1. Introduction
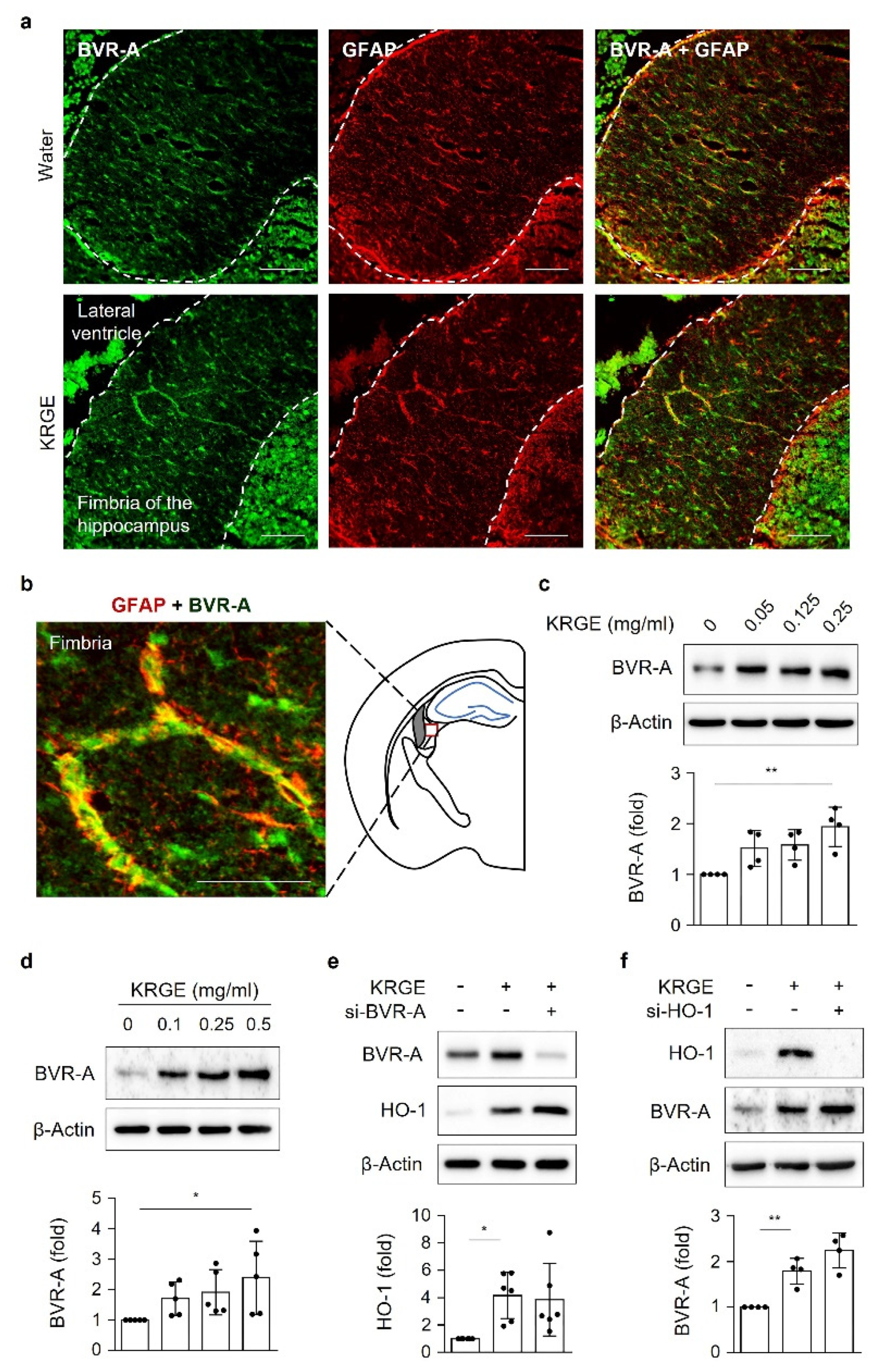
Under physiological conditions, Korean red ginseng extract (KRGE) promotes astrocyte proliferation in the brain of young adult mice [
1]. Enhanced mitochondrial function is associated with cell proliferation [
2], possibly through the heme oxygenase (HO)-mediated pathway [
1]. Reactive oxygen species can be produced in the process of oxidative phosphorylation (OXPHOS) in mitochondria. Superoxide dismutase 2 (SOD2) protects mitochondria from undesirable oxidative effects [
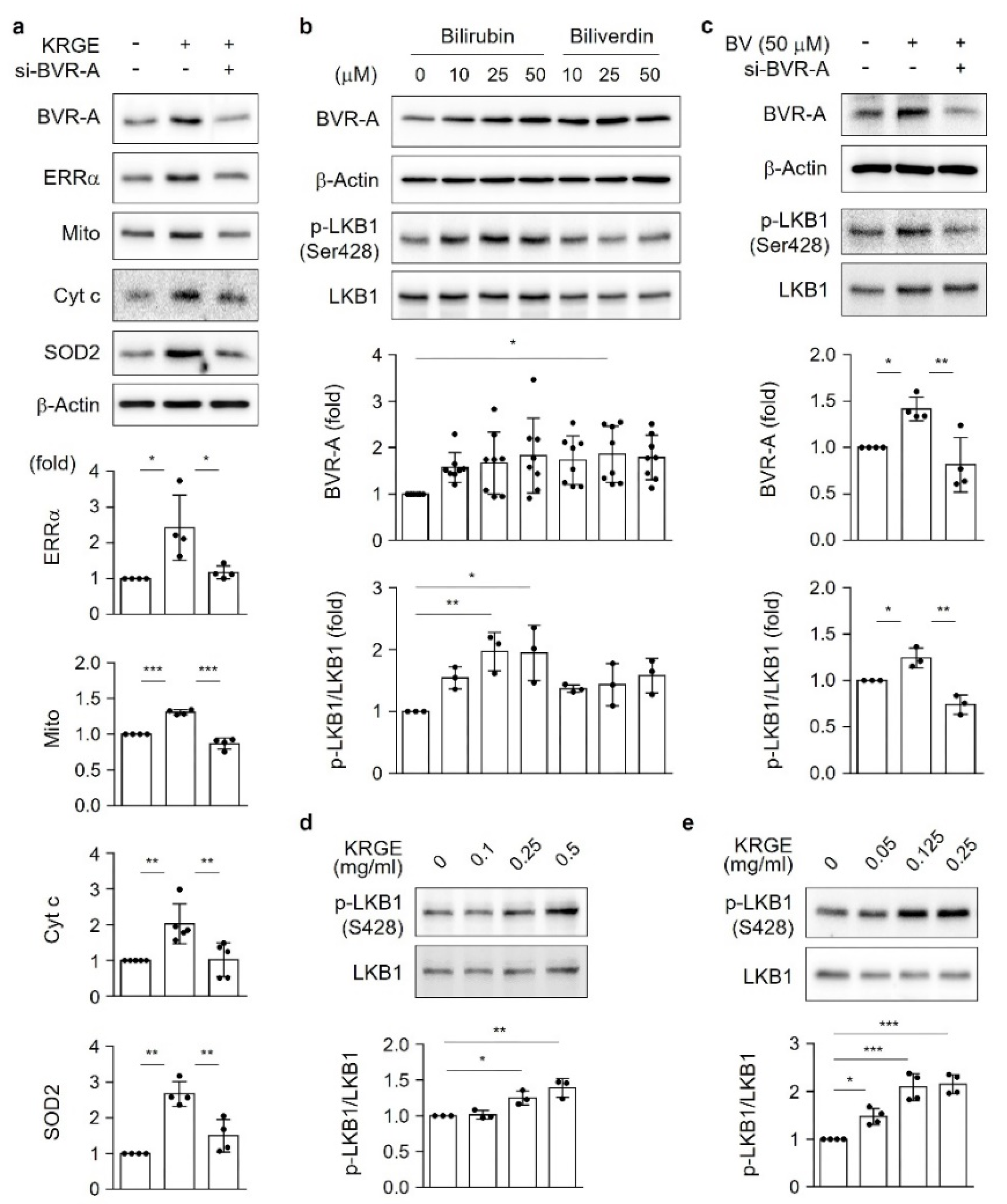
3]. Biliverdin reductase A (BVR-A) is an enzyme that converts biliverdin into bilirubin [
4]; however, its specific functions in adults have not been clarified. The physiological antioxidant effects of bilirubin depend on the expression and activity of BVR-A [
