NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Echocardiography
2.3. Electrophysiological Study
2.4. Blood Pressure
2.5. Cell Culture, siRNA Transfection, and Treatment
2.6. Histology
2.7. ROS Detection
2.8. Western Blot Analysis
2.9. Statistical Analysis
3. Results
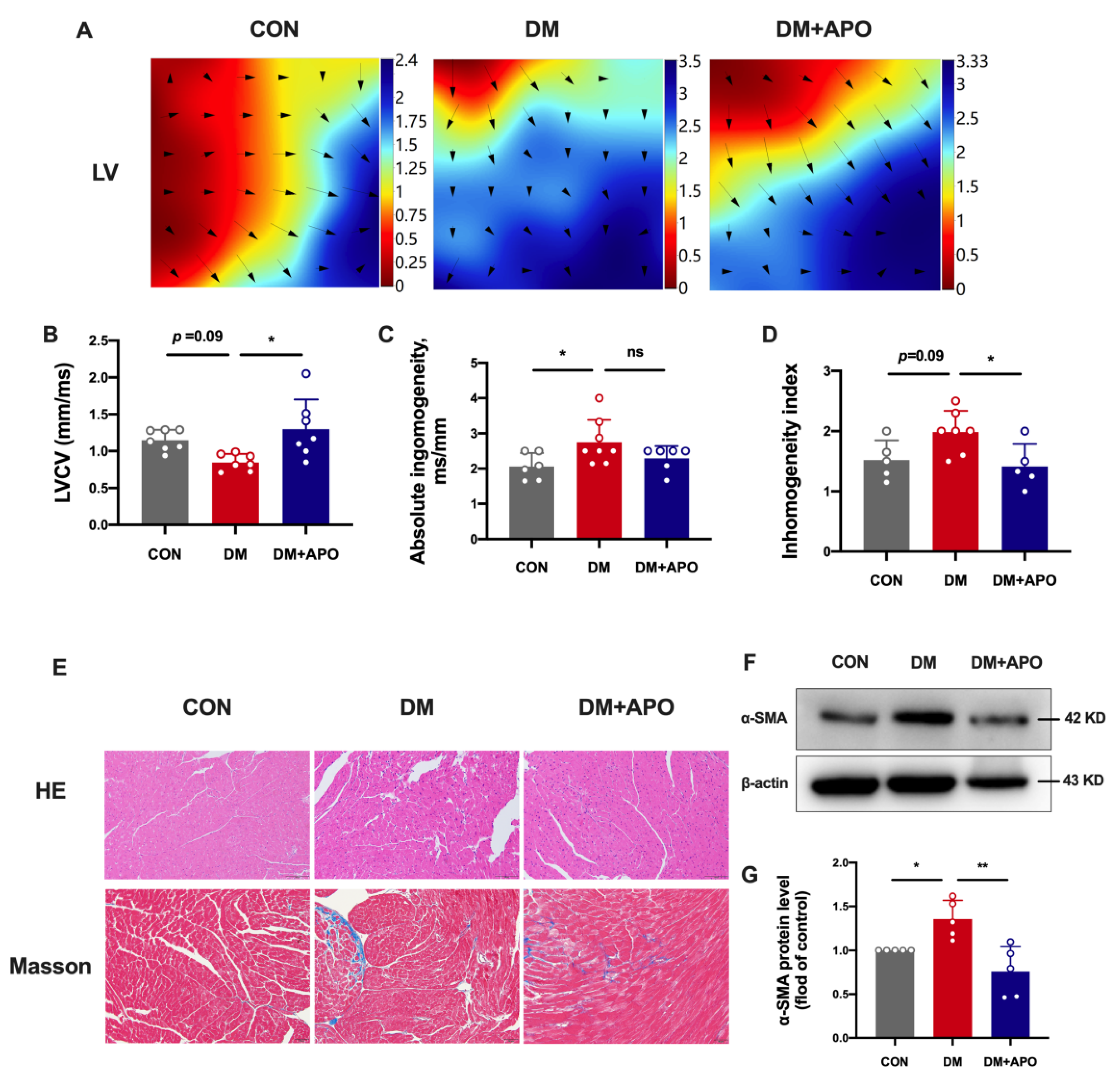
3.1. Apocynin Improves Cardiac Function in Diabetic Mice
3.2. Apocynin Improves the Ventricular Structural and Electrical Modeling in Diabetic Mice
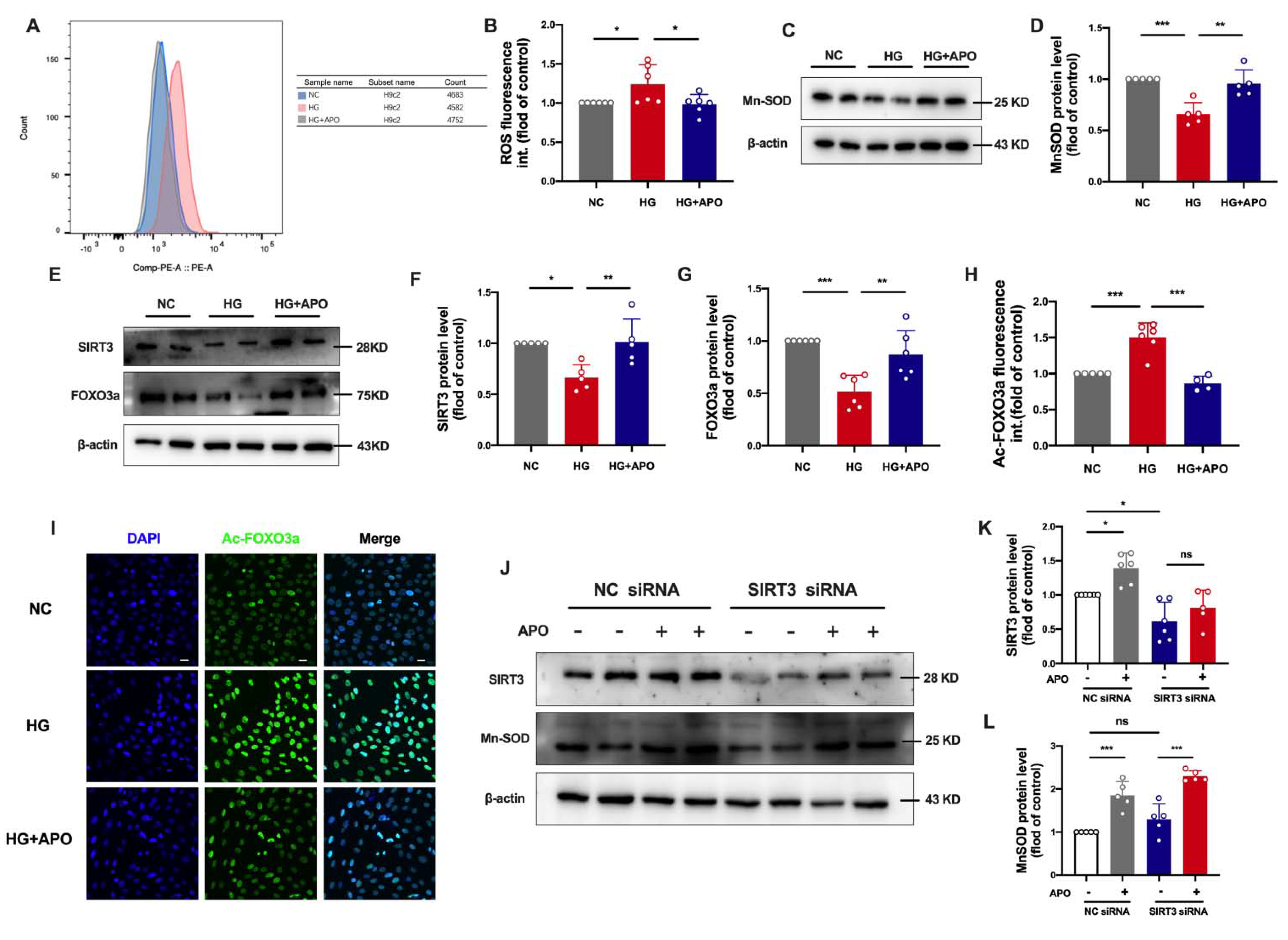
3.3. Apocynin Decreases ROS and Improves Mitochondrial Function of Diabetic Cardiomyopathy
3.4. Apocynin Regulates SIRT3/FOXO3a Signaling Pathway in Diabetic Cardiomyopathy
3.5. Apocynin Attenuates Oxidative Stress via SIRT3/FOXO3a Pathway in H9c2 Cells
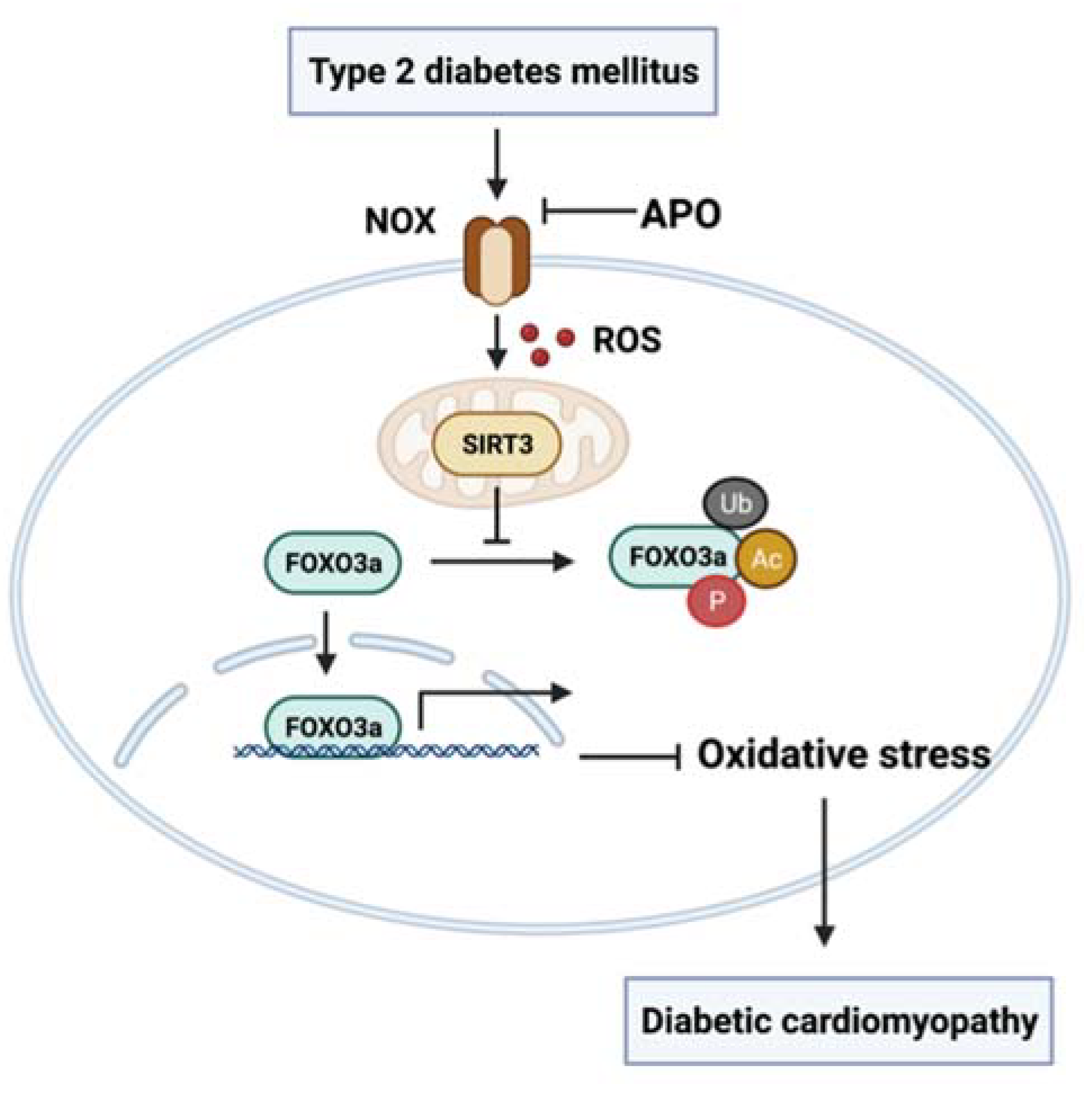
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Wang, L.; He, J.; Bi, Y.; Li, M.; Wang, T.; Wang, L.; Jiang, Y.; Dai, M.; Lu, J.; et al. Prevalence and control of diabetes in Chinese adults. JAMA 2013, 310, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; de Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wang, P.; Dong, C.; Zhang, J.; Wang, X.; Pei, H. Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy. Oxid. Med. Cell Longev. 2022, 2022, 5913374. [Google Scholar] [CrossRef]
- Petronio, M.S.; Zeraik, M.L.; Fonseca, L.M.; Ximenes, V.F. Apocynin: Chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules 2013, 18, 2821–2839. [Google Scholar] [CrossRef]
- Volpe, C.M.O.; Villar-Delfino, P.H.; Dos Anjos, P.M.F.; Nogueira-Machado, J.A. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell Death Dis. 2018, 9, 119. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Z.; Zhao, Y.; Jiang, N.; Qiu, J.; Yang, Y.; Li, J.; Liang, X.; Wang, X.; Tse, G.; et al. Alogliptin, a Dipeptidyl Peptidase-4 Inhibitor, Alleviates Atrial Remodeling and Improves Mitochondrial Function and Biogenesis in Diabetic Rabbits. J. Am. Heart Assoc. 