Physiological and RNA-Seq Analyses on Exogenous Strigolactones Alleviating Drought by Improving Antioxidation and Photosynthesis in Wheat (Triticum aestivum L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Plant Treatment and Sampling
2.3. Measurement of Dry Weight and Root Phenotype
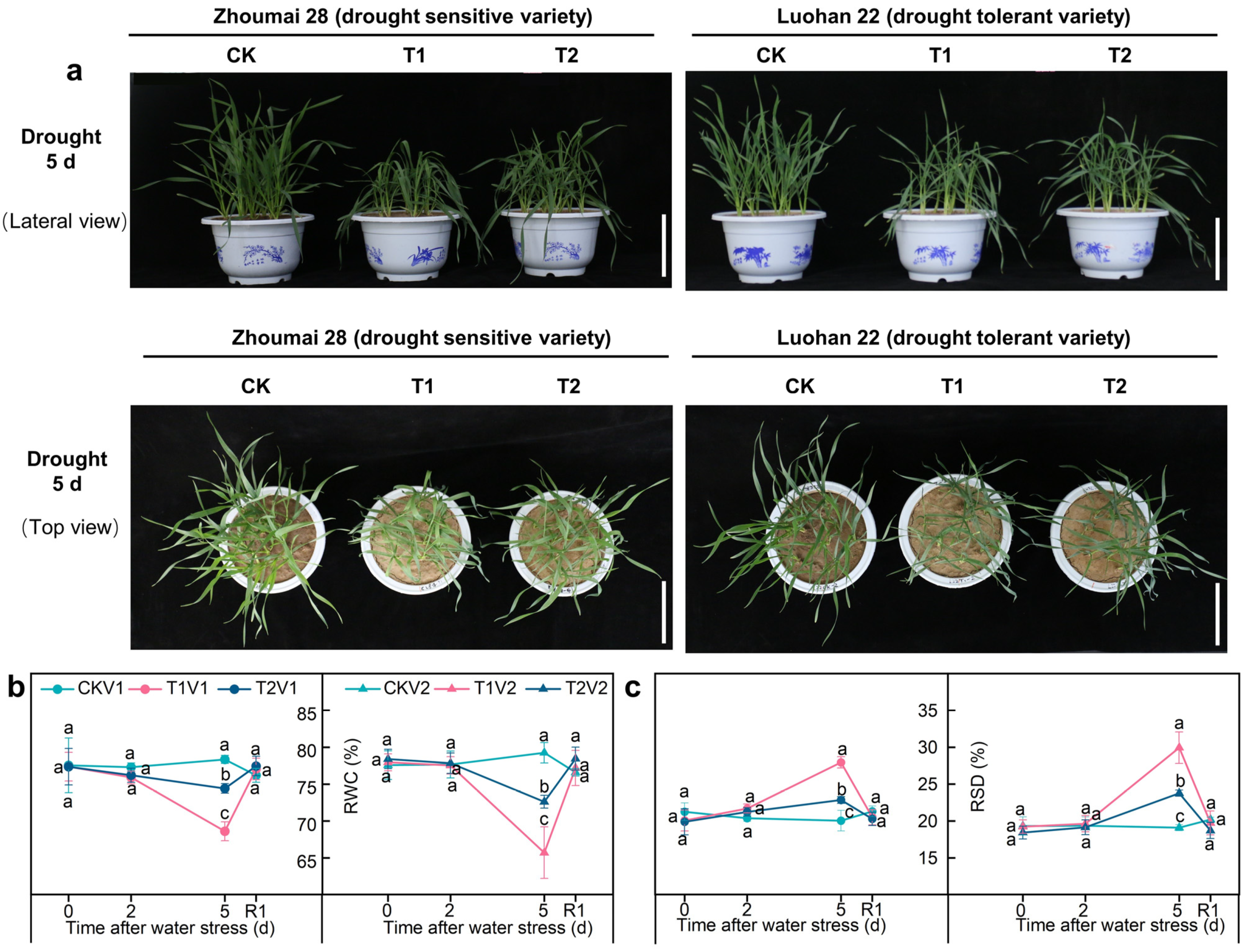
2.4. Determination of Leaf Water Relations
2.5. Determination of Content of Chlorophyll
2.6. Measurement of Photosynthetic Parameters
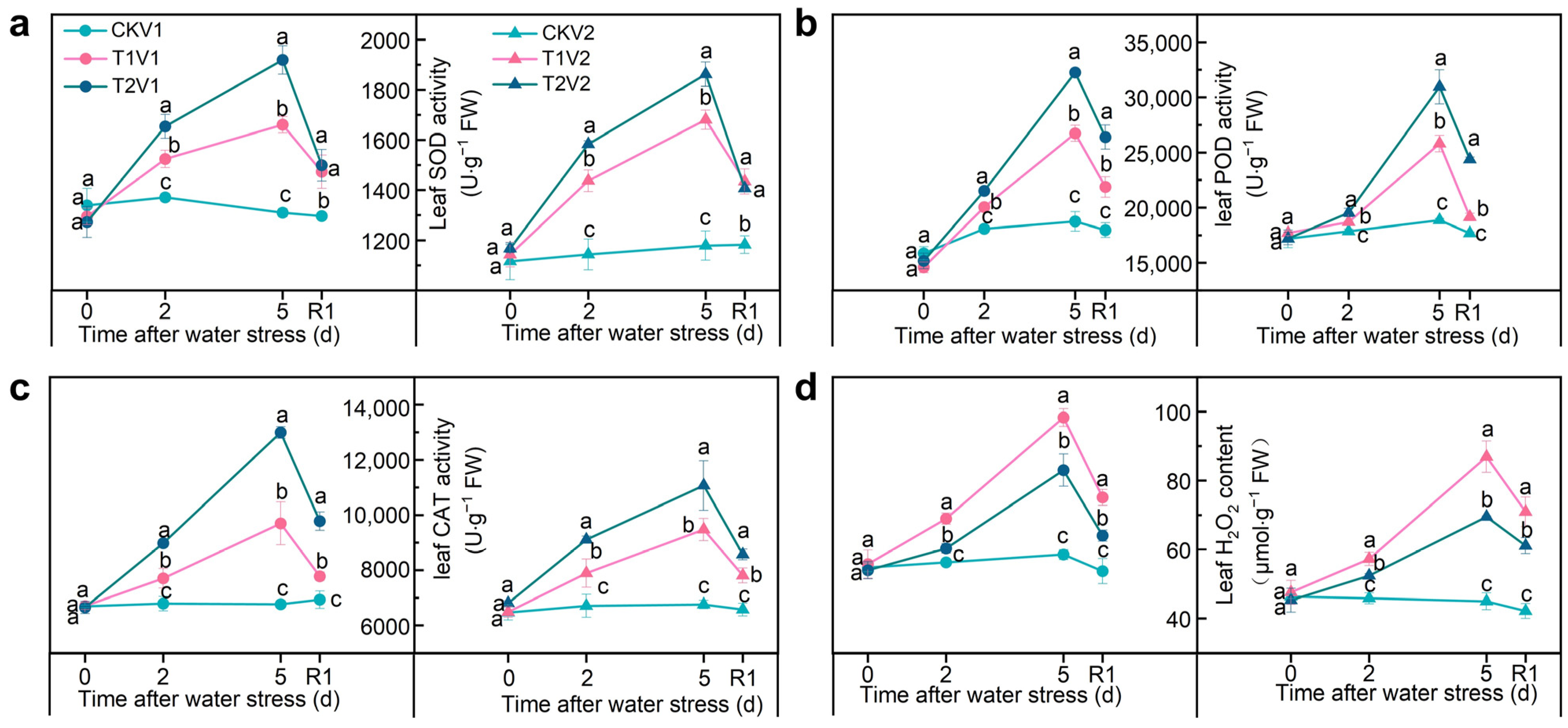
2.7. Determination of Enzymatic Antioxidant Activity and H2O2 and MDA Content in Leaves
2.8. RNA Sequencing
2.9. Verification of Gene Expression by qRT-PCR
2.10. Statistical Analysis
3. Results
3.1. SLs Improving Wheat Phenotypes and Leaf Water Relations under Drought Stress
3.2. SLs Increasing Chlorophyll Content and Photosynthesis Rate
3.3. SLs Improving Antioxidant Capacity to Scavenge ROS under Drought Stress
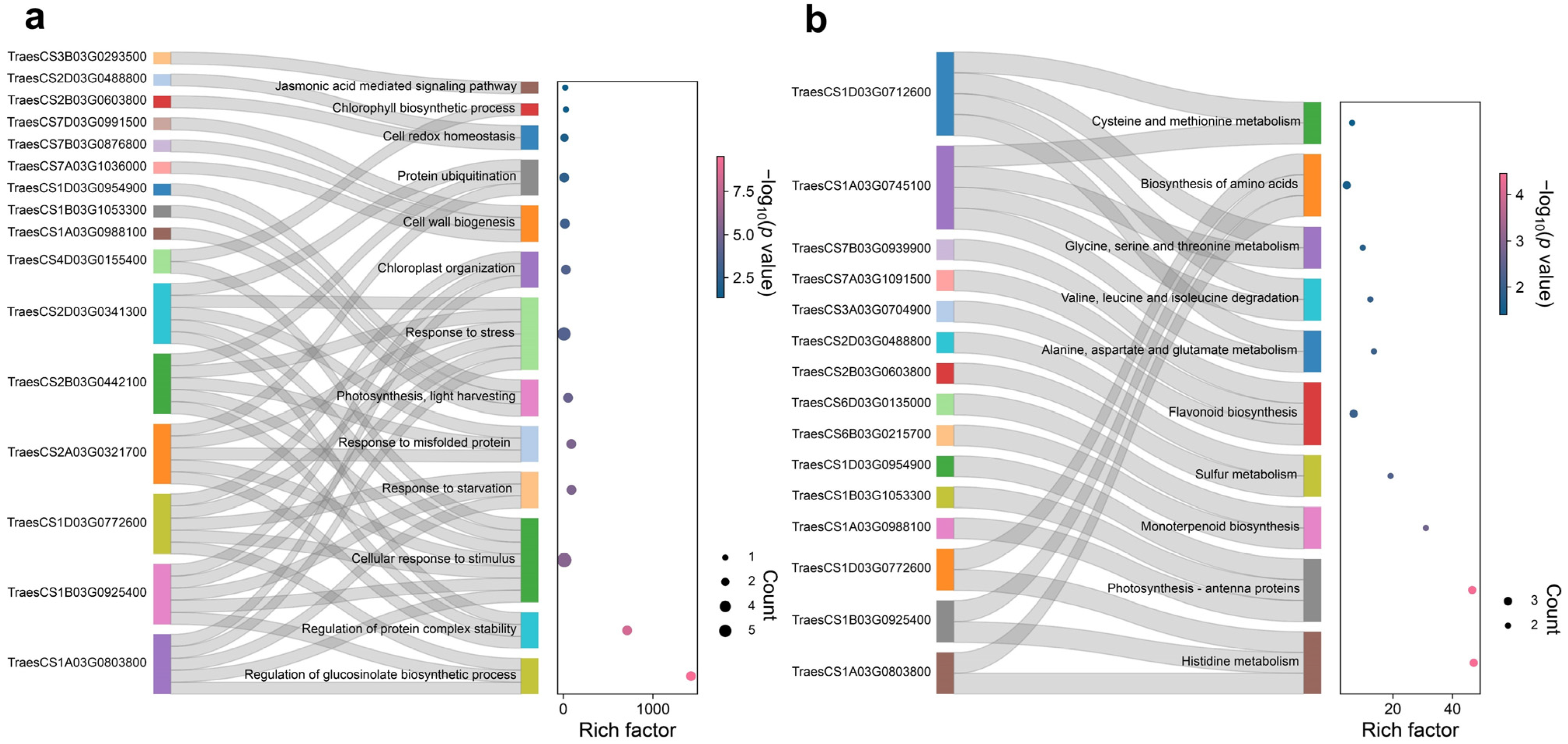
3.4. Transcriptional Regulation of SLs-Mediated Drought Relief in Wheat Leaves
3.5. K-Means Cluster Identifying the Gene Clusters Associated with Drought Tolerance
3.6. Identification of WGCNA Modules Associated with Drought Tolerance in Wheat Leaves
3.7. SLs Upregulating Expression of Antioxidant-Related Genes
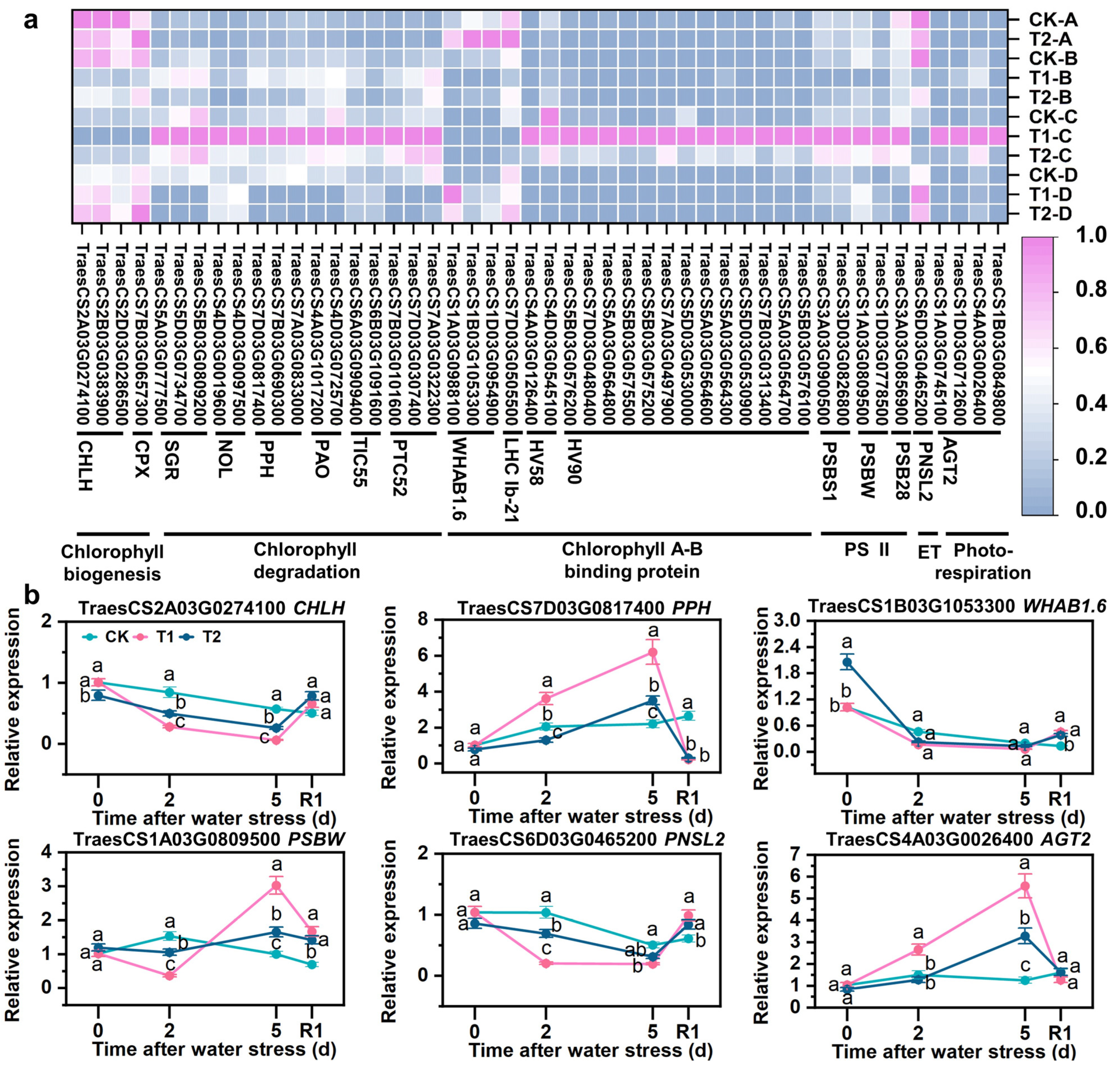
3.8. SLs Improved Expression of Chlorophyll- and Photosynthesis-Related Genes
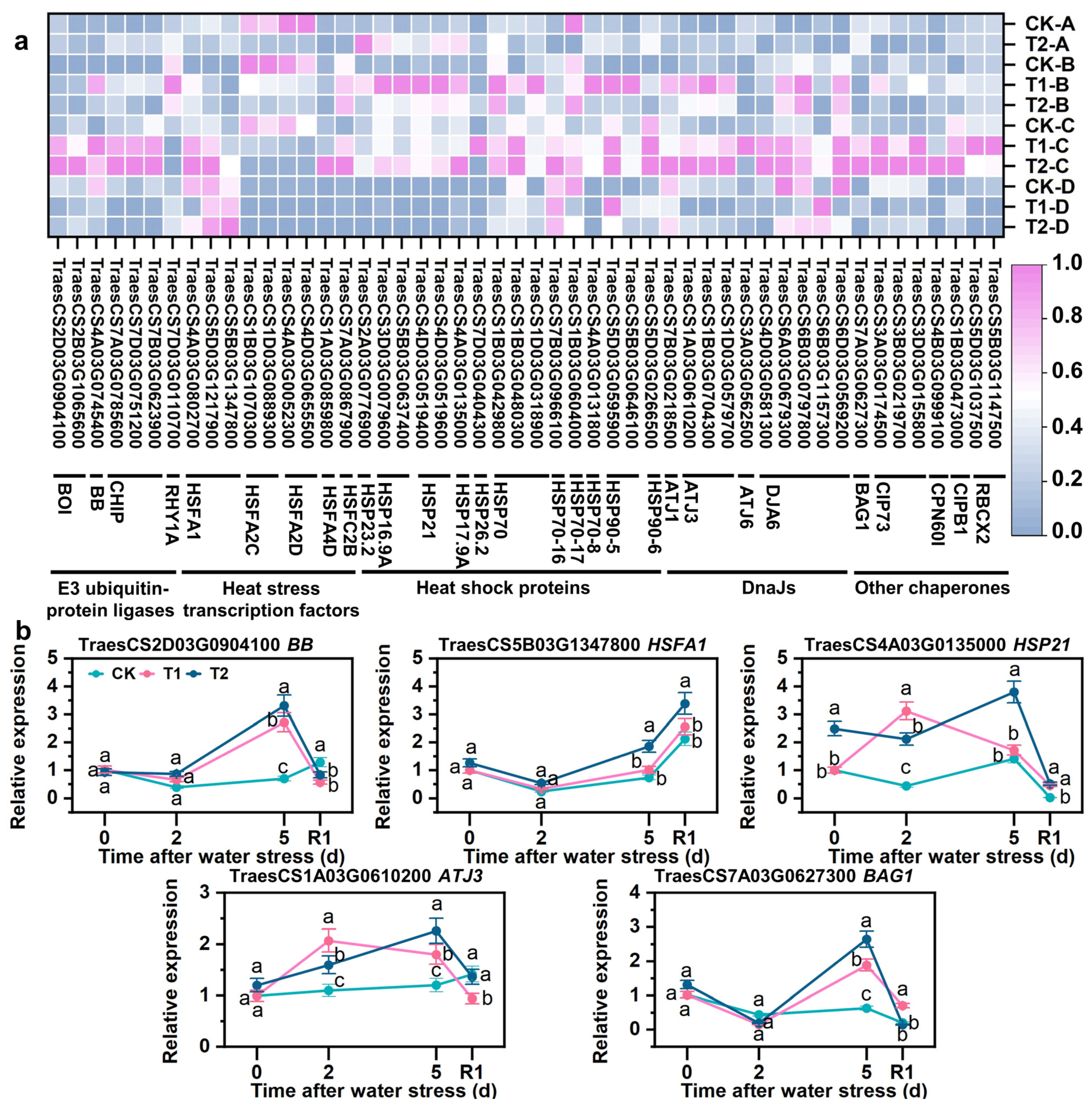
3.9. SLs Upregulating Expression of Repair Protein Misfolding-Related Genes
4. Discussion
4.1. SLs Improved Scavenging Capacity of ROS Responding to Drought Stress
4.2. SLs Restrained Degradation of Chlorophylls and Increased Biosynthesis
4.3. SLs Promoted Photosynthesis by Increasing Electron Transport and Inhibiting Photorespiration
4.4. HSFs and Chaperones Collaborated to Maintain the Turnover of Proteins
4.5. Physiological, Phenotypic, and Transcriptome Analysis Revealed the Comprehensive Mechanism of Sls Alleviating Drought Stress in Wheat
