1. Introduction
Around 200 drugs may cause hearing loss [
1,
2]. Among them, two groups with high ototoxic potential, aminoglycoside antibiotics and cisplatin and its derivates, are widely used in different therapeutical applications [
2].
Aminoglycoside antibiotics are the first choice in the treatment of many resistant infections caused by Gram
- bacteria. They are relatively inexpensive to produce, being extensively used in world regions with limited resources [
3,
4]. Besides their bactericidal power, they are also used to treat genetic diseases caused by abnormal stop codons, such as Duchenne muscular dystrophy, due to their ability to shift protein reading frames in the ribosome [
4,
5]. Despite their recognized ototoxicity [
2,
6], the benefits of aminoglycosides encourage protection from hearing loss [
6], instead of abandonment, as therapeutic agents.
Coordination complexes of platinum, particularly cisplatin, are extensively used as anti-neoplastic drugs. Despite advances in targeted cancer therapies, cisplatin, alone or in combined chemotherapy cycles, is still an essential part of oncotherapy. This includes treatment of solid tumors, notably testicular, ovarian and neck cancer as well as pediatric malignancies such as medulloblastoma, osteosarcoma or germ cell tumors [
7,
8].
For both types of drugs, much knowledge has accumulated on mechanisms of ototoxicity, although they are not yet fully worked out [
6,
9,
10]. Clearly, pharmacological and ototoxicity mechanisms are linked. In the case of aminoglycosides, bactericidal effects are mediated by interference with protein synthesis through binding to one of the subunits of the prokaryotic cell ribosome. One key to ototoxicity is that in eukaryotic cells, aminoglycosides interact with mitochondrial rRNA in ribosome subunits, which is evolutionarily related with its prokaryotic counterparts. Mitochondrial protein synthesis is then altered [
11,
12], and the activity of enzymes in the electron transport chain involved in oxidative phosphorylation is compromised, as shown in the cochlea [
10,
13], leading to faulty energy metabolism. Oxidative imbalance leads to excess accumulation of incompletely reduced, and thus avidly reactive, oxygen-related radicals or reactive oxygen species (ROS) [
14,
15,
16,
17]. Among other things, they readily combine with nitrogenated compounds, giving rise to nitrogen-related radicals or reactive nitrogen species (RNS). Therefore, ROS and RNS are free radicals that contribute fundamentally to unchecked oxidative stress, which finally overrides cellular “antioxidant defenses” [
4,
16,
17,
18]. Thus, oxidative stress self-perpetuates, irreversibly altering gene expression, as DNA and RNA are prone to oxidative damage, and the molecular structure, metabolism and interactions of proteins and complex lipids, which disorganizes cell membranes and membrane compartments [
16,
17,
18]. Altogether, this results in the activation of pathways leading to cell death, notably, although not exclusively, apoptosis, as shown in the cochlea [
19]. In such an oxidative imbalance context, the elevated metabolic demands of electromechanical transduction in the auditory organ may contribute to ototoxicity by more easily tipping out of balance the scale of oxidative stress [
12].
Regarding cisplatin, its antitumoral mechanisms involve the ability to crosslink with DNA, which blocks DNA replication and repair. Mitochondrial DNA, which lacks histones, easily forms such DNA adducts with cisplatin. The mitochondrial function is therefore compromised in tumoral cells, resulting in excess accumulation of ROS/RNS, leading to oxidative stress and activation of pathways leading to cell death [
3,
7]. Other mechanisms such as direct inactivation of antioxidant enzymes, may also contribute importantly to cisplatin antitumoral cytotoxicity [
20]. However, by virtue of its mechanisms of action, antitumoral cisplatin cytotoxicity is accompanied by unwanted cytotoxicity on normal cells. Ototoxicity is a major secondary effect of cisplatin treatment, which may lead to irreversible deafness in a very significant number of patients [
4,
21]. Damaging cisplatin-DNA adducts form in cochlear sensory and supporting cells [
21,
22]. The disruption of mitochondrial integrity and buildup of ROS/RNS with ensuing oxidative stress are also central to cisplatin ototoxicity [
4,
8,
10]. Such as in the case of aminoglycosides, the delicate metabolic energy balance in the auditory receptor also contributes to cisplatin ototoxicity. In addition, specific factors, such as the binding of cisplatin to the NADPH-oxidase NOX3, which is almost exclusively expressed in the inner ear, make the auditory receptor particularly prone to cisplatin toxicity [
10,
23].
In sum, much evidence supports that unchecked oxidative stress is at the core of irreversible hearing loss caused by aminoglycosides and cisplatin and other ototoxic drugs, as well as acoustic trauma and auditory aging [
12]. Physiologically, oxidative stress is kept within narrow margins by non-enzymatic and enzymatic mechanisms. “Antioxidant” non-enzymatic mechanisms include free radical scavengers, which are compounds with high potential for redox reactions with free radicals, preventing them from further intracellular redox interactions, deleterious for the function of essential macromolecules. Such “antioxidants” include glutathione and related compounds as well as some vitamins [
17]. Enzymatic mechanisms include enzyme systems that catalyze redox reactions that regenerate free radical scavengers or inactivate free radicals such as ROS/RNS. Among them, Glutathione peroxidase 1 (Gpx1), Catalase (Cat) and the superoxide dismutase (SOD, particularly SOD1) have a central role in controlling oxidative stress. SOD catalyzes the conversion of O
2-.to H
2O
2, whereas Cat catalyzes the conversion of H
2O
2 in H
2O and O
2. Gpx1 also catalyzes the reduction of H
2O
2 and other peroxides, as well as the “regeneration” of reduced glutathione to its oxidized form after H
2O
2 conversion [
17]. There is evidence showing involvement of these enzymes in ototoxicity. For instance, changes in GPx1, catalase or SOD1 expression or activities, linked to aminoglycoside or cisplatin hearing loss, have been reported [
10,
24,
25]. However, it is not known whether the expression and cellular distribution of these canonical antioxidant enzymes are identically affected regardless of the nature of the ototoxic agent, or whether there are differences. This is due in part to limited availability of experimental studies in animal models “in vivo,” in which the sequence of ototoxic damage in relation to the expression of antioxidant enzymes is compared directly side by side, for aminoglycosides and cisplatin. The question is relevant because differences or similarities may have implications for the mechanistic understanding of ototoxic damage, which may potentially translate into improved therapeutic strategies.
Therefore, we have compared in the Wistar rat model the ototoxicity of the aminoglycoside antibiotic kanamycin with that of cisplatin in relation to the generation of oxidative stress and the expression and tissue localization of the antioxidant enzymes Gpx1, Cat and SOD1. For this, we have used a combination of RT-qPCR, quantitative histology and immunocytochemistry with antibody markers of oxidative stress and antioxidant enzymes, along with acoustically evoked auditory brainstem response recordings (ABRs).
2. Materials and Methods
2.1. Experimental Animals
Male Wistar rats, aged from 11 to 13 weeks old at the beginning of the experiments, were used. Animals were provided by Charles River Laboratories (Barcelona, Spain) and were housed in the Animal House facility of the University of Castilla-La Mancha-UCLM in Albacete (Spain) under controlled temperature (22–23 °C) and humidity (60% ± 5%) in a 12-h light/dark cycle with ad libitum access to food and water. The handling and care of animals followed current national (Spain R.D. 53/2013; Law 32/2007) and EU (Directive 2010/63/EU) regulations on the protection and care of animals used for scientific purposes. Procedures were validated and supervised by the institutional Ethics Committee for Animal Experimentation and were authorized under registry PR-2016-04-11.
2.2. Induction of Ototoxicity by Kanamycin and Cisplatin
Kanamycin (Sigma-Aldrich, St. Louis, MO, USA, No. Cat. K4000) in 0.9% saline was injected daily subcutaneously at 400 mg/kg, from 3 to 15 days, depending on the survival time to which animals were randomly assigned (see below). Animals in the control group received saline vehicle injections.
