Differential Neuroprotective Effects of N-Acetylcysteine against Dithianon Toxicity in Glutamatergic, Dopaminergic, and GABAergic Neurons: Assessment Using Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance
2.2. Chemical Treatment
2.3. Bright-Field Imaging and Survival Test
2.4. Locomotive Behavior Analysis
2.5. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.6. Reactive Oxygen Species Detection
2.7. Statistical Analysis
3. Results
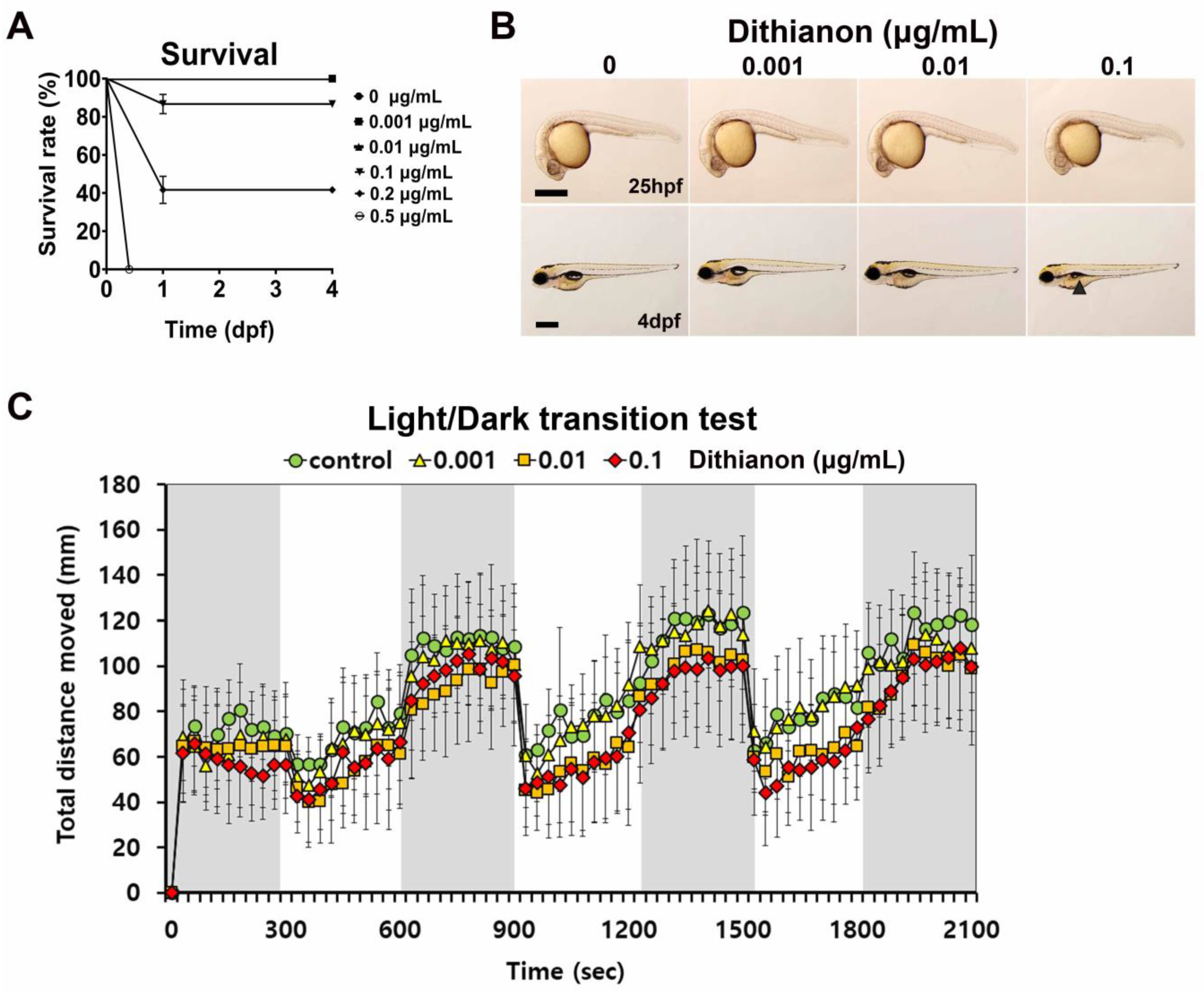
3.1. Effects of Dithianon Exposure on Zebrafish Larval Development
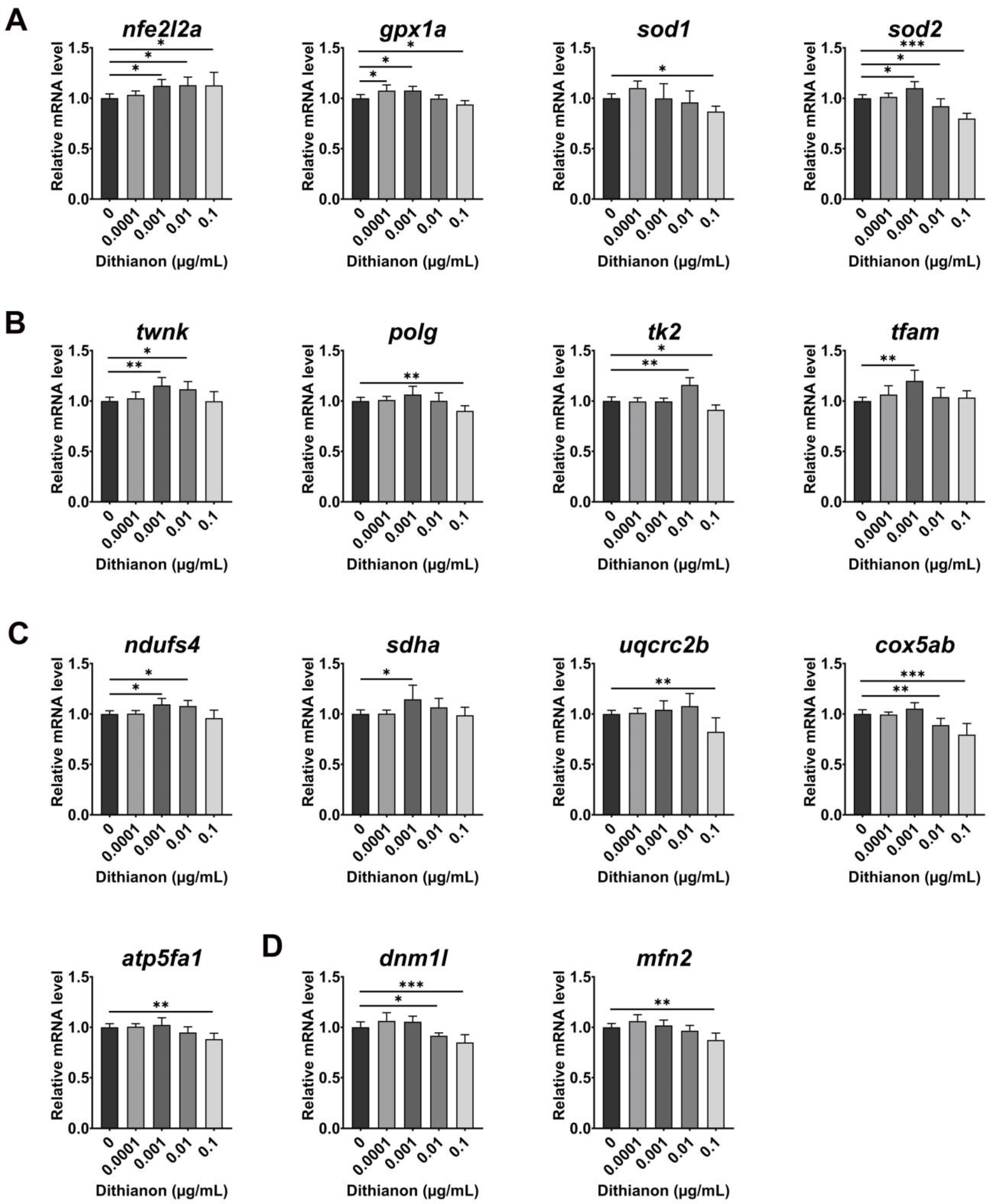
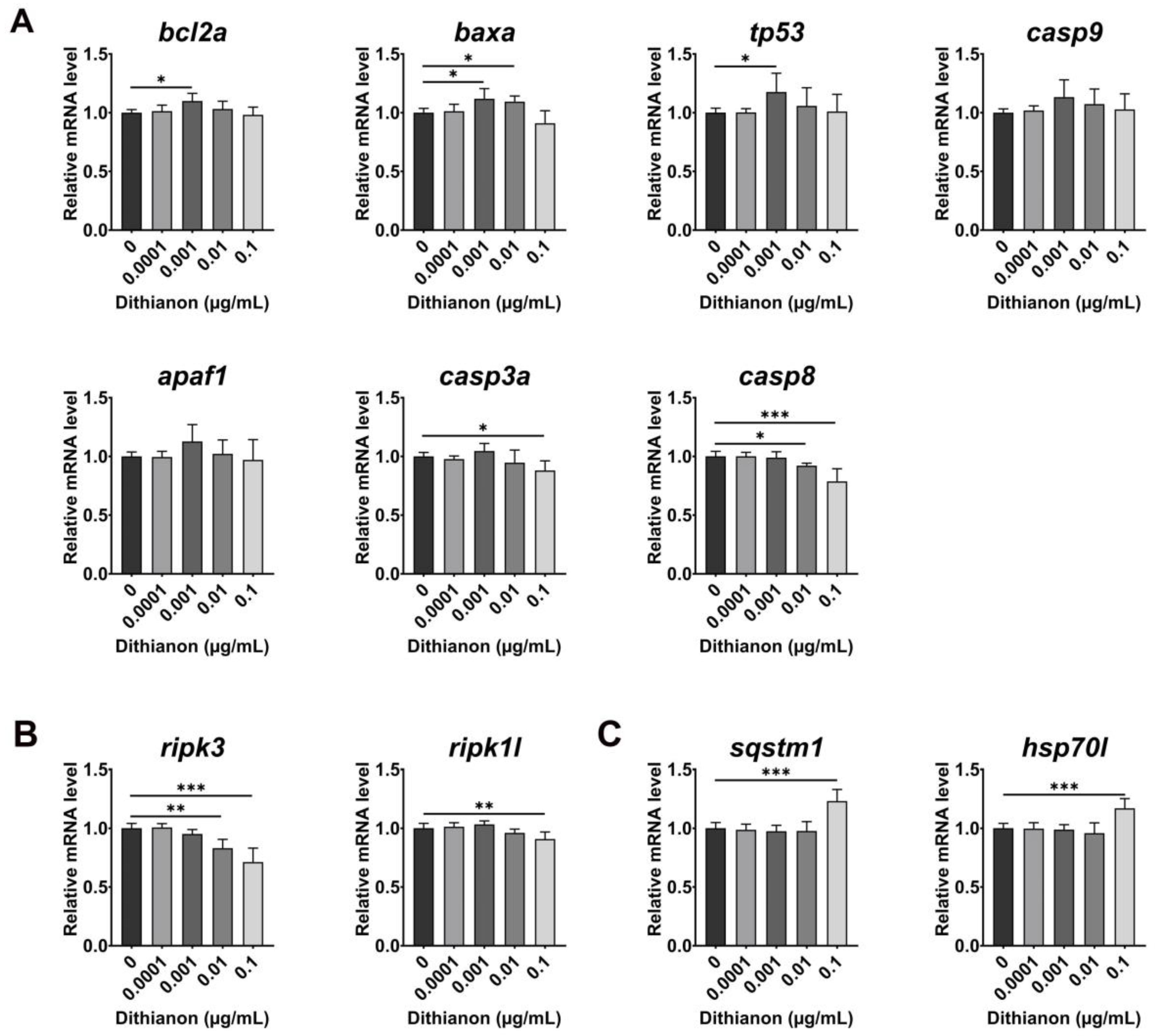
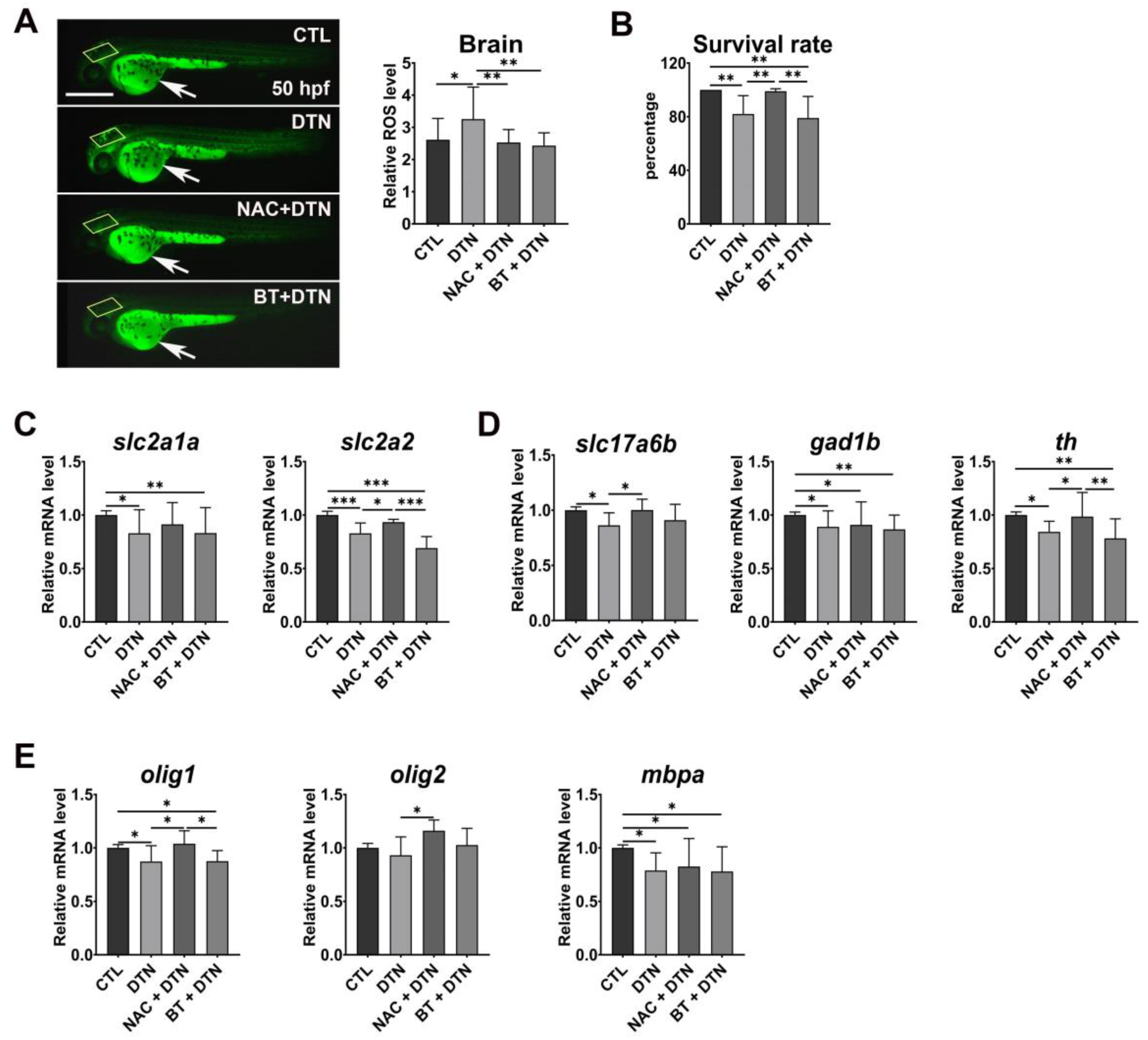
3.2. Effects of Dithianon Exposure on Antioxidant Response and Cellular Homeostasis
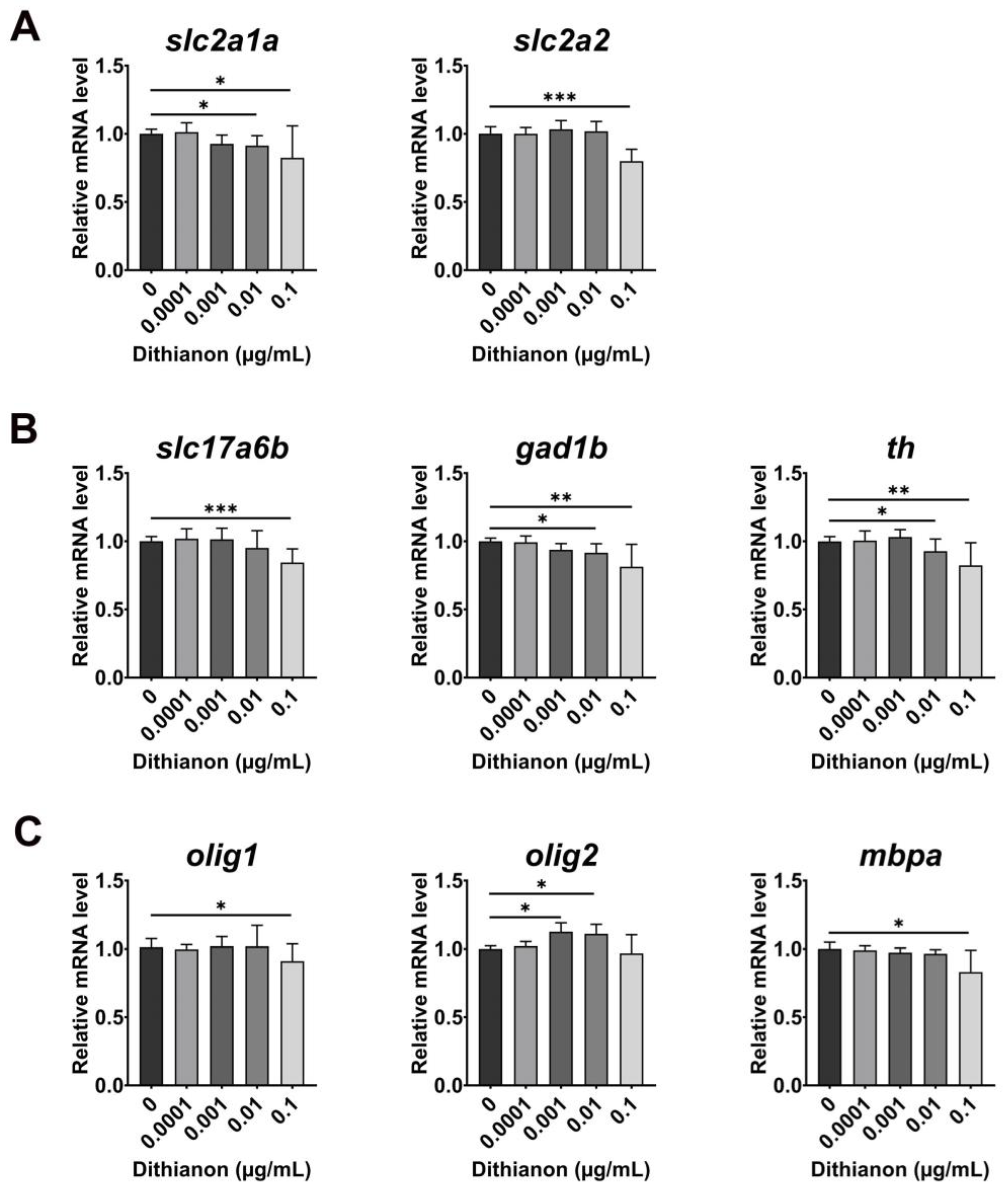
3.3. Effects of Dithianon Exposure on Neural and Glial Development
3.4. Protection Potency of N-Acetylcysteine and Betaine on Dithianon-Induced Toxicity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munze, R.; Hannemann, C.; Orlinskiy, P.; Gunold, R.; Paschke, A.; Foit, K.; Becker, J.; Kaske, O.; Paulsson, E.; Peterson, M.; et al. Pesticides from wastewater treatment plant effluents affect invertebrate communities. Sci. Total Environ. 2017, 599–600, 387–399. [Google Scholar] [CrossRef] [PubMed]
