Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings
Abstract
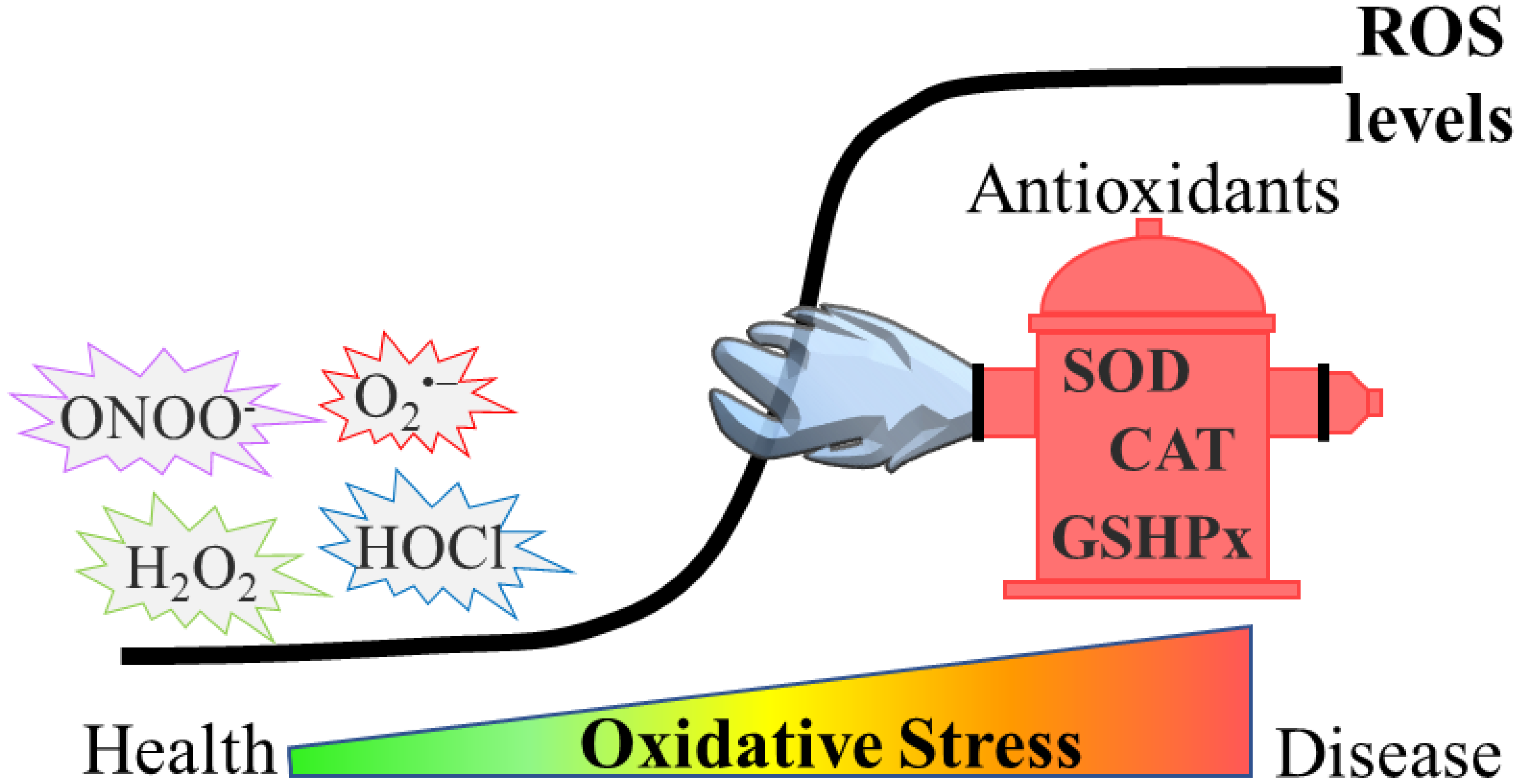
1. Introduction
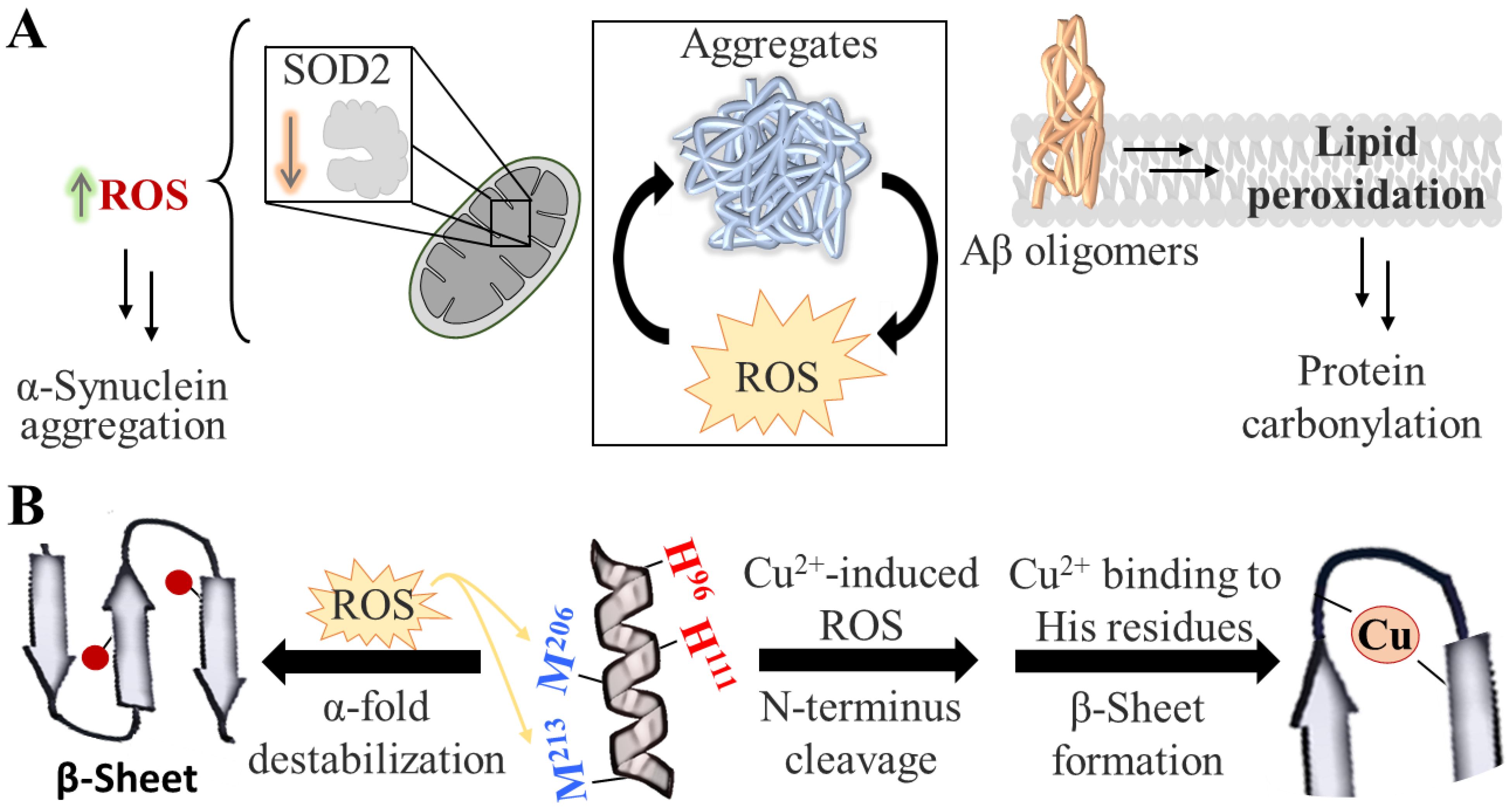
2. Protein Aggregation
3. ROS Generation and ROS-Related Processes
3.1. Major ROS Sources
3.2. Ferroptosis
3.3. Parthanatos
4. Recent Developments for Evaluating Oxidative Stress
4.1. Direct Probing of ROS
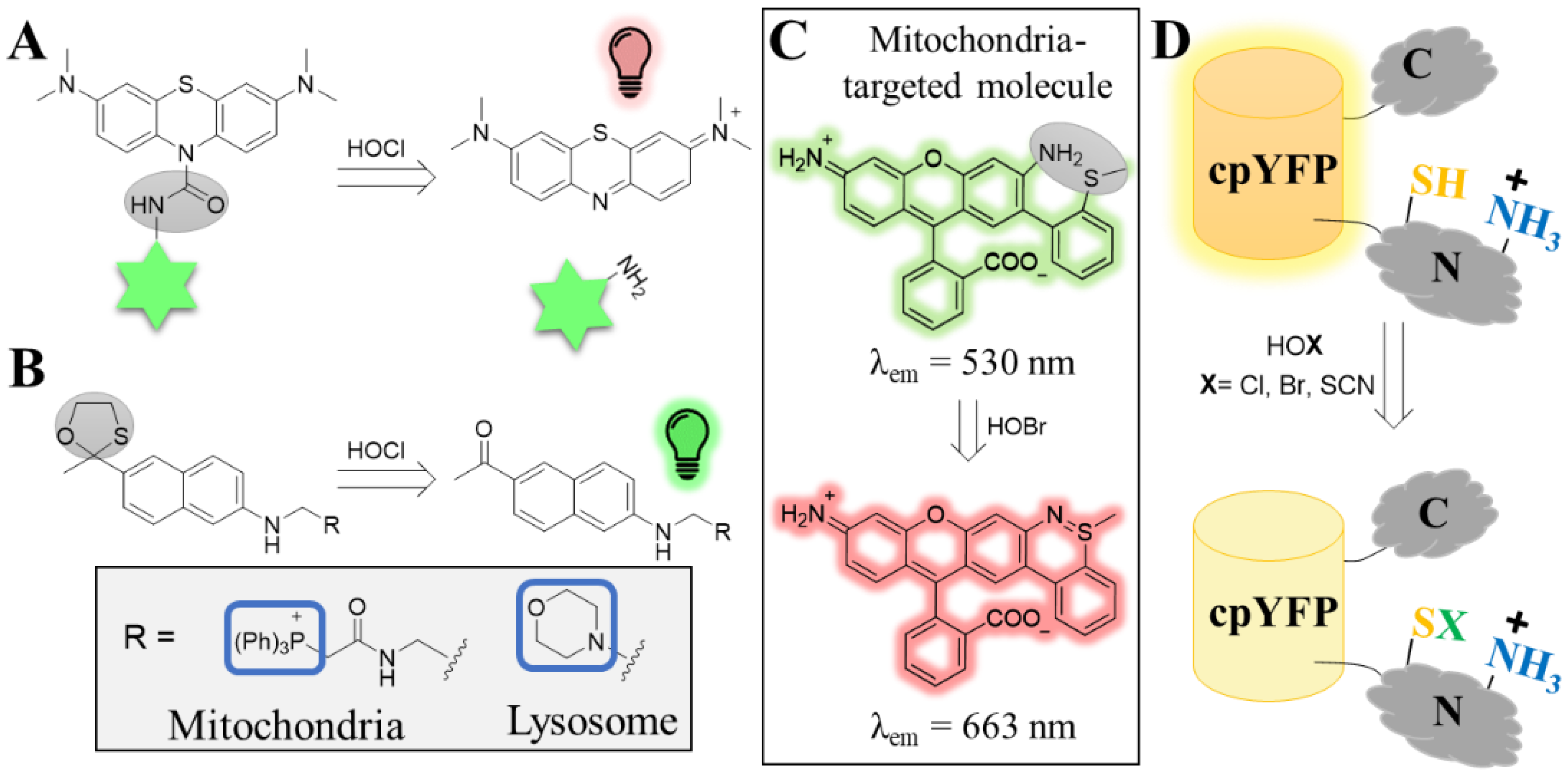
4.1.1. Hypohalous Acids (HOX)
4.1.2. Superoxide Anion (O2•−)
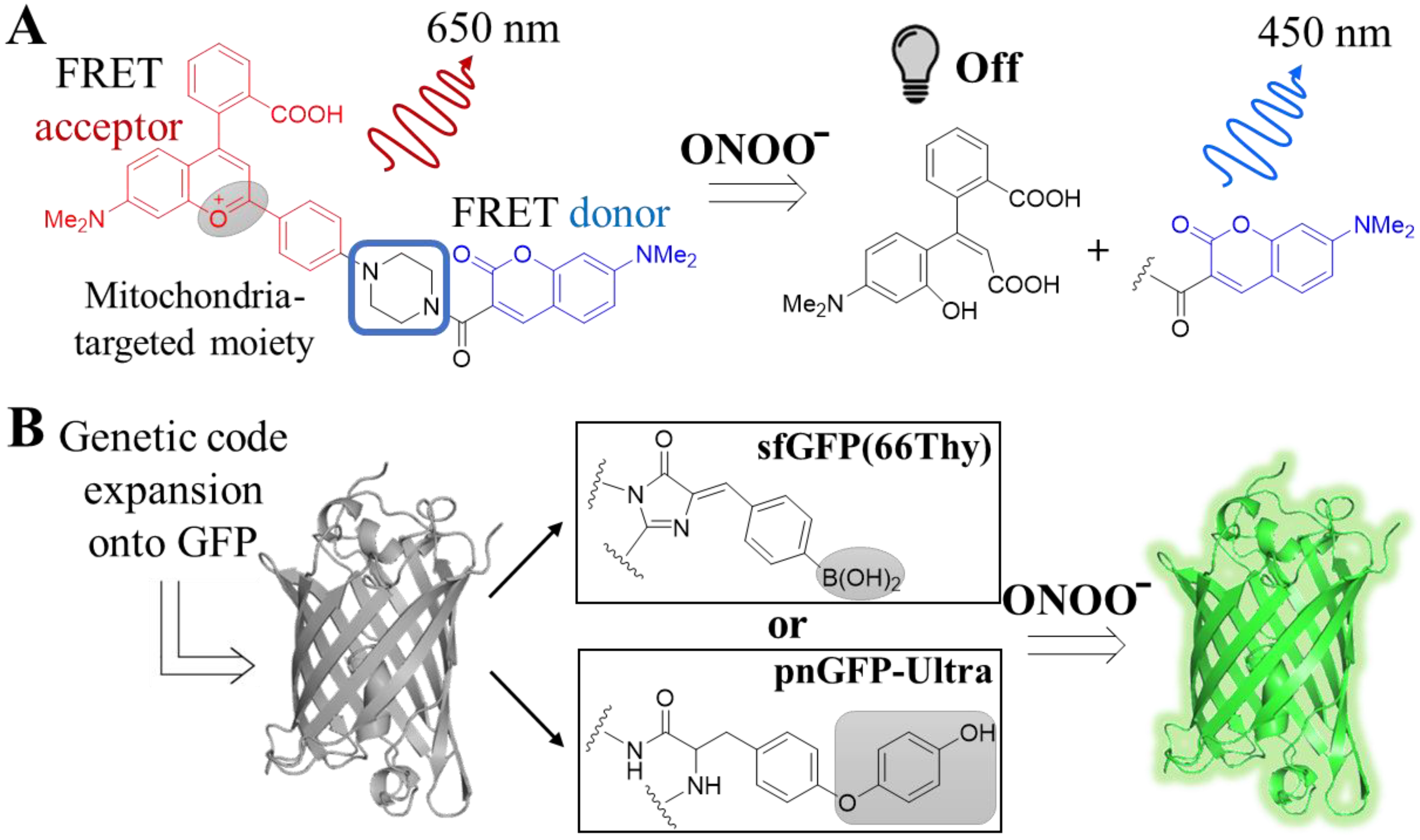
4.1.3. Peroxynitrite (ONOO−)
4.1.4. Hydrogen Peroxide (H2O2)
4.2. Mapping Protein Oxidative Damage
4.2.1. Oxidized Methionine
4.2.2. Oxidized Cysteine
4.2.3. Protein Carbonylation
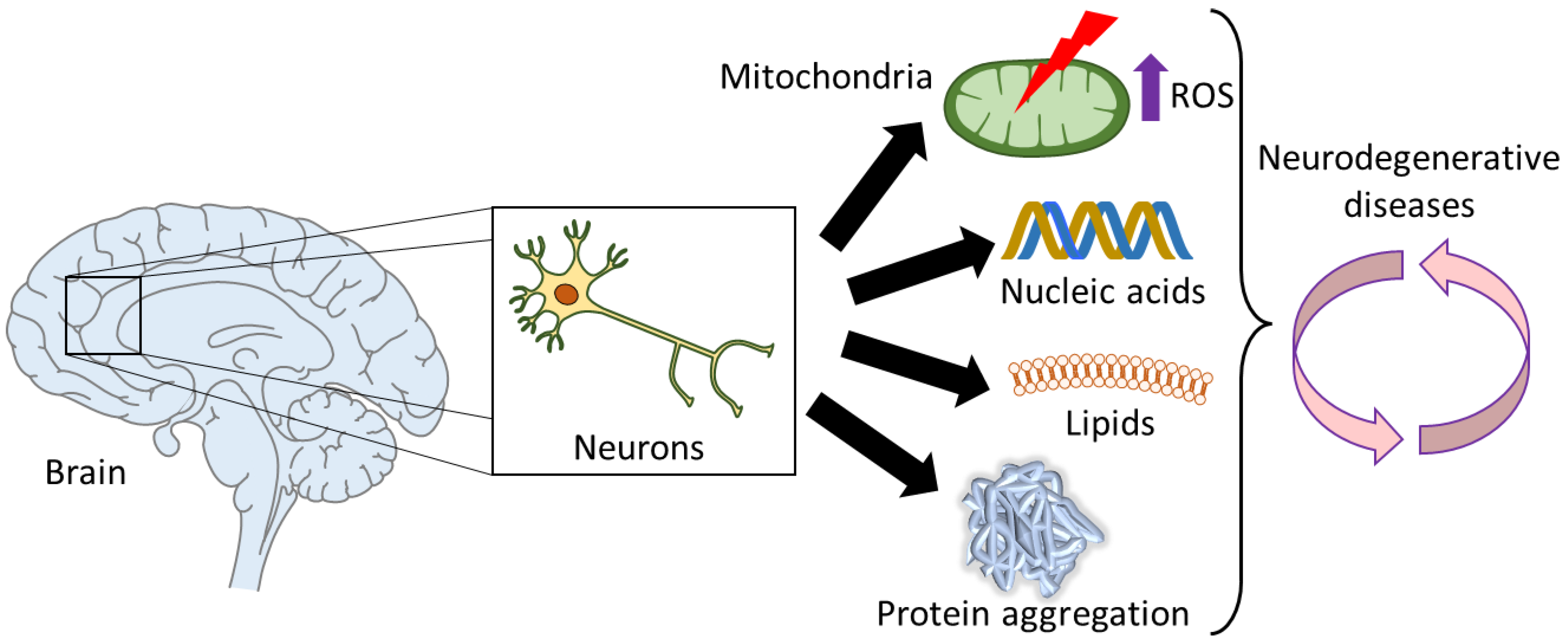
5. Oxidative Stress in Ageing and Age-Related Neurodegenerative Diseases
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lambeth, J.D. NOX Enzymes and the Biology of Reactive Oxygen. Nat. Rev. Immunol. 2004, 4, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular Mechanisms and Physiological Consequences of Redox-Dependent Signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Ray, S.; Abugable, A.A.; Parker, J.; Liversidge, K.; Palminha, N.M.; Liao, C.; Acosta-Martin, A.E.; Souza, C.D.S.; Jurga, M.; Sudbery, I.; et al. A Mechanism for Oxidative Damage Repair at Gene Regulatory Elements. Nature 2022, 609, 1038–1047. [Google Scholar] [CrossRef]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef]
- Dickinson, B.C.; Chang, C.J. Chemistry and Biology of Reactive Oxygen Species in Signaling or Stress Responses. Nat. Chem. Biol. 2011, 7, 504–511. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H. Targeting Oxidative Stress in Disease: Promise and Limitations of Antioxidant Therapy. Nat. Rev. Drug Discov. 2021, 20, 689–709. [Google Scholar] [CrossRef]
- Scudamore, O.; Ciossek, T. Increased Oxidative Stress Exacerbates α-Synuclein Aggregation In Vivo. J. Neuropathol. Exp. Neurol. 2018, 77, 443–453. [Google Scholar] [CrossRef]
- Wolschner, C.; Giese, A.; Kretzschmar, H.A.; Huber, R.; Moroder, L.; Budisa, N. Design of Anti- and pro-Aggregation Variants to Assess the Effects of Methionine Oxidation in Human Prion Protein. Proc. Natl. Acad. Sci. USA 2009, 106, 7756–7761. [Google Scholar] [CrossRef]
- Zuo, X.; Zhou, J.; Li, Y.; Wu, K.; Chen, Z.; Luo, Z.; Zhang, X.; Liang, Y.; Esteban, M.A.; Zhou, Y.; et al. TDP-43 Aggregation Induced by Oxidative Stress Causes Global Mitochondrial Imbalance in ALS. Nat. Struct. Mol. Biol. 2021, 28, 132–142. [Google Scholar] [CrossRef]
- van Dam, L.; Dansen, T.B. Cross-Talk between Redox Signalling and Protein Aggregation. Biochem. Soc. Trans. 2020, 48, 379–397. [Google Scholar] [CrossRef]
- Daiber, A.; Oelze, M.; Steven, S.; Kröller-Schön, S.; Münzel, T. Taking up the Cudgels for the Traditional Reactive Oxygen and Nitrogen Species Detection Assays and Their Use in the Cardiovascular System. Redox Biol. 2017, 12, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.A.; Poirier, M.A. Protein Aggregation and Neurodegenerative Disease. Nat. Med. 2004, 10, S10–S17. [Google Scholar] [CrossRef] [PubMed]
- Meisl, G.; Knowles, T.P.J.; Klenerman, D. Mechanistic Models of Protein Aggregation Across Length-Scales and Time-Scales: From the Test Tube to Neurodegenerative Disease. Front. Neurosci. 2022, 16, 909861. [Google Scholar] [CrossRef]
- Tutar, Y.; Özgür, A.; Tutar, L.; Tutar, Y.; Özgür, A.; Tutar, L. Role of Protein Aggregation in Neurodegenerative Diseases. Neurodegenerative Diseases; InTech: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Boyd-Kimball, D. Redox Proteomics and Amyloid β-Peptide: Insights into Alzheimer Disease. J. Neurochem. 2019, 151, 459–487. [Google Scholar] [CrossRef]
