Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro
Abstract
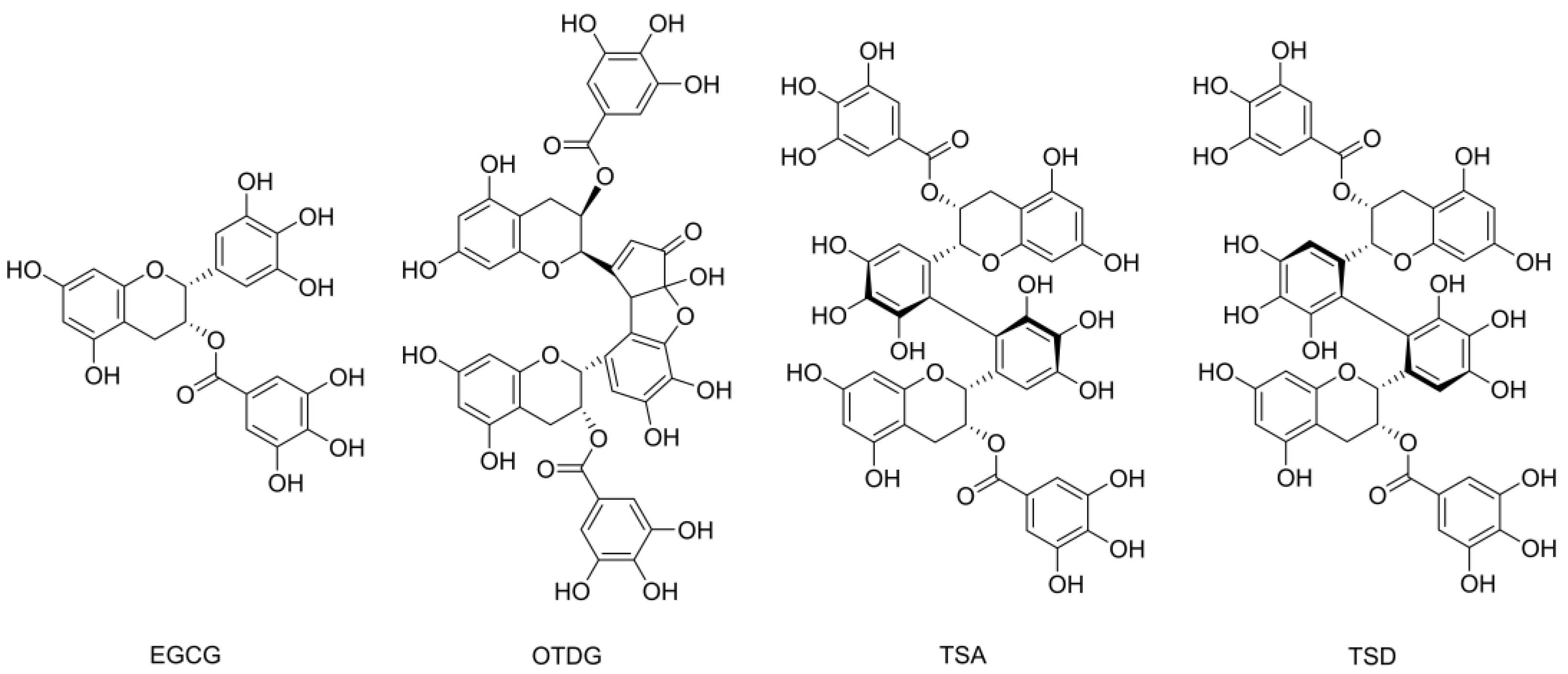
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. HPLC-MS/MS Method Settings, Calibration, Validation and Data Handling
2.3. Formation of EGCG Oxidation Products under Cell-Free Cell Culture Conditions
2.4. Determination of HepG2 Cellular Uptake
2.5. Evaluation of Antioxidant Capacity In Vitro Using a Modified Dichlorofluoresceine Diacetate (DCFH2-DA) Assay
2.6. Software
3. Results
3.1. Stability of EGCG and Formation of EGCG Oxidation Products under Cell-Free Cell Culture Conditions
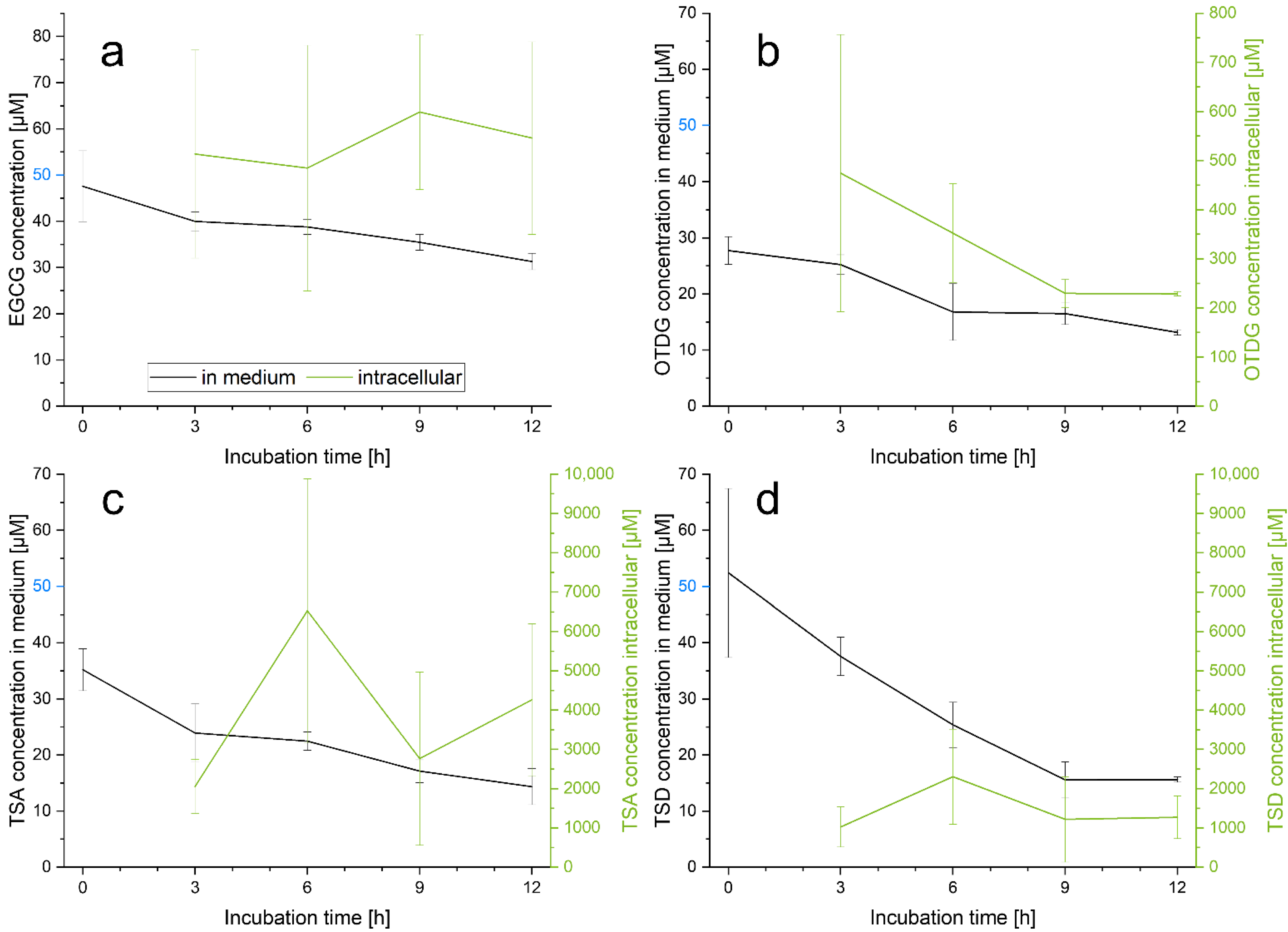
3.2. Cellular Uptake of EGCG and EGCG Oxidation Products in HepG2 Cells
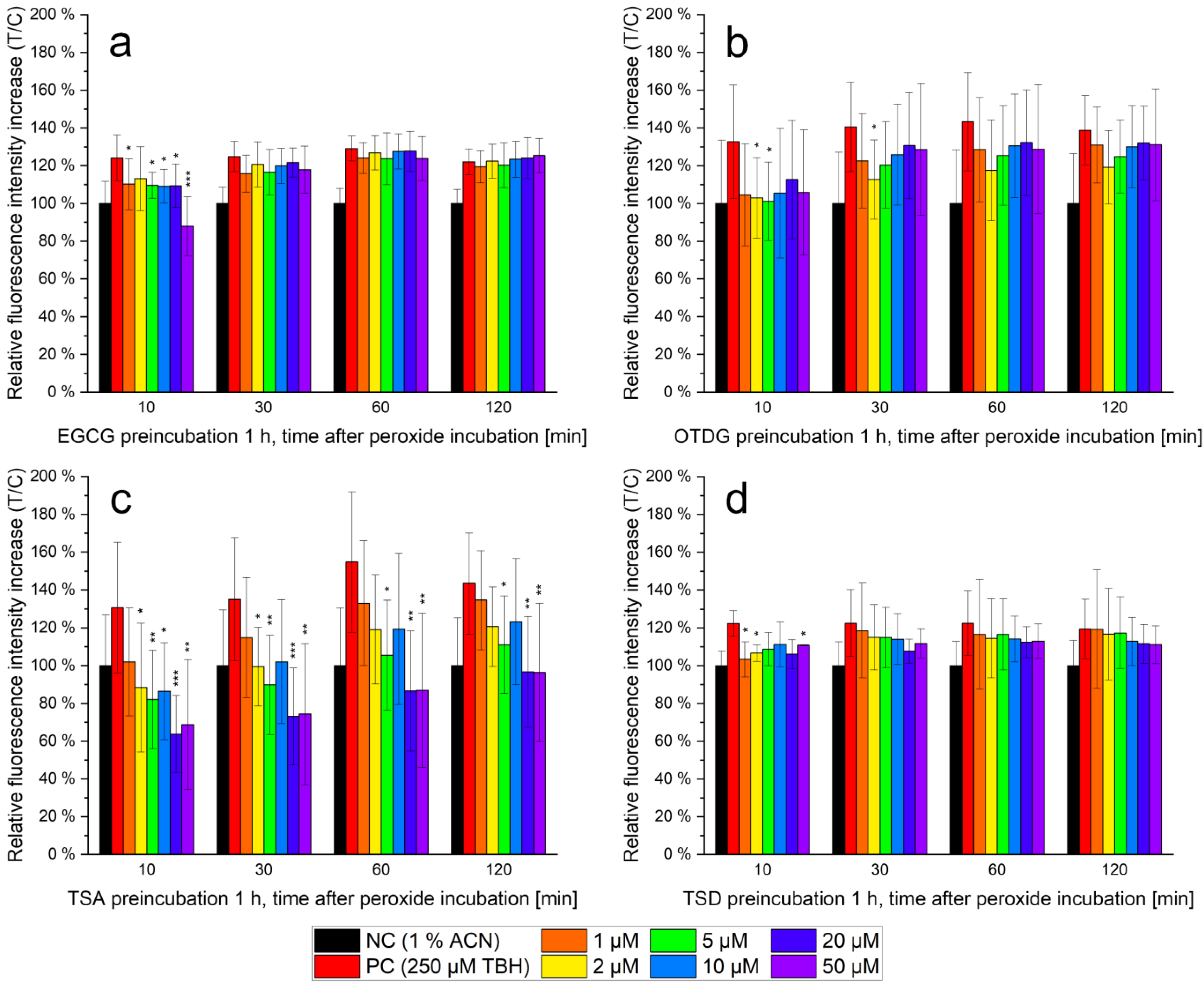
3.3. Antioxidant Capacity of EGCG and EGCG Oxidation Products In Vitro
4. Discussion
4.1. Stability of EGCG and Formation of EGCG Oxidation Products under Cell-Free Cell Culture Conditions
4.2. Cellular Uptake of EGCG and EGCG Oxidation Products in HepG2 Cells
4.3. Antioxidant Capacity of EGCG and EGCG Oxidation Products In Vitro
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, X.; Du, J.; Zhao, W.; Guo, Z. Epigallocatechin-3-gallate (EGCG): Mechanisms and the combined applications. Comb. Chem. High Throughput Screen. 2017, 20, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic effects of EGCG: A patent review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, H.; Liu, C.; Huang, J.; Liu, Z. A review for physiological activities of EGCG and the role in improving fertility in humans/mammals. Biomed. Pharmacother. 2020, 127, 110186. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Shankar, S.; Srivastava, R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): Mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011, 82, 1807–1821. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef]
