.; Chavez-Moreno, A.; Koh, W.; Akar, J.G.; Akar, F.G. Oxidative stress and inflammation as central mediators of atrial fibrillation in obesity and diabetes. Cardiovasc. Diabetol. 2017, 16, 120. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, L.; Jia, H.; Xu, L.; Cao, Y.; Zhai, M.; Li, K.; Xia, L.; Jiang, L.; Li, X.; et al. Tetrahydrocurcumin improves lipopolysaccharide-induced myocardial dysfunction by inhibiting oxidative stress and inflammation via JNK/ERK signaling pathway regulation. Phytomedicine 2022, 104, 154283. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, D.; Li, C.; Huang, J.; Li, W.; Qiu, Y.; Mao, A.; Zhou, M.; Xue, L. Lic regulates JNK-mediated cell death in Drosophila. Cell Prolif. 2019, 52, e12593. [Google Scholar] [CrossRef]
- Cui, N.; Li, H.; Dun, Y.; Ripley-Gonzalez, J.W.; You, B.; Li, D.; Liu, Y.; Qiu, L.; Li, C.; Liu, S. Exercise inhibits JNK pathway activation and lipotoxicity via macrophage migration inhibitory factor in nonalcoholic fatty liver disease. Front Endocrinol 2022, 13, 961231. [Google Scholar] [CrossRef]
- Xu, D.; Liu, L.; Zhao, Y.; Yang, L.; Cheng, J.; Hua, R.; Zhang, Z.; Li, Q. Melatonin protects mouse testes from palmitic acid-induced lipotoxicity by attenuating oxidative stress and DNA damage in a SIRT1-dependent manner. J. Pineal. Res. 2020, 69, e12690. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Sung, J.; Yang, J.; Ham, H.; Kim, Y.; Jeong, H.S.; Lee, J. Inhibitory effect of esculetin on free-fatty-acid-induced lipid accumulation in human HepG2 cells through activation of AMP-activated protein kinase. Food Sci. Biotechnol. 2017, 26, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Karnewar, S.; Vasamsetti, S.B.; Gopoju, R.; Kanugula, A.K.; Ganji, S.K.; Prabhakar, S.; Rangaraj, N.; Tupperwar, N.; Kumar, J.M.; Kotamraju, S. Mitochondria-targeted esculetin alleviates mitochondrial dysfunction by AMPK-mediated nitric oxide and SIRT3 regulation in endothelial cells: Potential implications in atherosclerosis. Sci. Rep. 2016, 6, 24108. [Google Scholar] [CrossRef] [PubMed]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free. Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Guo, X.; Yin, X.; Liu, Z.; Wang, J. Non-Alcoholic Fatty Liver Disease (NAFLD) Pathogenesis and Natural Products for Prevention and Treatment. Int. J. Mol. Sci. 2022, 23, 15489. [Google Scholar] [CrossRef]
- de Vries, J.E.; Vork, M.M.; Roemen, T.H.; de Jong, Y.F.; Cleutjens, J.P.; van der Vusse, G.J.; van Bilsen, M. Saturated but not mono-unsaturated fatty acids induce apoptotic cell death in neonatal rat ventricular myocytes. J. Lipid. Res. 1997, 38, 1384–1394. [Google Scholar] [CrossRef]
- Welters, H.J.; Tadayyon, M.; Scarpello, J.H.; Smith, S.A.; Morgan, N.G. Mono-unsaturated fatty acids protect against beta-cell apoptosis induced by saturated fatty acids, serum withdrawal or cytokine exposure. FEBS. Lett. 2004, 560, 103–108. [Google Scholar] [CrossRef]
- Gómez-Lechón, M.J.; Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Castell, J.V.; O’Connor, J.E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, R.; Xia, C.; Chen, Y.; Dong, Z.; Huang, B.; Li, R.; Li, M.; Xu, C. Effects of different fatty acids on BRL3A rat liver cell damage. J. Cell. Physiol. 2020, 235, 6246–6256. [Google Scholar] [CrossRef]
- Lipke, K.; Kubis-Kubiak, A.; Piwowar, A. Molecular Mechanism of Lipotoxicity as an Interesting Aspect in the Development of Pathological States-Current View of Knowledge. Cells 2022, 11, 844. [Google Scholar] [CrossRef] [PubMed]
