Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
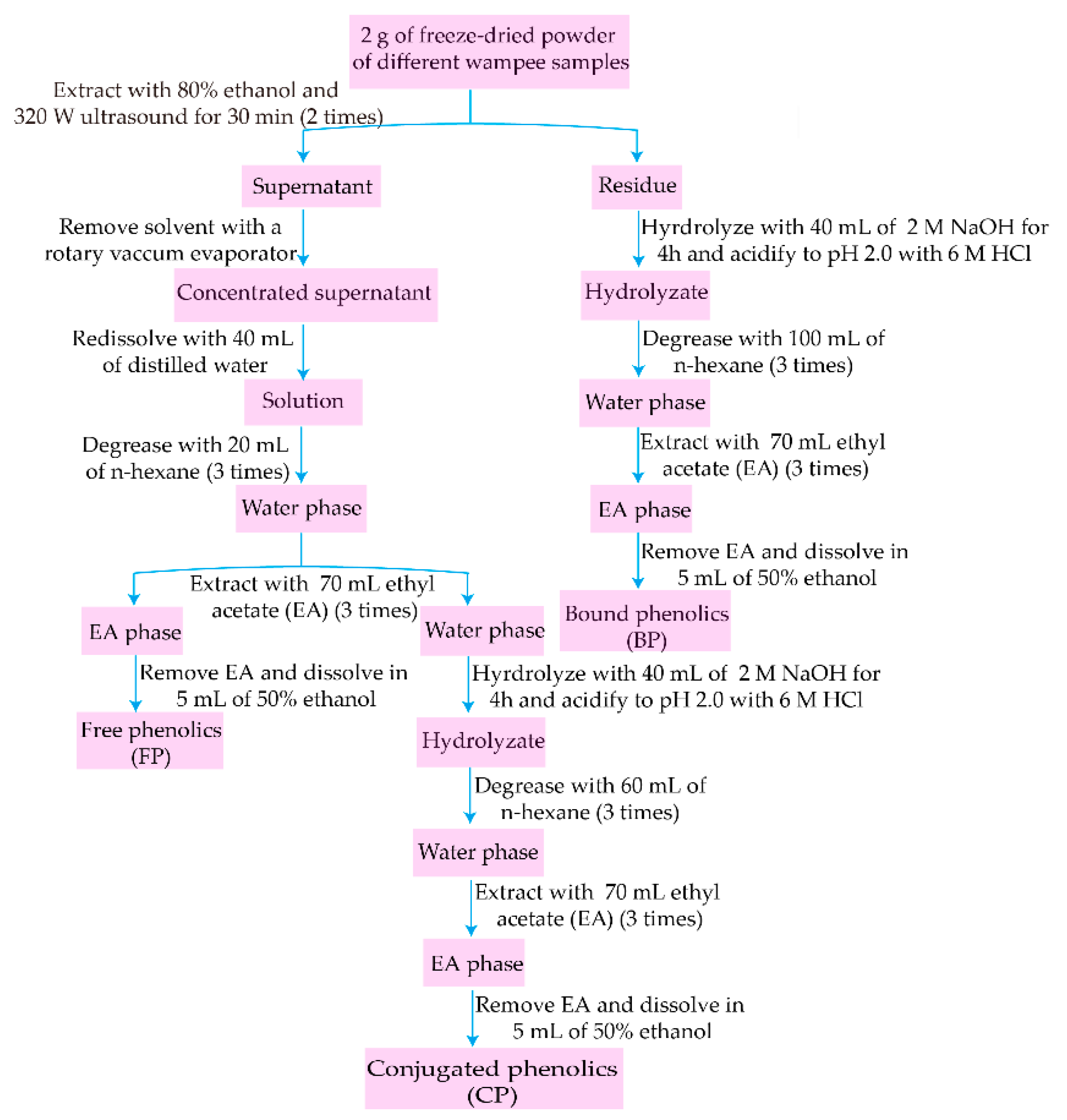
2.2. Extraction of FP, CP, and BP Fractions
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Analysis of Polyphenol Composition
2.4.1. Identification of Phenolic Compounds
2.4.2. Quantification of Phenolic Compounds
2.5. Evaluation of Antioxidant Activities in Four In Vitro Models
2.6. Determination of α-Glucosidase Inhibitory Activity
2.7. Statistical Analysis
3. Results and Discussion
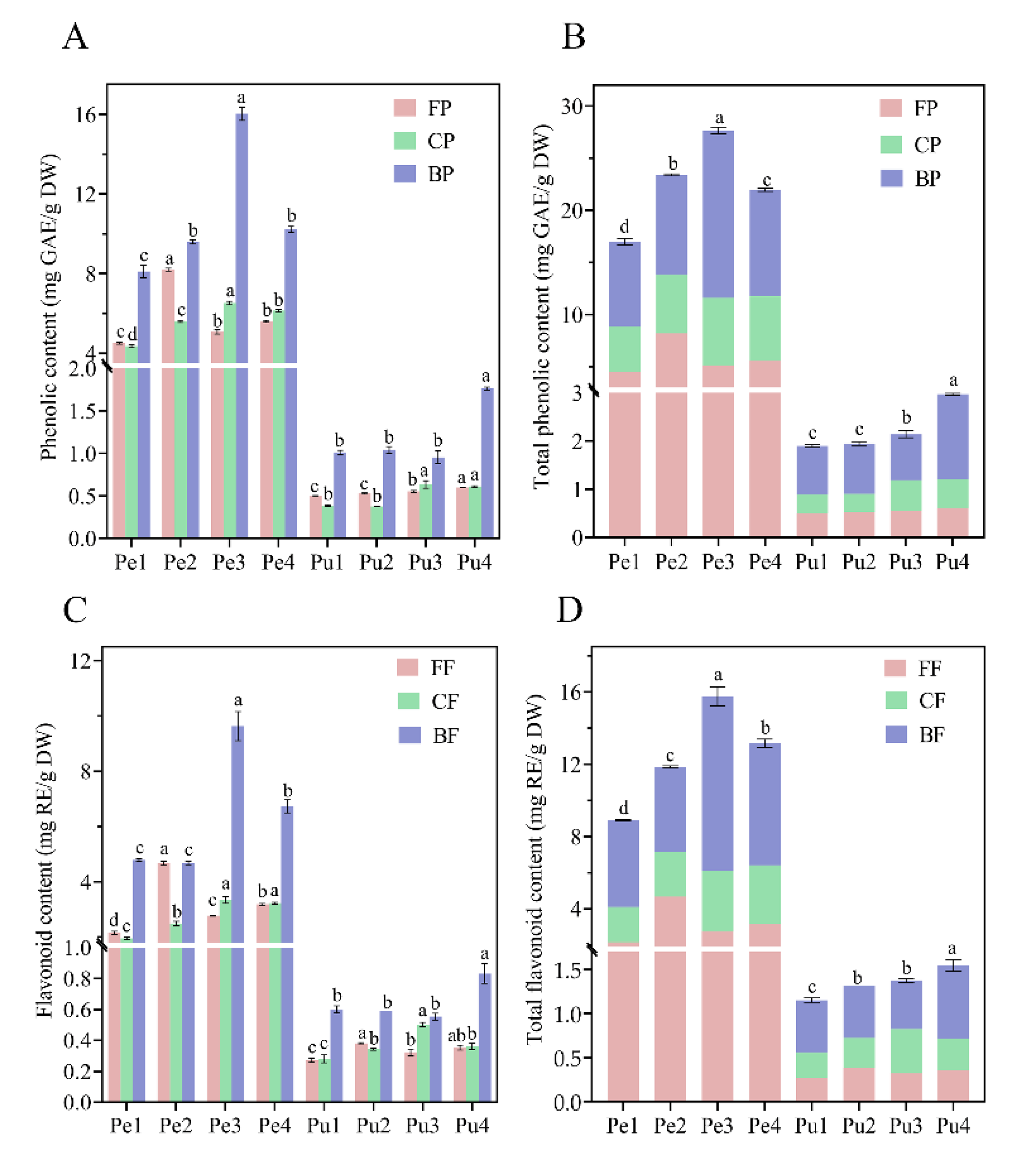
3.1. Total Phenolic Contents (TPC) and Total Flavonoid Contents (TFC)
3.2. Identification of Phenolic Compounds
3.3. Quantitative Analysis of Phenolic Composition
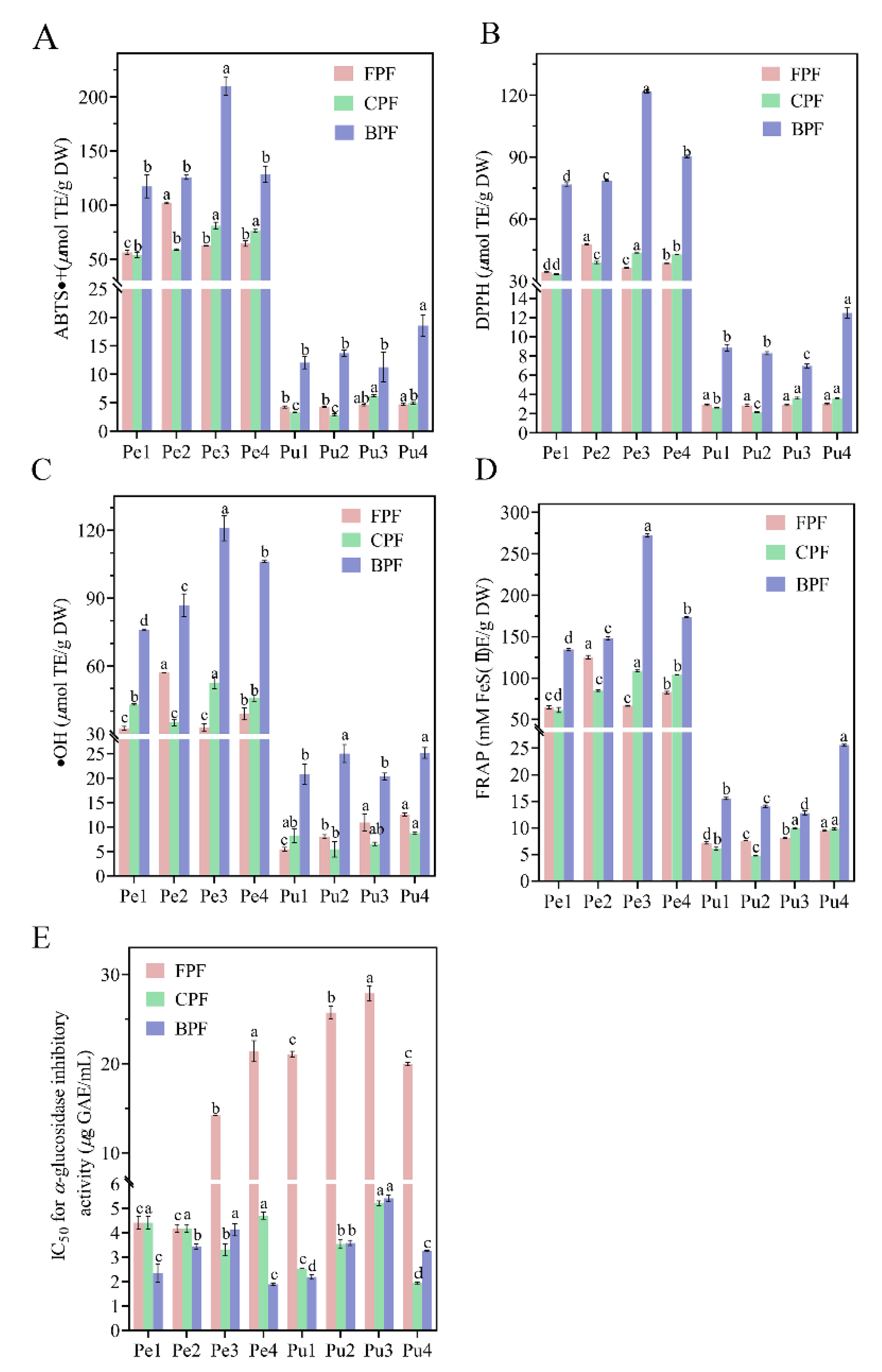
3.4. Antioxidant Activities
3.5. α-Glucosidase Inhibitory Activity
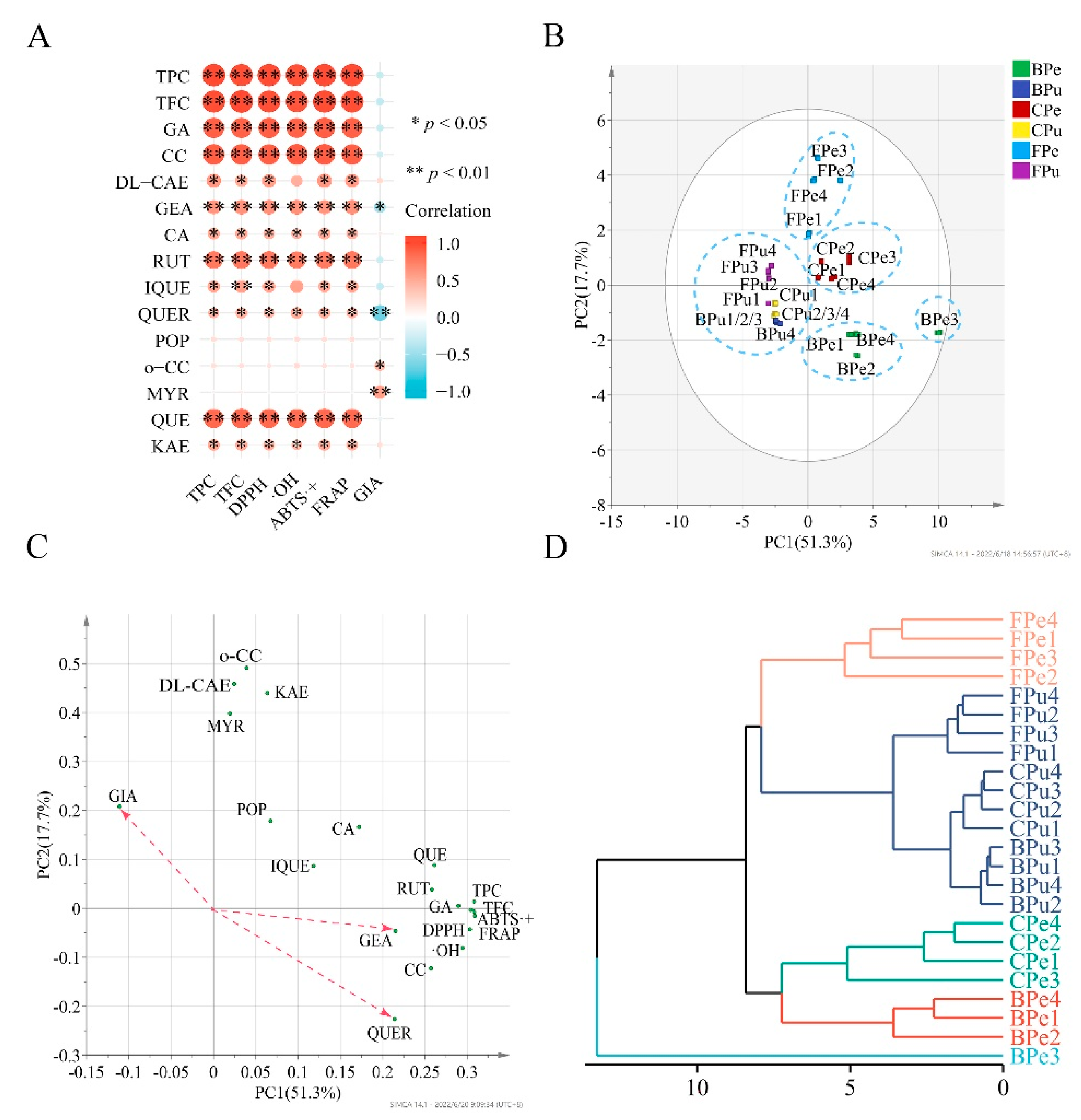
3.6. Correlation Analysis and Multivariate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| W | wampee |
| Pe | peel |
| Pu | pulp |
| DW | dry weight |
| FP | free phenolic |
| CP | conjugated phenolic |
| BP | bound phenolic |
| FF | free flavonoid |
| CF | conjugated flavonoid |
| BF | bound flavonoid |
| TPC | total phenolic content |
| TFC | total flavonoid content |
| GAE | gallic acid equivalents |
| RE | rutin equivalents |
| TE | Trolox equivalents |
References
- Prasad, K.N.; Hao, J.; Yi, C.; Zhang, D.; Qiu, S.; Jiang, Y.; Zhang, M.; Chen, F. Antioxidant and anticancer activities of wampee (Clausena lansium (Lour.) Skeels) peel. J. Biomed. Biotechnol. 2009, 2009, 612805. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sahu, S.K.; Yang, T.; Mu, W.; Wei, J.; Cheng, L.; Yang, J.; Liu, J.; Zhao, Y.; Lisby, M.; et al. The Clausena lansium (Wampee) genome reveal new insights into the carbazole alkaloids biosynthesis pathway. Genomics 2021, 113, 3696–3704. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Vallipuram, K.; Adebajo, A.C.; Reisch, J. 2,7-dihydroxy-3-formyl-1-(3′-methyl-2′-butenyl) carbazole from Clausena lansium. Phytochemistry 1995, 40, 1563–1565. [Google Scholar] [CrossRef]
- Adebajo, A.C.; Iwalewa, E.O.; Obuotor, E.M.; Ibikunle, G.F.; Omisore, N.O.; Adewunmi, C.O.; Obaparusi, O.O.; Klaes, M.; Adetogun, G.E.; Schmidt, T.J.; et al. Pharmacological properties of the extract and some isolated compounds of Clausena lansium stem bark: Anti-trichomonal, antidiabetic, anti-inflammatory, hepatoprotective and antioxidant effects. J. Ethnopharmacol. 2009, 122, 10–19. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.-J.; Yang, J.-Z.; Ning, N.; Si, Y.-K.; Li, L.; Chen, N.-H.; Zhao, Q.; Zhang, D.-M. Carbazole alkaloids from the stems of Clausena lansium. J. Nat. Prod. 2012, 75, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-S.; Mcchesney, J.D.; El-Feraly, F.S. Carbazole alkaloids from Clausena lansium. Phytochemistry 1991, 30, 343–346. [Google Scholar] [CrossRef]