- Colombo, G.; Meli, M.; Morra, G.; Gabizon, R.; Gasset, M. Methionine Sulfoxides on Prion Protein Helix-3 Switch on the α-Fold Destabilization Required for Conversion. PLoS ONE 2009, 4, e4296. [Google Scholar] [CrossRef]
- Pushie, M.J.; Rauk, A.; Jirik, F.R.; Vogel, H.J. Can Copper Binding to the Prion Protein Generate a Misfolded Form of the Protein? BioMetals 2009, 22, 159–175. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-Aspartate Receptor-Dependent Mechanism That Is Blocked by the Alzheimer Drug Memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef]
- Du, F.; Yu, Q.; Kanaan, N.M.; Yan, S.S. Du Mitochondrial Oxidative Stress Contributes to the Pathological Aggregation and Accumulation of Tau Oligomers in Alzheimer’s Disease. Hum. Mol. Genet. 2022, 31, 2498–2507. [Google Scholar] [CrossRef]
- Deas, E.; Cremades, N.; Angelova, P.R.; Ludtmann, M.H.R.; Yao, Z.; Chen, S.; Horrocks, M.H.; Banushi, B.; Little, D.; Devine, M.J.; et al. Alpha-Synuclein Oligomers Interact with Metal Ions to Induce Oxidative Stress and Neuronal Death in Parkinson’s Disease. Antioxidants Redox Signal. 2016, 24, 376–391. [Google Scholar] [CrossRef]
- Carija, A.; Navarro, S.; de Groot, N.S.; Ventura, S. Protein Aggregation into Insoluble Deposits Protects from Oxidative Stress. Redox Biol. 2017, 12, 699–711. [Google Scholar] [CrossRef]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Tran, D.H.; Kesavan, R.; Rion, H.; Soflaee, M.H.; Solmonson, A.; Bezwada, D.; Vu, H.S.; Cai, F.; Phillips, J.A.; DeBerardinis, R.J.; et al. Mitochondrial NADP+ Is Essential for Proline Biosynthesis during Cell Growth. Nat. Metab. 2021, 3, 571–585. [Google Scholar] [CrossRef]
- Ciccarese, F.; Ciminale, V. Escaping Death: Mitochondrial Redox Homeostasis in Cancer Cells. Front. Oncol. 2017, 7, 117. [Google Scholar] [CrossRef]
- Balsa, E.; Perry, E.A.; Bennett, C.F.; Jedrychowski, M.; Gygi, S.P.; Doench, J.G.; Puigserver, P. Defective NADPH Production in Mitochondrial Disease Complex I Causes Inflammation and Cell Death. Nat. Commun. 2020, 11, 2714. [Google Scholar] [CrossRef]
- Chakravorty, A.; Jetto, C.T.; Manjithaya, R. Dysfunctional Mitochondria and Mitophagy as Drivers of Alzheimer’s Disease Pathogenesis. Front. Aging Neurosci. 2019, 11, 311. [Google Scholar] [CrossRef]
- Curtis, W.M.; Seeds, W.A.; Mattson, M.P.; Bradshaw, P.C. NADPH and Mitochondrial Quality Control as Targets for a Circadian-Based Fasting and Exercise Therapy for the Treatment of Parkinson’s Disease. Cells 2022, 11, 2416. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Vila, M.; Perier, C. Mitochondrial Quality Control in Neurodegenerative Diseases: Focus on Parkinson’s Disease and Huntington’s Disease. Front. Neurosci. 2018, 12, 342. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kumar, V.; Sharma, D.; Devi, B.; Kapil, L.; Singh, C.; Singh, A. The Role of Mitophagy in Various Neurological Diseases as a Therapeutic Approach. Cell. Mol. Neurobiol. 2022. [Google Scholar] [CrossRef]
- Kim, J.; Bai, H. Peroxisomal Stress Response and Inter-Organelle Communication in Cellular Homeostasis and Aging. Antioxidants 2022, 11, 192. [Google Scholar] [CrossRef]
- Salvador, G.A. Iron in Neuronal Function and Dysfunction. BioFactors 2010, 36, 103–110. [Google Scholar] [CrossRef]
- Muñoz, P.; Humeres, A. Iron Deficiency on Neuronal Function. BioMetals 2012, 25, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Li, L.B.; Chai, R.; Zhang, S.; Xu, S.F.; Zhang, Y.H.; Li, H.L.; Fan, Y.G.; Guo, C. Iron Exposure and the Cellular Mechanisms Linked to Neuron Degeneration in Adult Mice. Cells 2019, 8, 198. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, F.; Yin, H.L.; Huang, Z.J.; Lin, Z.T.; Mao, N.; Sun, B.; Wang, G. Ferroptosis: Past, Present and Future. Cell Death Dis. 2020, 11, 88. [Google Scholar] [CrossRef] [PubMed]
