Abstract
The chemical composition, antioxidant properties, and sensory aspects of sponge cakes with the addition of flours from edible insects (buffalo worm, cricket, and mealworm) were evaluated. The addition of edible-insect flours increased the protein, fat, and dietary fiber content in all cases. The utilization of edible insects demonstrated a notable augmentation in the phenolic compounds (especially protocatechuic acid and protocatechuic aldehyde, and syringic, ferulic, and sinapic acids). This resulted in an increase in the antioxidant activity measured against the ABTS radical cation, the DPPH radical, and ferric ions. The antioxidant potential, assessed by four different methods, unequivocally confirmed that the aforementioned polyphenolic compounds found in edible insects provide significant radical-scavenging and antioxidant activity in sponge cakes containing them. The polyunsaturated fatty acid contents were significantly lower in cakes with insect flour compared to the standard wheat cakes. Products and raw materials exhibited high values of the n − 6/n − 3 ratio, which may be associated with negative health effects, with a high oleic acid content. The amino acid score (AAS) for the essential amino acids exceeded 100% for all obtained products. The sponge cakes were accepted by consumers and the taste was the most important predictor for overall acceptability, whereas the structure and appearance had less impact.
1. Introduction
An increasing number of institutions are emphasizing the importance of a sustainable food economy. The rising population is exerting greater pressure on ecosystems as a result of heightened demand for food, a fundamental human requirement [1,2]. One of the key issues is the production of sufficient amounts of protein, which is essential for the human body to carry out life processes properly [3]. An alternative to proteins derived from traditional animal and plant production are proteins obtained from edible insects. There is an increasing number of studies on the possibility of using edible-insect preparations in various food products [4,5,6] and their acceptance by consumers [7]. It seems that the sphere of consumer acceptance in particular poses many challenges, especially within the so-called Western culture, where eating insects has clearly negative connotations. However, it is worth emphasizing that an increasing number of studies indicate great potential in this area, especially among young people [2]. It is indicated that products containing edible insects are more widely accepted if they contain the additive in powdered form and not in the form of whole insects (consumer acceptance increases when insects are processed and are not visible in the final product) [7,8]. However, consumer acceptance in Western and developed countries is still limited. Food neophobia, disgust, familiarity, the visibility of insects, and taste seem to be the most significant factors impeding consumers from the consumption of insects as food [8].
Approximately 2300 insect species are edible. However, currently, the European Union (EU) permits only four insect species that comply with the Novell Food Regulations (Reg. 2015/2283) for human consumption. Among them are the larvae of mealworms (Tenebrio molitor L.), house crickets (Acheta domestica L.), migratory locust (Locusta migratoria), and, commencing from 5 March 2023, the larvae of the buffalo worms (Alphitobius diaperinus P). The Commission Implementing Regulation (EU) 2017/2470 was released on 30th December 2017. This regulation establishes a European Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and Council on novel foods (OJ L 351, 30 December 2017, p.72). In addition, the European Commission’s Catalogue of Novel Foods provides information on individual component statuses. This catalog contains the names of food ingredients and the information available at the time regarding their status; it is not a definitive guide [9].
In the case of insects such as mealworms or buffalo worms, their larval form is used for food purposes. The dried and ground larvae of these insects contain about 45–49% protein and 14–28% fat [5], and can be used to enrich such products as bread [6], protein bars [10], snack products [11], or as a meat substitute in hamburgers and other meat products [12]. The house cricket (Acheta domestica L.) is an insect whose adult form (imago) is used for feed or food. Similar to the previously mentioned insects, it can be widely used for the supplementation of pastry products [6] and the production of pancakes [13].
Edible insects are a good source of protein and fatty acids [5], and also provide such nutrients as minerals (phosphor, potassium, iron) and vitamins, especially ascorbic acid, thiamine, riboflavin, and niacin [5]. All of this means that products with the participation of edible insects can contribute to improving their nutritional status, and in a broader perspective, help fight malnutrition [14].
The aim of this study was to determine the effect of the powders of three species of edible insects (Tenebrio molitor L., Acheta domestica L., and Alphitobius diaperinus P.) on the physicochemical, functional properties, and consumer acceptance of sponge cakes supplemented with the addition of them. The research hypothesis assumes that the addition of the abovementioned edible-insect meals will increase the nutritional value of the obtained products, while maintaining consumer acceptance at an appropriate level.
2. Materials and Methods
2.1. Materials
Sponge cakes (S) and sponge cakes in which part of the pure refined wheat flour (GoodMills Polska Sp. z o.o., Stradunia, Poland) was replaced with an appropriate insect flour at a ratio of 15% and 30% of the original weight of the wheat flour were used. The following insect flours were used—mealworm, Tenebrio molitor (TF) (ZIRP Insects, Wien, Austria); buffalo worm, Alphitobius diaperinus (BF) (Isaac Nutrition GmbH, Cologne, Germany); cricket, Acheta domesticus (CF) (Sens Food LTD, London, UK). Sponge cakes were obtained under laboratory conditions.
The sponge cakes were made using the cold method with an eggs:sugar:flour ratio of 2:1:1 (2 kg:1 kg:1 kg). The dough was prepared using an Aristan 7 mixer (Kitchen Aid, Orlando, FL, USA), as follows: in the mixing bowl, the egg whites were whipped for 5 min (speed level 10), powdered sugar was added and whipped for 3 min (speed level 8), and the egg yolks were then added and mixed for 1 min (speed level 3). Sieved flour was then added and mixed for 1 min (speed level 2). The dough was formed using a confectionery sleeve into silicone-cake-tin (30 mm diameter) cakes. The cakes were baked in a Miwe roll-in oven (Germany) at 180 °C for 15 min. Cakes were cooled down for half an hour at room temperature and used for further analyses.
2.2. Methods
2.2.1. Chemical Analysis
The wheat and edible-insect flour were assessed for the following parameters: ash content (AOAC 923.03) and protein content (AOAC 950.36), with the protein content calculated using an appropriate conversion. In the case of the standard sponge cakes, a conversion factor of 6.25 was used, while in the case of products containing insect flour, conversion factors of 6.25 and 4.76 were used in accordance with the proportions of the individual ingredients in the recipe [15]. The crude fat content (AOAC 935.38), water content (AOAC 925.10), and total, soluble, and insoluble dietary fiber contents (AOAC 991.43) (AOAC, 2006) [16] were also assessed. The analyses were performed in triplicate.
2.2.2. Amino Acid Analysis
Amino acid analysis was performed according to the method of Moore and Stein (1951) [17], Davidson (2003) [18], and Smith (2003) [19]. The samples were freeze-dried and then hydrolyzed using liquid hydrochloric acid (6 M HCl containing 0.5% phenol at 110 °C) for 24 h in an argon atmosphere. The obtained sample was lyophilized again, then dissolved (sodium citrate buffer pH 2.2) and filtered through a 0.45 μm syringe filter before being analyzed using an amino acid analyzer. Ion-exchange chromatography, with a cation ion exchanger and sodium-citrate elution buffer system, followed by postcolumn derivatization with ninhydrin and spectrophotometric detection at 570 and 440 nm, according to the standard protocol of the manufacturer (Ingos, Praha, Czech Republic), were used for the amino acid determination. Sulfur-containing amino acids were analyzed as oxidation products obtained by performic acid oxidation, followed by a standard hydrolysis procedure with HCl. Calibration was done using a standard solution of amino acids (Sigma, St. Louis, MO, USA). The obtained data were assessed using the chromatographic device software CHROMuLAN v 0.91 (Chromulan, Pikron, Ustecky, Czech Republic). As it is destroyed during acid hydrolysis, tryptophan was not determined. Asparagine and glutamine have been designated as aspartic acid and glutamic acid (into which they transform). Analyses were performed in four repetitions.