- Kong, F.; Su, Z.; Guo, X.; Zeng, F.; Bi, Y. Antidiabetic and lipid-lowering effects of the polyphenol extracts from the leaves of Clausena lansium (Lour.) Skeels on streptozotocin-induced type 2 diabetic rats. J. Food Sci. 2018, 83, 212–220. [Google Scholar] [CrossRef]
- Fan, R.; Peng, C.; Zhang, X.; Qiu, D.; Mao, G.; Lu, Y.; Zeng, J. A comparative UPLC-Q-Orbitrap-MS untargeted metabolomics investigation of different parts of Clausena lansium (Lour.) Skeels. Food Sci. Nutr. 2020, 8, 5811–5822. [Google Scholar] [CrossRef]
- Ao, H.; Jia, X.; Dong, L.; Zhang, R.; Liu, L.; Huang, F.; Wang, L.; Zhang, M. A new benzofuran glycoside from the fruit of Clausena lansium. Nat. Prod. Res. 2020, 36, 501–507. [Google Scholar] [CrossRef]
- Sanford, S.L.; Welfer, G.A.; Freudenthal, B.D.; Opresko, P.L. Mechanisms of telomerase inhibition by oxidized and therapeutic dNTPs. Nat. Commun. 2020, 11, 5288. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative stress and inflammation: What polyphenols can do for us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.P.E.; Abd El Mohsen, M.M.; Minihane, A.-M.; Mathers, J.C. Biomarkers of the intake of dietary polyphenols: Strengths, limitations and application in nutrition research. Brit. J. Nutr. 2007, 99, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Chiva-Blanch, G.; Badimon, L. Effects of polyphenol intake on metabolic syndrome: Current evidences from human trials. Oxid. Med. Cell. Longev. 2017, 2017, 5812401. [Google Scholar] [CrossRef] [PubMed]
- Cardozo, L.F.M.F.; Pedruzzi, L.M.; Stenvinkel, P.; Stockler-Pinto, M.B.; Daleprane, J.B.; Leite, M.; Mafra, D. Nutritional strategies to modulate inflammation and oxidative stress pathways via activation of the master antioxidant switch Nrf2. Biochimie 2013, 95, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN Diversity Screen. J. Agri. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef]
- Gao, Y.; Ping, H.; Li, B.; Li, Y.; Zhao, F.; Ma, Z. Characterization of free, conjugated, and bound phenolics in early and late ripening kiwifruit cultivars. J. Sci. Food Agr. 2021, 101, 4743–4750. [Google Scholar] [CrossRef]
- Dou, Z.; Chen, C.; Fu, X. Bioaccessibility, antioxidant activity and modulation effect on gut microbiota of bioactive compounds from Moringa oleifera Lam. leaves during digestion and fermentation in vitro. Food Funct. 2019, 10, 5070–5079. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, J.; Li, C.; Liu, S.; Wang, L. Morinda citrifolia L. leaves extracts obtained by traditional and eco-friendly extraction solvents: Relation between phenolic compositions and biological properties by multivariate analysis. Ind. Crops Prod. 2020, 153, 112586. [Google Scholar] [CrossRef]
