Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. General Procedures for Resveratrol Oxidation
2.3. Reaction with PIFA in Acetonitrile (Ox1)
2.4. Reaction of Resveratrol with AAPH and NaIO4 (Ox2)
2.5. Reaction of Resveratrol with PIDA in Acetonitrile (Ox3)
2.6. Reaction with PIFA in Ethanol (Ox4)
2.7. Reaction of Resveratrol with Periodic Acid and Oxone in Ethanol (Ox5)
2.8. Reaction of Resveratrol with FeCl3 and H5IO6 in Acetonitrile (Ox6)
2.9. Reaction of Resveratrol with FeCl3 and H5IO6 in Ethanol (Ox7)
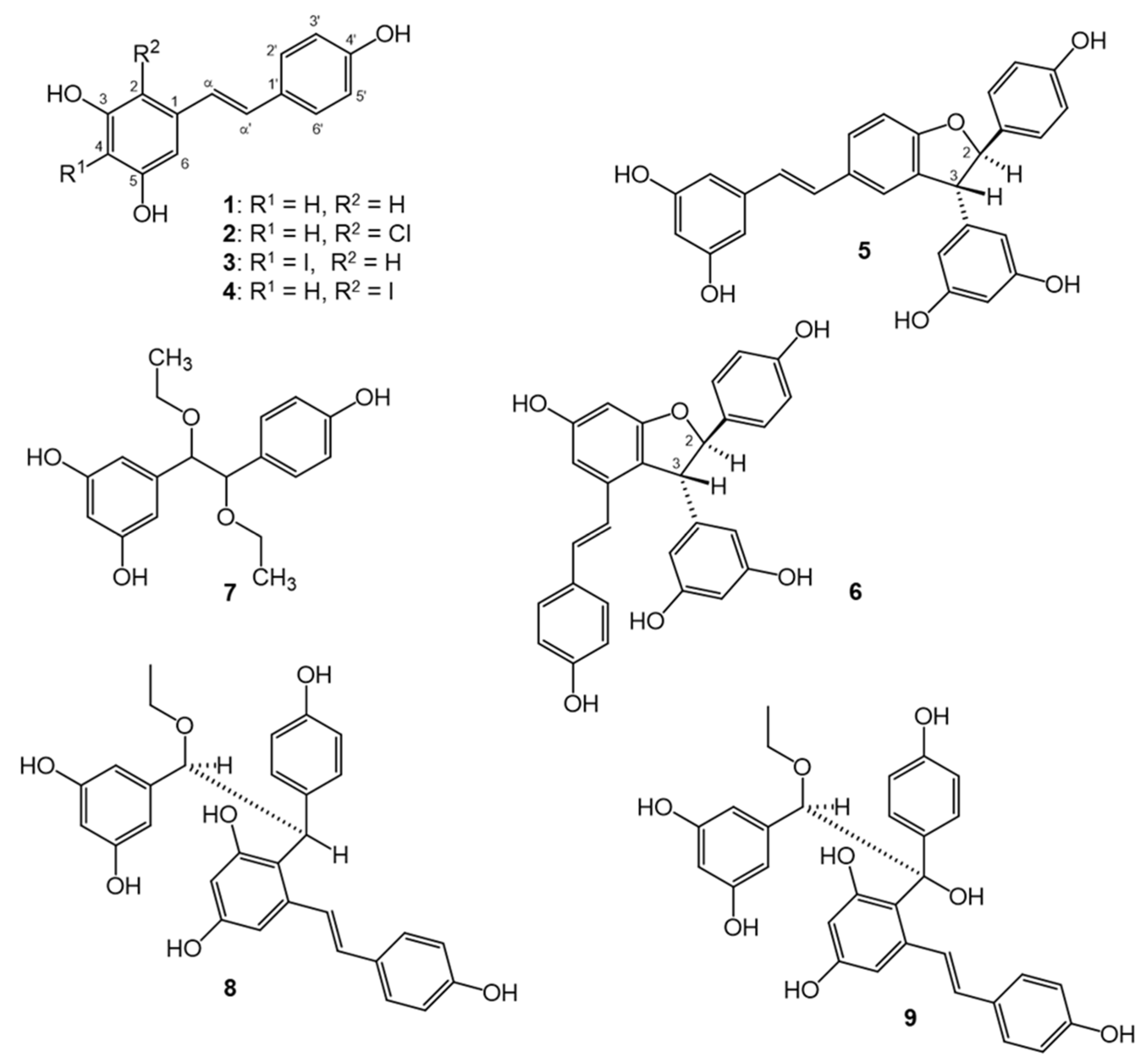
2.10. Structure Elucidation
2.11. Bioactivity Studies
2.11.1. Xanthine Oxidase Inhibitory Activity
2.11.2. DPPH Radical Scavenging Activity
2.11.3. Oxygen Radical Absorbance Capacity (ORAC) Assay
3. Results and Discussion
3.1. Preparation and Evaluation of Oxidized Resveratrol Metabolites
3.2. Structure Elucidation of the Isolated Compounds
3.3. Bioactivity of the Isolated Compounds
3.3.1. Xanthine Oxidase Inhibitory Activity
3.3.2. Free Radical Scavenging Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dybkowska, E.; Sadowska, A.; Swiderski, F.; Rakowska, R.; Wysocka, K. The occurrence of resveratrol in foodstuffs and its potential for supporting cancer prevention and treatment. A review. Rocz. Panstw. Zakl. Hig. 2018, 69, 5–14. [Google Scholar] [PubMed]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed Pharm. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Jaklova Dytrtova, J.; Straka, M.; Belonoznikova, K.; Jakl, M.; Ryslava, H. Does resveratrol retain its antioxidative properties in wine? Redox behaviour of resveratrol in the presence of Cu(II) and tebuconazole. Food Chem. 2018, 262, 221–225. [Google Scholar] [CrossRef]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef] [PubMed]
- Kelley, E.E.; Khoo, N.K.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Joshi, G.; Kler, H.; Kalra, S.; Kaur, M.; Arya, R. Toward an Understanding of Structural Insights of Xanthine and Aldehyde Oxidases: An Overview of their Inhibitors and Role in Various Diseases. Med. Res. Rev. 2018, 38, 1073–1125. [Google Scholar] [CrossRef]
