Inhibitory Effects of Ehretia tinifolia Extract on the Excessive Oxidative and Inflammatory Responses in Lipopolysaccharide-Stimulated Mouse Kupffer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Methanol Extraction of E. tinifolia
2.2. Ultra-HPLC-Quadrupole Time-of-Flight Mass Spectrometry (UPLC-Q-TOF-MS) Analysis
2.3. Radical Scavenging (DPPH) Assay
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Lactate Dehydrogenase (LDH) Release Assay
2.7. Glutathione (GSH) Assay
2.8. Measurement of Nitric Oxide (NO) Production
2.9. Western Blotting
2.10. Enzyme-Linked Immunosorbent Assay (ELISA)
2.11. Fractionation of Nuclear and Cytoplasm Proteins
2.12. Immunofluorescence Staining
2.13. Statistical Analysis
3. Results
3.1. Rosmarinic Acid Is a Major Consituent of ETME
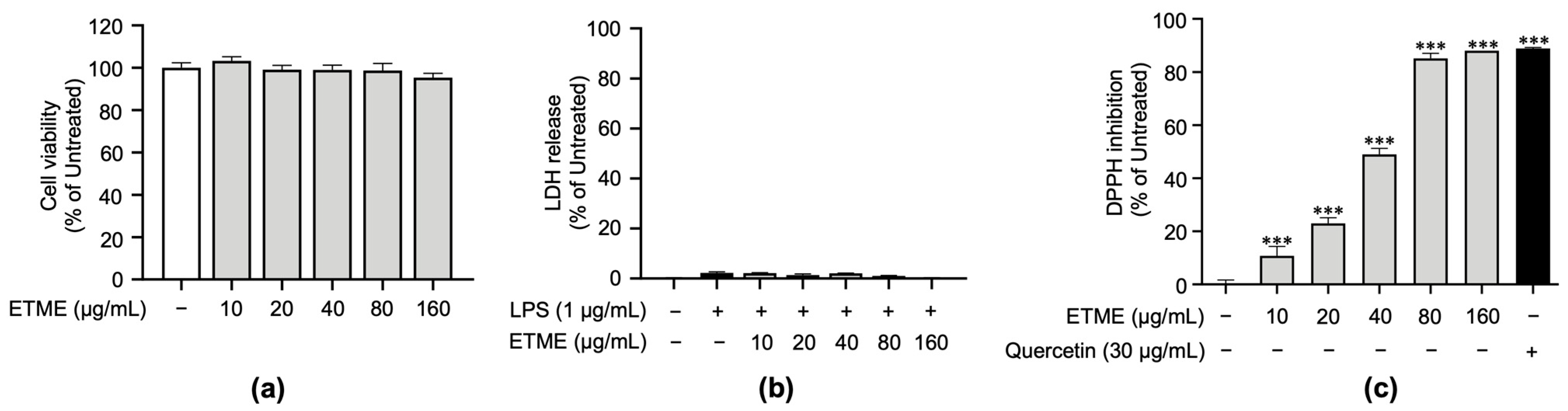
3.2. ETME Exerted Antioxidant Properties under Non-Cytotoxic Concentrations
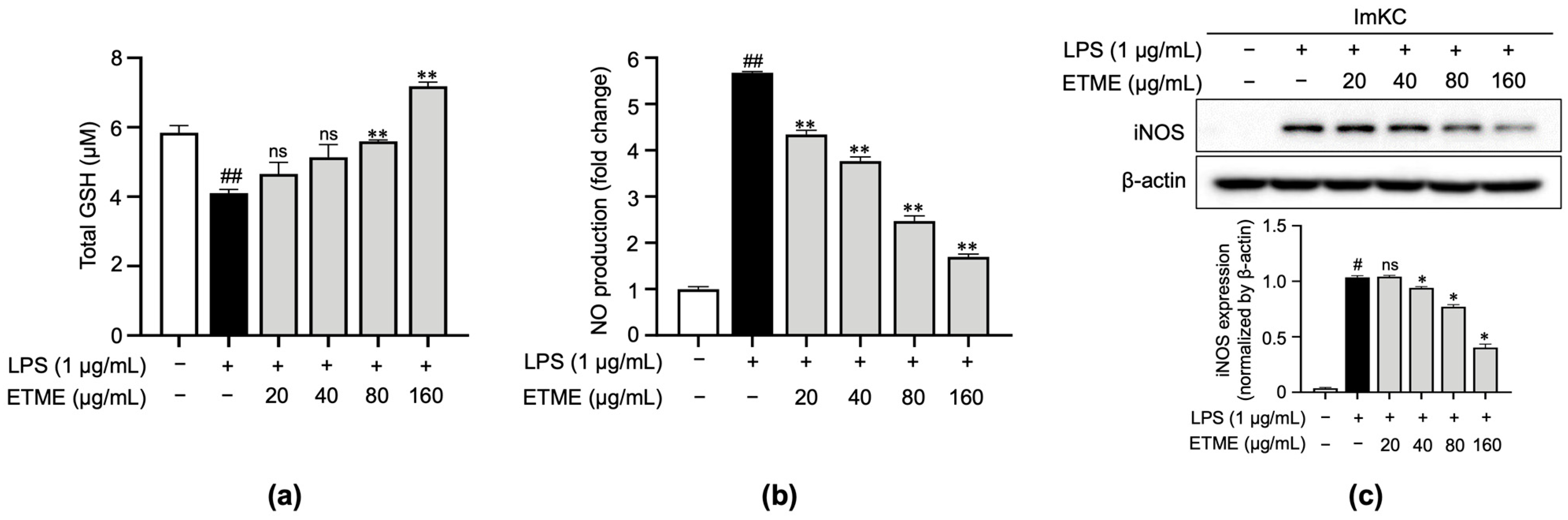
3.3. ETME Rescued GSH Levels and Inhibited NO Production
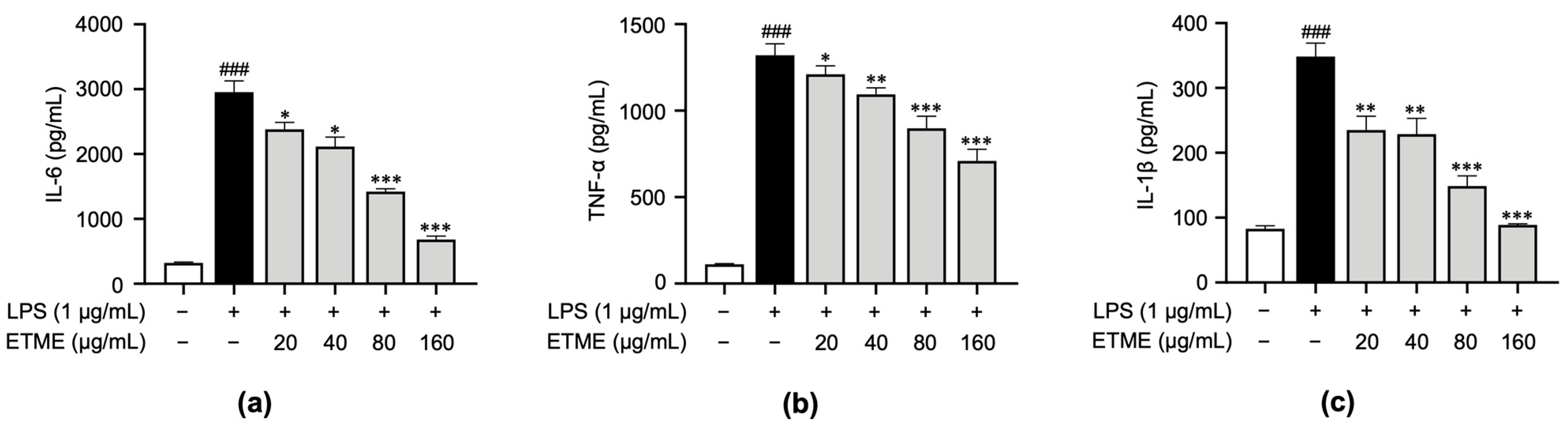
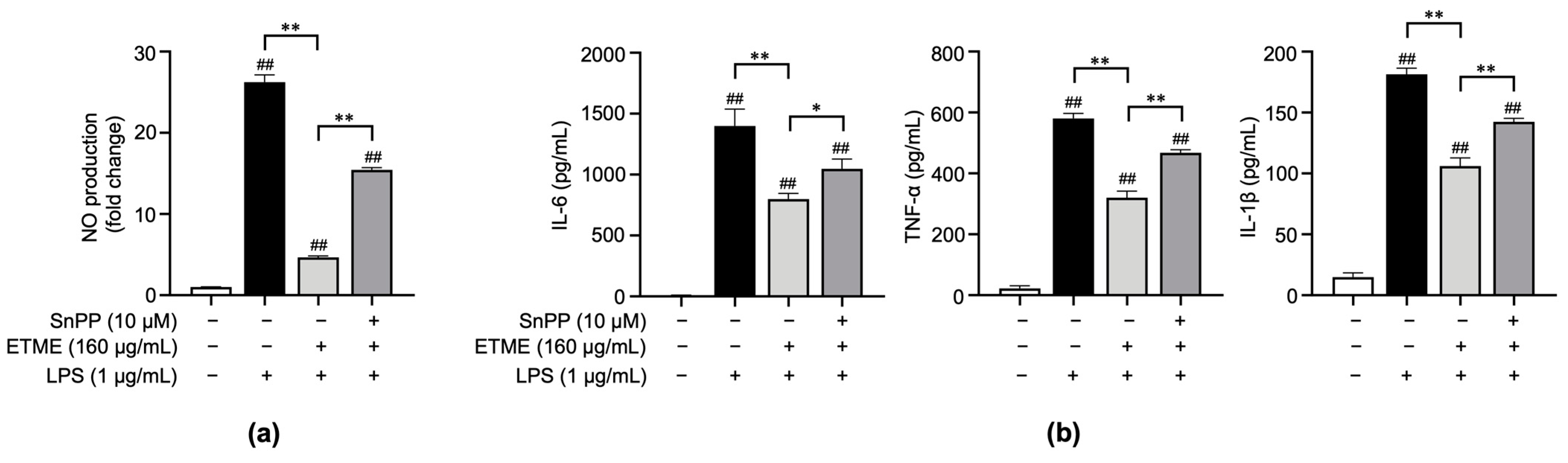
3.4. ETME Inhibited Pro-Inflammatory Cytokine Production
3.5. ETME Exerted Anti-Inflammatory Activity by Activating HO-1
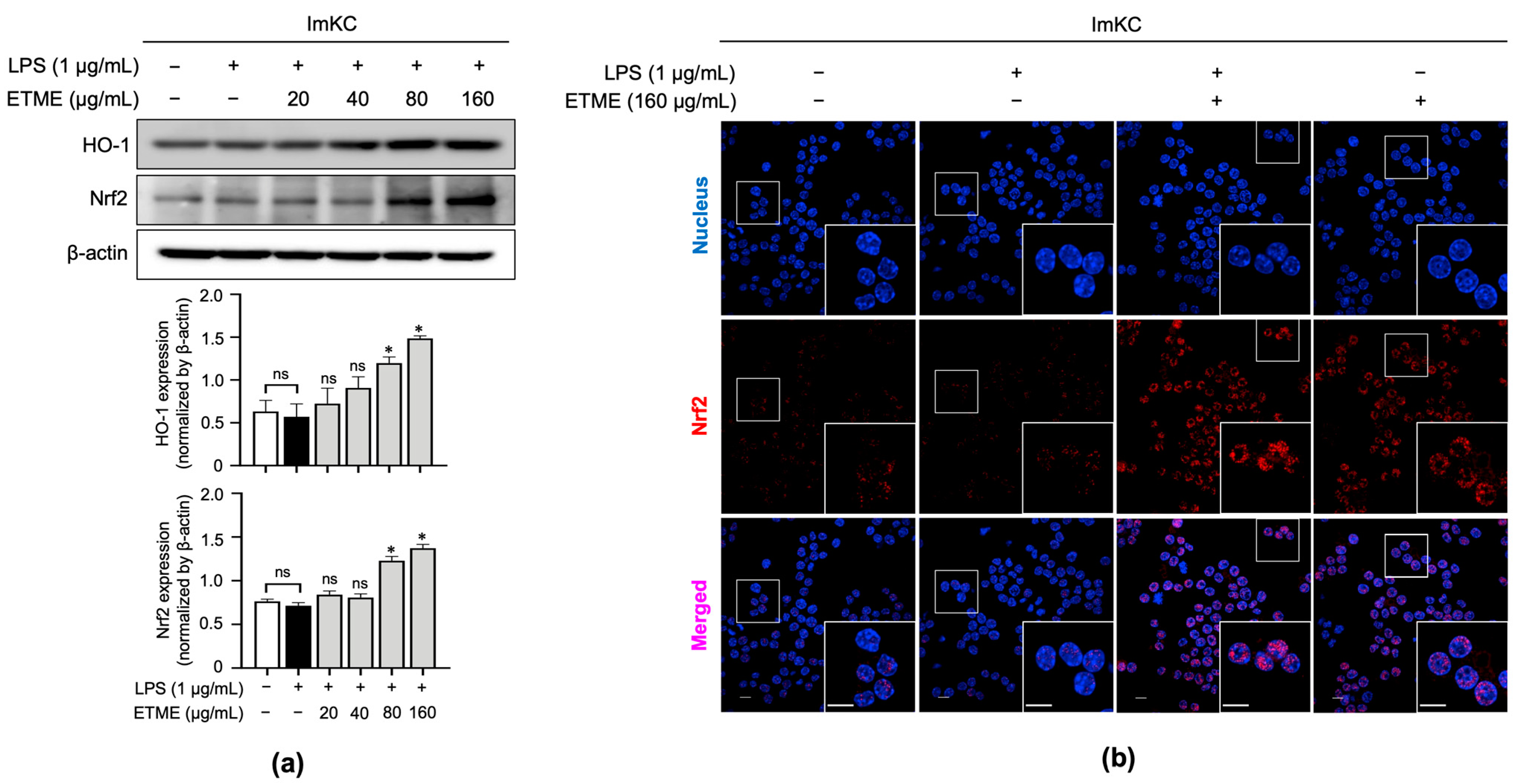
3.6. ETME Increased HO-1 and Nrf2 Expression Thereby Promoting Its Antioxidant Activity
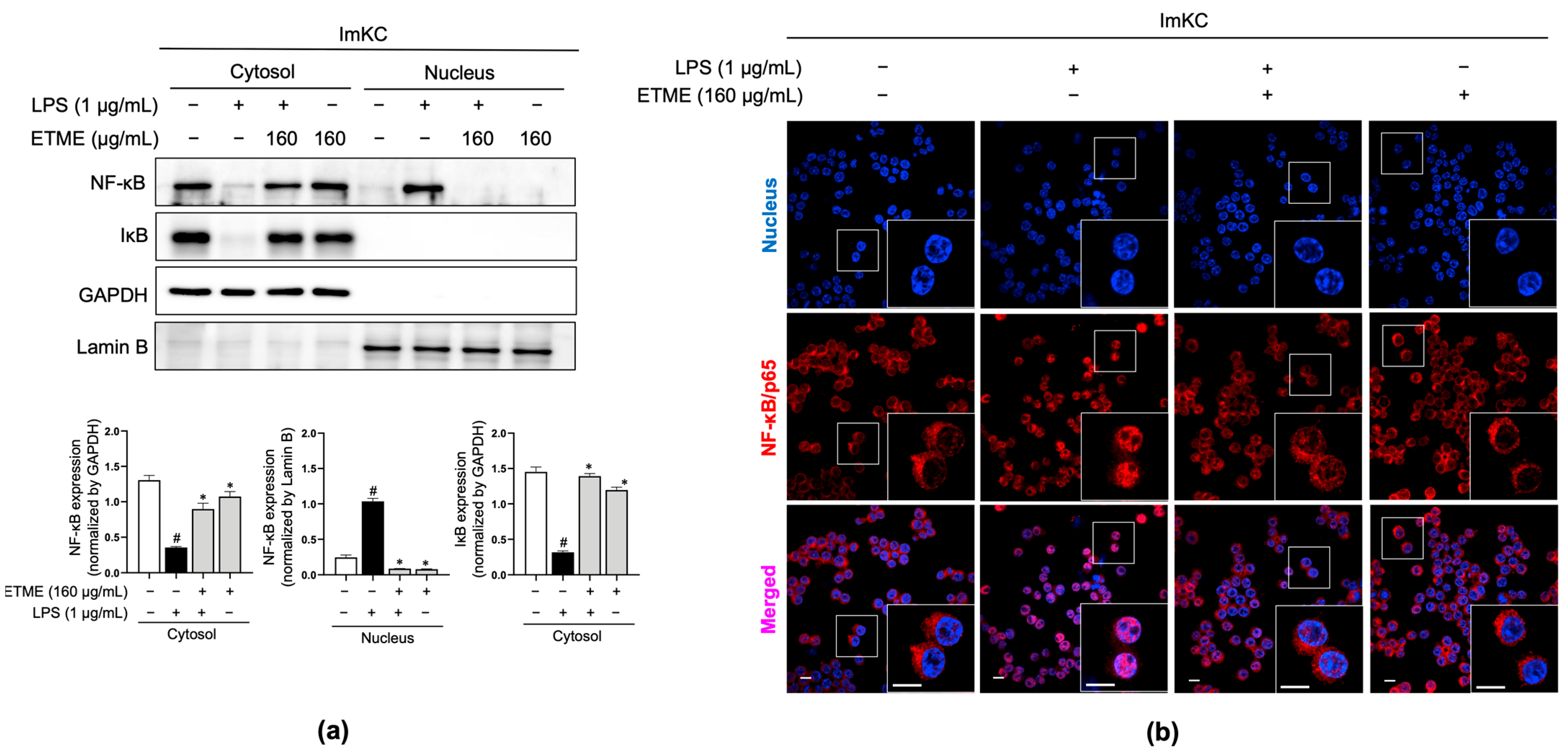
3.7. ETME Inhibited NF-κB/p65 Nuclear Translocation
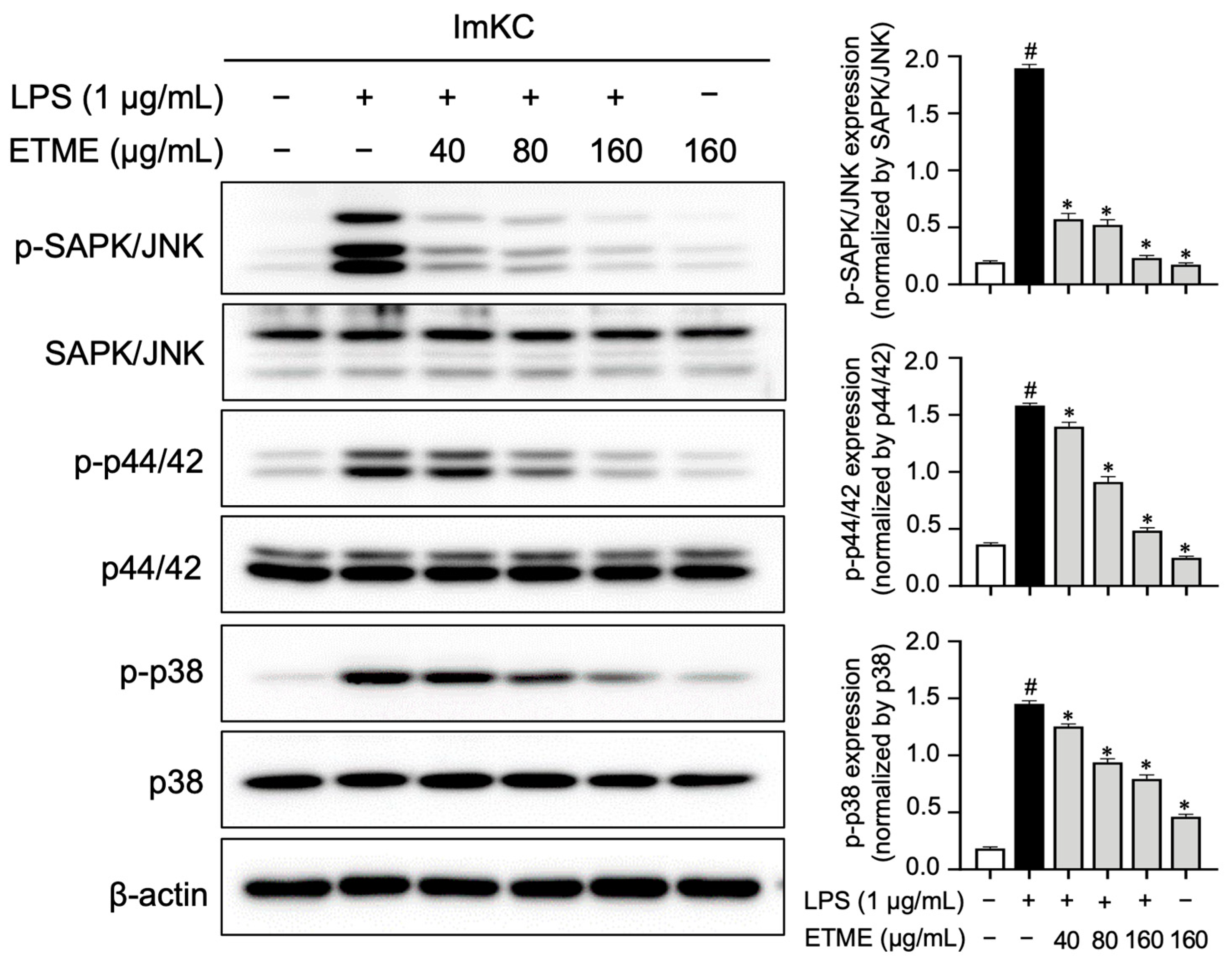
3.8. ETME Inhibited MAPK Phospohrylation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences, T.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.S.; Lee, S.H.; Lee, S.R.; Lim, H.-J.; Roh, Y.-S.; Won, E.J.; Cho, N.; Chun, C.; Cho, Y.-C. Inhibitory effects of Aucklandia lappa decne. Extract on inflammatory and oxidative responses in LPS-treated macrophages. Molecules 2020, 25, 1336. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, Y.-C.; Lim, J.S. Costunolide, a sesquiterpene lactone, suppresses skin cancer via induction of apoptosis and blockage of cell proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef]
- Hammer, K.D.; Hillwig, M.L.; Solco, A.K.; Dixon, P.M.; Delate, K.; Murphy, P.A.; Wurtele, E.S.; Birt, D.F. Inhibition of prostaglandin E2 production by anti-inflammatory hypericum perforatum extracts and constituents in RAW264.7 Mouse Macrophage Cells. J. Agric. Food Chem. 2007, 55, 7323–7331. [Google Scholar] [CrossRef]
- Li, C.; Wang, M.H. Anti-inflammatory effect of the water fraction from hawthorn fruit on LPS-stimulated RAW 264.7 cells. Nutr. Res. Pract. 2011, 5, 101–106. [Google Scholar] [CrossRef]