- Authority, E.F.S.; Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Crivellente, F. Updated peer review of the pesticide risk assessment for the active substance dithianon in light of confirmatory data submitted. EFSA J. 2020, 18, e06189. [Google Scholar] [CrossRef]
- Berker, J.; Hierholzer, O.; Mohr, G. Dithianon, a New Organic Compound with Fungicidal Properties; E. Merck AG: Darmstadt, Germany, 1963; pp. 351–366. [Google Scholar]
- Sturdik, E.; Drobnica, L. Effect of 2,3-dinitrilo-1,4-dithia-9,10-antraquinone on Ehrlich ascites carcinoma and yeast cells. Chem. Biol. Interact. 1980, 30, 105–114. [Google Scholar] [CrossRef]
- Amponsah, N.T.; Jones, E.; Ridgway, H.J.; Jaspers, M.V. Evaluation of fungicides for the management of Botryosphaeria dieback diseases of grapevines. Pest Manag. Sci. 2012, 68, 676–683. [Google Scholar] [CrossRef]
- Scariot, F.J.; Jahn, L.; Delamare, A.P.L.; Echeverrigaray, S. Necrotic cell death induced by dithianon on Saccharomyces cerevisiae. Pestic. Biochem. Physiol. 2018, 149, 137–142. [Google Scholar] [CrossRef]
- Parny, M.; Coste, A.; Aubouy, A.; Rahabi, M.; Prat, M.; Pipy, B.; Treilhou, M. Differential immunomodulatory effects of six pesticides of different chemical classes on human monocyte-derived macrophage functions. Food Chem. Toxicol. 2022, 163, 112992. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Choi, S.; Kim, K.W. Dithianon exposure induces dopaminergic neurotoxicity in Caenorhabditis elegans. Ecotoxicol. Environ. Saf. 2023, 255, 114752. [Google Scholar] [CrossRef]