- Tang, X.; Tang, P.; Ma, L.; Liu, L. Screening and Evaluation of Xanthine Oxidase Inhibitors from Gnetum parvifolium in China. Molecules 2019, 24, 2671. [Google Scholar] [CrossRef]
- Jia, Z.; Zhu, H.; Misra, B.R.; Mahaney, J.E.; Li, Y.; Misra, H.P. EPR studies on the superoxide-scavenging capacity of the nutraceutical resveratrol. Mol. Cell Biochem. 2008, 313, 187–194. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Hunyadi, A. The mechanism(s) of action of antioxidants: From scavenging reactive oxygen/nitrogen species to redox signaling and the generation of bioactive secondary metabolites. Med. Res. Rev. 2019, 39, 2505–2533. [Google Scholar] [CrossRef] [PubMed]
- Springer, M.; Moco, S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients 2019, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Shingai, Y.; Fujimoto, A.; Nakamura, M.; Masuda, T. Structure and function of the oxidation products of polyphenols and identification of potent lipoxygenase inhibitors from Fe-catalyzed oxidation of resveratrol. J. Agric. Food Chem. 2011, 59, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Hunyadi, A.; Agbadua, O.G.; Takács, G.; Balogh, G.T. Scavengome of an Antioxidant. ChemRxiv. Cambridg Camb. Open Engag. 2022. [Google Scholar] [CrossRef]
- Duddeck, H.; Dietrich, W.; Tóth, G. Structure Elucidation by Modern NMR; Steinkopff Springer: Darmstadt, Germany, 1998. [Google Scholar] [CrossRef]
- Pretsch, E.; Tóth, G.; Munk, E.M.; Badertscher, M. Computer-Aided Structure Elucidation; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Duong, N.T.; Vinh, P.D.; Thuong, P.T.; Hoai, N.T.; Thanh, L.N.; Bach, T.T.; Nam, N.H.; Anh, N.H. Xanthine oxidase inhibitors from Archidendron clypearia (Jack.) I.C. Nielsen: Results from systematic screening of Vietnamese medicinal plants. Asian Pac. J. Trop. Med. 2017, 10, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Fasi, L.; Di Meo, F.; Kuo, C.Y.; Stojkovic Buric, S.; Martins, A.; Kusz, N.; Beni, Z.; Dekany, M.; Balogh, G.T.; Pesic, M.; et al. Antioxidant-Inspired Drug Discovery: Antitumor Metabolite Is Formed in Situ from a Hydroxycinnamic Acid Derivative upon Free-Radical Scavenging. J. Med. Chem. 2019, 62, 1657–1668. [Google Scholar] [CrossRef]