Protein Nutritional Quality
The protein nutritional value was assessed by calculating the amino acid score (AAS) in accordance with FAO/WHO guidelines, based on the quantity and composition of the amino acids [20] (Equation (1)):
where * is the recommended amino acid scoring patterns for adolescents and adults, according to the FAO [21] (2013).
2.2.3. Determination of Fatty Acid Profile
The fat fraction was obtained according to the AOAC method 935.38, while the determination of the total fatty acid profile was performed according to the AOAC-approved method 991.39 (AOAC, 2006) [16]. To obtain the methanol esters of the fatty acids, saponification (0.5 N KOH methanol solution) and esterification (12% BF3 methanol solution) of the fat sample were performed. The methyl esters were then transferred to the organic phase (hexane). This process was supported by the addition of a saturated NaCl solution. The fat phase was collected into vials and analyzed using a Shimadzu GC2010Plus chromatograph (Shimadzu Corp., Kioto, Japan) with a flame ionization detector (FID). The operating parameters were as follows: FID detector temp. 240 °C; temperature dispenser 240 °C; oven temp. 195 °C to 240 °C (5 °C/min) (240 °C, 10 min). An SH-FAME column (30 m–0.32 mm–0.25 μm) was used, and the carrier gas helium was 1.6 mL/min. The split ratio was 100. Individual fatty acid methyl esters were identified by comparison to the standard mixture of the Supelco 37 component FAME Mix and of CLA isomers (Sigma-Aldrich Co., St. Louis, MO, USA). The analyses were performed in four repetitions.
2.2.4. Color Analysis
The color of the bread crust and crumb was assessed employing the CIELab (L*, a*, b*) system using the Konica Minolta CM-5 spectrophotometer (Konica Minolta Sensing, Osaka, Japan). A measurement angle of 10° and an illuminant of D65 with an 8 mm shutter were used. Ten different locations on the crust and crumb surface were measured to ensure accuracy. The test temperature was maintained at 21 °C throughout. The average color parameters obtained from the measurements were utilized to compute the overall color variation of wheat bread as determined by the formula [22] (Equation (2)):
where:
- ΔL = brightness difference;
- Δa = redness difference;
- Δb = yellowness difference.
2.2.5. Antioxidant Properties
The extraction procedure: approximately 0.6 g of the sample was shaken in 30 mL of 80 g/100 g ethanol for 120 min (electric shaker type WB22, Memmert, Schwabach, Germany). The obtained extracts were centrifuged (15 min, 4500 rpm; 1050× g) (MPW MED, Instruments, Warsaw, Poland). The supernatant was decanted and stored at −20 °C for further analyses.
Phenolic Compound Analysis by UHPLC–DAD–ESI–MS/MS
The phenolic compounds in the extracts obtained from the edible-insect flour and sponge cakes were determined using the UHPLC–DAD–ESI–MS/MS method according to the procedure described in detail by Oracz et al. [23].
Analysis of Total Polyphenol Content, Flavonoid Content, and Antioxidant Potential by Four Different Methods
The Folin–Ciocalteu reagent method, as described by Singleton et al. [24], was used to measure the total polyphenol content. A 5 mL sample of the extract was diluted to a volume of 50 mL with distilled water in the flask, and 5 mL of the extract was then mixed with 0.25 mL of the Folin–Ciocalteau reagent and 0.5 mL 7% Na2CO3. The contents were mixed using a vortex (WF2, Janke & Kunkel, Staufen, Germany) and stored for 30 min in the dark. The absorbance was measured at 760 nm using a Helios Gamma 100–240 spectrophotometer (Runcorn, UK). The results were reported in mg catechin/100 g of dry matter of the sample.
The flavonoid content was determined using the spectrophotometric method described by El Hariri et al. [25], using the following method: 0.5 mL of the extract was combined with 0.2 mL 2-aminoethyldiphenylborate reagent with 1.8 mL distilled water into a test tube. The absorbance was measured at a wavelength of 404 nm (Jenway spectrophotometer 6405 UV/Vis, Staffordshire, UK) and the flavonoid content was then expressed in mg rutin/100 g dry matter of the samples.
Free-Radical-Scavenging Activity by DPPH
The free-radical-scavenging activity of the samples was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) according to the method of Sánchéz-Moreno et al. [26]. The extract (0.4 mL) was combined with 3.6 mL of the DPPH solution (0.025 g DPPH in 100 mL methanol). The absorbance of the reaction mixture was determined using a Jenway spectrophotometer (6405 UV/Vis, England) at 515 nm within 6 min after adding the DPPH radical to the extract. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) was used as the standard, and the results were expressed in mg/g Trolox equivalents.
Antiradical Activity by ABTS
Antiradical activity, using the synthetic radical cation ABTS, was determined according to Re et al. [27]. The radical-scavenging activity was estimated by the ABTS (2,2, azinobis (3-ethylbenzthiazoline-6-sulfuric acid)) radical-cation discoloration assay. The ABTS+• stock solution was diluted with PBS buffer to obtain an Abs of 0.700 ± 0.05. Volumes of 2.00 mL of the ABTS+• were added to the diluted sample extracts. The ABTS+• bleaching was monitored at 30 °C and the discoloration after 6 min was used as the measure of antiradical activity. The bleaching rate of the ABTS+• in the presence of the sample was monitored at 734 nm using a Helios Gamma 100–240 spectrophotometer (Runcorn, UK). The results were expressed in mg/g Trolox equivalents
Ferric-Reducing Antioxidant Power (FRAP)
The Oyaizu method [28] was used to evaluate the reducing power of the samples against potassium ferricyanide. An amount of 1 mL of the extract was combined with 5 mL PBS (phosphate buffer with a pH of 6.6) and 5 mL of 1% potassium ferricyanide in the test tube. The mixture was stirred thoroughly and heated in a water bath for 20 min at 50 °C. After cooling, 5 mL of 10% trichloroacetic acid was then added. An amount of 5 mL of the mixture was pipetted into the test tube and combined with 5 mL of distilled water and 1 mL of the 0.1% ferric chloride solution. The absorbance at 700 nm was measured using a Jenway spectrophotometer (6405 UV/VIS, England). The results were expressed in mg/g Trolox equivalents.
Determination of Ferrous Ion Chelating Activity
The chelation of Fe(II) ions by the ethanol extracts was determined as described by Gu et al. [29]. An amount of 1 mL of the extract or blank was combined with 1.85 mL of high-purity deionized water and 50 µL of 2.0 mM FeCl2. The solution was mixed for 30 s, and then 100 µL 5 mM ferrozine was added. The reaction mixture was mixed and left to stand at 23 °C for 15 min. The absorbance of the solution was estimated by the spectrophotometric method at 562 nm with a Helios Gamma 100–240 spectrophotometer (Runcorn, UK). A calibration curve was constructed using disodium ethylenediaminetetraacetate dihydrate (EDTA). The results were expressed as mg EDTA/g.
2.2.6. Analysis of Volatile Compounds Using an Electronic Nose
The analysis of volatile flavor compounds using an electronic nose (e-nose) was conducted following the method described by Kowalski et al. [9].
