Valorization of the Antioxidant Effect of Mantua PGI Pear By-Product Extracts: Preparation, Analysis and Biological Investigation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pear Extract Preparation
2.2.1. Starting Biomass
2.2.2. Extractions
Solvent-Based Extraction
Supercritical Fluid Extraction
- p = 150 bar, T = 40 °C (dCO2 = 780.6 kg/m3). The alternation of 3 static/dynamic cycles was enough to exhaust the extractables in these conditions, and no evident mass gain was further achieved (SF1).
- p = 300 bar, T = 40 °C (dCO2 = 909.3 kg/m3). These conditions were not able to provide any extract mass gain.
- p = 300 bar, T= 60 °C (dCO2 = 829.5 kg/m3). These conditions were not able to provide any extract mass gain.
- p = 300 bar, T= 80 °C (dCO2 = 746.1 kg/m3). The alternation of 1 static/dynamic cycle was enough to exhaust the extractables in these conditions, and no evident mass gain was further achieved.
- p = 300 bar, T= 60 °C, cosolvent = ethanol (10% v EtOH/v sc-CO2). An alternation of 7 static/dynamic cycles was carried out (SF6).
2.2.3. Waxes Removal
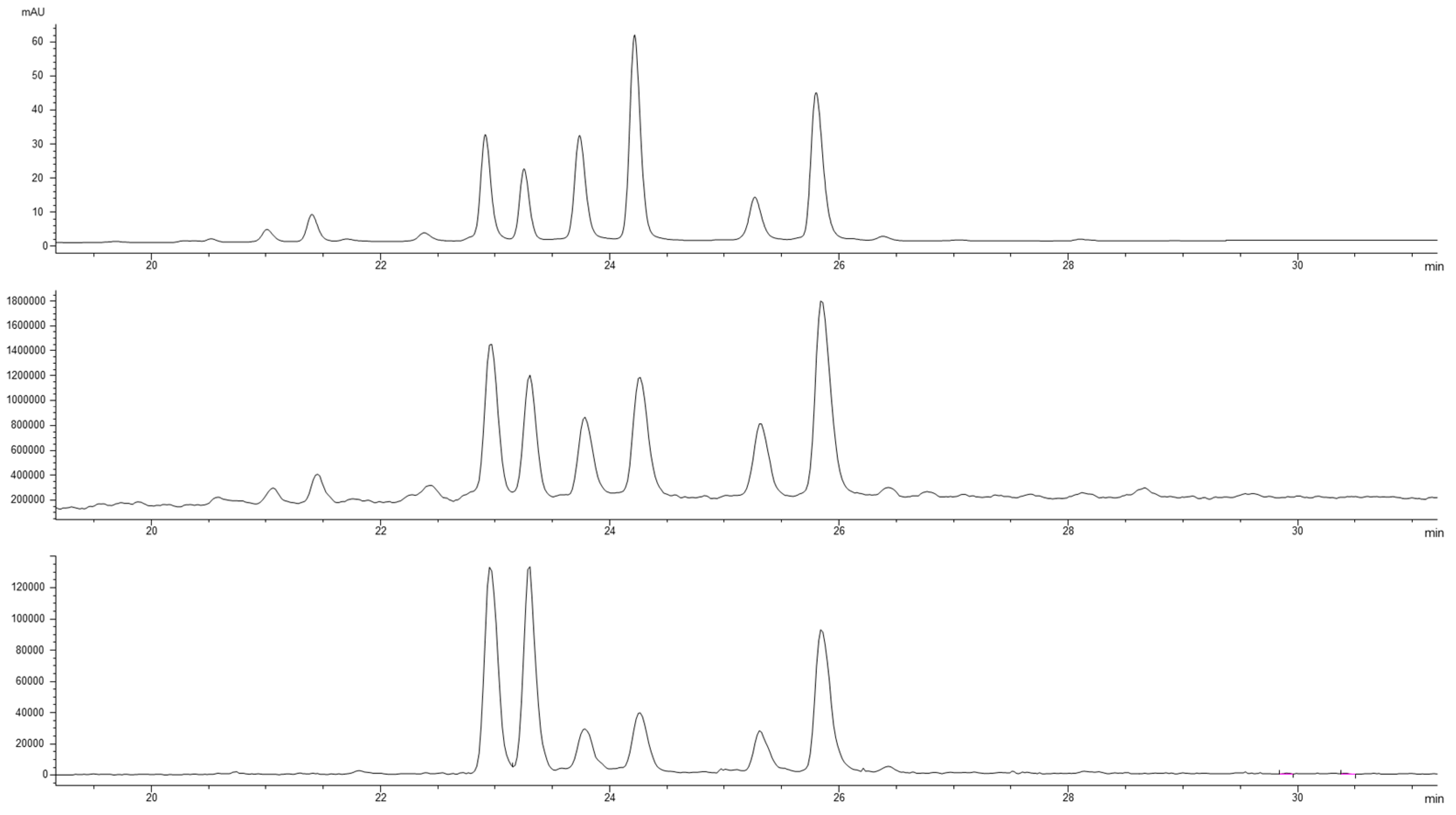
2.3. HPLC-DAD-MS Analysis of Pear Extracts
2.4. DPPH (2,2-Diphenyl-1-picrylhydrazyl Radical Scavenging) Assay
2.5. TEAC Assay
2.6. FRAP Assay
2.7. Cell Culture
2.8. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide (MTT) Assay
2.9. Nitric Oxide Level Evaluation on Caco-2 Cells
2.10. Fluorometric Intracellular ROS Assay