- Qnais, E.Y.; Abu-Dieyeh, M.; Abdulla, F.A.; Abdalla, S.S. The antinociceptive and anti-inflammatory effects of Salvia officinalis leaf aqueous and butanol extracts. Pharm. Biol. 2010, 48, 1149–1156. [Google Scholar] [CrossRef]
- Guardia, T.; Rotelli, A.E.; Juarez, A.O.; Pelzer, L.E. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin and hesperidin on adjuvant arthritis in rat. Il Farmaco 2001, 56, 683–687. [Google Scholar] [CrossRef]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-Inflammatory Activity of Natural Products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Hadjichambis, A.; Paraskeva-Hadjichambi, D.; Della, A.; Giusti, M.E.; De Pasquale, C.; Lenzarini, C.; Censorii, E.; Gonzales-Tejero, M.R.; Sanchez-Rojas, C.P.; Ramiro-Gutierrez, J.M.; et al. Wild and semi-domesticated food plant consumption in seven circum-Mediterranean areas. Int. J. Food Sci. Nutr. 2008, 59, 383–414. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S. A Revision of the New World Species of Ehretia (Boraginaceae). Ann. Mo. Bot. Gard. 1989, 76, 1050–1076. [Google Scholar] [CrossRef]
- Emes Boronda, M.; Ochurte Espinoza, C.; Castañeda Silva, G.; Peralta González, B. Flora medicinal Indígena de México: Treinta y Cinco Monografías del Atlas de las Plantas de la Medicina Tradicional Mexicana; Instituto Nacional Indigenista: México, DF, Mexico, 1994; 3 Volumes. [Google Scholar]
- Martínez, M. Las plantas medicinales de México. In Las plantas medicinales de México; Ediciones Botas: México, Mexico, 1991; p. 656. [Google Scholar]
- Lentz, D.L.; Dickau, R. Seeds of the Central America and Southern Mexico: The Economic Species; New York Botanical Garden: New York, NY, USA, 2005. [Google Scholar]
- Liogier, H. Plantas medicinales de Puerto Rico y del Caribe.; Iberoamericana de Ediciones: San Juan, Puerto Rico, 1990; Volume 566. [Google Scholar]
- Dzib-Guerra, W.D.; Escalante-Erosa, F.; Garcia-Sosa, K.; Derbre, S.; Blanchard, P.; Richomme, P.; Pena-Rodriguez, L.M. Anti-Advanced Glycation End-product and Free Radical Scavenging Activity of Plants from the Yucatecan Flora. Pharmacogn. Res. 2016, 8, 276–280. [Google Scholar] [CrossRef]
- Pío-León, J.F.; Díaz-Camacho, S.P.; López, M.G.; Montes-Avila, J.; López-Angulo, G.; Delgado-Vargas, F. Physicochemical, nutritional, and antioxidant characteristics of the fruit of Ehretia tinifolia. Rev. Mex. Biodivers. 2012, 83, 273–280. [Google Scholar] [CrossRef]