- Angelova, P.R.; Choi, M.L.; Berezhnov, A.V.; Horrocks, M.H.; Hughes, C.D.; De, S.; Rodrigues, M.; Yapom, R.; Little, D.; Dolt, K.S.; et al. Alpha Synuclein Aggregation Drives Ferroptosis: An Interplay of Iron, Calcium and Lipid Peroxidation. Cell Death Differ. 2020, 27, 2781–2796. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhao, J.; Zhao, Y.; Zhou, L.; Qiao, H.; Xu, Q.; Liu, Y. The Role of Ferroptosis in Neurodegenerative Diseases. Mol. Biol. Rep. 2022. [Google Scholar] [CrossRef]
- Onukwufor, J.O.; Dirksen, R.T.; Wojtovich, A.P. Iron Dysregulation in Mitochondrial Dysfunction and Alzheimer’s Disease. Antioxidants 2022, 11, 692. [Google Scholar] [CrossRef]
- Huang, X.T.; Liu, X.; Ye, C.Y.; Tao, L.X.; Zhou, H.; Zhang, H.Y. Iron-Induced Energy Supply Deficiency and Mitochondrial Fragmentation in Neurons. J. Neurochem. 2018, 147, 816–830. [Google Scholar] [CrossRef]
- Lee, D.G.; Kam, M.K.; Lee, S.R.; Lee, H.J.; Lee, D.S. Peroxiredoxin 5 Deficiency Exacerbates Iron Overload-Induced Neuronal Death via ER-Mediated Mitochondrial Fission in Mouse Hippocampus. Cell Death Dis. 2020, 11, 204. [Google Scholar] [CrossRef]
- Li, J.; Jia, B.; Cheng, Y.; Song, Y.; Li, Q.; Luo, C. Targeting Molecular Mediators of Ferroptosis and Oxidative Stress for Neurological Disorders. Oxid. Med. Cell. Longev. 2022, 2022, 3999083. [Google Scholar] [CrossRef]
- Huang, P.; Chen, G.; Jin, W.; Mao, K.; Wan, H.; He, Y. Molecular Mechanisms of Parthanatos and Its Role in Diverse Diseases. Int. J. Mol. Sci. 2022, 23, 7292. [Google Scholar] [CrossRef]
- Koehler, R.C.; Dawson, V.L.; Dawson, T.M. Targeting Parthanatos in Ischemic Stroke. Front. Neurol. 2021, 12, 622. [Google Scholar] [CrossRef]
- Abo, M.; Weerapana, E. Chemical Probes for Redox Signaling and Oxidative Stress. Antioxid. Redox Signal. 2019, 30, 1369–1386. [Google Scholar] [CrossRef]
- Matsuoka, Y.; Yamada, K. Detection and Structural Analysis of Lipid-Derived Radicals in Vitro and in Vivo. Free Radic. Res. 2021, 55, 441–449. [Google Scholar] [CrossRef]
- Su, D.; Wang, X.; Zhang, W.; Li, P.; Tang, B. Fluorescence Imaging for Visualizing the Bioactive Molecules of Lipid Peroxidation within Biological Systems. TrAC Trends Anal. Chem. 2022, 146, 116484. [Google Scholar] [CrossRef]
- Guo, C.; Ding, P.; Xie, C.; Ye, C.; Ye, M.; Pan, C.; Cao, X.; Zhang, S.; Zheng, S. Potential Application of the Oxidative Nucleic Acid Damage Biomarkers in Detection of Diseases. Oncotarget 2017, 8, 75767. [Google Scholar] [CrossRef]
- Chao, M.-R.; Evans, M.D.; Hu, C.-W.; Ji, Y.; Møller, P.; Rossner, P.; Cooke, M.S. Biomarkers of Nucleic Acid Oxidation—A Summary State-of-the-Art. Redox Biol. 2021, 42, 101872. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and Methods to Measure Oxidative Stress in Clinical Samples: Research Applications in the Cancer Field. Oxid. Med. Cell. Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Gaggini, M.; Sabatino, L.; Vassalle, C. Conventional and Innovative Methods to Assess Oxidative Stress Biomarkers in the Clinical Cardiovascular Setting. Biotechniques 2020, 68, 223–231. [Google Scholar] [CrossRef]
- Wei, P.; Yuan, W.; Xue, F.; Zhou, W.; Li, R.; Zhang, D.; Yi, T. Deformylation Reaction-Based Probe for in Vivo Imaging of HOCl. Chem. Sci. 2018, 9, 495–501. [Google Scholar] [CrossRef]
