Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients
Abstract
1. The Immune System and Metabolic Disorders
2. Characteristics of Vitamin E
2.1. Different Forms of Vitamin E
2.2. Bioavailability of Vitamin E
2.3. Hepatic Metabolism of Vitamin E
2.4. Biological Activity and Physiological Relevance of Vitamin E
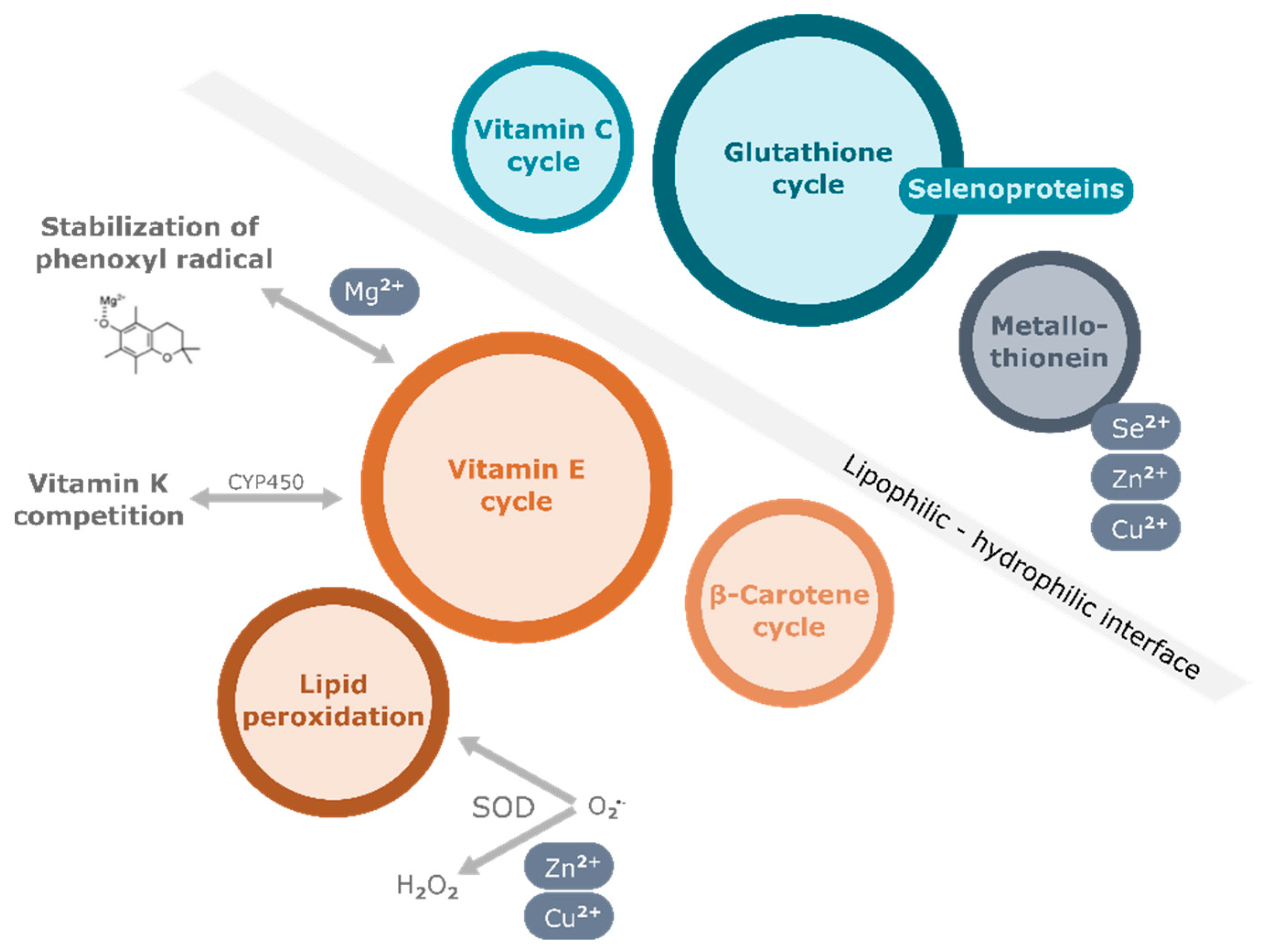
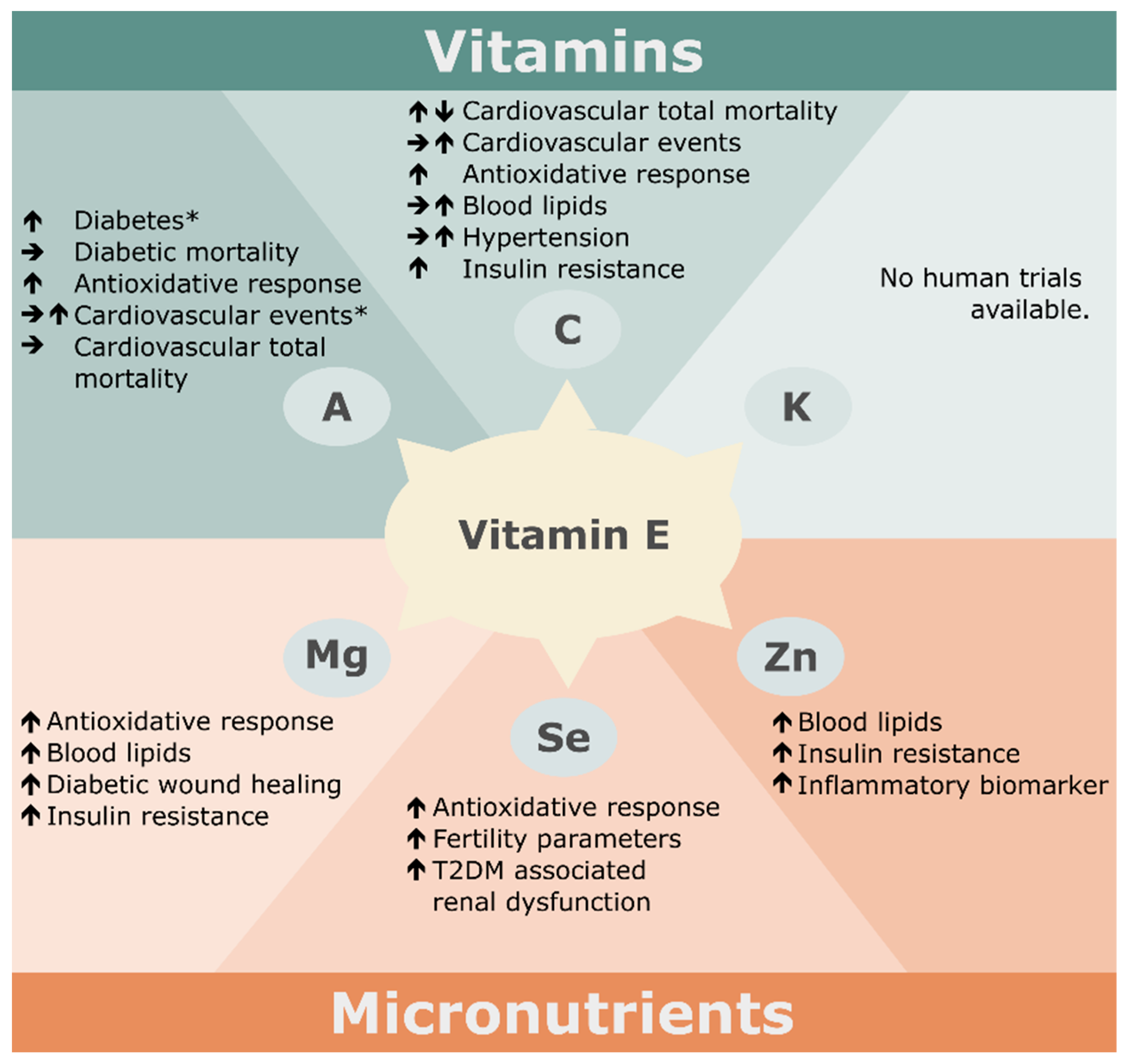
3. Nutritional Factors Interfering with the Effects of Vitamin E Supplementation
3.1. Vitamins
3.1.1. Vitamin C
3.1.2. Vitamin A
3.1.3. Vitamin K
3.2. Minerals
3.2.1. Magnesium
3.2.2. Selenium
3.2.3. Zinc
4. Biological Activities of Long-Chain Metabolites of Vitamin E
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Parasher, A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad. Med. J. 2021, 97, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Mendy, A.; Apewokin, S.; Wells, A.A.; Morrow, A.L. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Deng, G.; Yin, M.; Chen, X.; Zeng, F. Clinical determinants for fatality of 44,672 patients with COVID-19. Crit. Care 2020, 24, 179. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Steenblock, C.; Schwarz, P.E.H.; Ludwig, B.; Linkermann, A.; Zimmet, P.; Kulebyakin, K.; Tkachuk, V.A.; Markov, A.G.; Lehnert, H.; de Angelis, M.H.; et al. COVID-19 and metabolic disease: Mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021, 9, 786–798. [Google Scholar] [CrossRef]
- Galmés, S.; Serra, F.; Palou, A. Current State of Evidence: Influence of Nutritional and Nutrigenetic Factors on Immunity in the COVID-19 Pandemic Framework. Nutrients 2020, 12, 2738. [Google Scholar] [CrossRef]
- Pecora, F.; Persico, F.; Argentiero, A.; Neglia, C.; Esposito, S. The Role of Micronutrients in Support of the Immune Response against Viral Infections. Nutrients 2020, 12, 3198. [Google Scholar] [CrossRef]
- Linneberg, A.; Kampmann, F.B.; Israelsen, S.B.; Andersen, L.R.; Jørgensen, H.L.; Sandholt, H.; Jørgensen, N.R.; Thysen, S.M.; Benfield, T. The Association of Low Vitamin K Status with Mortality in a Cohort of 138 Hospitalized Patients with COVID-19. Nutrients 2021, 13, 1985. [Google Scholar] [CrossRef]
- Dofferhoff, A.S.M.; Piscaer, I.; Schurgers, L.J.; Visser, M.P.J.; van den Ouweland, J.M.W.; de Jong, P.A.; Gosens, R.; Hackeng, T.M.; van Daal, H.; Lux, P.; et al. Reduced vitamin K status as a potentially modifiable risk factor of severe COVID-19. Clin. Infect. Dis. 2020, 73, e4039-46. [Google Scholar] [CrossRef]
- Jovic, T.H.; Ali, S.R.; Ibrahim, N.; Jessop, Z.M.; Tarassoli, S.P.; Dobbs, T.D.; Holford, P.; Thornton, C.A.; Whitaker, I.S. Could Vitamins Help in the Fight Against COVID-19? Nutrients 2020, 12, 2550. [Google Scholar] [CrossRef]
- Dharmalingam, K.; Birdi, A.; Tomo, S.; Sreenivasulu, K.; Charan, J.; Yadav, D.; Purohit, P.; Sharma, P. Trace Elements as Immunoregulators in SARS-CoV-2 and Other Viral Infections. Indian J. Clin. Biochem. 2021, 36, 416–426. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, R.; Nowicka, B.; Kruk, J. Vitamin E—Occurrence, Biosynthesis by Plants and Functions in Human Nutrition. Mini Rev. Med. Chem. 2017, 17, 1039–1052. [Google Scholar] [CrossRef]
- Evans, H.M.; Bishop, K.S. On the existence of a hitherto unrecognized dietary factor essential for reproduction. Science 1922, 56, 650–651. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef]
- Zingg, J.-M. Vitamin E: Regulatory Role on Signal Transduction. IUBMB life 2019, 71, 456–478. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, Y.; Qi, G.; Brand, D.; Zheng, S.G. Role of Vitamin A in the Immune System. J. Clin. Med. 2018, 7, 258. [Google Scholar] [CrossRef]
- Wallert, M.; Schmölz, L.; Galli, F.; Birringer, M.; Lorkowski, S. Regulatory metabolites of vitamin E and their putative relevance for atherogenesis. Redox Biol. 2014, 2, 495–503. [Google Scholar] [CrossRef]
- Grebenstein, N.; Schumacher, M.; Graeve, L.; Frank, J. α-Tocopherol transfer protein is not required for the discrimination against γ-tocopherol in vivo but protects it from side-chain degradation in vitro. Mol. Nutr. Food Res. 2014, 58, 1052–1060. [Google Scholar] [CrossRef]
- Hosomi, A.; Arita, M.; Sato, Y.; Kiyose, C.; Ueda, T.; Igarashi, O.; Arai, H.; Inoue, K. Affinity for alpha-tocopherol transfer protein as a determinant of the biological activities of vitamin E analogs. FEBS Lett. 1997, 409, 105–108. [Google Scholar] [CrossRef]
- Azzi, A. Many tocopherols, one vitamin E. Mol. Aspects Med. 2018, 61, 92–103. [Google Scholar] [CrossRef]
- Schmölz, L.; Birringer, M.; Lorkowski, S.; Wallert, M. Complexity of vitamin E metabolism. World J. Biol. Chem. 2016, 7, 14–43. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Mah, E.; Sapper, T.N.; Chitchumroonchokchai, C.; Failla, M.L.; Schill, K.E.; Clinton, S.K.; Bobe, G.; Traber, M.G.; Bruno, R.S. α-Tocopherol bioavailability is lower in adults with metabolic syndrome regardless of dairy fat co-ingestion: A randomized, double-blind, crossover trial. Am. J. Clin. Nutr. 2015, 102, 1070–1080. [Google Scholar] [CrossRef]
- Ortega, R.M.; Requejo, A.M.; López-Sobaler, A.M.; Andrés, P.; Navia, B.; Perea, J.M.; Robles, F. Cognitive Function in Elderly People Is Influenced by Vitamin E Status. J. Nutr. 2002, 132, 2065–2068. [Google Scholar] [CrossRef]
- Malik, A.; Eggersdorfer, M.; Trilok-Kumar, G. Vitamin E status in healthy population in Asia: A review of current literature. Int. J. Vitam. Nutr. Res. 2021, 91, 356–369. [Google Scholar] [CrossRef]
- Abe, C.; Uchida, T.; Ohta, M.; Ichikawa, T.; Yamashita, K.; Ikeda, S. Cytochrome P450-dependent metabolism of vitamin E isoforms is a critical determinant of their tissue concentrations in rats. Lipids 2007, 42, 637–645. [Google Scholar] [CrossRef]
- Schubert, M.; Kluge, S.; Schmölz, L.; Wallert, M.; Galli, F.; Birringer, M.; Lorkowski, S. Long-Chain Metabolites of Vitamin E: Metabolic Activation as a General Concept for Lipid-Soluble Vitamins? Antioxidants 2018, 7, 10. [Google Scholar] [CrossRef]
- Bartolini, D.; Marinelli, R.; Giusepponi, D.; Galarini, R.; Barola, C.; Stabile, A.M.; Sebastiani, B.; Paoletti, F.; Betti, M.; Rende, M.; et al. Alpha-Tocopherol Metabolites (the Vitamin E Metabolome) and Their Interindividual Variability during Supplementation. Antioxidants 2021, 10, 173. [Google Scholar] [CrossRef]
- Taylor, L.; Krueger, N.; Malysheva, O.; Atkinson, J.; Parker, R.S. ω-Hydroxylation of α-tocopheryl quinone reveals a dual function for cytochrome P450-4F2 in vitamin E metabolism. Bioorganic Med. Chem. 2018, 26, 5555–5565. [Google Scholar] [CrossRef]
- Birringer, M.; Lington, D.; Vertuani, S.; Manfredini, S.; Scharlau, D.; Glei, M.; Ristow, M. Proapoptotic effects of long-chain vitamin E metabolites in HepG2 cells are mediated by oxidative stress. Free Radic. Biol. Med. 2010, 49, 1315–1322. [Google Scholar] [CrossRef]
- Wallert, M.; Mosig, S.; Rennert, K.; Funke, H.; Ristow, M.; Pellegrino, R.M.; Cruciani, G.; Galli, F.; Lorkowski, S.; Birringer, M. Long-chain metabolites of α-tocopherol occur in human serum and inhibit macrophage foam cell formation in vitro. Free Radic. Biol. Med. 2014, 68, 43–51. [Google Scholar] [CrossRef]
- Ciffolilli, S.; Wallert, M.; Bartolini, D.; Krauth, V.; Werz, O.; Piroddi, M.; Sebastiani, B.; Torquato, P.; Lorkowski, S.; Birringer, M.; et al. Human serum determination and in vitro anti-inflammatory activity of the vitamin E metabolite α-(13’-hydroxy)-6-hydroxychroman. Free Radic. Biol. Med. 2015, 89, 952–962. [Google Scholar] [CrossRef]