Rats were randomly distributed in the following groups: Kanamycin treatment, 3 days (D3;
n = 12), 11 days (D11;
n = 12), 12 days (D12;
n = 3), 13 days (D13;
n = 3), 14 days (D14;
n = 3) and 15 days (D15;
n = 11). The saline vehicle-injected control group was 15 days (
n = 10). Prior to the first administration of the drug, auditory brainstem response (ABR) recordings were carried out to confirm normal baseline from controls, as previously published [
26]. Immediately before euthanasia, a second ABR recording was performed to analyze changes in hearing thresholds after the corresponding days of treatment. After recording ABRs at the specified treatment days, eight cochleae from 8 animals in the D3, D11 and D15 survival groups were processed for OHC counts and apoptosis detection with the TUNEL method, whereas the other eight cochleae were processed for immunocytochemical detection of 3-NT and Gpx1 (see below), as described below. In addition, to establish the timeline of OHC loss, the cochleae of the 3 animals from the D12, D13 and D14 survival groups were processed for OHC counts and TUNEL staining (see below). Controls were cochleae from 6 animals in the saline vehicle-injected group.
The cochleae from the remaining 4 animals in the D3, D11 and D15 survival groups along with 4 animals in the control group were used for the gene expression analysis of antioxidant enzymes using RT-qPCR, as described in the corresponding section below.
Cisplatin (Sigma-Aldrich, Cat. N. P4394) was administered at 16 mg/kg in 0.9% saline through a single intraperitoneal injection [
27]. Animals were randomly distributed in two survival groups: cisplatin 24 h (24 h;
n = 9) and cisplatin 72 h (72 h;
n = 9). The control group was injected intraperitoneally with saline vehicle and allowed to survive 72 h (
n = 10). Prior to cisplatin injection, ABR recordings were performed to confirm hearing sensitivity within the Wistar rat normal range [
26]. Prior to sacrifice (i.e., 24 or 72 h after administration of cisplatin) a second ABR recording was performed to test the effects of cisplatin on hearing function. Five cochleae from 5 animals in each survival group (24 and 72 h) were used for OHC cell counts and TUNEL staining. The other five cochleae from the animals in each survival group were used for 3-NT and Gpx1 immunocytochemistry. Five saline-injected animals were used as controls. The cochleae of the remaining four animals in each survival group and from five control animals were used for RT-qPCR. The final sample number was pooled from cisplatin injection protocols with mortality rates from 32% to 44%, within the range or lower than previously reported with similar experimental protocols in rats [
24].
2.3. Auditory Brainstem Response Recordings (ABRs)
ABR recordings were carried out in a sound-proof booth (Incotron Eymasa S.L., Barcelona, Spain) inside a sound-attenuation room, as previously described [
26,
28]. Animals were anesthetized with isoflurane at a flow rate from 1 L/min O
2 at 4% for induction and 1.5–2% for maintenance. During the recordings, body temperature was regulated at 37.5 °C with a non-electrical thermal pad. Temperature was monitored and controlled with a rectal probe. The subdermal electrodes (Rochester Electro-Medical, Tampa, FL, USA) were placed at the cranial vertex (non-inverted) and on the right (inverted) and left (ground) mastoid apophysis. Sound stimulation and recordings were carried out using a BioSig System III (Tucker-Davis Technologies, Alachua, FL, USA).
The stimuli were generated digitally with SigGenRP software (Tucker-Davis Technologies) and an RX6 Piranha Multifunction Processor hardware (Tucker-Davis Technologies). Stimuli were tone bursts (5 ms rise/fall without a plateau, 20 times/second) at seven different frequencies (0.5, 1, 2, 4, 8, 16 and 32 kHz), which were applied into the right external ear canal using an ED1 electrostatic speaker controller (Tucker-Davis Technologies) through an EC-1 electrostatic speaker (Tucker-Davis Technologies). The stimuli were calibrated prior to experiments with SigCal software (Tucker-Davis Technologies) and an ER-10B+ low-noise microphone (Etymotic Research Inc., Elk Grove, IL, USA).
Auditory thresholds were obtained for each tested frequency. To determine threshold level, the evoked responses were recorded in 5 dB steps descending from a maximum stimulus intensity of 80 dB SPL. The background activity was measured before the stimulus onset. The auditory threshold was defined as the stimulus intensity that evoked waveforms with a peak-to-peak voltage greater than 2 standard deviations (SD) of the background activity [
26,
28]. The maximum level of stimulus intensity was established at 80 dB as a safe limit for noise overstimulation [
28,
29]. Following treatments, if no evoked responses were obtained at 80 dB, the auditory thresholds were recorded at that value for statistical analysis [
29].
The threshold shift was determined for each of the frequencies studied by subtracting the auditory thresholds at the different survival times after treatments from auditory thresholds obtained prior to the initiation of treatment.
2.4. Real Time Quantitative PCR
Animals intended for the analysis of gene expression in the cochlea received an overdose of sodium pentobarbital (700 mg/kg, intraperitoneal). The cochleae were extracted, quickly frozen in liquid nitrogen, and stored at −80 °C until they were used for real time quantitative PCR (RT-qPCR).
2.4.1. RNA Extraction and cDNA Synthesis for RT-qPCR
First, 1 mL of cold TRIzol reagent (Thermo-Fisher Scientific, Waltham, MA, USA) was added to each frozen cochlea, according to the manufacturer’s instructions. Then, they were then quickly homogenized using a Polytron PT 2100 homogenizer (Kinematica-Fisher Scientific, Malters, Switzerland) for 30–40 s. The pre-cleaned Polytron rotor was treated with RNaseZap (Sigma-Aldrich, St. Louis, MO, USA) and cooled with dry ice for approximately 1 min. After 5 min incubation at room temperature, 0.2 mL chloroform was added, followed by re-incubation for 3 min at room temperature. Samples were then centrifuged for 15 min at 12,000× g at 4 °C. After this, the upper aqueous phase was transferred to a new tube to which 0.5 mL of isopropanol was added. Incubation proceeded for 10 min at room temperature. Samples were then centrifuged at 12,000× g for 10 min at 4 °C to precipitate RNA, the supernatant was removed, and the pellet was resuspended in 1 mL of 75% ethanol. After vortex-washing, the pellet was re-centrifuged at 7500× g for 5 min at 4 °C, the resulting supernatant was removed, and the pellet was air-dried for 5–10 min. Finally, the pellet was re-suspended in 20 mL of RNAse-free H2O and incubated at 55 °C for 10 min. The quantity and quality of RNA were tested by spectrophotometry (Nanodrop ND-1000, Thermo Scientific). All RNA samples showed appropriate A260/A280 ratios. The RNA was stored at −80 °C.
For cDNA synthesis, RevertAid First Strand kit (Thermo Fisher Scientific), was used, with 1 g of RNA with oligo–(dT)18 as a primer. Reactions were carried out on a DNA Engine® Peltier thermocycler (Bio-Rad, Hercules, CA, USA) at a final volume of 20 mL. The reaction conditions were as follows: 65 °C for 5 min, 37 °C for 5 min, 42 °C for 1 h, 72 °C for 10 min and 4 °C until tube collection. The reaction product was diluted 1:10 in ultrapure water for qPCR use and remained at 4 °C for immediate use or at −20 °C for long-term storage. All qPCR experiments were conducted with the same batch of cDNAs. Controls without reverse transcription (–RT) were carried out to verify the absence of genomic DNA contamination.
2.4.2. RT-qPCR
RT-qPCR was carried according to previously published methods [
30]. A StepOnePlus Real-Time PCR System (Applied Biosystems, Waltham, MA, USA) was used, with 96-well plates and Fast SYBR Green Master Mix (Applied Biosystems) as the detection reagent. The reaction mixture in each well consisted of 2.8 mL of ultrapure water, 0.1 mL of each primer (final concentration of 100 nM), 5 mL of Fast SYBR Green Master Mix and 2 mL of 1:10 cDNA. The plate was centrifuged at 1200 rpm for 2 min. PCR amplification was performed under the following conditions: initial activation at 95 °C for 20 s, followed by 40 cycles of 95 °C for 6 s and 60 °C for 45 s. To confirm that the primers amplified a single specific PCR product, a melting curve was generated by an initial denaturation step at 95 °C for 20 s, followed by a gradual heating of 60 °C to 95 °C, in increments of 0.3 °C. All qPCR plates included controls without cDNA (NTC) that generated values of Cq > 35. The experiments were performed in triplicate.