- Wei, P.; Liu, L.; Wen, Y.; Zhao, G.; Xue, F.; Yuan, W.; Li, R.; Zhong, Y.; Zhang, M.; Yi, T. Release of Amino- or Carboxy-Containing Compounds Triggered by HOCl: Application for Imaging and Drug Design. Angew. Chemie Int. Ed. 2019, 58, 4547–4551. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, L.; Agrawalla, B.K.; Park, S.-J.; Zhu, H.; Sivaraman, B.; Peng, J.; Xu, Q.-H.; Chang, Y.-T. Development of Targetable Two-Photon Fluorescent Probes to Image Hypochlorous Acid in Mitochondria and Lysosome in Live Cell and Inflamed Mouse Model. J. Am. Chem. Soc. 2015, 137, 5930–5938. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zheng, A.; Luan, D.; Wang, X.; Kong, F.; Tong, L.; Xu, K.; Tang, B. High-Quantum-Yield Mitochondria-Targeting Near-Infrared Fluorescent Probe for Imaging Native Hypobromous Acid in Living Cells and in Vivo. Anal. Chem. 2017, 89, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, A.I.; Tossounian, M.-A.; Panova, A.S.; Thauvin, M.; Raevskii, R.I.; Ezeriņa, D.; Wahni, K.; Van Molle, I.; Sergeeva, A.D.; Vertommen, D.; et al. Hypocrates Is a Genetically Encoded Fluorescent Biosensor for (Pseudo)Hypohalous Acids and Their Derivatives. Nat. Commun. 2022, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xu, W.; Yang, Z.; Li, S.; Wang, Y.; Hua, J. A Turn-on Mitochondria-Targeted near-Infrared Fluorescent Probe with a Large Stokes Shift for Detecting and Imaging Endogenous Superoxide Anion in Cells. J. Photochem. Photobiol. A Chem. 2021, 415, 113304. [Google Scholar] [CrossRef]
- Li, R.-Q.; Mao, Z.-Q.; Rong, L.; Wu, N.; Lei, Q.; Zhu, J.-Y.; Zhuang, L.; Zhang, X.-Z.; Liu, Z.-H. A Two-Photon Fluorescent Probe for Exogenous and Endogenous Superoxide Anion Imaging in Vitro and in Vivo. Biosens. Bioelectron. 2017, 87, 73–80. [Google Scholar] [CrossRef]
- Wang, R.; Han, X.; You, J.; Yu, F.; Chen, L. Ratiometric Near-Infrared Fluorescent Probe for Synergistic Detection of Monoamine Oxidase B and Its Contribution to Oxidative Stress in Cell and Mice Aging Models. Anal. Chem. 2018, 90, 4054–4061. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Pan, Y.; Wang, L.; Zeng, Z.; Yuan, L.; Zhang, X.; Chang, Y.-T. Selective Visualization of the Endogenous Peroxynitrite in an Inflamed Mouse Model by a Mitochondria-Targetable Two-Photon Ratiometric Fluorescent Probe. J. Am. Chem. Soc. 2017, 139, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, B.; Kobayashi, T.; Yu, B.; Liu, J.; Wang, L. Genetically Encoding Thyronine for Fluorescent Detection of Peroxynitrite. Bioorg. Med. Chem. 2020, 28, 115665. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Li, X.; Ai, H. A High-Performance Genetically Encoded Fluorescent Biosensor for Imaging Physiological Peroxynitrite. Cell Chem. Biol. 2021, 28, 1542–1553. [Google Scholar] [CrossRef]
- Zhu, H.; Tamura, T.; Fujisawa, A.; Nishikawa, Y.; Cheng, R.; Takato, M.; Hamachi, I. Imaging and Profiling of Proteins under Oxidative Conditions in Cells and Tissues by Hydrogen-Peroxide-Responsive Labeling. J. Am. Chem. Soc. 2020, 142, 15711–15721. [Google Scholar] [CrossRef]
- Sugiura, K.; Mihara, S.; Fu, N.; Hisabori, T. Real-Time Monitoring of the in Vivo Redox State Transition Using the Ratiometric Redox State Sensor Protein FROG/B. Proc. Natl. Acad. Sci. USA 2020, 117, 16019–16026. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Yang, X.; Jia, S.; Weeks, A.M.; Hornsby, M.; Lee, P.S.; Nichiporuk, R.V.; Iavarone, A.T.; Wells, J.A.; Toste, F.D.; et al. Redox-Based Reagents for Chemoselective Methionine Bioconjugation. Science 2017, 355, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Yang, J.; Liebler, D.C.; Carroll, K.S. Diverse Redoxome Reactivity Profiles of Carbon Nucleophiles. J. Am. Chem. Soc. 2017, 139, 5588–5595. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Fu, L.; Yang, J.; Carroll, K.S. Wittig Reagents for Chemoselective Sulfenic Acid Ligation Enables Global Site Stoichiometry Analysis and Redox-Controlled Mitochondrial Targeting. Nat. Chem. 2021, 13, 1140–1150. [Google Scholar] [CrossRef]