2.2.7. Analysis of Chemical Compounds Using an Electronic Tongue
Instrumental taste analysis of the tested samples was carried out using the Alpha MOS ASTREE II electronic tongue (e-tongue) from Alpha MOS, Toulouse, France, according to the method described by Kowalski et al. [9]. The e-tongue system included a 48-position autosampler, a liquid sensor array (set #5) consisting of seven sensors (SRS, GPS, STS, SPS, UMS, SWS, and BRS), and a reference electrode (Ag/AgCl). The sensor set #5, designed for food and beverage applications, was employed based on [30]. The analysis of each water extract was performed five times. The taste screening analysis was carried out using AlphaSoft software version 14.2 from Alpha MOS (Toulouse, France). This test was used to rank the samples based on taste attributes using a scale from 0 to 12 for intensity. Principal component analysis (PCA) was conducted to differentiate the response signals from the seven sensors.
2.2.8. Differential Scanning Calorimetry
The thermal properties of the sponge cakes were determined by differential scanning calorimetry (DSC) [31]. For this purpose, a MICRO DSC III differential scanning calorimeter from Setaram Instrumentation (Caluire, France) was used. Sponge cake samples (approximately 40 mg) placed in a high-pressure stainless-steel ‘batch’ cell were heated from 15 to 200 °C at 3 °C min−1 and then cooled to 15 °C. The analysis was performed in triplicate. The onset (To), peak (Tp), and conclusion (Tc) temperatures, depolymerization temperature range of the RS starch ΔT = (Tc − To), and enthalpy change (ΔH), expressed in J g−1 sample, were calculated from thermograms.
2.2.9. Consumer Acceptance Analysis
Acceptability of Insect-Based Sponge Cake
The sponge cakes were offered to participants right after they were baked. The study comprised 64 volunteers aged between 19 and 23 years, chosen from the Medical University of Gdańsk’s database of willing participants. Only those without food neophobia were included in the study. Each sponge cake variety, including the control sample, was tested for acceptability on alternate days. The study was conducted as a double-blind study. Each probant received one biscuit with an assessment card, which was appropriately coded. Each participant received a unique code to facilitate the compilation of data from all days. The subjects knew that they were sponge cakes with the addition of insect flour, while the amount of the additive and the type of edible insect were not known to the probants or the staff who conducted the study. The respondents were healthy, did not take any medications or supplements, did not follow any special diets, and did not smoke cigarettes. All participants provided informed consent to take part in the study, which was approved by the Independent Institutional Research Ethics Committee at the Medical University of Gdańsk (NKBBN/346/2021). Prior to the study, an assessment of food neophobia was conducted on all subjects using the Food Neophobia Scale (FNS) [32]. Appearance, aroma, taste, structure, and overall acceptance were evaluated using 7-point visual scale with extreme indicators of “I dislike it entirely” and “I fully appreciate it” [33].
2.2.10. Statistical Analysis
Statistica 13.0, developed by Tibco Software in Palo Alto, was utilized to conduct the statistical analysis. Sponge cake features were tested through a two-way ANOVA on the flour type, percentage of substitution, and their interaction, with a significance level of p ≤ 0.05. In cases where the Levene test indicated significant differences, a post hoc least-significant-difference (LSD) Fisher’s test was conducted. The mean ± standard deviation was used to present the results. During the planning phase of the experiment (on the acceptability of the sponge cakes made with insects), an investigation was conducted into the sample size selection at a level that could produce a statistical conclusion with adequate accuracy and confidence. The probability of detecting the effects of a specific size through test power analysis and interval estimation was also explored. The parameters of multiple regression were estimated using the REGLINP command in an Excel 2010 PL spreadsheet considering the concept of shared variability.
3. Results and Discussion
3.1. Nutrient Composition
The chemical composition of food determines its nutritional value, which affects its usefulness in human nutrition. Both the raw materials and individual sponge cakes differed in the content of specific nutrients (Table 1). Insect flours were characterized by a significantly higher content of all the analyzed ingredients compared to the wheat flour. Among the insect flours, cricket had the highest content of protein (50.68%), lipids (29.01%), and dietary fiber (soluble, 0.24; chitin, 9.92; insoluble, 18.48; total, 18.72%). This resulted in an increase in the content of these compounds in the sponge cakes with insect flours compared to the control sample (Table 1). In other studies, an increase in the content of individual nutrients as a result of supplementation with flour from edible insects in products such as gluten-free bread, pancakes, muffins, or cupcakes was also observed [12,13,34].

Table 1.
Chemical composition of the raw materials and products (g/100 g).
3.2. Amino Acid Profile and Protein Nutritional Quality
Insect flours were characterized by a significantly higher content of all essential amino acids compared to the wheat flour (Table 2). Sponge cakes made with insect flours also showed a significantly higher content of most essential amino acids compared to the standard product (Table 2).

Table 2.
Essential amino acid content of the raw materials and sponge cakes (mg/g of protein).
Table 2.
Essential amino acid content of the raw materials and sponge cakes (mg/g of protein).
| Samples | Essential Amino Acids (mg/g of Protein) | Total EAA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| His | Ile | Leu | Lys | Met | Phe | Thr | Val | ||
| WF | 22.02 ± 0.16 f | 32.94 ± 0.50 d | 63.89 ± 1.85 e | 21.33 ± 0.57 e | 24.10 ± 3.96 de | 46.39 ± 1.46 c | 25.67 ± 0.70 e | 38.75 ± 1.17 f | 275.09 ± 10.09 g |
| BF | 44.76 ± 0.13 a | 53.75 ± 1.25 a | 82.94 ± 2.01 ba | 81.15 ± 2.36 a | 24.63 ± 2.98 dc | 54.08 ± 1.87 a | 49.89 ± 1.12 a | 71.20 ± 1.39 b | 462.40 ± 8.78 a |
| CF | 28.15 ± 0.18 c | 48.12 ± 0.29 b | 86.17 ± 0.63 a | 62.34 ± 0.70 c | 21.44 ± 0.93 e | 40.40 ± 0.34 e | 44.68 ± 0.46 b | 68.20 ± 0.83 c | 399.52 ± 2.58 c |
| TF | 39.15 ± 0.51 b | 52.18 ± 0.86 a | 85.31 ± 1.41 a | 67.01 ± 1.08 b | 22.18 ± 2.29 e | 40.60 ± 0.62 e | 48.33 ± 0.95 a | 74.46 ± 1.25 a | 429.22 ± 4.47 b |
| S | 22.68 ± 2.17 fe | 45.82 ± 4.33 b | 79.18 ± 3.76 ba | 49.69 ± 5.58 d | 29.17 ± 1.29 a | 51.92 ± 4.67 a | 41.07 ± 3.68 cd | 55.85 ± 5.02 de | 375.37 ± 31.85 d |
| BF15 | 23.00 ± 0.25 e | 43.09 ± 0.27 cb | 72.34 ± 0.61 c | 50.49 ± 1.99 d | 28.26 ± 0.80 ab | 47.14 ± 0.39 cb | 38.78 ± 0.23 d | 53.09 ± 0.24 de | 356.20 ± 2.93 e |
| BF30 | 23.98 ± 0.48 de | 41.36 ± 0.64 c | 68.80 ± 1.15 d | 50.15 ± 1.12 d | 25.83 ± 0.45 cd | 45.06 ± 1.81 d | 38.21 ± 0.39 d | 52.21 ± 0.44 e | 345.60 ± 5.29 f |
| CF15 | 21.69 ± 0.32 f | 43.11 ± 0.26 cb | 75.25 ± 0.35 cb | 49.50 ± 1.18 d | 28.02 ± 0.21 ab | 46.87 ± 1.69 cb | 39.64 ± 0.19 cd | 54.81 ± 0.46 ed | 358.87 ± 2.99 e |
| CF30 | 21.45 ± 0.29 f | 42.19 ± 0.87 c | 72.96 ± 1.33 cd | 48.07 ± 0.94 d | 27.08 ± 1.09 ba | 43.48 ± 0.90 e | 39.41 ± 0.41 cd | 54.67 ± 1.20 ed | 349.32 ± 6.30 f |
| TF15 | 24.54 ± 1.29 d | 45.49 ± 2.17 b | 78.89 ± 3.81 ba | 49.48 ± 3.56 d | 27.50 ± 0.83 ba | 48.66 ± 2.34 b | 41.69 ± 2.06 c | 58.14 ± 2.08 d | 374.37 ± 16.61 de |
| TF30 | 23.41 ± 1.68 de | 42.01 ± 2.68 c | 72.14 ± 4.74 cd | 48.76 ± 3.65 d | 27.03 ± 0.62 ba | 42.53 ± 2.64 e | 39.61 ± 2.09 cd | 54.44 ± 3.22 de | 349.95 ± 20.93 fe |
Explanatory notes: meaning of the symbols as in Table 1. Values in the same column marked with different letters are statistically significantly different at p < 0.05 ± SD. EAA—essential amino acids. For all sponge cakes made with insect flour, the amino acid score (AAS) surpassed 100% (Table 3). This proves the increased nutritional value of the products obtained.