- Pein, H.; Ville, A.; Pace, S.; Temml, V.; Garscha, U.; Raasch, M.; Alsabil, K.; Viault, G.; Dinh, C.-P.; Guilet, D.; et al. Endogenous metabolites of vitamin E limit inflammation by targeting 5-lipoxygenase. Nat. Commun. 2018, 9, 3834. [Google Scholar] [CrossRef]
- Schmölz, L.; Schubert, M.; Kirschner, J.; Kluge, S.; Galli, F.; Birringer, M.; Wallert, M.; Lorkowski, S. Long-chain metabolites of vitamin E: Interference with lipotoxicity via lipid droplet associated protein PLIN2. Biochim. Biophys. Acta 2018, 1863, 919–927. [Google Scholar] [CrossRef]
- Kluge, S.; Schubert, M.; Börmel, L.; Lorkowski, S. The vitamin E long-chain metabolite α-13’-COOH affects macrophage foam cell formation via modulation of the lipoprotein lipase system. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158875. [Google Scholar] [CrossRef]
- Willems, S.; Gellrich, L.; Chaikuad, A.; Kluge, S.; Werz, O.; Heering, J.; Knapp, S.; Lorkowski, S.; Schubert-Zsilavecz, M.; Merk, D. Endogenous vitamin E metabolites mediate allosteric PPARγ activation with unprecedented co-regulatory interactions. Cell Chem. Biol. 2021, 28, 1489–1500.e8. [Google Scholar] [CrossRef]
- Birringer, M.; Pfluger, P.; Kluth, D.; Landes, N.; Brigelius-Flohé, R. Identities and differences in the metabolism of tocotrienols and tocopherols in HepG2 cells. J. Nutr. 2002, 132, 3113–3118. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, antioxidant and nothing more. Free Radic. Biol. Med. 2007, 43, 4–15. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Tocopherol isoforms (α-, γ-, and δ-) show distinct capacities to control Nrf-2 and NfκB signaling pathways that modulate inflammatory response in Caco-2 intestinal cells. Mol. Cell. Biochem. 2015, 404, 123–131. [Google Scholar] [CrossRef]
- Wallert, M.; Börmel, L.; Lorkowski, S. Inflammatory Diseases and Vitamin E-What Do We Know and Where Do We Go? Mol. Nutr. Food Res. 2021, 65, e2000097. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.; Kattoor, A.J.; Saldeen, T.; Mehta, J.L. Vitamin E and its anticancer effects. Crit. Rev. Food Sci. Nutr. 2019, 59, 2831–2838. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Han, S.N. Vitamin E: Regulatory role on gene and protein expression and metabolomics profiles. IUBMB Life 2019, 71, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Lewis, E.D.; Meydani, S.N.; Wu, D. Regulatory role of vitamin E in the immune system and inflammation. IUBMB Life 2019, 71, 487–494. [Google Scholar] [CrossRef]
- Ziegler, M.; Wallert, M.; Lorkowski, S.; Peter, K. Cardiovascular and Metabolic Protection by Vitamin E: A Matter of Treatment Strategy? Antioxidants 2020, 9, 935. [Google Scholar] [CrossRef] [PubMed]
- Baburao Jain, A.; Anand Jain, V. Vitamin E, Its Beneficial Role in Diabetes Mellitus (DM) and Its Complications. J. Clin. Diagn. Res. 2012, 6, 1624–1628. [Google Scholar] [CrossRef] [PubMed]
- Browne, D.; McGuinness, B.; Woodside, J.V.; McKay, G.J. Vitamin E and Alzheimer’s disease: What do we know so far? Clin. Interv. Aging 2019, 14, 1303–1317. [Google Scholar] [CrossRef]
- Akinloye, O.; Adaramoye, O.; Kareem, O. Changes in antioxidant status and lipid peroxidation in Nigerian patients with prostate carcinoma. Pol. Arch. Med. Wewn. 2009, 119, 526–532. [Google Scholar] [CrossRef]
- Adaramoye, O.A.; Akinloye, O.; Olatunji, I.K. Trace elements and vitamin E status in Nigerian patients with prostate cancer. Afr. Health Sci. 2010, 10, 2–8. [Google Scholar]
- Yang, C.S.; Luo, P.; Zeng, Z.; Wang, H.; Malafa, M.; Suh, N. Vitamin E and cancer prevention: Studies with different forms of tocopherols and tocotrienols. Mol. Carcinog. 2020, 59, 365–389. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, J.; Guo, H.; Liu, Z.; Liu, Q.; Li, Y.; Zhang, D.; Liang, J. Associations of Dietary and Circulating Vitamin E Level with Metabolic Syndrome. A Meta-Analysis of Observational Studies. Front. Nutr. 2021, 8, 783990. [Google Scholar] [CrossRef]
- Schwarzova, M. Vitamin E: Recommended Intake. In Vitamin E in Health and Disease; Fatrcova-Sramkova, K., Ed.; IntechOpen: Rijeka, Hrvatska, 2021; Chapter 9; ISBN 978-1-83968-838-6. [Google Scholar]
- 52. In Ergebnisbericht Teil 2 Nationale Verzehrsstudie II: Wie sich Verbraucher in Deutschland Ernähren; Bundesforschungsinstitut für Ernährung und Lebensmittel, Ed.; Max Rubner-Institut: Karlsruhe, Germany, 2008. [Google Scholar]
- Bailey, R.L.; Gahche, J.J.; Miller, P.E.; Thomas, P.R.; Dwyer, J.T. Why US adults use dietary supplements. JAMA Intern. Med. 2013, 173, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Hoeft, B.; McBurney, M.; Eggersdorfer, M.; Weber, P. Dietary surveys indicate vitamin intakes below recommendations are common in representative Western countries. Br. J. Nutr. 2012, 108, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.A.; Chun, O.K. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int. J. Mol. Sci. 2016, 17, 1328. [Google Scholar] [CrossRef]
- Agarwal, A.; Hager, D.N.; Sevransky, J.E. Any Role of High-Dose Vitamin C for Septic Shock in 2021? Semin. Respir. Crit. Care Med. 2021, 42, 672–682. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; de Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 5. [Google Scholar] [CrossRef]
- Zhao, B.; Ling, Y.; Li, J.; Peng, Y.; Huang, J.; Wang, Y.; Qu, H.; Gao, Y.; Li, Y.; Hu, B.; et al. Beneficial aspects of high dose intravenous vitamin C on patients with COVID-19 pneumonia in severe condition: A retrospective case series study. Ann. Palliat. Med. 2021, 10, 1599–1609. [Google Scholar] [CrossRef]
- Traber, M.G.; Stevens, J.F. Vitamins C and E: Beneficial effects from a mechanistic perspective. Free Radic. Biol. Med. 2011, 51, 1000–1013. [Google Scholar] [CrossRef]
- Kagan, V.E.; Serbinova, E.A.; Forte, T.; Scita, G.; Packer, L. Recycling of vitamin E in human low density lipoproteins. J. Lipid Res. 1992, 33, 385–397. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef]
- Hamilton, I.M.; Gilmore, W.S.; Benzie, I.F.; Mulholland, C.W.; Strain, J.J. Interactions between vitamins C and E in human subjects. Br. J. Nutr. 2000, 84, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Jungert, A.; Neuhäuser-Berthold, M. Interrelation between Plasma Concentrations of Vitamins C and E along the Trajectory of Ageing in Consideration of Lifestyle and Body Composition: A Longitudinal Study over Two Decades. Nutrients 2020, 12, 2944. [Google Scholar] [CrossRef] [PubMed]
- Albanes, D.; Heinonen, O.P.; Taylor, P.R.; Virtamo, J.; Edwards, B.K.; Rautalahti, M.; Hartman, A.M.; Palmgren, J.; Freedman, L.S.; Haapakoski, J.; et al. Alpha-Tocopherol and beta-carotene supplements and lung cancer incidence in the alpha-tocopherol, beta-carotene cancer prevention study: Effects of base-line characteristics and study compliance. J. Natl. Cancer Inst. 1996, 88, 1560–1570. [Google Scholar] [CrossRef]
- Hemilä, H. Vitamin E and Mortality in Male Smokers of the ATBC Study: Implications for Nutritional Recommendations. Front. Nutr. 2020, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Hemilä, H.; Kaprio, J. Modification of the effect of vitamin E supplementation on the mortality of male smokers by age and dietary vitamin C. Am. J. Epidemiol. 2009, 169, 946–953. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 2012, CD007176. [Google Scholar] [CrossRef]