- Ferreira, R.B.; Fu, L.; Jung, Y.; Yang, J.; Carroll, K.S. Reaction-Based Fluorogenic Probes for Detecting Protein Cysteine Oxidation in Living Cells. Nat. Commun. 2022, 13, 5522. [Google Scholar] [CrossRef]
- Lo Conte, M.; Lin, J.; Wilson, M.A.; Carroll, K.S. A Chemical Approach for the Detection of Protein Sulfinylation. ACS Chem. Biol. 2015, 10, 1825–1830. [Google Scholar] [CrossRef]
- Akter, S.; Fu, L.; Jung, Y.; Conte, M.L.; Lawson, J.R.; Lowther, W.T.; Sun, R.; Liu, K.; Yang, J.; Carroll, K.S. Chemical Proteomics Reveals New Targets of Cysteine Sulfinic Acid Reductase. Nat. Chem. Biol. 2018, 14, 995–1004. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Lan, T.; Qin, W.; Zhu, Y.; Qin, K.; Gao, J.; Wang, H.; Hou, X.; Chen, N.; et al. Quantitative Profiling of Protein Carbonylations in Ferroptosis by an Aniline-Derived Probe. J. Am. Chem. Soc. 2018, 140, 4712–4720. [Google Scholar] [CrossRef]
- Mukherjee, K.; Chio, T.I.; Gu, H.; Sackett, D.L.; Bane, S.L.; Sever, S. A Novel Fluorogenic Assay for the Detection of Nephrotoxin-Induced Oxidative Stress in Live Cells and Renal Tissue. ACS Sens. 2021, 6, 2523–2528. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J.; Hawkins, C.L. Reactions and Reactivity of Myeloperoxidase-Derived Oxidants: Differential Biological Effects of Hypochlorous and Hypothiocyanous Acids. Free Radic. Res. 2012, 46, 975–995. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Obinger, C. Reaction of Myeloperoxidase Compound I with Chloride, Bromide, Iodide, and Thiocyanate. Biochemistry 1998, 37, 17923–17930. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zhong, G.; Zhao, Y.; Zhang, P.; Fu, Y.; Shen, B. Recent Development of Synthetic Probes for Detection of Hypochlorous Acid/Hypochlorite. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118545. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Lu, Y.; Saredy, J.; Wang, X.; Drummer IV, C.; Shao, Y.; Saaoud, F.; Xu, K.; Liu, M.; Yang, W.Y.; et al. ROS Systems Are a New Integrated Network for Sensing Homeostasis and Alarming Stresses in Organelle Metabolic Processes. Redox Biol. 2020, 37, 101696. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Reconciling the Chemistry and Biology of Reactive Oxygen Species. Nat. Chem. Biol. 2008, 4, 278–286. [Google Scholar] [CrossRef]
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine Is an Essential Trace Element for Assembly of Collagen IV Scaffolds in Tissue Development and Architecture. Cell 2014, 157, 1380–1392. [Google Scholar] [CrossRef]
- Xu, K.; Luan, D.; Wang, X.; Hu, B.; Liu, X.; Kong, F.; Tang, B. An Ultrasensitive Cyclization-Based Fluorescent Probe for Imaging Native HOBr in Live Cells and Zebrafish. Angew. Chemie Int. Ed. 2016, 55, 12751–12754. [Google Scholar] [CrossRef]
- Gray, M.J.; Li, Y.; Leichert, L.I.-O.; Xu, Z.; Jakob, U. Does the Transcription Factor NemR Use a Regulatory Sulfenamide Bond to Sense Bleach? Antioxid. Redox Signal. 2015, 23, 747–754. [Google Scholar] [CrossRef]
- Wang, W.; Fang, H.; Groom, L.; Cheng, A.; Zhang, W.; Liu, J.; Wang, X.; Li, K.; Han, P.; Zheng, M.; et al. Superoxide Flashes in Single Mitochondria. Cell 2008, 134, 279–290. [Google Scholar] [CrossRef]
- Schwarzländer, M.; Wagner, S.; Ermakova, Y.G.; Belousov, V.V.; Radi, R.; Beckman, J.S.; Buettner, G.R.; Demaurex, N.; Duchen, M.R.; Forman, H.J.; et al. The ‘Mitoflash’ Probe CpYFP Does Not Respond to Superoxide. Nature 2014, 514, E12–E14. [Google Scholar] [CrossRef]