2.11. Lipid Peroxidation (MDA) Assay
2.12. Western Blot Analysis
2.13. Statical Analysis
3. Results
3.1. Preparation of Pear Extract Exploiting Traditional and Green Extraction Methods
- p = 150 bar, T = 40 °C. An overall extraction yield of 0.10 ± 0.02% was achieved.
- p = 300 bar, T = 40 °C. These conditions were not able to provide any extract mass gain.
- p = 300 bar, T = 60 °C. These conditions were not able to provide any extract mass gain.
- p = 300 bar, T = 80 °C. The extraction yield was slightly incremented to 0.11% (0.01% yield gain).
- p = 300 bar, T = 60°, cosolvent = ethanol (10% v EtOH/v sc-CO2). After the alternation of seven static/dynamic cycles, the final yield was incremented up to 1.1 ± 0.1% (1.0% yield gain).
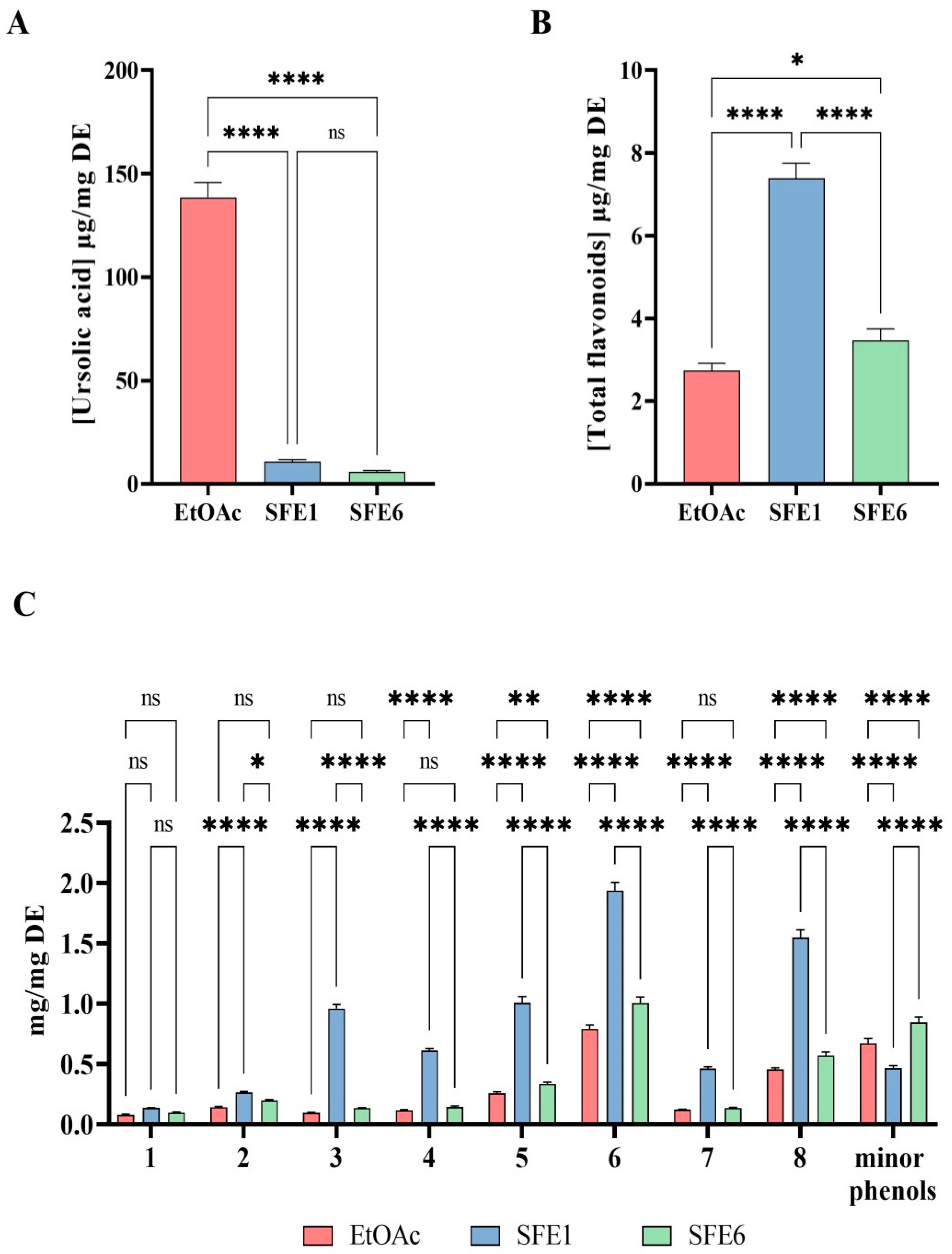
3.2. Analysis of the Pear Extracts
3.3. Evaluation of the Direct Antioxidant Activity of EtOAc, SFE1 and SFE6
3.4. Evaluation of Caco-2 Cells Viability
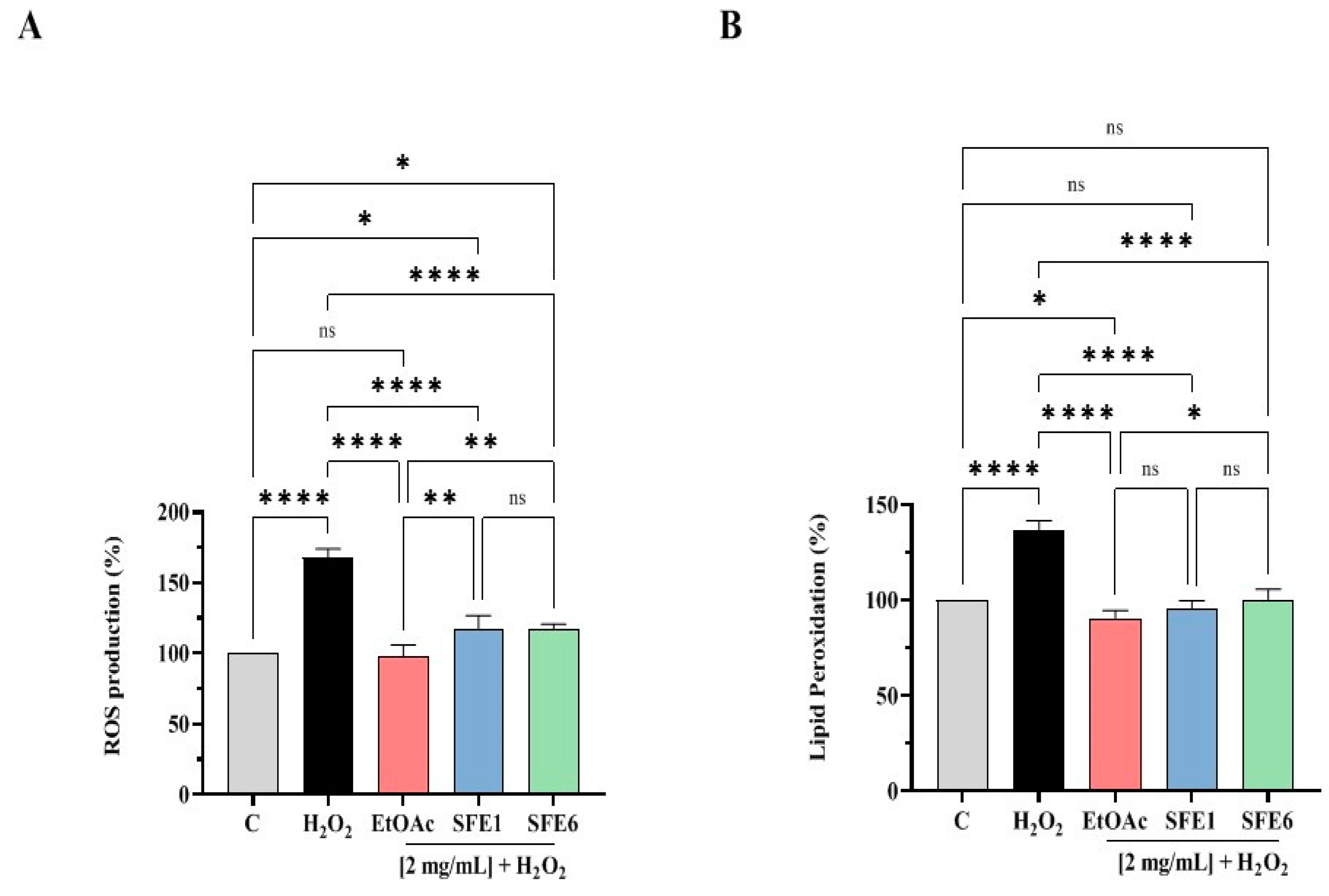
3.5. EtOAc, SFE1 and SFE6 Decrease the H2O2-Induced ROS and Lipid Peroxidation Levels in Human Intestinal Caco-2 Cells
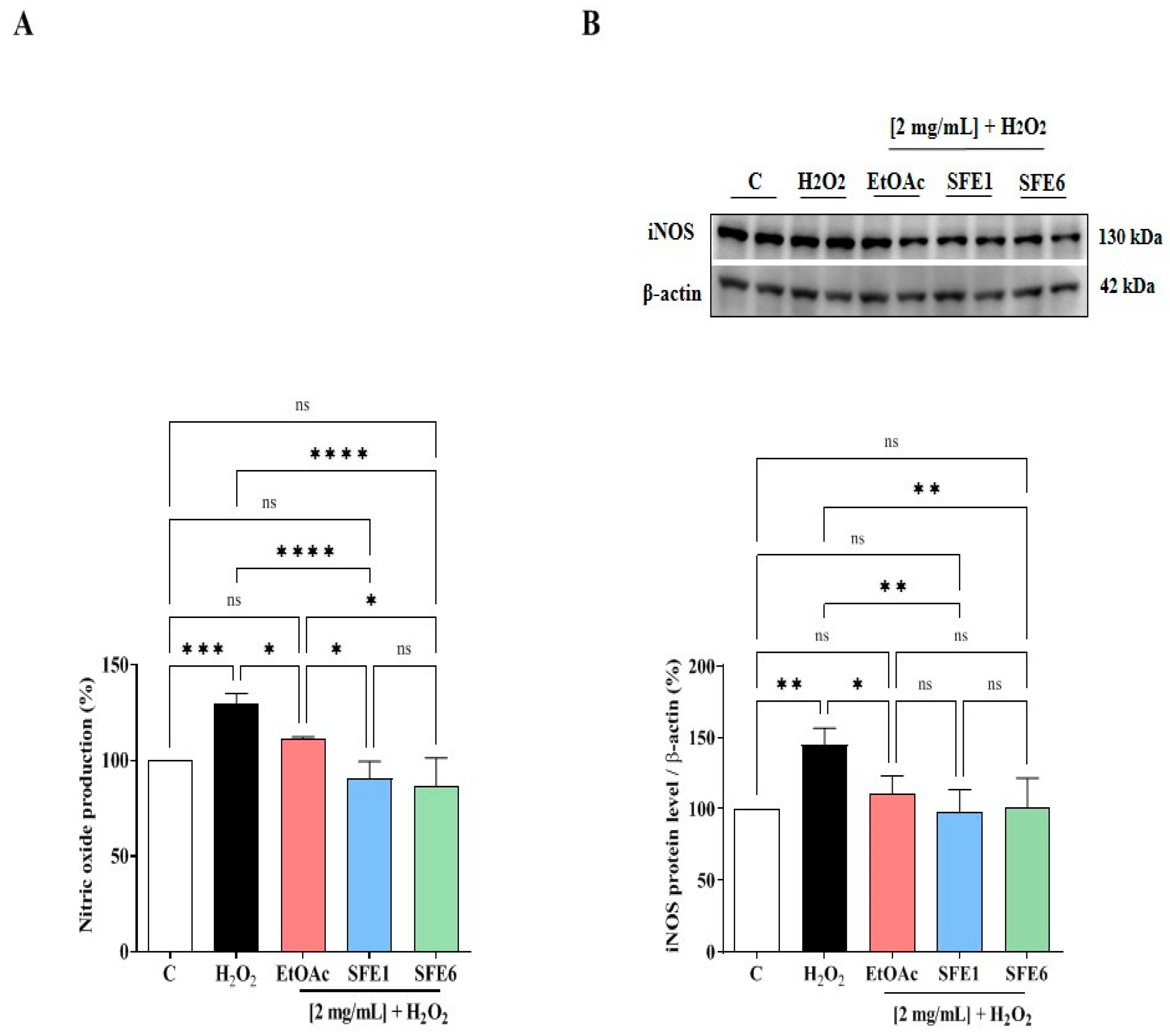
3.6. EtOAc, SFE1 and SFE6 Modulate the H2O2-Induced NO Level Production via the iNOS Protein Modulation in Caco-2 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pawlowska, E.; Szczepańska, J.; Koskela, A.; Kaarniranta, K.; Blasiak, J. Dietary Polyphenols in Age-Related Macular Degeneration: Protection against Oxidative Stress and Beyond. Oxidative Med. Cell. Longev. 2019, 2019, 9682318. [Google Scholar] [CrossRef]