- Aldini, G.; Yeum, K.-J.; Carini, M.; Krinsky, N.I.; Russell, R.M. (−)-Epigallocatechin-(3)-gallate prevents oxidative damage in both the aqueous and lipid compartments of human plasma. Biochem. Biophys. Res. Commun. 2003, 302, 409–414. [Google Scholar] [CrossRef]
- Kim, H.-S.; Quon, M.J.; Kim, J.-A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014, 2, 187–195. [Google Scholar] [CrossRef]
- Moravcova, A.; Cervinkova, Z.; Kucera, O.; Mezera, V.; Lotkova, H. Antioxidative effect of epigallocatechin gallate against D-galactosamine-induced injury in primary culture of rat hepatocytes. Acta Med. 2014, 57, 3–8. [Google Scholar] [CrossRef]
- Hong, J.; Lu, H.; Meng, X.; Ryu, J.-H.; Hara, Y.; Yang, C.S. Stability, cellular uptake, biotransformation, and efflux of tea polyphenol (−)-epigallocatechin-3-gallate in HT-29 human colon adenocarcinoma cells. Cancer Res. 2002, 62, 7241–7246. [Google Scholar]
- Wu, Q.-Q.; Liang, Y.-F.; Ma, S.-B.; Li, H.; Gao, W.-Y. Stability and stabilization of (−)-gallocatechin gallate under various experimental conditions and analyses of its epimerization, auto-oxidation, and degradation by LC-MS. J. Sci. Food Agric. 2019, 99, 5984–5993. [Google Scholar] [CrossRef]
- Long, L.H.; Clement, M.V.; Halliwell, B. Artifacts in cell culture: Rapid generation of hydrogen peroxide on addition of (−)-epigallocatechin, (−)-epigallocatechin gallate, (+)-catechin, and quercetin to commonly used cell culture media. Biochem. Biophys. Res. Commun. 2000, 273, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of tea polyphenol (−)-epigallocatechin-3-gallate and formation of dimers and epimers under common experimental conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Alfke, J.; Kampermann, U.; Kalinina, S.; Esselen, M. Isolation and structural elucidation of dimeric epigallocatechin-3-gallate autoxidation products and their antioxidant capacity. Eur. Food Res. Technol. 2021, 247, 2961–2975. [Google Scholar] [CrossRef]