- McKay, G.J.; Lyner, N.; Linden, G.J.; Kee, F.; Moitry, M.; Biasch, K.; Amouyel, P.; Dallongeville, J.; Bongard, V.; Ferrières, J.; et al. Association of low plasma antioxidant levels with all-cause mortality and coronary events in healthy middle-aged men from France and Northern Ireland in the PRIME study. Eur. J. Nutr. 2021, 60, 2631–2641. [Google Scholar] [CrossRef]
- Losonczy, K.G.; Harris, T.B.; Havlik, R.J. Vitamin E and vitamin C supplement use and risk of all-cause and coronary heart disease mortality in older persons: The Established Populations for Epidemiologic Studies of the Elderly. Am. J. Clin. Nutr. 1996, 64, 190–196. [Google Scholar] [CrossRef]
- Amini, L.; Chekini, R.; Nateghi, M.R.; Haghani, H.; Jamialahmadi, T.; Sathyapalan, T.; Sahebkar, A. The Effect of Combined Vitamin C and Vitamin E Supplementation on Oxidative Stress Markers in Women with Endometriosis: A Randomized, Triple-Blind Placebo-Controlled Clinical Trial. Pain Res. Manag. 2021, 2021, 5529741. [Google Scholar] [CrossRef]
- Busso, D.; David, A.; Penailillo, R.; Echeverría, G.; Rigotti, A.; Kovalskys, I.; Gómez, G.; Cortés Sanabria, L.Y.; Yépez García, M.C.; Pareja, R.G.; et al. Intake of Vitamin E and C in Women of Reproductive Age: Results from the Latin American Study of Nutrition and Health (ELANS). Nutrients 2021, 13, 1954. [Google Scholar] [CrossRef] [PubMed]
- Rafighi, Z.; Shiva, A.; Arab, S.; Mohd Yousof, R. Association of dietary vitamin C and E intake and antioxidant enzymes in type 2 diabetes mellitus patients. Glob. J. Health Sci. 2013, 5, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Plantinga, Y.; Ghiadoni, L.; Magagna, A.; Giannarelli, C.; Franzoni, F.; Taddei, S.; Salvetti, A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am. J. Hypertens. 2007, 20, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Mellyana, O.; Widajat, R.; Sekarwana, N. Combined supplementation with α-tocopherol and vitamin C improves the blood pressure of pediatric idiopathic nephrotic syndrome patients. Clin. Nutr. Exp. 2019, 23, 116–121. [Google Scholar] [CrossRef][Green Version]
- Sesso, H.D.; Buring, J.E.; Christen, W.G.; Kurth, T.; Belanger, C.; MacFadyen, J.; Bubes, V.; Manson, J.E.; Glynn, R.J.; Gaziano, J.M. Vitamins E and C in the prevention of cardiovascular disease in men: The Physicians’ Health Study II randomized controlled trial. JAMA 2008, 300, 2123–2133. [Google Scholar] [CrossRef]
- Montero, D.; Walther, G.; Stehouwer, C.D.A.; Houben, A.J.H.M.; Beckman, J.A.; Vinet, A. Effect of antioxidant vitamin supplementation on endothelial function in type 2 diabetes mellitus: A systematic review and meta-analysis of randomized controlled trials. Obes. Rev. 2014, 15, 107–116. [Google Scholar] [CrossRef]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–665S. [Google Scholar] [CrossRef]
- Sarohan, A.R.; Kızıl, M.; İnkaya, A.Ç.; Mahmud, S.; Akram, M.; Cen, O. A novel hypothesis for COVID-19 pathogenesis: Retinol depletion and retinoid signaling disorder. Cell. Signal. 2021, 87, 110121. [Google Scholar] [CrossRef]
- Trasino, S.E. A role for retinoids in the treatment of COVID-19? Clin. Exp. Pharmacol. Physiol. 2020, 47, 1765–1767. [Google Scholar] [CrossRef]
- Palace, V.P.; Khaper, N.; Qin, Q.; Singal, P.K. Antioxidant potentials of vitamin A and carotenoids and their relevance to heart disease. Free Radic. Biol. Med. 1999, 26, 746–761. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.M. Antioxidant and Prooxidant Properties of Carotenoids. Arch. Biochem. Biophys. 2001, 385, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Greenberg, E.R.; Baron, J.A.; Karagas, M.R.; Stukel, T.A.; Nierenberg, D.W.; Stevens, M.M.; Mandel, J.S.; Haile, R.W. Mortality associated with low plasma concentration of beta carotene and the effect of oral supplementation. JAMA 1996, 275, 699–703. [Google Scholar] [CrossRef] [PubMed]
- Hennekens, C.H.; Buring, J.E.; Manson, J.E.; Stampfer, M.; Rosner, B.; Cook, N.R.; Belanger, C.; LaMotte, F.; Gaziano, J.M.; Ridker, P.M.; et al. Lack of effect of long-term supplementation with beta carotene on the incidence of malignant neoplasms and cardiovascular disease. N. Engl. J. Med. 1996, 334, 1145–1149. [Google Scholar] [CrossRef] [PubMed]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Upritchard, J.E.; Schuurman, C.R.W.C.; Wiersma, A.; Tijburg, L.B.M.; Coolen, S.A.J.; Rijken, P.J.; Wiseman, S.A. Spread supplemented with moderate doses of vitamin E and carotenoids reduces lipid peroxidation in healthy, nonsmoking adults. Am. J. Clin. Nutr. 2003, 78, 985–992. [Google Scholar] [CrossRef]
- Kataja-Tuomola, M.K.; Kontto, J.P.; Männistö, S.; Albanes, D.; Virtamo, J.R. Effect of alpha-tocopherol and beta-carotene supplementation on macrovascular complications and total mortality from diabetes: Results of the ATBC Study. Ann. Med. 2010, 42, 178–186. [Google Scholar] [CrossRef]
- Törnwall, M.E.; Virtamo, J.; Korhonen, P.A.; Virtanen, M.J.; Taylor, P.R.; Albanes, D.; Huttunen, J.K. Effect of alpha-tocopherol and beta-carotene supplementation on coronary heart disease during the 6-year post-trial follow-up in the ATBC study. Eur. Heart J. 2004, 25, 1171–1178. [Google Scholar] [CrossRef]
- Shargorodsky, M.; Debby, O.; Matas, Z.; Zimlichman, R. Effect of long-term treatment with antioxidants (vitamin C, vitamin E, coenzyme Q10 and selenium) on arterial compliance, humoral factors and inflammatory markers in patients with multiple cardiovascular risk factors. Nutr. Metab. 2010, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Tadzic, R.; Vcev, A.; Rucevic, S.; Drenjancevic, I. Blood Pressure Reduction is Associated with the Changes in Oxidative Stress and Endothelial Activation in Hypertension, Regardless of Antihypertensive Therapy. Kidney Blood Press. Res. 2016, 41, 721–735. [Google Scholar] [CrossRef] [PubMed]
- Karajibani, M.; Hashemi, M.; Montazerifar, F.; Dikshit, M. Effect of vitamin E and C supplements on antioxidant defense system in cardiovascular disease patients in Zahedan, southeast Iran. J. Nutr. Sci. Vitaminol. 2010, 56, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Altoum, A.E.A.; Osman, A.L.; Babker, A.M. Comparative Study of Levels of Selective Oxidative Stress Markers (Malondialdehyde Zinc and Antioxidant Vitamins A E and C) in Ischemic and Non-Ischemic Heart Disease Patients Suffering from Type- 2 Diabetes. Asian J. Pharm. Clin. Res. 2018, 11, 508–510. [Google Scholar] [CrossRef]
- Zitouni, K.; Steyn, M.R.C.P.; Lyka, E.; Kelly, F.J.; Cook, P.; Ster, I.C.; Earle, K.A. Derepression of glomerular filtration, renal blood flow and antioxidant defence in patients with type 2 diabetes at high-risk of cardiorenal disease. Free Radic. Biol. Med. 2020, 161, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Safiyeh, F.D.; Mojgan, M.; Parviz, S.; Sakineh, M.A.; Behnaz, S.O. The effect of selenium and vitamin E supplementation on anti-Mullerian hormone and antral follicle count in infertile women with occult premature ovarian insufficiency: A randomized controlled clinical trial. Complement. Ther. Med. 2021, 56, 102533. [Google Scholar] [CrossRef] [PubMed]
- Guertin, K.A.; Grant, R.K.; Arnold, K.B.; Burwell, L.; Hartline, J.; Goodman, P.J.; Minasian, L.M.; Lippman, S.M.; Klein, E.; Cassano, P.A. Effect of long-term vitamin E and selenium supplementation on urine F2-isoprostanes, a biomarker of oxidative stress. Free Radic. Biol. Med. 2016, 95, 349–356. [Google Scholar] [CrossRef]