- Syromyatnikov, M.Y.; Kokina, A.V.; Lopatin, A.V.; Starkov, A.A.; Popov, V.N. Evaluation of the toxicity of fungicides to flight muscle mitochondria of bumblebee (Bombus terrestris L.). Pestic. Biochem. Physiol. 2017, 135, 41–46. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H.J. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Schneider, R., Jr.; Santos, C.F.; Clarimundo, V.; Dalmaz, C.; Elisabetsky, E.; Gomez, R. N-acetylcysteine prevents behavioral and biochemical changes induced by alcohol cessation in rats. Alcohol 2015, 49, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Liu, W.; Wu, J.; Yang, X.; Xu, H. Chemoprotective effect of N-acetylcysteine (NAC) on cellular oxidative damages and apoptosis induced by nano titanium dioxide under UVA irradiation. Toxicol. In Vitro 2011, 25, 110–116. [Google Scholar] [CrossRef]
- Day, C.R.; Kempson, S.A. Betaine chemistry, roles, and potential use in liver disease. BBA Gen. Subj. 2016, 1860, 1098–1106. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Rogers, D.D., 2nd; Mailliard, M.E.; Siford, G.L.; Barak, A.J.; Beckenhauer, H.C.; Sorrell, M.F.; Tuma, D.J. Role of elevated S-adenosylhomocysteine in rat hepatocyte apoptosis: Protection by betaine. Biochem. Pharmacol. 2005, 70, 1883–1890. [Google Scholar] [CrossRef]