- Abudureheman, B.; Yu, X.; Fang, D.; Zhang, H. Enzymatic Oxidation of Tea Catechins and Its Mechanism. Molecules 2022, 27, 942. [Google Scholar] [CrossRef]
- Alfke, J.; Kampermann, U.; Esselen, M. HPLC-MS/MS Quantification of Flavan-3-ols, Xanthines, and Epigallocatechin-3-Gallate Oxidation Products in Tea Samples and New Insights into Their Cytotoxicity. ACS Food Sci. Technol. 2022, 2, 925–935. [Google Scholar] [CrossRef]
- Xiao, J.; Högger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Hong, J.; Yang, C. Effects of tea constituents on intracellular level of the major tea catechin, (−)-epigallocatechin-3-gallate. Food Sci. Biotechnol. 2007, 16, 167–170. [Google Scholar]
- Negri, A.; Naponelli, V.; Rizzi, F.; Bettuzzi, S. Molecular Targets of Epigallocatechin-Gallate (EGCG): A Special Focus on Signal Transduction and Cancer. Nutrients 2018, 10, 1936. [Google Scholar] [CrossRef]
- Chen, X.; Zhong, Z.; Xu, Z.; Chen, L.; Wang, Y. 2’,7’-Dichlorodihydrofluorescein as a fluorescent probe for reactive oxygen species measurement: Forty years of application and controversy. Free Radic. Res. 2010, 44, 587–604. [Google Scholar] [CrossRef]
- Bellion, P.; Digles, J.; Will, F.; Dietrich, H.; Baum, M.; Eisenbrand, G.; Janzowski, C. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef]
- Hong, J.; Lambert, J.D.; Lee, S.H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (-)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Neilson, A.P.; Song, B.J.; Sapper, T.N.; Bomser, J.A.; Ferruzzi, M.G. Tea catechin auto-oxidation dimers are accumulated and retained by Caco-2 human intestinal cells. Nutr. Res. 2010, 30, 327–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.; Guan, R.; Chen, X.; Tao, M.; Ma, J.; Zhao, J. Optimization on condition of epigallocatechin-3-gallate (EGCG) nanoliposomes by response surface methodology and cellular uptake studies in Caco-2 cells. Nanoscale Res. Lett. 2014, 9, 291. [Google Scholar] [CrossRef]