- Afzali, H.; Jafari Kashi, A.H.; Momen-Heravi, M.; Razzaghi, R.; Amirani, E.; Bahmani, F.; Gilasi, H.R.; Asemi, Z. The effects of magnesium and vitamin E co-supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. Wound Rep. Reg. 2019, 27, 277–284. [Google Scholar] [CrossRef]
- Shokrpour, M.; Asemi, Z. The Effects of Magnesium and Vitamin E Co-Supplementation on Hormonal Status and Biomarkers of Inflammation and Oxidative Stress in Women with Polycystic Ovary Syndrome. Biol. Trace Elem. Res. 2019, 191, 54–60. [Google Scholar] [CrossRef]
- Jamilian, M.; Sabzevar, N.K.; Asemi, Z. The Effect of Magnesium and Vitamin E Co-Supplementation on Glycemic Control and Markers of Cardio-Metabolic Risk in Women with Polycystic Ovary Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Horm. Metab. Res. 2019, 51, 100–105. [Google Scholar] [CrossRef]
- Maktabi, M.; Jamilian, M.; Amirani, E.; Chamani, M.; Asemi, Z. The effects of magnesium and vitamin E co-supplementation on parameters of glucose homeostasis and lipid profiles in patients with gestational diabetes. Lipids Health Dis. 2018, 17, 163. [Google Scholar] [CrossRef]
- Ostadmohammadi, V.; Samimi, M.; Mobini, M.; Zarezade Mehrizi, M.; Aghadavod, E.; Chamani, M.; Dastorani, M.; Asemi, Z. The effect of zinc and vitamin E cosupplementation on metabolic status and its related gene expression in patients with gestational diabetes. J. Matern. Fetal Neonatal Med. 2019, 32, 4120–4127. [Google Scholar] [CrossRef]
- Alpha-Tocopherol Beta Carotene Cancer Prevention Study Group. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N. Engl. J. Med. 1994, 330, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Sesso, H.D.; Christen, W.G.; Bubes, V.; Smith, J.P.; MacFadyen, J.; Schvartz, M.; Manson, J.E.; Glynn, R.J.; Buring, J.E. Multivitamins in the Prevention of Cancer in Men: The Physicians’ Health Study II Randomized Controlled Trial. JAMA 2012, 308, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Galan, P.; Preziosi, P.; Bertrais, S.; Mennen, L.; Malvy, D.; Roussel, A.-M.; Favier, A.; Briançon, S. The SU.VI.MAX Study: A randomized, placebo-controlled trial of the health effects of antioxidant vitamins and minerals. Arch. Intern. Med. 2004, 164, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Hercberg, S.; Kesse-Guyot, E.; Druesne-Pecollo, N.; Touvier, M.; Favier, A.; Latino-Martel, P.; Briançon, S.; Galan, P. Incidence of cancers, ischemic cardiovascular diseases and mortality during 5-year follow-up after stopping antioxidant vitamins and minerals supplements: A postintervention follow-up in the SU.VI.MAX Study. Int. J. Cancer 2010, 127, 1875–1881. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cook, N.R.; Albert, C.M.; van Denburgh, M.; Manson, J.E. Effects of vitamins C and E and beta-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: A randomized controlled trial. Am. J. Clin. Nutr. 2009, 90, 429–437. [Google Scholar] [CrossRef]
- Ye, Y.; Li, J.; Yuan, Z. Effect of antioxidant vitamin supplementation on cardiovascular outcomes: A meta-analysis of randomized controlled trials. PLoS ONE 2013, 8, e56803. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.U.; Riaz, H.; Valavoor, S.; Zhao, D.; Vaughan, L.; Okunrintemi, V.; Riaz, I.B.; Khan, M.S.; Kaluski, E.; et al. Effects of Nutritional Supplements and Dietary Interventions on Cardiovascular Outcomes: An Umbrella Review and Evidence Map. Ann. Intern. Med. 2019, 171, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Mondul, A.M.; Sampson, J.N.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Virtamo, J.; Albanes, D. Metabolomic profile of response to supplementation with β-carotene in the Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 2013, 98, 488–493. [Google Scholar] [CrossRef]
- Card, D.J.; Gorska, R.; Cutler, J.; Harrington, D.J. Vitamin K metabolism: Current knowledge and future research. Mol. Nutr. Food Res. 2014, 58, 1590–1600. [Google Scholar] [CrossRef]
- Shioi, A.; Morioka, T.; Shoji, T.; Emoto, M. The Inhibitory Roles of Vitamin K in Progression of Vascular Calcification. Nutrients 2020, 12, 583. [Google Scholar] [CrossRef]
- Vlasschaert, C.; Goss, C.J.; Pilkey, N.G.; McKeown, S.; Holden, R.M. Vitamin K Supplementation for the Prevention of Cardiovascular Disease: Where Is the Evidence? A Systematic Review of Controlled Trials. Nutrients 2020, 12, 2909. [Google Scholar] [CrossRef] [PubMed]
- van Ballegooijen, A.J.; Beulens, J.W. The Role of Vitamin K Status in Cardiovascular Health: Evidence from Observational and Clinical Studies. Curr. Nutr. Rep. 2017, 6, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Wheldon, G.H.; Bhatt, A.; Keller, P.; Hummler, H. d,1-alpha-Tocopheryl acetate (vitamin E): A long term toxicity and carcinogenicity study in rats. Int. J. Vitam. Nutr. Res. 1983, 53, 287–296. [Google Scholar] [PubMed]
- Glynn, R.J.; Ridker, P.M.; Goldhaber, S.Z.; Zee, R.Y.L.; Buring, J.E. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: Report from the Women’s Health Study. Circulation 2007, 116, 1497–1503. [Google Scholar] [CrossRef]
- Lee, I.-M.; Cook, N.R.; Gaziano, J.M.; Gordon, D.; Ridker, P.M.; Manson, J.E.; Hennekens, C.H.; Buring, J.E. Vitamin E in the primary prevention of cardiovascular disease and cancer: The Women’s Health Study: A randomized controlled trial. JAMA 2005, 294, 56–65. [Google Scholar] [CrossRef]
- Traber, M.G. Vitamin E and K interaction—A 50-year-old problem. Nutr. Rev. 2008, 66, 624–629. [Google Scholar] [CrossRef]
- McDonald, M.G.; Rieder, M.J.; Nakano, M.; Hsia, C.K.; Rettie, A.E. CYP4F2 is a vitamin K1 oxidase: An explanation for altered warfarin dose in carriers of the V433M variant. Mol. Pharmacol. 2009, 75, 1337–1346. [Google Scholar] [CrossRef]
- Hanzawa, F.; Sakuma, E.; Nomura, S.; Uchida, T.; Oda, H.; Ikeda, S. Excess α-tocopherol decreases extrahepatic phylloquinone in phylloquinone-fed rats but not menaquinone-4 in menaquinone-4-fed rats. Mol. Nutr. Food Res. 2014, 58, 1601–1609. [Google Scholar] [CrossRef]
- Shearer, M.J.; Newman, P. Recent trends in the metabolism and cell biology of vitamin K with special reference to vitamin K cycling and MK-4 biosynthesis. J. Lipid Res. 2014, 55, 345–362. [Google Scholar] [CrossRef]
- Farley, S.M.; Leonard, S.W.; Taylor, A.W.; Birringer, M.; Edson, K.Z.; Rettie, A.E.; Traber, M.G. ω-Hydroxylation of phylloquinone by CYP4F2 is not increased by α-tocopherol. Mol. Nutr. Food Res. 2013, 57, 1785–1793. [Google Scholar] [CrossRef]
- Watanabe, H.; Yamaori, S.; Kamijo, S.; Aikawa, K.; Ohmori, S. In Vitro Inhibitory Effects of Sesamin on CYP4F2 Activity. Biol. Pharm. Bull. 2020, 43, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.-P.; Ma, C.-Y.; Liu, C.-L.; Lin, Y.-H.; Hu, M.-L.; Chen, J.-J.; Hung, D.-Z.; Hsieh, W.-T.; Huang, J.-D. Sesamin: A Naturally Occurring Lignan Inhibits CYP3A4 by Antagonizing the Pregnane X Receptor Activation. Evid. Based Complement. Alternat. Med. 2012, 2012, 242810. [Google Scholar] [CrossRef] [PubMed]
- Hanzawa, F.; Nomura, S.; Sakuma, E.; Uchida, T.; Ikeda, S. Dietary sesame seed and its lignan, sesamin, increase tocopherol and phylloquinone concentrations in male rats. J. Nutr. 2013, 143, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Farley, S.M.; Leonard, S.W.; Labut, E.M.; Raines, H.F.; Card, D.J.; Harrington, D.J.; Mustacich, D.J.; Traber, M.G. Vitamin E decreases extra-hepatic menaquinone-4 concentrations in rats fed menadione or phylloquinone. Mol. Nutr. Food Res. 2012, 56, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Guo, L.; Bu, C. Vitamin K status and cardiovascular events or mortality: A meta-analysis. Eur. J. Prev. Cardiol. 2019, 26, 549–553. [Google Scholar] [CrossRef]