- Kharbanda, K.K.; Vigneswara, V.; McVicker, B.L.; Newlaczyl, A.U.; Bailey, K.; Tuma, D.; Ray, D.E.; Carter, W.G. Proteomics reveal a concerted upregulation of methionine metabolic pathway enzymes, and downregulation of carbonic anhydrase-III, in betaine supplemented ethanol-fed rats. Biochem. Biophys. Res. Commun. 2009, 381, 523–527. [Google Scholar] [CrossRef]
- Lee, I. Betaine is a positive regulator of mitochondrial respiration. Biochem. Biophys. Res. Commun. 2015, 456, 621–625. [Google Scholar] [CrossRef]
- Ryan, R.M.; Ingram, S.L.; Scimemi, A. Regulation of Glutamate, GABA and Dopamine Transporter Uptake, Surface Mobility and Expression. Front. Cell. Neurosci. 2021, 15, 670346. [Google Scholar] [CrossRef]
- Sheffler, Z.M.; Reddy, V.; Pillarisetty, L.S. Physiology, neurotransmitters. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Falk, S.; Gotz, M. Glial control of neurogenesis. Curr. Opin. Neurobiol. 2017, 47, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Choi, J.; Yoon, B.E. Neuron-Glia Interactions in Neurodevelopmental Disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef]