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Sustain. Agric. 2009, 29, 153–188. [Google Scholar]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Feng, S.; Semenov, M.A.; Olesen, J.E.; Kersebaum, K.C.; Rötter, R.P.; Semerádová, D.; Klem, K.; Huang, W.; Ruiz-Ramos, M.; et al. Mitigation efforts will not fully alleviate the increase in water scarcity occurrence probability in wheat-producing areas. Sci. Adv. 2019, 5, eaau2406. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 01147. [Google Scholar] [CrossRef] [PubMed]
- Ru, C.; Hu, X.; Chen, D.; Wang, W.; Zhen, J. Photosynthetic, antioxidant activities, and osmoregulatory responses in winter wheat differ during the stress and recovery periods under heat, drought, and combined stress. Plant Sci. 2023, 327, 111557. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.; Dang, P.; Gao, L.; Wang, J.; Tao, J.; Qin, X.; Feng, B.; Gao, J. How does the environment affect wheat yield and protein content response to drought? a meta-analysis. Front. Plant Sci. 2022, 13, 896985. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Balzer, C.; Hill, J.; Befort, B.L. Global food demand and the sustainable intensification of agriculture. Proc. Natl. Acad. Sci. USA 2011, 108, 20260–20264. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Chen, J.; Wang, X.; Cai, J.; Zhou, Q.; Dai, T.; Cao, W.; Jiang, D. Parental drought-priming enhances tolerance to post-anthesis drought in offspring of wheat. Front. Plant Sci. 2018, 9, 00261. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Sun, M.; Shafiq, F.; Khalilzadeh, R.; Gao, Z. Soil water content and wheat yield in response to tillage and precipitation in the dry, normal, and wet years at the Loess Plateau. Int. J. Plant Prod. 2021, 15, 655–668. [Google Scholar] [CrossRef]
- Ahmadian, K.; Jalilian, J.; Pirzad, A. Nano-fertilizers improved drought tolerance in wheat under deficit irrigation. Agric. Water Manag. 2021, 244, 106544. [Google Scholar] [CrossRef]