- Szabó, C.; Ischiropoulos, H.; Radi, R. Peroxynitrite: Biochemistry, Pathophysiology and Development of Therapeutics. Nat. Rev. Drug Discov. 2007, 6, 662–680. [Google Scholar] [CrossRef]
- Xiong, J.; Wang, W.; Wang, C.; Zhong, C.; Ruan, R.; Mao, Z.; Liu, Z. Visualizing Peroxynitrite in Microvessels of the Brain with Stroke Using an Engineered Highly Specific Fluorescent Probe. ACS Sens. 2020, 5, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ryu, J.-C.; Chung, Y.W.; Lee, D.; Ryu, J.-H.; Yoon, J.-H.; Yoon, J. A Far-Red-Emitting Fluorescence Probe for Sensitive and Selective Detection of Peroxynitrite in Live Cells and Tissues. Anal. Chem. 2017, 89, 10924–10931. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Gong, D.; Ru, J.; Guo, Y.; Cao, T.; Liu, W.; Iqbal, A.; Iqbal, K.; Qin, W.; Guo, H. A Naphthalimide-Based Lysosome-Targeting Fluorescent Probe for the Selective Detection and Imaging of Endogenous Peroxynitrite in Living Cells. Anal. Bioanal. Chem. 2019, 411, 3929–3939. [Google Scholar] [CrossRef] [PubMed]
- Davis, L.; Chin, J.W. Designer Proteins: Applications of Genetic Code Expansion in Cell Biology. Nat. Rev. Mol. Cell Biol. 2012, 13, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Davis, L.; Baxter, K.; Tynan, A.; Goutou, A.; Greiss, S. Using a Quadruplet Codon to Expand the Genetic Code of an Animal. Nucleic Acids Res. 2022, 50, 4801–4812. [Google Scholar] [CrossRef]
- Lee, S.-R.; Kwon, K.-S.; Kim, S.-R.; Rhee, S.G. Reversible Inactivation of Protein-Tyrosine Phosphatase 1B in A431 Cells Stimulated with Epidermal Growth Factor. J. Biol. Chem. 1998, 273, 15366–15372. [Google Scholar] [CrossRef]
- He, D.; Feng, H.; Sundberg, B.; Yang, J.; Powers, J.; Christian, A.H.; Wilkinson, J.E.; Monnin, C.; Avizonis, D.; Thomas, C.J.; et al. Methionine Oxidation Activates Pyruvate Kinase M2 to Promote Pancreatic Cancer Metastasis. Mol. Cell 2022, 82, 3045–3060.e11. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Wright, A.T.; Kozarich, J.W. Activity-Based Protein Profiling: From Enzyme Chemistry to Proteomic Chemistry. Annu. Rev. Biochem. 2008, 77, 383–414. [Google Scholar] [CrossRef]
- Hung, R.-J.; Pak, C.W.; Terman, J.R. Direct Redox Regulation of F-Actin Assembly and Disassembly by Mical. Science 2011, 334, 1710–1713. [Google Scholar] [CrossRef]
- Mnatsakanyan, R.; Markoutsa, S.; Walbrunn, K.; Roos, A.; Verhelst, S.H.L.; Zahedi, R.P. Proteome-Wide Detection of S-Nitrosylation Targets and Motifs Using Bioorthogonal Cleavable-Linker-Based Enrichment and Switch Technique. Nat. Commun. 2019, 10, 2195. [Google Scholar] [CrossRef]
- Madian, A.G.; Diaz-Maldonado, N.; Gao, Q.; Regnier, F.E. Oxidative Stress Induced Carbonylation in Human Plasma. J. Proteom. 2011, 74, 2395–2416. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Guo, C.; Kong, J. Oxidative Stress in Neurodegenerative Diseases. Neural Regen. Res. 2012, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, S.K. Anti-Oxidant and Anti-Aging Effects of Phlorizin Are Mediated by DAF-16-Induced Stress Response and Autophagy in Caenorhabditis Elegans. Antioxidants 2022, 11, 1996. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.B.; Zhang, Z.P.; Bai, X.; Wang, S.S.; Chen, X.H.; Liu, X.; Su, P.J.; Zhi, D.J.; Fei, D.Q.; Zhang, Z.X.; et al. Cryptotanshinone Alleviates Oxidative Stress and Reduces the Level of Abnormally Aggregated Protein in Caenorhabditis Elegans AD Models. Int. J. Mol. Sci. 2022, 23, 10030. [Google Scholar] [CrossRef] [PubMed]
- Wink, M.; Duangjan, C.; Zhang, S.; Song, X.; Wang, X. Exendin-4 Alleviates β-Amyloid Peptide Toxicity via DAF-16 in a Caenorhabditis Elegans Model of Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 955113. [Google Scholar] [CrossRef]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative Stress and Parkinson’s Disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef]