- Lee, G.; Banik, A.; Eum, J.; Hwang, B.J.; Kwon, S.H.; Kee, Y. Ipconazole disrupts mitochondrial homeostasis and alters GABAergic neuronal development in zebrafish. Int. J. Mol. Sci. 2022, 24, 496. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.P.; Choi, S.Y.; Kee, Y.; Kim, M.J.; Kim, S.H.; Lee, Y.; Park, H.C.; Ro, H. Transgenic fluorescent zebrafish lines that have revolutionized biomedical research. Lab. Anim. Res. 2021, 37, 26. [Google Scholar] [CrossRef]
- Chowdhury, M.A.U.; Raslan, A.A.; Lee, E.; Eum, J.; Hwang, B.J.; Kwon, S.H.; Kee, Y. Histopathological assessment of laterality defects in zebrafish development. Anim. Cells Syst. 2021, 25, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Ki, S.; Kwon, S.H.; Eum, J.; Raslan, A.A.; Kim, K.N.; Hwang, B.J.; Kee, Y. 3D light-sheet assay assessing novel valproate-associated cardiotoxicity and folic acid relief in zebrafish embryogenesis. Chemosphere 2019, 227, 551–560. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Costa-Silva, D.G.D.; Leandro, L.P.; Vieira, P.B.; de Carvalho, N.R.; Lopes, A.R.; Schimith, L.E.; Nunes, M.E.M.; de Mello, R.S.; Martins, I.K.; de Paula, A.A.; et al. N-acetylcysteine inhibits Mancozeb-induced impairments to the normal development of zebrafish embryos. Neurotoxicol. Teratol. 2018, 68, 1–12. [Google Scholar] [CrossRef]
- Kim, M.J. Betaine enhances the cellular survival via mitochondrial fusion and fission factors, MFN2 and DRP1. Anim. Cells Syst. 2018, 22, 289–298. [Google Scholar] [CrossRef]