5].
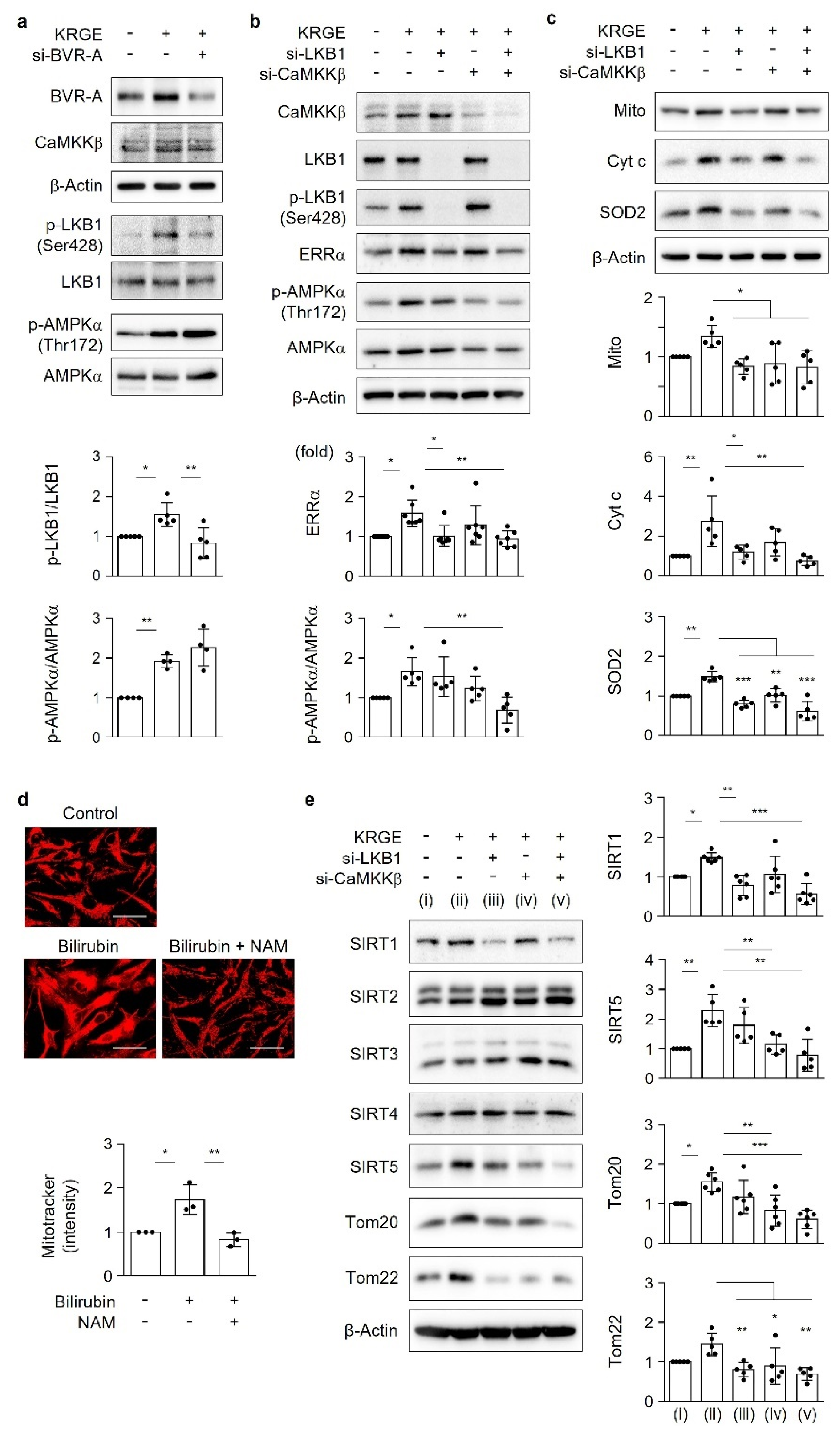
The serine/threonine kinase liver kinase B1 (LKB1), a principal regulator of energy metabolism, inhibits cancer cell growth under energy stress conditions through the modulation of 14 kinases that act on various processes, such as cell attachment and metabolism [
6]. One of the target proteins regulated by LKB1 is the AMP-activated protein kinase α (AMPKα) [
7], which is important for mitochondrial biogenesis activated by HO metabolites (e.g., carbon monoxide (CO)) [
8,
9]. After CO pretreatment, followed by a recovery phase (CO/R), AMPKα can activate the peroxisome-proliferator-activating receptor-γ coactivator-1α (PGC-1α)-estrogen-related receptor α (ERRα) pathway in astrocytes [
8].