Table 3.
Nutritional value of the protein of raw material and enriched products.
Table 3.
Nutritional value of the protein of raw material and enriched products.
| Sample | AAS (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| His | Ile | Leu | Lys | Thr | Val | AAA | SAA | |
| WF | 137.65 | 109.79 | 104.73 | 44.43 | 102.69 | 96.89 | 171.88 | 200.91 |
| BF | 279.76 | 179.15 | 135.97 | 169.06 | 199.56 | 178.00 | 375.63 | 162.04 |
| CF | 175.94 | 160.39 | 141.26 | 129.88 | 178.73 | 170.51 | 254.22 | 143.92 |
| TF | 244.68 | 173.93 | 139.85 | 139.60 | 193.33 | 186.16 | 299.37 | 148.99 |
| S | 141.75 | 152.73 | 129.81 | 103.52 | 164.28 | 139.62 | 199.16 | 222.33 |
| BF15 | 143.76 | 143.65 | 118.59 | 105.20 | 155.14 | 132.72 | 206.40 | 206.77 |
| BF30 | 149.86 | 137.86 | 112.79 | 104.48 | 152.86 | 130.52 | 214.13 | 189.36 |
| CF15 | 135.59 | 143.67 | 123.35 | 103.12 | 158.53 | 137.03 | 201.47 | 206.55 |
| CF30 | 134.09 | 140.65 | 119.60 | 100.15 | 157.63 | 136.67 | 195.57 | 198.21 |
| TF15 | 153.34 | 151.64 | 129.32 | 103.07 | 166.75 | 145.35 | 216.43 | 204.78 |
| TF30 | 146.33 | 140.04 | 118.27 | 101.59 | 158.45 | 136.10 | 205.48 | 199.27 |
Explanatory notes: meaning of the symbols as in Table 1. AAA—aromatic amino acids; SAA—sulfur amino acids.
3.3. Fatty Acid Profile
In the fatty acid profile, both insect flours and sponge cakes with their inclusion were characterized by the dominance of monounsaturated fatty acids (MUFAs), ranging from 38.78 in CF30 to 47.97 g/100 g in TF15, and saturated fatty acids (SFAs), ranging from 34.38 for BF30 to 38.27 g/100 g for CF30. The addition of insect flour as an animal-derived ingredient contributed to a reduction in the nutritional value of the final product. Except for the BF15, TF15, and TF30 sponge cakes, the MUFA content in the sponge cakes was significantly lower compared to the standard. Furthermore, the PUFA content and the ratio of total PUFAs to total SFAs were significantly lower in the sponge cakes with the insect flour compared to the standard, except for the BF30 sponge cakes. The use of insect flours also led to a significant increase in the n6/n3 ratio (Table 4). The recommended n-6/n-3 ratio should range from 1:1 to 5:1, while a ratio of 10:1 or higher is associated with negative health effects. A low PUFA/SFA ratio in diets is considered a risk factor for increased blood cholesterol levels. In this study, both the raw materials and the sponge cakes exhibited high values of the n-6/n-3 ratio, indicating an imbalance in the lipid fractions (Table 4).

Table 4.
Fatty acid profile of the sponge cakes and insect flour (g/100 g).
However, evaluating the nutritional value of insect lipids based solely on fat indexes and the ratio of individual fractions is challenging, as certain fatty acids, like oleic acid, found in higher amounts in sponge cakes with edible-insect flour, have been shown to have beneficial effects on insulin sensitivity [35]. Oleic acid not only prevents insulin resistance but also inhibits endoplasmic reticulum stress, exhibits anti-inflammatory effects, prevents the attenuation of the insulin-signaling pathway, and enhances β cell survival [35]. Studies by Perona et al. (2004) have demonstrated the effectiveness of oils rich in oleic acid in reducing blood pressure and LDL cholesterol levels [36].
3.4. Color Analysis
Color is one of the attributes of bakery products, such as cakes, that can influence consumer acceptance. The addition of insect flour has contributed to significant changes in the CIELab parameters and total color change compared to the standard sponge cakes (Table 5). Insect flour has led to a decrease in lightness (L*) as well as a reduction in the levels of red (a*) and yellow (b*) color components. Similar effects on color-parameter values were observed by Pauter et al. [34], who used cricket flour in muffin production. On the other hand, Kowalski et al. [6] observed an increase in the levels of red and yellow color components and total color change in sponge cakes containing mealworms. In that case, chicken eggs were substituted with a plant-based replacement, which may have contributed to the different content of individual color components [6].

Table 5.
Color parameters of the sponge cakes.
3.5. Antioxidant Composition and Properties
The characteristics of the raw materials used (insect flours) were presented in a previous publication by Gumul et al. [10]. The utilization of ground edible insects at varying levels between 15% and 30% demonstrated a notable augmentation in the overall polyphenol content of sponge cakes, ranging from 89% to 258%, compared to the standard. Similarly, there was a considerable rise in flavonoid levels, ranging from 3% to 150%, in comparison to the control sample. It is worth emphasizing that the increase in total polyphenols exhibited a direct correlation with the quantity of edible insects employed, with crickets displaying the highest increase and mealworms displaying the lowest. These findings align with the edible-insect characteristics elucidated in the study conducted by Gumul et al. [10], in which cricket flour exhibited the highest polyphenol content, followed by buffalo worm flour and mealworm flour with comparatively lower amounts. By incorporating a substantial proportion of edible insects, wheat sponge cakes displayed an elevation of flavonoid content by up to 2.5 times, particularly when utilizing a 30% inclusion of mealworm in comparison to the control. However, other edible insects did not yield similarly remarkable outcomes (Table 6). In the case of sponge cakes, a 15% inclusion of buffalo and cricket flours resulted in a comparable flavonoid content to that of the control sample. Nonetheless, a 30% inclusion of the flour of these edible insects exhibited an approximately 40% increase in flavonoids relative to the control. The highest flavonoid quantities were observed in sponge cakes incorporating the mealworm flour, particularly at the 30% inclusion level.