- Cozzolino, M.; Mangano, M.; Galassi, A.; Ciceri, P.; Messa, P.; Nigwekar, S. Vitamin K in Chronic Kidney Disease. Nutrients 2019, 11, 168. [Google Scholar] [CrossRef]
- Simes, D.C.; Viegas, C.S.B.; Araújo, N.; Marreiros, C. Vitamin K as a Diet Supplement with Impact in Human Health: Current Evidence in Age-Related Diseases. Nutrients 2020, 12, 138. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium, inflammation, and obesity in chronic disease. Nutr. Rev. 2010, 68, 333–340. [Google Scholar] [CrossRef]
- Kruse, H.D.; Orent, E.R.; McCollum, E.V. Studies on Magnesium Deficiency in Animals I. Symptomatology Resulting from Magnesium Deprivation. Nutr. Rev. 1979, 37, 145–148. [Google Scholar] [CrossRef]
- Oka, R.K.; Alley, H.F. Differences in nutrition status by body mass index in patients with peripheral artery disease. J. Vasc. Nurs. 2012, 30, 77–87. [Google Scholar] [CrossRef]
- Adrian, M.; Chanut, E.; Laurant, P.; Gaume, V.; Berthelot, A. A long-term moderate magnesium-deficient diet aggravates cardiovascular risks associated with aging and increases mortality in rats. J. Hypertens. 2008, 26, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Manning, P.J.; Sutherland, W.H.F.; Walker, R.J.; Williams, S.M.; de Jong, S.A.; Ryalls, A.R.; Berry, E.A. Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care 2004, 27, 2166–2171. [Google Scholar] [CrossRef] [PubMed]
- Meng, C. Nitric oxide (NO) levels in patients with polycystic ovary syndrome (PCOS): A meta-analysis. J. Int. Med. Res. 2019, 47, 4083–4094. [Google Scholar] [CrossRef] [PubMed]
- Dehbalaei, M.G.; Ashtary-Larky, D.; Amarpoor Mesrkanlou, H.; Talebi, S.; Asbaghi, O. The effects of magnesium and vitamin E co-supplementation on some cardiovascular risk factors: A meta-analysis. Clin. Nutr. ESPEN 2021, 41, 110–117. [Google Scholar] [CrossRef]
- Farvid, M.S.; Jalali, M.; Siassi, F.; Saadat, N.; Hosseini, M. The impact of vitamins and/or mineral supplementation on blood pressure in type 2 diabetes. J. Am. Coll. Nutr. 2004, 23, 272–279. [Google Scholar] [CrossRef]
- Farvid, M.S.; Siassi, F.; Jalali, M.; Hosseini, M.; Saadat, N. The impact of vitamin and/or mineral supplementation on lipid profiles in type 2 diabetes. Diabetes Res. Clin. Pract. 2004, 65, 21–28. [Google Scholar] [CrossRef]
- Farvid, M.S.; Jalali, M.; Siassi, F.; Hosseini, M. Comparison of the effects of vitamins and/or mineral supplementation on glomerular and tubular dysfunction in type 2 diabetes. Diabetes Care 2005, 28, 2458–2464. [Google Scholar] [CrossRef]
- Nakanishi, I.; Fukuhara, K.; Shimada, T.; Ohkubo, K.; Iizuka, Y.; Inami, K.; Mochizuki, M.; Urano, S.; Itoh, S.; Miyata, N.; et al. Effects of magnesium ion on kinetic stability and spin distribution of phenoxyl radical derived from a vitamin E analogue: Mechanistic insight into antioxidative hydrogen-transfer reaction of vitamin E. J. Chem. Soc. Perkin Trans. 2 2002, 9, 1520–1524. [Google Scholar] [CrossRef]
- Hoffmann, P.R.; Berry, M.J. The influence of selenium on immune responses. Mol. Nutr. Food Res. 2008, 52, 1273–1280. [Google Scholar] [CrossRef]
- Avery, J.C.; Hoffmann, P.R. Selenium, Selenoproteins, and Immunity. Nutrients 2018, 10, 1203. [Google Scholar] [CrossRef]
- Shrimali, R.K.; Irons, R.D.; Carlson, B.A.; Sano, Y.; Gladyshev, V.N.; Park, J.M.; Hatfield, D.L. Selenoproteins mediate T cell immunity through an antioxidant mechanism. J. Biol. Chem. 2008, 283, 20181–20185. [Google Scholar] [CrossRef]
- Hawkes, W.C.; Kelley, D.S.; Taylor, P.C. The Effects of Dietary Selenium on the Immune System in Healthy Men. Biol. Trace Elem. Res. 2001, 81, 189–213. [Google Scholar] [CrossRef]
- Nelson, S.M.; Lei, X.; Prabhu, K.S. Selenium levels affect the IL-4-induced expression of alternative activation markers in murine macrophages. J. Nutr. 2011, 141, 1754–1761. [Google Scholar] [CrossRef]
- Carlson, B.A.; Yoo, M.-H.; Shrimali, R.K.; Irons, R.; Gladyshev, V.N.; Hatfield, D.L.; Park, J.M. Role of selenium-containing proteins in T-cell and macrophage function. Proc. Nutr. Soc. 2010, 69, 300–310. [Google Scholar] [CrossRef]
- Safir, N.; Wendel, A.; Saile, R.; Chabraoui, L. The effect of selenium on immune functions of J774.1 cells. Clin. Chem. Lab. Med. 2003, 41, 1005–1011. [Google Scholar] [CrossRef]
- Köse, S.A.; Nazıroğlu, M. Selenium reduces oxidative stress and calcium entry through TRPV1 channels in the neutrophils of patients with polycystic ovary syndrome. Biol. Trace Elem. Res. 2014, 158, 136–142. [Google Scholar] [CrossRef]
- Kiremidjian-Schumacher, L.; Roy, M.; Wishe, H.I.; Cohen, M.W.; Stotzky, G. Supplementation with selenium augments the functions of natural killer and lymphokine-activated killer cells. Biol. Trace Elem. Res. 1996, 52, 227–239. [Google Scholar] [CrossRef]
- Kim, H.-Y. The methionine sulfoxide reduction system: Selenium utilization and methionine sulfoxide reductase enzymes and their functions. Antioxid. Redox Signal. 2013, 19, 958–969. [Google Scholar] [CrossRef]
- Lee, B.C.; Péterfi, Z.; Hoffmann, F.W.; Moore, R.E.; Kaya, A.; Avanesov, A.; Tarrago, L.; Zhou, Y.; Weerapana, E.; Fomenko, D.E.; et al. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol. Cell 2013, 51, 397–404. [Google Scholar] [CrossRef]
- Dewell, A.; Tsao, P.; Rigdon, J.; Gardner, C.D. Antioxidants from diet or supplements do not alter inflammatory markers in adults with cardiovascular disease risk. A pilot randomized controlled trial. Nutr. Res. 2018, 50, 63–72. [Google Scholar] [CrossRef]
- Murer, S.B.; Aeberli, I.; Braegger, C.P.; Gittermann, M.; Hersberger, M.; Leonard, S.W.; Taylor, A.W.; Traber, M.G.; Zimmermann, M.B. Antioxidant supplements reduced oxidative stress and stabilized liver function tests but did not reduce inflammation in a randomized controlled trial in obese children and adolescents. J. Nutr. 2014, 144, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Passerieux, E.; Hayot, M.; Jaussent, A.; Carnac, G.; Gouzi, F.; Pillard, F.; Picot, M.-C.; Böcker, K.; Hugon, G.; Pincemail, J.; et al. Effects of vitamin C, vitamin E, zinc gluconate, and selenomethionine supplementation on muscle function and oxidative stress biomarkers in patients with facioscapulohumeral dystrophy: A double-blind randomized controlled clinical trial. Free Radic. Biol. Med. 2015, 81, 158–169. [Google Scholar] [CrossRef]
- Singh, N.; Ahuja, V.; Sachdev, V.; Upadhyay, A.D.; Goswami, R.; Ramakrishnan, L.; Dwivedi, S.; Saraya, A. Antioxidants for Pancreatic Functions in Chronic Pancreatitis: A Double-blind Randomized Placebo-controlled Pilot Study. J. Clin. Gastroenterol. 2020, 54, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zeng, C.; Gong, Q.; Li, X.; Lei, G.; Yang, T. Associations between Dietary Antioxidant Intake and Metabolic Syndrome. PLoS ONE 2015, 10, e0130876. [Google Scholar] [CrossRef] [PubMed]
- Rosário, P.W.; Batista, K.C.S.; Calsolari, M.R. Radioiodine-induced oxidative stress in patients with differentiated thyroid carcinoma and effect of supplementation with vitamins C and E and selenium (antioxidants). Arch. Endocrinol. Metab. 2016, 60, 328–332. [Google Scholar] [CrossRef]