Cq data were obtained using Step One v2.3 software (Applied Biosystems). The level of expression of a target gene was obtained following the ∆∆Cq method [
31]. It was normalized to the average level of the reference gene, in our case
Gapdh [
30,
32], obtaining the value of ∆Cq for each pair of genes in the samples (control and treatment). The ∆∆Cq of each gene was calculated according to:
Where the “treatment group” corresponds to each experimental treatment group: cisplatin 24 h and 72 h; kanamycin D3, D11 and D15. Relative expression was calculated according to:
qPCRs were performed using pairs of specific primers to amplify the transcription of genes related to antioxidant enzymes. Pairs of primers (
Table 1) were designed using Primer3 Plus software (
http://www.bioinformatics.nl/cgi-bin/primer3plus/primer3plus.cgi/, accessed date 4 July 2022) and were produced by Thermo Fisher Scientific. Specificities were tested using BLAST analysis (NIH, NCBI, USA). Using Ensembl databases, it was verified that primers covered at least two exons with a large intron in between to prevent false amplification resulting from genomic DNA contamination.
2.5. Cochlear Histology
Animals intended for histological analysis of the cochlea were anesthetized with sodium pentobarbital (Dolethal, Vetoquinol, Madrid, Spain; i.p. 700 mg/kg) after ABR recordings. Tissue fixation by transcardial perfusion was initiated with a vascular flush with 0.9% saline solution, followed by 4% paraformaldehyde fixative (PFA 4%) in 0.1 M phosphate buffer (PB, pH 7.3). After perfusion, the cochleae were dissected out from the temporal bones, after which they were washed three times in phosphate-buffered saline (PBS) for 5 min and decalcified in 50% RDO (Rapid Decalcifier Solution, Apex Engineering Products Corporation, IL, USA). The right cochleae, used for quantification of outer hair cells (OHCs) and apoptosis, were maintained in RDO for 1 h. The left cochleae, used for immunohistochemistry (
Table 2) in 20 μm-thick cochlear sections, were maintained in RDO for 2 h. After decalcification, the cochleae were washed in PBS 3 times for 5 min.
2.5.1. Cochlear Spiral Surface Preparations for Hair Cell Quantification and TUNEL Method for Detection of Apoptosis
The right cochleae were dissected under an Olympus SZX10 stereoscopic microscope (Olympus, Tokyo, Japan) with the help of 2 thin-tipped dissection tweezers and a surgical blade. First, a cut was made with the blade perpendicular to the modiolus, dividing the total length of the cochlea in two equal halves. For the apical half, the decalcified bone capsule was removed with the help of tweezers. Then, the lateral wall was excised and the modiolus and the tectorial membrane were removed. For the basal half, the procedure was similar, with special care when removing lateral wall structures from the bone in the most basal segment. Each dissected cochlear turn fragment (between three and four for the full cochlear length) with the organ of Corti exposed, was immersed in PBS in a single well of a multi-well plate (Sigma-Aldrich).
To visualize and count OHCs (see below), immunofluorescence for the detection of myosin VIIa was performed on the cochlear segments “in toto”. The TUNEL method for detection of apoptosis (Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling) was used simultaneously.
Cochlear turn fragments were first incubated in phosphate-buffered saline with 1% TritonX-100 and 5% bovine serum albumin (PBS-Tx100 1%-BSA 5%) for 1h. They were then incubated with an anti-myosin-VIIa primary antibody (Proteus BioSciences, Ramona, CA, USA) diluted 1:1000 in PBS-Tx100 0.2%-BSA 3%. The incubation proceeded overnight. The next day, the primary antibody solution was removed, and the cochlear turns were washed in PBS 3 × 5 min. This was followed by incubation in anti-rabbit Alexa Fluor 594 secondary antibody (Thermo Fisher Scientific) diluted 1:200 in PBS-Tx100 0.2%-BSA 3% for 1 h at room temperature in darkness. After removing the secondary antibody, the cochlear turns were washed once in PBS-Tx100 0.2% for 5 min, and 3 times more in PBS for 5 min. After washing, TUNEL was carried out on the same samples, using the DeadEnd kit™ Fluorometric TUNEL System (Promega, Madison, WI, USA). After removing the PBS from the wells, 50 mL of “equilibration” buffer was added, and the cochlear turns were incubated for 10 min. After removing the “equilibration” buffer, 25 mL of reaction mix (TdT reaction mix: “equilibration buffer”, 22.5 mL, nucleotide mixture, 2.5 mL, rTdT enzyme, 0.5 L) were used to incubate for 50 min at 37 °C in the darkness and in a moist chamber. The reaction was stopped by adding 400 mL of SSC 2X (StopReaction Buffer) and incubating for 15 min. Finally, the cochlear turns were washed 3 × 5 min with PBS and mounted with Vectashield with DAPI for nuclear counterstaining (Vector Labs, Burlingame, CA, USA).
Surface preparations of the cochlear turns were observed and photographed under a Zeiss 710 confocal microscope (Zeiss, Oberkochen, Germany). OHC and TUNEL+ cell nuclei counts were carried out, as further detailed.
2.5.2. Immunohistochemistry in Cochlear Sections
Decalcified and washed cochleae were immersed for cryoprotection in 30% sucrose in phosphate buffer (PB) for 48–72 h. Afterwards, the cochleae were embedded in gelatin diluted in 30% sucrose in PB. Gelatin blocks containing the cochleae were quickly frozen at −70 °C using 2-propanol (Sigma-Aldrich) and dry ice and stored at −80 °C. Paramodiolar cochlear Sections 20 mm-thick were obtained using a Leica CM3050 S cryostat (Leica, Wetzlar, Germany). Immunofluorescence detections were performed for the localization of the oxidative stress marker 3-NT and the key oxidative stress enzyme Gpx-1. Antibodies and dilutions are detailed in
Table 2.
The cochlear sections, stored at −80 °C, were tempered for 30 min at room temperature and then post-fixed with PFA 4% for 8 min. After washing the slides 3 times for 5 min with PBS, 1 mL of blocking and permeabilization solution (PBS-Tx100 0.25%-BSA 2%) was added, keeping it at room temperature for 1 h. Incubation with the primary antibodies was carried out overnight at 4 °C. The next day, the slides with the sections were washed 3 times for 5 min with PBS and incubation with the secondary antibody was then carried out for 1 h at room temperature in darkness. All antibodies were diluted in PBS-BSA 0.5%. After washing 3 times for 5 min with PBS, the slides were cover slipped with Vectashield (Vector Labs) with DAPI and observed and analyzed in a Zeiss 880 (Zeiss) confocal microscope.
2.5.3. Quantification of OHCs and TUNEL-Positive Nuclei
Quantitative analysis was carried out using ImageJ software (US National Institutes of Health, Bethesda, MD, USA). OHC counts were performed according to previously published protocols [
33] adapted to fluorescent labeling of cochlear turns (see above). To quantify OHCs, each labeled cochlear spiral, sectioned in three to four pieces as described above, was photographed under the confocal microscope. Images were orderly arranged in the apical to basal direction. Using ImageJ, 0.1 mm-long segments were delineated through the apical portion of inner hair cells with the software
“segmented line” tool. In each of these segments, the number of myosin VIIa-immunolabeled OHCs was counted, and the percentage of lost cells per 0.1 mm cochlear length was determined. In addition, the number of TUNEL positive nuclei for every 0.1 mm of organ of Corti’s length was also counted.
2.5.4. Semi-Quantitative Analysis of the Intensity of Immunofluorescence in Cochlear Sections
Photomicrographs were obtained from immunolabeled sections of the cochlea with a confocal fluorescence microscope. Pictures were taken under the same conditions (incubation protocols, confocal settings, software parameters: exposure time, digital zoom, brightness), so that all were comparable to each other in terms of relative fluorescence signal strength. Photomicrographs were analyzed with ImageJ software. The region of interest (OHC region, excluding nuclei, or stria vascularis, excluding blood vessel profiles) was delimited, using ImageJ’s “polygon selections” tool. Once this was achieved, the “mean gray value” was obtained exclusively for the previously delimited area, so that the average intensity of fluorescence in that area, in grayscale, is obtained. Thus, semi-quantitative comparison of relative immunofluorescence intensities between different conditions can be made.
2.6. Statistical Analysis
For a comparison of differences of means between groups, one-factor ANOVA was used. In cases where the average of three or more groups was compared and statistical significance was obtained, a Tukey–Kramer post hoc test was performed to determine paired groups between which statistically significant differences existed. Data are expressed as mean±standard deviation (SD.) Statistical significance was defined as * p < 0.05, ** p < 0.005, *** p < 0.001.
4. Discussion
We report results of a side-by-side comparison of two commonly used experimental protocols reproducing kanamycin and cisplatin ototoxicity in the rat [
33,
34]. The metrics included auditory threshold shifts in ABRs, in correlation with rates of OHC loss and spatial patterns of apoptosis, along with the involvement of oxidative stress and enzymatic oxidative “defenses” in cochlear damage mediated by these two drugs. These may be relevant to interpret future therapeutical outcomes, in particular with antioxidation therapies.