- Shemi, R.; Wang, R.; Gheith, E.S.M.S.; Hussain, H.A.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Role of exogenous-applied salicylic acid, zinc and glycine betaine to improve drought-tolerance in wheat during reproductive growth stages. BMC Plant Biol. 2021, 21, 574. [Google Scholar] [CrossRef] [PubMed]
- Valluru, R.; Davies, W.J.; Reynolds, M.P.; Dodd, I.C. Foliar abscisic acid-to-ethylene accumulation and response regulate shoot growth sensitivity to mild drought in wheat. Front. Plant Sci. 2016, 7, 00461. [Google Scholar] [CrossRef] [PubMed]
- Javadipour, Z.; Balouchi, H.; Movahhedi, M.; Yadavi, A. Roles of methyl jasmonate in improving growth and yield of two varieties of bread wheat (Triticum aestivum) under different irrigation regimes. Agric. Water Manag. 2019, 222, 336–345. [Google Scholar] [CrossRef]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Aliche, E.B.; Screpanti, C.; De Mesmaeker, A.; Munnik, T.; Bouwmeester, H.J. Science and application of strigolactones. New Phytol. 2020, 227, 1001–1011. [Google Scholar] [CrossRef]
- Gamir, J.; Torres-Vera, R.; Rial, C.; Berrio, E.; de Souza Campos, P.M.; Varela, R.M.; Macías, F.A.; Pozo, M.J.; Flors, V.; López-Ráez, J.A. Exogenous strigolactones impact metabolic profiles and phosphate starvation signalling in roots. Plant Cell Environ. 2020, 43, 1655–1668. [Google Scholar] [CrossRef]
- Chen, C.; Xu, L.K.; Zhang, X.; Wang, H.; Nisa, Z.-u.; Jin, X.; Yu, L.; Jing, L.; Chen, C. Exogenous strigolactones enhance tolerance in soybean seedlings in response to alkaline stress. Physiol. Plant. 2022, 174, e13784. [Google Scholar] [CrossRef]
- Sedaghat, M.; Emam, Y.; Mokhtassi-Bidgoli, A.; Hazrati, S.; Lovisolo, C.; Visentin, I.; Cardinale, F.; Tahmasebi-Sarvestani, Z. The potential of the synthetic strigolactone analogue GR24 for the maintenance of photosynthesis and yield in winter wheat under drought: Investigations on the mechanisms of action and delivery modes. Plants 2021, 10, 1223. [Google Scholar] [CrossRef]