- Monroy-García, I.N.; Carranza-Torres, I.E.; Carranza-Rosales, P.; Oyón-Ardoiz, M.; García-Estévez, I.; Ayala-Zavala, J.F.; Morán-Martínez, J.; Viveros-Valdez, E. Phenolic Profiles and Biological Activities of Extracts from Edible Wild Fruits Ehretia tinifolia and Sideroxylon lanuginosum. Foods 2021, 10, 2710. [Google Scholar] [CrossRef]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory effect of rosmarinic acid and an extract of Rosmarinus officinalis in rat models of local and systemic inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Krenkel, O.; Tacke, F. Liver macrophages in tissue homeostasis and disease. Nat. Rev. Immunol. 2017, 17, 306–321. [Google Scholar] [CrossRef]
- Su, G.L. Lipopolysaccharides in liver injury: Molecular mechanisms of Kupffer cell activation. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 283, G256–G265. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, L.J.; He, Z.D.; Yang, Q.Q.; Peng, Y.; Xiao, P.G. Chemical study on ethyl acetate portion of Ehretia thyrsiflora, boraginaceae species of Kudingcha. Zhongguo Zhong Yao Za Zhi 2008, 33, 2121–2123. [Google Scholar] [PubMed]
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocârlan, N.; Spinu, M. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef]
- Koley, S.; Chu, K.L.; Gill, S.S.; Allen, D.K. An efficient LC-MS method for isomer separation and detection of sugars, phosphorylated sugars, and organic acids. J. Exp. Bot. 2021, 73, 2938–2952. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.-Z.; Yu, Y.-L.; Zhu, S.-C.; Cao, J.; Ye, L.-H. Nontargeted metabonomics-assisted two-dimensional ion mobility mass spectrometry point imaging to identify plant teas. LWT 2022, 167, 113852. [Google Scholar] [CrossRef]
- Yang, J.-M.; Ip, S.-P.P.; Yeung, H.-K.J.; Che, C.-T. HPLC-MS analysis of Schisandra lignans and their metabolites in Caco-2 cell monolayer and rat everted gut sac models and in rat plasma. Acta Pharm. Sin. B 2011, 1, 46–55. [Google Scholar] [CrossRef]
- Le Gall, G.; DuPont, M.S.; Mellon, F.A.; Davis, A.L.; Collins, G.J.; Verhoeyen, M.E.; Colquhoun, I.J. Characterization and Content of Flavonoid Glycosides in Genetically Modified Tomato (Lycopersicon esculentum) Fruits. J. Agric. Food Chem. 2003, 51, 2438–2446. [Google Scholar] [CrossRef]
- Abdallah, H.M.; Al-Abd, A.M.; Asaad, G.F.; Abdel-Naim, A.B.; El-halawany, A.M. Isolation of Antiosteoporotic Compounds from Seeds of Sophora japonica. PLoS ONE 2014, 9, e98559. [Google Scholar] [CrossRef]
- Jung, C.H.; Kim, Y.; Kim, M.S.; Lee, S.; Yoo, S.H. The establishment of efficient bioconversion, extraction, and isolation processes for the production of phyllodulcin, a potential high intensity sweetener, from sweet hydrangea leaves (Hydrangea macrophylla Thunbergii). Phytochem. Anal. 2016, 27, 140–147. [Google Scholar] [CrossRef]