For kanamycin, the first statistically significant threshold increase, around 15 dB, was observed at days 12 or 13 of treatment, at the highest frequencies tested of 16 kHz and 32 kHz. At day 14, threshold shifts increased further, up to 23 dBs, at these same frequencies. The 8 kHz frequency was also affected, with threshold shifts over 15 dB. At day 15, threshold shifts were even more evident in the same frequency range, and extended further down to 4 kHz, where threshold shifts of over 15 dB were recorded. Loss of hearing sensitivity centered initially at high frequencies and progressively extending to lower frequencies is a landmark of aminoglycoside ototoxicity [
1,
33,
35]. Our results demonstrate that such hearing loss in the Wistar rat concentrates in the last four days of treatment, progressing quickly from higher to lower frequencies down to 4 kHz.
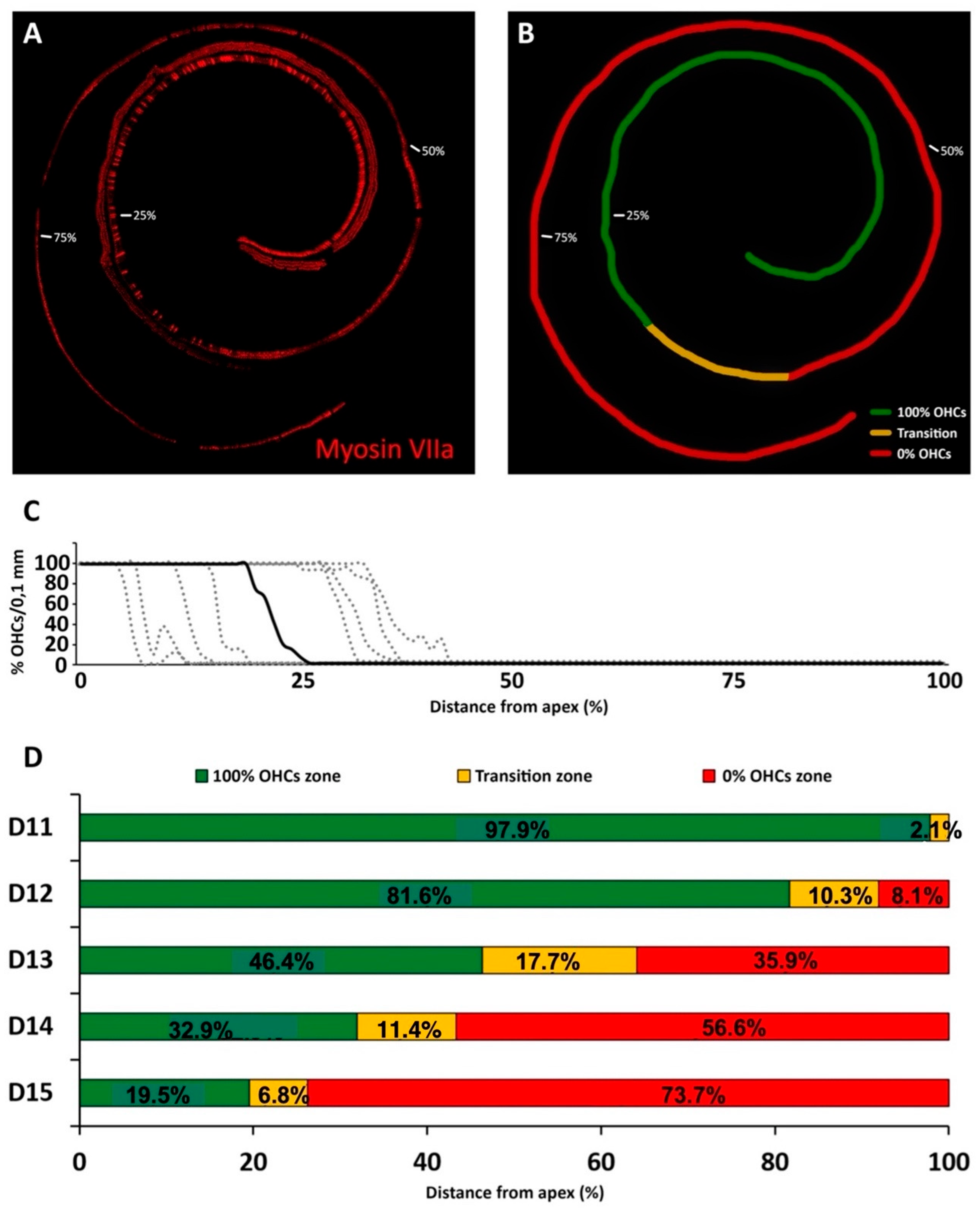
High frequency threshold elevations at day 12 after treatment corresponded with missing OHCs in the most basal 8.1% of the total cochlear length. An adjoining stretch in which ongoing apoptosis was detectable with the TUNEL method, the “transition zone,” spanned an average of 10.3% of cochlear length. Therefore, by the time the first significant auditory threshold elevations were recorded during kanamycin treatment, 18.4% length of the cochlear neuroepithelium was damaged, as judged by the total or partial loss of OHCs. Coincident with threshold shifts, from day 12 to day 14 after kanamycin treatment there was a fast progression of OHC loss. The average percent cochlear length showing OHC absence at day 14 (56.6%) was almost 6.7 times larger than at day 12 (8.1%). Threshold elevations were correspondingly larger and spanned a wider tested frequency range, down to 8 KHz. By day 15, the last kanamycin post-treatment timepoint assessed, the absence of OHCs affected 73.9% of the cochlear length starting at the basal end, which corresponded with increased thresholds at frequencies down to 4 kHz. Although the end of kanamycin treatment at day 15 is somehow arbitrary, it has been demonstrated that at doses of 300 to 500 mg/kg in the rat, OHCs in the cochlear apex are largely preserved even after three weeks of the end of a 15-day treatment [
33].
The fast progression of the cochlear length affected by complete loss of OHCs was paralleled by a progressive decrease in the length of the adjoining transition zone, from 17.3% at day 13 to 6.3% at day 15. This suggests that apoptotic cochlear cell death mediated by kanamycin evolves on a spatially ordered fashion starting at the basal end, as shown by the identification of a transition zone, with undergoing nuclear DNA fragmentation, as detected by the TUNEL method. As ototoxicity progresses, the final stages of apoptosis downstream of DNA fragmentation, therefore not detectable with TUNEL, may predominate. Consequently, more length of the cochlear spiral will be contained within the region devoid of OHCs. In this regard, the reported quick shortening of the transition zone to a length of just 6.3% of the cochlear spiral at day 15 post-kanamycin treatment, may anticipate the spatial “fading away” of kanamycin-mediated OHC apoptosis towards the cochlear apex. At this endpoint of kanamycin treatment, the most apical 19.5% end of the cochlea was preserved. Because the cochlear span showing apoptosis with TUNEL labeling shortens dramatically between days 13 and 15 after treatment, regardless of the participation of other cell death pathways such as necroptosis [
19], it is conceivable that such a 19.5% length of cochlear apex at day 15 post-treatment was relatively better preserved [
33], representing a relevant functional region of “residual hearing” [
36], tuned approximately at 2 to 0.5 kHz in the rat.