- Krishnan, V. Is There a Role for Reactive Oxygen Species in Zebrafish Embryonic Development? Northeastern University: Boston, MA, USA, 2013. [Google Scholar]
- Lee, S.; Eum, J.; Park, S.; Ki, S.; Hwang, B.J.; Kee, Y.; Chae, J.H. TNNT1 myopathy with novel compound heterozygous mutations. Neuromuscul. Disord. 2022, 32, 176–184. [Google Scholar] [CrossRef]
- Fernandes, R.; Hosoya, K.; Pereira, P. Reactive oxygen species downregulate glucose transport system in retinal endothelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C927–C936. [Google Scholar] [CrossRef]
- Graham, J.M. GLUT1 deficiency syndrome as a cause of encephalopathy that includes cognitive disability, treatment-resistant infantile epilepsy and a complex movement disorder. Eur. J. Med. Genet. 2012, 55, 332–334. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jurcovicova, J. Glucose transport in brain—Effect of inflammation. Endocr. Regul. 2014, 48, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.P.; Romme, E.; van der Spek, P.J.; Dirven, C.M.; Willemsen, R.; Kros, J.M. Glut1/SLC2A1 is crucial for the development of the blood-brain barrier in vivo. Ann. Neurol. 2010, 68, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Thorens, B. GLUT2, glucose sensing and glucose homeostasis. Diabetologia 2015, 58, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Drobnica, E.; Majtán, V.; Šturdík, E.; Miko, M. Effect of 2, 3-dinitrilo-1, 4-dithia-9, 10-anthraquinone on Mycobacterium smegmatis. Folia Microbiol. 