- Lin, X.; Chen, S.; Wang, R.; Li, C.; Wang, L. Fabrication, characterization and biological properties of pectin and/or chitosan-based films incorporated with noni (Morinda citrifolia) fruit extract. Food Hydrocolloids 2023, 134, 108025. [Google Scholar] [CrossRef]
- Wu, L.; Chen, Z.; Li, S.; Wang, L.; Zhang, J. Eco-friendly and high-efficient extraction of natural antioxidants from Polygonum aviculare leaves using tailor-made deep eutectic solvents as extractants. Sep. Purif. Technol. 2021, 262, 118339. [Google Scholar] [CrossRef]
- Cai, Y.; Wu, L.; Lin, X.; Hu, X.; Wang, L. Phenolic profiles and screening of potential α-glucosidase inhibitors from Polygonum aviculare L. leaves using ultra-filtration combined with HPLC-ESI-qTOF-MS/MS and molecular docking analysis. Ind. Crops Prod. 2020, 154, 112673. [Google Scholar] [CrossRef]
- Chang, X.; Ye, Y.; Pan, J.; Lin, Z.; Qiu, J.; Peng, C.; Guo, X.; Lu, Y. Comparative analysis of phytochemical profiles and antioxidant activities between sweet and sour wampee (Clausena lansium) fruits. Foods 2022, 11, 1230. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Deng, Z.; Liu, R.; Zhang, H.; Zhu, H.; Jiang, L.; Tsao, R. A comprehensive profiling of free, conjugated and bound phenolics and lipophilic antioxidants in red and green lentil processing by-products. Food Chem. 2020, 325, 126925. [Google Scholar] [CrossRef] [PubMed]
- Bei, Q.; Liu, Y.; Wang, L.; Chen, G.; Wu, Z. Improving free, conjugated, and bound phenolic fractions in fermented oats (Avena sativa L.) with Monascus anka and their antioxidant activity. J. Funct. Foods 2017, 32, 185–194. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F. Dietary fiber as a carrier of dietary antioxidants: An essential physiological function. J. Agric. Food Chem. 2010, 59, 43–49. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, M.; Dong, L.; Jia, X.; Liu, L.; Ma, Y.; Huang, F.; Zhang, R. Phytochemical profile, bioactivity, and prebiotic potential of bound phenolics released from rice bran dietary fiber during in vitro gastrointestinal digestion and colonic fermentation. J. Agric. Food Chem. 2019, 67, 12796–12805. [Google Scholar] [CrossRef]
- Arruda, H.S.; Pereira, G.A.; de Morais, D.R.; Eberlin, M.N.; Pastore, G.M. Determination of free, esterified, glycosylated and insoluble-bound phenolics composition in the edible part of araticum fruit (Annona crassiflora Mart.) and its by-products by HPLC-ESI-MS/MS. Food Chem. 2018, 245, 738–749. [Google Scholar] [CrossRef]