- Gan, N.; Wakayama, C.; Inubushi, S.; Kunihisa, T.; Mizumoto, S.; Baba, M.; Tanino, H.; Ooya, T. Size Dependency of Selective Cellular Uptake of Epigallocatechin Gallate-modified Gold Nanoparticles for Effective Radiosensitization. ACS Appl. Bio Mater. 2022, 5, 355–365. [Google Scholar] [CrossRef]
- Lu, Y.-C.; Luo, P.-C.; Huang, C.-W.; Leu, Y.-L.; Wang, T.-H.; Wei, K.-C.; Wang, H.-E.; Ma, Y.-H. Augmented cellular uptake of nanoparticles using tea catechins: Effect of surface modification on nanoparticle-cell interaction. Nanoscale 2014, 6, 10297–10306. [Google Scholar] [CrossRef] [PubMed]
- Marwah, M.; Perrie, Y.; Badhan, R.K.S.; Lowry, D. Intracellular uptake of EGCG-loaded deformable controlled release liposomes for skin cancer. J. Liposome Res. 2020, 30, 136–149. [Google Scholar] [CrossRef] [PubMed]
- Hisanaga, A.; Ishida, H.; Sakao, K.; Sogo, T.; Kumamoto, T.; Hashimoto, F.; Hou, D.-X. Anti-inflammatory activity and molecular mechanism of Oolong tea theasinensin. Food Funct. 2014, 5, 1891–1897. [Google Scholar] [CrossRef]
- Hung, W.-L.; Yang, G.; Wang, Y.-C.; Chiou, Y.-S.; Tung, Y.-C.; Yang, M.-J.; Wang, B.-N.; Ho, C.-T.; Wang, Y.; Pan, M.-H. Protective effects of theasinensin A against carbon tetrachloride-induced liver injury in mice. Food Funct. 2017, 8, 3276–3287. [Google Scholar] [CrossRef]
- Ohgitani, E.; Shin-Ya, M.; Ichitani, M.; Kobayashi, M.; Takihara, T.; Kawamoto, M.; Kinugasa, H.; Mazda, O. Significant Inactivation of SARS-CoV-2 In Vitro by a Green Tea Catechin, a Catechin-Derivative, and Black Tea Galloylated Theaflavins. Molecules 2021, 26, 3572. [Google Scholar] [CrossRef]
- Hou, D.-X.; Masuzaki, S.; Tanigawa, S.; Hashimoto, F.; Chen, J.; Sogo, T.; Fujii, M. Oolong tea theasinensins attenuate cyclooxygenase-2 expression in lipopolysaccharide (LPS)-activated mouse macrophages: Structure-activity relationship and molecular mechanisms. J. Agric. Food Chem. 2010, 58, 12735–12743. [Google Scholar] [CrossRef]