- Fasi, L.; Latif, A.D.; Zupko, I.; Levai, S.; Dekany, M.; Beni, Z.; Konczol, A.; Balogh, G.T.; Hunyadi, A. AAPH or Peroxynitrite-Induced Biorelevant Oxidation of Methyl Caffeate Yields a Potent Antitumor Metabolite. Biomolecules 2020, 10, 1537. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Lee, D.; Bhat, K.P.; Fong, H.H.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Aromatase inhibitors from Broussonetia papyrifera. J. Nat. Prod. 2001, 64, 1286–1293. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Wei, X.; Zhang, C.J.; Jin, X.L.; Tang, J.J.; Fan, G.J.; Zhou, B. Hypohalous acid-mediated halogenation of resveratrol and its role in antioxidant and antimicrobial activities. Food Chem. 2012, 135, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, J.W.; Lee, B.H.; Chung, K.H.; Chi, D.Y. Syntheses and radical scavenging activities of resveratrol derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 463–466. [Google Scholar] [CrossRef]
- D’Orsi, R.; Morrongiello, F.; Laurita, T.; Funicello, M.; Lupattelli, P.; Chiummiento, L. Regio- and Diastereo-Selective Biomimetic Synthesis of (±)-ϵ-Viniferin by NIS and Resveratrol. ChemistrySelect 2021, 6, 6863–6866. [Google Scholar] [CrossRef]
- Ngoc, T.M.; Minh, P.T.; Hung, T.M.; Thuong, P.T.; Lee, I.; Min, B.S.; Bae, K. Lipoxygenase inhibitory constituents from rhubarb. Arch. Pharm. Res. 2008, 31, 598–605. [Google Scholar] [CrossRef]
- Pezet, R.; Perret, C.; Jean-Denis, J.B.; Tabacchi, R.; Gindro, K.; Viret, O. Delta-viniferin, a resveratrol dehydrodimer: One of the major stilbenes synthesized by stressed grapevine leaves. J. Agric. Food Chem. 2003, 51, 5488–5492. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.B.; Han, X.Z.; Lou, H.X. Oligomers of resveratrol and ferulic acid prepared by peroxidase-catalyzed oxidation and their protective effects on cardiac injury. J. Agric. Food Chem. 2007, 55, 7753–7757. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, A.E.G.; Öberg, C.T.; Hillgren, J.M.; Elofsson, M. Total Synthesis of the Resveratrol Oligomers (±)-Ampelopsin B and (±)-ϵ-Viniferin. Eur. J. Org. Chem. 2016, 2016, 426–429. [Google Scholar] [CrossRef]