- Wang, R.; He, R.; Li, Z.; Wang, L. LC-Q-Orbitrap-MS/MS characterization, antioxidant activity, and alpha-glucosidase-inhibiting activity with in silico analysis of extract from Clausena indica (Datz.) oliv fruit pericarps. Front. Nutr. 2021, 8, 727087. [Google Scholar] [CrossRef]
- Li, W.; Yang, R.; Ying, D.; Yu, J.; Sanguansri, L.; Augustin, M.A. Analysis of polyphenols in apple pomace: A comparative study of different extraction and hydrolysis procedures. Ind. Crops Prod. 2020, 147, 112250. [Google Scholar] [CrossRef]
- Bhagya, N.; Chandrashekar, K.R. Identification and quantification of cytotoxic phenolic acids and flavonoids in Ixora brachiata by UHPLC-DAD and UHPLC-ESI-MS/MS. Int. J. Mass. Spectrom. 2020, 450, 116290. [Google Scholar] [CrossRef]
- Yin, Q.-C.; Ji, J.-B.; Zhang, R.-H.; Duan, Z.-W.; Xie, H.; Chen, Z.; Hu, F.-C.; Deng, H. Identification and verification of key taste components in wampee using widely targeted metabolomics. Food Chem. X 2022, 13, 100261. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Zhang, R.; Wu, G.; Chen, Z.; Deng, H. Comparative metabolomics analysis reveals the taste variations among three selected wampee cultivars. Plant Food. Hum. Nutr. 2022, 77, 250–257. [Google Scholar] [CrossRef]
- Albishi, T.; John, J.A.; Al-Khalifa, A.S.; Shahidi, F. Phenolic content and antioxidant activities of selected potato varieties and their processing by-products. J. Funct. Foods 2013, 5, 590–600. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- Kan, C.; Pedersen, N.L.; Christensen, K.; Bornstein, S.R.; Licinio, J.; MacCabe, J.H.; Ismail, K.; Rijsdijk, F. Genetic overlap between type 2 diabetes and depression in Swedish and Danish twin registries. Mol. Psychiatr. 2016, 21, 903–909. [Google Scholar] [CrossRef]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pr. 2019, 157. [Google Scholar] [CrossRef]
- Bjornstad, P.; Hughan, K.; Kelsey, M.M.; Shah, A.S.; Lynch, J.; Nehus, E.; Mitsnefes, M.; Jenkins, T.; Xu, P.; Xie, C.; et al. Effect of surgical versus medical therapy on diabetic kidney disease over 5 years in severely obese adolescents with type 2 diabetes. Diabetes Care 2020, 43, 187–195. [Google Scholar] [CrossRef]
- Chen, X.; Guo, H.; Qiu, L.; Zhang, C.; Deng, Q.; Leng, Q. Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury. Front. Immunol. 2020, 11, 2056. [Google Scholar] [CrossRef]
- Begg, D.P.; May, A.A.; Mul, J.D.; Liu, M.; D’Alessio, D.A.; Seeley, R.J.; Woods, S.C. Insulin detemir is transported from blood to cerebrospinal fluid and has prolonged central anorectic action relative to NPH insulin. Diabetes 2015, 64, 2457–2466. [Google Scholar] [CrossRef]