- Gayer, B.A.; Avendano, E.E.; Edelson, E.; Nirmala, N.; Johnson, E.J.; Raman, G. Effects of Intake of Apples, Pears, or Their Products on Cardiometabolic Risk Factors and Clinical Outcomes: A Systematic Review and Meta-Analysis. Curr. Dev. Nutr. 2019, 3, nzz109. [Google Scholar] [CrossRef] [PubMed]
- Kolniak-Ostek, J.; Kłopotowska, D.; Rutkowski, K.P.; Skorupińska, A.; Kruczyńska, D.E. Bioactive Compounds and Health-Promoting Properties of Pear (Pyrus communis L.) Fruits. Molecules 2020, 25, 4444. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K.; Hernandez, F. Comparison of bioactive compounds and health promoting properties of fruits and leaves of apple, pear and quince. Sci. Rep. 2021, 11, 20253. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A Novel Method for Measuring Antioxidant Capacity and its Application to Monitoring the Antioxidant Status in Premature Neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Lammi, C.; Bollati, C.; Gelain, F.; Arnoldi, A.; Pugliese, R. Enhancement of the Stability and Anti-DPPIV Activity of Hempseed Hydrolysates Through Self-Assembling Peptide-Based Hydrogels. Front. Chem. 2019, 6, 670. [Google Scholar] [CrossRef]