In the outer mitochondrial membrane, the translocase of the outer mitochondrial membrane (TOM) complex leads to the primary entry of nuclear-encoded proteins into mitochondria [
10]. Tom20 and Tom22, together with other TOM complexes, bind to the outer membrane of mitochondria [
6] and play key roles in the regulation of the mitochondrial membrane potential and protein import [
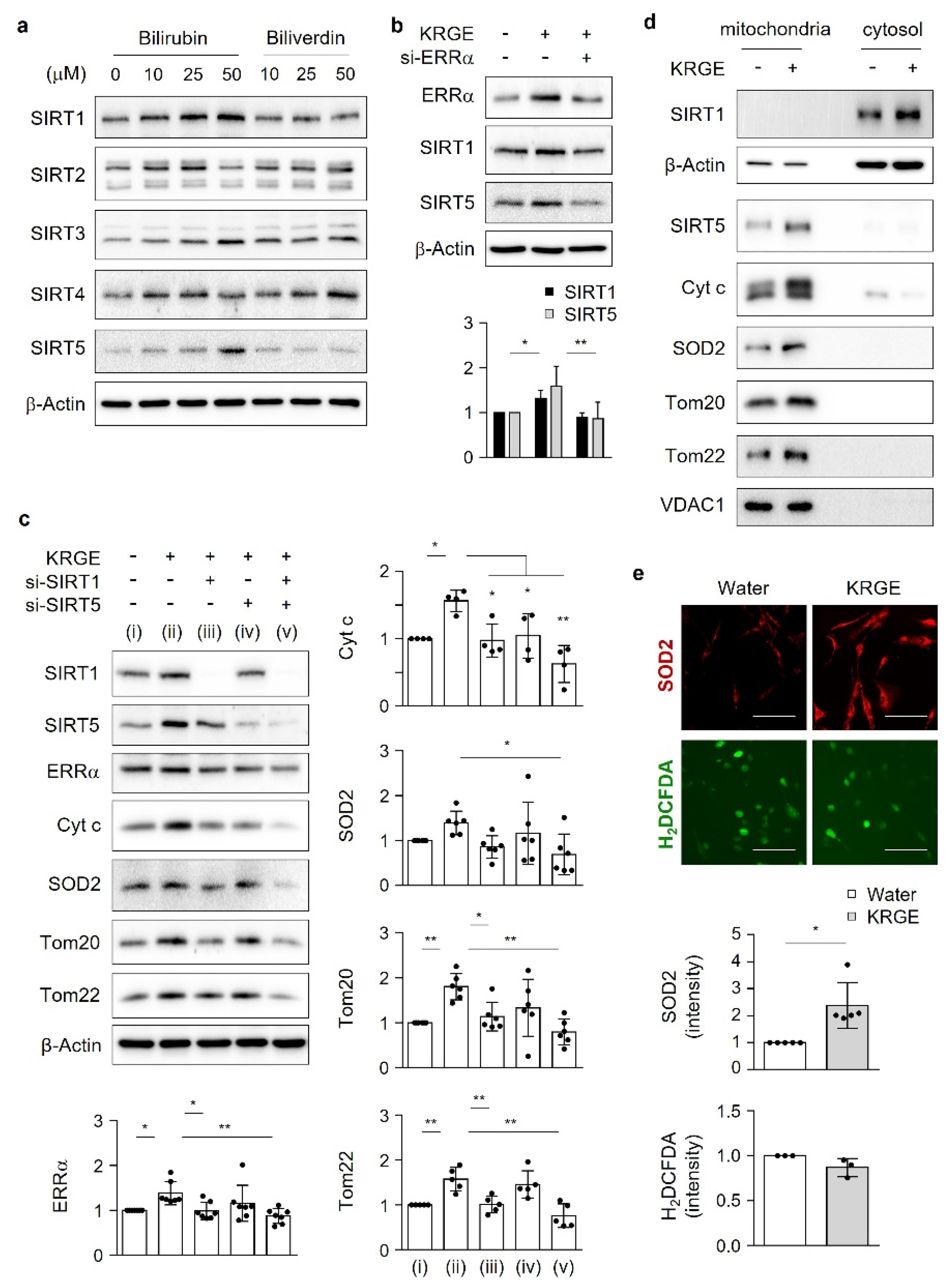
11]. Mitochondrial quality control stems from sirtuins (SIRTs). In mammals, seven SIRTs are classified as SIRT1–7; specifically, SIRT1 is localized in the cytosol and the nucleus, SIRT2 and SIRT4 are localized in the cytosol and mitochondria, SIRT3 and SIRT5 are localized in the mitochondria, and SIRT6 and SIRT7 are localized in the nucleus [
12,
13,
14,
15]. SIRTs are nicotinamide adenine dinucleotide (NAD
+)-dependent deacetylases [
15].