- Velu, S.S.; Di Meo, F.; Trouillas, P.; Sancho-Garcia, J.C.; Weber, J.F. Regio- and stereocontrolled synthesis of oligostilbenoids: Theoretical highlights at the supramolecular level. J. Nat. Prod. 2013, 76, 538–546. [Google Scholar] [CrossRef]
- Bonechi, C.; Martini, S.; Magnani, A.; Rossi, C. Stacking interaction study of trans-resveratrol (trans-3,5,4′-trihydroxystilbene) in solution by Nuclear Magnetic Resonance and Fourier Transform Infrared Spectroscopy. Magn. Reson. Chem. 2008, 46, 625–629. [Google Scholar] [CrossRef]
- Naiker, M.; Anderson, S.; Johnson, J.B.; Mani, J.S.; Wakeling, L.; Bowry, V. Loss of trans-resveratrol during storage and ageing of red wines. Aust. J. Grape Wine Res. 2020, 26, 385–387. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, P.; Yu, H.; Li, J.; Wang, M.-W.; Zhao, W. Anti-Helicobacter pylori and Thrombin Inhibitory Components from Chinese Dragon’s Blood, Dracaena cochinchinensis. J. Nat. Prod. 2007, 70, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-B.; Dong, M.-J.; Zhang, J. A holistic in silico approach to develop novel inhibitors targeting ErbB1 and ErbB2 kinases. Trop. J. Pharm. Res. 2016, 15, 231–239. [Google Scholar] [CrossRef]
- Hunyadi, A.; Martins, A.; Danko, B.; Chuang, D.-W.; Trouillas, P.; Chang, F.-R.; Wu, Y.-C.; Falkay, G. Discovery of the first non-planar flavonoid that can strongly inhibit xanthine oxidase: Protoapigenone 1′-O-propargyl ether. Tetrahedron Lett. 2013, 54, 6529–6532. [Google Scholar] [CrossRef]
- Cao, H.; Pauff, J.M.; Hille, R. Substrate orientation and catalytic specificity in the action of xanthine oxidase: The sequential hydroxylation of hypoxanthine to uric acid. J. Biol. Chem. 2010, 285, 28044–28053. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.M.G.; Fernandes, H.S.; Maia, L.B.; Sousa, S.F.; Moura, J.J.G.; Cerqueira, N.M.F.S.A. The complete catalytic mechanism of xanthine oxidase: A computational study. Inorg. Chem. Front. 2021, 8, 405–416. [Google Scholar] [CrossRef]
- Nagao, A.; Seki, M.; Kobayashi, H. Inhibition of xanthine oxidase by flavonoids. Biosci. Biotechnol. Biochem. 1999, 63, 1787–1790. [Google Scholar] [CrossRef]