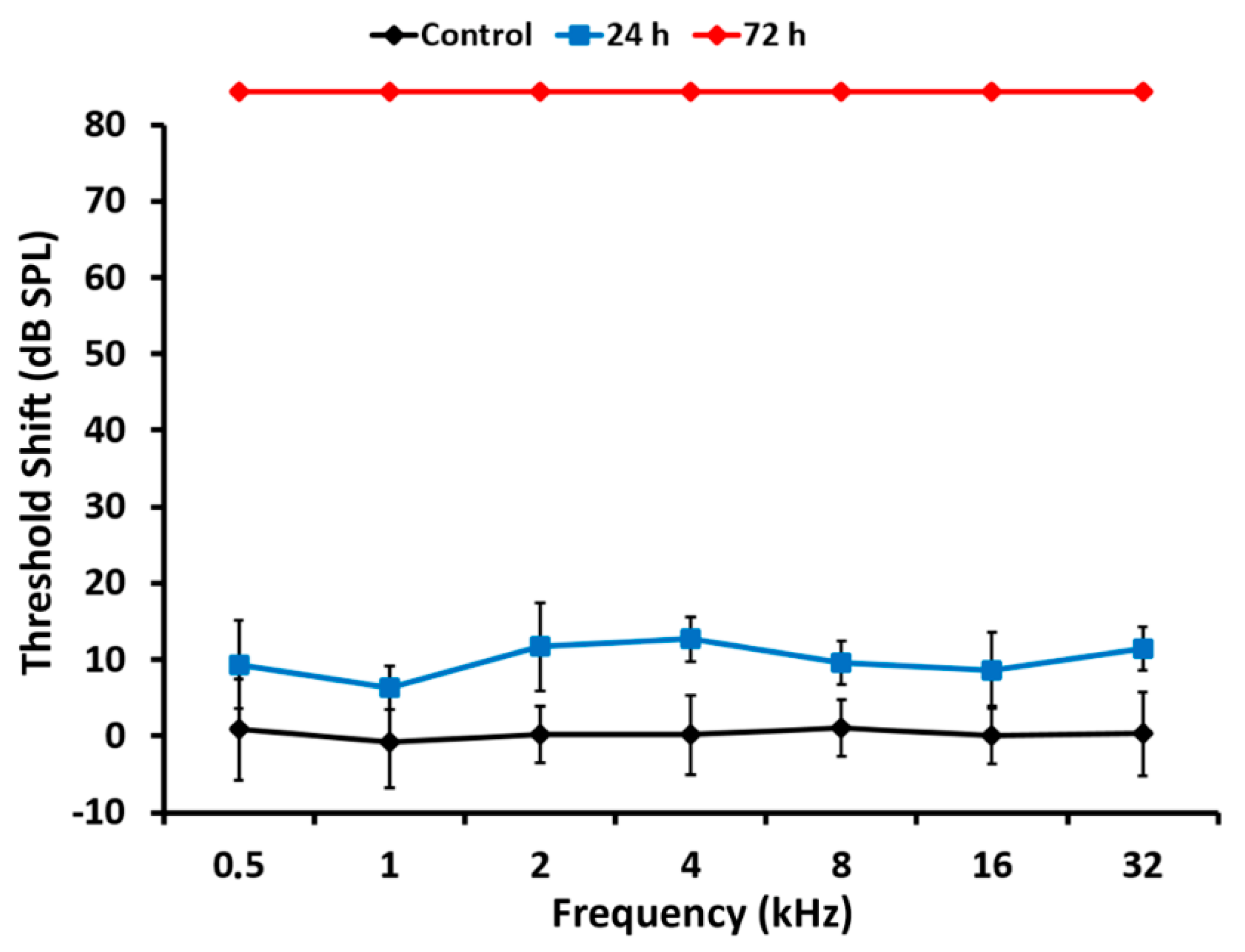
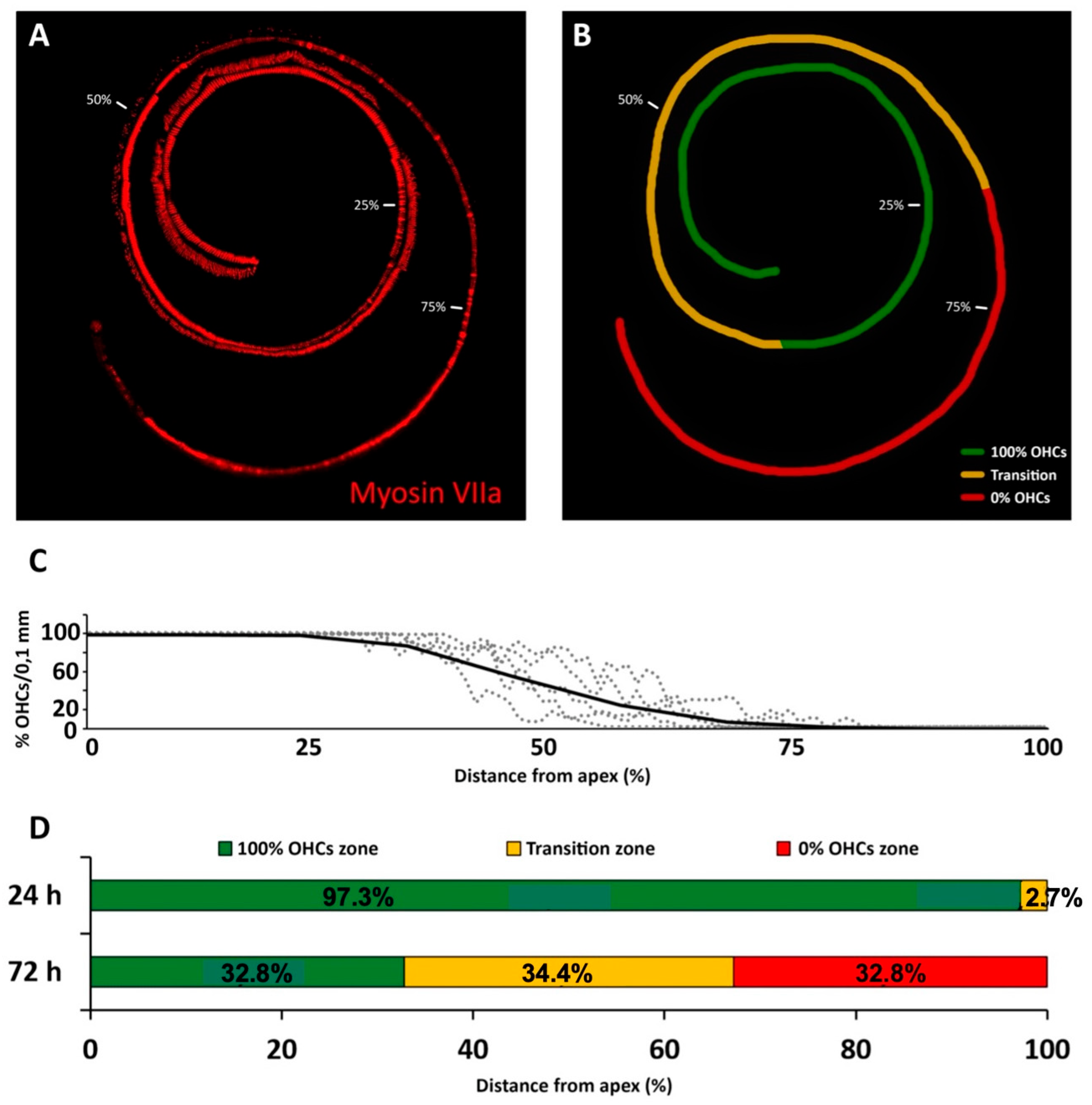
As expected, the loss of hearing sensitivity with a single subcutaneous injection of cisplatin at 16 mg/kg differed in pattern from that of kanamycin. Within 24 h after the injection, there was no significant threshold shift in ABRs at any tested frequency. Seventy-two hours after the injection, however, evoked activity that was not recordable, precluding accurate threshold determination at any tested frequency. High threshold elevations across the entire frequency range, in occasions at the limit of precise determination, have been reported previously with comparable treatment protocols [
37]. Such extreme loss of hearing sensitivity facilitates further insights in relation with the corresponding OHC loss. After 24 h of cisplatin administration, when ABR recordings are still within normal range, OHCs were present on 97.3% of the cochlear length, with the most basal 2.7% showing apoptosis. These do not differ much to those found with kanamycin after 11 days of treatment, supporting similarities for both drugs in the sensitivity of the receptor lamina at the outset of ototoxicity. However, 72 h after the administration of cisplatin, when auditory evoked potentials were no longer recordable, roughly the most apical third of the receptor lamina length was preserved as far as OHCs are concerned, whereas the most basal third concentrates OHC loss. In the intervening third, the transition zone, TUNEL
+ nuclei coexisted with recognizable OHCs. Such as with kanamycin, counts of TUNEL
+ nuclei suggest that cell death progressed in a spatially ordered fashion from base to apex. Moreover, comparatively lower numbers of TUNEL
+ nuclei with cisplatin suggest different rates of ototoxic cell death.