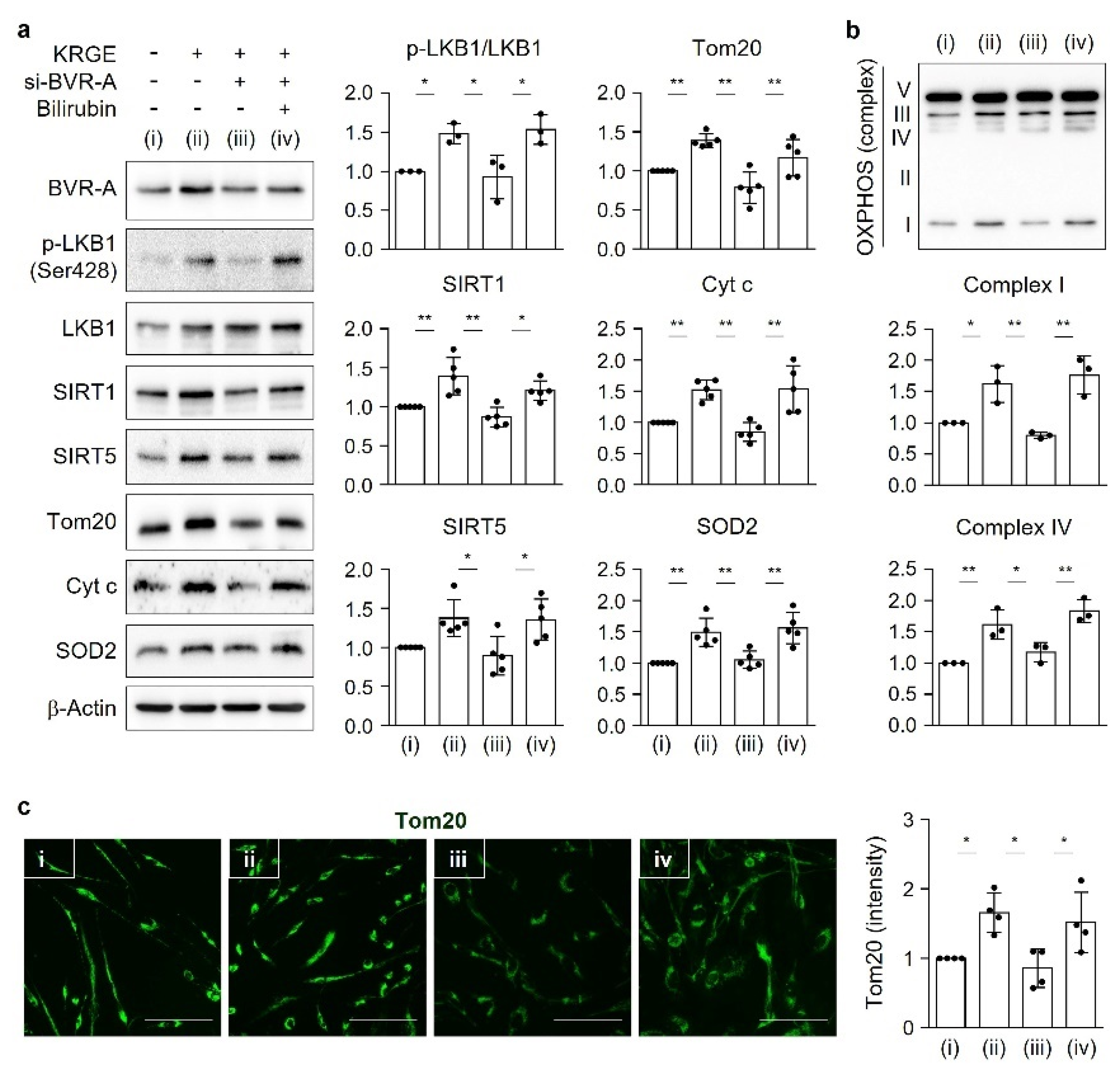
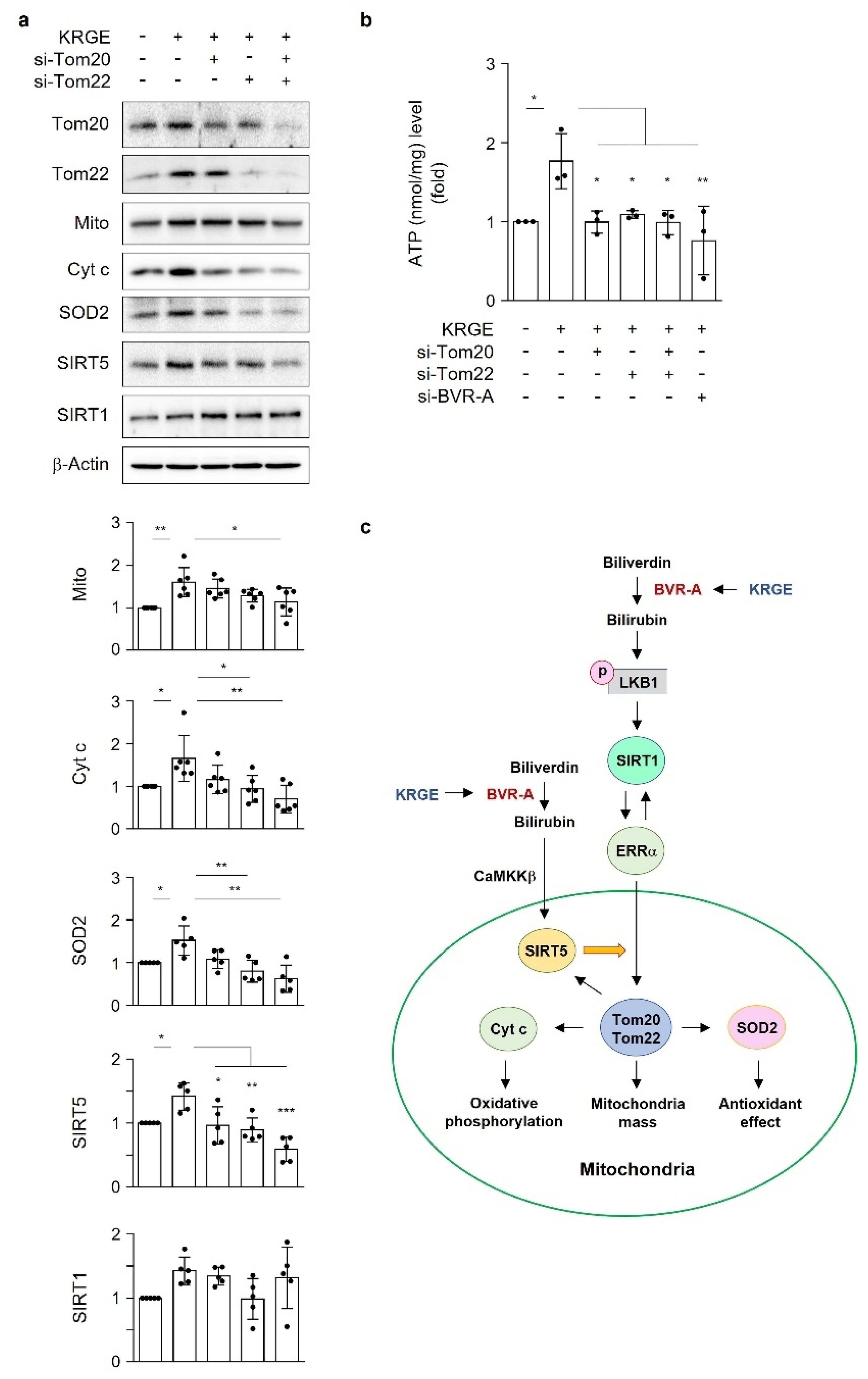
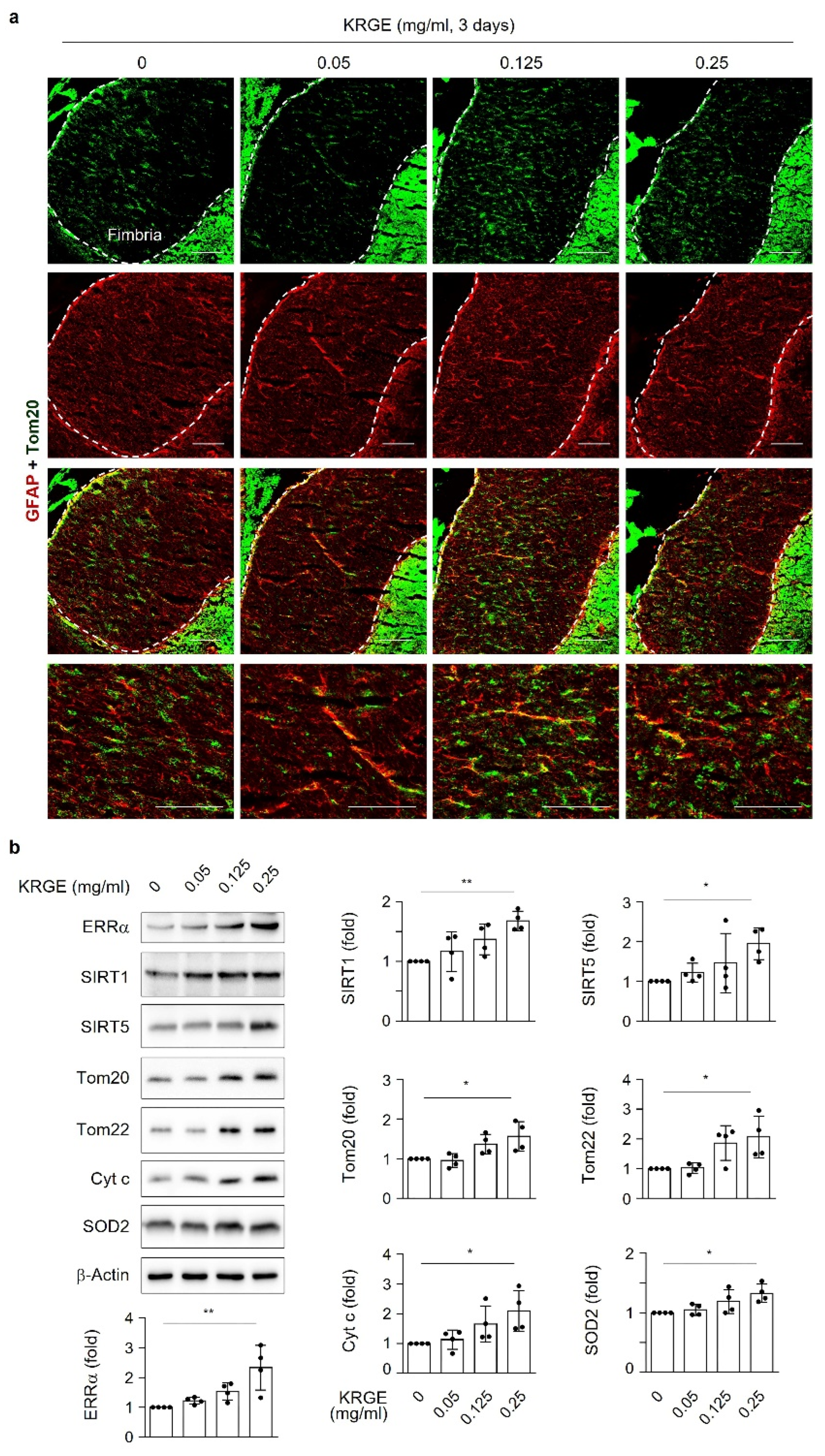
Here, we investigate the roles of KRGE in mitochondrial functions (i.e., mitochondrial membrane potential, mitochondrial mass, and expression of OXPHOS, Tom20, Tom22, cytochrome c (Cyt c), and SOD2) under physiological conditions. Our novel findings show that KRGE induces BVR-A expression in astrocytes in vitro and hippocampal astrocytes, especially in the fimbria region, in vivo. BVR-A-derived bilirubin is associated with KRGE-induced mitochondrial function changes via the LKB1–SIRT1–ERRα pathway. The interplay between cytosolic localization of SIRT1 and mitochondrial localization of SIRT5 may facilitate mitochondrial functions by regulating Tom20 and Tom22, consequently upregulating Cyt c and SOD2. In addition, the combined functions of Tom20 and Tom22 facilitate SIRT5 expression. Taken together, these results indicate that KRGE-induced BVR-A/bilirubin is critical for astrocytic mitochondrial function.