Table 6.
Total polyphenol and flavonoid content in the sponge cakes.
Control samples and sponge cakes with the 15% and 30% inclusion of ground edible insects were subjected to HPLC analysis. In the control sample, the presence of phenolic acid derivatives, which may have come from wheat flour used for baking, such as ferulic acid, caffeic acid, p-coumaric acid (hydroxycinnamic acids), and protocatechuic aldehyde (hydroxybenzoic acids), were detected (Table 7). These findings are consistent with the results from other studies that have reported wheat flour as a source of the aforementioned acids [37]. Substituting this flour with edible-insect flour, which is characterized by a low amount of hydroxycinnamic acid derivatives [10], resulted in a decrease in their content in wheat sponge cakes containing edible-insect powder (Table 7). In contrast, hydroxybenzoic acids were predominant in sponge cakes with edible insects due to the fact that edible insects themselves contain significant amounts of these acids [10].

Table 7.
Profile of the phenolic compounds of the sponge cakes.
The presence of hydroxybenzoic acids in the sponge cakes with edible-insect flour can be influenced by multiple factors. It may partially result from the thermal breakdown of quercetin derivatives, especially rutinoside quercetin, which generates phenolic acids [38]. Considering the presence of quercetin derivatives in edible insects (except for buffalo worms) [10], their thermal degradation may contribute to the increase in the phenolic acid content in sponge cakes. A clear example is the presence of protocatechuic acid in sponge cakes with crickets and mealworms, which was not found in these particular edible insects but appears in the sponge cakes due to the thermal degradation of quercetin derivatives during baking (Table 7).
When analyzing the phenolic acids in sponge cakes with the addition of edible insects, it can be observed that there is a decrease in these compounds (Table 7) due to the thermal decarboxylation of these compounds, including the formation of 4-vinyl guaiacol, during the baking process [39]. Although some of these compounds may originate from the thermal breakdown of quercetin derivatives, or manifest at different stages of sponge cake production, their overall content decreases during baking.
Considering the quantities of hydroxybenzoic and hydroxycinnamic acids, it is evident that the amount of hydroxybenzoic acid is significantly higher than that of the hydroxycinnamic acids in sponge cakes with edible-insect flour. Moreover, a higher inclusion of edible insects results in a greater quantity of these phenolic acids (Table 7). This can be attributed to the dominant presence of hydroxybenzoic acids in edible insects [10]. In summary, it can be stated that, although the baking process leads to losses (up to 66%) of certain phenolic compounds [40], mainly due to the thermal, enzymatic, oxidative degradation, and decarboxylation of phenolic acids [40], it is suggested that the increase in phenolic compounds (primarily phenolic acids) in sponge cakes with edible insects compared to the control is attributed to the addition of these ingredients. It should also be noted that flavonols are completely degraded during baking in sponge cakes; hence, their absence in the product containing edible insects (Table 7). Additionally, the losses of phenolic compounds may be caused by the formation of complexes with polysaccharides [41].
The antioxidant potential, as assessed by four different methods, unequivocally confirmed that the aforementioned polyphenolic compounds found in edible insects provide significant radical-scavenging and antioxidant activity in sponge cakes containing them. Moreover, it was observed that the antioxidant potential, determined by the four methods, was significantly lower in sponge cakes with the 15% inclusion of edible insects compared to those with the 30% inclusion (Table 8). Among all the samples analyzed, sponge cakes with the 30% inclusion of cricket flour exhibited the highest in vitro antioxidant potential (Table 8). This observation can be attributed to the notable presence of hydroxycinnamic acids, such as ferulic acid, caffeic acid, and sinapic acid (Table 7), which have been reported to exhibit superior efficiency in scavenging free radicals [42].

Table 8.
Antioxidant activity of the sponge cakes.
A strong positive correlation was observed between the total polyphenol content (TPC) in the analyzed sponge cakes and ABTS (R2 = 0.924), TPC, and DPPH (R2 = 0.939), as well as between TPC and FRAP (R2 = 0.925), and TPC and iron reduction (R2 = −0.859). Furthermore, it can be suggested that, in addition to polyphenols, newly formed compounds during the baking process, such as Maillard-reaction products, may also contribute partially to the high radical-scavenging and antioxidant activity observed [43]. These compounds are likely to significantly influence the radical-scavenging and antioxidant activity of the sponge cakes due to their increased exposure to temperature and the presence of Maillard-reaction substrates, such as sugars, in the sponge cake recipe (Table 8).
3.6. Analysis of Volatile Compounds Using the Electronic Nose
An electronic nose was utilized to analyze the volatile compounds present in the control and insect-flour-enriched sponge cakes. Technical abbreviations have been explained upon their initial use. The results are shown in Table 9, revealing thirty-four distinguishable volatile compounds, including aldehydes, ketones, alcohols, esters, pyrazines, lactones, phenols, acids, and pyranones, present in both the control and insect-flour-enriched sponge cakes.

Table 9.
The content of volatile compounds (%) in the sponge cakes.
The main flavor and fragrance compounds in both the control and insect-flour-enriched samples were 3-methylbutanal, 2,3-pentanedione, butanal, and 2-propanol, which generate the aromas of malt, sweetness, butter, and a sharp, musty scent, respectively [44]. Nevertheless, it was also observed that the share of different insect flours contributed to a notable change in the concentrations of volatile compounds. The sponge cakes fortified with 30% of cricket and mealworm flours had significantly higher contents of furfural, heptanal, hexanal, 2-propanol, butanoic acid, eugenol, guaiol, and trimethyl pyrazine, but significantly lower contents of acetaldehyde, 3-methylbutanal, 2,3-pentanedione, 2,5-dimethylpyrazine, 2,3-dimethylpyrazine, and 2-phenylethanol compared to the control and buffalo worm-flour-substituted sponge cakes. The presence of aldehydes, ketones, and alcohols in cakes may be due to thermal reactions during baking, such as nonenzymatic Maillard reactions and sugar caramelization. Similarly, these compounds can also be formed by lipid oxidation [45]. The pyrazines are also known to be produced by Maillard and Strecker reactions, and a high amount of protein in the insect flours may intensify the formation of those compounds. These findings indicate that the substitution of wheat flour with different insect flours can influence the aroma profile of baked products. However, the flavor of the final product was greatly influenced by the type of insect flour used, as well as the proportion of each flour in the recipe. For instance, the increased amount of hexanal with the increasing substitution of mealworm flour could be explained by the high fat content in this flour compared to buffalo worm and cricket flours, which could lead to the increased formation of compounds generated by lipid oxidation during thermal processing [44,45]. Moreover, fascinatingly, furfural, a compound formed via the 1,2-enolisation pathway through 3-deoxyosone, was present in significant quantities in the mealworm- and cricket-flour-enriched sponge cakes compared to the other samples. However, almost all of the enriched sponge cakes exhibited high levels of desirable volatile compounds, including 3-methylbutanal, 2,3-pentanedione, and butanal, which provide malt and sweet notes.
