Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques
Abstract
:1. Introduction
2. Materials and Methods
2.1. D. officinale Leaves and Drying
2.2. Moisture Content, Rehydration Ratio, and Color Measurements
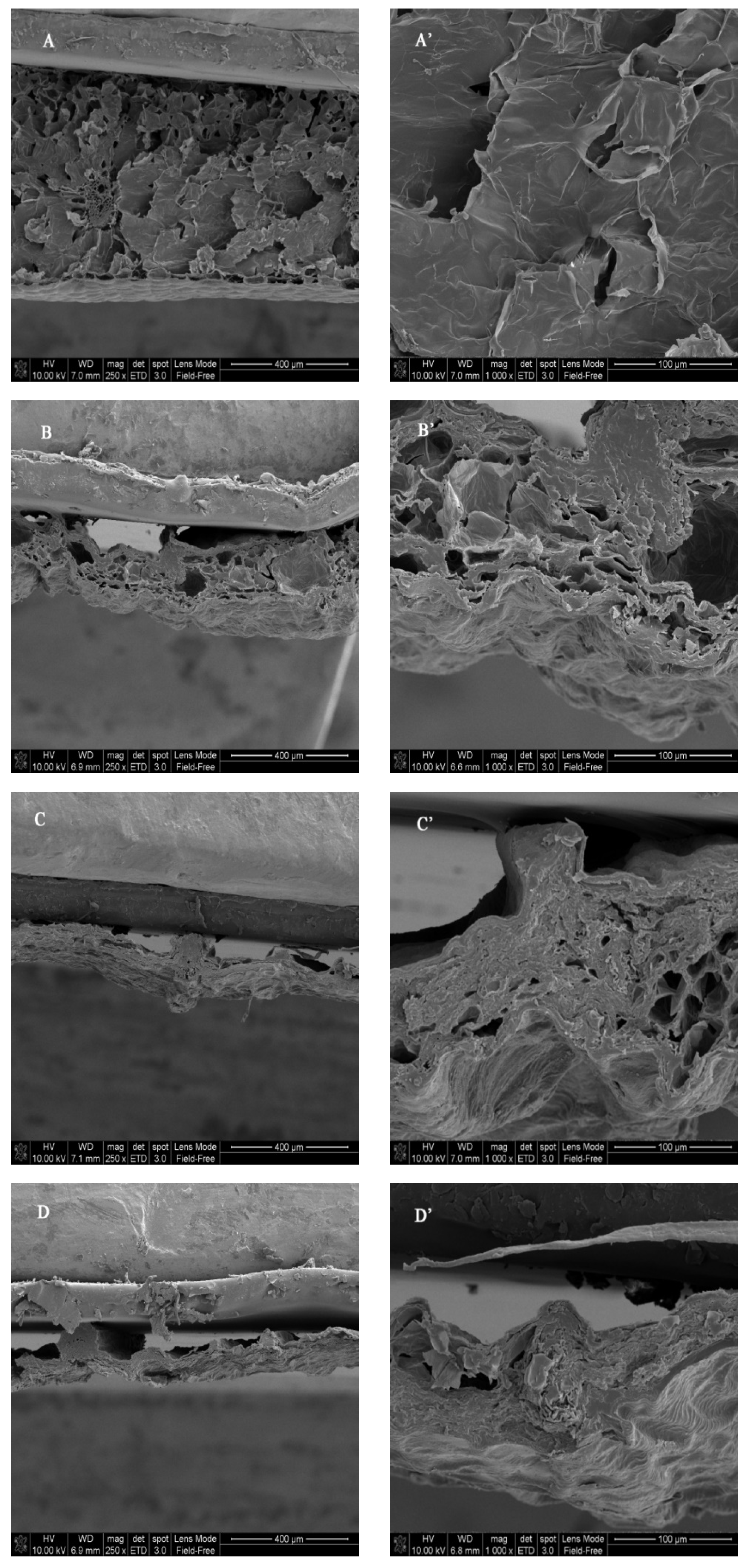
2.3. Microstructure Observation
2.4. Phenolic Compound Content Measurements
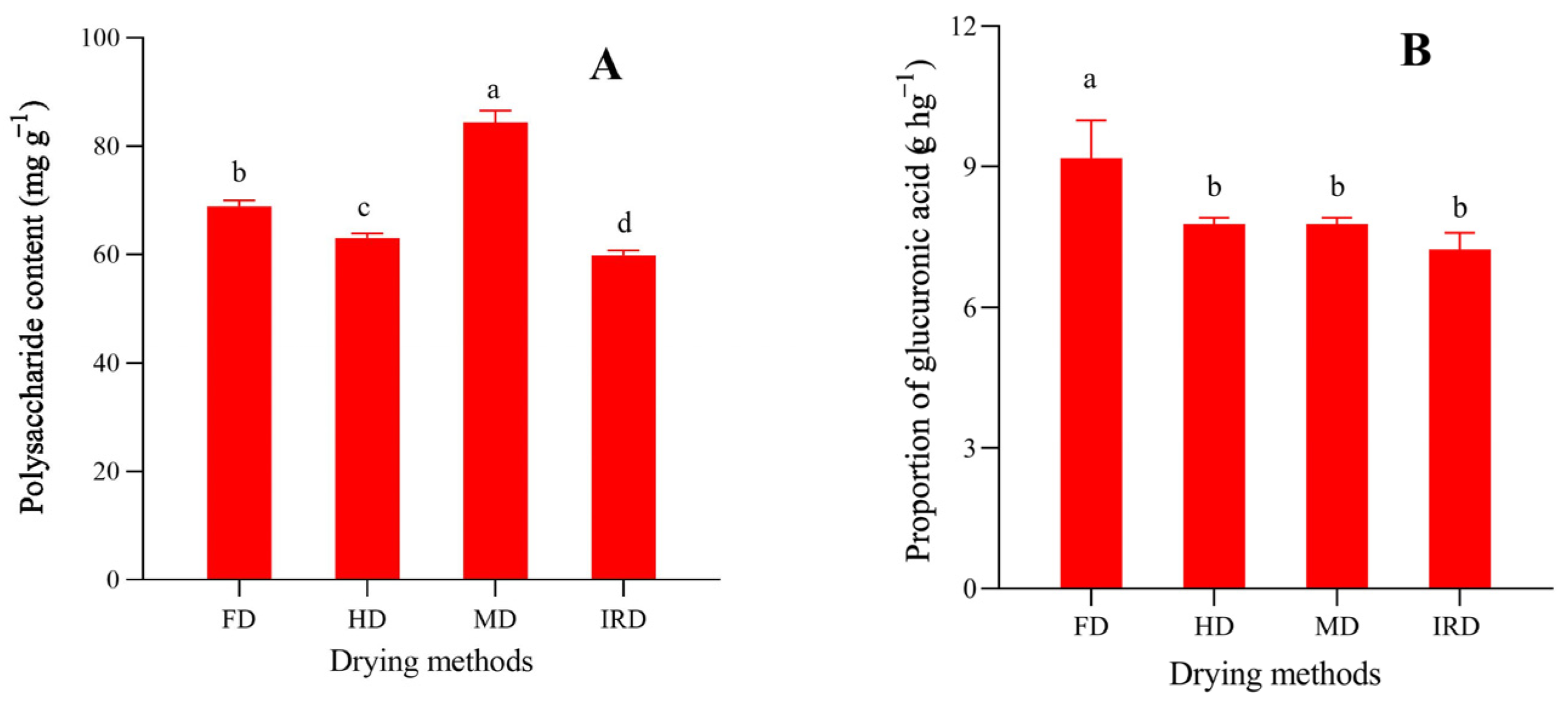
2.5. Measurements of Total Polysaccharide and Uronic Acid Contents
2.6. Antioxidant Activity Measurements
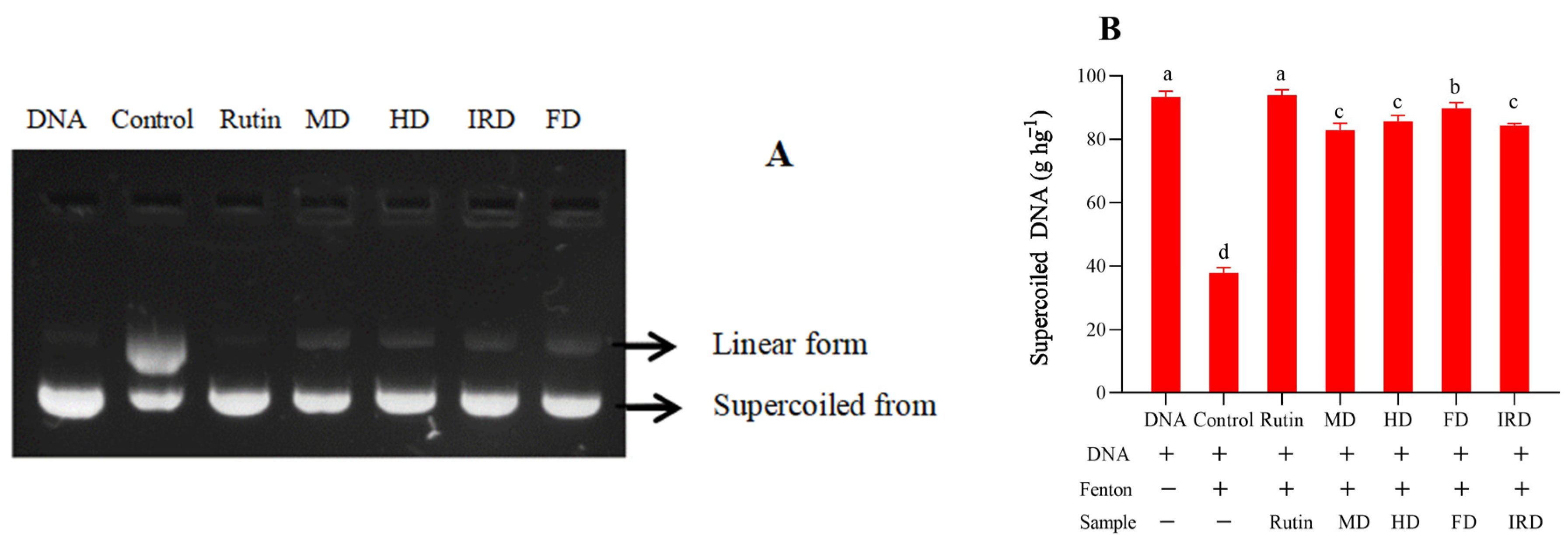
2.7. DNA Damage Protective Activity Measurement
2.8. Enzyme Inhibitory Ability Measurements
2.9. Cell Experiments
2.10. Data Analysis
3. Results and Discussion
3.1. Drying Time, Color, Microstructure, and Rehydration
3.2. Phenolic Compounds
3.3. Polysaccharides and Their Uronic Acid Content
3.4. Antioxidant Activity
3.5. Protective Activity against DNA Oxidation
3.6. Inhibition Activities against α-Amylase and α-Glucosidase
3.7. Glucose Metabolism in HL-7702 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, H.; Er-bu, A.G.A.; Dongzhi, Z.; Xie, H.; Ye, B.; He, J. Alkaloids from Dendrobium and their biosynthetic pathway, biological activity and total synthesis. Phytomedicine 2022, 102, 154132. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, L.; Liu, J.; Liang, J.; Si, J.; Wu, S. Dendrobium officinale leaves as a new antioxidant source. J. Funct. Foods 2017, 37, 400–415. [Google Scholar] [CrossRef]
- Liu, J.J.; Liu, Z.P.; Zhang, X.F.; Si, J.P. Effects of various processing methods on the metabolic profile and antioxidant activity of Dendrobium catenatum Lindley leaves. Metabolites 2021, 11, 351. [Google Scholar] [CrossRef]
- Wang, Y.H. Traditional uses, chemical constituents, pharmacological activities, and toxicological effects of Dendrobium leaves: A review. J. Ethnopharmacol. 2021, 270, 113851. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Lin, Y.; Xie, H.; Farag, M.A.; Feng, S.; Li, J.; Shao, P. Dendrobium officinale leaf polysaccharides ameliorated hyperglycemia and promoted gut bacterial associated SCFAs to alleviate type 2 diabetes in adult mice. Food Chem. X 2022, 13, 100207. [Google Scholar] [CrossRef]