- Zakaria, Z.A.; Balan, T.; Azemi, A.; Omar, M.; Mohtarrudin, N.; Ahmad, Z.; Abdullah, M.; Mohd Desa, M.N.; Teh, L.K.; Salleh, M.Z. Mechanism(s) of action underlying the gastroprotective effect of ethyl acetate fraction obtained from the crude methanolic leaves extract of Muntingia calabura. BMC Complement. Altern. Med. 2016, 16, 78. [Google Scholar] [CrossRef]
- Damašius, J.; Venskutonis, R.; Kaškonienė, V.; Maruska, A. Fast Screening of the Main Phenolic Acids with Antioxidant Properties in Common Spices Using On-Line HPLC/UV/DPPH Radical Scavenging Assay. Anal. Methods 2014, 6, 2774–2779. [Google Scholar] [CrossRef]
- Jakšić, D.; Puel, O.; Canlet, C.; Kopjar, N.; Kosalec, I.; Klarić, M. Cytotoxicity and genotoxicity of versicolorins and 5-methoxysterigmatocystin in A549 cells. Arch. Toxicol. 2012, 86, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Avasthi, A.S.; Bhatnagar, M.; Sarkar, N.; Kitchlu, S.; Ghosal, S. Bioassay guided screening, optimization and characterization of antioxidant compounds from high altitude wild edible plants of Ladakh. J. Food Sci. Technol. 2016, 53, 3244–3252. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cao, T.Q.; Liu, Z.; Dong, L.; Lee, H.; Ko, W.; Vinh, L.B.; Tuan, N.Q.; Kim, Y.C.; Sohn, J.H.; Yim, J.H.; et al. Identification of Potential Anti-Neuroinflammatory Inhibitors from Antarctic Fungal Strain Aspergillus sp. SF-7402 via Regulating the NF-κB Signaling Pathway in Microglia. Molecules 2022, 27, 2851. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Da, S.; Lopes, A.; De, M.; Agra, M.D.F.; Vasconcelos, E.; Da-Cunha, L.; Barbosa Filho, J.; Silva, M.; Braz-Filho, R. A new arylnaphthalene type lignan from Cordia rufescens A. DC. (Boraginaceae). Arkivoc 2004, 2004, 54–58. [Google Scholar] [CrossRef]
- De Taeye, C.; Caullet, G.; Eyamo Evina, V.J.; Collin, S. Procyanidin A2 and Its Degradation Products in Raw, Fermented, and Roasted Cocoa. J. Agric. Food Chem. 2017, 65, 1715–1723. [Google Scholar] [CrossRef] [PubMed]
- Katragunta, K.; Siva, B.; Kondepudi, N.; Vadaparthi, P.R.R.; Rama Rao, N.; Tiwari, A.K.; Suresh Babu, K. Estimation of boswellic acids in herbal formulations containing Boswellia serrata extract and comprehensive characterization of secondary metabolites using UPLC-Q-Tof-MS(e). J. Pharm. Anal. 2019, 9, 414–422. [Google Scholar] [CrossRef]
- Habib, H.; Finno, C.J.; Gennity, I.; Favro, G.; Hales, E.; Puschner, B.; Moeller, B.C. Simultaneous quantification of vitamin E and vitamin E metabolites in equine plasma and serum using LC-MS/MS. J. Vet. Diagn. Investig. 2021, 33, 506–515. [Google Scholar] [CrossRef]