1980, 25, 403–411. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banik, A.; Eum, J.; Hwang, B.J.; Kee, Y. Differential Neuroprotective Effects of N-Acetylcysteine against Dithianon Toxicity in Glutamatergic, Dopaminergic, and GABAergic Neurons: Assessment Using Zebrafish. Antioxidants 2023, 12, 1920. https://doi.org/10.3390/antiox12111920
Banik A, Eum J, Hwang BJ, Kee Y. Differential Neuroprotective Effects of N-Acetylcysteine against Dithianon Toxicity in Glutamatergic, Dopaminergic, and GABAergic Neurons: Assessment Using Zebrafish. Antioxidants. 2023; 12(11):1920. https://doi.org/10.3390/antiox12111920
Chicago/Turabian StyleBanik, Amit, Juneyong Eum, Byung Joon Hwang, and Yun Kee. 2023. "Differential Neuroprotective Effects of N-Acetylcysteine against Dithianon Toxicity in Glutamatergic, Dopaminergic, and GABAergic Neurons: Assessment Using Zebrafish" Antioxidants 12, no. 11: 1920. https://doi.org/10.3390/antiox12111920
APA StyleBanik, A., Eum, J., Hwang, B. J., & Kee, Y. (2023). Differential Neuroprotective Effects of N-Acetylcysteine against Dithianon Toxicity in Glutamatergic, Dopaminergic, and GABAergic Neurons: Assessment Using Zebrafish. Antioxidants, 12(11), 1920. https://doi.org/10.3390/antiox12111920