- Li, Y.; Aiello, G.; Bollati, C.; Bartolomei, M.; Arnoldi, A.; Lammi, C. Phycobiliproteins from Arthrospira Platensis (Spirulina): A New Source of Peptides with Dipeptidyl Peptidase-IV Inhibitory Activity. Nutrients 2020, 12, 794. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is There Room for Improving the Nutraceutical Composition of Apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef]
- He, W.; Laaksonen, O.; Tian, Y.; Haikonen, T.; Yang, B. Chemical Composition of Juices Made from Cultivars and Breeding Selections of European Pear (Pyrus communis L.). J. Agric. Food Chem. 2022, 70, 16. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and quantification of fruit phenolic compounds of European and Tunisian pear cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.-Y.; Lansky, E.; Kang, S.-S.; Yang, M. A review of pears (Pyrus spp.), ancient functional food for modern times. BMC Complement. Med. Ther. 2021, 21, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Canavari, M. Marketing Research on Fruit Branding: The Case of the Pear Club Variety “Angelys”. In Case Studies in the Traditional Food Sector: A Volume in the Consumer Science and Strategic Marketing Series; Woodhead Publishing: Sawston, UK, 2018; ISBN 9780081012604. [Google Scholar]
- Azuma, K.; Osaki, T.; Ifuku, S.; Saimoto, H.; Morimoto, M.; Takashima, O.; Tsuka, T.; Imagawa, T.; Okamoto, Y.; Minami, S. Anti-inflammatory effects of cellulose nanofiber made from pear in inflammatory bowel disease model. Bioact. Carbohydrates Diet. Fibre 2013, 3, 1–10. [Google Scholar] [CrossRef]