In sum, after 72 h of cisplatin administration, there was OHC preservation in 35% of the length receptor lamina at its apical end with no recordable ABRs. This is in sharp contrast with kanamycin; at the 15 day-endpoint of treatment, as already discussed, 19.5% of the apical end contained preserved OHCs, with normal auditory thresholds in the 0.5–2 kHz range. The percent length of the preserved auditory neuroepithelium in the cochlear apex at the end of cisplatin treatment was 1.6 times larger than with kanamycin. Thus, the complete loss of hearing sensitivity with high doses of cisplatin does not necessarily involve a massive loss of receptor cells. Even with approximately 1/3 of the cochlear length containing identifiable OHCs at the endpoint of cisplatin treatment, there was no recordable activity in ABRs. This supports that a considerable fraction of OHCs may survive immediately after cisplatin ototoxicity but are not able to generate receptor potentials. As discussed further, this may be because, as compared with kanamycin, the structure and function of the stria vascularis, involved in ion concentration homeostasis necessary for the electrical activity of sensory receptor cells, is more compromised with cisplatin [
10,
21,
38,
39]. With this cisplatin ototoxicity protocol, it is not possible to know the final fate of the surviving OHCs past day three of treatment, despite which it seems safe to conclude that their function is compromised from early stages of ototoxicity. The fact that they are still structurally preserved may have implications for establishing a recovery window.
In this regard, the immunohistological distribution of the oxidative stress marker 3-NT provides additional information about possible differences in ototoxic mechanisms and progression between kanamycin and cisplatin. 3-NT accumulates because of extensive nitrosylation of tyrosine by excess nitrogen free radicals, mainly peroxynitrites. This renders structurally and functionally altered proteins in the chain of events leading to oxidative damage. 3-NT immunolabeling was detectable in the cochlea, particularly in the organ of Corti and lateral wall structures, both after kanamycin and cisplatin treatment. This supports oxidative stress mediating ototoxicity by both drugs, as previously reported [
4,
6,
8,
10,
14,
15]. However, we found different cochlear distribution of 3-NT immunolabeling, pointing to differences in mechanisms involving oxidative stress. It is important to point out that no conclusions can be drawn from comparing relative differences in 3-NT cochlear immunolabeling intensities