- Petermann-Rocha, F.; Wirth, M.D.; Boonpor, J.; Parra-Soto, S.; Zhou, Z.; Mathers, J.C.; Livingstone, K.; Forrest, E.; Pell, J.P.; Ho, F.K.; et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: A prospective study of 171,544 UK Biobank participants. BMC. Med. 2023, 21, 123. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Diao, S.; Fan, Z. The role and mechanism of mitochondrial functions and energy metabolism in the function regulation of the mesenchymal stem cells. Stem. Cell. Res. Ther. 2021, 12, 140. [Google Scholar] [CrossRef]
- Middleton, P.; Vergis, N. Mitochondrial dysfunction and liver disease: Role, relevance, and potential for therapeutic modulation. Therap. Adv. Gastroenterol. 2021, 14, 17562848211031394. [Google Scholar] [CrossRef]
- Yuzefovych, L.; Wilson, G.; Rachek, L. Different effects of oleate vs. palmitate on mitochondrial function, apoptosis, and insulin signaling in L6 skeletal muscle cells: Role of oxidative stress. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E1096–E1105. [Google Scholar] [CrossRef]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental Nonalcoholic Steatohepatitis and Liver Fibrosis Are Ameliorated by Pharmacologic Activation of Nrf2 (NF-E2 p45-Related Factor 2). Cell. Mol. Gastroenterol. Hepatol. 2017, 5, 367–398. [Google Scholar] [CrossRef]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food. Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and Oxidative Stress: A General Overview of Mechanisms and Implications in Human Disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef]
- Palma, F.R.; He, C.; Danes, J.M.; Paviani, V.; Coelho, D.R.; Gantner, B.N.; Bonini, M.G. Mitochondrial Superoxide Dismutase: What the Established, the Intriguing, and the Novel Reveal About a Key Cellular Redox Switch. Antioxid. Redox. Signal. 2020, 32, 701–714. [Google Scholar] [CrossRef]
- Di Stasi, L.C. Natural Coumarin Derivatives Activating Nrf2 Signaling Pathway as Lead Compounds for the Design and Synthesis of Intestinal Anti-Inflammatory Drugs. Pharmaceuticals 2023, 16, 511. [Google Scholar] [CrossRef] [PubMed]
- Levada, K.; Guldiken, N.; Zhang, X.; Vella, G.; Mo, F.R.; James, L.P.; Haybaeck, J.; Kessler, S.M.; Kiemer, A.K.; Ott, T.; et al. Hsp72 protects against liver injury via attenuation of hepatocellular death, oxidative stress, and JNK signaling. J. Hepatol. 2018, 68, 996–1005. [Google Scholar] [CrossRef] [PubMed]
- Win, S.; Than, T.A.; Le, B.H.; García-Ruiz, C.; Fernandez-Checa, J.C.; Kaplowitz, N. Sab (Sh3bp5) dependence of JNK mediated inhibition of mitochondrial respiration in palmitic acid induced hepatocyte lipotoxicity. J. Hepatol. 2015, 62, 1367–1374. [Google Scholar] [CrossRef]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V., Jr. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, G.; Du, X.; Shi, Z.; Jin, M.; Sha, X.; Li, X.; Wang, Z.; Li, X. Expression patterns of hepatic genes involved in lipid metabolism in cows with subclinical or clinical ketosis. J. Dairy. Sci. 2019, 102, 1725–1735. [Google Scholar] [CrossRef]
- Miquilena-Colina, M.E.; Lima-Cabello, E.; Sánchez-Campos, S.; García-Mediavilla, M.V.; Fernández-Bermejo, M.; Lozano-Rodríguez, T.; Vargas-Castrillón, J.; Buqué, X.; Ochoa, B.; Aspichueta, P.; et al. Hepatic fatty acid translocase CD36 upregulation is associated with insulin resistance, hyperinsulinaemia and increased steatosis in non-alcoholic steatohepatitis and chronic hepatitis C. Gut 2011, 60, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed]