3.7. Electronic Tongue Analysis
The sensory attributes of water extracts from the control sponge cakes and those substituted with edible-insect flour were assessed for taste quality and intensity using an electronic tongue equipped with seven cross-selectivity sensors. The results showed that the substitution of wheat flour with insect flour significantly affected the sensory properties of the samples studied (Figure 1A). Sponge cakes made with insect flours were found to have significantly higher bitter and umami taste, irrespective of the kind and amount of the insect flour used. Furthermore, the addition of insect flour led to a slight increase in the intensities of sour, sweet, and salty tastes in almost all the substituted sponge cakes in comparison to the control samples. With the increase in the quantity of insect flour added, the intensities of these five basic tastes also increased. The bitterness and sweetness of the sponge cakes increased the most with a 30% proportion of cricket flour and buffalo worm flour, respectively. Sponge cakes with 15% and 30% buffalo worm flour had the highest scores for the sour and salty taste. The most prominent change in the intensity of umami taste was noted in sponge cakes supplemented with mealworm flour, irrespective of the share size. Principal component analysis (PCA) was conducted to visualize the relationship between the samples and variables (taste scores) in order to establish any differences and similarities. Figure 1B demonstrates that PC1 and PC2 encompass 67.35% of the entire variance in the data. Of these two principal components, PC1 accounted for 39.50% of the total variation and PC2 explained 27.84% of the variation.
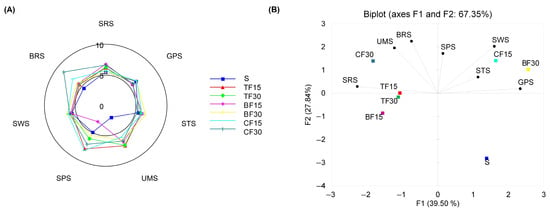
Figure 1.
Radar chart representing taste scores of sponge cakes (A) and PCA graph showing the relationship among samples and tastes (B). SRS (sourness), GPS (metallic), STS (saltiness), SPS (spiciness), UMS (umami), SWS (sweetness), and BRS (bitterness).
The biplot shows that the tested sponge cake samples and their tastes fall into three distinct clusters. The initial cluster consisted exclusively of control samples, exhibiting less variability in the taste profile and the lowest intensity of the umami taste. The second cluster comprised sponge cakes made with the 15% cricket flour and 30% buffalo worm flour. These samples were notably sweeter and saltier than the other sponge cakes. The remaining sponge cakes that were fortified with insect flours (TF15, TF30, BF15, CF30) were grouped into the third cluster due to their noticeable deviation in relation to the other examined samples, particularly with regard to their umami and bitter taste. The correlation between the increase in umami, bitterness, sourness, saltiness, and sweetness, and the substitution of wheat flour with insect flour, may be associated with the relatively higher protein, mineral, and organic acid content in the insect flours as compared to the wheat flour. Consequently, the findings of this study suggest that the composition and quantity of ingredients used in the production of sponge cakes could influence their sensory properties.
3.8. Differential Scanning Calorimetry
Based on the obtained results of thermal analysis of the tested products, it was noted that a single peak was visible in the obtained endotherms in the temperature range from approximately 45 °C to 160 °C, indicating the presence of resistant starch (RS) in those products (Table 10).

Table 10.
Thermal parameters of the obtained sponge cakes depending on the type and concentration of insect flour.
Depending on the type of flour used, and its proportion in the recipe, it was found that the amount of RS formed (expressed as enthalpy value) in the sponge cakes ranged from an average of 18% to 53% (i.e., 1.2 to 1.5 times) higher compared to its content in the control sample without insect flour. A significant impact of the type of insect flour used on the amount of RS formed was demonstrated. The highest amount of RS was present in sponge cakes with the mealworm flour, followed by the buffalo worm and cricket flours, regardless of the concentration of these flours. The obtained ∆T values indicate that the RS in the sponge cakes produced with insect flours has a less-crystalline structure compared to the ∆T value obtained for the control sample, suggesting a different influence of the present components in the insect flour, such as proteins and fats, on the retrogradation process of starch. It is known that the interaction between starch and certain nonstarch components, including proteins and lipids, may affect the formation of RS, which exhibits health-promoting properties. It has been observed that protein, as the main food component, plays a significant role in reducing starch digestibility, similar to other components of the food matrix, such as hydrocolloids and phenolic compounds, partly by forming complex compounds with starch [46].
3.9. Acceptability of Insect-Based Sponge Cake
The ANOVA results indicate that the quantity of the additive had a significant impact on several sensory assessment factors, whereas the type of additive did not. Only the structure of the sponge cake was affected by the type and quantity of the additive. It is worth noting that the assessed attributes were mainly influenced by the quantity of the additive and not by the type used. Sponge cakes without the addition of insect flour received the highest score from respondents (6.19), while the acceptance for sponge cakes with the insect flour substitution was lower, ranging from 4.63 to 5.56. Increasing the content of the added meal resulted in lower ratings for all parameters assessed by the evaluation team. Table 11 displays the results. The sponge cake supplemented with the 15% insect meal achieved a high level of acceptability on a seven-point scale, irrespective of the insect species used.

Table 11.
The results of the consumer organoleptic evaluation of the sponge cakes.
Equations were formulated using the component factors that influenced the acceptability for every variant analyzed in the data study to establish which of the examined sensory parameters had the most significant effect on overall acceptability. Table 12 shows the resulting multiple general-acceptability equations.

Table 12.
Multiple equations of the overall sponge cake acceptability.
Preliminary regression analysis indicated that the results of the sensory characteristics can be generalized by omitting the amount of added insect flour. Multiple regression analysis showed that, for sponge cakes with the addition of insect meal, the smell turned out to be insignificant and had no effect on the overall acceptability of the tested sponge cakes, or had only a slight effect. The results obtained demonstrate that the acceptability level was determined by the predictors incorporated in the regression equation, with taste being the most significant factor for overall acceptability, followed by structure and appearance, to a lesser extent. The correlation coefficient R2 measured for all tested samples was high at 0.99, thereby highlighting the model’s ability to explain almost 99% of the dependent variable’s variability. The remaining 10% of the variability was attributed to unanalyzed parameters. The regression analysis identified taste as the critical factor in determining the overall acceptability. It was observed that the greater the inclusion of insect meal, the lower the taste rating. The high x1 coefficient, exceeding 0.5 each time (0.73 for T. molitor), made the taste the most important element in determining acceptability and the final rating set by the testers.
The current results find partial confirmation in the literature. However, sensory evaluation depends on the type of product and the amount of additive used. Roncolini et al. [47] observed a decline in the consumer acceptability of bread enriched with T. molitor flour. However, they did not find any significant impact on overall product acceptability when varying the additive level (5% and 10%). In the authors’ previous study [6], a decrease was observed in the organoleptic quality of sponge cakes regardless of the proportion of mealworm flour incorporated into them. Ruszkowska et al. [11] observed that, in extruded corn snacks, the inclusion of up to 6% cricket flour allowed for the production of sensory-appealing snacks.