- Ci, X.; Ren, R.; Xu, K.; Li, H.; Yu, Q.; Song, Y.; Wang, D.; Li, R.; Deng, X. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation 2010, 33, 126–136. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, H.; Xia, X.; Yang, L.; He, J. Kaempferol 3-O-(2G-glucosylrutinoside)-7-O-glucoside isolated from the flowers of Hosta plantaginea exerts anti-inflammatory activity via suppression of NF-κB, MAPKs and Akt pathways in RAW 264.7 cells. Biomed. Pharmacother. 2022, 153, 113295. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Gao, H.; Yuan, R.; Han, S.; Li, X.X.; Tang, M.; Dong, B.; Li, J.X.; Zhao, L.C.; Feng, J.; et al. Procyanidin A2, a polyphenolic compound, exerts anti-inflammatory and anti-oxidative activity in lipopolysaccharide-stimulated RAW264.7 cells. PLoS ONE 2020, 15, e0237017. [Google Scholar] [CrossRef]
- Frankel, E.N. The antioxidant and nutritional effects of tocopherols, ascorbic acid and beta-carotene in relation to processing of edible oils. Bibl. Nutr. Dieta 1989, 43, 297–312. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Xue, Q.; Yan, Y.; Zhang, R.; Xiong, H. Regulation of iNOS on Immune Cells and Its Role in Diseases. Int. J. Mol. Sci. 2018, 19, 3805. [Google Scholar] [CrossRef]
- Yang, G.; Nguyen, X.; Ou, J.; Rekulapelli, P.; Stevenson, D.K.; Dennery, P.A. Unique effects of zinc protoporphyrin on HO-1 induction and apoptosis. Blood 2001, 97, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef]
- Sharif, O.; Bolshakov, V.N.; Raines, S.; Newham, P.; Perkins, N.D. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007, 8, 1. [Google Scholar] [CrossRef]
- Hobbs, S.; Reynoso, M.; Geddis, A.V.; Mitrophanov, A.Y.; Matheny, R.W., Jr. LPS-stimulated NF-kappaB p65 dynamic response marks the initiation of TNF expression and transition to IL-10 expression in RAW 264.7 macrophages. Physiol. Rep. 2018, 6, e13914. [Google Scholar] [CrossRef]
- Moens, U.; Kostenko, S.; Sveinbjornsson, B. The Role of Mitogen-Activated Protein Kinase-Activated Protein Kinases (MAPKAPKs) in Inflammation. Genes 2013, 4, 101–133. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Riches, D.W. IFN-gamma + LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38(mapk) in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 2001, 280, C441–C450. [Google Scholar] [CrossRef] [PubMed]
- Clemens, M.G. Nitric oxide in liver injury. Hepatology 1999, 30, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Milbourne, E.A.; Bygrave, F.L. Does nitric oxide play a role in liver function? Cell. Signal. 1995, 7, 313–318. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Anavi, S.; Eisenberg-Bord, M.; Hahn-Obercyger, M.; Genin, O.; Pines, M.; Tirosh, O. The role of iNOS in cholesterol-induced liver fibrosis. Lab. Investig. 2015, 95, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Naftaly, M.; Friedman, S.L. Current status of novel antifibrotic therapies in patients with chronic liver disease. Therap. Adv. Gastroenterol. 2011, 4, 391–417. [Google Scholar] [CrossRef]