2017, 6, e005945. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Q.; Wang, X.; Yuan, M.; Zhang, Y.; Xu, Z.; Li, G.; Liu, T. Reactive oxygen species mediated oxidative stress links diabetes and atrial fibrillation. Mol. Med. Rep. 2018, 17, 4933–4940. [Google Scholar] [CrossRef]
- Gong, M.; Yuan, M.; Meng, L.; Zhang, Z.; Tse, G.; Zhao, Y.; Zhang, Y.; Yuan, M.; Liang, X.; Fan, G.; et al. Wenxin Keli Regulates Mitochondrial Oxidative Stress and Homeostasis and Improves Atrial Remodeling in Diabetic Rats. Oxid. Med. Cell Longev. 2020, 2020, 2468031. [Google Scholar] [CrossRef]
- Sundaresan, N.R.; Gupta, M.; Kim, G.; Rajamohan, S.B.; Isbatan, A.; Gupta, M.P. Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J. Clin. Investig. 2009, 119, 2758–2771. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Liu, Y.; Wang, Z.; Liu, D.; Xie, B.; Zhang, Y.; Yuan, M.; Tse, G.; Li, G.; Xu, G.; et al. Activation of NADPH oxidase mediates mitochondrial oxidative stress and atrial remodeling in diabetic rabbits. Life Sci. 2021, 272, 119240. [Google Scholar] [CrossRef]
- Bause, A.S.; Haigis, M.C. SIRT3 regulation of mitochondrial oxidative stress. Exp. Gerontol. 2013, 48, 634–639. [Google Scholar] [CrossRef]
- Sun, W.; Liu, C.; Chen, Q.; Liu, N.; Yan, Y.; Liu, B. SIRT3: A New Regulator of Cardiovascular Diseases. Oxid. Med. Cell Longev. 2018, 2018, 7293861. [Google Scholar] [CrossRef]
- Lee, S.; Liu, T.; Zhou, J.; Zhang, Q.; Wong, W.T.; Tse, G. Predictions of diabetes complications and mortality using hba1c variability: A 10-year observational cohort study. Acta Diabetol. 2021, 58, 171–180. [Google Scholar] [CrossRef]