- Xie, H.; Fang, J.; Farag, M.A.; Li, Z.; Sun, P.; Shao, P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chem. X 2022, 13, 100235. [Google Scholar] [CrossRef]
- Chen, D.; Xing, B.; Yi, H.; Li, Y.; Zheng, B.; Wang, Y.; Shao, Q. Effects of different drying methods on appearance, microstructure, bioactive compounds and aroma compounds of saffron (Crocus sativus L.). LWT 2020, 120, 108913. [Google Scholar] [CrossRef]
- Saikia, J.; Sarkar, A.; Washmin, N.; Borah, T.; Das, B.; Konwar, P.; Siga, A.; Banik, D. Effect of postharvest drying on physicochemical properties, volatile yield, composition, and sensory attributes of Alpinia zerumbet (shell ginger) rhizome. Ind. Crop. Prod. 2023, 198, 116719. [Google Scholar] [CrossRef]
- Lemus-Mondaca, R.; Vega-Gálvez, A.; Rojas, P.; Stucken, K.; Delporte, C.; Valenzuela-Barra, G.; Jagus, R.J.; Agüero, M.V.; Pasten, A. Antioxidant, antimicrobial and anti-inflammatory potential of Stevia rebaudiana leaves: Effect of different drying methods. J. Appl. Res. Med. Aromat. Plants 2018, 11, 37–46. [Google Scholar] [CrossRef]
- Zhao, T.; Dong, Q.; Zhou, H.; Yang, H. Drying kinetics, physicochemical properties, antioxidant activity and antidiabetic potential of Sargassum fusiforme processed under four drying techniques. LWT 2022, 163, 113578. [Google Scholar] [CrossRef]
- Si, C.; Zeng, D.; Yu, Z.; Teixeira da Silva, J.A.; Duan, J.; He, C.; Zhang, J. Transcriptomic and metabolomic analyses reveal the main metabolites in Dendrobium officinale leaves during the harvesting period. Plant Physiol. Biochem. 2022, 190, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Feng, K.L.; Wei, S.Y.; Xiang, X.R.; Ding, Y.; Li, H.Y.; Zhao, L.; Qin, W.; Gan, R.-Y.; Wu, D.T. Comparison of structural characteristics and bioactivities of polysaccharides from loquat leaves prepared by different drying techniques. Int. J. Biol. Macromol. 2020, 145, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Zheng, E.; Zhao, T.; Hu, S.; Yang, H. Evaluation of physicochemical properties, equivalent umami concentration and antioxidant activity of Coprinus comatus prepared by different drying methods. LWT 2022, 162, 113479. [Google Scholar] [CrossRef]