- Stahl, W.; Matejovic, M.; Radermacher, P. Inhibition of nitric oxide synthase during sepsis: Revival because of isoform selectivity? Shock 2010, 34, 321–322. [Google Scholar] [CrossRef]
- Nathan, C. Is iNOS beginning to smoke? Cell 2011, 147, 257–258. [Google Scholar] [CrossRef]
- Immenschuh, S.; Ramadori, G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000, 60, 1121–1128. [Google Scholar] [CrossRef]
- Campbell, N.K.; Fitzgerald, H.K.; Dunne, A. Regulation of inflammation by the antioxidant haem oxygenase 1. Nat. Rev. Immunol. 2021, 21, 411–425. [Google Scholar] [CrossRef]
- Kapturczak, M.H.; Wasserfall, C.; Brusko, T.; Campbell-Thompson, M.; Ellis, T.M.; Atkinson, M.A.; Agarwal, A. Heme oxygenase-1 modulates early inflammatory responses: Evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 2004, 165, 1045–1053. [Google Scholar] [CrossRef]
- Yachie, A.; Niida, Y.; Wada, T.; Igarashi, N.; Kaneda, H.; Toma, T.; Ohta, K.; Kasahara, Y.; Koizumi, S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Investig. 1999, 103, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Poss, K.D.; Tonegawa, S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc. Natl. Acad. Sci. USA 1997, 94, 10925–10930. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, N.; Yadav, S.P.; Sachdeva, A.; Pruthi, P.K.; Sawhney, S.; Piplani, T.; Wada, T.; Yachie, A. Human heme oxygenase-1 deficiency presenting with hemolysis, nephritis, and asplenia. J. Pediatr. Hematol. Oncol. 2011, 33, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Biswas, S.K.; Kirkham, P.A. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem. Pharmacol. 2006, 72, 1439–1452. [Google Scholar] [CrossRef]
- Wang, P.; Geng, J.; Gao, J.; Zhao, H.; Li, J.; Shi, Y.; Yang, B.; Xiao, C.; Linghu, Y.; Sun, X.; et al. Macrophage achieves self-protection against oxidative stress-induced ageing through the Mst-Nrf2 axis. Nat. Commun. 2019, 10, 755. [Google Scholar] [CrossRef]
- Villeneuve, N.F.; Lau, A.; Zhang, D.D. Regulation of the Nrf2-Keap1 antioxidant response by the ubiquitin proteasome system: An insight into cullin-ring ubiquitin ligases. Antioxid. Redox Signal. 2010, 13, 1699–1712. [Google Scholar] [CrossRef]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzyme Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef]
- Singh, A.; Venkannagari, S.; Oh, K.H.; Zhang, Y.Q.; Rohde, J.M.; Liu, L.; Nimmagadda, S.; Sudini, K.; Brimacombe, K.R.; Gajghate, S.; et al. Small Molecule Inhibitor of NRF2 Selectively Intervenes Therapeutic Resistance in KEAP1-Deficient NSCLC Tumors. ACS Chem. Biol. 2016, 11, 3214–3225. [Google Scholar] [CrossRef]