2. Materials and Methods
2.1. Materials
We obtained mannitol, sucrose, MOPS, and ethylene-diamine-tetra acetic acid (EDTA) from Sigma-Aldrich (St. Louis, MO, USA). Fetal bovine serum (FBS) was obtained from HyClone Laboratories (Omaha, NE, USA). The proteinase inhibitor cocktail was purchased from Thermo Fisher Scientific (Waltham, MA, USA). The SIRT activity inhibitor nicotinamide (NAM) was purchased from Merck Millipore (Darmstadt, Germany). Bilirubin, biliverdin, CORM-2 ([Ru(CO)3Cl2]2, a CO-releasing compound), and FeCl2 were purchased from Sigma-Aldrich. Bilirubin powder was dissolved in 0.1 M NaOH to make a 50 mM stock solution and stored at −25 °C in the dark. CORM-2 (100 mM stock) and biliverdin (50 mM stock) were dissolved in DMSO (Sigma-Aldrich) and stored at −75 °C. KRGE was obtained from the Korea Ginseng Corporation and stored at 4 °C. Stock solutions were prepared by filtering distilled water. The aliquoted stock solutions (250 mg/mL) were stored at −25 °C in the dark.
2.2. Cell Culture
Primary human brain astrocytes were acquired from Applied Cell Biology Research Institute (Kirkland, WA, USA). Astrocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM, HyClone, Omaha, NE, USA) supplemented with 10% FBS (Corning, NY, USA) and 1% penicillin/streptomycin (HyClone).
2.3. Treatment of Cells with Reagents
Astrocytes were cultured to reach a density of 80%, and the medium was changed to 0% FBS (serum-free) DMEM for 3 h. Then, 50 µM bilirubin, 50 µM biliverdin, 50 µM CORM-2, or 50 µM FeCl2 was added for 4 h.
2.4. Transfection
At 70% confluency, astrocytes were transfected with small interfering RNAs (siRNAs) against SIRT1, SIRT5, ERRα, HO-1, LKB1, Ca2+/calmodulin-dependent protein kinase kinase α (CaMKKβ), Tom20, Tom22, BVR-A (50 nM, Santa Cruz Biotechnology, Dallas, TX, USA), or a negative control (50 nM; Thermo Fisher Scientific) using RNAiMax (Thermo Fisher Scientific). After approximately 16 h of recovery, cells were incubated with or without KRGE for 24 h in serum-free DMEM.
2.5. MitoTracker Staining
Intracellular active mitochondrial levels were measured by quantitative fluorescence imaging using the mitochondrial membrane potential-sensitive dye, MitoTracker
® Deep Red FM (Thermo Fisher Scientific). Astrocytes plated on 25 mm round coverslips in 6-well plates were cultured until they reached 80% confluence. The medium was changed to serum-free DMEM for 3 h. Cells were then treated with 50 µM HO metabolites (biliverdin, CORM-2 (CO), or FeCl
2 (Fe
2+)) and 50 µM bilirubin for 4 h. After 3 h of starvation, astrocytes were incubated with 5 mM NAM for 30 min and 50 µM bilirubin for 3.5 h, followed by 0.5 µM MitoTracker-Red (Thermo Fisher Scientific) for 0.5 h. Phosphate-buffered saline (PBS) was used for washing the cells. Fluorescent images of live cells were obtained using a confocal microscope (Olympus FV1000), and the intensity was obtained and analyzed using ImageJ (
https://rsb.info.nih.gov/ij/ accessed on 1 March 2022). The average intensity of five randomly chosen cells from each image was determined.
2.6. Mitochondria-Enriched Fraction
Cells in four 100 mm dishes were used for each group. Water or 0.5 mg/mL KRGE was added for 24 h, and the cells were washed with PBS and collected using mannitol–sucrose buffer containing 210 mM mannitol, 70 mM sucrose, 5 mM MOPS, 1 mM EDTA, and a protease inhibitor cocktail at pH 7.4. After the collected cells were homogenized (with a 27-gauge 1/2-inch needle, the homogenates were centrifuged at 800× g for 10 min at 4 °C to pellet the nuclei. The supernatants were used as total lysates or centrifuged at 10,000× g for 20 min at 4 °C to collect mitochondria-enriched fraction pellets. The pellets were washed with mannitol–sucrose buffer and centrifuged at 10,000× g for 20 min at 4 °C, and the supernatants were filtered with a 3 kDa centrifugal filter (Merck Millipore, Darmstadt, Germany) at 4000× g for 2 h (cytosolic protein). The mitochondria-enriched fraction (pellet) was suspended in 30 µL Protein Extraction Solution (RIPA buffer; Elpis-Biotech, Daejeon, South Korea), incubated for 20 min on ice, and centrifuged at 15,000× g for 20 min at 4 °C. Mitochondrial protein (10 µg, supernatant solution) and cytosolic protein (10 µg) were subjected to Western blotting after quantification using Pierce BCA protein assay reagent (Thermo Fisher Scientific). Antibodies directed against the following proteins were used: SIRT1, SIRT5, Tom20, Tom22, SOD2, voltage-dependent anion channel 1 (Santa Cruz Biotechnology), β-actin (Sigma-Aldrich), and Cyt c (BD Pharmingen).