4. Conclusions
The addition of insect flours had a significant impact on the nutritional properties and consumer acceptance of the obtained sponge cakes. Both the raw materials and sponge cakes exhibited high values of the n-6/n-3 ratio, indicating an imbalance in the lipid fractions; however, with a relatively high oleic acid content. For all the analyzed sponge cakes’ essential amino acids, the AAS exceeded 100%. The range was from 100.15% (lysine in CF30) to 216.43% (for AAA in the TF15 sample). The introduction of edible insects to the biscuit recipe also increased their antioxidant potential, although one should be aware that this type of addition will not be crucial in increasing the antioxidant value of the product as much as plant additives. Sponge cakes supplemented with flour from edible insects are also characterized by an increase in the content of dietary fiber. This increase is not only due to the fiber content of the flours themselves, but also due to the interaction with starch and the formation of resistant starch. Products with the addition of edible insects are generally well-accepted by consumers, and the most important quality factor affecting acceptance is taste. The conducted research indicates that the use of edible insects in the form of flours added to confectionary products may both gain consumer acceptance and contribute to the improvement of the prohealth properties of these products.
Author Contributions
Conceptualization, S.K., A.M. and D.G.; methodology, S.K., D.G., J.O., A.M., B.M. and M.S.; validation, S.K., D.G., B.M., A.M. and M.S.; formal analysis, S.K., D.G., J.O., J.R.-K., A.M., B.M., M.S. and M.Z.; investigation, S.K., D.G., J.O., J.R.-K., A.M., B.M., M.S. and M.Z.; resources, S.K., D.G., J.O., J.R.-K., A.M. and M.Z.; data curation, S.K. and D.G., writing—original draft preparation, S.K., D.G., J.O., A.M., B.M. and M.S.; writing—S.K., D.G., J.O., A.M. and B.M.; visualization, S.K., D.G. and J.O.; supervision, S.K. and B.M.; funding acquisition, S.K. and D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical approval for the involvement of 597 human subjects in this study was granted by the Independent Bioethical Committee for Scientific Research at the Medical University of Gdansk, reference number (NKBBN/346/2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Acknowledgments
This research was financed by the Ministry of Education and Science of the Republic of Poland. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviation
WF—wheat flour; BF—buffalo worm flour; CF—cricket flour; TF—mealworm flour; S—standard sponge cake; BF15, BF30—sponge cake with 15 and 30% addition of buffalo worm flour; CF15, CF30—sponge cake with 15 and 30% addition of cricket flour; TF15, TF30—sponge cake with 15 and 30% addition of mealworm flour.
References
- Food and Agriculture Organization of the United Nations (FAO). Building a Common Vision for Sustainable Food and Agriculture: Principles and Approaches; FAO: Rome, Italy, 2014. [Google Scholar]
- Guiné, R.P.F.; Florença, S.G.; Anjos, O.; Boustani, N.M.; Chuck-Hernández, C.; Sarić, M.M.; Ferreira, M.; Costa, C.A.; Bartkiene, E.; Cardoso, A.P.; et al. Are Consumers Aware of Sustainability Aspects Related to Edible Insects? Results from a Study Involving 14 Countries. Sustainability 2022, 14, 14125. [Google Scholar] [CrossRef]
- Reeds, P.J. Dispensable and Indispensable Amino Acids for Humans. J. Nutr. 2000, 130, 1835S–1840S. [Google Scholar] [CrossRef] [PubMed]
- García-Segovia, P.; Igual, M.; Noguerol, A.T.; Martínez-Monzó, J. Use of Insects and Pea Powder as Alternative Protein and Mineral Sources in Extruded Snacks. Eur. Food Res. Technol. 2020, 246, 703–712. [Google Scholar] [CrossRef]
- Jantzen da Silva Lucas, A.; Menegon de Oliveira, L.; da Rocha, M.; Prentice, C. Edible Insects: An Alternative of Nutritional, Functional and Bioactive Compounds. Food Chem. 2020, 311, 126022. [Google Scholar] [CrossRef]
- Kowalski, S.; Mikulec, A.; Skotnicka, M.; Mickowska, B.; Makarewicz, M.; Sabat, R.; Wywrocka-Gurgul, A.; Mazurek, A. Effect of the Addition of Edible Insect Flour from Yellow Mealworm (Tenebrio molitor) on the Sensory Acceptance, and the Physicochemical and Textural Properties of Sponge Cake. Pol. J. Food Nutr. Sci. 2022, 72, 393–405. [Google Scholar] [CrossRef]
- Skotnicka, M.; Karwowska, K.; Kłobukowski, F.; Borkowska, A.; Pieszko, M. Possibilities of the Development of Edible Insect-Based Foods in Europe. Foods 2021, 10, 766. [Google Scholar] [CrossRef]
- Alhujaili, A.; Nocella, G.; Macready, A. Insects as Food: Consumers’ Acceptance and Marketing. Foods 2023, 12, 886. [Google Scholar] [CrossRef] [PubMed]
- European Union. Regulation (EU) 2015/2283. Regulation (EU) 2015/2283 of the European Parliament and of the Council of 25 November 2015 on novel foods, amending Regulation (EU) No 1169/2011 of the European Parliament and of the Council and repealing Regulation (EC) No 258/97 of the European Parliament and of the Council and Commission Regulation (EC) No 1852/2001. Off. J. Eur. Union 2015, L327, 1–22. [Google Scholar]
- Gumul, D.; Oracz, J.; Kowalski, S.; Mikulec, A.; Skotnicka, M.; Karwowska, K.; Areczuk, A. Bioactive Compounds and Antioxidant Composition of Nut Bars with Addition of Various Edible Insect Flours. Molecules 2023, 28, 3556. [Google Scholar] [CrossRef]
- Ruszkowska, M.; Tańska, M.; Kowalczewski, P.Ł. Extruded Corn Snacks with Cricket Powder: Impact on Physical Parameters and Consumer Acceptance. Sustainability 2022, 14, 16578. [Google Scholar] [CrossRef]
- Smarzyński, K.; Sarbak, P.; Musiał, S.; Jeżowski, P.; Piątek, M.; Kowalczewski, P.Ł. Nutritional Analysis and Evaluation of the Consumer Acceptance of Pork Pâté Enriched with Cricket Powder-Preliminary Study. Open Agric. 2019, 4, 159–163. [Google Scholar] [CrossRef]
- Mazurek, A.; Palka, A.; Skotnicka, M.; Kowalski, S. Consumer Attitudes and Acceptability of Wheat Pancakes with the Addition of Edible Insects: Mealworm (Tenebrio molitor), Buffalo Worm (Alphitobius diaperinus), and Cricket (Acheta domesticus). Foods 2023, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Matiza Ruzengwe, F.; Nyarugwe, S.P.; Manditsera, F.A.; Mubaiwa, J.; Cottin, S.; Matsungo, T.M.; Chopera, P.; Ranawana, V.; Fiore, A.; Macheka, L. Contribution of Edible Insects to Improved Food and Nutrition Security: A Review. Int. J. Food Sci. Technol. 2022, 57, 6257–6269. [Google Scholar] [CrossRef]
- Janssen, R.H.; Vincken, J.P.; van den Broek, L.A.; Fogliano, V.; Lakemond, C.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Official Methods of Analysis of AOAC International-18th Edition, Revision 3. Available online: https://www.techstreet.com/standards/official-methods-of-analysis-of-aoac-international-18th-edition-revision-3?product_id=1678986 (accessed on 15 February 2021).