- Morales-Martínez, A.; Martínez-Gómez, P.A.; Martinez-Fong, D.; Villegas-Rojas, M.M.; Pérez-Severiano, F.; Del Toro-Colín, M.A.; Delgado-Minjares, K.M.; Blanco-Alvarez, V.M.; Leon-Chavez, B.A.; Aparicio-Trejo, O.E.; et al. Oxidative Stress and Mitochondrial Complex I Dysfunction Correlate with Neurodegeneration in an α-Synucleinopathy Animal Model. Int. J. Mol. Sci. 2022, 23, 11394. [Google Scholar] [CrossRef]
- Aoyama, T.; Peters, J.M.; Iritani, N.; Nakajima, T.; Furihata, K.; Hashimoto, T.; Gonzalez, F.J. Altered Constitutive Expression of Fatty Acid-Metabolizing Enzymes in Mice Lacking the Peroxisome Proliferator-Activated Receptor α (PPARα). J. Biol. Chem. 1998, 273, 5678–5684. [Google Scholar] [CrossRef]
- Cuttler, K.; de Swardt, D.; Engelbrecht, L.; Kriel, J.; Cloete, R.; Bardien, S. Neurexin 2 p.G849D Variant, Implicated in Parkinson’s Disease, Increases Reactive Oxygen Species, and Reduces Cell Viability and Mitochondrial Membrane Potential in SH-SY5Y Cells. J. Neural Transm. 2022, 129, 1435–1446. [Google Scholar] [CrossRef]
- Sasazawa, Y.; Souma, S.; Furuya, N.; Miura, Y.; Kazuno, S.; Kakuta, S.; Suzuki, A.; Hashimoto, R.; Hirawake-Mogi, H.; Date, Y.; et al. Oxidative Stress-Induced Phosphorylation of JIP4 Regulates Lysosomal Positioning in Coordination with TRPML1 and ALG2. EMBO J. 2022, 41, e111476. [Google Scholar] [CrossRef]
- Abubakar, M.B.; Sanusi, K.O.; Ugusman, A.; Mohamed, W.; Kamal, H.; Ibrahim, N.H.; Khoo, C.S.; Kumar, J. Alzheimer’s Disease: An Update and Insights Into Pathophysiology. Front. Aging Neurosci. 2022, 14, 234. [Google Scholar] [CrossRef] [PubMed]
- Misrani, A.; Tabassum, S.; Yang, L. Mitochondrial Dysfunction and Oxidative Stress in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 57. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhang, L.; Zhou, F.; Wang, Y.; Zhang, X.; Fan, J.; Li, S.; Li, X.; Li, Y. Scavenging Reactive Oxygen Species Decreases Amyloid-β Levels via Activation of PI3K/Akt/GLUT1 Pathway in N2a/APP695swe Cells. J. Alzheimers Dis. 2022, 90, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Villegas, L.; Nørremølle, A.; Freude, K.; Vilhardt, F. Nicotinamide Adenine Dinucleotide Phosphate Oxidases Are Everywhere in Brain Disease, but Not in Huntington’s Disease? Front. Aging Neurosci. 2021, 13, 736734. [Google Scholar] [CrossRef]
- Smatlikova, P.; Askeland, G.; Vaskovicova, M.; Klima, J.; Motlik, J.; Eide, L.; Ellederová, Z. Age-Related Oxidative Changes in Primary Porcine Fibroblasts Expressing Mutated Huntingtin. Neurodegener. Dis. 2019, 19, 22–34. [Google Scholar] [CrossRef]
- Machiela, E.; Jeloka, R.; Caron, N.S.; Mehta, S.; Schmidt, M.E.; Baddeley, H.J.E.; Tom, C.M.; Polturi, N.; Xie, Y.; Mattis, V.B.; et al. The Interaction of Aging and Cellular Stress Contributes to Pathogenesis in Mouse and Human Huntington Disease Neurons. Front. Aging Neurosci. 2020, 12, 524369. [Google Scholar] [CrossRef]
- Machiela, E.; Southwell, A.L. Biological Aging and the Cellular Pathogenesis of Huntington’s Disease. J. Huntingt. Dis. 2020, 9, 115–128. [Google Scholar] [CrossRef]
- Pinho, B.R.; Reis, S.D.; Hartley, R.C.; Murphy, M.P.; Oliveira, J.M.A. Mitochondrial Superoxide Generation Induces a Parkinsonian Phenotype in Zebrafish and Huntingtin Aggregation in Human Cells. Free Radic. Biol. Med. 2019, 130, 318–327. [Google Scholar] [CrossRef]