2.7. Immunocytochemistry
For ERRα immunocytochemistry, human astrocytes were seeded on coverslips in 12-well (Falcon) incubation plates. Astrocytes were fixed in 4% formaldehyde for 20 min at 23 ± 2 °C, washed gently with 0.1% (v/v) Tween 20 in PBS for 10 min each, blocked with 3% bovine serum albumin (BSA) (USBiological Life Sciences, Salem, MA, USA), diluted in 0.1% Triton X-100 (Sigma-Aldrich) in PBS for 1 h at room temperature, and incubated with the ERRα primary antibody (1:150, Santa Cruz Biotechnology) overnight at 4 °C, followed by incubation with an Alexa Fluor antibody (1:600, Thermo Fisher Scientific). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific). Images were obtained using a confocal microscope (Olympus FV1000; Tokyo, Japan). For SOD2 and Tom20 immunoreactivity, samples were incubated with rabbit anti-SOD2 (1:300, Santa Cruz Biotechnology) and mouse anti-Tom20 (1:500, Abcam, Cambridge, UK) overnight at 4 °C. After washing, the secondary antibody was diluted in 0.3% BSA and added to each well. The samples were incubated with a mixture of tetramethylrhodamine (TRITC)-conjugated donkey immunoglobulin G (IgG) (1:300; Jackson ImmunoResearch, West Grove, PA, USA) and fluorescein isothiocyanate (FITC)-conjugated donkey IgG (1:300; Jackson ImmunoResearch, West Grove, PA, USA) for 1 h at room temperature. After the final wash, the cells were mounted on microslides using Fluoro-Gel II with DAPI mounting solution (Electron Microscopy Sciences, Hatfield, PA, USA). The upper side of the coverslips was reversed to allow for attachment to the DAPI mounting solution. Images were acquired using an inverted microscope (Eclipse Ti2-U; Nikon, Tokyo, Japan).
2.8. Detection of Oxidative Phosphorylation
Protein (10–15 µg) from the cell lysates (lysed by RIPA) was combined with sodium dodecyl sulfate (SDS) sample buffer (10% glycerol (v/v), Tris-Cl pH 6.8, 2% SDS (w/v), 1% β-mercaptoethanol (v/v), and bromophenol blue) and heated at 37 °C for 5 min. Protein samples were then subjected to Western blot analysis. OXPHOS antibody (1:8000; Abcam, Cambridge, UK) was used. The OXPHOS antibody detects the oxidative phosphorylation complex I (NDUFB8, NADH: ubiquinone oxidoreductase subunit B8), complex II (succinate dehydrogenase complex iron-sulfur subunit B), complex III (UQCRC2, ubiquinol-cytochrome c reductase core protein 2), complex IV (MTCO1, cytochrome c oxidase subunit I), and complex V (ATP5A, ATP synthase).
2.9. Western Blot Analysis
RIPA buffer was used for cell lysis. Brain tissue samples were obtained from bregma −1.1 to −1.3, and cells were lysed in RIPA buffer. Selected amounts of protein from the cell lysates were combined with SDS sample buffer (10% glycerol (v/v), Tris-Cl pH 6.8, 2% SDS (w/v), 1% β-mercaptoethanol (v/v), and bromophenol blue) and incubated at 100 °C for 5 min. Protein samples were then separated using SDS polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes (MilliporeSigma, Burlington, MA, USA), and blocked using Tris-buffered saline containing 0.1% Tween 20 and 5% skim milk (BD Difco, BD, Franklin Lakes, NJ, USA). The membranes were incubated with primary antibodies at 4 °C overnight. The primary antibodies used in this study were as follows: Tom20 (1:1000, Santa Cruz Biotechnology, Dallas, TX, USA; 1:5000, Abcam), Tom22 (1:1000, Santa Cruz Biotechnology), Cyt c (1:3000, BD Pharmingen, San Jose, CA, USA), SIRT1 (1:1000, Santa Cruz Biotechnology), SIRT2 (1:5000, Abcam), SIRT3 (1:1000, Santa Cruz Biotechnology), SIRT4 (1:3000, Thermo Fisher Scientific), SIRT5 (1:1000, Santa Cruz Biotechnology), ERRα (1:3000, Novus Biologicals, Littleton, CO, USA), p-LKB1 (Ser428) (1:2000, Cell Signaling Technology, Danvers, MA, USA), LKB1 (1:2000, Cell Signaling Technology), CaMKKβ (1:1000, Santa Cruz Biotechnology), AMPKα (1:2000, Cell Signaling Technology), p-AMPKα (Thr172) (1:2000, Cell Signaling Technology), SOD2 (1:1000, Santa Cruz Biotechnology), mitochondria (1:10,000, Abcam), HO-1 (1:2000, BD Biosciences, Franklin Lakes, NJ, USA), BVR-A (1:1000, Santa Cruz Biotechnology; 1:3000, Enzo Life Sciences, Farmingdale, NY, USA), and anti-β-actin (1:8000, Sigma-Aldrich). After washing, the membranes were incubated with peroxidase-conjugated secondary antibodies (Thermo Fisher Scientific) and visualized using enhanced chemiluminescence (Elpis-Biotech) with the appropriate Western blot detection equipment (Fusion Solo, Vilber, Collégien, France).
2.10. Cellular Reactive Oxygen Species (ROS) Detection
For ROS detection, human astrocytes were seeded into 12-well plates (Falcon, Glendale, CA, USA) and transfected with specific siRNAs. After approximately 16 h, the cells were treated with water or 0.5 mg/mL KRGE for 23.5 h in serum-free DMEM media. Then, the astrocytes were treated with 20 μM H2DCFDA (Thermo Fisher Scientific) for 30 min at 23 ± 2 °C. Finally, the cells were washed gently with PBS for 10 min. Images were captured using an inverted microscope (Eclipse Ti2-U; Nikon).
2.11. Intracellular ATP Levels
An ATP colorimetric assay kit (Bio-Vision, Milpitas, CA, USA) was used for measuring intracellular ATP levels. Human astrocytes cultured on a 60 mm dish (Falcon) were transfected with indicated siRNAs and exposed to water or 0.5 mg/mL KRGE for 24 h in serum-free DMEM media. After trypsinization, the cells were transferred to Eppendorf tubes. The cell pellet was lysed in 120 μL of ATP assay buffer and incubated for 5 min at room temperature. The tubes were centrifuged at 15,000 rpm for 5 min at 4 °C. Next, the supernatant (50 μL) was mixed with reaction mixture reagents (50 μL) on 96-well plates (Falcon). After a 30 min incubation with blocking light at room temperature, absorbance was measured at 570 nm (Epoch Microplate Spectrophotometer, BioTek, Santa Clara, CA, USA). Lysed cells were quantified with BCA reagents (Thermo Fisher Scientific, Carthage, MO, USA). ATP levels and protein amount (nmol ATP/mg protein) in the control group were set as “1”, and the other groups were adjusted to the control group.