- Sedaghat, M.; Sarvestani, Z.T.; Emam, Y.; Bidgoli, A.M.; Sorooshzadeh, A. Foliar-applied GR24 and salicylic acid enhanced wheat drought tolerance. Russ. J. Plant Physiol. 2020, 67, 733–739. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Yang, X.; Li, X.; Zang, H.; Fang, B. Effects of wheat grain filling and yield formation by exogenous strigolactone under drought condition. J. Biobased Mater. Bioenergy 2021, 15, 218–223. [Google Scholar] [CrossRef]
- Ahmed, K.; Shabbir, G.; Ahmed, M.; Shah, K.N. Phenotyping for drought resistance in bread wheat using physiological and biochemical traits. Sci. Total Environ. 2020, 729, 139082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Ma, C.; Su, M.; Wang, J.; Zheng, S.; Zhang, T. Exogenous strigolactones alleviate the photosynthetic inhibition and oxidative damage of cucumber seedlings under salt stress. Sci. Hortic. 2022, 297, 110962. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Han, Z.; Feng, H.; Wang, Y.; Kang, J.; Han, X.; Wang, L.; Wang, C.; Li, H.; et al. Analysis of physiological indicators associated with drought tolerance in wheat under drought and re-watering conditions. Antioxidants 2022, 11, 2266. [Google Scholar] [CrossRef] [PubMed]
- Vuković, R.; Čamagajevac, I.Š.; Vuković, A.; Šunić, K.; Begović, L.; Mlinarić, S.; Sekulić, R.; Sabo, N.; Španić, V. Physiological, biochemical and molecular response of different winter wheat varieties under drought stress at germination and seedling growth stage. Antioxidants 2022, 11, 693. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Al Mahmud, J.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Borhannuddin Bhuyan, M.H.M.; Anee, T.I.; Parvin, K.; Nahar, K.; Al Mahmud, J.; Fujita, M. Regulation of ascorbate-glutathione pathway in mitigating oxidative damage in plants under abiotic stress. Antioxidants 2019, 8, 384. [Google Scholar] [CrossRef]
- Sainz, M.M.; Filippi, C.V.; Eastman, G.; Sotelo-Silveira, J.; Borsani, O.; Sotelo-Silveira, M. Analysis of thioredoxins and glutaredoxins in soybean: Evidence of translational regulation under water restriction. Antioxidants 2022, 11, 1622. [Google Scholar] [CrossRef]