- Hills, A.P.; Arena, R.; Khunti, K.; Yajnik, C.S.; Jayawardena, R.; Henry, C.J.; Street, S.J.; Soares, M.J.; Misra, A. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018, 6, 966–978. [Google Scholar] [CrossRef]
- Paolillo, S.; Marsico, F.; Prastaro, M.; Renga, F.; Esposito, L.; De Martino, F.; Di Napoli, P.; Esposito, I.; Ambrosio, A.; Ianniruberto, M.; et al. Diabetic Cardiomyopathy: Definition, Diagnosis, and Therapeutic Implications. Heart Fail. Clin. 2019, 15, 341–347. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, J.; Wong, W.T.; Liu, T.; Wu, W.K.K.; Wong, I.C.K.; Zhang, Q.; Tse, G. Glycemic and lipid variability for predicting complications and mortality in diabetes mellitus using machine learning. BMC Endocr. Disord. 2021, 21, 94. [Google Scholar] [CrossRef]
- Lakhani, I.; Gong, M.; Wong, W.T.; Bazoukis, G.; Lampropoulos, K.; Wong, S.H.; Wu, W.K.K.; Wong, M.C.S.; Ong, K.L.; Liu, T.; et al. Fibroblast growth factor 21 in cardio-metabolic disorders: A systematic review and meta-analysis. Metabolism 2018, 83, 11–17. [Google Scholar] [CrossRef]
- Gomez-Samano, M.A.; Grajales-Gomez, M.; Zuarth-Vazquez, J.M.; Navarro-Flores, M.F.; Martinez-Saavedra, M.; Juarez-Leon, O.A.; Morales-Garcia, M.G.; Enriquez-Estrada, V.M.; Gomez-Perez, F.J.; Cuevas-Ramos, D. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Jeevaratnam, K.; Liu, T.; Chang, D.; Chang, C.; Wong, W.T.; Wong, I.C.K.; Lip, G.Y.H.; Tse, G. Risk stratification of cardiac arrhythmias and sudden cardiac death in type 2 diabetes mellitus patients receiving insulin therapy: A population-based cohort study. Clin. Cardiol. 2021, 44, 1602–1612. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, J.; Guo, C.L.; Wong, W.T.; Liu, T.; Wong, I.C.K.; Jeevaratnam, K.; Zhang, Q.; Tse, G. Predictive scores for identifying patients with type 2 diabetes mellitus at risk of acute myocardial infarction and sudden cardiac death. Endocrinol. Diabetes Metab. 2021, 4, e00240. [Google Scholar] [CrossRef]