- Teles Fujishima, M.A.; Silva, N.; Ramos, R.D.S.; Batista Ferreira, E.F.; Santos, K.; Silva, C.; Silva, J.O.D.; Campos Rosa, J.M.; Santos, C. An Antioxidant Potential, Quantum-Chemical and Molecular Docking Study of the Major Chemical Constituents Present in the Leaves of Curatella americana Linn. Pharmaceuticals 2018, 11, 72. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, L.; Ren, L.; Xie, Y. Advances in structures required of polyphenols for xanthine oxidase inhibition. Food Front. 2020, 1, 152–167. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Antioxidant property of coffee components: Assessment of methods that define mechanisms of action. Molecules 2014, 19, 19180–19208. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Lin, J.; Li, X.; Chen, B.; Wei, G.; Chen, D. E-Configuration Improves Antioxidant and Cytoprotective Capacities of Resveratrols. Molecules 2018, 23, 1790. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Spadaro, A.; Lorenti, M.; Panico, A.; Siciliano, E.A.; Barbagallo, S.; Macaluso, B.; Kamble, S.H.; Modica, M.N.; Montenegro, L. In Vitro Antioxidant and Anti-Glycation Activity of Resveratrol and Its Novel Triester with Trolox. Antioxidants 2020, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Nanah, C.N.; Held, R.A.; Clark, A.R.; Huynh, U.G.; Maraskine, M.C.; Uzarski, R.L.; McCracken, J.; Sharma, A. Effect of electron donating groups on polyphenol-based antioxidant dendrimers. Biochimie 2015, 111, 125–134. [Google Scholar] [CrossRef]
- Lee, C.Y.; Sharma, A.; Semenya, J.; Anamoah, C.; Chapman, K.N.; Barone, V. Computational Study of Ortho-Substituent Effects on Antioxidant Activities of Phenolic Dendritic Antioxidants. Antioxidants 2020, 9, 189. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef]
- Li, C.; Xu, X.; Tao, Z.; Wang, X.J.; Pan, Y. Resveratrol dimers, nutritional components in grape wine, are selective ROS scavengers and weak Nrf2 activators. Food Chem. 2015, 173, 218–223. [Google Scholar] [CrossRef]
- Lopez-Alarcon, C.; Fuentes-Lemus, E.; Figueroa, J.D.; Dorta, E.; Schoneich, C.; Davies, M.J. Azocompounds as generators of defined radical species: Contributions and challenges for free radical research. Free Radic. Biol. Med. 2020, 160, 78–91. [Google Scholar] [CrossRef]
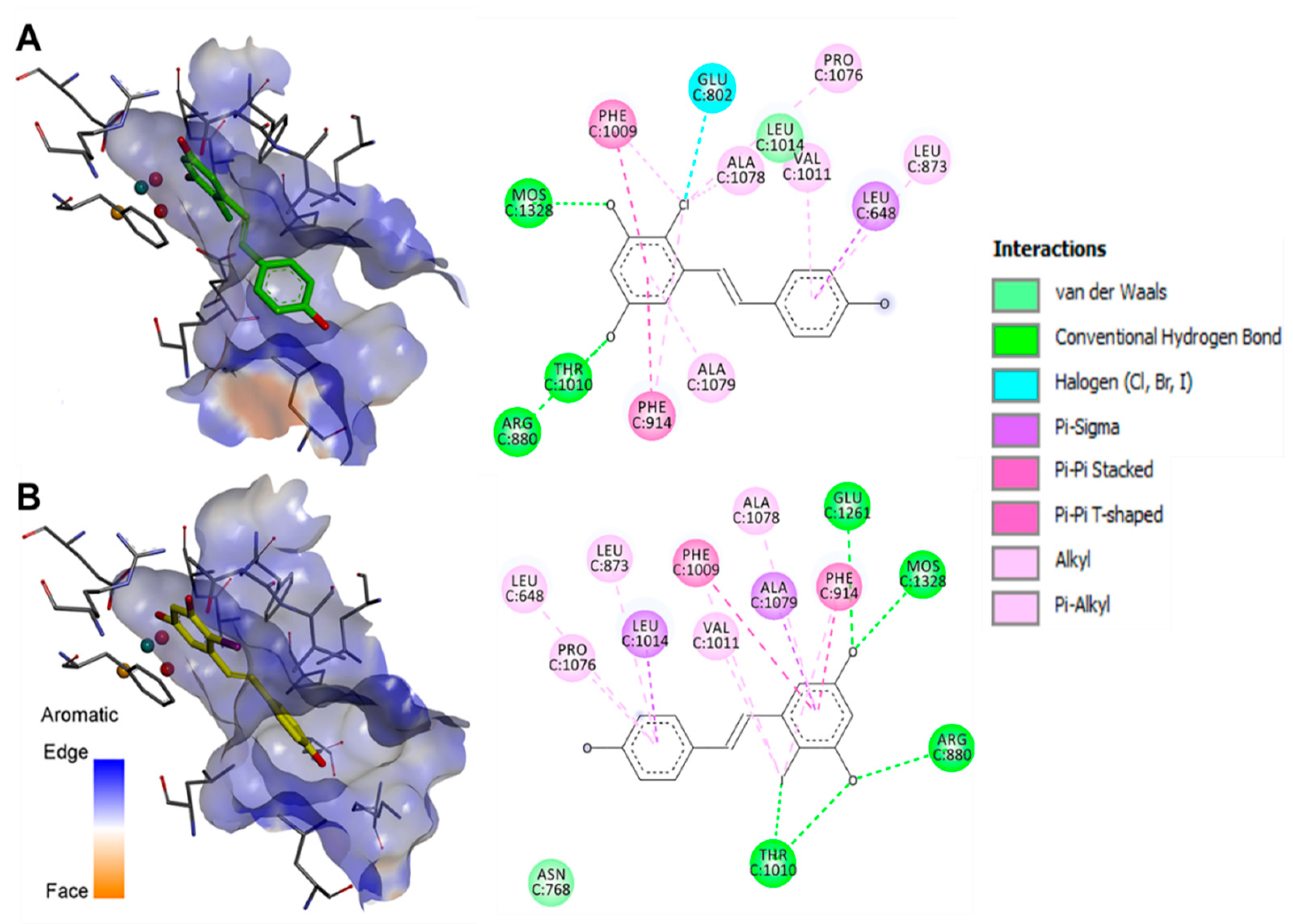
| ID | Oxidants | Experimental Conditions a | XO Inh. (%) | Compound Isolated |