- Li, P.; Zhou, L.; Mou, Y.; Mao, Z. Extraction optimization of polysaccharide from Zanthoxylum bungeanum using RSM and its antioxidant activity. Int. J. Biol. Macromol. 2015, 72, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Zengin, G.; Sarikurkcu, C.; Gunes, E.; Uysal, A.; Ceylan, R.; Uysal, S.; Gungor, H.; Aktumsek, A. Two Ganoderma species: Profiling of phenolic compounds by HPLC-DAD, antioxidant, antimicrobial and inhibitory activities on key enzymes linked to diabetes mellitus, Alzheimer’’s disease and skin disorders. Food Funct. 2015, 6, 2794–2802. [Google Scholar] [CrossRef]
- Gu, J.F.; Zheng, Z.Y.; Yuan, J.R.; Zhao, B.J.; Wang, C.F.; Zhang, L.; Xu, Q.Y.; Yin, G.W.; Feng, L.; Jia, X.B. Comparison on hypoglycemic and antioxidant activities of the fresh and dried Portulaca oleracea L. in insulin-resistant HepG2 cells and streptozotocin-induced C57BL/6J diabetic mice. J. Ethnopharmacol. 2015, 161, 214–223. [Google Scholar] [CrossRef]
- Bai, C.; Chen, R.; Tan, L.; Bai, H.; Tian, L.; Lu, J.; Gao, M.; Sun, H.; Chi, Y. Effects of multi-frequency ultrasonic on the physicochemical properties and bioactivities of polysaccharides from different parts of ginseng. Int. J. Biol. Macromol. 2022, 206, 896–910. [Google Scholar] [CrossRef]
- Fan, X.; Jiao, G.; Pang, T.; Wen, T.; He, Z.; Han, J.; Zhang, F.; Chen, W. Ameliorative effects of mangiferin derivative TPX on insulin resistance via PI3K/AKT and AMPK signaling pathways in human HepG2 and HL-7702 hepatocytes. Phytomedicine 2023, 114, 154740. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, D.; Gao, H. Kinetics, physicochemical properties, and antioxidant activities of Angelica keiskei processed under four drying conditions. LWT 2018, 98, 349–357. [Google Scholar] [CrossRef]