- Khalifa, O.A.; Al Wakeel, R.A.; Hemeda, S.A.; Abdel-Daim, M.M.; Albadrani, G.M.; El Askary, A.; Fadl, S.E.; Elgendey, F. The impact of vitamin E and/or selenium dietary supplementation on growth parameters and expression levels of the growth-related genes in broilers. BMC Vet. Res. 2021, 17, 251. [Google Scholar] [CrossRef]
- Dalia, A.M.; Loh, T.C.; Sazili, A.Q.; Jahromi, M.F.; Samsudin, A.A. Effects of vitamin E, inorganic selenium, bacterial organic selenium, and their combinations on immunity response in broiler chickens. BMC Vet. Res. 2018, 14, 249. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Sodhi, S.; Kaur, R. Effects of dietary supplements of selenium, vitamin E or combinations of the two on antibody responses of broilers. Br. Poult. Sci. 2006, 47, 714–719. [Google Scholar] [CrossRef]
- Swain, B.K.; Johri, T.S.; Majumdar, S. Effect of supplementation of vitamin E, selenium and their different combinations on the performance and immune response of broilers. Br. Poult. Sci. 2000, 41, 287–292. [Google Scholar] [CrossRef]
- Saito, Y. Diverse cytoprotective actions of vitamin E isoforms- role as peroxyl radical scavengers and complementary functions with selenoproteins. Free Radic. Biol. Med. 2021, 175, 121–129. [Google Scholar] [CrossRef]
- Saito, Y. Lipid peroxidation products as a mediator of toxicity and adaptive response—The regulatory role of selenoprotein and vitamin E. Arch. Biochem. Biophys. 2021, 703, 108840. [Google Scholar] [CrossRef]
- May, J.M.; Burk, R.F. Interactions of selenium, vitamin E, and vitamin C in atherosclerosis. Adv. Cell Aging Gerontol. 2002, 11, 337–348. [Google Scholar]
- Fischer, A.; Pallauf, J.; Gohil, K.; Weber, S.U.; Packer, L.; Rimbach, G. Effect of selenium and vitamin E deficiency on differential gene expression in rat liver. Biochem. Biophys. Res. Commun. 2001, 285, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Güney, M. Selenium-vitamin E combination modulates endometrial lipid peroxidation and antioxidant enzymes in streptozotocin-induced diabetic rat. Biol. Trace Elem. Res. 2012, 149, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Del Maria Bas, J.; Rodríguez, B.; Puiggròs, F.; Mariné, S.; Rodríguez, M.A.; Moriña, D.; Armengol, L.; Caimari, A.; Arola, L. Hepatic accumulation of S-adenosylmethionine in hamsters with non-alcoholic fatty liver disease associated with metabolic syndrome under selenium and vitamin E deficiency. Clin. Sci. 2019, 133, 409–423. [Google Scholar] [CrossRef]
- Zulet, M.A.; Puchau, B.; Hermsdorff, H.H.M.; Navarro, C.; Martínez, J.A. Dietary selenium intake is negatively associated with serum sialic acid and metabolic syndrome features in healthy young adults. Nutr. Res. 2009, 29, 41–48. [Google Scholar] [CrossRef]
- Al-Busafi, S.A.; Bhat, M.; Wong, P.; Ghali, P.; Deschenes, M. Antioxidant therapy in nonalcoholic steatohepatitis. Hepat. Res. Treat. 2012, 2012, 947575. [Google Scholar] [CrossRef]
- Hussein, A.M.; Saleh, H.A.; Mustafa, H.N. Effect of sodium selenite and vitamin E on the renal cortex in rats: An ultrastructure study. Tissue Cell 2014, 46, 170–177. [Google Scholar] [CrossRef]
- Amraoui, W.; Adjabi, N.; Bououza, F.; Boumendjel, M.; Taibi, F.; Boumendjel, A.; Abdennour, C.; Messarah, M. Modulatory Role of Selenium and Vitamin E, Natural Antioxidants, against Bisphenol A-Induced Oxidative Stress in Wistar Albinos Rats. Toxicol. Res. 2018, 34, 231–239. [Google Scholar] [CrossRef]
- Feriani, A.; Hachani, R.; Tir, M.; Ghazouani, L.; Mufti, A.; Borgi, M.A.; Allagui, M.S. Bifenthrin exerts proatherogenic effects via arterial accumulation of native and oxidized LDL in rats: The beneficial role of vitamin E and selenium. Environ. Sci. Pollut. Res. Int. 2020, 27, 5651–5660. [Google Scholar] [CrossRef]
- Bardas, E.; Arslan, Y.K.; Polat, S.; Erisir, M.; Uslu, G.A.; Cetin, N.; Cicek, B. Vitamin E and Selenium Reduce Prednisolone Side Effects in Rat Hearts. Int. J. Vitam. Nutr. Res. 2020, 90, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R. Critical Role of Zinc as Either an Antioxidant or a Prooxidant in Cellular Systems. Oxid. Med. Cell. Longev. 2018, 2018, 9156285. [Google Scholar] [CrossRef] [PubMed]
- Shkembi, B.; Huppertz, T. Influence of Dairy Products on Bioavailability of Zinc from Other Food Products: A Review of Complementarity at a Meal Level. Nutrients 2021, 13, 4253. [Google Scholar] [CrossRef]
- Maret, W. The Function of Zinc Metallothionein: A Link between Cellular Zinc and Redox State. J. Nutr. 2000, 130, 1455S–1458S. [Google Scholar] [CrossRef]
- Krężel, A.; Maret, W. The Functions of Metamorphic Metallothioneins in Zinc and Copper Metabolism. Int. J. Mol. Sci. 2017, 18, 1237. [Google Scholar] [CrossRef]
- Kim, B.; Lee, W.-W. Regulatory Role of Zinc in Immune Cell Signaling. Mol. Cells 2021, 44, 335–341. [Google Scholar] [CrossRef]
- Gammoh, N.Z.; Rink, L. Zinc in Infection and Inflammation. Nutrients 2017, 9, 624. [Google Scholar] [CrossRef]
- Wang, X.; Dong, W.; Yuan, B.; Yang, Y.; Yang, D.; Lin, X.; Chen, C.; Zhang, W. Vitamin E confers cytoprotective effects on cardiomyocytes under conditions of heat stress by increasing the expression of metallothionein. Int. J. Mol. Med. 2016, 37, 1429–1436. [Google Scholar] [CrossRef]
- Hajiani, M.; Razi, F.; Golestani, A.; Frouzandeh, M.; Owji, A.A.; Khaghani, S.; Ghannadian, N.; Shariftabrizi, A.; Pasalar, P. Time- and dose-dependent differential regulation of copper-zinc superoxide dismutase and manganese superoxide dismutase enzymatic activity and mRNA level by vitamin E in rat blood cells. Redox Rep. 2012, 17, 101–107. [Google Scholar] [CrossRef]
- Bruno, R.S.; Song, Y.; Leonard, S.W.; Mustacich, D.J.; Taylor, A.W.; Traber, M.G.; Ho, E. Dietary zinc restriction in rats alters antioxidant status and increases plasma F2 isoprostanes. J. Nutr. Biochem. 2007, 18, 509–518. [Google Scholar] [CrossRef]
- Farias, M.S.; Budni, P.; Ribeiro, C.M.; Parisotto, E.B.; Santos, C.E.I.; Dias, J.F.; Dalmarco, E.M.; Fröde, T.S.; Pedrosa, R.C.; Wilhelm Filho, D. Antioxidant supplementation attenuates oxidative stress in chronic hepatitis C patients. Gastroenterol. Hepatol. 2012, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Czernichow, S.; Vergnaud, A.-C.; Galan, P.; Arnaud, J.; Favier, A.; Faure, H.; Huxley, R.; Hercberg, S.; Ahluwalia, N. Effects of long-term antioxidant supplementation and association of serum antioxidant concentrations with risk of metabolic syndrome in adults. Am. J. Clin. Nutr. 2009, 90, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, H.; Wu, M.; Liu, M. Serum and dietary antioxidant status is associated with lower prevalence of the metabolic syndrome in a study in Shanghai, China. Asia Pac. J. Clin. Nutr. 2013, 22, 60–68. [Google Scholar] [CrossRef]
- Karamali, M.; Heidarzadeh, Z.; Seifati, S.M.; Samimi, M.; Tabassi, Z.; Hajijafari, M.; Asemi, Z.; Esmaillzadeh, A. Zinc supplementation and the effects on metabolic status in gestational diabetes: A randomized, double-blind, placebo-controlled trial. J. Diabetes Complicat. 2015, 29, 1314–1319. [Google Scholar] [CrossRef]
- Rimbach, G.; Moehring, J.; Huebbe, P.; Lodge, J.K. Gene-regulatory activity of alpha-tocopherol. Molecules 2010, 15, 1746–1761. [Google Scholar] [CrossRef]