|---|---|---|---|---|
| Resveratrol | - | - | 49.9 ± 6.9 | - |
| Ox1 | PIFA | CH3CN, r.t. 5 h | 42.5 ± 3.3 | 6 |
| Ox2 | AAPH, NaIO4 | CH3CN, 65 °C, 23 h b | 37.6 ± 5.3 | 3, 5 |
| Ox3 | PIDA | CH3CN, r.t., 2 h b | 32.0 ± 2.1 * | 5 |
| Ox4 | PIFA | EtOH, 2 h b | 47.1 ± 1.6 | 7, 8, 9 |
| Ox5 | H5IO6, Oxone | EtOH, r.t, 7 h b | 55.8 ± 0.9 | 3, 4 |
| Ox6 | H5IO6, FeCl3 | CH3CN, r.t. 17 h b | 67.8 ± 1.8 * | 2, 3, 4 |
| Ox7 | H5IO6, FeCl3 | EtOH, r.t., 17 h b | 75.7 ± 4.9 * | 3 |
| Compounds | XO Inh (%) | XO IC50 (µM) |
|---|---|---|
| Resveratrol (1) | 55.6 ± 1.1 | 119.4 ± 2.0 |
| 2 | 90.6 ± 4.4 | 4.8 ± 0.8 |
| 3 | 97.2 ± 4.9 * | 15.3 ± 1.4 |
| 4 | 93.8 ± 1.3 * | 6.4 ± 0.5 |
| 5 | 77.4 ± 2.1 | 16.4 ± 1.3 |
| 6 | 69.1 ± 3.1 | 22.8 ± 6.3 |
| 7 | 14.4 ± 3.0 * | >1000 |
| 8 | 15.1 ± 1.7 | 233.3 ± 5.1 |
| 9 | 31.5 ± 4.8 | 234.1 ± 4.8 |
| Allopurinol | 97.3 ± 0.9 * | 5.9 ± 0.9 |
| Compounds | DPPH IC50 (µM) | ORAC (Trolox Equivalents, TE) |
|---|---|---|
| Resveratrol (1) | 27.7 ± 1.4 | 8.9 ± 0.2 |
| 2 | 15.8 ± 1.0 | 9.9 ± 0.5 |
| 3 | 41.0 ± 1.5 | 11.6 ± 0.2 * |
| 4 | 51.7 ± 1.6 | 12.5 ± 0.7 * |
| 5 | 340.7 ± 2.1 | 6.3 ± 0.3 |
| 6 | 92.1 ± 1.6 | 15.2 ± 0.5 * |
| 7 | >500 | 8.1 ± 0.5 |
| 8 | 53.1 ± 1.5 | 13.9 ± 0.1 * |
| 9 | 22.6 ± 1.9 | 9.5 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbadua, O.G.; Kúsz, N.; Berkecz, R.; Gáti, T.; Tóth, G.; Hunyadi, A. Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants 2022, 11, 1832. https://doi.org/10.3390/antiox11091832
Agbadua OG, Kúsz N, Berkecz R, Gáti T, Tóth G, Hunyadi A. Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants. 2022; 11(9):1832. https://doi.org/10.3390/antiox11091832
Chicago/Turabian StyleAgbadua, Orinamhe G., Norbert Kúsz, Róbert Berkecz, Tamás Gáti, Gábor Tóth, and Attila Hunyadi. 2022. "Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors" Antioxidants 11, no. 9: 1832. https://doi.org/10.3390/antiox11091832
APA StyleAgbadua, O. G., Kúsz, N., Berkecz, R., Gáti, T., Tóth, G., & Hunyadi, A. (2022). Oxidized Resveratrol Metabolites as Potent Antioxidants and Xanthine Oxidase Inhibitors. Antioxidants, 11(9), 1832. https://doi.org/10.3390/antiox11091832