- Matsui, T. Condensed catechins and their potential health-benefits. Eur. J. Pharmacol. 2015, 765, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, W.; Bartosz, G. 2,7-dichlorofluorescin oxidation and reactive oxygen species: What does it measure? Cell Biol. Int. 2000, 24, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) is the most effective cancer chemopreventive polyphenol in green tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef] [PubMed]
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef]
- Hashimoto, F.; Ono, M.; Masuoka, C.; Ito, Y.; Sakata, Y.; Shimizu, K.; Nonaka, G.-I.; Nishioka, I.; Nohara, T. Evaluation of the anti-oxidative effect (In Vitro) of tea polyphenols. Biosci. Biotechnol. Biochem. 2003, 67, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Krupkova, O.; Ferguson, S.J.; Wuertz-Kozak, K. Stability of (−)-epigallocatechin gallate and its activity in liquid formulations and delivery systems. J. Nutr. Biochem. 2016, 37, 1–12. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfke, J.; Esselen, M. Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants 2022, 11, 1746. https://doi.org/10.3390/antiox11091746
Alfke J, Esselen M. Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants. 2022; 11(9):1746. https://doi.org/10.3390/antiox11091746
Chicago/Turabian StyleAlfke, Julian, and Melanie Esselen. 2022. "Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro" Antioxidants 11, no. 9: 1746. https://doi.org/10.3390/antiox11091746
APA StyleAlfke, J., & Esselen, M. (2022). Cellular Uptake of Epigallocatechin Gallate in Comparison to Its Major Oxidation Products and Their Antioxidant Capacity In Vitro. Antioxidants, 11(9), 1746. https://doi.org/10.3390/antiox11091746