- Birringer, M.; Lorkowski, S. Vitamin E: Regulatory role of metabolites. IUBMB Life 2019, 71, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Bartolini, D.; de Franco, F.; Torquato, P.; Marinelli, R.; Cerra, B.; Ronchetti, R.; Schon, A.; Fallarino, F.; de Luca, A.; Bellezza, G.; et al. Garcinoic Acid Is a Natural and Selective Agonist of Pregnane X Receptor. J. Med. Chem. 2020, 63, 3701–3712. [Google Scholar] [CrossRef]
- Devaraj, S.; Tang, R.; Adams-Huet, B.; Harris, A.; Seenivasan, T.; De Lemos, J.A.; Jialal, I. Effect of high-dose alpha-tocopherol supplementation on biomarkers of oxidative stress and inflammation and carotid atherosclerosis in patients with coronary artery disease. Am. J. Clin. Nutr. 2007, 86, 1392–1398. [Google Scholar] [CrossRef]
- Robinson, I.; de Serna, D.G.; Gutierrez, A.; David, S. Schade. Review Article. Economica 2003, 70, 691–697. [Google Scholar] [CrossRef]
- Zuo, S.; Wang, G.; Han, Q.; Xiao, H.; Santos, H.O.; Avelar Rodriguez, D.; Khani, V.; Tang, J. The effects of tocotrienol supplementation on lipid profile: A meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 52, 102450. [Google Scholar] [CrossRef]
- Jiang, Q. Natural forms of vitamin E and metabolites-regulation of cancer cell death and underlying mechanisms. IUBMB Life 2019, 71, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Schubert, M.; Kluge, S.; Brunner, E.; Pace, S.; Birringer, M.; Werz, O.; Lorkowski, S. The α-tocopherol-derived long-chain metabolite α-13′-COOH mediates endotoxin tolerance and modulates the inflammatory response via MAPK and NFκB pathways. Free Radic. Biol. Med. 2021, 178, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Park, N.-Y.; Rostgaard-Hansen, A.L.; Huang, J.; Jiang, Q. Vitamin E metabolite 13′-carboxychromanols inhibit pro-inflammatory enzymes, induce apoptosis and autophagy in human cancer cells by modulating sphingolipids and suppress colon tumor development in mice. Free Radic. Biol. Med. 2016, 95, 190–199. [Google Scholar] [CrossRef]
- Wallert, M.; Schmölz, L.; Koeberle, A.; Krauth, V.; Glei, M.; Galli, F.; Werz, O.; Birringer, M.; Lorkowski, S. α-Tocopherol long-chain metabolite α-13′-COOH affects the inflammatory response of lipopolysaccharide-activated murine RAW264.7 macrophages. Mol. Nutr. Food Res. 2015, 59, 1524–1534. [Google Scholar] [CrossRef]
- Alsabil, K.; Suor-Cherer, S.; Koeberle, A.; Viault, G.; Lavaud, A.; Temml, V.; Waltenberger, B.; Schuster, D.; Litaudon, M.; Lorkowski, S.; et al. Semisynthetic and Natural Garcinoic Acid Isoforms as New mPGES-1 Inhibitors. Planta Med. 2016, 82, 1110–1116. [Google Scholar] [CrossRef]
- Olajide, O.A.; Iwuanyanwu, V.U.; Lepiarz-Raba, I.; Al-Hindawi, A.A.; Aderogba, M.A.; Sharp, H.L.; Nash, R.J. Garcinia kola and garcinoic acid suppress SARS-CoV-2 spike glycoprotein S1-induced hyper-inflammation in human PBMCs through inhibition of NF-κB activation. Phytother. Res. 2021, 35, 6963. [Google Scholar] [CrossRef]
- Birringer, M.; EyTina, J.H.; Salvatore, B.A.; Neuzil, J. Vitamin E analogues as inducers of apoptosis: Structure-function relation. Br. J. Cancer 2003, 88, 1948–1955. [Google Scholar] [CrossRef]
- Schmölz, L.; Wallert, M.; Rozzino, N.; Cignarella, A.; Galli, F.; Glei, M.; Werz, O.; Koeberle, A.; Birringer, M.; Lorkowski, S. Structure-Function Relationship Studies In Vitro Reveal Distinct and Specific Effects of Long-Chain Metabolites of Vitamin E. Mol. Nutr. Food Res. 2017, 61, 1700562. [Google Scholar] [CrossRef] [PubMed]
- Birringer, M.; Siems, K.; Maxones, A.; Frank, J.; Lorkowski, S. Natural 6-hydroxy-chromanols and -chromenols: Structural diversity, biosynthetic pathways and health implications. RSC Adv. 2018, 8, 4803–4841. [Google Scholar] [CrossRef]
- Podszun, M.C.; Jakobi, M.; Birringer, M.; Weiss, J.; Frank, J. The long chain α-tocopherol metabolite α-13′-COOH and γ-tocotrienol induce P-glycoprotein expression and activity by activation of the pregnane X receptor in the intestinal cell line LS 180. Mol. Nutr. Food Res. 2017, 61, 1600605. [Google Scholar] [CrossRef]
- Landes, N.; Pfluger, P.; Kluth, D.; Birringer, M.; Rühl, R.; Böl, G.-F.; Glatt, H.; Brigelius-Flohé, R. Vitamin E activates gene expression via the pregnane X receptor. Biochem. Pharmacol. 2003, 65, 269–273. [Google Scholar] [CrossRef]
- Cárcamo, J.M.; Pedraza, A.; Bórquez-Ojeda, O.; Golde, D.W. Vitamin C Suppresses TNFα-Induced NFκB Activation by Inhibiting IκBα Phosphorylation. Biochemistry 2002, 41, 12995–13002. [Google Scholar] [CrossRef]
- Kawade, N.; Murai, A.; Suzuki, W.; Tokuda, Y.; Kobayashi, M.; Horio, F. Ascorbic acid deficiency increases hepatic expression of acute phase proteins through the intestine-derived IL-6 and hepatic STAT3 pathway in ODS rats. J. Nutr. Biochem. 2019, 70, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Austenaa, L.M.; Carlsen, H.; Hollung, K.; Blomhoff, H.K.; Blomhoff, R. Retinoic acid dampens LPS-induced NF-κB activity: Results from human monoblasts and in vivo imaging of NF-κB reporter mice. J. Nutr. Biochem. 2009, 20, 726–734. [Google Scholar] [CrossRef]
- Supasai, S.; Aimo, L.; Adamo, A.M.; Mackenzie, G.G.; Oteiza, P.I. Zinc deficiency affects the STAT1/3 signaling pathways in part through redox-mediated mechanisms. Redox Biol. 2017, 11, 469–481. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.J.; Sarkar, F.H. Zinc up-regulates NF-kappaB activation via phosphorylation of IkappaB in HUT-78 (Th0) cells. FEBS Lett. 2007, 581, 4507–4511. [Google Scholar] [CrossRef]
- Lee, B.C.; Lee, S.-G.; Choo, M.-K.; Kim, J.H.; Lee, H.M.; Kim, S.; Fomenko, D.E.; Kim, H.-Y.; Park, J.M.; Gladyshev, V.N. Selenoprotein MsrB1 promotes anti-inflammatory cytokine gene expression in macrophages and controls immune response in vivo. Sci. Rep. 2017, 7, 5119. [Google Scholar] [CrossRef]

| Author | Type of Study | No. of Participants | Subjects | Endpoints | Vitamin E Dosage | Co-Supplement Dosage | Duration |
|---|---|---|---|---|---|---|---|
| Vitamin C | |||||||
| Jungert et al. [64] | Observational | 399 | Participants aged ≥ 60 years | Stable positive interrelation was shown between plasma concentrations of VC and VE with aging | - | - | 12 years |
| Hemilä [66] | Observational | 27,111 | Smoking males aged 50–69 years | In older participants (66–69) who had VC intakes > 90 mg/d and dietary vitamin E intakes > 12 mg/d, vitamin E supplementation was significantly beneficial on mortality rate | - | - | 10 years |
| Losonczy et al. [71] | Interventional | 11,178 | Participants aged 67–105 years | Reduced risk of all-cause of mortality and risk of CVD mortality in vitamin E group; lower risk of total mortality and CVD mortality by VE and VC | Use of supplement including different dosages of VE and/or VC | 10 years | |
| Hamilton et al. [63] | Interventional | 30 | Healthy participants | Plasma α-TOH increased with vitamin C supplementation (~10%); plasma ascorbic acid also increased with vitamin E supplementation (>12%); plasma antioxidant power and glutathione peroxidase activity increased, total cholesterol and triglyceride concentrations decreased in both supplementation groups | 73.5 mg/d RRR-α-TOH acetate | 500 mg/d VC | 6 weeks |
| Shargorodsky et al. [91] | Interventional | 70 | Patients with cardiovascular risk factors | Significant decline of HbA1C and increase in HDL-cholesterol were also observed by co-supplementation. | 134 mg (200 IU)/d α-TOH | 500 mg/d VC | 6 months |
| Sesso et al. [77] | Interventional | 14,641 | Males aged > 50 with low risk of cardiovascular disease | Vitamin E/C supplementation has no effect on the incidence of major cardiovascular events; co-supplementation had no beneficial effects neither | 268 mg (400 IU)/two days α-TOH | 500 mg/d VC | 8 years |