- An, N.N.; Sun, W.H.; Li, B.Z.; Wang, Y.; Shang, N.; Lv, W.Q.; Li, D.; Wang, L.J. Effect of different drying techniques on drying kinetics, nutritional components, antioxidant capacity, physical properties and microstructure of edamame. Food Chem. 2022, 373, 131412. [Google Scholar] [CrossRef]
- Altay, K.; Hayaloglu, A.A.; Dirim, S.N. Determination of the drying kinetics and energy efficiency of purple basil (Ocimum basilicum L.) leaves using different drying methods. Heat Mass Transf. 2019, 55, 2173–2184. [Google Scholar] [CrossRef]
- Yang, S.J.; Sun, M.; Yang, Q.Y.; Ma, R.Y.; Zhang, J.L.; Zhang, S.B. Two strategies by epiphytic orchids for maintaining water balance: Thick cuticles in leaves and water storage in pseudobulbs. AoB Plants 2016, 8, plw046. [Google Scholar] [CrossRef] [PubMed]
- Polat, S.; Guclu, G.; Kelebek, H.; Keskin, M.; Selli, S. Comparative elucidation of colour, volatile and phenolic profiles of black carrot (Daucus carota L.) pomace and powders prepared by five different drying methods. Food Chem. 2022, 369, 130941. [Google Scholar] [CrossRef] [PubMed]
- Telfser, A.; Gómez Galindo, F. Effect of reversible permeabilization in combination with different drying methods on the structure and sensorial quality of dried basil (Ocimum basilicum L.) leaves. LWT 2019, 99, 148–155. [Google Scholar] [CrossRef]
- Wang, P.; Tian, B.; Ge, Z.; Feng, J.; Wang, J.; Yang, K.; Sun, P.; Cai, M. Ultrasound and deep eutectic solvent as green extraction technology for recovery of phenolic compounds from Dendrobium officinale leaves. Process Biochem. 2023, 128, 1–11. [Google Scholar] [CrossRef]
- Sim, Y.Y.; Nyam, K.L. Effect of different drying methods on the physical properties and antioxidant activities of Hibiscus cannabinus leaves. J. Food Meas. Charact. 2019, 13, 1279–1286. [Google Scholar] [CrossRef]
- Li, R.; Shang, H.; Wu, H.; Wang, M.; Duan, M.; Yang, J. Thermal inactivation kinetics and effects of drying methods on the phenolic profile and antioxidant activities of chicory (Cichorium intybus L.) leaves. Sci. Rep. 2018, 8, 9529. [Google Scholar] [CrossRef]