2.12. Animals and Brain Tissue Immunohistochemistry
Male C57BL/6 mice were purchased from Joong Ah Bio Inc. (Suwon, South Korea) and maintained under standard conditions, with water and food available ad libitum. KRGE (0.05, 0.125, or 0.25 mg/mL) was administered via the drinking water for 3 days. The control group was administered only water. For histological analysis, mice were anesthetized using isoflurane (1.5%) and N2O gas and then transcardially perfused with saline. Using an optimal cutting temperature compound, frozen brain tissues were sliced into 20 μm sections on a cryostat (HM525 NX, Thermo Fisher Scientific). The sections were incubated with 4% paraformaldehyde for 15 min, washed three times in PBS, and then incubated with 3% BSA for 1 h. The sections were then incubated with rabbit anti-Tom20 (1:300, Abcam), mouse anti-glial fibrillary acidic protein (GFAP; 1:300, BD Bioscience), and anti-BVR-A (1:200, Enzo Life Sciences) antibodies in 0.1% Triton X-100 in PBS at 4 °C overnight. After washing, the sections were incubated in a mixture of TRITC-conjugated donkey anti-rabbit IgG (1:300, Jackson ImmunoResearch) and FITC-conjugated donkey anti-mouse IgG (1:300, Jackson ImmunoResearch) for 1 h at room temperature. Between incubations, tissues were washed with 0.1% Tween 20 in PBS. The sections were visualized using a mounting solution (Fluoro-Gel II with DAPI; Electron Microscopy Sciences). The stained sections were examined under an inverted phase-contrast microscope (Eclipse Ti2eU, Nikon).
2.13. Data Analysis
Quantification of the intensity of protein expression in Western blotting and immunocytochemistry was performed using ImageJ. GraphPad Prism 6 was used for statistical analyses. Multiple comparisons were evaluated using one-way analysis of variance (ANOVA) and Tukey’s post hoc test (mean ± standard deviation).
4. Discussion
KRGE plays a role in health prophylaxis; however, the molecular mechanisms of active mitochondrial conditions induced by KRGE have not been well established. Our findings suggest that KRGE stimulates astrocytic mitochondrial function partly through bilirubin production. BVR-A can produce bilirubin from biliverdin, which is an HO metabolite. Compared to biliverdin, bilirubin has a more potent effect on mitochondrial membrane potential and mass as well as ERRα activation, consequently enhancing the expression of OXPHOS, Cyt c, and SOD2. SOD2 exists in homotetramers and localizes to the mitochondrial matrix, regulating mitochondrial oxidative stress [
22]. KRGE may have antioxidant effects by upregulating the levels of bilirubin, a strong antioxidant, and, consequently, suppressing undesirable oxidative stress through SOD2.
Canto and Auwerx [
23] suggest that AMPK, SIRT1, and PGC-1α act as an orchestrated network to improve metabolic fitness and control energy expenditure. Based on this research, we expanded our study to signaling proteins such as ERRα, LKB1, Tom20, Tom22, and SIRT1-5, which are related to mitochondrial functions. The energy metabolism kinase LKB1 helps maintain ATP levels and is critical for neuronal survival following mitochondrial dysfunction [
19]. LKB1-deficient astrocytes exhibit impaired metabolic function and enhanced inflammatory activation [
18]. Under physiological circumstances, KRGE activates the LKB1–SIRT1–ERRα pathway, thereby stimulating Tom20/Tom22 complex-mediated SIRT5 expression. SIRT5 augments KRGE-induced SIRT1–ERRα signaling. Our results show that the SIRT1/SIRT5 combination is more potent in inducing the expression of mitochondrial proteins such as Cyt c, SOD2, Tom20, and Tom22 than SIRT1 alone. In this study, we found that bilirubin activated LKB1, leading to discrete SIRT1 expression in astrocytes. This signaling pathway was dependent on BVR-A-induced bilirubin generation in KRGE-treated astrocytes.
We previously found that CO/R-induced HO-1 initially increases intracellular Ca
2+ concentrations by activating L-type voltage-gated Ca
2+ channels, subsequently leading to CaMKKβ-mediated AMPKα activation [
8]. This signaling pathway promotes the expression of hypoxia-inducible factor-1α (HIF-1α), PGC-1α, ERRα, and the vascular endothelial growth factor (VEGF) [
8,
24]. However, in the present study, KRGE-stimulated BVR-A induction promoted ATP production but did not alter AMPKα phosphorylation. Instead, the BVR-A/bilirubin pathway enhanced LKB1 phosphorylation and consequently increased the levels of SIRT1, Mito, Cyt c, and SOD2. CaMKKβ in KRGE-treated astrocytes appeared to facilitate LKB1-mediated changes in mitochondrial function. The combination of LKB1 and CaMKKβ synergistically regulated KRGE-induced SIRT5, Cyt c, SOD2, Tom20, and Tom22 levels. Because KRGE also stimulates the CaMKKβ–AMPKα pathway, we cannot rule out a possible influence of the Ca
2+–CaMKKβ–AMPKα–PGC-1α pathway on mitochondrial functions. Additionally, our previous study demonstrated that KRGE improves astrocytic mitochondrial functions such as mitochondrial O
2 consumption and ATP generation by triggering the HO-1-HIF-1α signaling cascade [
1]. In the current study, BVR-A did not alter KRGE-induced HO-1 protein levels. Thus, we speculated that KRGE stimulates multiple signaling pathways involved in mitochondrial biogenesis and activity. Thus, our next study will include KRGE-induced Ca
2+ signaling and its effects on HIF-1α, VEGF, PGC-1α, and AMPKα.