- Ritchie, R.H.; Abel, E.D. Basic Mechanisms of Diabetic Heart Disease. Circ. Res. 2020, 126, 1501–1525. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; Yuan, M.; Liu, X.; et al. Hyperglycemia Induces Endoplasmic Reticulum Stress in Atrial Cardiomyocytes, and Mitofusin-2 Downregulation Prevents Mitochondrial Dysfunction and Subsequent Cell Death. Oxid. Med. Cell. Longev. 2020, 2020, 6569728. [Google Scholar] [CrossRef]
- Sumimoto, H. Structure, regulation and evolution of Nox-family NADPH oxidases that produce reactive oxygen species. FEBS J. 2008, 275, 3249–3277. [Google Scholar] [CrossRef]
- Huang, X.; Sun, M.; Li, D.; Liu, J.; Guo, H.; Dong, Y.; Jiang, L.; Pan, Q.; Man, Y.; Wang, S.; et al. Augmented NADPH oxidase activity and p22phox expression in monocytes underlie oxidative stress of patients with type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011, 91, 371–380. [Google Scholar] [CrossRef]
- Wang, K.; Zhu, Z.F.; Chi, R.F.; Li, Q.; Yang, Z.J.; Jie, X.; Hu, X.L.; Han, X.B.; Wang, J.P.; Li, B.; et al. The NADPH oxidase inhibitor apocynin improves cardiac sympathetic nerve terminal innervation and function in heart failure. Exp. Physiol. 2019, 104, 1638–1649. [Google Scholar] [CrossRef]
- Stolk, J.; Hiltermann, T.J.; Dijkman, J.H.; Verhoeven, A.J. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am. J. Respir. Cell Mol. Biol. 1994, 11, 95–102. [Google Scholar] [CrossRef]
- Johnson, D.K.; Schillinger, K.J.; Kwait, D.M.; Hughes, C.V.; McNamara, E.J.; Ishmael, F.; O’Donnell, R.W.; Chang, M.M.; Hogg, M.G.; Dordick, J.S.; et al. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium 2002, 9, 191–203. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R. ROS-induced ROS release in plant and animal cells. Free Radic. Biol. Med. 2018, 122, 21–27. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Azman Ali, R.; Mohamed Ibrahim, N.; Ahmadiani, A. Mitochondrial Dysfunction and Biogenesis in Neurodegenerative diseases: Pathogenesis and Treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Sebastian, D.; Hernandez-Alvarez, M.I.; Segales, J.; Sorianello, E.; Munoz, J.P.; Sala, D.; Waget, A.; Liesa, M.; Paz, J.C.; Gopalacharyulu, P.; et al. Mitofusin 2 (Mfn2) links mitochondrial and endoplasmic reticulum function with insulin signaling and is essential for normal glucose homeostasis. Proc. Natl. Acad. Sci. USA 2012, 109, 5523–5528. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; Tang, D.; Gao, E.; Li, C.; Wang, K.; Qi, B.; Qiu, J.; Zhao, H.; Chang, P.; et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics 2019, 9, 3687–3706. [Google Scholar] [CrossRef]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.T.; et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 1999, 96, 846–851. [Google Scholar] [CrossRef]
- Yu, W.; Gao, B.; Li, N.; Wang, J.; Qiu, C.; Zhang, G.; Liu, M.; Zhang, R.; Li, C.; Ji, G.; et al. Sirt3 deficiency exacerbates diabetic cardiac dysfunction: Role of Foxo3A-Parkin-mediated mitophagy. Biochim. Biophys. Acta Mol. Basis. Dis. 2017, 1863, 1973–1983. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. Sirtuins and Type 2 Diabetes: Role in Inflammation, Oxidative Stress, and Mitochondrial Function. Front. Endocrinol. 2019, 10, 187. [Google Scholar] [CrossRef]
- Porter, G.A.; Urciuoli, W.R.; Brookes, P.S.; Nadtochiy, S.M. SIRT3 deficiency exacerbates ischemia-reperfusion injury: Implication for aged hearts. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1602–H1609. [Google Scholar] [CrossRef]
- Qiu, X.; Brown, K.; Hirschey, M.D.; Verdin, E.; Chen, D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010, 12, 662–667. [Google Scholar] [CrossRef]