- Li, C.; Lu, X.; Liu, Y.; Xu, J.; Yu, W. Strigolactone alleviates the adverse effects of salt stress on seed germination in cucumber by enhancing antioxidant capacity. Antioxidants 2023, 12, 1043. [Google Scholar] [CrossRef]
- Sattar, A.; Ul-Allah, S.; Ijaz, M.; Sher, A.; Butt, M.; Abbas, T.; Irfan, M.; Fatima, T.; Alfarraj, S.; Alharbi, S.A. Exogenous application of strigolactone alleviates drought stress in maize seedlings by regulating the physiological and antioxidants defense mechanisms. Cereal Res. Commun. 2022, 50, 263–272. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Haider, G.; Awan, M.I.; Gohar, M.; Munsif, F.; Ahmad, I. Strigolactone-mediated oxidative stress alleviation in Brassica rapa through upregulating antioxidant system under water deficit conditions. J. Plant Growth Regul. 2023, 42, 4675–4687. [Google Scholar] [CrossRef]
- Wang, W.N.; Min, Z.; Wu, J.R.; Liu, B.C.; Xu, X.L.; Fang, Y.L.; Ju, Y.L. Physiological and transcriptomic analysis of Cabernet Sauvginon (Vitis vinifera L.) reveals the alleviating effect of exogenous strigolactones on the response of grapevine to drought stress. Plant Physiol. Biochem. 2021, 167, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gu, M.; Lu, J.; Li, X.; Liu, D.; Li, X.; Wang, L. GR24 alleviates the adverse effects of drought stress on physiology and photosystem II function in alfalfa (Medicago sativa L.). Grassl. Sci. 2022, 69, 113–119. [Google Scholar] [CrossRef]
- Moosavi, S.S.; Abdi, F.; Abdollahi, M.R.; Tahmasebi-Enferadi, S.; Maleki, M. Phenological, morpho-physiological and proteomic responses of Triticum boeoticum to drought stress. Plant Physiol. Biochem. 2020, 156, 95–104. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, Y.; Zhang, J.; Chao, M.; Xie, K.; Zhang, C.; Sun, F.; Liu, S.; Xi, Y. Proteomic analysis of the similarities and differences of soil drought and polyethylene glycol stress responses in wheat (Triticum aestivum L.). Plant Mol. Biol. 2019, 100, 391–410. [Google Scholar] [CrossRef]