- Li, S.; Wang, J.; Zhang, B.; Li, X.; Liu, Y. Diabetes mellitus and cause-specific mortality: A population-based study. Diabetes Metab. J. 2019, 43, 319–341. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, R.; Hu, X.; Li, C.; Wang, L. Bio-affinity ultra-filtration combined with HPLC-ESI-qTOF-MS/MS for screening potential α-glucosidase inhibitors from Cerasus humilis (Bge.) Sok. leaf-tea and in silico analysis. Food Chem. 2021, 373, 131528. [Google Scholar] [CrossRef] [PubMed]
| Abbre. | Collect Location | Type | Color | Length (mm) | Width (mm) | Single Weight (g) | Photo |
|---|---|---|---|---|---|---|---|
| W1 | Yunfu, Guangdong | Seedless wampee | Bright yellow | 31.12 ± 2.78 ac | 25.24 ± 2.26 a | 10.62 ± 2.72 a |  |
| W2 | Danzhou, Hainan | Wild wampee | Dark yellow | 23.61 ± 2.71 c | 23.22 ± 1.93 ab | 7.15 ± 1.29 b |  |
| W3 | Haikou, Hainan | Jixin wampee | Light dark yellow | 31.14 ± 2.69 a | 21.83 ± 1.81 b | 7.63 ± 1.67 b |  |
| W4 | Qingzhou, Guangxi | Heipi wampee | Brown | 27.48 ± 2.94 b | 23.18 ± 2.19 ab | 8.38 ± 2.53 ab |  |
| Peak No. | Retention Time (Min) | Compound Name | Formula | Mw | Molecular Ion (m/z) | MS/MS (m/z) | Error | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.38 | o-Coumaric acid | C9H8O3 | 164 | 165.0549 [M+H]+ | 165.0549, 123.0444, | 1.89 | Standard, MS/MS |
| 2 | 1.63 | Gallic acid | C7H6O5 | 170 | 169.0136 [M-H]− | 169.0136, 125.0234 | −3.73 | Standard, MS/MS |
| 3 | 1.95 | Gentisic acid | C7H6O4 | 154 | 153.0185 [M-H]− | 153.0185, 109.0283 | −5.14 | Standard, MS/MS |
| 4 | 2.00 | Epigallocatechin | C15H14O7 | 306 | 305.0669 [M-H]− | 305.0669, 137.0229, 125.0234 | 0.91 | MS/MS |
| 5 | 2.18 | Chlorogenic acid | C16H18O9 | 354 | 353.0883 [M-H]− | 353.0883, 191.0543 | 1.42 | Standard, MS/MS |
| 6 | 2.37 | (+/−)-Taxifolin | C15H12O7 | 304 | 303.0514 [M-H]− | 303.0514, 285.0435, 125.0233 | 1.29 | MS/MS |
| 7 | 2.47 | m-Salicylic acid | C7H6O3 | 138 | 139.0392 [M+H]+ | 139.0392, 121.0284, 95.0493 | 1.73 | MS/MS |
| 8 | 2.56 | DL-Catechin | C15H14O6 | 290 | 289.0721 [M-H]− | 289.0721, 245.0809, 205.0492, 109.0286 | 1.32 | Standard, MS/MS |
| 9 | 2.96 | Isoquercitrin | C21H20O12 | 464 | 465.1037 [M+H]+ | 465.1037, 303.0498, 285.0414 | 1.97 | Standard, MS/MS |
| 10 | 3.03 | Caffeic acid | C9H8O4 | 180 | 179.0344 [M-H]- | 179.0344, 135.0441 | −2.91 | Standard, MS/MS |
| 11 | 3.29 | (+)-Epicatechin | C15H14O6 | 290 | 335.0777 [M+FA-H]- | 335.0777, 179.0342, 135.0441 | 1.55 | MS/MS |
| 12 | 3.64 | Myricetin | C15H10O8 | 318 | 319.0453 [M+H]+ | 319.0453, 245.0468, 217.0505, 153.0187 | 1.88 | Standard, MS/MS |
| 13 | 3.98 | Quercetin | C15H10O7 | 302 | 303.0504 [M+H]+ | 303.0504, 229.0503, 153.0817, 137.0232 | 1.76 | Standard, MS/MS |