| Amini et al. [72] | Interventional | 60 | Females in reproductive age with endometriosis | Combined deficient intake of both vitamins was observed in 33.7% of participants before intervention; co-supplementation decreased oxidative stress marker (MDA and ROS level), no influence of TAC, improvement of endometriosis syndrome (pain, blooding) shown | 537 mg (800 IU)/d α-TOH | 1000 mg/d VC | 8 weeks |
| Plantinga et al. [75] | Interventional | 30 | Males with untreated essential hypertension | Co-supplementation had beneficial effects on endothelium-dependent vasodilation and arterial stiffness in untreated, essential hypertensive patients; effect was associated with changes in plasma markers of oxidative stress. | 268 mg (400 IU)/d α-TOH | 1000 mg/d ascorbic acid | 8 weeks |
| Mihalj et al. [92] | Interventional | 57 | Patients with treated hypertension | Co-supplementation showed no BP-lowering effect; not effective in reducing oxidative biomarker (8-iso-PGF2α) | 537 mg (800 IU)/d α-TOH | 500 mg/d ascorbic acid | 8 weeks |
| Rafighi et al. [74] | Interventional | 170 | Patients with T2DM aged 30–60 years | Co-supplementation decreased blood glucose concentration, oxidative stress and insulin resistance by increasing antioxidant capacity (SOD and GST enzyme activity) | 201 mg (300 IU)/d α-TOH | 266.7 mg/d ascorbic acid | 3 months |
| Mellyana et al. [76] | Interventional | 42 | Patients with pediatric idiopathic nephrotic syndrome aged 1–15 years | Co-supplements improved blood pressure parameters (decreased number of patients in pre-hypertensive to hypertensive stage) | 10–15 mg/kg/d α-TOH | 10–15 mg/kg/d ascorbic acid | 12 weeks |
| Karajibani et al. [93] | Interventional | 40 | Patients with CVD | Reduced lipid peroxidation and strengthened the antioxidant defense system by (elevated SOD, GPx-activity, induced TAC and decreased MDA) | 268 mg (400 IU)/d α-TOH | 500 mg/d ascorbic acid | 8 weeks |
| Bjelakovic et al. [69] | Interventional (meta-analysis) | 296,707 | Participants with low bias from 78 studies | Antioxidant supplements with vitamin E and C has no evidence to support primary or secondary prevention but may increase significantly mortality compared to placebo groups. | Co-supplementation including different dosages of vitamin E and C | - | |
| Vitamin A | |||||||
| Altoum et al. [94] | Observational | 400 | Healthy participants/patients with CVD/non-CVD patients with T2DM | Lower antioxidant vitamin A, E and C concentrations are associated with increased diabetes risk. For those who already had diabetes, lower concentrations of vitamin E and A were associated significantly with a higher risk of CVD. | - | - | - |
| Upritchard et al. [88] | Interventional | 105 | Healthy adults | Co-supplementation improved dose-dependently the lipid peroxidation by increasing antioxidant capacity | 43/111 mg/d α-TE | 0.45/1.24 mg/d carotenoids | 11 weeks |
| Törnwall et al. [90] | Interventional | 27,271 | Smoking males aged 50–60 years | Co-supplementation showed no significant influence on risk of major coronary events, and no reduction of myocardial infarction and CVD mortality | 50 mg/d RRR-α-TOH acetate | 20 mg/d β-carotene | 5–8 years (median: 6.1 years) |
| Kataja-Tuomola et al. [89] | Interventional | 1700 | Smoking males aged 50–60 years | Co-supplementation showed no effect on diabetes mortality during the intervention | 50 mg/d RRR-α-tocopherol acetate | 20 mg/d β-carotene | 5–8 years (median: 6.1 years) |
| Se | |||||||
| Zitouni et al. [95] | Interventional | 170 | Patients with T2DM caused renal diseases | Co-supplementation improved renal function (glomerular filtration, renal blood flow); GPx3 was shown to be involved in the restoration of renal function. | 268 mg (400 IU)/d α-TOH | 200 μg/d selenium | 12 months |
| Safiyeh et al. [96] | Interventional | 70 | Females with OPOI | Co-supplementation improved fertility parameters (number of antral follicles and ovarian volume) | 268 mg (400 IU)/d α-TOH | 200 μg/d selenium | 12 weeks |
| Guertin et al. [97] | Interventional | 312 | Smoking males | Co-supplementation lowered plasma biomarkers of oxidative stress (8-iso-PGF2α) in vitamin E group; no effect in vitamin E plus Se or Se only group | 268 mg (400 IU)/d α-TOH | 200 μg/d selenium | 3 years |
| Mg | |||||||
| Afzali et al. [98] | Interventional | 57 | Patients with grade 3 DFU | Co-supplementation improved diabetic wound healing by reduced ulcer size; decreased insulin resistance and improved glycemic control; ameliorated blood lipid profile (TG, LDL-cholesterol, HDL-cholesterol); improved inflammatory biomarker (hs-CRP); improved oxidative stress biomarker (TAC, MDA) | 268 mg (400 IU)/d α-TOH | 250 mg/d magnesium oxide | 12 weeks |
| Shokrpour et al. [99] | Interventional | 60 | Females with PCOS aged 18–40 | Co-supplementation reduced plasma inflammatory biomarker (hs-CRP); improved oxidative stress biomarker (plasma NO level; TAC) but no effect on plasma GSH and MDA | 268 mg (400 IU)/d α-TOH | 250 mg/d magnesium oxide | 12 weeks |
| Jamilian et al. [100] | Interventional | 60 | Females with PCOS aged 18–40 | Co-supplementation improved insulin metabolism (insulin concentrations, homeostatic model of assessment for insulin resistance, quantitative insulin sensitivity check index); improved blood lipide profile (TG, VLDL) | 268 mg (400 IU)/d α-TOH | 250 mg/d magnesium oxide | 12 weeks |
| Maktabi et al. [101] | Interventional | 60 | Females with gestational diabetes aged 18–40 | Co-supplementation improved glycemic control (lower blood glucose and insulin concentrations, homeostatic model of assessment for insulin resistance and higher quantitative insulin sensitivity check index); improved blood lipide profile (TG, VLDL, LDL- and total or HDL-cholesterol) | 268 mg (400 IU)/d α-TOH | 250 mg/d magnesium oxide | 6 weeks |
| Zn | |||||||
| Ostadmohammadi et al. [102] | Interventional | 54 | Females with gestational diabetes | Co-supplementation lowered insulin resistance (increased insulin plasma concentrations, homeostasis model of assessment; insulin sensitivity check index); improved blood profile parameter (reduced total and LDL-cholesterol); increased gene expression of lipid metabolism regulatory genes (PPARγ, LDLR) | 268 mg (400 IU)/d α-TOH | 233 mg/d zinc gluconate | 6 weeks |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, S.; Omage, S.O.; Börmel, L.; Kluge, S.; Schubert, M.; Wallert, M.; Lorkowski, S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants 2022, 11, 1785. https://doi.org/10.3390/antiox11091785
Liao S, Omage SO, Börmel L, Kluge S, Schubert M, Wallert M, Lorkowski S. Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants. 2022; 11(9):1785. https://doi.org/10.3390/antiox11091785
Chicago/Turabian StyleLiao, Sijia, Sylvia Oghogho Omage, Lisa Börmel, Stefan Kluge, Martin Schubert, Maria Wallert, and Stefan Lorkowski. 2022. "Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients" Antioxidants 11, no. 9: 1785. https://doi.org/10.3390/antiox11091785
APA StyleLiao, S., Omage, S. O., Börmel, L., Kluge, S., Schubert, M., Wallert, M., & Lorkowski, S. (2022). Vitamin E and Metabolic Health: Relevance of Interactions with Other Micronutrients. Antioxidants, 11(9), 1785. https://doi.org/10.3390/antiox11091785