- Ren, D.; Villeneuve, N.F.; Jiang, T.; Wu, T.; Lau, A.; Toppin, H.A.; Zhang, D.D. Brusatol enhances the efficacy of chemotherapy by inhibiting the Nrf2-mediated defense mechanism. Proc. Natl. Acad. Sci. USA 2011, 108, 1433–1438. [Google Scholar] [CrossRef]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Zhang, X.J.; Yang, Y.; Zhang, C.; Zhu, C.H.; Miao, J.Y.; Chen, R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018, 13, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Domitrovic, R.; Skoda, M.; Vasiljev Marchesi, V.; Cvijanovic, O.; Pernjak Pugel, E.; Stefan, M.B. Rosmarinic acid ameliorates acute liver damage and fibrogenesis in carbon tetrachloride-intoxicated mice. Food Chem. Toxicol. 2013, 51, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Li, W.; Tsubouchi, R.; Haneda, M.; Murakami, K.; Takeuchi, F.; Nisimoto, Y.; Yoshino, M. Rosmarinic acid inhibits the formation of reactive oxygen and nitrogen species in RAW264.7 macrophages. Free Radic. Res. 2005, 39, 995–1003. [Google Scholar] [CrossRef]
- Chen, C.P.; Lin, Y.C.; Peng, Y.H.; Chen, H.M.; Lin, J.T.; Kao, S.H. Rosmarinic Acid Attenuates the Lipopolysaccharide-Provoked Inflammatory Response of Vascular Smooth Muscle Cell via Inhibition of MAPK/NF-kappaB Cascade. Pharmaceuticals 2022, 15, 437. [Google Scholar] [CrossRef]
- Siddiqui, M.Z. Boswellia serrata, a potential antiinflammatory agent: An overview. Indian J. Pharm. Sci. 2011, 73, 255–261. [Google Scholar] [CrossRef]
- Zhang, P.Y.; Yu, B.; Men, W.J.; Bai, R.Y.; Chen, M.Y.; Wang, Z.X.; Zeng, T.; Zhou, K. Acetyl-alpha-boswellic acid and Acetyl-beta-boswellic acid protects against caerulein-induced pancreatitis via down-regulating MAPKs in mice. Int. Immunopharmacol. 2020, 86, 106682. [Google Scholar] [CrossRef]
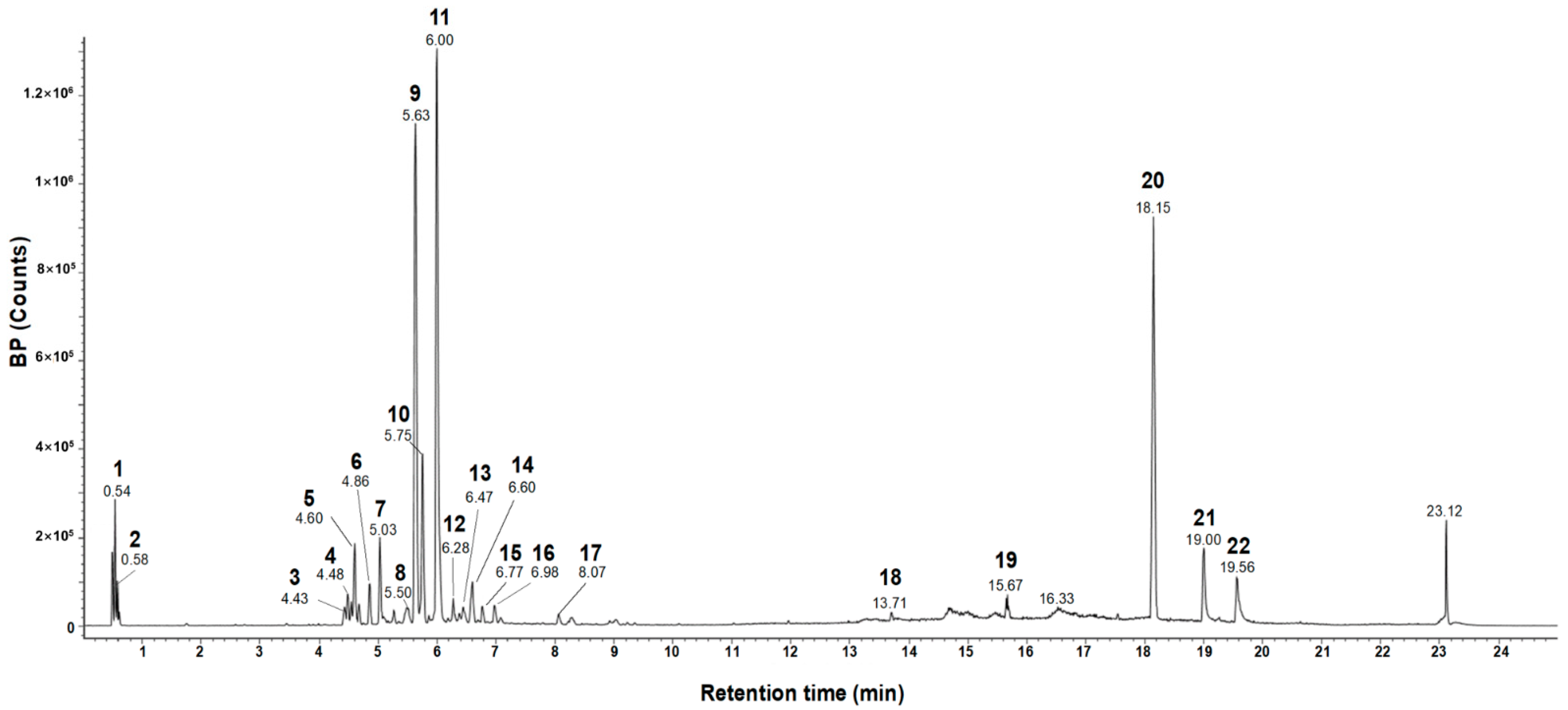
| # | Observed RT (min) | Neutral Mass (Da) | Observed Neutral Mass (Da) | Observed (m/z) | Adducts | Component Name |
|---|---|---|---|---|---|---|
| 1 | 0.54 | 504.16903 | 504.1691 | 503.1619 | −H, +HCOO | Raffinose [28] |
| 2 | 0.58 | 150.05282 | 150.0527 | 195.0509 | +HCOO, −H | Pentose (TML) |
| 3 | 4.43 | 242.09429 | 242.0938 | 241.0865 | −H | Flavanthrinin [29] |
| 4 | 4.48 | 536.20463 | 536.2039 | 581.2022 | +HCOO | Schisantherin A [30] |
| 5 | 4.60 | 610.15338 | 610.1547 | 609.1475 | −H | Kaempferol-3,7-diglucoside [31] |
| 6 | 4.86 | 594.15847 | 594.1591 | 593.1519 | −H | Genistein-7,4′-di-O-β-D-glucoside [32] |
| 7 | 5.03 | 286.08412 | 286.0831 | 285.0759 | −H | Phyllodulcin [33] |