- Roig-Oliver, M.; Fullana-Pericàs, M.; Bota, J.; Flexas, J. Adjustments in photosynthesis and leaf water relations are related to changes in cell wall composition in Hordeum vulgare and Triticum aestivum subjected to water deficit stress. Plant Sci. 2021, 311, 111015. [Google Scholar] [CrossRef]
- Min, Z.; Li, R.; Chen, L.; Zhang, Y.; Li, Z.; Liu, M.; Ju, Y.; Fang, Y. Alleviation of drought stress in grapevine by foliar-applied strigolactones. Plant Physiol. Biochem. 2019, 135, 99–110. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Feng, Q.; Zhang, J.; Han, X.; Zhang, L.; Yang, F.; Zhou, J. Effects of exogenous Strigolactone on the physiological and ecological characteristics of Pennisetum purpureum Schum. Seedlings under drought stress. BMC Plant Biol. 2022, 22, 578. [Google Scholar] [CrossRef]
- Feller, U. Drought stress and carbon assimilation in a warming climate: Reversible and irreversible impacts. J. Plant Physiol. 2016, 203, 84–94. [Google Scholar] [CrossRef]
- Yang, Y.; Gu, M.; Chen, J.; Zhang, R.; Liu, Z.; Shi, Y.; Liu, D.; Wang, L. Comparative transcriptomes reveal the mitigation effect of GR24 in alfalfa under drought stress. J. Plant Growth Regul. 2022, 42, 3150–3161. [Google Scholar] [CrossRef]
- Redekop, P.; Sanz-Luque, E.; Yuan, Y.; Villain, G.; Petroutsos, D.; Grossman, A.R. Transcriptional regulation of photoprotection in dark-to-light transition—More than just a matter of excess light energy. Sci. Adv. 2022, 8, abn1832. [Google Scholar] [CrossRef] [PubMed]
- García-Cerdán, J.G.; Kovács, L.; Tõth, T.; Kereïche, S.; Aseeva, E.; Boekema, E.J.; Mamedov, F.; Funk, C.; Schröder, W.P. The PsbW protein stabilizes the supramolecular organization of photosystem II in higher plants. Plant J. 2011, 65, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Vijayalakshmi, J.; Peisach, D.; Hulsebus, B.; Olsen, L.J.; Saper, M.A. Crystal structure of photorespiratory alanine:glyoxylate aminotransferase 1 (AGT1) from Arabidopsis thaliana. Front. Plant Sci. 2019, 10, 01229. [Google Scholar] [CrossRef]