- Wu, J.; Zeng, Z.; Zhang, W.; Deng, Z.; Wan, Y.; Zhang, Y.; An, S.; Huang, Q.; Chen, Z. Emerging role of SIRT3 in mitochondrial dysfunction and cardiovascular diseases. Free Radic. Res. 2019, 53, 139–149. [Google Scholar] [CrossRef]



| CON Group (n = 6) | DM Group (n = 6) | DM + APO Group (n = 6) | |
|---|---|---|---|
| HR (bpm) | 522.33 ± 46.04 | 528.33 ± 57.49 | 552.00 ± 50.53 |
| IVS (mm) | 0.83 ± 0.17 | 0.95 ± 0.12 | 0.86 ± 0.15 |
| LVEDD (mm) | 4.02 ± 0.24 | 3.71 ± 0.28 | 3.61 ± 0.18 |
| LVESD (mm) | 2.92 ± 0.18 | 2.47 ± 0.22 * | 2.61 ± 0.19 |
| LVPW (mm) | 0.88 ± 0.26 | 0.97 ± 0.17 | 0.89 ± 0.18 |
| E/A | 2.36 ± 0.76 | 1.53 ± 0.49 | 1.77 ± 0.56 |
| LVEF (%) | 55.20 ± 5.33 | 48.13 ± 5.6 | 64.68 ± 5.04 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, J.; Liu, D.; Li, P.; Zhou, L.; Zhou, L.; Liu, X.; Zhang, Y.; Yuan, M.; Tse, G.; Li, G.; et al. NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice. Antioxidants 2022, 11, 1745. https://doi.org/10.3390/antiox11091745
Qiu J, Liu D, Li P, Zhou L, Zhou L, Liu X, Zhang Y, Yuan M, Tse G, Li G, et al. NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice. Antioxidants. 2022; 11(9):1745. https://doi.org/10.3390/antiox11091745
Chicago/Turabian StyleQiu, Jiuchun, Daiqi Liu, Pengsha Li, Lingling Zhou, Lu Zhou, Xing Liu, Yue Zhang, Meng Yuan, Gary Tse, Guangping Li, and et al. 2022. "NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice" Antioxidants 11, no. 9: 1745. https://doi.org/10.3390/antiox11091745
APA StyleQiu, J., Liu, D., Li, P., Zhou, L., Zhou, L., Liu, X., Zhang, Y., Yuan, M., Tse, G., Li, G., & Liu, T. (2022). NADPH Oxidase Mediates Oxidative Stress and Ventricular Remodeling through SIRT3/FOXO3a Pathway in Diabetic Mice. Antioxidants, 11(9), 1745. https://doi.org/10.3390/antiox11091745