between kanamycin and cisplatin, mainly because antibody incubation times and controls were optimized for each ototoxic treatment, thus limiting the interpretation of such comparisons between both drugs. However, the regional or spatial distribution of cochlear 3-NT immunolabeling intensity can be tested individually for each ototoxic drug and compared. We found that relative 3-NT immunolabeling intensity distribution differs regionally between kanamycin and cisplatin, at least in the stria vascularis. For kanamycin, relative levels of 3-NT immunofluorescence intensity were increased in regions where OHCs could still be identified based on topographical localization of nuclei. In these regions, 3-NT immunolabeling at 3, 11 and 15 days after treatment was significantly more intense than in controls. In the stria vascularis, kanamycin treatment also resulted in increased 3-NT immunolabeling levels, but with noticeable regional differences. Basal regions showed increased 3-NT immunolabeling at all post-treatment times analyzed. In middle regions, significantly elevated 3-NT immunolabeling intensity was detected at 11 and 15 days after treatment. In contrast, in apical regions there was no detectable increase in 3-NT immunolabeling relative to controls at any time post-treatment. Therefore, the apical portions of the stria vascularis seem to be comparatively less sensitive to oxidative stress damage induced by kanamycin. In the case of cisplatin, such as with kanamycin, regions of interest with identifiable OHCs in the cochlear apex also had increased 3-NT immunolabeling throughout treatment time points. In contrast to kanamycin, however, in the stria vascularis increased levels of 3-NT immunolabeling were observed in all turns, both at 24 and 72 h of cisplatin administration. This supports that, different to kanamycin, the whole stria vascularis is overall more sensitive to oxidative stress damage mediated by cisplatin. Long term accumulation of cisplatin has been reported in the stria vascularis [
39]. Therefore, the extreme loss of hearing sensitivity observed with cisplatin may be related to massive oxidative damage throughout the stria vascularis. This results in the inability to keep ion concentrations in the endolymph, particularly K
+, within the range needed to preserve the endocochlear potential, with subsequent blockade of evoked activity, even with a considerable fraction of preserved OHCs at the end of treatment, in comparison with kanamycin.
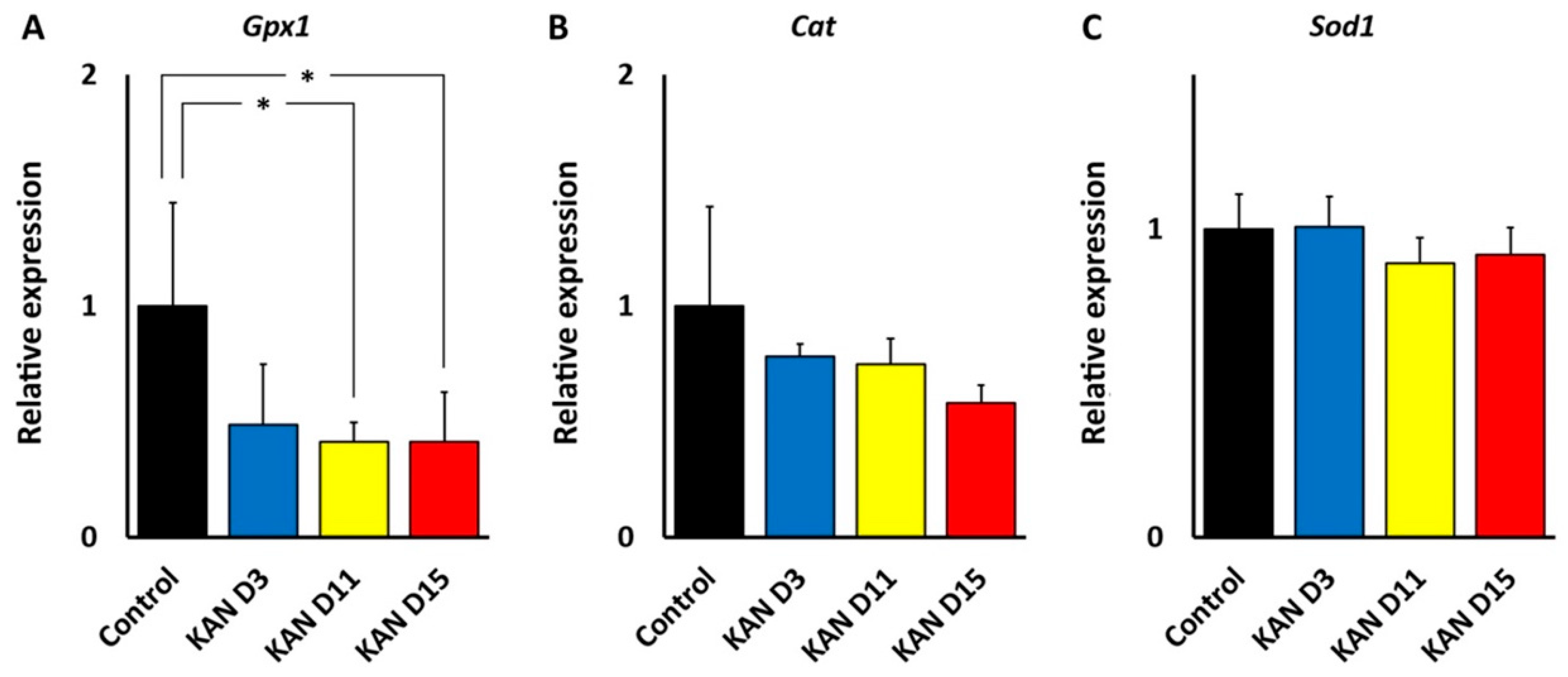
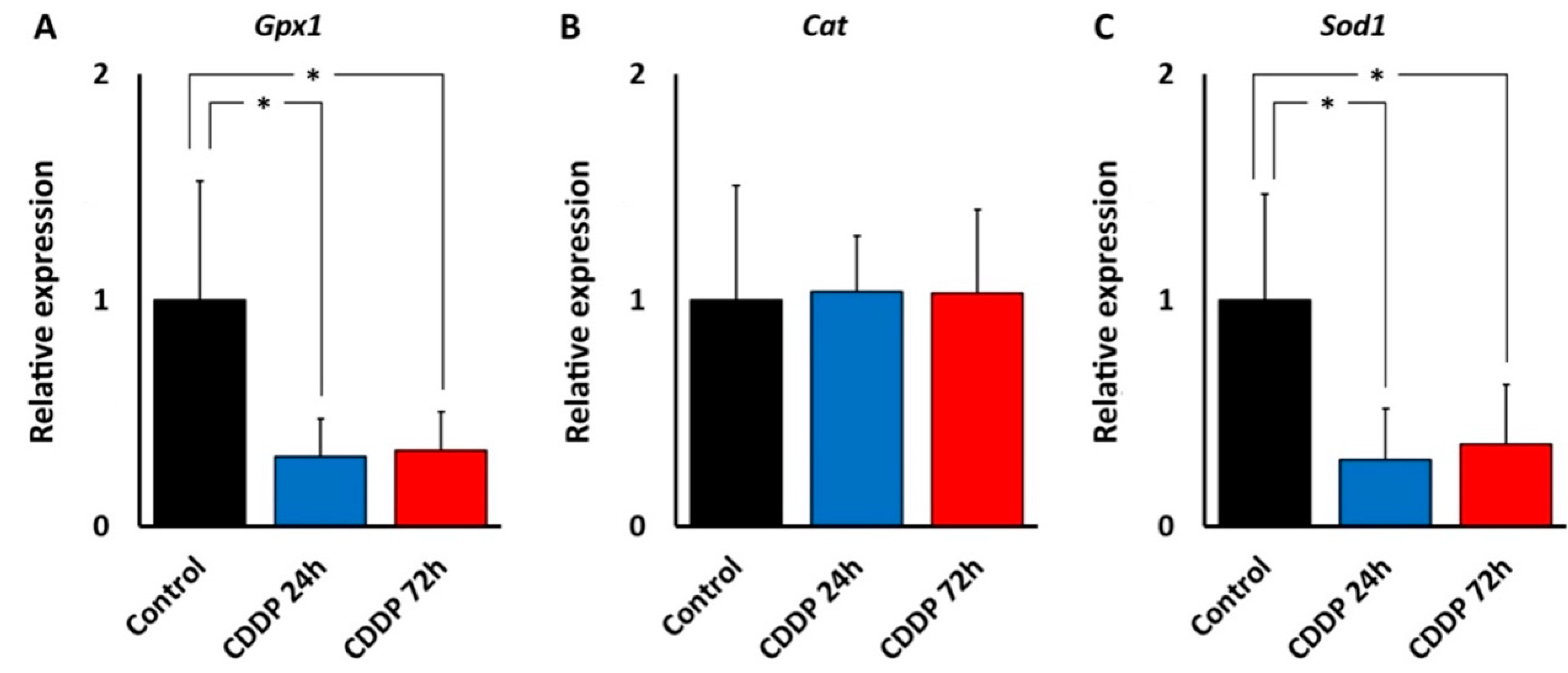
Thus, regional cochlear differences in susceptibility to oxidative stress may be responsible for differences in the ototoxicity profiles of kanamycin and cisplatin. The central position of oxidative stress in ototoxicity mechanisms is also observed in the expression and localization of enzymes involved in “antioxidation defense.” We found that, in the cochlea, both kanamycin and cisplatin systemic treatment changed the expression levels of genes coding for antioxidation enzymes, with relevant differences between both ototoxic drugs, as observed with qRT-PCR. With kanamycin, mRNA expression levels for Gpx1 dropped significantly after 11 days of treatment and remained diminished at 15 days, whereas mRNA levels for Cat and Sod1, the two other key enzymes for antioxidation, remained unchanged. Down-regulation of Gpx1 in the cochlea after kanamycin treatment has been reported [