- Zhong, C.; Tian, W.; Chen, H.; Yang, Y.; Xu, Y.; Chen, Y.; Chen, P.; Zhu, S.; Li, P.; Du, B. Structural characterization and immunoregulatory activity of polysaccharides from Dendrobium officinale leaves. J. Food Biochem. 2022, 46, e14023. [Google Scholar] [CrossRef]
- Yang, K.; Lu, T.; Zhan, L.; Zhou, C.; Zhang, N.; Lei, S.; Wang, Y.; Yang, J.; Yan, M.; Lv, G.; et al. Physicochemical characterization of polysaccharide from the leaf of Dendrobium officinale and effect on LPS induced damage in GES-1 cell. Int. J. Biol. Macromol. 2020, 149, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; He, D.; Ni, X.; Zhou, H.; Yang, H. Comparative study on phenolic compounds, triterpenoids, and antioxidant activity of Ganoderma lucidum affected by different drying methods. J. Food Meas. Charact. 2019, 13, 3198–3205. [Google Scholar] [CrossRef]
- An, K.; Wu, J.; Xiao, H.; Hu, T.; Yu, Y.; Yang, W.; Xiao, G.; Xu, Y. Effect of various drying methods on the physicochemical characterizations, antioxidant activities and hypoglycemic activities of lychee (Litchi chinensis Sonn.) pulp polysaccharides. Int. J. Biol. Macromol. 2022, 220, 510–519. [Google Scholar] [CrossRef]
- Yan, J.K.; Wu, L.X.; Qiao, Z.R.; Cai, W.D.; Ma, H. Effect of different drying methods on the product quality and bioactive polysaccharides of bitter gourd (Momordica charantia L.) slices. Food Chem. 2019, 271, 588–596. [Google Scholar] [CrossRef]
- Saifullah, M.; McCullum, R.; McCluskey, A.; Van Vuong, Q. Effect of drying techniques and operating conditions on the retention of color, phenolics, and antioxidant properties in dried lemon scented tea tree (Leptospermum petersonii) leaves. J. Food Process. Preserv. 2021, 45, e15257. [Google Scholar] [CrossRef]
- Guo, Y.R.; An, Y.M.; Jia, Y.X.; Xu, J.G. Effect of drying methods on chemical composition and biological activity of essential oil from Cumin (Cuminum cyminum L.). J. Essent. Oil Bear. Plants 2018, 21, 1295–1302. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Lu, S.; Tang, W.; Zhou, Y.; Quek, S.Y. Effects of different drying methods on phenolic components and in vitro hypoglycemic activities of pulp extracts from two Chinese bayberry (Myrica rubra Sieb. et Zucc.) cultivars. Food Sci. Hum. Wellness 2022, 11, 366–373. [Google Scholar] [CrossRef]