- Shikanai, T. Chloroplast NDH: A different enzyme with a structure similar to that of respiratory NADH dehydrogenase. Biochim. Biophys. Acta Bioenerg. 2016, 1857, 1015–1022. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Jonwal, S.; Verma, N.; Sinha, A.K. Regulation of photosynthetic light reaction proteins via reversible phosphorylation. Plant Sci. 2022, 321, 111312. [Google Scholar] [CrossRef]
- Gupta, R. The oxygen-evolving complex: A super catalyst for life on earth, in response to abiotic stresses. Plant Signal. Behav. 2020, 15, 1824721. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Guo, M.; Liu, J.H.; Ma, X.; Luo, D.X.; Gong, Z.H.; Lu, M.H. The plant heat stress transcription factors (HSFs): Structure, regulation, and function in response to abiotic stresses. Front. Plant Sci. 2016, 7, 114. [Google Scholar] [CrossRef]
- Priya, M.; Dhanker, O.P.; Siddique, K.H.M.; Hanumantha Rao, B.; Nair, R.M.; Pandey, S.; Singh, S.; Varshney, R.K.; Prasad, P.V.V.; Nayyar, H. Drought and heat stress-related proteins: An update about their functional relevance in imparting stress tolerance in agricultural crops. Theor. Appl. Genet. 2019, 132, 1607–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Qi, J.; Chi, Y.; Fan, B.; Yu, J.Q.; Chen, Z. E3 ubiquitin ligase CHIP and NBR1-mediated selective autophagy protect additively against proteotoxicity in plant stress re-sponses. PLoS Genet. 2014, 10, e1004116. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Y.; Tang, X.; Zhao, D.; Zhang, D.; Li, C.; Li, W.; Li, T.; Jiang, L. TaSINA2B, interacting with TaSINA1D, positively regulates drought tolerance and root growth in wheat (Triticum aestivum L.). Plant Cell Environ. 2023, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.L.; Yuan, S.N.; Zhang, H.N.; Zhang, Y.Y.; Zhang, Y.J.; Wang, G.Y.; Li, Y.Q.; Li, G.L. Heat-response patterns of the heat shock transcription factor family in advanced development stages of wheat (Triticum aestivum L.) and thermotolerance-regulation by TaHsfA2-10. BMC Plant Biol. 2020, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Poonia, A.K.; Mishra, S.K.; Sirohi, P.; Chaudhary, R.; Kanwar, M.; Germain, H.; Chauhan, H. Overexpression of wheat transcription factor (TaHsfA6b) provides thermotolerance in barley. Planta 2020, 252, 53. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, X.; Li, J.; Su, X.; Liu, J. Overexpression of a tobacco J-domain protein enhances drought tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2014, 83, 100–106. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Hu, W.; Xiang, Y.; Yan, M.; Wang, J. Overexpressing heat-shock protein OsHSP50.2 improves drought tolerance in rice. Plant Cell Rep. 2018, 37, 1585–1595. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Hu, N.; Zhou, S.; Xie, S.; Yang, J.; Ma, W.; Teng, Z.; Liang, W.; Wang, C.; Bu, M.; et al. Physiological and RNA-Seq Analyses on Exogenous Strigolactones Alleviating Drought by Improving Antioxidation and Photosynthesis in Wheat (Triticum aestivum L.). Antioxidants 2023, 12, 1884. https://doi.org/10.3390/antiox12101884
Song M, Hu N, Zhou S, Xie S, Yang J, Ma W, Teng Z, Liang W, Wang C, Bu M, et al. Physiological and RNA-Seq Analyses on Exogenous Strigolactones Alleviating Drought by Improving Antioxidation and Photosynthesis in Wheat (Triticum aestivum L.). Antioxidants. 2023; 12(10):1884. https://doi.org/10.3390/antiox12101884
Chicago/Turabian StyleSong, Miao, Naiyue Hu, Sumei Zhou, Songxin Xie, Jian Yang, Wenqi Ma, Zhengkai Teng, Wenxian Liang, Chunyan Wang, Mingna Bu, and et al. 2023. "Physiological and RNA-Seq Analyses on Exogenous Strigolactones Alleviating Drought by Improving Antioxidation and Photosynthesis in Wheat (Triticum aestivum L.)" Antioxidants 12, no. 10: 1884. https://doi.org/10.3390/antiox12101884
APA StyleSong, M., Hu, N., Zhou, S., Xie, S., Yang, J., Ma, W., Teng, Z., Liang, W., Wang, C., Bu, M., Zhang, S., Yang, X., & He, D. (2023). Physiological and RNA-Seq Analyses on Exogenous Strigolactones Alleviating Drought by Improving Antioxidation and Photosynthesis in Wheat (Triticum aestivum L.). Antioxidants, 12(10), 1884. https://doi.org/10.3390/antiox12101884