25]. Different to kanamycin, however, the cisplatin treatment resulted in diminished levels of Gpx1
and Sod1 gene expression. Down-regulation of the Sod1 gene with cisplatin but not with kanamycin suggests differences in antioxidation response mechanisms between both ototoxic drugs. On the other hand, Cat expression levels were not affected by either ototoxic, suggesting that the involvement of this enzyme in antioxidation response to both drugs does not require changes in gene expression. Thus, enzymatic antioxidation mechanisms may not be stereotypically affected by aminoglycosides and cisplatin. In this regard, overall diminished antioxidation enzymatic activity has been reported after amikacin ototoxicity in the guinea pig cochlea [
40], whereas changes in antioxidation enzymatic activity in the cochlea after cisplatin seem to be more complex, but with low Gpx activity predominating [
24]. It is tempting to speculate that diminished cochlear expression of both Gpx1 and Sod1 genes with cisplatin may manifest a larger “exhaustion” of the enzymatic defense systems than with kanamycin.
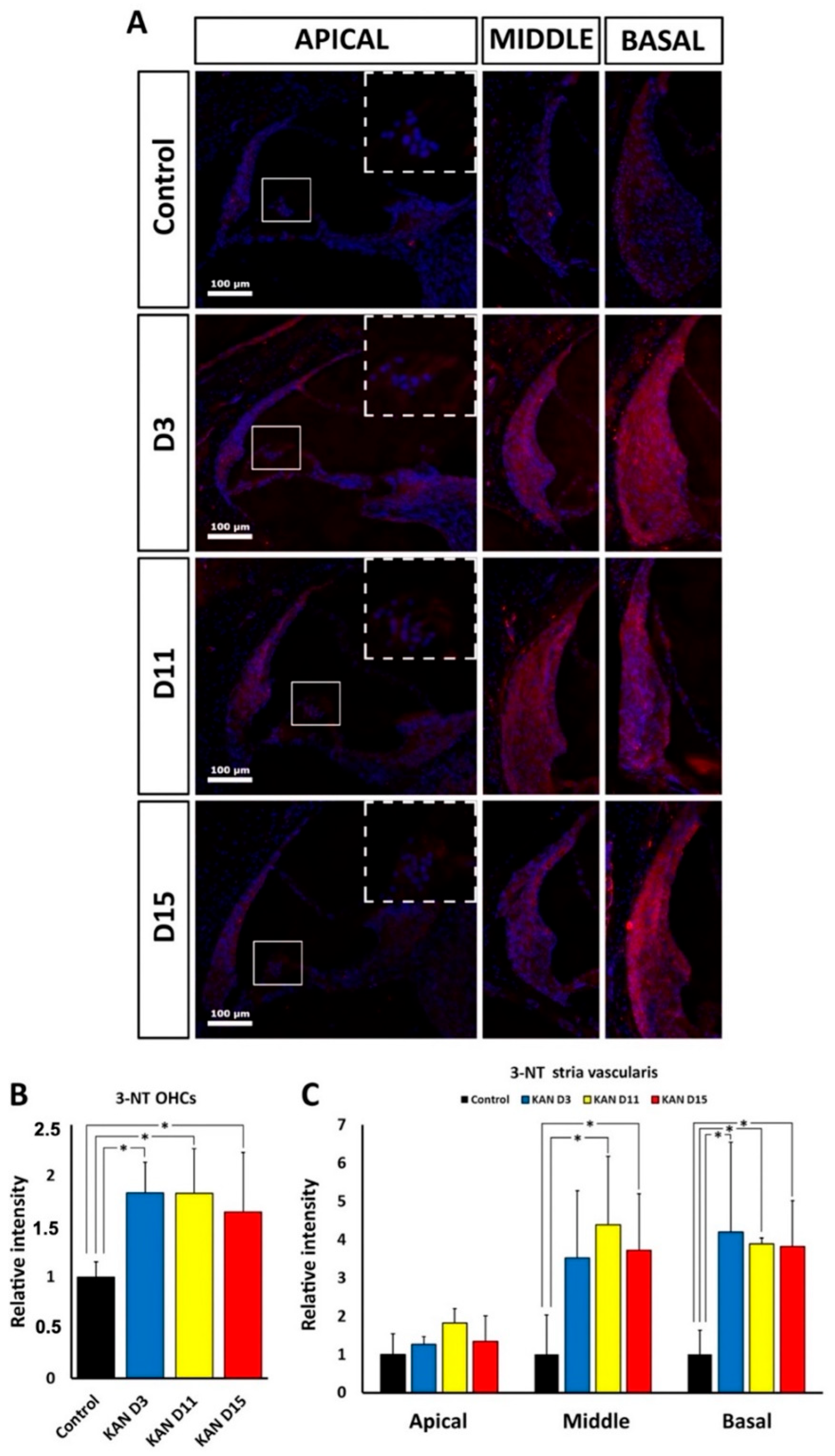
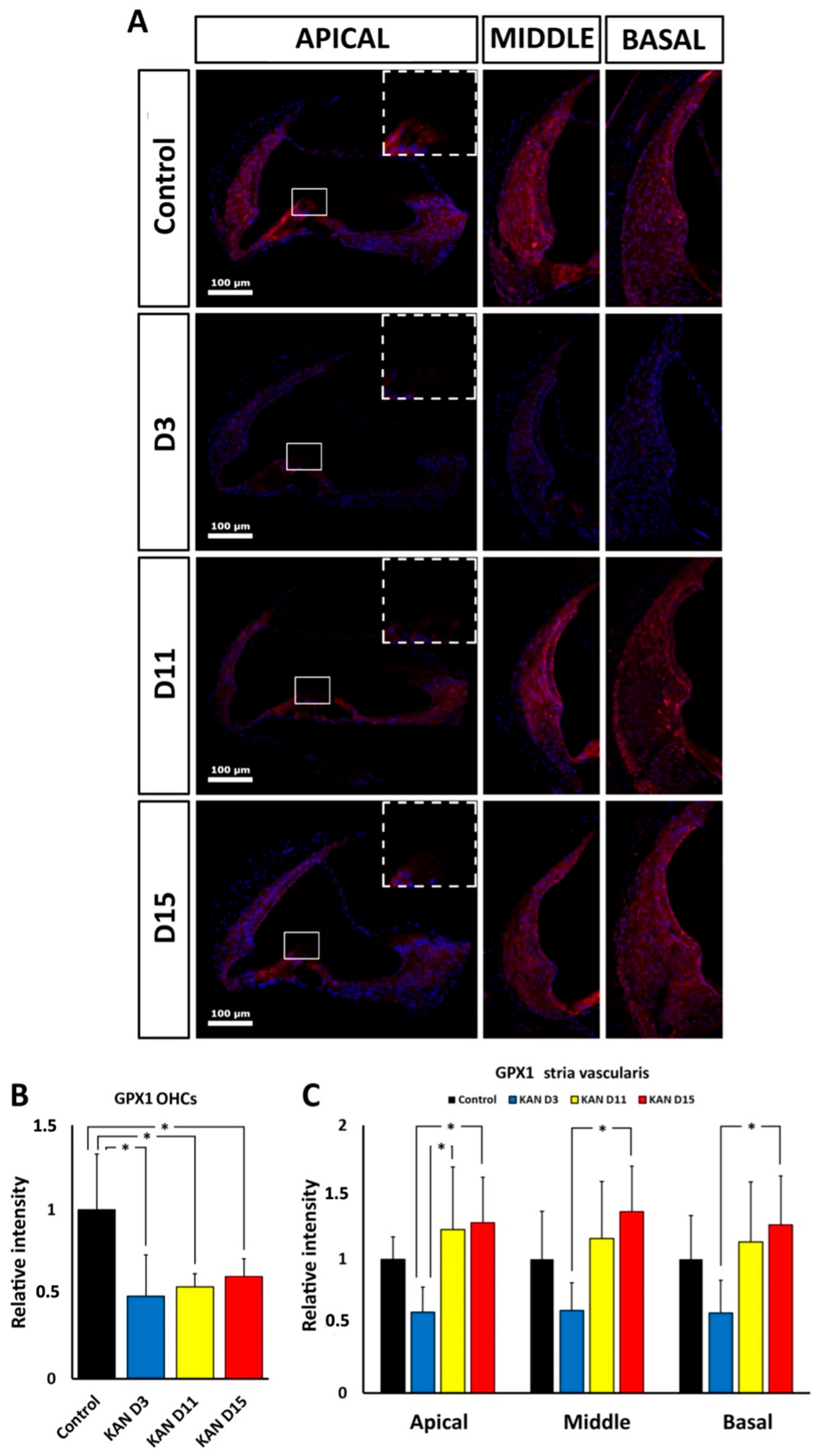
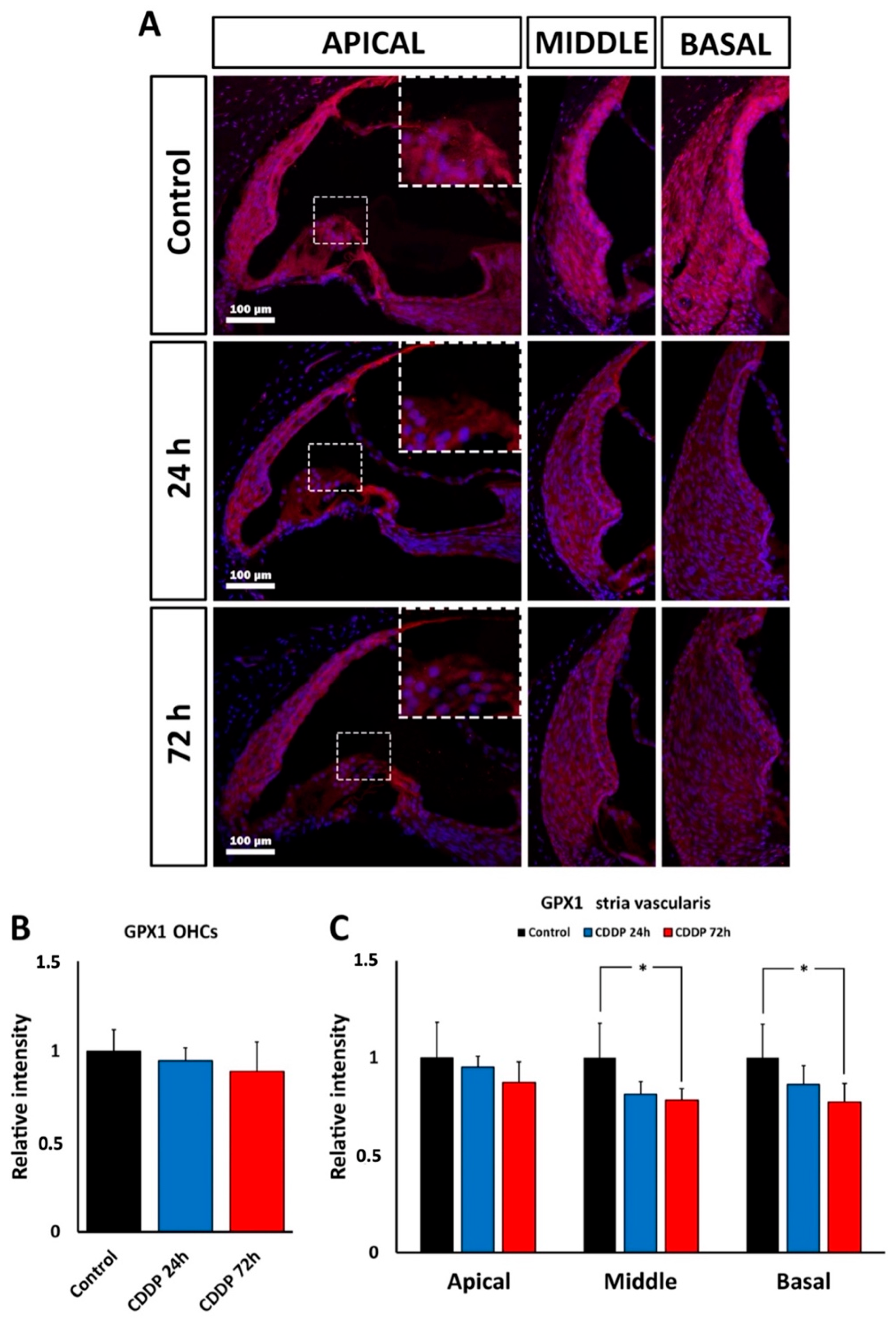
Cochlear distribution of immunolabeling for the Gpx1 protein product, whose gene expression is down-regulated both by kanamycin and cisplatin, provides additional clues about the involvement of oxidative stress in ototoxicity mediated by these drugs. Although mRNA levels may not bear simple correlations with levels of the corresponding protein [
41], patterns of cochlear immunolabeling for Gpx1 support distinct ototoxic oxidative stress responses for kanamycin and cisplatin. With kanamycin, immunolabeling for Gpx1 was diminished in the OHC region throughout treatment, whereas in the stria vascularis, intensity levels at the end of treatment at days 11 and 15 were comparable to controls. In the case of cisplatin, Gpx1 immunolabeling intensity was significantly diminished in the stria vascularis, particularly in the middle and basal turns, at the end of treatment 72 h after the injection, whereas OHCs did not show significant differences with controls. Altogether, this suggests that the GPx1 expression level is downregulated during kanamycin and cisplatin ototoxicity. In the case of kanamycin such down-regulation persists throughout treatment in the OHC region, whereas in the case of cisplatin it seems to more affect the stria vascularis.