| 14 | 3.97 | Rutin | C27H30O16 | 610 | 611.1617 [M+H]+ | 611.1617, 303.0501 | 1.81 | Standard, MS/MS |
| 15 | 4.41 | Fisetin | C15H10O6 | 286 | 287.0555 [M+H]+ | 287.0555, 213.0554, 137.0242, 121.0289 | 1.59 | MS/MS |
| 16 | 4.52 | Populnin | C21H20O11 | 448 | 449.1086 [M+H]+ | 449.1086, 431.0988, 287.0529 | 1.77 | Standard, MS/MS |
| 17 | 4.53 | Quercitrin | C21H20O11 | 448 | 447.0938 [M-H]+ | 447.0938, 300.0272, 271.0249, 255.0298 | 1.60 | Standard, MS/MS |
| 18 | 5.27 | Kaempferol | C15H10O6 | 286 | 287.0555 [M+H]+ | 287.0555, 153.0185, 121.0281 | 1.56 | Standard, MS/MS |
| 19 | 5.64 | (+/−)-Naringenin | C15H12O5 | 272 | 271.0615 [M-H]− | 271.0615, 151.0030, 119.0492 | 1.06 | MS/MS |
| Phenolic Compounds | Status | Contents (µg/g DW) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pe1 | Pe2 | Pe3 | Pe4 | Pu1 | Pu2 | Pu3 | Pu4 | ||
| Gallic acid (GA) | FP | 56.09 ± 1.01 b | 87.63 ± 0.83 a | 57.81 ± 4.23 b | 56.20 ± 1.40 b | 25.46 ± 0.05 c | 24.05 ± 0.19 cd | 22.23 ± 0.05 d | 26.20 ± 0.15 c |
| CP | 45.44 ± 1.65 b | 44.48 ± 0.02 b | 51.09 ± 0.77 a | 41.23 ± 0.70 c | 16.70 ± 0.32 e | 18.02 ± 0.03 e | 20.31 ± 0.04 d | 25.11 ± 0.43 c | |
| BP | 102.93 ± 1.86 b | 68.96 ± 1.97 d | 215.43 ± 1.80 a | 97.68 ± 2.97 c | ND | ND | ND | ND | |
| TP | 204.47 ± 0.80 b | 201.08 ± 1.12 b | 324.32 ± 5.27 a | 195.10 ± 3.68 c | 42.16 ± 0.27 e | 42.07 ± 0.22 e | 42.53 ± 0.08 e | 51.31 ± 0.28 d | |
| Chlorogenic acid (CC) | FP | 316.72 ± 1.14 a | 270.71 ± 2.49 b | 236.04 ± 11.41 d | 255.95 ± 2.83 c | 59.15 ± 4.47 e | 64.27 ± 4.64 e | 58.17 ± 0.83 e | 66.28 ± 1.00 e |
| CP | 234.14 ± 10.93 a | 110.65 ± 5.98 d | 194.52 ± 7.14 b | 165.00 ± 8.19 c | 56.84 ± 0.61 f | 55.30 ± 0.76 f | 53.15 ± 0.20 f | 86.97 ± 1.68 e | |
| BP | 626.39 ± 18.87 c | 832.93 ± 36.47 b | 2611.36 ± 27.81 a | 412.30 ± 9.71 d | 107.20 ± 0.81 f | 126.31 ± 0.67 ef | 112.65 ± 0.17 ef | 144.65 ± 5.08 e | |
| TP | 1177.25 ± 30.94 b | 1214.29 ± 32.98 b | 3041.91 ± 46.35 a | 833.25 ± 1.31 c | 223.19 ± 4.27 e | 245.88 ± 3.21 e | 223.97 ± 0.46 e | 297.90 ± 5.76 d | |
| Gentisic acid (GEA) | FP | 251.29 ± 14.39 d | 280.42 ± 1.00 c | 445.50 ± 5.30 a | 357.52 ± 19.08 b | 141.54 ± 0.85 g | 192.69 ± 10.07 e | 119.14 ± 0.35 h | 177.92 ± 0.69 f |
| CP | 1287.14 ± 56.18 c | 1412.22 ± 5.30 b | 1905.12 ± 228.96 a | 1798.06 ± 160.06 a | 556.71 ± 25.60 d | 285.97 ± 2.48 g | 394.77 ± 20.01 f | 433.76 ± 1.12 e | |
| BP | 713.60 ± 21.20 d | 1068.70 ± 47.70 b | 1423.05 ± 19.96 a | 921.54 ± 3.03 c | 385.18 ± 0.08 e | ND | 317.51 ± 0.42 f | 252.42 ± 1.06 g | |
| TP | 2252.03 ± 20.59 d | 2761.34 ± 52.00 c | 3773.67 ± 214.31 a | 3077.13 ± 176.11 b | 1083.42 ± 24.84 e | 478.66 ± 12.55 h | 831.41 ± 19.94 g | 864.10 ± 0.75 f | |