- Moore, S.; Stein, W.H. Chromatography of Amino Acids on Sulfonated Polystyrene Resins. J. Biol. Chem. 1951, 192, 663–681. [Google Scholar] [CrossRef] [PubMed]
- Davidson, I. Hydrolysis of Samples for Amino Acid Analysis. In Protein Sequencing Protocols; Smith, B.J., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2003; pp. 111–122. ISBN 978-1-59259-342-2. [Google Scholar]
- Smith, A.J. Post Column Amino Acid Analysis. In Protein Sequencing Protocols; Smith, B.J., Ed.; Methods in Molecular BiologyTM; Humana Press: Totowa, NJ, USA, 2003; pp. 133–141. ISBN 978-1-59259-342-2. [Google Scholar]
- Neira, G. Protein Quality Evaluation Report of Joint FAO/WHO Expert Consultation 51 food and agricul ture organization of the united nations. FAO Food Nutr. Pap. 1991, 51, 247. [Google Scholar]
- FAO Dietary Protein Quality Evaluation in Human Nutrition. Report of an FAQ Expert Consultation. FAO Food Nutr. Pap. 2013, 92, 1–66.
- Fernandez-Artigas, P.; Guerra-Hernandez, E.; Garcia-Villanova, B. Browning Indicators in Model Systems and Baby Cereals. J. Agric. Food Chem. 1999, 47, 2872–2878. [Google Scholar] [CrossRef]
- Oracz, J.; Nebesny, E.; Żyżelewicz, D. Identification and Quantification of Free and Bound Phenolic Compounds Contained in the High-Molecular Weight Melanoidin Fractions Derived from Two Different Types of Cocoa Beans by UHPLC-DAD-ESI-HR-MSn. Food Res. Int. 2019, 115, 135–149. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. In Methods in Enzymology; Oxidants and Antioxidants Part A; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- El Hariri, B.; Sallé, G.; Andary, C. Involvement of Flavonoids in the Resistance of Two Poplar Cultivars to Mistletoe (Viscum album L.). Protoplasma 1991, 162, 20–26. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. A Procedure to Measure the Antiradical Efficiency of Polyphenols. J. Sci. Food Agric. 1998, 76, 270–276. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Oyaizu, M. Studies on Products of Browning Reaction. Antioxidative Activities of Products of Browning Reaction Prepared from Glucosamine. Jpn. J. Nutr. Diet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Gu, F.L.; Kim, J.M.; Abbas, S.; Zhang, X.M.; Xia, S.Q.; Chen, Z.X. Structure and antioxidant activity of high molecular weight Maillard reaction products from casein–glucose. Food Chem. 2010, 120, 505–511. [Google Scholar] [CrossRef]
- Raithore, S.; Bai, J.; Plotto, A.; Manthey, J.; Irey, M.; Baldwin, E. Electronic Tongue Response to Chemicals in Orange Juice That Change Concentration in Relation to Harvest Maturity and Citrus Greening or Huanglongbing (HLB) Disease. Sensors 2015, 15, 30062–30075. [Google Scholar] [CrossRef] [PubMed]
- Kapusniak, K.; Lubas, K.; Wojcik, M.; Rosicka-Kaczmarek, J.; Pavlyuk, V.; Kluziak, K.; Gonçalves, I.; Lopes, J.; Coimbra, M.A.; Kapusniak, J. Effect of Continuous and Discontinuous Microwave-Assisted Heating on Starch-Derived Dietary Fiber Production. Molecules 2021, 26, 5619. [Google Scholar] [CrossRef] [PubMed]
- Ritchey, P.N.; Frank, R.A.; Hursti, U.-K.; Tuorila, H. Validation and Cross-National Comparison of the Food Neophobia Scale (FNS) Using Confirmatory Factor Analysis. Appetite 2003, 40, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Jebb, A.T.; Ng, V.; Tay, L. A Review of Key Likert Scale Development Advances: 1995–2019. Front. Psychol. 2021, 12, 637547. [Google Scholar] [CrossRef] [PubMed]
- Pauter, P.; Różańska, M.; Wiza, P.; Dworczak, S.; Grobelna, N.; Sarbak, P.; Kowalczewski, P.Ł. Effects of the Replacement of Wheat Flour with Cricket Powder on the Characteristics of Muffins. Acta Sci. Pol. Technol. Aliment. 2018, 17, 227–233. [Google Scholar] [CrossRef]
- Palomer, X.; Pizarro-Delgado, J.; Barroso, E.; Vázquez-Carrera, M. Palmitic and Oleic Acid: The Yin and Yang of Fatty Acids in Type 2 Diabetes Mellitus. Trends Endocrinol. Metab. 2018, 29, 178–190. [Google Scholar] [CrossRef]
- Perona, J.S.; Cañizares, J.; Montero, E.; Sánchez-Domínguez, J.M.; Catalá, A.; Ruiz-Gutiérrez, V. Virgin Olive Oil Reduces Blood Pressure in Hypertensive Elderly Subjects. Clin. Nutr. 2004, 23, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yao, Y.; He, Z.; Wang, D.; Liu, A.; Zhang, Y. Determination of Phenolic Acid Concentrations in Wheat Flours Produced at Different Extraction Rates. J. Cereal Sci. 2013, 57, 67–72. [Google Scholar] [CrossRef]
- Barnes, J.S.; Foss, F.W., Jr.; Schug, K.A. Thermally Accelerated Oxidative Degradation of Quercetin Using Continuous Flow Kinetic Electrospray-Ion Trap-Time of Flight Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of Antioxidant Activity during Kilning: Role of Insoluble Bound Phenolic Acids of Barley and Malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Alvarez-Jubete, L.; Arendt, E.K.; Gallagher, E. Nutritive Value and Chemical Composition of Pseudocereals as Gluten-Free Ingredients. Int. J. Food. Sci. Nutr. 2009, 60 (Suppl. S4), 240–257. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of Bread Dough with Added Fiber Polysaccharides and Phenolic Antioxidants: A Review. J. Food. Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- Karamać, M.; Kosińska, A.; Pegg, R.B. Comparison of Radical-Scavenging Activities for Selected Phenolic Acids. Pol. J. Food Nutr. Sci. 2005, 55, 165–170. [Google Scholar]
- Sakač, M.; Torbica, A.; Sedej, I.; Hadnađev, M. Influence of Breadmaking on Antioxidant Capacity of Gluten Free Breads Based on Rice and Buckwheat Flours. Food Res. Int. 2011, 9, 2806–2813. [Google Scholar] [CrossRef]
- Yoon, S.; Jeong, H.; Jo, S.M.; Hong, S.J.; Kim, Y.J.; Kim, J.K.; Shin, E.-C. Chemosensoric Approach for Microwave- or Oven-Roasted Coffea arabica L. (Cv. Yellow Bourbon) Using Electronic Sensors. LWT 2022, 167, 113844. [Google Scholar] [CrossRef]
- Man, S.M.; Stan, L.; Păucean, A.; Chiş, M.S.; Mureşan, V.; Socaci, S.A.; Pop, A.; Muste, S. Nutritional, Sensory, Texture Properties and Volatile Compounds Profile of Biscuits with Roasted Flaxseed Flour Partially Substituting for Wheat Flour. Appl. Sci. 2021, 11, 4791. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D.; Gawlik-Dziki, U. Starch and Protein Analysis of Wheat Bread Enriched with Phenolics-Rich Sprouted Wheat Flour. Food Chem. 2017, 228, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Roncolini, A.; Milanović, V.; Cardinali, F.; Osimani, A.; Garofalo, C.; Sabbatini, R.; Clementi, F.; Pasquini, M.; Mozzon, M.; Foligni, R.; et al. Protein Fortification with Mealworm (Tenebrio molitor L.) Powder: Effect on Textural, Microbiological, Nutritional and Sensory Features of Bread. PLoS ONE 2019, 14, e0211747. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).