- Liu, L.; Bai, J.; Liu, F.; Xu, Y.; Zhao, M.; Zhao, C.; Zhou, Z. Cross-Talking Pathways of Forkhead Box O1 (FOXO1) Are Involved in the Pathogenesis of Alzheimer’s Disease and Huntington’s Disease. Oxid. Med. Cell. Longev. 2022, 2022, 7619255. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Stefanatos, R.; Sanz, A. The Role of Mitochondrial ROS in the Aging Brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Gammon, K. Neurodegenerative Disease: Brain Windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fu, L.; Liu, K.; Tian, C.; Wu, Z.; Jung, Y.; Ferreira, R.B.; Carroll, K.S.; Blackwell, T.K.; Yang, J. Global Profiling of Distinct Cysteine Redox Forms Reveals Wide-Ranging Redox Regulation in C. Elegans. Nat. Commun. 2021, 12, 1415. [Google Scholar] [CrossRef] [PubMed]

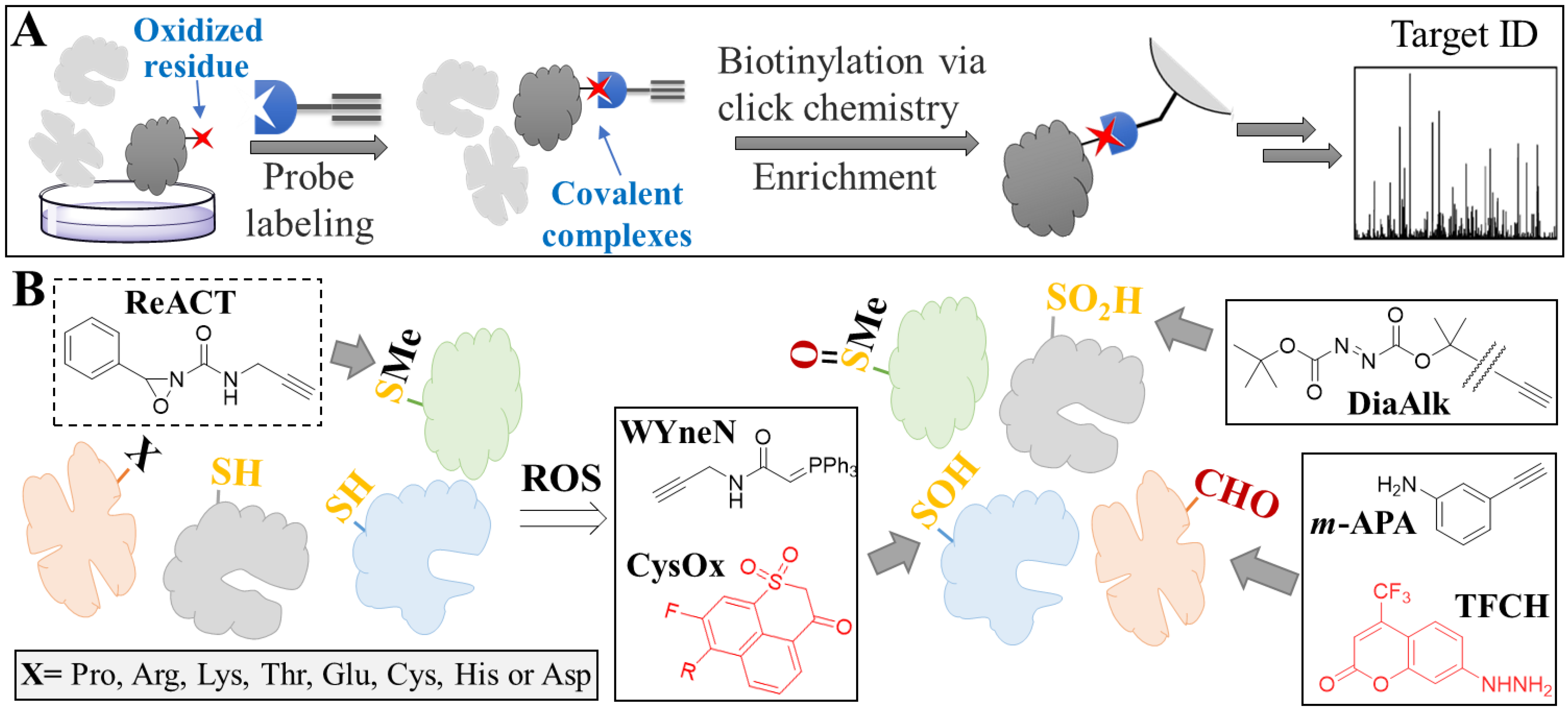
| Evaluation Method | Detection | Tool | Application | Key Feature | Notes | Ref |
|---|---|---|---|---|---|---|
| ROS | Hypochlorous acid (HOCl) | FDOCl-1 a | Imaging | Near-infrared (NIR) emission, turn-on probe | [50] | |
| FDOCl-18 a | Visualization in the off state (additionally to the above) | [51] | ||||
| LYSO- & MITO-TP a | Targeted imaging of mitochondria or lysosome | [52] | ||||
| Hypobromous acid (HOBr) | RhSN-mito a | Ratiometric probe | Selective over HOCl | [53] | ||
| All hypohalous acids (HOX) | Hypocrates b | First biosensor specific for HOX, ratiometric imaging | [54] | |||
| Superoxide anion (O2•−) | DMPS-O a | Turn-on, mitochondria targeting probe with NIR emission | [55] | |||
| HQ a | Two-photon excitability | [56] | ||||
| MitoHCy-NH2 a | Detection of ROS generated by a certain protein | [57] | ||||
| Peroxynitrite (ONOO−) | MITO-CC a | FRET-based, mitochondria targeting probe with two-photon excitability | [58] | |||
| sfGFP(66Thy) b | Turn-on reporters with non-natural amino acids | [59] | ||||
| pnGFP-Ultra b | [60] | |||||
| Hydrogen peroxide (H2O2) | Hyp-L a | Imaging/ Proteomics | Labeling of H2O2-surrounding proteins | [61] | ||
| FROG/B b | Imaging | Excitation state intramolecular proton transfer (ESIPT) mechanism | Requires a single excitation wavelength but its emission is redox-dependent | [62] | ||
| Damaged proteins | Oxidized methionine | ReACT a | Proteomics | First selective probe for methionine | Note that results regarding methionine oxidation are by elimination | [63] |
| Sulfonic acid | BTD a | High reactivity towards sulfenic acid-modified proteins | Commercial | [64] | ||
| WYneN a | 10-fold increase in kinetics (compared to BTD) | [65] | ||||
| CysOx a | Imaging | Fluorogenic probe for live cell imaging | [66] | |||
| Sulfinic acid | NO-Bio a | Proteomics | High selectivity over sulfenic acid | Commercial | [67] | |
| DiaAlk a | Additional selectivity (compared to NO-Bio) over cysteine | Commercial | [68] | |||
| Carbonylation | m-APA a | High reaction kinetics and selectivity | [69] | |||
| TFCH a | Imaging | High sensitivity and suitable for live cell imaging | [70] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korovesis, D.; Rubio-Tomás, T.; Tavernarakis, N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants 2023, 12, 131. https://doi.org/10.3390/antiox12010131
Korovesis D, Rubio-Tomás T, Tavernarakis N. Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants. 2023; 12(1):131. https://doi.org/10.3390/antiox12010131
Chicago/Turabian StyleKorovesis, Dimitris, Teresa Rubio-Tomás, and Nektarios Tavernarakis. 2023. "Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings" Antioxidants 12, no. 1: 131. https://doi.org/10.3390/antiox12010131
APA StyleKorovesis, D., Rubio-Tomás, T., & Tavernarakis, N. (2023). Oxidative Stress in Age-Related Neurodegenerative Diseases: An Overview of Recent Tools and Findings. Antioxidants, 12(1), 131. https://doi.org/10.3390/antiox12010131