| Caffeic acid (CA) | FP | 1.71 ± 0.01 b | 3.77 ± 0.49 a | 0.21 ± 0.08 c | 3.81 ± 0.11 a | ND | ND | ND | ND |
| CP | ND | ND | 8.93 ± 0.31 a | ND | ND | ND | ND | ND | |
| BP | ND | ND | 5.43 ± 0.45 a | ND | ND | ND | ND | ND | |
| TP | 1.71 ± 0.01 c | 3.77 ± 0.49 b | 14.57 ± 0.68 a | 3.81 ± 0.11 b | ND | ND | ND | ND | |
| Rutin (RUT) | FP | 276.61 ± 8.05 c | 813.88 ± 33.73 a | 227.27 ± 0.77 d | 395.19 ± 11.93 b | 30.35 ± 0.78 f | 8.37 ± 0.04 h | 22.74 ± 0.03 g | 36.41 ± 0.06 e |
| CP | 895.04 ± 6.70 b | 988.24 ± 4.92 a | 1048.47 ± 2.12 c | 1173.74 ± 96.77 d | 141.62 ± 8.94 h | 255.67 ± 2.30 g | 279.93 ± 15.23 f | 154.02 ± 0.34 e | |
| BP | 609.19 ± 4.10 c | 659.99 ± 8.22 b | 1197.04 ± 39.47 a | 536.07 ± 0.43 d | 97.78 ± 2.79 f | 128.96 ± 1.05 e | 147.69 ± 4.05 e | 95.85 ± 0.44 f | |
| TP | 1780.84 ± 10.65 c | 2462.11 ± 46.87 a | 2472.77 ± 40.82 a | 2015.00 ± 85.27 b | 269.75 ± 5.36 e | 392.99 ± 1.21 d | 450.36 ± 19.26 d | 286.28 ± 0.17 e | |
| Isoquercitrin (IQUE) | FP | 128.43 ± 7.33 b | 216.31 ± 5.46 a | 221.65 ± 0.15 a | 222.77 ± 5.93 a | 10.90 ± 0.40 e | 21.06 ± 0.91 d | 22.43 ± 0.10 d | 40.41 ± 0.02 c |
| CP | 1172.76 ± 8.93 c | 1295.64 ± 6.52 b | 1395.58 ± 17.67 a | 1363.47 ± 49.30 a | 179.10 ± 11.78 e | 329.47 ± 3.03 d | 361.52 ± 20.15 d | 195.58 ± 0.43 e | |
| BP | 192.00 ± 1.07 d | 264.96 ± 2.82 b | 286.55 ± 9.23 a | 212.90 ± 0.76 c | ND | ND | 25.20 ± 2.13 f | 37.51 ± 0.02 e | |
| TP | 1493.18 ± 0.52 c | 1776.91 ± 14.80 b | 1903.78 ± 8.29 a | 1799.13 ± 56.00 b | 190.00 ± 11.38 g | 350.53 ± 2.13 e | 409.16 ± 22.39 d | 273.50 ± 0.43 f | |
| Quercitrin (QUER) | FP | 4510.19 ± 286.30 d | 20775.72 ± 107.48 a | 15001.01 ± 193.94 c | 16552.39 ± 371.56 b | 518.29 ± 15.12 g | 896.19 ± 30.23 e | 826.66 ± 6.05 f | 887.27 ± 5.59 e |
| CP | 2558.98 ± 30.23 a | 578.75 ± 15.12 d | 790.38 ± 15.11 b | 639.22 ± 15.30 c | ND | ND | ND | ND | |
| BP | 66900.24 ± 1247.09 c | 124417.57 ± 1776.16 a | 90709.88 ± 2116.28 b | 21936.13 ± 744.58 d | 3463.24 ± 121.53 g | 3577.77 ± 12.85 g | 4419.79 ± 86.46 f | 8331.27 ± 48.37 e | |
| TP | 73969.41 ± 1503.16 c | 145772.05 ± 1868.52 a | 106501.28 ± 1937.45 b | 39127.74 ± 357.91 d | 3981.52 ± 106.42 h | 4473.96 ± 17.38 g | 5246.45 ± 80.42 f | 9218.55 ± 53.97 e | |
| Populnin (POP) | FP | 2.20 ± 0.09 b | 5.57 ± 0.91 a | 1.81 ± 0.09 b | ND | ND | ND | ND | ND |
| CP | 34.20 ± 0.25 a | 16.06 ± 0.61 c | 16.96 ± 0.09 bc | 17.86 ± 1.00 b | 3.62 ± 0.24 d | ND | 1.74 ± 0.18 f | 2.93 ± 0.09 e | |
| BP | ND | ND | ND | ND | ND | ND | ND | ND | |
| TP | 36.40 ± 0.16 a | 21.63 ± 0.30 b | 18.77 ± 0.01 c | 17.86 ± 1.00 c | 3.62 ± 0.24 d | ND | 1.74 ± 0.18 f | 2.93 ± 0.09 e | |