| 8 | 5.50 | 314.07904 | 314.0787 | 313.0714 | −H | Ermanin [34] |
| 9 | 5.63 | 360.08452 | 360.0848 | 359.0775 | −H | Rosmarinic acid [35] |
| 10 | 5.75 | 312.06339 | 312.0631 | 357.0613 | +HCOO, −H | 2-Acetyl emodin (TML) |
| 11 | 6.00 | 286.06887 | 286.0688 | 321.0399 | +Cl | Uralenneoside (TML) |
| 12 | 6.28 | 340.0583 | 340.0584 | 339.0511 | −H | Versicolorin B [36] |
| 13 | 6.47 | 374.10017 | 374.1001 | 373.0928 | −H | Methyl rosmarinate [26] |
| 14 | 6.60 | 354.07395 | 354.0741 | 353.0668 | −H | 5-Methoxysterigmatocystn [38] |
| 15 | 6.77 | 336.027 | 336.0268 | 335.0195 | −H | Rufescidride [39] |
| 16 | 6.99 | 518.32435 | 518.3231 | 517.3159 | −H | 2-Hydroxyesculentic acid (TML) |
| 17 | 8.07 | 576.12678 | 576.124 | 575.1167 | −H | Procyanidin A2 [40] |
| 18 | 13.71 | 256.24023 | 256.2403 | 255.233 | −H | Methyl pentadecanote (TML) |
| 19 | 15.67 | 510.17373 | 510.1744 | 555.1726 | +HCOO | Globularinin (TML) |
| 20 | 18.15 | 498.37091 | 498.3713 | 497.3641 | −H, +HCOO | Acetyl-β-boswellic acid [41] |
| 21 | 19.00 | 206.16707 | 206.1668 | 205.1595 | −H | Longicamphenylone (TML) |
| 22 | 19.57 | 416.36543 | 416.3664 | 461.3646 | +HCOO | γ-Tocopherol [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.S.; Lee, S.H.; Yun, H.; Lee, D.Y.; Cho, N.; Yoo, G.; Choi, J.U.; Lee, K.Y.; Bach, T.T.; Park, S.-J.; et al. Inhibitory Effects of Ehretia tinifolia Extract on the Excessive Oxidative and Inflammatory Responses in Lipopolysaccharide-Stimulated Mouse Kupffer Cells. Antioxidants 2023, 12, 1792. https://doi.org/10.3390/antiox12101792
Lim JS, Lee SH, Yun H, Lee DY, Cho N, Yoo G, Choi JU, Lee KY, Bach TT, Park S-J, et al. Inhibitory Effects of Ehretia tinifolia Extract on the Excessive Oxidative and Inflammatory Responses in Lipopolysaccharide-Stimulated Mouse Kupffer Cells. Antioxidants. 2023; 12(10):1792. https://doi.org/10.3390/antiox12101792
Chicago/Turabian StyleLim, Jae Sung, Sung Ho Lee, Hyosuk Yun, Da Young Lee, Namki Cho, Guijae Yoo, Jeong Uk Choi, Kwang Youl Lee, Tran The Bach, Su-Jin Park, and et al. 2023. "Inhibitory Effects of Ehretia tinifolia Extract on the Excessive Oxidative and Inflammatory Responses in Lipopolysaccharide-Stimulated Mouse Kupffer Cells" Antioxidants 12, no. 10: 1792. https://doi.org/10.3390/antiox12101792
APA StyleLim, J. S., Lee, S. H., Yun, H., Lee, D. Y., Cho, N., Yoo, G., Choi, J. U., Lee, K. Y., Bach, T. T., Park, S.-J., & Cho, Y.-C. (2023). Inhibitory Effects of Ehretia tinifolia Extract on the Excessive Oxidative and Inflammatory Responses in Lipopolysaccharide-Stimulated Mouse Kupffer Cells. Antioxidants, 12(10), 1792. https://doi.org/10.3390/antiox12101792