- Lv, M.; Liang, Q.; He, X.; Du, X.; Liu, Y.; Liu, Y.; Fang, C. Hypoglycemic effects of dendrobium officinale leaves. Front. Pharmacol. 2023, 14, 1163028. [Google Scholar] [CrossRef]
- Gowd, V.; Xie, L.; Sun, C.; Chen, W. Phenolic profile of bayberry followed by simulated gastrointestinal digestion and gut microbiota fermentation and its antioxidant potential in HepG2 cells. J. Funct. Foods 2020, 70, 103987. [Google Scholar] [CrossRef]
- Shen, W.; Xu, Y.; Lu, Y.H. Inhibitory effects of citrus flavonoids on starch digestion and antihyperglycemic effects in HepG2 Cells. J. Agric. Food Chem. 2012, 60, 9609–9619. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Chen, Y.; Li, W.; Lu, Y. Anti-aging properties of Dendrobium nobile Lindl.: From molecular mechanisms to potential treatments. J. Ethnopharmacol. 2020, 257, 112839. [Google Scholar] [CrossRef] [PubMed]



| Drying Method | L* | a* | b* | ΔE | Rehydration Ratio |
|---|---|---|---|---|---|
| Fresh | 30.49 ± 0.14 a | −4.28 ± 0.25 e | 13.69 ± 0.88 a | - | - |
| HD | 25.38 ± 0.14 b | 2.17 ± 0.10 c | 12.07 ± 0.19 b | 8.43 ± 0.32 c | 3.74 ± 0.10 c |
| MD | 19.60 ± 0.77 d | 2.55 ± 0.15 b | 5.43 ± 0.11 d | 15.31 ± 0.55 a | 3.63 ± 0.21 d |
| IRD | 22.89 ± 0.22 c | 3.43 ± 0.08 a | 11.48 ± 0.28 c | 11.09 ± 0.05 b | 4.03 ± 0.14 b |
| FD | 30.52 ± 0.85 a | −1.46 ± 0.17 d | 13.27 ± 0.35 a | 2.95 ± 0.07 d | 5.59 ± 0.07 a |
| Assays | Drying Methods | |||
|---|---|---|---|---|
| HD | IRD | MD | FD | |
| Rutin (μg g−1) | 2311.96 ± 4.35 c | 2516.91 ± 7.07 b | 2375.84 ± 103.38 c | 4267.30 ± 21.51 a |
| Vicenin II (μg g−1) | 298.84 ± 0.52 c | 438.66 ± 1.32 b | 267.30 ± 9.37 d | 540.49 ± 2.18 a |
| Vicenin I (μg g−1) | 195.74 ± 4.10 c | 302.26 ± 2.46 b | 170.24 ± 0.49 d | 375.54 ± 11.58 a |
| Schaftoside (μg g−1) | 179.58 ± 0.75 c | 236.45 ± 1.84 b | 163.88 ± 11.50 d | 295.83 ± 4.22 a |
| Isoschaftoside (μg g−1) | 171.21 ± 0.27 c | 255.67 ± 1.24 b | 153.94 ± 3.90 d | 310.63 ± 1.81 a |