| o-Coumaric acid (o-CC) | FP | 8570.31 ± 276.71 d | 21464.95 ± 102.74 a | 15214.21 ± 194.52 b | 14089.54 ± 267.13 c | ND | 6493.58 ± 268.50 e | 5533.30 ± 6.85 f | 5999.06 ± 6.85 ef |
| CP | 8041.54 ± 210.96 c | 12621.03 ± 116.44 a | 12515.55 ± 63.01 a | 10345.67 ± 142.47 b | 5875.77 ± 327.40 d | ND | 5845.63 ± 321.92 d | 7716.88 ± 28.77 c | |
| BP | ND | ND | ND | ND | ND | ND | ND | ND | |
| TP | 16611.86 ± 487.68 d | 34085.98 ± 13.70 a | 27729.76 ± 257.53 b | 24435.21 ± 409.59 c | 5875.77 ± 327.40 g | 6493.58 ± 268.50 g | 11378.93 ± 315.07 f | 13715.94 ± 35.62 e | |
| Myricetin (MYR) | FP | 12524.73 ± 137.64 c | 2323.11 ± 204.17 f | 17451.74 ± 16.67 a | 16729.17 ± 463.96 b | 619.87 ± 24.67 h | 4092.03 ± 34.20 e | 681.29 ± 2.72 g | 6524.66 ± 13.78 d |
| CP | ND | ND | ND | ND | ND | ND | ND | ND | |
| BP | 2185.55 ± 66.35 b | ND | 2499.72 ± 24.31 a | ND | ND | ND | ND | ND | |
| TP | 14710.28 ± 71.29 c | 2323.11 ± 204.17 f | 19951.46 ± 7.63 a | 16729.17 ± 463.96 b | 619.87 ± 24.67 h | 4092.03 ± 34.20 e | 681.29 ± 2.72 g | 6524.66 ± 13.78 d | |
| Quercetin (QUE) | FP | 12.93 ± 0.10 a | 7.85 ± 0.69 c | 10.94 ± 0.22 b | 3.89 ± 0.07 d | ND | ND | ND | ND |
| CP | 3.31 ± 0.05 c | 2.15 ± 0.04 c | 18.73 ± 1.95 a | 8.89 ± 0.73 b | ND | ND | ND | ND | |
| BP | 16.36 ± 0.52 b | 6.58 ± 0.12 d | 35.03 ± 0.88 a | 10.86 ± 0.01 c | ND | ND | ND | ND | |
| TP | 32.60 ± 0.37 b | 16.58 ± 0.61 d | 64.70 ± 2.61 a | 23.64 ± 0.81 c | ND | ND | ND | ND | |
| Kaempferol (KAE) | FP | 5.59 ± 0.17 c | 34.93 ± 0.71 a | 2.04 ± 0.15 d | 26.17 ± 0.51 b | ND | ND | ND | ND |
| CP | ND | 2.85 ± 0.18 | ND | ND | ND | ND | ND | ND | |
| BP | ND | ND | 4.26 ± 0.09 | ND | ND | ND | ND | ND | |
| TP | 5.59 ± 0.17 c | 37.78 ± 0.89 a | 6.30 ± 0.05 c | 26.17 ± 0.51 b | ND | ND | ND | ND | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Shi, Y.; Wen, P.; Hu, X.; Wang, L. Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis. Antioxidants 2022, 11, 1831. https://doi.org/10.3390/antiox11091831
Lin X, Shi Y, Wen P, Hu X, Wang L. Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis. Antioxidants. 2022; 11(9):1831. https://doi.org/10.3390/antiox11091831
Chicago/Turabian StyleLin, Xue, Yousheng Shi, Pan Wen, Xiaoping Hu, and Lu Wang. 2022. "Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis" Antioxidants 11, no. 9: 1831. https://doi.org/10.3390/antiox11091831
APA StyleLin, X., Shi, Y., Wen, P., Hu, X., & Wang, L. (2022). Free, Conjugated, and Bound Phenolics in Peel and Pulp from Four Wampee Varieties: Relationship between Phenolic Composition and Bio-Activities by Multivariate Analysis. Antioxidants, 11(9), 1831. https://doi.org/10.3390/antiox11091831