- Day, E.A.; Ford, R.J.; Steinberg, G.R. AMPK as a Therapeutic Target for Treating Metabolic Diseases. Trends Endocrinol. Metab. 2017, 28, 545–560. [Google Scholar] [CrossRef]
- Diniz, T.A.; de Lima Junior, E.A.; Teixeira, A.A.; Biondo, L.A.; da Rocha, L.A.F.; Valadão, I.C.; Silveira, L.S.; Cabral-Santos, C.; de Souza, C.O.; Rosa Neto, J.C. Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life. Sci. 2021, 266, 118868. [Google Scholar] [CrossRef]
- Chyau, C.C.; Wang, H.F.; Zhang, W.J.; Chen, C.C.; Huang, S.H.; Chang, C.C.; Peng, R.Y. Antrodan Alleviates High-Fat and High-Fructose Diet-Induced Fatty Liver Disease in C57BL/6 Mice Model via AMPK/Sirt1/SREBP-1c/PPARγ Pathway. Int. J. Mol. Sci. 2020, 21, 360. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Yu, Y.; Zhang, J.; Li, H.; Weng, Q.; Jiang, S.; Tian, S.; Xu, T.; Hu, S.; Yang, G.; et al. Cordycepin Ameliorates Nonalcoholic Steatohepatitis by Activation of the AMP-Activated Protein Kinase Signaling Pathway. Hepatology 2021, 74, 686–703. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.I.; Yun, K.W.; Seo, K.I.; Kim, M.J.; Lee, M.K. Scopoletin prevents alcohol-induced hepatic lipid accumulation by modulating the AMPK-SREBP pathway in diet-induced obese mice. Metabolism 2014, 63, 593–601. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Li, F.; Zou, J.; Li, X.; Xu, M.; Yu, D.; Ma, Y.; Huang, W.; Sun, X.; et al. Psoralen Suppresses Lipid Deposition by Alleviating Insulin Resistance and Promoting Autophagy in Oleate-Induced L02 Cells. Cells 2022, 11, 1067. [Google Scholar] [CrossRef] [PubMed]



| Gene | Sense 5′–3′ | Antisense 5′–3′ | Probe 5′–3′ | Accession Number |
|---|---|---|---|---|
| 18s | CGGCTACCACATCCAAGGA | CCAATTACAGGGCCTCGAAA | CGCGCAAATT ACCCACTCCCGA | X01117 |
| Srebp1c | GGAGCCATGGATTGCACATT | CCTGTCTCACCCCCAGCATA | CAGCTCATCAACAACCAAGACAGTGACTTCC | XM_213329 |
| Cd36 | GATCGGAACTGTGGGCTCAT | GGTTCCTTCTTCAAGGACAACTTC | AGAATGCCTCCAAACACAGCCAGGAC | NM_031561 |
| Pparα | CACCCTCTCTCCAGCTTCCA | GCCTTGTCCCCAC ATATTCG | TCCCCACCAGTACAGATGAGTCCCCTG | NM_013196 |
| Nrf2 | AGCCCAGCACATCCAGACA | TGTCTCTGCCAAAAGCTGCAT | TCAGCTACTCCCAGGTTGCCCACATTC | NM_031789 |
| Sod1 | CAGGACCTCATTTTAATCCTCACTC | GTCTCCAACATGCCTCTCTTCA | CCGCTGGACCG CCATGTTTCTT | NM_017050 |
| Gpx1 | GGACATCAGGAGAATGGCAAGA | CGCACTTCTCAAACAATGTAAAGTTG | TTCCCTCAAGTATGTCCGACCCGGTG | NM_030826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, M.; Wu, Z.; Wang, J.; Buist-Homan, M.; Moshage, H. The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation. Antioxidants 2023, 12, 1922. https://doi.org/10.3390/antiox12111922
Xia M, Wu Z, Wang J, Buist-Homan M, Moshage H. The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation. Antioxidants. 2023; 12(11):1922. https://doi.org/10.3390/antiox12111922
Chicago/Turabian StyleXia, Mengmeng, Zongmei Wu, Junyu Wang, Manon Buist-Homan, and Han Moshage. 2023. "The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation" Antioxidants 12, no. 11: 1922. https://doi.org/10.3390/antiox12111922
APA StyleXia, M., Wu, Z., Wang, J., Buist-Homan, M., & Moshage, H. (2023). The Coumarin-Derivative Esculetin Protects against Lipotoxicity in Primary Rat Hepatocytes via Attenuating JNK-Mediated Oxidative Stress and Attenuates Free Fatty Acid-Induced Lipid Accumulation. Antioxidants, 12(11), 1922. https://doi.org/10.3390/antiox12111922