| Isovitexin (μg g−1) | 32.01 ± 0.20 d | 39.93 ± 0.45 b | 33.78 ± 1.19 c | 60.64 ± 0.79 a |
| Apigenin 6-C-α-L-arabinopyranosyl-8-C-β-D-xylopyranoside (μg g−1) | 52.33 ± 0.20 d | 73.83 ± 0.19 b | 55.04 ± 1.59 c | 91.28 ± 0.51 a |
| Kaempferol-3-rutinoside (μg g−1) | 42.94 ± 0.47 c | 54.31 ± 0.90 b | 40.68 ± 0.85 c | 112.36 ± 10.15 a |
| Ferulic acid (μg g−1) | 2.45 ± 0.18 d | 7.06 ± 0.16 b | 3.67 ± 0.37 c | 11.17 ± 0.16 a |
| Quercetin (μg g−1) | Nd | Nd | Nd | Nd |
| Total (μg g−1) | 3287.06 ± 11.04 | 3925.08 ± 15.63 | 3264.37 ± 132.64 | 6065.24 ± 52.91 |
| DPPH radical scavenging (mg AsAE g−1) | 5.69 ± 0.08 c | 6.44 ± 0.07 b | 6.43 ± 0.11 b | 7.26 ± 0.12 a |
| ABTS radical scavenging (mg AsAE g−1) | 7.13 ± 0.02 b | 6.13 ± 0.16 c | 6.06 ± 0.09 c | 7.43 ± 0.19 a |
| Reducing Fe3+ power (mg AsAE g−1) | 0.88 ± 0.01 b | 0.90 ± 0.01 b | 0.85 ± 0.01 c | 0.98 ± 0.02 a |
| Chelating Fe2+ ability (mg EDTAE g−1) | 66.26 ± 2.02 b | 65.37 ± 1.15 b | 67.29 ± 0.66 b | 83.65 ± 2.05 a |
| α-amylase inhibitory (mg ACE g−1) | 13.32 ± 0.33 c | 14.14 ± 0.06 b | 14.36 ± 0.14 b | 17.65 ± 0.48 a |
| α-glucosidase inhibitory (mg ACE g−1) | 3.83 ± 0.46 d | 25.33 ± 2.55 b | 8.44 ± 1.01 c | 44.87 ± 1.93 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, G.; Dong, H.; Liu, S.; Wu, W.; Yang, H. Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques. Antioxidants 2023, 12, 1911. https://doi.org/10.3390/antiox12111911
Cai G, Dong H, Liu S, Wu W, Yang H. Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques. Antioxidants. 2023; 12(11):1911. https://doi.org/10.3390/antiox12111911
Chicago/Turabian StyleCai, Gonglin, Hangmeng Dong, Shoulong Liu, Weijie Wu, and Hailong Yang. 2023. "Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques" Antioxidants 12, no. 11: 1911. https://doi.org/10.3390/antiox12111911
APA StyleCai, G., Dong, H., Liu, S., Wu, W., & Yang, H. (2023). Comparative Evaluation of the Physiochemical Properties, and Antioxidant and Hypoglycemic Activities of Dendrobium officinale Leaves Processed Using Different Drying Techniques. Antioxidants, 12(11), 1911. https://doi.org/10.3390/antiox12111911