- Oak, M.-H.; Yi, E.; Sharma, K.; Kang, S.; Gong, D.; Oh, S.-H.; Park, E.-Y. Combination of Garcinia cambogia extract and pear pomace extract additively suppresses adipogenesis and enhances lipolysis in 3T3-L1 cells. Pharmacogn. Mag. 2018, 14, 220–226. [Google Scholar] [CrossRef]
- Sarkar, D.; Ankolekar, C.; Pinto, M.; Shetty, K. Dietary functional benefits of Bartlett and Starkrimson pears for potential management of hyperglycemia, hypertension and ulcer bacteria Helicobacter pylori while supporting beneficial probiotic bacterial response. Food Res. Int. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- De Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- de Castro, M.L. Where is supercritical fluid extraction going? TrAC Trends Anal. Chem. 2000, 19, 223–228. [Google Scholar] [CrossRef]
- Vona, R.; Pallotta, L.; Cappelletti, M.; Severi, C.; Matarrese, P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants 2021, 10, 201. [Google Scholar] [CrossRef]
- Jiang, H.; Wu, F.; Jiang, X.; Pu, Y.-F.; Shen, L.-R.; Wu, C.-Y.; Bai, H.-J. Antioxidative, cytoprotective and whitening activities of fragrant pear fruits at different growth stages. Front. Nutr. 2022, 9, 855. [Google Scholar] [CrossRef] [PubMed]
- Musto, G.; Laurenzi, V.; Annunziata, G.; Novellino, E.; Stornaiuolo, M. Genotoxic Assessment of Nutraceuticals Obtained from Agricultural Biowaste: Where Do We “AMES”? Antioxidants 2022, 11, 1197. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bollati, C.; Marzorati, S.; Cecchi, L.; Bartolomei, M.; Li, J.; Bellumori, M.; d’Adduzio, L.; Verotta, L.; Piazza, L.; Arnoldi, A.; et al. Valorization of the Antioxidant Effect of Mantua PGI Pear By-Product Extracts: Preparation, Analysis and Biological Investigation. Antioxidants 2023, 12, 144. https://doi.org/10.3390/antiox12010144
Bollati C, Marzorati S, Cecchi L, Bartolomei M, Li J, Bellumori M, d’Adduzio L, Verotta L, Piazza L, Arnoldi A, et al. Valorization of the Antioxidant Effect of Mantua PGI Pear By-Product Extracts: Preparation, Analysis and Biological Investigation. Antioxidants. 2023; 12(1):144. https://doi.org/10.3390/antiox12010144
Chicago/Turabian StyleBollati, Carlotta, Stefania Marzorati, Lorenzo Cecchi, Martina Bartolomei, Jianqiang Li, Maria Bellumori, Lorenza d’Adduzio, Luisella Verotta, Laura Piazza, Anna Arnoldi, and et al. 2023. "Valorization of the Antioxidant Effect of Mantua PGI Pear By-Product Extracts: Preparation, Analysis and Biological Investigation" Antioxidants 12, no. 1: 144. https://doi.org/10.3390/antiox12010144
APA StyleBollati, C., Marzorati, S., Cecchi, L., Bartolomei, M., Li, J., Bellumori, M., d’Adduzio, L., Verotta, L., Piazza, L., Arnoldi, A., Mulinacci, N., & Lammi, C. (2023). Valorization of the Antioxidant Effect of Mantua PGI Pear By-Product Extracts: Preparation, Analysis and Biological Investigation. Antioxidants, 12(1), 144. https://doi.org/10.3390/antiox12010144













